Abstract
Progressive carcinogenesis of a gastric polyp with transformation to gastric adenocarcinoma and subsequent development of leptomeningeal carcinomatosis is described in an adult male Scottish terrier. Presenting clinical signs consisted of vomiting with intermittent hematemesis. Surgical biopsies over the course of 14 months documented the progression from gastric polyp to minimally invasive gastric carcinoma to invasive gastric adenocarcinoma, a pathogenesis not previously documented in veterinary oncology. The patient ultimately developed neurologic pathology and was euthanized, and necropsy evaluation identified widespread carcinomatosis with accompanying leptomeningeal metastasis. As in humans, gastric polyps in dogs rarely have malignant potential.
Keywords: carcinogenesis, carcinoma, dog, stomach
Abbreviations
- cm
centimeter
- GC
gastric carcinoma
- LMC
leptomeningeal carcinomatosis
- mm
millimeter
1. INTRODUCTION
Gastric carcinoma (GC) is a highly aggressive neoplasm with a devastating clinical course in both humans and dogs and ranks as a leading cause of cancer‐related death in humans. 1 Gastric carcinoma has often progressed to an advanced stage at the time of presentation with more than half of human patients 2 and 60% to 75% of dogs 3 , 4 having detectable metastases. Despite treatment with surgery and chemotherapy, overall prognosis remains poor with 5‐year survival rates of only 25% to 30% in the Western world. 5 , 6 , 7 The prognosis of GC in dogs is similarly poor, with a median survival time of 6 months after surgical removal with or without adjuvant chemotherapy. 8 Naturally occurring GC in dogs recapitulates many aspects of GC in humans. Aggressive local disease, high rates of nodal metastasis, and similar treatment options make dogs a potentially clinically relevant translational model for GC in humans, and highlight the need to further understand the pathogenesis of this disease in dogs. 9
Gastric carcinogenesis in humans is multifactorial and remains incompletely understood. Several distinct risk factors for development of gastric carcinoma have been described, including hereditary factors, specific sporadic mutations, smoking, alcohol consumption, diets high in salt and fats, and Helicobacter pylori infection. 10 , 11 In humans, males and people of Eastern Asian heritage are overrepresented. 12 Although breed predispositions and a male predilection are recognized, the etiology of GC in dogs is similarly complex and poorly defined. 9 , 13 , 14 In contrast to humans, the role of Helicobacter species in the gut mucosa driving the pathogenesis of GC has not been firmly established in dogs. 15 , 16 Furthermore, although GC has been reported in association with hypertrophic gastritis (Ménétrier‐like disease) in dogs, 17 , 18 gastric polyps have been previously found to lack features suggestive of neoplastic transformation. 19 , 20 In dogs, gastric polyps are considered benign without progression or predisposition towards neoplasia, 19 , 20 , 21 whereas they are recognized as an important risk factor for development of GC in humans. 22 , 23 , 24 , 25
Leptomeningeal carcinomatosis (LMC) is an uncommon end‐stage disease involving the pia mater and arachnoid mater (meninges) documented in various types of cancers in humans. The incidence of LMC in humans with GC is relatively low, occurring in 0.16% to 0.69% of GC patients, compared with an incidence of 12% to 34% in breast cancers and 10% to 26% in lung cancers. 26 , 27 , 28 However, cases of LMC secondary to GC have been noted with increased frequency coinciding with more effective locoregional therapeutic options. 28 , 29 Leptomeningeal carcinomatosis in humans often is treated by intrathecal chemotherapy, but prognosis remains poor with a median survival time of 6 to 8 weeks from the time of LMC diagnosis. 30 , 31 Reports of LMC in the veterinary literature are limited to rare reports of disseminated mammary carcinoma, colonic carcinoma, and 1 report of unknown primary origin. 32 , 33 , 34 , 35
Herein we document malignant transformation of a gastric polyp and the first reported case of LMC secondary to GC in a dog.
2. CASE HISTORY
A 6‐year‐old, male castrated, Scottish terrier dog was evaluated for a 3‐month history of intermittent emesis and occasional hematemesis. Physical examination identified no clinically relevant abnormalities. Complete blood count and serum biochemistry results were within normal reference ranges aside from a previously documented increase in alkaline phosphatase activity (721 U/L; reference range, 20‐155 U/L). Abdominal ultrasound examination identified a thickened gastric wall extending from the lesser curvature of the stomach, pyloric thickening, and hyperechoic peri‐gastric fat. A barium study further defined thickened rugal folds at the body of the stomach and thickening of the pyloric sphincter. The patient was treated with outpatient gastrointestinal supportive care using maropitant citrate (Cerenia, Zoetis Inc, Kalamazoo, MI) and famotidine (Aurobindo Pharma Limited, Mahabubnagar, India).
Repeat abdominal ultrasound examination and endoscopy were performed 2 months later because of persistent clinical signs, and detected a gastric mass extending from the mucosal layer of the antrum of the stomach (Figure 1A). Gross appearance of the surface of the mass was similar to the adjacent gastric mucosa and the mass was partially freely movable with a serpentine portion fixed to the mucosa (Figure 1B). Endoscopic and subsequent excisional biopsy identified hyperplastic gastric glandular epithelium overlying a dense fibrous connective tissue stroma with abundant lymphoplasmacytic and lesser eosinophilic infiltrates, consistent with the diagnosis of gastric polyp with complete excision on histopathologic examination (Figure 2A). Histopathology of samples collected from the nearby stomach mucosa indicated moderate to severe chronic lymphocytic, plasmacytic, and eosinophilic gastritis, and a duodenal biopsy sample was consistent with inflammatory bowel disease. Histopathologic evaluation of a gastric lymph node collected at the time of polyp excision identified lymphoid hyperplasia, consistent with chronic antigenic stimulation. The liver was also biopsied, and histopathologic evaluation did not identify any clinically relevant lesions. Evidence of neoplasia was not present in any of the tissues examined. All clinical signs resolved after surgical removal of the gastric polyp and no new health concerns were reported over the next year.
FIGURE 1.
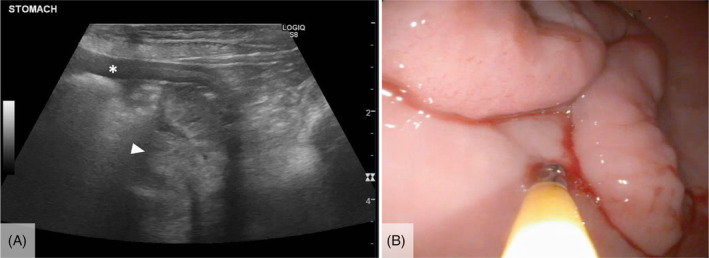
Abdominal ultrasound (A) and endoscopic (B) images of a serpentine gastric mass that was subsequently removed via gastrectomy and determined to be a gastric polyp on excisional biopsy. The gastric wall (asterisk) and gastric mass (arrowhead) on the ultrasonographic image (A) are identified.
FIGURE 2.
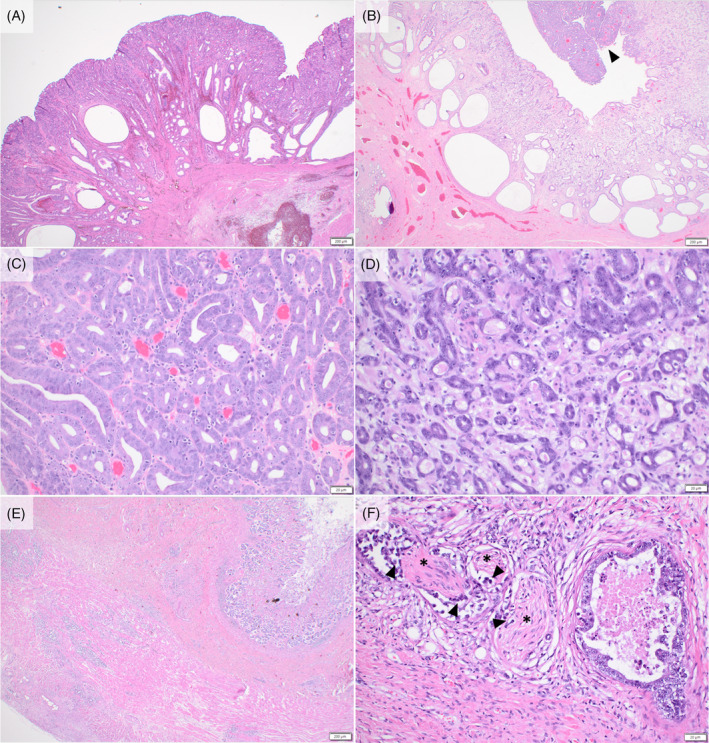
Histopathologic progression of gastric lesions. The initial mass excised from the stomach was a benign gastric polyp (A). A mass in the region a year later had similar morphology (B) with a clear focus on neoplastic cells (arrowhead). Neoplastic cells form irregular tubules with increased nuclear: cytoplasmic ratio, loss of polarity, prominent nucleoli, and increased mitotic activity (C). Revisional surgery of the site 1 month later revealed noticeably greater pleomorphism (D). On necropsy, the neoplastic cells were present throughout all levels of the gastric wall and serosa (E). Histologically, neoplastic cells were frequently seen within lymphatic vessels along with perineural invasion (arrowheads) of the nerves (asterisks) (F).
One year after surgical excision of the gastric polyp, the dog was presented for recurrent vomiting, hematemesis, and weight loss. Abdominal ultrasound examination identified a 3‐cm diameter, broad‐based gastric mass over the lesser curvature of the stomach near the site of the prior polyp removal. Surgical excision by partial gastrectomy and histopathology initially resulted in a diagnosis of incompletely excised gastric carcinoma in situ, but deeper recuts of the sample later showed a small focus of submucosal invasion with a revised diagnosis of gastric adenocarcinoma with minimal invasion (Figure 2B,C). Postoperative staging was recommended, and thoracic radiographs and abdominal ultrasound examination indicated no evidence of tumor recurrence or metastasis. Because of residual microscopic disease, a revision surgery was performed to achieve wider surgical margins 1 month later, and a 2 cm × 6 cm diameter mass overlying the previous surgical site was noted intraoperatively and removed. This mass was diagnosed as gastric adenocarcinoma with complete but narrow surgical margins and lymphatic invasion (Figure 2D).
The patient received adjuvant chemotherapy consisting of carboplatin (Eugia Pharma Specialities Limited, Medchal‐Malkajgiri District, India) and 5‐fluorouracil (5‐FU, Fresenius Kabi, Lake Zurich, IL), given concurrently every 3 weeks for a total of 6 treatments as previously described. 36 Overall, chemotherapy was well tolerated with low‐grade gastrointestinal and hematologic toxicity. 37 Serial abdominal ultrasonography and thoracic radiography during and after chemotherapy indicated no evidence of cancer recurrence or metastasis until 5 months after chemotherapy completion (approximately 10 months after surgery and diagnosis of gastric adenocarcinoma) when pancreaticoduodenal lymphadenopathy (9 mm × 5 mm), gastric lymphadenopathy (5 mm × 3 mm), and a suspected peritoneal metastatic lesion (hypoechoic nodule in the left cranial abdomen, 19 mm × 11 mm) were noted. Because of presumed metastasis, toceranib phosphate (Palladia, Pfizer, Ascoli, Italy, UK) was initiated at 2.8 mg/kg PO every Monday, Wednesday, and Friday. Three months after initiation of toceranib, the lymph nodes and peritoneal lesion were stable in size on ultrasound examination, but new findings included splenomegaly. Because the patient continued to experience a good quality of life as perceived by the owner, toceranib treatment was continued.
Five months after initiation of toceranib, the dog developed nonweight bearing lameness of the right forelimb that progressed to shifting forelimb lameness and cognitive decline over the course of 2 weeks. Neurologic consultation confirmed dull mentation, ataxia, hemiparesis, head bob, weak gag reflex, asymmetric optic nerve size, and spinal discomfort, resulting in a multifocal primarily left‐sided brainstem localization with consideration to C1‐T2 and T3‐L3 involvement. Euthanasia was elected and a necropsy was performed 841 days after the initial diagnosis of gastric polyp and 406 days after the diagnosis of invasive gastric carcinoma.
On necropsy, a region of the lesser curvature of the stomach was grossly thickened and pale tan with reddened serosa. Histologically, this region contained frequent aggregates of carcinoma cells forming rare tubules with dense desmoplasia and lymphovascular and perineural invasion (Figure 2E,F). Several thin fibrous adhesions extended between loops of small intestine and from the intestine to the body wall. The parietal peritoneum, diaphragm, and abdominal viscera had several white‐to‐tan plaques of carcinomatosis. Multiple mesenteric and other intraabdominal lymph nodes were expanded by firm, pale tan nodules of metastatic carcinoma, with similar nodules in the lungs. The spleen was diffusely small and irregular with near complete infarction (Figure 3A) and multiple foci of metastasis. Histologically, the vascular and lymphatic vessels to the spleen were nearly obliterated by neoplastic cells. Of note, abundant tumor cells infiltrated the perineurium of nerves accompanying the affected splenic vessels (Figure 3B) as well as nerves and vessels of the gastrosplenic ligament. Examination of the central nervous system identified numerous aggregates of carcinoma throughout the leptomeninges, including over the cerebrum, cerebellum, brainstem, and the entire length of the spinal cord (Figure 4A–D). Spirochete bacteria, such as Helicobacter spp., were not identified histologically on any biopsy or necropsy sections.
FIGURE 3.
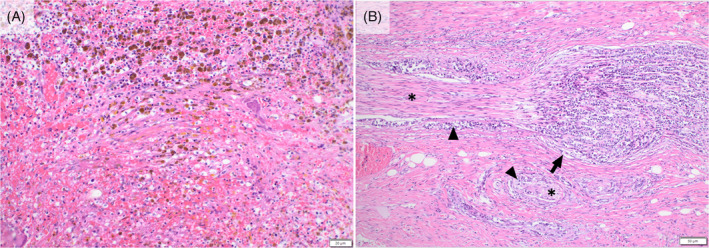
The spleen at necropsy demonstrated near complete coagulative necrosis with frequent hemosiderophages, presumed secondary to vascular occlusion by neoplastic cells (A). Vessels and nerves (asterisks) entering the spleen are infiltrated (arrowheads) to nearly obliterated (arrow) by neoplastic cells (B).
FIGURE 4.
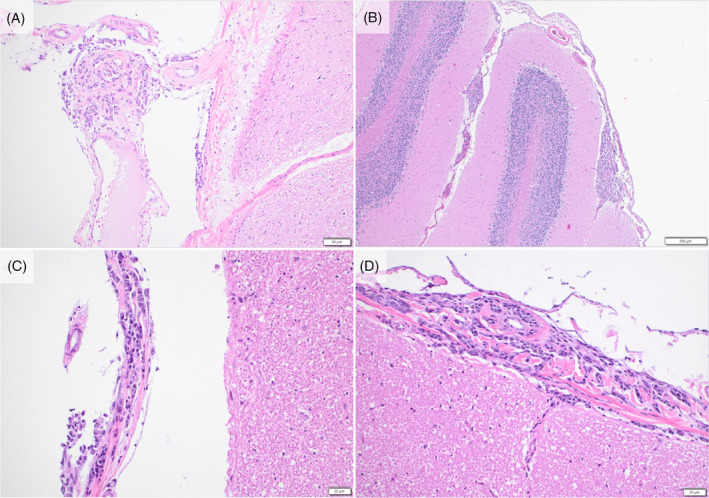
Gastric adenocarcinoma in the leptomeninges over the cerebrum (A), cerebellum (B), brainstem (C), and spinal cord (D) at necropsy.
3. DISCUSSION
Progression from mucosal hyperplasia to malignancy is described in several types of cancers, but an association between gastric polyps and neoplasia has not been previously described in dogs. This case documents progression of a gastric polyp to minimally‐invasive GC to GC in a dog over 14 months. Previously, gastric polyps have been considered proliferative and hyperplastic lesions that lack potential for neoplastic transformation. 19 Surgery generally is considered curative, and although recurrence or additional lesions may develop, progression to GC and development of metastasis have not been reported previously. 20 , 21 Our case report indicates that gastric polyps in dogs have malignant potential, similar to their counterpart in humans, and future studies to define appropriate treatment and monitoring of gastric polyps in dogs should be considered.
The role of chemotherapy in the management of GC in dogs is unclear, but adjuvant chemotherapy has been found to be positively associated with survival after surgical resection. 8 Because of the high metastatic potential, adjuvant chemotherapy with carboplatin and 5‐FU, a protocol with described activity against a variety of carcinomas in dogs, was recommended in this case. 36 The use of apatinib, a small molecule inhibitor targeting vascular endothelial growth factor receptor 2 (VEGFR2), is well described in humans with advanced GC. 38 , 39 Toceranib blocks the function of several receptor tyrosine kinases, including VEGFR2, and has documented clinical activity against various types of carcinomas in dogs. 40 Therefore, subsequent treatment with toceranib was initiated after cancer progression. Although our patient experienced prolonged survival after the diagnosis of GC, the clinical benefit of adjuvant chemotherapy is unclear and there remains no widely accepted standard of care chemotherapy for metastatic GC in veterinary patients.
Another unusual feature of our case was the extensive neurotropism in the stomach and spleen noted at the time of necropsy. Neoplastic invasion of nerves, described as perineural invasion in the human medical literature, is a feature associated with poor patient outcome in various carcinomas, including increased risk of local recurrence, decreased disease‐free interval, and decreased survival time. 41 Perineural invasion is increasingly recognized as a distinct pathophysiologic process involving cellular signaling and interactions between the neoplasm and the cells of the nervous tissues, particularly microglia. 42 , 43 Infiltration into nerves allows extension of the neoplasm beyond grossly visible tumor margins, and in some cases, perineural invasion may lead to retrograde spread from peripheral nerves into the central nervous system. 44
The presence of small foci of neoplastic cells distributed throughout the leptomeninges of the entire central nervous system in our patient is consistent with LCM. Leptomeningeal carcinomatosis occurs when a carcinoma that has entered the central nervous system seeds throughout the leptomeninges, similar to pleural and peritoneal carcinomatosis. This end‐stage manifestation of metastatic disease occurs with increased frequency in some cancers of humans, particularly in mammary and pulmonary carcinomas, although it has rarely been reported secondary to GC. 29 , 45 , 46 Reports of LCM are exceedingly rare in veterinary medicine, with few cases reported in dogs 32 , 34 , 35 and other species. 47 , 48 Ours is the first documentation of LMC of a GC in a dog.
4. CONCLUSION
This case helps advance our current understanding of GC pathogenesis by documenting malignant transformation of a gastric polyp to minimally invasive gastric carcinoma, and ultimately, invasive gastric carcinoma with multiorgan and leptomeningeal metastasis. It is possible that an association between gastric polyps and GC exists in dogs, but may be unrecognized because of late‐stage diagnosis. Evaluation of the transformative potential of gastric polyps and underlying gastric inflammation in the predisposition to gastric cancer in dogs, as occurs in human medicine, is needed. Our case indicates the neoplastic potential of a diagnosis that previously has been considered benign and provides rationale for future investigations of the frequency with which polyps may transform, because such data may alter the therapeutic approach to such patients.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
No funding was received for this study.
Didehvar DS, Lanza MR, Atherton MJ, Lenz JA. Malignant transformation and subsequent leptomeningeal carcinomatosis of a gastric polyp in a dog. J Vet Intern Med. 2024;38(3):1744‐1750. doi: 10.1111/jvim.17072
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Deng JY, Liang H. Clinical significance of lymph node metastasis in gastric cancer. World J Gastroenterol. 2014;20:3967‐3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Swann HM, Holt DE. Canine gastric adenocarcinoma and leiomyosarcoma: a retrospective study of 21 cases (1986‐1999) and literature review. J Am Anim Hosp Assoc. 2002;38:157‐164. [DOI] [PubMed] [Google Scholar]
- 4. Sullivan M, Lee R, Fisher EW, Nash A, McCandlish I. A study of 31 cases of gastric carcinoma in dogs. Vet Rec. 1987;120:79‐83. [DOI] [PubMed] [Google Scholar]
- 5. Verdecchia A, Francisci S, Brenner H, et al. Recent cancer survival in Europe: a 2000‐02 period analysis of EUROCARE‐4 data. Lancet Oncol. 2007;8:784‐796. [DOI] [PubMed] [Google Scholar]
- 6. American Cancer Society . Cancer Facts & Figures 2022. Atlanta, GA: American Cancer Society; 2022. [Google Scholar]
- 7. Ajani JA, D'Amico TA, Bentrem DJ, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:167‐192. [DOI] [PubMed] [Google Scholar]
- 8. Abrams B, Wavreille VA, Husbands BD, et al. Perioperative complications and outcome after surgery for treatment of gastric carcinoma in dogs: a Veterinary Society of Surgical Oncology retrospective study of 40 cases (2004‐2018). Vet Surg. 2019;48:923‐932. [DOI] [PubMed] [Google Scholar]
- 9. Hugen S, Thomas RE, German AJ, Burgener IA, Mandigers PJJ. Gastric carcinoma in canines and humans, a review. Vet Comp Oncol. 2017;15:692‐705. [DOI] [PubMed] [Google Scholar]
- 10. Gonzalez CA, Lujan‐Barroso L, Bueno‐de‐Mesquita HB, et al. Fruit and vegetable intake and the risk of gastric adenocarcinoma: a reanalysis of the European Prospective Investigation into Cancer and Nutrition (EPIC‐EURGAST) study after a longer follow‐up. Int J Cancer. 2012;131:2910‐2919. [DOI] [PubMed] [Google Scholar]
- 11. Wadhwa R, Song S, Lee JS, Yao Y, Wei Q, Ajani JA. Gastric cancer‐molecular and clinical dimensions. Nat Rev Clin Oncol. 2013;10:643‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69‐90. [DOI] [PubMed] [Google Scholar]
- 13. Hardas A, Suarez‐Bonnet A, Beck S, et al. Canine gastric carcinomas: a histopathological and Immunohistochemical study and similarities with the human counterpart. Animals. 2021;11:1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seim‐Wikse T, Jorundsson E, Nodtvedt A, et al. Breed predisposition to canine gastric carcinoma – a study based on the Norwegian canine cancer register. Acta Vet Scand. 2013;55:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amorim I, Smet A, Alves O, et al. Presence and significance of Helicobacter spp. in the gastric mucosa of Portuguese dogs. Gut Pathogens. 2015;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taillieu E, Chiers K, Amorim I, et al. Gastric helicobacter species associated with dogs, cats and pigs: significance for public and animal health. Vet Res. 2022;53:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Munday JS, Aberdein D, Cullen GD, French AF. Menetrier disease and gastric adenocarcinoma in 3 Cairn terrier littermates. Vet Pathol. 2012;49:1028‐1031. [DOI] [PubMed] [Google Scholar]
- 18. Lecoindre P, Bystricka M, Chevallier M, Peyron C. Gastric carcinoma associated with Menetrier's‐like disease in a West Highland white terrier. J Small Anim Pract. 2012;53:714‐718. [DOI] [PubMed] [Google Scholar]
- 19. Flores AR, Lemos I, Rema A, et al. Tn and Sialyl‐Tn antigens in canine gastric tissues. Vet Comp Oncol. 2020;18:615‐625. [DOI] [PubMed] [Google Scholar]
- 20. Amorim I, Taulescu MA, Ferreira A, et al. An immunohistochemical study of canine spontaneous gastric polyps. Diagn Pathol. 2014;9:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taulescu MA, Valentine BA, Amorim I, et al. Histopathological features of canine spontaneous non‐neoplastic gastric polyps – a retrospective study of 15 cases. Histol Histopathol. 2014;29:65‐75. [DOI] [PubMed] [Google Scholar]
- 22. Hu H, Zhang Q, Chen G, Pritchard DM, Zhang S. Risk factors and clinical correlates of neoplastic transformation in gastric hyperplastic polyps in Chinese patients. Sci Rep. 2020;10:2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joao M, Areia M, Alves S, et al. Gastric hyperplastic polyps: a benign entity? Analysis of recurrence and neoplastic transformation in a cohort study. GE Port J Gastroenterol. 2021;28:328‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Orlowska J, Kupryjanczyk J. Malignant transformation of gastric hyperplastic polyps. Am J Clin Pathol. 2002;117:165‐166. [PubMed] [Google Scholar]
- 25. Imura J, Hayashi S, Ichikawa K, et al. Malignant transformation of hyperplastic gastric polyps: an immunohistochemical and pathological study of the changes of neoplastic phenotype. Oncol Lett. 2014;7:1459‐1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim M. Intracranial involvement by metastatic advanced gastric carcinoma. J Neurooncol. 1999;43:59‐62. [DOI] [PubMed] [Google Scholar]
- 27. Kim NH, Kim JH, Chin HM, Jun KH. Leptomeningeal carcinomatosis from gastric cancer: single institute retrospective analysis of 9 cases. Ann Surg Treat Res. 2014;86:16‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tomita H, Yasui H, Boku N, et al. Leptomeningeal carcinomatosis associated with gastric cancer. Int J Clin Oncol. 2012;17:361‐366. [DOI] [PubMed] [Google Scholar]
- 29. Lee JL, Kang YK, Kim TW, et al. Leptomeningeal carcinomatosis in gastric cancer. J Neurooncol. 2004;66:167‐174. [DOI] [PubMed] [Google Scholar]
- 30. Bulut G, Erden A, Karaca B, et al. Leptomeningeal carcinomatosis of gastric adenocarcinoma. Turk J Gastroenterol. 2011;22:195‐198. [DOI] [PubMed] [Google Scholar]
- 31. Oh SY, Lee SJ, Lee J, et al. Gastric leptomeningeal carcinomatosis: multi‐center retrospective analysis of 54 cases. World J Gastroenterol. 2009;15:5086‐5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mandara MT, Rossi F, Lepri E, Angeli G. Cerebellar leptomeningeal carcinomatosis in a dog. J Small Anim Pract. 2007;48:504‐507. [DOI] [PubMed] [Google Scholar]
- 33. Behling‐Kelly E, Petersen S, Muthuswamy A, Webb JL, Young KM. Neoplastic pleocytosis in a dog with metastatic mammary carcinoma and meningeal carcinomatosis. Vet Clin Pathol. 2010;39:247‐252. [DOI] [PubMed] [Google Scholar]
- 34. Mateo I, Lorenzo V, Munoz A, et al. Meningeal carcinomatosis in a dog: magnetic resonance imaging features and pathological correlation. J Small Anim Pract. 2010;51:43‐48. [DOI] [PubMed] [Google Scholar]
- 35. Stampley ASD, Prasse K. Meningeal carcinomatosis secondary to a colonic signet‐ring cell carcinoma in a dog. J Am Anim Hosp Assoc. 1987;23:655‐658. [Google Scholar]
- 36. Menard K, Flesner BK, Glahn A, Boudreaux B, Bryan JN. Concurrent 5‐fluorouracil and carboplatin for the treatment of canine carcinomas. Vet Comp Oncol. 2018;16:590‐595. [DOI] [PubMed] [Google Scholar]
- 37. LeBlanc AK, Atherton M, Bentley RT, et al. Veterinary Cooperative Oncology Group‐Common Terminology Criteria for Adverse Events (VCOG‐CTCAE v2) following investigational therapy in dogs and cats. Vet Comp Oncol. 2021;19:311‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scott LJ. Apatinib: a review in advanced gastric cancer and other advanced cancers. Drugs. 2018;78:747‐758. [DOI] [PubMed] [Google Scholar]
- 39. Li J, Qin S, Wen L, et al. Safety and efficacy of apatinib in patients with advanced gastric or gastroesophageal junction adenocarcinoma after the failure of two or more lines of chemotherapy (AHEAD): a prospective, single‐arm, multicenter, phase IV study. BMC Med. 2023;21:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. London C, Mathie T, Stingle N, et al. Preliminary evidence for biologic activity of toceranib phosphate (Palladia([R])) in solid tumours. Vet Comp Oncol. 2012;10:194‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379‐3391. [DOI] [PubMed] [Google Scholar]
- 42. Cavel O, Shomron O, Shabtay A, et al. Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor. Cancer Res. 2012;72:5733‐5743. [DOI] [PubMed] [Google Scholar]
- 43. Chen SH, Zhang BY, Zhou B, Zhu CZ, Sun LQ, Feng YJ. Perineural invasion of cancer: a complex crosstalk between cells and molecules in the perineural niche. Am J Cancer Res. 2019;9:1‐21. [PMC free article] [PubMed] [Google Scholar]
- 44. Raleigh JS, Lanza MR, Perry JA. Apocrine gland anal sac adenocarcinoma with perineural metastasis in a cat. JFMS Open Rep. 2018;4(2):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee HG, Lee B, Kim SM, Suh BJ, Yu HJ. A case of gastric adenocarcinoma presenting as meningeal carcinomatosis. Korean J Intern Med. 2007;22:304‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Park KK, Yang SI, Seo KW, Kim YO, Yoon KY. A case of metastatic leptomeningeal carcinomatosis from early gastric carcinoma. World J Surg Oncol. 2012;10:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Posporis C, Grau‐Roma L, Travetti O, Oliveira M, Polledo L, Wessmann A. Meningeal carcinomatosis and spinal cord infiltration caused by a locally invasive pulmonary adenocarcinoma in a cat. JFMS Open Rep. 2017;3(2):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wright JA, Giles CJ. Diffuse carcinomatosis involving the meninges of a horse. Equine Vet J. 1986;18:147‐150. [DOI] [PubMed] [Google Scholar]


