Abstract
Trypanosoma cruzi infection in dogs can cause heart failure and sudden death with few treatment options available. A litter of 4 dogs living in a T cruzi endemic area were randomized to prophylaxis and nonprophylaxis groups as part of a study evaluating a modified benznidazole dosing regimen administered twice weekly to prevent T cruzi infection during a vector transmission season. The 2 dogs that received prophylaxis remained healthy without T cruzi infection or cardiac disease for >2 years. One dog that did not receive prophylaxis died unexpectedly with acute T cruzi–induced pancarditis, and the second dog tested positive for T cruzi and developed complex arrhythmias with markedly increased cardiac troponin I and improved with a higher benznidazole treatment dose. Although the small sample size precludes definitive conclusions, we describe the potential clinical benefit of prophylactic and early treatment with modified benznidazole dosing regimens for dogs with T cruzi infection.
Keywords: Chagas disease, myocarditis, prophylaxis, sudden death, troponin
Abbreviations
- Ct
cycle threshold I
- cTnI
cardiac troponin I
- DTU
discrete typing unit
- FETCH
Functional Evaluation of Cardiac Health
- IFA
indirect fluorescent antibody
- PCR
polymerase chain reaction
1. INTRODUCTION
Chagas disease is a zoonotic parasitic infection caused by the protozoan hemoflagellate, Trypanosoma cruzi, and transmitted by triatomine insect vectors, commonly known as “kissing bugs.” Infection with T cruzi can cause cardiomyopathy, heart failure, and sudden death in dogs and is endemic in the southern United States and the temperate areas of southern South America. 1 , 2 , 3 , 4 Benznidazole, a nitroimidazole prodrug with trypanocidal activity, is routinely used to treat T cruzi infection in humans. Administration of benznidazole at a dosage of 5 to 7 mg/kg PO q12h for 30 to 60 days has been the standard treatment for humans and dogs, with variable effects reported. 5 , 6 , 7 , 8 , 9 Improvements in parasitological cure rates were shown using a contemporary modified treatment regimen of less frequent (twice weekly), higher dose (approximately 20 mg/kg) benznidazole in mice, dogs, and macaques. 10 We recently evaluated benznidazole as prophylaxis by administering approximately 10 mg/kg twice weekly for 24 weeks during an insect vector transmission season when vectors are most active. 11 Although initial attempts did not prevent T cruzi infection, the potential clinical benefits of the prophylactic modified dosing protocol were not the objective of the study.
Therefore, from 2021 to 2023, we monitored clinical and clinicopathological findings in a litter of 4 English Cocker spaniels enrolled in a study to evaluate the modified benznidazole prophylactic regimen. 11 One of the dogs received a modified treatment regimen of benznidazole. The dogs were born to 2 dogs with chronic asymptomatic T cruzi infection in a private multidog kennel environment in south Texas, an area known for intense transmission pressure and increased risk of exposure to T cruzi. 12
At enrollment (visit 1), congenital infection was ruled out by negative real‐time blood PCR, negative T cruzi serology using indirect fluorescent antibody (IFA) assay, and no response on Luminex‐based multiplex serology performed on all 4 puppies at 9.1 weeks of age. The dogs were randomly assigned to prophylaxis and nonprophylaxis groups with 2 dogs in each group. All 4 dogs apparently were healthy with a functional evaluation of cardiac health (FETCH) score of 0 indicating the absence of clinical signs. 13 All 4 dogs were maintained in the same kennel with exposure to outdoors throughout the study period.
The dogs were reevaluated at as many as 6 time points: visit 2 (dogs 1‐4, 2.5 months after enrollment), visit 3 (dogs 1, 2, and 4, 3.3 months after enrollment), visit 4 (dog 4, 4.2 months after enrollment; 0.9 months after starting treatment), visit 5 (dogs 1, 2, and 4, 5.3 months after enrollment; 2.0 months after starting treatment for dog 4), visit 6 (dog 4, 10.3 months after enrollment; 7.0 months after starting treatment), and visit 7 (dogs 2 and 4, 16.4 months after enrollment; 13.1 months after starting treatment for dog 4). During these visits, a FETCH score was recorded, and some of the following diagnostic tests were performed based on the clinical evaluation of each dog, as described in detail below, including cardiac auscultation, SNAP 4Dx Plus testing (IDEXX, Westbrook, Maine) for detection of Dirofilaria immitis antigen, and antibodies to Ehrlichia canis, E ewingii, Borrelia burgdorferi, Anaplasma phagocytophilum, and A platys, serum biochemistry panel, serum cardiac troponin I (cTnI) concentration (ADVIA Centaur TnI‐Ultra assay, Siemens Medical Solutions USA, Inc, Malvern, Pennsylvania, USA), echocardiogram, 5‐minute standard ECG, and 24‐hour ambulatory ECG. Tests for T cruzi infection included real‐time blood PCR for T cruzi DNA with discrete typing unit (DTU), a classification of the T cruzi parasite genotype, if PCR‐positive based on cycle threshold (Ct) value <35 cycles, 10 IFA (Texas A&M Veterinary Medical Diagnostic Lab, College Station, TX), and Luminex‐based multiplex immunoassay to detect antibodies. 10
2. CASE HISTORY
2.1. Prophylaxis group: Dogs 1 and 2
Dog 1, an 11.1 kg female, and dog 2, a 12.6 kg male, were randomly assigned to receive a prophylactic schedule of 125 mg of benznidazole (11.2 and 9.9 mg/kg, respectively) administered as a 125 mg capsule PO twice weekly for 24 weeks throughout the predicted exposure season for transmission of T cruzi from May to October 2021. At visit 2, both dogs were blood PCR negative for T cruzi with FETCH scores of 0. Serum cTnI concentrations were 0.068 ng/mL for dog 1 and < 0.006 ng/mL for dog 2 (reference range, <0.128 ng/mL). 14 At visit 3, both dogs had FETCH scores of 0 with normal cardiac auscultation, normal biochemistry panel results, negative T cruzi IFA and SNAP 4Dx Plus results, normal sinus rhythm with normal complex morphology and intervals on a 5‐minute ECG, and normal echocardiogram results. On a 24‐hour ambulatory ECG, dog 1 had rare supraventricular premature beats, and dog 2 had no abnormalities detected. At visit 5, both dogs had FETCH scores of 0 and remained blood PCR negative with serum cTnI concentrations <0.006 ng/mL. At visit 7, both dogs had FETCH scores of 0. Only dog 2 was available for examination and was normal on cardiac auscultation, had negative blood PCR and serology (IFA, Luminex) results, serum cTnI concentration <0.006 ng/mL, normal sinus rhythm with normal complex morphology and intervals on a 5‐minute ECG, and normal echocardiogram results. Two years after enrollment, both dogs were alive and clinically well.
2.2. Nonprophylaxis group: Dog 3
Dog 3, a 12.6 kg male, was randomly assigned to the nonprophylaxis control group. At visit 2, the FETCH score was 0 and blood PCR was strongly positive (Ct value 14 cycles) with DTU cardiac troponin I (TcIV). Serum cTnI concentration was increased at 2.59 ng/mL. The dog died suddenly 10 days after visit 2 before the investigators received the PCR and cTnI results. Histopathologic evaluation of the heart, performed by a board‐certified veterinary pathologist, showed large amounts of inflammation multifocally infiltrating the myocardium, occasionally extending to the endocardial and epicardial surfaces. The inflammation consisted of plasma cells, lymphocytes, and histiocytes. Scattered cardiomyocytes contained cytoplastic pseudocysts filled with abundant amastigotes of T cruzi. These structures measured approximately 2 μm in diameter and had central nuclei with occasionally visible, perpendicularly oriented kinetoplasts. In heavily inflamed regions, cardiomyocyte necrosis and edema were present with scattered small foci of mineralization. Diffuse moderate congestion of the lungs was observed. No abnormal findings were noted in other organs. The histopathologic diagnosis was severe, multifocal to coalescing, acute, lymphohistiocytic, necrotizing pancarditis with intralesional amastigotes, morphologically consistent with T cruzi. These findings confirmed a diagnosis of acute Chagas disease because the infection was identified within 3 months of confirming a negative status.
2.3. Nonprophylaxis group treatment: Dog 4
Dog 4, a 14.3 kg male, was randomly assigned to the untreated, control group. At visit 2, dog 4 had a FETCH score of 0, a markedly increased serum cTnI concentration of 14.88 ng/mL and positive blood PCR for T cruzi (Ct value 18 cycles) with DTU TcIV. A comprehensive diagnostic evaluation was scheduled. At visit 3, the FETCH score remained 0, and cardiac auscultation disclosed a heart rate of 70 beats/min, grade 2/6 left apical systolic and right mid‐heart murmurs, and an irregular heart rhythm. Serum biochemistry panel results were normal, SNAP 4Dx Plus test results were negative, and T cruzi antibody titer by IFA was positive at 1:1280. A 5‐min standard ECG recording disclosed frequent, multiform ventricular premature beats occurring as singles and in a bigeminal pattern. A 24‐hour ambulatory ECG identified frequent multiform ventricular arrhythmias occurring as singles, in couplets, in a bigeminal pattern, and in short paroxysms of ventricular tachycardia (Table 1). Also noted were a period of sinus arrest with escape beats and a short period of supraventricular tachycardia followed by sinus arrest and rare supraventricular premature beats. Echocardiographic findings included normal chamber size, normal ventricular systolic function, and mild tricuspid valve regurgitation with normal regurgitant jet velocity. Once the test results were compiled, the dog was started on benznidazole at a dosage of 17.5 mg/kg PO twice weekly for 12 months. At visits 4 to 6, the FETCH scores remained 0 with decreased, but still positive, blood PCR at visits 5 (Ct value 29 cycles) and 6 (Ct value 28 cycles), and with progressive continued decreases in serum cTnI concentration of 1.27, 0.506, and 0.08 ng/mL, respectively (Figure 1). Also at visit 4, echocardiographic findings included normal chamber size, normal ventricular systolic function, and no valve regurgitation. A 24‐hour ambulatory ECG disclosed a similar percentage of ventricular ectopic beats with rare single supraventricular premature beats compared with the previous evaluation. However, the severity was improved based on a higher number of singles and fewer number of couplets and runs of ventricular tachycardia. At visit 7, the FETCH score remained 0, and no murmurs or arrhythmias were noted, blood PCR remained positive (Ct value 26 cycles), IFA titer was 1:320, and serum cTnI concentration remained low at 0.081 ng/mL. Echocardiographic findings remained normal. A 24‐hour ambulatory ECG identified even fewer ventricular arrhythmias occurring mainly as singles with rare couplets, rare runs of ventricular tachycardia, and occasional single supraventricular premature beats. Two years after enrollment, dog 4 was alive without clinical signs.
TABLE 1.
Selected 24‐hour ambulatory ECG (Holter) results obtained for dog 4.
| Parameter | Visit 3 | Visit 4 | Visit 7 |
|---|---|---|---|
| Total heart beats | 127 159 | 148 410 | 140 087 |
| Ventricular ectopic beats | 11 296 | 13 048 | 1944 |
| Ventricular ectopic beats as percent of total heart beats | 9% | 9% | 1% |
| Ventricular ectopic beats—singlets | 7812 | 12 356 | 1924 |
| Ventricular ectopic beats—couplets | 531 | 106 | 4 |
| Ventricular tachycardia runs | 372 | 100 | 3 |
| Supraventricular ectopic beats—singlets | 2 | 21 | 308 |
Note: A modified treatment dose regimen of benznidazole was started after testing positive for Trypanosoma cruzi at visit 2.
FIGURE 1.
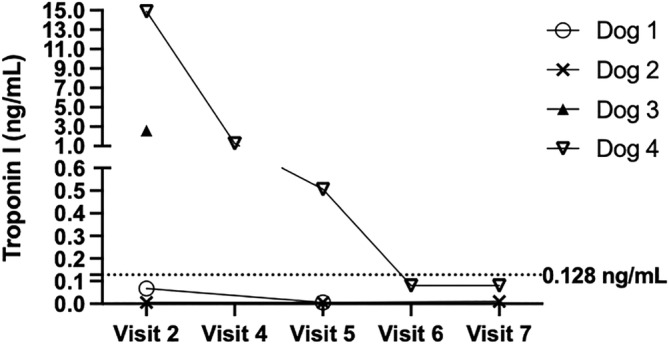
Serum cardiac troponin I results for the 4 dogs. Dogs 1 and 2 were in the prophylaxis group, dog 3 was in the nonprophylaxis group with death after visit 2 and dog 4 was in the nonprophylaxis group and subsequently started on a modified treatment dose regimen of benznidazole. The upper limit of the reference range (0.128 ng/mL) is represented by the dotted line.
3. DISCUSSION
We describe the clinical findings in a litter of 4 dogs, 3 of which received modified dosing regimens of benznidazole for prophylaxis or early treatment of T cruzi infection. These dogs were exposed to the same management practices and environment, including pressure from triatomine vectors, thus presenting a semicontrolled natural experiment to discover clinical outcomes with benznidazole treatment. Notably, the 2 dogs that received benznidazole prophylaxis for T cruzi infection did not become infected or develop evidence of heart disease. In addition, in both dogs that received prophylaxis, serum cTnI concentration, a marker of myocardial damage, remained low. Conversely, the 2 dogs that did not receive prophylaxis developed severe acute T cruzi infections; 1 dog died suddenly, and the other had markedly increased serum cTnI concentration and complex arrhythmias despite FETCH scores of 0 in both. These findings highlight the potential challenge of using the absence of clinical signs when monitoring for the development of Chagas disease. Ventricular arrhythmias in dogs with Chagas disease and cTnI concentration >1.0 ng/mL have been associated with decreased survival times and poor prognosis. 3 , 15 A treatment regimen of benznidazole using the modified dosing protocol did not result in parasitic cure in dog 4 but was associated with decreased serum cTnI concentration from severely increased to normal concentrations and marked improvement in ventricular arrhythmias (ie, improved total number and complexity of ventricular arrhythmias without antiarrhythmic treatment). These findings are consistent with previous findings in experimentally infected dogs and in infected humans treated with benznidazole, where the parasite may not be completely eliminated but potential clinical benefits include limiting myocardial fibrosis, cardiac abnormalities, and disease progression. 5 , 9 , 16
Standard benznidazole dosing regimens in experimentally infected dogs have been shown to limit but not prevent myocardial damage and ECG abnormalities while decreasing parasitic load in some dogs. 5 , 6 , 7 Treatment initiated soon after inoculation in experimentally infected dogs prevented or decreased fibrosis in the right atrium, prolongation of the PR interval, and other ECG abnormalities typically observed in infected dogs. 5 Although the observed trypanocidal effects and limitation of cardiac damage, ECG, and echocardiographic abnormalities are encouraging, none of the studies has reported clinical outcomes related to progression of heart failure, mortality rates, survival time, or quality of life in affected dogs. 5 , 17 , 18 Investigation into the role of benznidazole treatment for T cruzi infection in naturally infected dogs has been limited partly because of the lack of availability of the medication, knowledge of which animals will benefit from treatment, and lack of veterinary awareness of Chagas disease. 4 , 17 Consequently, the role of benznidazole treatment for T cruzi infection in dogs has not been well established, and treatment options remain limited.
The objective of using a modified dosing protocol is to administer benznidazole at a higher dose for a longer duration of time to treat actively replicating and dormant parasites. 10 , 11 , 19 In mice, a modified dosing regimen with weekly or twice weekly administration of benznidazole at a dose 2.5 to 5 times the standard daily dose successfully eradicated established T cruzi infection. 19 A similar regimen also cured a proportion of dogs and nonhuman primates. 10 Our recent study investigated the prophylactic use of a similar protocol for preventing T cruzi infection in dogs. 11 Although the attempts did not prevent new infection, the positive clinical outcomes observed in dogs in our report demonstrate potential clinical benefits of the modified dosing protocol. Given the increased understanding of the severe burden that T cruzi places on dogs, the risk of sudden death, and the poor prognosis with acute myocarditis, and chronic cardiomyopathy, further investigation of benznidazole incorporated into preventative care may be worthy of future study in dogs at high risk of T cruzi infection living in or entering an endemic area. Similarly in veterinary medicine, routine administration of macrocyclic lactones is recommended for eliminating Dirofilaria immitis infection. In human medicine, preexposure or postexposure chemoprophylaxis is a feasible strategy for the presumptive treatment of some endemic parasites in immigrants and travelers. 20 , 21 The use of chemoprophylaxis generally is based on the high risk of acquiring infection, high clinical impact of infection, low ecological impact, high cost‐effectiveness, and low frequency of adverse drug effects. 21 Administration of benznidazole can cause adverse effects in humans including hypersensitivity dermatitis, gastrointestinal upset, polyneuritis, and rarely bone marrow suppression and hepatopathy. 22 In dogs, dose‐dependent neurologic signs have been reported at dosages >20 mg/kg/day. 23 The modified dosing regimens of benznidazole administered twice weekly were not associated with adverse effects in the 3 dogs in our study or in dogs in previous studies. 10 , 11
Dog 4 had persistently PCR‐positive blood, but with a marked decrease in blood parasite burden as evidenced by increasing Ct values that coincided with benznidazole treatment. The failure of this treatment to achieve parasitological cure may relate to the markedly low initial Ct value potentially signaling a very high infectious dose. In a recent work in similar multidog kennel environments in central and south Texas, most serologically positive T cruzi–infected dogs were negative on blood PCR tests. 12 The blood parasite burden likely varies based on initial inoculation dose, time since infection, and route of transmission. For example, PO transmission in children, potentially an important route for dogs, can inoculate the patient with a higher dose of the parasite compared with contact with infected insect vector feces, posing a higher risk of acute myocarditis associated with a more efficient route of infection. 24
The complexity of T cruzi infection related to parasite load, strain, and duration of time infected combined with variable host immune response leads to unpredictable outcomes and persistent challenges in identifying successful antiparasitic treatments. Modified treatment protocols, such as that described here, and combination treatment are options being further explored. 17 , 25 , 26
In conclusion, prophylactic and early benznidazole treatment using a modified dosing regimen may not prevent or cure T cruzi infection but potentially can ameliorate the clinical impact of T cruzi infection by decreasing morbidity and mortality related to cardiac disease, and such treatment was well tolerated by the dogs of our study. The extremely small sample size and descriptive nature of the study preclude definitive conclusions, and a larger study to evaluate modified dosing protocols of benznidazole is necessary to clarify the potential clinical benefits of these protocols in dogs, particularly for those in high‐risk environments.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by Texas A&M University IACUC, number 2018‐0460.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
Funding provided by a grant from the American Veterinary Medical Foundation (AVMF) and Veterinary Pharmacology Research Foundation (VPRF), a Clinical and Translational Science (CTSA) Pilot Award supported by NIH Award: 1UL1TR003163‐01A1, and the George Robinson Foundation. The authors thank the kennel owner and dog manager who cared for the dogs and supported the study of Chagas disease, as well as the Mundo Sano Foundation for donation of benznidazole, Madeline Droog, PharmD at Texas A&M University's Small Animal Veterinary Medical Teaching Hospital Pharmacy for formulating benznidazole capsules, Erin Edwards, DVM, MS, DACVP at Texas A&M Veterinary Medical Diagnostic Laboratory for assistance with histopathology, Lisa Auckland for assistance with PCR, Elizabeth Malcolm, DVM and Briana Wilson. MS, VMD for their assistance with study data management, and Kristen Flitcroft and Cyneen Santoya for their cardiology technical support.
Lim S, Collins S, Hamer SA, Tarleton RL, Saunders AB. Positive clinical outcome using a modified dosing regimen of benznidazole in dogs at high risk for infection or acutely infected with Trypanosoma cruzi . J Vet Intern Med. 2024;38(3):1725‐1729. doi: 10.1111/jvim.17028
REFERENCES
- 1. Barr SC. Canine Chagas' disease (American trypanosomiasis) in North America. Vet Clin North Am Small Anim Pract. 2009;39:1055‐1064. [DOI] [PubMed] [Google Scholar]
- 2. Hamer SA, Saunders AB. Veterinary Chagas disease (American trypanosomiasis) in the United States. Vet Clin North Am Small Anim Pract. 2022;52:1267‐1281. [DOI] [PubMed] [Google Scholar]
- 3. Matthews DJ, Saunders AB, Meyers AC, Gordon SG, Hamer SA. Cardiac diagnostic test results and outcomes in 44 dogs naturally infected with Trypanosoma cruzi . J Vet Intern Med. 2021;35:1800‐1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gavic EA, Achen SE, Fox PR, et al. Trypanosoma cruzi infection diagnosed in dogs in nonendemic areas and results from a survey suggest a need for increased Chagas disease awareness in North America. J Am Vet Med Assoc. 2023;261:1‐8. [DOI] [PubMed] [Google Scholar]
- 5. Caldas IS, da Matta Guedes PM, dos Santos FM, et al. Myocardial scars correlate with eletrocardiographic changes in chronic Trypanosoma cruzi infection for dogs treated with benznidazole. Trop Med Int Health. 2013;18:75‐84. [DOI] [PubMed] [Google Scholar]
- 6. Santos FM, Mazzeti AL, Caldas S, et al. Chagas cardiomyopathy: the potential effect of benznidazole treatment on diastolic dysfunction and cardiac damage in dogs chronically infected with Trypanosoma cruzi . Acta Trop. 2016;161:44‐54. [DOI] [PubMed] [Google Scholar]
- 7. Cunha ELA, Torchelsen FKVDS, Cunha LM, et al. Benznidazole, itraconazole and their combination in the treatment of acute experimental chagas disease in dogs. Exp Parasitol. 2019;204:107711‐107719. [DOI] [PubMed] [Google Scholar]
- 8. Bern C, Messenger LA, Whitman JD, Maguire JH. Chagas disease in the United States: a public health approach. Clin Microbiol Rev. 2019;33:e00023‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hasslocher‐Moreno AM, Saraiva RM, Sangenis LHZ, et al. Benznidazole decreases the risk of chronic Chagas disease progression and cardiovascular events: a long‐term follow up study. E Clin Med. 2023;31:100694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bustamante JM, White BE, Wilkerson GK, et al. Frequency variation and dose modification of benznidazole administration for the treatment of Trypanosoma cruzi infection in mice, dogs, and nonhuman primates. Antimicrob Agents Chemother. 2023;67:e0013223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bustamante JM, Padilla AM, White B, et al. Prophylactic low‐dose, bi‐weekly benznidazole treatment fails to prevent Trypanosoma cruzi infection in dogs under intense transmission pressure. PLoS Negl Trop Dis. 2022;16:e0010688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Busselman RE, Meyers A, Zecca I, et al. High incidence of Trypanosoma cruzi infections in dogs directly detected through longitudinal tracking at 10 multi‐dog kennels, Texas, USA. PLoS Negl Trop Dis. 2021;15:e0009935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freeman LM, Rush JE, Farabaugh AE, Must A. Development and evaluation of a questionnaire for assessing health‐related quality of life in dogs with cardiac disease. J Am Vet Med Assoc. 2005;226:1864‐1868. [DOI] [PubMed] [Google Scholar]
- 14. Winter RL, Saunders AB, Gordon SG, et al. Analytical validation and clinical evaluation of a commercially available high‐sensitivity immunoassay for the measurement of troponin I in humans for use in dogs. J Vet Cardiol. 2014;16:81‐89. [DOI] [PubMed] [Google Scholar]
- 15. Fonfara S, Loureiro J, Swift S, James R, Cripps P, Dukes‐McEwan J. Cardiac troponin I as a marker for severity and prognosis of cardiac disease in dogs. Vet J. 2010;184:334‐339. [DOI] [PubMed] [Google Scholar]
- 16. Machado‐de‐Assis GF, Diniz GA, Montoya RA, et al. A serological, parasitological and clinical evaluation of untreated Chagas disease patients and those treated with benznidazole before and thirteen years after intervention. Mem Inst Oswaldo Cruz. 2013;108:873‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Lana M, Giunchetti RC. Dogs as a model for chemotherapy of Chagas disease and leishmaniasis. Curr Pharm des. 2021;27:1741‐1756. [DOI] [PubMed] [Google Scholar]
- 18. Santos FM, Lima WG, Gravel AS, et al. Cardiomyopathy prognosis after benznidazole treatment in chronic canine Chagas' disease. J Antimicrob Chemother. 2012;67:1987‐1995. [DOI] [PubMed] [Google Scholar]
- 19. Bustamante JM, Sanchez‐Valdez F, Padilla AM, White B, Wang W, Tarleton RL. A modified drug regimen clears active and dormant trypanosomes in mouse models of Chagas disease. Sci Transl Med. 2020;12:e0010688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Noack S, Harrington J, Carithers DS, Kaminsky R, Selzer PM. Heartworm disease—overview, intervention, and industry perspective. Int J Parasitol Drugs Drug Resist. 2021;16:65‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stauffer WM, Cantey PT, Montgomery S, et al. Presumptive treatment and medical screening for parasites in refugees resettling to the United States. Curr Infect Dis Rep. 2013;15:222‐231. [DOI] [PubMed] [Google Scholar]
- 22. Viotti R, Vigliano C, Lococo B, et al. Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Rev Anti Infect Ther. 2009;7:157‐163. [DOI] [PubMed] [Google Scholar]
- 23. Flores‐Vieira CLL, Barreira AA. Experimental benznidazole encephalopathy: I. clinical‐neurological alterations. J Neurol Sci. 1997;150:3‐11. [DOI] [PubMed] [Google Scholar]
- 24. Noya BA, Gonzalez ON. An ecological overview on the factors that drives to Trypanosoma cruzi oral transmission. Acta Trop. 2015;151:94‐102. [DOI] [PubMed] [Google Scholar]
- 25. Cunha ELA, Torchelsen FKVDS, Fonseca KDS, et al. Benznidazole, itraconazole, and their combination for the treatment of chronic experimental Chagas disease in dogs. Exp Parasitol. 2022;238:108266. [DOI] [PubMed] [Google Scholar]
- 26. Lascano F, Bournissen FG, Altcheh J. Review of pharmacological options for the treatment of Chagas disease. Br J Clin Pharmacol. 2022;88:383‐402. [DOI] [PubMed] [Google Scholar]


