Abstract
Smoldering multiple myeloma (SMM) is an asymptomatic precursor to multiple myeloma. Here we define the epidemiological characteristics of SMM in the general population in Iceland. The iStopMM study (ClinicalTrials.gov ID: NCT03327597) is a nationwide screening study for multiple myeloma precursors where all residents in Iceland 40 years or older were invited to participate. SMM was defined as 10–60% bone marrow plasma cells and/or monoclonal (M) protein concentration ≥3 g dl−1, in the absence of myeloma-defining events. Of the 80,759 who gave informed consent to participate, 75,422 (93%) were screened. The prevalence of SMM in the total population was 0.53% (95% confidence interval (CI) = 0.49–0.57%) in individuals 40 years or older. In men and women, the prevalence of SMM was 0.67% (95% CI = 0.62–0.73%) and 0.39% (95% CI = 0.35–0.43%), respectively; it increased with age in both sexes. For the 193 individuals with SMM, median age was 70 years (range 44–92 years) and 60% were males. The mean M protein concentration of individuals with SMM was 0.62 g dl−1 (range 0.01–3.5 g dl−1) and 73% had 11–20% bone marrow plasma cell infiltration. The high prevalence of SMM has implications for future treatment policies in multiple myeloma as the evidence supporting treatment initiation at the SMM stage is emerging.
First identified in a small case series published in 1980, smoldering multiple myeloma (SMM) is defined as an asymptomatic precursor condition to multiple myeloma1. The diagnostic criteria for SMM requires the presence of 10–59% plasma cell infiltration in the bone marrow and/or monoclonal (M) protein concentration in serum >3 g dl−1, in the absence of myeloma-defining events2. SMM is distinguished from monoclonal gammopathy of undetermined significance (MGUS) by a much higher risk of progression to multiple myeloma, with an average risk of progression of 10% annually for the first 5 years after diagnosis3. The prevalence of SMM in the general population has not been investigated because this requires screening the population with blood samples and bone marrow biopsies. The incidence of SMM has previously been estimated based on incidentally diagnosed SMM in the Swedish Cancer Registry where the incidence of SMM was 0.44 cases per 100,000 individuals4. However, because only a minority of SMM cases progress to multiple myeloma per year, it is unlikely that SMM is less common than multiple myeloma, which has an incidence of around 5 cases per 100,000 individuals, and this might therefore be an underestimation5.
SMM is generally an asymptomatic condition that is usually detected incidentally when individuals seek healthcare because of other unrelated symptomatic conditions. Once an individual is diagnosed with SMM, most current clinical guidelines recommend risk stratification to determine the risk of progression from SMM to multiple myeloma and thereafter lifelong monitoring for progression to multiple myeloma6. Several risk stratification models have been proposed in the literature7–11. For example, the Programa de Estudio y Tratamiento de las Hemopatías Malignas (PETHEMA) criteria stratify patients into low-risk, intermediate-risk and high-risk groups based on suppression (versus normal levels) of uninvolved immunoglobulins (referred to as immunoparesis) and the percentage of aberrant (versus normal) plasma cells in the bone marrow according to multiparameter flow cytometry7. The Mayo Clinic 2/20/20 risk stratification model incorporates three factors (serum M protein >2 g dl−1, involved to uninvolved free light chain (FLC) ratio >20 and bone marrow plasma cell (BMPC) infiltration >20%) to stratify patients as having low risk, intermediate risk and high risk of progression to multiple myeloma11. These and other risk models were developed using cohorts of incidentally diagnosed SMM cases that might not represent the underlying population with SMM in the general population. Therefore, the distribution of the different risk groups in the population and the clinical utility of the risk scores in stratifying the SMM population are unknown.
Emerging data from clinical trials indicate that, compared to watchful monitoring, initiation of therapy at the SMM stage might further improve outcomes in multiple myeloma. Mateos et al.12,13 found that patients with high-risk SMM treated with lenalidomide and dexamethasone, compared to no treatment, had delayed progression to active multiple myeloma and had better overall survival 3 years after the initiation of therapy12,13. These findings were confirmed by Lonial et al.14, who showed that lenalidomide monotherapy delayed progression to active multiple myeloma in intermediate- to high-risk SMM14. In light of these findings, although observation or enrollment in clinical trials is generally advised and there are not yet any US Food and Drug Administration or European Medicines Agency drug approvals for the indication of SMM, some clinicians now recommend early treatment in high-risk SMM15,16. However, only a minority of patients with multiple myeloma (3–6%) are diagnosed at a precursor stage, limiting the practical implementation of these recommendations17,18.
We were motivated to define the epidemiological and clinical characteristics of SMM in the general population using a large (n > 75,000) population-based screening study. The iStopMM study (Iceland Screens, Treats, or Prevents Multiple Myeloma) is a prospective nationwide screening study in Iceland investigating the potential benefits and harms of screening for precursors of multiple myeloma (ClinicalTrials.gov ID: NCT03327597) where 75,422 (51%) individuals over 40 years old in Iceland have been screened for multiple myeloma precursor conditions19. The aims of the study were to map the epidemiological and clinical features of SMM in the population-based screened cohort and describe the true prevalence of SMM.
Results
Baseline and laboratory characteristics
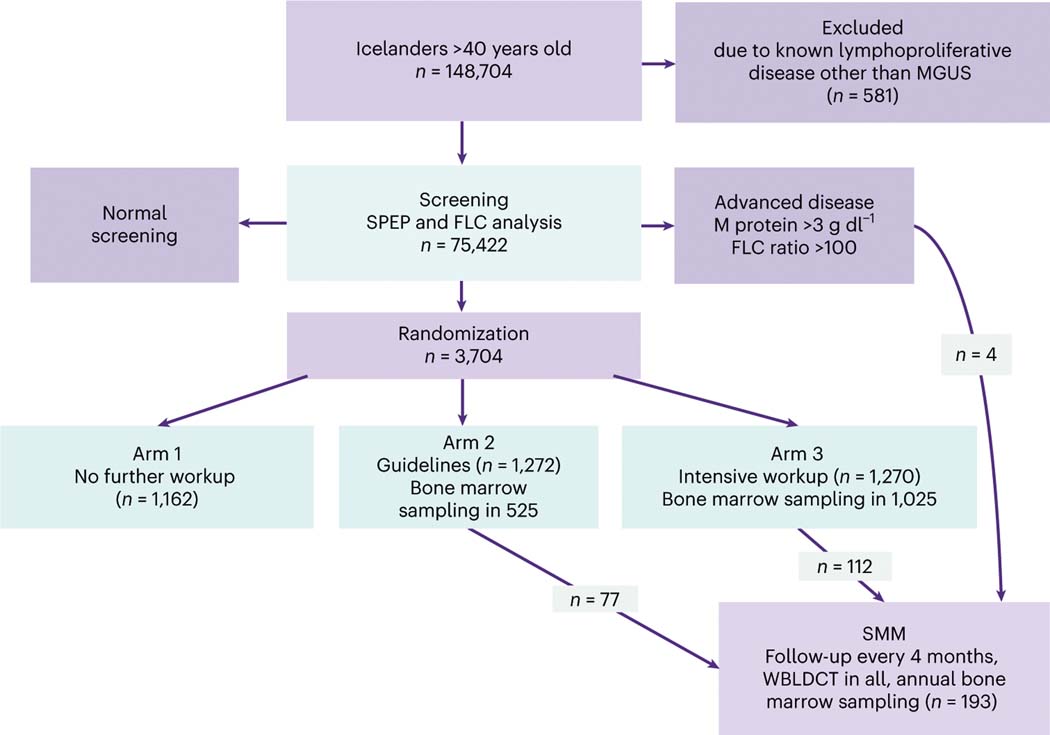
Of the 148,704 individuals over 40 years of age in Iceland, 75,422 (51%) were screened for abnormal M protein concentration and FLC ratio in the serum. The median age of all screened participants was 61 years (range 41–103 years), 34,619 (46%) were male and 40,803 (54%) were female. A total of 3,704 participants were identified with abnormal screening results and were randomized to one of the three arms of different follow-up strategies per study protocol (Fig. 1). Participants in arm 1 continued care in the Icelandic healthcare system as though they were never screened. Participants in arms 2 and 3 were evaluated at the study clinic for initial assessment and follow-up, with arm 2 receiving care according to current guidelines7 including bone marrow sampling for all except low-risk MGUS. In arm 3, bone marrow testing was offered to all participants. Bone marrow sampling was performed in 1,562 individuals, 525 (41%) in arm 2 and 1,025 (82%) in arm 3, and in 12 individuals that were not randomized due to advanced disease at screening. Thus, 90% of bone marrow sampling indicated by the protocol was completed. The randomized controlled trial (RCT) of follow-up strategies is still ongoing; in this article, we report the findings from the initial blood sample and bone marrow screening.
Fig. 1 |. The iStopMM study design.
All Icelanders >40 years old residing in Iceland on 9 September 2016 (148,704 individuals) were invited to participate. The only exclusion criterion was previously known lymphoproliferative disease other than MGUS or SMM. Participants with abnormal screening tests based on a positive M protein result on SPEP or an abnormal FLC analysis entered an RCT. The RCT was divided into three arms. Participants in arm 1 continued care in the Icelandic healthcare system as though they were never screened. Participants in arms 2 and 3 were evaluated at the study clinic for initial assessment and followup, with participants in arm 2 receiving care according to current guidelines6, including bone marrow sampling and skeletal surveys for all except those with low-risk MGUS. In arm 3, bone marrow testing and WBLDCT were offered to all participants. Participants with previously known MGUS were randomized only to arms 2 and 3. Participants with an M protein concentration ≥3.0 g dl−1 or an FLC ratio ≥100 were not randomized but were all called in for evaluation. Participants diagnosed with SMM were enrolled to more intensive follow-up at least every 4 months, with annual bone marrow sampling and WBLDCT. In this article, we report the results from the initial blood and bone marrow screening. The RCT is still ongoing.
After the initial screening and first visit at the study clinic, a total of 193 individuals in the screened cohort were diagnosed with SMM, of whom 116 (60%) were male, 77 (40%) were female and the median age was 70 years (range 44–92 years; Table 1). Of these individuals, 178 (92%) patients had a detectable M protein at the time of SMM diagnosis with a mean M protein concentration of 0.62 g dl−1 (range 0.01–3.5 g dl−1) with 1 patient having an M protein concentration >3 g dl−1. The most common isotype was IgG in 109 (56%) patients (64 kappa and 45 lambda) and 49 (25%) patients had IgA (27 kappa and 22 lambda), 2 had IgM at time of SMM diagnosis (1 kappa and 1 lambda; both had transient IgM and later developed other isotypes), and 5 (2%) had biclonal M proteins (1 IgM and IgG, 2 IgA and IgG, 1 with 2 IgG bands and 1 with 2 IgA bands). The FLC ratio was abnormal in 122 (63%) patients and was >20 in 28 (15%) patients. A total of 25 (13%) patients had light chain SMM (17 kappa and 8 lambda). Three patients (2%) had a negative serum protein electrophoresis (SPEP) result and normal FLC analysis at the time of SMM diagnosis despite abnormal results at screening, but all had kappa peaks on mass spectrometry; two of these patients had 11–20% plasma cell infiltration in the bone marrow and one had 21–30% infiltration.
Table 1 |.
Baseline characteristics of patients with SMM diagnosed in the study
| Variable | All patients with SMM (n = 193) | Patients with SMM in arm 3 (n = 112) |
|---|---|---|
| Median (range) age, years | 70 (44–92) | 70 (44–92) |
| Sex | ||
| Male | 116 (60) | 69 (62) |
| Female | 77 (40) | 43 (38) |
| Ethnicity | ||
| White | 173 (90) | 109 (97) |
| Asian | 1 (1) | 1 (1) |
| Unknown | 19 (10) | 2 (2) |
| Median (range) hemoglobin, g l−1 (range) | 138 (81–169) | 139 (81–169) |
| Median (range) creatinine, mmol l−1 (range) | 86 (42–577) | 86 (50–577) |
| Median (range) calcium, mmol l −1 (range) | 2.37 (2.15–2.81) | 2.36 (2.16–2.61) |
| Median (range) β−2 microglobulin, mg l−1 (range) | 7.2 (4.3–43.3) | 7.3 (4.8–26.5) |
| M protein (n) | 178 | 102 |
| Mean (range), g dl−1 | 0.62 (0.01–3.5) | 0.52 (0.01–2.1) |
| >1.0 g dl−1 | 37 (19) | 17 (15) |
| >2.0 g dl−1 | 4 (2) | 1 (1) |
| >3.0 g dl−1 | 1 (0.5) | 0 |
| FLC ratio | ||
| >1.65 or <0.26 | 122 (63) | 63 (56) |
| >20 or <0.05 | 28 (15) | 15 (13) |
| Isotype | ||
| IgG | 109 (56) | 61 (54) |
| Kappa | 64 | 30 |
| Lambda | 45 | 30 |
| IgA | 49 (25) | 32 (28) |
| Kappa | 27 | 18 |
| Lambda | 22 | 14 |
| IgM | 2 (1) | 0 |
| Biclonal | 5 (2) | 1 (1) |
| Light chain | 25 (13) | 18 (16) |
| Kappa | 17 | 10 |
| Lambda | 8 | 7 |
| Normal SPEP and FLC analysis | 3 (2) | 1 (1) |
| BMPCs | ||
| 11–20% | 141 (73) | 87 (78) |
| 21–30% | 33 (17) | 16 (14) |
| 31–40% | 11 (6) | 4 (4) |
| 41–50% | 8 (4) | 5 (4) |
| Immunoparesis | 46 (24) | 22 (20) |
| Risk score (2/20/20 risk model) | ||
| Low risk | 126 (65) | 76 (68) |
| Intermediate risk | 52 (27) | 30 (27) |
| High risk | 15 (8) | 6 (5) |
| Bone disease excluded with: | ||
| MR | 28 (14) | 12 (11) |
| CT | 143 (74) | 97 (87) |
| Skeletal survey | 13 (7) | 0 (0) |
| No imaging | 9 (5) | 2 (2) |
Data are shown as n (%) unless indicated otherwise.
All individuals with SMM had BMPC infiltration >10%; 141 (73%) patients had 11–20% BMPCs at SMM diagnosis, 33 (17%) had 21–30%, 11 (6%) had 31–40% and 8 (4%) had 41–50%. Bone disease was excluded with imaging in 184 (95%) patients (142 with whole-body low-dose computed tomography (WBLDCT), 28 with magnetic resonance imaging (MRI), 13 with skeletal survey and 1 with fluorodeoxyglucose positron emission tomography/CT); 9 (5%) patients did not have bone imaging performed because of patient refusal, comorbidities or death. Patient characteristics for all patients with SMM and patients with SMM diagnosed in arm 3 are presented in Table 1.
Prevalence of SMM
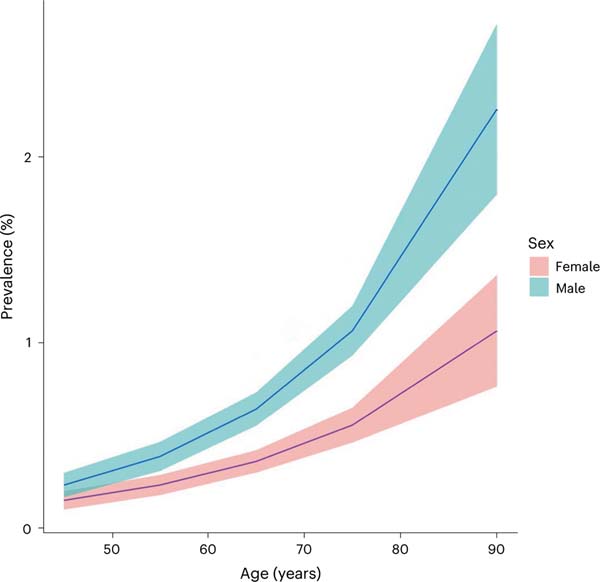
Of the 1,270 patients randomized to arm 3, bone marrow sampling was performed in 1,025 (81%), whereof 112 were diagnosed with SMM (10.9%). The prevalence of SMM in the total population was 0.53% (95% confidence interval (CI) = 0.49–0.57%) in individuals 40 years of age or older. In men and women, the prevalence of SMM was 0.67% (95% CI = 0.62–0.73%) and 0.39% (95% CI = 0.35–0.43%), respectively. SMM prevalence increased with age and was 1.08% (95% CI = 0.94–1.21%) in individuals over 70 years and 1.59% (95% CI = 1.33–1.86%) in individuals over 80 years old (Table 2 and Fig. 2).
Table 2 |.
Prevalence of SMM in iStopMM according to age group and sex
| Age group | iStopMM | Randomized | Arm 3 | Arm 3 with BMB | SMM arm 3 | SMM arm 3 with BMB | Prevalence (95% CI) of SMM in the whole populationa |
|---|---|---|---|---|---|---|---|
| Females | |||||||
| 40–49 | 7,474 | 98 | 32 | 31 | 2 | 6% | 0.15% (0.1–0.21%) |
| 50–59 | 12,061 | 288 | 103 | 92 | 11 | 12% | 0.24% (0.18–0.29%) |
| 60–69 | 11,356 | 457 | 163 | 141 | 13 | 9% | 0.36% (0.3–0.42%) |
| 70–79 | 7,236 | 513 | 169 | 134 | 11 | 8% | 0.55% (0.46–0.64%) |
| 80+ | 3,106 | 347 | 122 | 72 | 6 | 8% | 1.03% (0.74–1.33%) |
| Males | |||||||
| 40–49 | 5,214 | 93 | 30 | 26 | 2 | 8% | 0.24% (0.17–0.31%) |
| 50–59 | 8,881 | 289 | 90 | 84 | 7 | 8% | 0.39% (0.31–0.47%) |
| 60–69 | 10,186 | 578 | 187 | 168 | 24 | 14% | 0.64% (0.55–0.73%) |
| 70–79 | 6,950 | 623 | 224 | 195 | 26 | 13% | 1.04% (0.91–1.17%) |
| 80+ | 2,958 | 418 | 150 | 82 | 10 | 12% | 2.16% (1.71–2.61%) |
| Total | 75,422 | 3,704 | 1,270 | 1,025 | 112 | 11% | 0.53% (0.49–0.57%) |
BMB, bone marrow biopsy.
Estimated with fitted values of age.
Fig. 2 |. Estimated prevalence of SMM by age in the population over 40 years old, using fitted values stratified by sex.
The error bands are the 95% CIs from the fit.
In individuals with a positive screening for M protein and/or FLC analysis randomized to arm 3 where bone marrow sampling was performed in all, the risk of being diagnosed with SMM (and not MGUS) did not increase with age (for a 10-year increase in age, the estimate was odds ratio (OR) = 1.01; 95% CI = 0.86–1.20; P = 0.66) and was not significantly different according to sex (estimate at age 68, OR = 0.72; 95% CI = 0.43–1.02; P = 0.11).
Of the 1,270 patients randomized to arm 3, we found that 492 (39%) had low-risk MGUS based on blood work done in the initial screening. Among these individuals, bone marrow sampling was performed in 420 and 28 (6.7%) were diagnosed with SMM based on the initial bone marrow biopsy (that is, the plasma cell infiltration of the bone marrow was between 10% and 59%).
Risk stratification
We next evaluated the risk profile of the patients with SMM. Two clinical risk stratification models were applied to stratify patients according to risk of progression to multiple myeloma. A total of 4 out of 193 (2%) patients had M protein concentration >2 g dl−1, 28 (15%) patients had an FLC ratio >20 and 52 (27%) patients had BMPCs >20% (Table 1). According to the proposed 2/20/20 clinical risk stratification model for SMM, 126 (65%) patients were low risk, 52 (27%) intermediate risk and 15 (8%) high risk. In all, 46 (24%) had immunoparesis at diagnosis and out of the 87 patients who underwent testing with flow cytometry of the bone marrow aspirates, 28 had >95% abnormal plasma cells. Using the PETHEMA SMM risk model on the 87 patients who underwent testing with flow cytometry of the bone marrow aspirates, 46 (53%) patients were low risk, 26 (30%) were intermediate risk and 15 (17%) were high risk. Among patients evaluated with both risk models (n = 87), the concordance between the two models was poor (overall agreement was 52%; Table 3).
Table 3 |.
Comparison of the Mayo Clinic 2/20/20 and PETHEMA risk stratification models in 87 patients with SMM evaluated with both models
| Mayo Clinic 2/20/20 model | PETHEMA model | ||
|---|---|---|---|
| Low risk | Intermediate risk | High risk | |
| Low risk | 31 | 11 | 4 |
| Intermediate risk | 14 | 9 | 6 |
| High risk | 1 | 6 | 5 |
| Overall agreement for 45 of 87 patients (52%) | |||
Discussion
In this large population-based iStopMM study, including over 75,000 individuals, we describe the prevalence of SMM in a screened population in Iceland and found SMM to be present in 0.53% of individuals who are 40 years of age or older. Furthermore, we found that the prevalence of SMM increased with age, with a prevalence of 1.1% in individuals 70 years of age or older and 1.6% in individuals 80 years of age or older. The iStopMM population represents over 50% of the Icelandic population over 40 years old for the screening of multiple myeloma precursors. Based on established clinical risk models, one-third of patients had SMM at intermediate to high risk for progression to multiple myeloma, suggesting that potential candidates for early treatment12,14 can be identified by systematic screening of the population.
The median age at SMM diagnosis was 70 years, which is in line with other population-based SMM cohorts20. The risk of being diagnosed with SMM among those with M protein or evidence of light chain disease identified after a positive screening did not increase with age, which suggests that age is not a risk factor for progression from MGUS to SMM. This is in accordance with results from previous studies where age at MGUS diagnosis did not predict progression to multiple myeloma and underlines that rate of progression in plasma cell disorders is predominantly dependent on the biology of the disease6,21. Furthermore, we found that 6.7% of individuals who fulfilled the diagnostic criteria for low-risk MGUS based on blood samples only were diagnosed with SMM based on the initial bone marrow biopsy. This highlights the influence of the degree of workup on the diagnosis, with direct impact on clinical management, although further follow-up is needed to determine the risk profile of these individuals.
In our screened iStopMM cohort with SMM, most patients had a low M protein concentration and plasma cell burden, which is in contrast with prior retrospective studies3,7,22. All cases of SMM in this screened cohort where multiple myeloma has been ruled out to a greater extent than previously, had over 10% of BMPCs and only three individuals had an M protein concentration >2 g dl−1. This finding raises the question of whether the current SMM diagnostic criteria of an M protein concentration of 3 g dl−1 or higher may need to be adjusted in future versions of the diagnostic criteria for SMM and suggests that previous studies on SMM may have included individuals with more advanced disease according to current definitions. Furthermore, the patients were at low risk of progression to multiple myeloma according to established clinical risk stratification models for progression from SMM to multiple myeloma3,9. As demonstrated previously23, we found considerable discordance between the 2/20/20 and PETHEMA SMM clinical risk models, suggesting that there is room for improvement and that the risk scores do not predict individual risk of progression because many clinical treatment trials developed for high-risk SMM use these risk models to determine patients’ eligibility for study participation12,14. The low-risk characteristics of our cohort and the relatively high prevalence of SMM indicate that some patients with SMM in the general population have an indolent disease biology that does not progress to multiple myeloma or progresses at a very slow rate. Going forward, improved and biology-oriented strategies are needed to accurately identify patients with a progressive myeloma precursor condition before clonal expansion. Such efforts would allow earlier initiation of therapy before onset of end-organ damage or other myeloma-defining biomarkers to avoid severe clinical complications and prevent oversurveillance and overtreatment of patients diagnosed with precursor conditions24–26. As part of the iStopMM study, we are currently conducting prospective analyses with whole-genome sequencing in serial samples taken from patients with MGUS, SMM and multiple myeloma to further characterize both progressive and stable disease (ClinicalTrials.gov ID: NCT03327597).
The strengths of our study include its population-based screening design with a very high participation rate in the Icelandic population. Furthermore, the study protocol includes accurate diagnostics with both bone marrow aspirates and trephine biopsies performed in a standardized manner. Additionally, the biopsy material from each patient was assessed by the same pathologist and/or hematologist. Other strengths of our study include complete data with extensive blood tests in all and bone imaging in 95% of the cohort. Lastly, the study protocol only had one exclusion criterion (previous lymphoproliferative disease) and therefore represents real-world data on the clinical characteristics of patients with SMM.
The limitations of our study include that the Icelandic population is genetically homogenous and almost exclusively white. The results may therefore not be representative of other populations because the epidemiology of both MGUS and multiple myeloma are associated with race and ethnicity27,28. Another limitation may be the use of WBLDCT to rule out bone disease. It is possible that a small number of patients would have been classified as having multiple myeloma (instead of SMM) had whole-body MRI been performed in all patients and evidence was found of focal lesions in the bones and/or bone marrow. Furthermore, if nonsecretory multiple myeloma is preceded by nonsecretory SMM, these individuals would not be detected by our screening. However, because nonsecretory multiple myeloma is very rare29, this is unlikely to have impacted our results.
In conclusion, based on the large population-based iStopMM screening study, we have characterized the population with SMM who are at this critical interface between precursor and active malignant disease. We have shown that the prevalence of SMM is 0.53% in persons 40 years or older. According to current clinical risk stratification models, around one-third of patients with SMM have an intermediate or high risk of progression to multiple myeloma. Our study is of public health relevance because the optimal timing of when to start therapy in multiple myeloma is being debated worldwide14,30–32; our results show that screening could identify possible candidates for early treatment. Although our findings are encouraging, until final results of the iStopMM study become available, including data on survival and quality of life, we advise against MGUS screening in healthy individuals. Importantly, improved and biology-oriented strategies are needed to accurately define the individual patient’s risk of progression26. Such efforts will allow clinicians to focus their care on those who benefit the most from surveillance and therapeutic interventions, preventing overtreatment and enabling early detection and early effective treatments, improving outcomes in individuals with SMM and multiple myeloma worldwide.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/s41591–022-02183–6.
Methods
Recruitment of participants for screening
The design of the iStopMM study has previously been described in detail and is shown in Fig. 1 (ref. 19). Briefly, all individuals born in 1975 and earlier residing in Iceland on 9 September 2016 (148,704 individuals) were invited to participate. A letter containing a detailed information brochure and consent form was mailed to them and an extensive campaign on social and conventional media was launched introducing the study to the Icelandic public. This campaign was followed by phone calls to those who had not yet signed up for the study. The only exclusion criterion was previously known lymphoproliferative disease other than MGUS (including previous multiple myeloma and SMM). A total of 80,759 participants (54.3% of the underlying Icelandic population) gave informed consent to participate in the screening; during the sampling phase, a total of 75,422 blood samples were collected.
Ethical approval
The iStopMM study protocol was approved by the Icelandic National Bioethics Committee (no. 16–022, 26 April 2016) and the Icelandic Data Protection Agency. All participants provided signed informed consent.
Original screening
All screening samples were sent to the clinical laboratory at Landspitali University Hospital in Reykjavik, Iceland. Serum was aliquoted into identical sample tubes and assigned an anonymous study identification number, using the TC automation and aliquoter (Thermo Fisher Scientific) for sample handling. Samples were then sent to The Binding Site laboratory in Birmingham, UK, where all samples were screened for M protein by capillary zone electrophoresis (CZE) (Helena Laboratories) and for FLC, immunoglobulins (IgG, IgA and IgM) and total protein by Freelite and Hevylite assays performed on an Optilite turbidimeter (The Binding Site). Immunofixation electrophoresis (Helena Laboratories) was performed on samples with clear or suspected M protein bands by CZE and/or abnormal FLC results. The CZE and immunofixation electrophoresis gels were assessed independently by at least two experienced observers. Participants with abnormal screening tests based on a positive M protein on SPEP result or an abnormal FLC analysis entered an RCT. The MGUS risk score was defined using current guidelines6. The RCT was divided into three arms. Arm 1 participants continued care in the Icelandic healthcare system as though they were never screened. Participants in arms 2 and 3 were evaluated at the study clinic for initial assessment and follow-up with arm 2 receiving care according to current guidelines7 including bone marrow sampling and skeletal surveys for all except low-risk MGUS. In arm 3, bone marrow testing and WBLDCT were offered to all participants. Participants with previously known MGUS were randomized only to arms 2 and 3. Participants with an M protein concentration ≥3.0 g dl−1 or an FLC ratio ≥100 were not randomized but were all called in for evaluation. Data collection was performed using REDCap v.12.0.29.
SMM diagnosis
Bone marrow sampling was performed by trained study nurses in all except individuals with low-risk MGUS in arm 2 and in all participants in arm 3 at their first visit to the study clinic; blood test were repeated, including SPEP and FLC assay. Bone marrow smears were stained with Giemsa stain and jointly evaluated by two senior hematologists. Trephine biopsies were stained with hematoxylin and eosin and for CD138 before being evaluated by two senior hematopathologists. The sample with the higher percentage of BMPC infiltration at each sampling time was used for diagnosis. SMM was defined by 10–59% bone marrow clonal plasma cells on a smear or trephine biopsy and/or M protein concentration in the serum ≥30 g l−1, in the absence of myeloma-defining events (hypercalcemia, renal insufficiency associated with plasma cell disease, anemia, osteolytic lesions or an FLC ratio >100), based on the International Myeloma Working Group 2014 diagnostic criteria.2 Participants diagnosed with SMM were enrolled to intensive follow-up, with annual bone marrow samples and WBLDCT, as well as MRI or fluorodeoxyglucose positron emission tomography/CT when clinically indicated. All WBLDCT images were reviewed independently by two physicians.
SMM risk stratification
Patients with SMM were stratified according to the Mayo Clinic 2/20/20 risk stratification model which incorporates serum M protein concentration >2 g dl−1, involved to uninvolved FLC ratio >20 and BMPC infiltration >20% to stratify patients into low-risk, intermediate-risk and high-risk groups (0 factors, 1 factor and 2–3 factors, respectively), and the PETHEMA risk model, which uses immunoparesis and the percentage of aberrant (versus normal) plasma cells in the bone marrow according to flow cytometry to stratify patients into low-risk, intermediate-risk and high-risk groups (that is, neither factor, one factor or both, respectively)7,11. Immunoparesis was defined as a reduction in one or two uninvolved immunoglobulins of more than 25%, compared with normal values at SMM diagnosis. In a fraction of patients with SMM, the EuroFlow next-generation flow multiple myeloma minimal residual disease (MRD) method33 was used to immunophenotype and quantify normal and abnormal plasma cells in bone marrow samples and estimate the proportion of abnormal BMPCs. Samples were acquired using a FACSCanto II flow cytometer (BD Biosciences) and flow cytometry data were analyzed using Infinicyt software (Cytognos). The multiple myeloma MRD Panel Kit was used for cell staining along with two EuroFlow recommended drop-in antibodies (CD27-BV510, clone O323, cat. no. 302836, BioLegend; CD138-BV421, clone MI15, cat. no. 562935, BD Biosciences). The kit (cat. no. CYT-MM-MRD, Cytognos) consists of a premixed six-color eight-antibody combination (CD19-PE-Cy7, clone SA287; CD38-FITC, multi-epitope; CD45-PerCp-Cy5.5, clone EO1; CD56-PE, clone C5.9; CD81-APC-C750, clone M38; CD117-APC, clone 104D2; CyIgKappa-APC, polyclonal; CyIgLambda-APC-C750, polyclonal) for staining in two separate tubes. Lyophilized multicolor antibody vials in the multiple myeloma MRD kit were reconstituted according to the manufacturer’s description. All antibodies were used without predilution to stain a target number of 5 million nucleated cells in 300 μl of staining buffer per tube after bulk red blood cell lysis of whole bone marrow samples. Participants who developed intermediate- to high-risk SMM or multiple myeloma were offered enrollment on an independent treatment trial (ClinicalTrials.gov ID: NCT03815279) and/or referred to a hematology unit in Iceland.
Statistical analysis
Arm 3 of the RCT, where both bone marrow biopsy and imaging were performed in all participants when possible, was used to calculate the prevalence of SMM, providing the most precise proportion of SMM in those with a positive SPEP and/or FLC analysis screening. The point prevalence of SMM was calculated based on the proportion of participants with a positive SPEP and/or FLC analysis at screening multiplied by the proportion of those randomized to arm 3 who underwent a bone marrow biopsy and were diagnosed with SMM (and not MGUS or multiple myeloma) at initial assessment at the study clinic. The underlying Icelandic population on 1 July 2018 was used to calculate the prevalence of SMM, chosen as the mean date of return of the blood sample used in SPEP and FLC analysis. Information on sex was collected from a central registry (Registers Iceland) and ethnicity was self-reported. These proportions were then used to estimate the number of patients with SMM in the age groups 40–49, 50–59, 60–69, 70–79 and >80 years old for both sexes in the population and to calculate the prevalence of SMM in the iStopMM population. The age- and sex-specific prevalence was determined and visualized in figures with a fitted binomial function corrected for age, sex and interaction between them. The standard error of the fit was used to calculate the 95% CIs of the prevalence. The fit was made within age groups using SMM diagnosed in arm 3 and samples screened. The estimate for each age group and corresponding standard error were used to report prevalence with 95% CI for each age group (Table 2). Age- and sex-specific prevalence of SMM in those who screened positive for M protein and/or FLC analysis randomized to arm 3 was fitted with a binomial model with interaction between age and sex. Statistical analysis was performed using R v.3.6.3.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary Material
Acknowledgements
The iStopMM study is funded by the Black Swan Research Initiative by the International Myeloma Foundation and the Icelandic Centre for Research (grant no. 173857). This project also received funding from the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant no. 716677), the International Myeloma Society, the Paula and Rodger Riney Foundation Translational Research Award and the Leukemia & Lymphoma Society Career Development Program Scholar in Clinical Research Award. Screening tests were performed by The Binding Site. Additional funding was provided by the University of Iceland, Landspítali University Hospital and the Icelandic Cancer Society. The funders had no role in study design, data collection, data analysis, data interpretation or manuscript writing. We thank all the participants in the iStopMM study.
Footnotes
Competing interests
O.B. and S.H. are currently employed by The Binding Site. The other authors declare no competing interests.
Code availability
The code used to generate the main results in this paper is publicly available on GitHub (https://github.com/blodskimun/SMM_prevalence).
Additional information
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41591-022-02183-6.
Peer review information Nature Medicine thanks Dickran Kazandjian, Roberto Mina, Niels Abildgaard and Ingemar Turesson for their contribution to the peer review of this work. Primary Handling Editor: Ming Yang, in collaboration with the Nature Medicine team.
Data availability
EuroFlow developed and validated a multiple myeloma MRD database containing representative flow cytometry datasets from normal healthy bone marrow samples processed in different standardized centers. The database (available through Infinicyt), when used with files that follow standardized operating procedures, allows for an automated analysis of the complete bone marrow sample (https://www.cytognos.com/euroflow-databases/resources/multiple-myeloma/). Due to Icelandic law on ethics in research, data privacy regulations and per informed consent from participants in this study, the patient-level data used for this study cannot be shared. We encourage researchers or parties interested in collaboration for noncommercial use to apply to the corresponding author. The request will be reviewed by the iStopMM team to verify whether data sharing is within the restrictions of the study’s ethical approval.
References
- 1.Kyle RA & Greipp PR Smoldering multiple myeloma. N. Engl. J. Med. 302, 1347–1349 (1980). [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar SV et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 15, e538–e548 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N. Engl. J. Med. 356, 2582–2590 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Kristinsson SY, Holmberg E. & Blimark C. Treatment for high-risk smoldering myeloma. N. Engl. J. Med. 369, 1762–1763 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Cowan AJ et al. Global burden of multiple myeloma: a systematic analysis for the global burden of disease study 2016. JAMA Oncol. 4, 1221–1227 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyle RA et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia 24, 1121–1127 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pérez-Persona E. et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood 110, 2586–2592 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Dispenzieri A. et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood 111, 785–789 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mateos M-V et al. International Myeloma Working Group risk stratification model for smoldering multiple myeloma (SMM). Blood Cancer J. 10, 102 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajkumar SV, Landgren O. & Mateos M-V Smoldering multiple myeloma. Blood 125, 3069–3075 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lakshman A. et al. Risk stratification of smoldering multiple myeloma incorporating revised IMWG diagnostic criteria. Blood Cancer J. 8, 59 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mateos M-V et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N. Engl. J. Med. 369, 438–447 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Mateos M-V et al. Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): long-term follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol. 17, 1127–1136 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Lonial S. et al. Randomized trial of lenalidomide versus observation in smoldering multiple myeloma. J. Clin. Oncol. 38, 1126–1137 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar SK et al. Multiple Myeloma, Version 3.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl Compr. Canc. Netw. 18, 1685–1717 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Lonial S, Rajkumar SV & Mateos MV Risk stratified management approaches for smouldering multiple myeloma: clinical research becomes clinical practice. Lancet Haematol. 9, e162–e165 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Sigurdardottir EE et al. The role of diagnosis and clinical follow-up of monoclonal gammopathy of undetermined significance on survival in multiple myeloma. JAMA Oncol. 1, 168–174 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Go RS, Gundrum JD & Neuner JM Determining the clinical significance of monoclonal gammopathy of undetermined significance: a SEER-Medicare population analysis. Clin. Lymphoma Myeloma Leuk. 15, 177–186.e4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rögnvaldsson S. et al. Iceland screens, treats, or prevents multiple myeloma (iStopMM): a population-based screening study for monoclonal gammopathy of undetermined significance and randomized controlled trial of follow-up strategies. Blood Cancer J. 11, 94 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sørrig R. et al. Smoldering multiple myeloma risk factors for progression: a Danish population-based cohort study. Eur. J. Haematol. 97, 303–309 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Kyle RA et al. Long-term follow-up of monoclonal gammopathy of undetermined significance. N. Engl. J. Med. 378, 241–249 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miguel JS et al. Updated risk stratification model for smoldering multiple myeloma (SMM) incorporating the revised IMWG diagnostic criteria. J. Clin. Oncol. 37, 8000 (2019). [Google Scholar]
- 23.Hill E. et al. Assessment of discordance among smoldering multiple myeloma risk models. JAMA Oncol. 7, 132–134 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maura F. et al. Moving from cancer burden to cancer genomics for smoldering myeloma: a review. JAMA Oncol. 6, 425–432 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maura F, Landgren O. & Morgan GJ Designing evolutionary based interception strategies to block the transition from precursor phases to multiple myeloma. Clin. Cancer Res. 27, 15–23 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landgren O. Advances in MGUS diagnosis, risk stratification, and management: introducing myeloma-defining genomic events. Hematology Am. Soc. Hematol. Educ. Program 2021, 662–672 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landgren O. et al. Prevalence of monoclonal gammopathy of undetermined significance among men in Ghana. Mayo Clin. Proc. 82, 1468–1473 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Iwanaga M, Tagawa M, Tsukasaki K, Kamihira S. & Tomonaga M. Prevalence of monoclonal gammopathy of undetermined significance: study of 52,802 persons in Nagasaki City, Japan. Mayo Clin. Proc. 82, 1474–1479 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Dimopoulos MA, Kastritis E. & Terpos E. Non-secretory myeloma: one, two, or more entities? Oncology 27, 930–932 (2013). [PubMed] [Google Scholar]
- 30.Mohyuddin GR, Ouchveridze E, Goodman A. & Prasad V. The landscape of trials for smoldering multiple myeloma: endpoints, trial design, and lessons learnt. Leuk. Lymphoma 62, 2793–2795 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Musto P. et al. 2021 European Myeloma Network review and consensus statement on smoldering multiple myeloma: how to distinguish (and manage) Dr. Jekyll and Mr. Hyde. Haematologica 106, 2799–2812 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapoor P. & Rajkumar SV Smoldering multiple myeloma: to treat or not to treat. Cancer J. 25, 65–71 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Flores-Montero J. et al. Next generation flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia 31, 2094–2103 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
EuroFlow developed and validated a multiple myeloma MRD database containing representative flow cytometry datasets from normal healthy bone marrow samples processed in different standardized centers. The database (available through Infinicyt), when used with files that follow standardized operating procedures, allows for an automated analysis of the complete bone marrow sample (https://www.cytognos.com/euroflow-databases/resources/multiple-myeloma/). Due to Icelandic law on ethics in research, data privacy regulations and per informed consent from participants in this study, the patient-level data used for this study cannot be shared. We encourage researchers or parties interested in collaboration for noncommercial use to apply to the corresponding author. The request will be reviewed by the iStopMM team to verify whether data sharing is within the restrictions of the study’s ethical approval.