Abstract
Comprehensive recommendations for maintenance therapy post-autologous stem cell transplantation (ASCT) for patients with multiple myeloma (MM) have yet to be defined. Bortezomib has been utilized as maintenance therapy after ASCT, but data attesting to the safety and efficacy of this agent compared to lenalidomide in the post-ASCT setting are limited. Therefore we retrospectively analyzed the outcomes of 102 MM patients who received maintenance therapy with bortezomib post-ASCT at Duke University’s Adult Bone Marrow Transplant Clinic (ABMT) between 2005 and 2015. Maintenance with bortezomib was initiated between 60 and 90 days after ASCT as a single agent 1.3 mg/m2 once every 2 weeks (n= 92), or in combination with lenalidomide (10 mg/day) (n= 10). The median age at ASCT was 64 (range 31 to 78). Of the 100 patients with molecular data available, 42% had high-risk cytogenetics [including d17p, t(4;14), +1q, and t(14;16) by FISH]. Overall, 46% of patients experienced side effects from maintenance therapy with 31% of all patients experiencing peripheral neuropathy. In total, 2% of patients required discontinuation of bortezomib maintenance due to adverse events. No secondary malignancies were reported from the therapy. The median progression-free survival (PFS) for patients receiving maintenance therapy with bortezomib post-ASCT was 36.5 months (95% confidence interval [CI], 21.3 to NA) and median overall survival was 72.7 months (95% CI, 63.9 to NA). PFS of patients with high-risk cytogenetics was not statistically significantly different to those with standard-risk cytogenetics, suggesting that maintenance with bortezomib may help overcome the impact of high-risk cytogenetics on early progression. These results indicate that maintenance therapy with bortezomib represents a safe, well tolerated, and efficacious option for patients with an inability to tolerate lenalidomide, high-risk cytogenetics, renal insufficiency, or a previous history of another cancer.
Keywords: Multiple Myeloma, Maintenance Therapy, Bortezomib, Autologous Stem Cell Transplantation
Introduction
High-dose induction chemotherapy followed by autologous stem cell transplantation (ASCT) is currently the standard of care for eligible patients diagnosed with multiple myeloma (MM) [1,2,3]. While studies have illustrated a benefit in both progression-free survival (PFS) and overall survival (OS) conferred by the use of ASCT, almost all patients eventually relapse after transplant [4,5]. Due to this high rate of relapse as well as the observed correlation between the depth of response achieved and overall outcome, several groups have investigated the role of maintenance therapy post-ASCT to achieve a durable remission and delay disease progression. Maintenance therapy is given for an extended period of time with the goal of prolonging the duration of response early in the disease course. Post-transplant maintenance therapy has even been suggested to be potentially more important than the choice of induction therapy for extending PFS [6–8]. While many studies evaluating the role of maintenance therapy indicate a clear PFS advantage, the benefit to overall survival remains unclear.
Initial studies involved the use of interferon and steroids as maintenance therapy. Although a PFS benefit was shown, these agents were associated with significant toxicities and poor tolerance; therefore, they are not widely used to date [9–12]. Several groups have studied the application of the first generation IMiD (immunomodulatory imide drug) thalidomide as maintenance therapy as well. These studies showed an improvement in PFS and response rates, however reports of significant cumulative toxicity and the possible risk of a resistant relapse has prevented the widespread use of thalidomide in the maintenance setting [13–16]. A few groups observed that patients on thalidomide maintenance with high-risk cytogenetics experienced shorter OS and additionally, some reported shorter survival post-relapse following thalidomide maintenance [16–18].
The standard of treatment for patients undergoing autologous transplant is to receive low dose post-transplant maintenance therapy with lenalidomide, which is considered a better candidate than thalidomide due to its more acceptable safety profile [6]. With respect to lenalidomide maintenance, all large randomized studies conducted to date have shown a PFS advantage, with one even showing an OS advantage [3,19–20]. However, in the study performed by Attal et al., PFS and OS after first progression were shorter in the cohort that received maintenance therapy with lenalidomide, suggesting that continuous therapy may act as a selective pressure for the dominance of more aggressive, drug-resistant sub-clones. Furthermore this study, similar to those investigating thalidomide maintenance, noted that patients with high risk cytogenetics (notably those with t(4;14) or d17p) did not derive a PFS or OS advantage from maintenance therapy with lenalidomide [7,19]. Two of these randomized studies also indicated an increase in secondary primary malignancies with long-term use of maintenance treatment with lenalidomide [19–20]. Due to the lack of benefit from maintenance with lenalidomide in patients with high-risk cytogenetics, as well as that patients with renal insufficiency, an inability to tolerate lenalidomide due to side effects, or a previous history of cancer require an alternative option for maintenance therapy, the use of bortezomib maintenance in the post-transplant setting has been suggested.
Bortezomib is a selective and reversible proteasome inhibitor associated with high response rates in newly diagnosed as well as relapsed patients [21]. The role of bortezomib as maintenance therapy post-transplant is less established with only two large randomized studies published to date, neither of which compared the use of bortezomib maintenance to a control group receiving no maintenance [22–23]. The study conducted by the HOVON/GMMG group showed the cohort that received induction with a bortezomib-based combination followed by bortezomib maintenance post-transplant derived both a PFS and OS advantage when compared to the arm that received non-bortezomib induction followed by transplant and thalidomide maintenance. Maintenance was terminated after a maximum of 2 years, regardless of whether patients had no evidence of disease progression [22]. A second large randomized study investigating bortezomib maintenance post-transplant conducted by the Spanish Myeloma Group, involved patients receiving maintenance post-transplant, and compared interferon alpha-2b, thalidomide, and bortezomib/thalidomide. This study demonstrated a PFS advantage in the cohort that received bortezomib/thalidomide maintenance. Unfortunately, some groups received induction with bortezomib while others did not, making it unclear whether the derived survival benefits were a result of the bortezomib induction or maintenance [23].
We do not have adequate guidelines for how long we should continue bortezomib in the maintenance setting, the optimal scheduling of the drug, and if it is truly comparable to lenalidomide. Large phase 3 studies aiming to optimize maintenance are ongoing, however results are not available at this point. Thus, one of the questions for physicians treating myeloma revolves around good alternative maintenance therapy options that impact PFS and OS for patients and are comparable or superior to lenalidomide. In this study we report the results of a single institution retrospective study of patients who received maintenance therapy with bortezomib post-autologous stem cell transplantation. Our goal was to define outcomes of patients on bortezomib maintenance and to determine whether this maintenance strategy is safe, efficacious, and well tolerated.
Methods
Patients
With institutional review board approval, we performed a systematic, retrospective review of medical charts of all myeloma patients who received an autologous transplant followed by maintenance therapy with bortezomib at the Adult Bone Marrow Transplant Clinic at Duke University between 2005 and December 2015. In total, 102 patients were identified.
Definitions
Maintenance therapy was defined as low dose bortezomib (1.3 mg/m2 once every two weeks) therapy given subcutaneously every other week alone or in combination with low dose lenalidomide (10 mg/day) initiated between 60 and 90 days post autologous transplant, until either disease progression or cumulative toxicity necessitated alternative therapy. Patients who received maintenance therapy with single agent lenalidomide post-transplant were excluded from this study. PFS was defined as the time from the date of initiation of maintenance to disease progression/relapse or death, whereas OS was defined from the date of initiation of maintenance to the date of death from any cause. High-risk patients were defined as bearing the presence of any of the following high-risk cytogenetic features: subgroups- d17p, t(4;14), +1q, and t(14;16) by FISH. Standard-risk patients were defined as having the absence of any of the aforementioned high-risk cytogenetic features.
Response
Response and progression were defined according to the International Myeloma Working Group criteria [24]. A complete response (CR) was defined as negative immunofixation of serum and urine, disappearance of soft tissue plasmacytoma, and <5% plasma cells in the bone marrow. Very good partial response (VGPR) was defined as more than 90% reduction of serum M-protein and urine M-protein less than 100mg/24 hours. A partial response (PR) was defined as more than 50% reduction of serum M-protein or reduction of urine M-spike less than 200mg/24 hours. Stable disease was defined as failure to meet any response criteria. Responses were assessed after induction, after transplant, at 2-month intervals during maintenance, as well as at progression/relapse. PFS was calculated from date of initiation of maintenance post-transplant until progression, relapse, or death, whichever came first. OS was measured from date of initiation of maintenance post-transplant until death from any cause. Patients alive at the date of most recent contact were censored.
Statistical Analysis
The primary goal of the study was to define outcomes for patients on bortezomib maintenance and compare PFS between the high risk and standard risk cohorts, as well as among subgroups within the high-risk cohort. The Kaplan-Meier plots of the survival curves for the overall survival and progression free survival were obtained for the high risk group and standard risk group, as well as the 1q+, d17p, t(4;14) and t(14,16) subgroups. The log rank test was used to test for the differences between groups. A two-sided p value <0.05 is considered to be significant. We also obtained the median survival times and their 95% confidence intervals. All statistical analyses were performed using R 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
Patient characteristics are outlined in Table 1. In total, 102 patients (51 males and 51 females) received post-transplant maintenance therapy with bortezomib at our institution from January 2005 to December 2015. Median age at transplant was 63 years (range, 31-75). The majority of patients received RVD (31%) and VCD (33%) as induction therapy prior to transplant. At time of transplant 72 patients (71%) achieved at least a VGPR. Post-transplant, 58 patients (57%) achieved a VGPR, 20 patients (20%) achieved a CR, and 18 patients (17%) achieved a sCR. All patients received post-transplant maintenance therapy incorporating bortezomib. In total, 92 patients (90%) received single agent bortezomib maintenance and 10 patients (10%) received a combination of lenalidomide and bortezomib. We identified 20 patients (20%) with history of other primary cancer. These patients were placed on bortezomib maintenance due to the potentially increased risk of developing secondary a cancer if placed on long-term maintenance with lenalidomide.
Table 1.
Patient Demographics and Disease Characteristics
| Characteristic | No. | % |
|---|---|---|
| Age at transplant, years | ||
| < 65 years of age | 53 | 52% |
| ≥ 65 years of age | 49 | 48% |
| Median | 64 | |
| Range | 31–78 | |
|
| ||
| Sex | ||
| Male | 51 | 50% |
| Female | 51 | 50% |
|
| ||
| Race | ||
| White | 73 | 71% |
| Black | 24 | 24% |
| Asian | 4 | 4% |
| Other | 0 | 0% |
| Missing | 1 | 1% |
|
| ||
| Immunochemical Subtype | ||
| IgG | 53 | 52% |
| IgA | 27 | 26% |
| IgD | 1 | 1% |
| LCM | 21 | 21% |
| Non-secretory | 0 | 0% |
|
| ||
| Light Chain Type | ||
| Kappa | 63 | 62% |
| Lambda | 39 | 38% |
|
| ||
| ISS Stage | ||
| Stage I | 40 | 39% |
| Stage II | 29 | 28% |
| Stage III | 23 | 23% |
| Unknown | 10 | 10% |
|
| ||
| Genetic Abnormality | ||
| High Risk | 43 | 42% |
| d17p | 20 | 20% |
| t(4;14) | 13 | 13% |
| +1q | 13 | 13% |
| t(14;16) | 5 | 5% |
| Standard Risk | 56 | 55% |
| Not Performed | 3 | 3% |
|
| ||
| Melphalan Dose | ||
| 200 mg/m2 | 78 | 76% |
| 140 mg/m2 | 24 | 24% |
|
| ||
| Induction Therapy | ||
| RVD | 32 | 31% |
| VCD | 34 | 33% |
| VD | 19 | 19% |
| RD | 10 | 10% |
| TD | 2 | 2% |
| VAD/similar | 5 | 5% |
|
| ||
| Disease Status at Transplant | ||
| PR | 30 | 29% |
| VGPR | 50 | 49% |
| CR | 5 | 5% |
| sCR | 17 | 17% |
|
| ||
| Type of Transplant | ||
| Single Auto | 97 | 95% |
| Tandem Auto | 5 | 5% |
|
| ||
| Post-transplant Maintenance Therapy | ||
| Lenalidomide + bortezomib | 10 | 10% |
| Bortezomib | 92 | 90% |
|
| ||
| Disease Status Post-Transplant | ||
| PR | 6 | 6% |
| VGPR | 58 | 57% |
| CR | 20 | 20% |
| sCR | 18 | 17% |
|
| ||
| Baseline Creatinine | ||
| ≤2mg/dl | 71 | 69% |
| > 2mg/dl | 18 | 18% |
| Unavail. | 13 | 13% |
|
| ||
| Number of skeletal lesions | ||
| 0 | 38 | 37% |
| 1–2 | 16 | 16% |
| >3 | 48 | 47% |
|
| ||
| History of another primary cancer | ||
| Positive | 20 | 20% |
| Negative | 82 | 80% |
|
| ||
| Median Beta-2 microglobulin | 3.23 | |
ISS indicates International Staging System; RVD, lenalidomide-bortezomib-dexamethasone; VCD, bortezomib-cyclophosphamide-dexamethasone; TD, thalidomide-dexamethasone; VAD, Vincristine-Adriamycin-Doxorubicin
Response and Survival
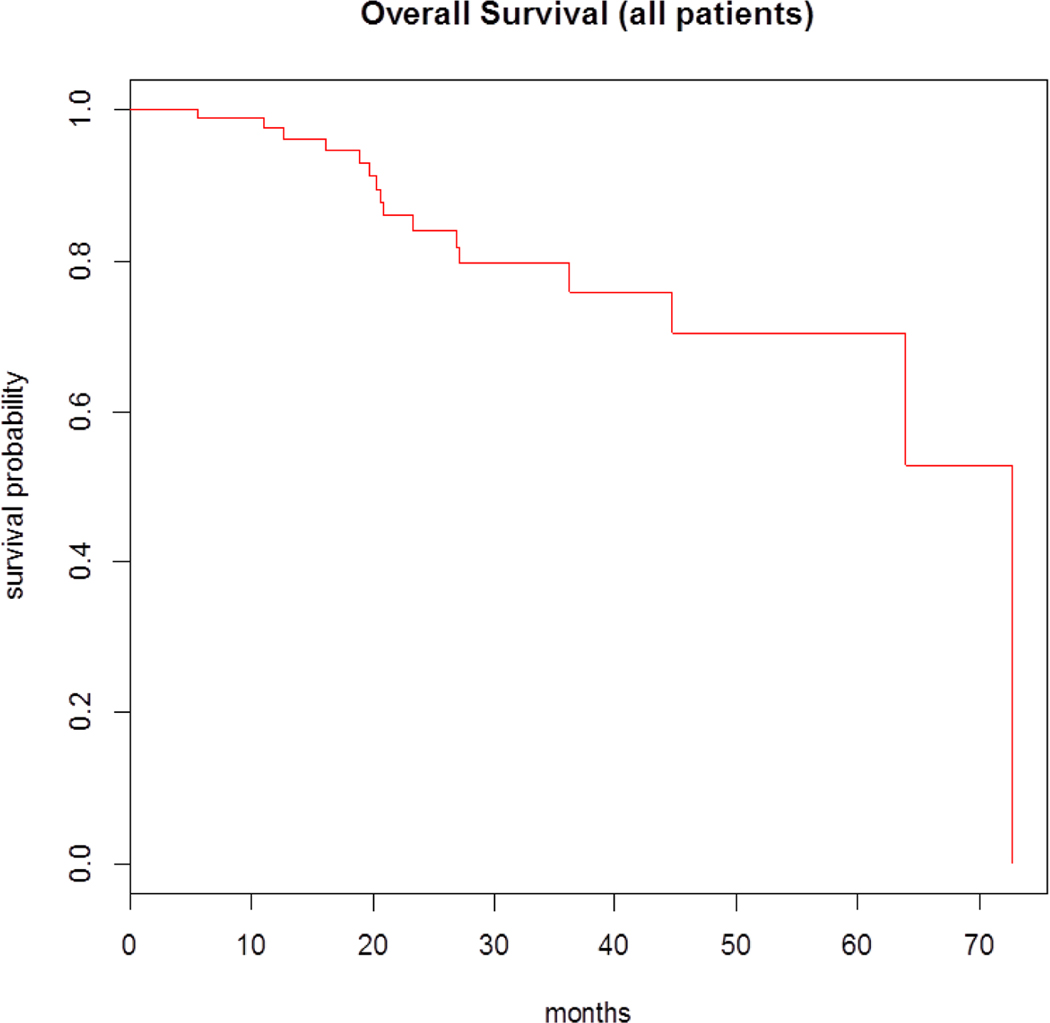
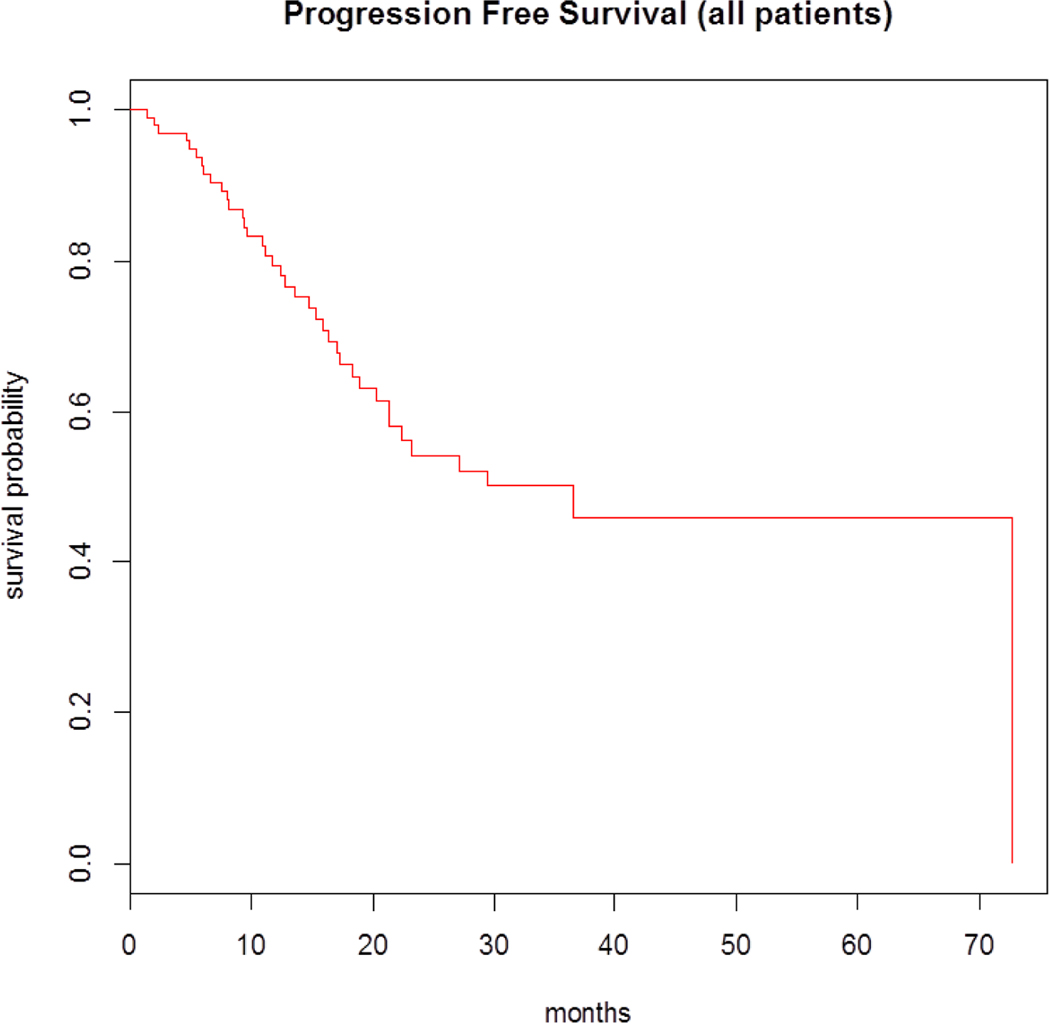
The median follow-up period was 18.8 months for survivors. In total, 35 patients (34%) had disease progression on maintenance. The median progression-free survival (PFS) for all patients who received maintenance therapy with bortezomib was 36.5 months (95% confidence interval [CI], 21.3 to NA). The median overall-survival (OS) was 72.7 months (95% CI, 63.9 to NA) (Figures 1 and 2). The safety profiles and most common toxicities are listed in Table 2. Peripheral neuropathy was the most commonly experienced toxicity (31%). The second most common toxicity was fatigue (15%). GI symptoms (5%), Rash (2%), Nausea (3%), and Neutropenia (1%) were less commonly observed. In 2 (2%) of 102 patients, treatment was discontinued due to peripheral neuropathy related to the bortezomib based maintenance therapy. At time of last follow up, 55 patients (54%) remained free of any reported toxicity, 2 patients (2%) discontinued bortezomib maintenance due to adverse effects, and no patients had died due to adverse events. There were no incidences of secondary malignancies reported as a result of maintenance therapy with bortezomib.
Figure 1.
OS for MM patients on maintenance therapy with bortezomib
Figure 2.
PFS for MM patients on maintenance therapy with bortezomib
Table 2.
Safety Profile & Toxicities from Maintenance Therapy
| Variable | No. | % |
|---|---|---|
| AE leading to discontinuation of bortezomib | 2 | 2% |
|
| ||
|
| ||
| Death from AE | 0 | 0% |
|
| ||
| Secondary Malignancy | 0 | 0% |
|
| ||
| Toxicities | ||
| Yes | 47 | 46% |
| No | 55 | 54% |
|
| ||
| Neutropenia | 1 | 1% |
|
| ||
| Fatigue | 15 | 15% |
|
| ||
| Peripheral Neuropathy | 32 | 31% |
|
| ||
| Nausea | 3 | 3% |
|
| ||
| Rash | 2 | 2% |
|
| ||
| GI symptoms | 5 | 5% |
AE, Adverse Event; GI, Gastrointestinal
Secondary Analyses
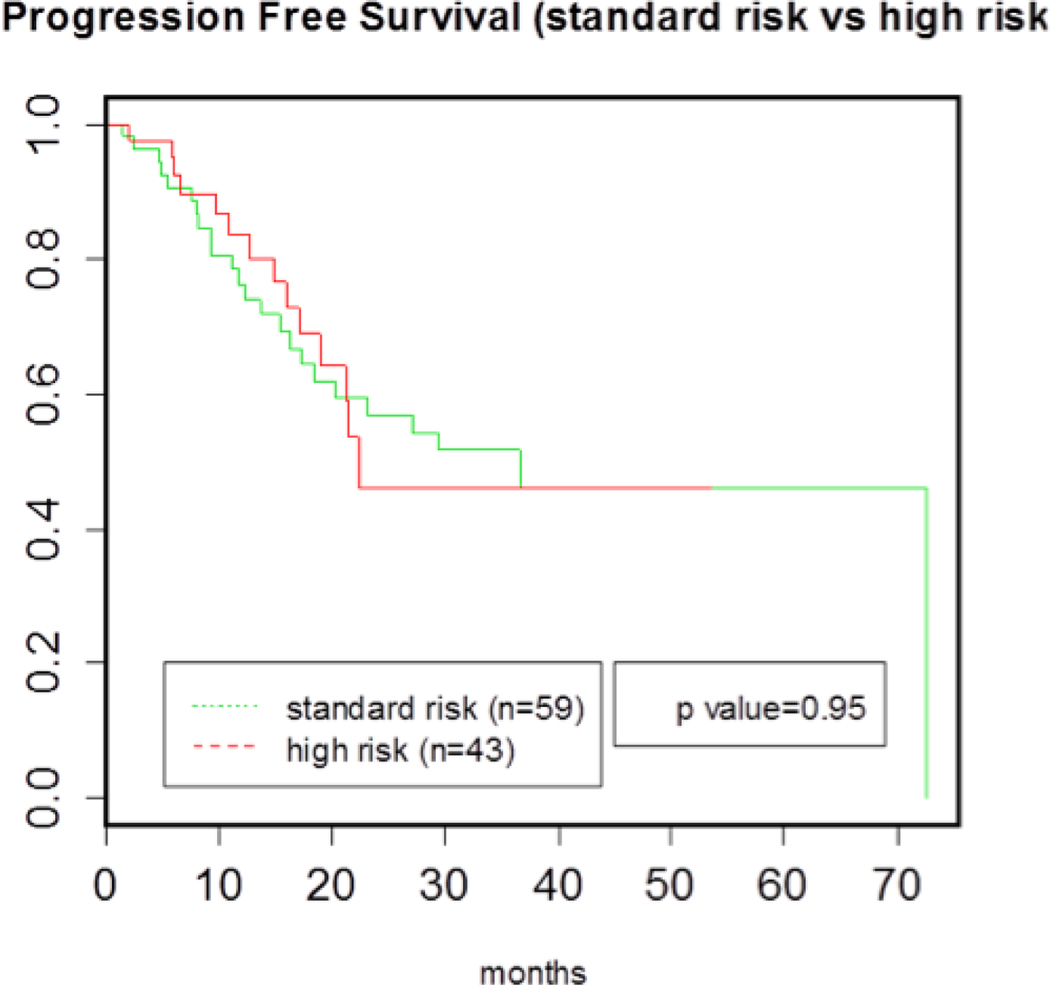
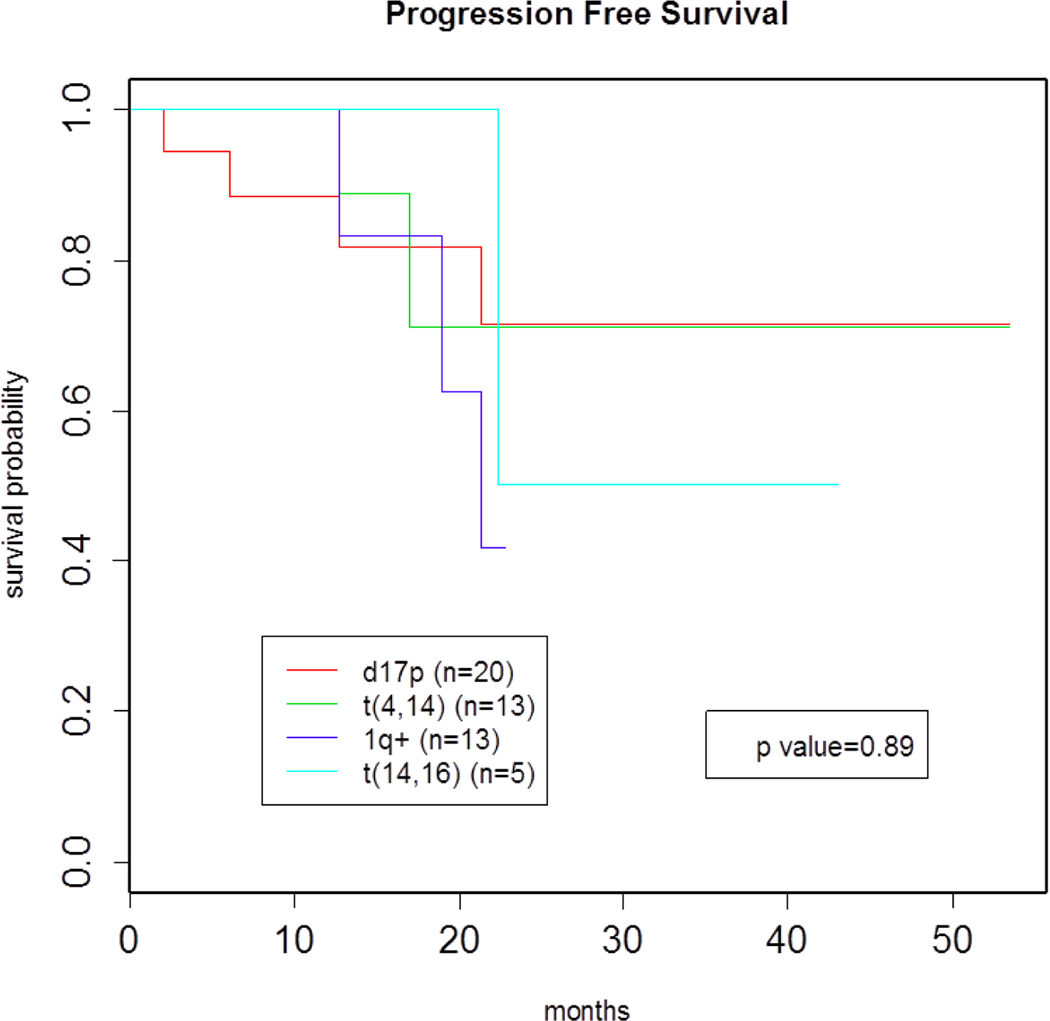
Fluorescent in situ hybridization was available in 99 patients. Of those 100 patients with available data, 42% had high-risk cytogenetics [d17p, t(4;14), +1q, t(14;16)] at time of diagnosis. When compared with patients who had standard-risk cytogenetics at diagnosis, those harboring high-risk cytogenetics receiving bortezomib maintenance therapy did not display a statistically significant inferior PFS (p=.95) (Figure 3). In order to evaluate a possible differential effect of bortezomib maintenance therapy based on patient cytogenetics, subgroup analyses were performed for FISH abnormalities, including t(4;14), d17p, +1q, and t(14;16). In these analyses, patients with various high risk FISH abnormalities were compared against each other and with standard-risk patients i.e. patients without these cytogenetic abnormalities. In analyses, there were no statistically significant differences in PFS for any of the high-risk subgroups when compared with each other and with the standard-risk cohort, though the sample sizes for the high-risk subgroups were small (Figure 4).
Figure 3.
PFS of high risk and standard risk cohorts
Figure 4.
PFS of high risk subgroups
Discussion
Despite significant progress in the treatment of Multiple Myeloma, disease relapse and the corresponding necessity for multiple sequential treatments remains a mainstay of the disease. In order to prolong PFS post-ASCT, a low dose maintenance therapy is often administered. The selection of maintenance therapy is a major decision for physicians, and currently we do not have conclusive guidelines regarding the use of these agents [25]. Therapy with low dose lenalidomide is the most widely used maintenance strategy for patients with MM post-transplant. Some patients are unable to tolerate lenalidomide, have high-risk myeloma, renal insufficiency, or a previous history of other cancer; thus, an alternative bortezomib based maintenance strategy has been explored. Unfortunately, we currently do not have adequate guidelines for how long to continue bortezomib in the maintenance setting as well as if it is truly comparable to lenalidomide [26].
This retrospective analysis indicates that that patients can benefit from post-transplant bortezomib maintenance, with median PFS from date of maintenance initiation of 36.5 months and OS of 72.7 months, which is comparable to the historical data reported by other studies exploring bortezomib in the maintenance setting [22, 23]. Additionally, we found that maintenance with bortezomib is well tolerated with minimal toxicities and that patients were able to stay on maintenance bortezomib until progression with no evidence of secondary malignancies. Peripheral neuropathy related to the therapy was the most prevalent toxicity experienced. Among those who received bortezomib maintenance, 31% experienced some degree of peripheral neuropathy. Overall this maintenance strategy was well tolerated, even in those with renal insufficiency, with only 2% of patients discontinuing therapy due to side effects. Patients who were cytogenetically defined as high-risk derived similar benefit from this maintenance strategy as those with standard-risk disease. Progression-free survival in the high-risk and standard-risk cohorts was not statistically significantly different, indicating that bortezomib maintenance impacted positively on high-risk patients, a cohort that historically when treated with lenalidomide maintenance, failed to achieve a PFS comparable to patients with standard-risk disease. Our subgroup analysis did not show a statistically significant difference in PFS among high-risk subgroups when compared with each other as well as with the standard-risk cohort, though our sample sizes for the high-risk subgroups were small.
It is important to acknowledge the limitations of this retrospective study. Primarily, that the sample size of 102 patients was relatively small, and the median follow-up time of 20.45 months was short. Additionally, the sample sizes for the high-risk cohorts were low enough such that we cannot draw any definite conclusions as to whether patients harboring certain types of high-risk cytogenetics benefit more or less from maintenance with bortezomib.
The prospective randomized trial evaluating bortezomib in the maintenance setting conducted by the HOVON/GMMG group has been updated to include a long-term follow-up and subgroup analysis. This follow-up asserted that bortezomib based induction followed by maintenance therapy with bortezomib post-ASCT significantly improved PFS and OS in patients harboring the d17p cytogenetic abnormality, and nearly completely overcame the negative impact conferred by this abnormality. However, subgroups harboring the t(4;14) and +1q abnormalities did not benefit to such a degree, and the negative impact conferred by these abnormalities remained significant. Additional studies are required to determine whether or not patients with t(4;14) and +1q truly benefit from maintenance therapy with bortezomib [27]. The role of maintenance therapy in the context of the current treatment landscape remains a topic that must be further explored. It is widely accepted that the use of post-transplant maintenance therapy extends time to progression post-transplant for patients with MM. However it has also been suggested that the imposition of long-term maintenance therapy may lead to the selection of more aggressive, drug resistant sub-clones, specifically in the study by Attal et al., in which PFS and OS after first progression were shorter in the cohort that received maintenance therapy with lenalidomide [19, 28].
Newly released agents are beginning to be considered for use as potential maintenance therapies post-ASCT. Particularly ixazomib, a second-generation proteasome inhibitor, represents a new option that may eventually replace bortezomib in the maintenance setting. Ixazomib is the first oral proteasome inhibitor to be approved by the FDA. Side effects appear to be more manageable than those of bortezomib, with a lower incidence of peripheral neuropathy. Its oral administration and side effect profile present an attractive and convenient option for patients who would have otherwise received post transplant maintenance with bortezomib [29–30]. An abstract from a phase II trial recently evaluated ixazomib given in combination with lenalidomide in the maintenance setting (ASH 2015). Overall, the incidence of adverse events was comparable to that of historical data on maintenance with lenalidomide alone. At time of abstract presentation, the median PFS had not been reached, and the estimated 2-year PFS was 83%. Next generation therapies, especially immunostimulatory monoclonal antibodies, should also be studied further to determine their potential benefit in the maintenance setting [31–32].
In summary, despite the benefits conferred by ASCT, nearly all patients relapse posttransplant. Maintenance therapy represents an important strategy by which to delay disease progression and relapse. Therefore, comprehensive guidelines for the use of maintenance therapy are important for physicians to effectively communicate the duration of therapy, potential toxicities, and PFS and OS benefits to patients. Our study concludes that bortezomib in the maintenance setting is well tolerated, efficacious, and can be continued without evidence of secondary malignancy until disease progression. Our data suggests that this maintenance strategy confers a PFS benefit to patients with high-risk cytogenetics, a population that has been reported to derive limited benefit from maintenance with lenalidomide. Next generation agents such as ixazomib are being explored in the maintenance setting and may further reduce maintenance-related toxicities and likely impact even more favorably on PFS and OS than our current strategies. Additional large-scale studies are required to evaluate treatment outcomes in patients with MM who received maintenance therapy compared to no maintenance post-ASCT as well as to evaluate the possibility of long-term maintenance eventually giving rise to more drug-resistant sub-clones, and finally to further evaluate subgroups that may benefit the most from one maintenance strategy over another.
Highlights.
Retrospective review analyzing outcomes of maintenance therapy with bortezomib post autologous stem cell transplantation (ASCT)
Total of 102 patients treated from 2005–2015 analyzed
Total of 2% of patients required discontinuation of bortezomib maintenance due to adverse events.
Bortezomib in the maintenance setting is well tolerated, efficacious, and can be continued without evidence of secondary malignancy until disease progression.
Acknowledgments
We gratefully acknowledge the work of the nurses of Adult Bone Marrow Transplant Clinic at Duke University Medical Center. Additionally, we acknowledge the work of Kenneth Glander and Leslie Digby for their efforts in supporting research and authorship. 5KL2TR001115-03.
Footnotes
Conflict of Interest
C.G. serves as a speaker/consultant/advisor to Celgene, Takeda Oncology, and Onyx, and has received research funding from Celgene. D.A.R. serves as a speaker to Celgene. All other authors have stated that they have no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 2.Palumbo A, Bringhen S, Petrucci MT, et al. Intermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: results of a randomized controlled trial. Blood. 2004;104(10):3052–3057. doi: 10.1182/blood-2004-02-0408. [DOI] [PubMed] [Google Scholar]
- 3.Palumbo A, Cavallo F, Gay F, et al. Autologous Transplantation and Maintenance Therapy in Multiple Myeloma. New England Journal of Medicine. 2014;371(10):895–905. doi: 10.1056/NEJMoa1402888. [DOI] [PubMed] [Google Scholar]
- 4.Kumar SK, Therneau TM, Gertz MA, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004;79(7):867–874. doi: 10.1016/S0025-6196(11)62152-6. [DOI] [PubMed] [Google Scholar]
- 5.Harousseau J-L, Moreau P. Autologous Hematopoietic Stem-Cell Transplantation for Multiple Myeloma. New England Journal of Medicine. 2009;360(25):2645–2654. doi: 10.1056/NEJMct0805626. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy PL, Hahn T. Strategies for induction, autologous hematopoietic stem cell transplantation, consolidation, and maintenance for transplantation-eligible multiple myeloma patients. Hematology Am Soc Hematol Educ Program. 2013;2013:496–503. doi: 10.1182/asheducation-2013.1.496. [DOI] [PubMed] [Google Scholar]
- 7.Morgan GJ, Walker BA, Davies FE. The genetic architecture of multiple myeloma. Nat Rev Cancer. 2012;12(5):335–348. doi: 10.1038/nrc3257. [DOI] [PubMed] [Google Scholar]
- 8.Cornell RF, Costa LJ, Kassim AA, et al. Post-Transplant Therapy Is More Important Than Induction Regimen Choice in Autologous Hematopoietic Cell Transplantation (AHCT) Recipients for Multiple Myeloma (MM). Blood. 2015;126(23):396-396. [Google Scholar]
- 9.Fritz E, Ludwig H. Interferon-alpha treatment in multiple myeloma: meta-analysis of 30 randomised trials among 3948 patients. Ann Oncol. 2000;11(11):1427–1436. [DOI] [PubMed] [Google Scholar]
- 10.Myeloma Trialists’ Collaborative Group. Interferon as therapy for multiple myeloma: an individual patient data overview of 24 randomized trials and 4012 patients. Br J Haematol. 2001;113(4):1020–1034. [DOI] [PubMed] [Google Scholar]
- 11.Berenson JR, Crowley JJ, Grogan TM, et al. Maintenance therapy with alternate-day prednisone improves survival in multiple myeloma patients. Blood. 2002;99(9):3163–3168. [DOI] [PubMed] [Google Scholar]
- 12.Shustik C, Belch A, Robinson S, et al. A randomised comparison of melphalan with prednisone or dexamethasone as induction therapy and dexamethasone or observation as maintenance therapy in multiple myeloma: NCIC CTG MY.7. Br J Haematol. 2007;136(2):203–211. doi: 10.1111/j.1365-2141.2006.06405.x. [DOI] [PubMed] [Google Scholar]
- 13.Attal M, Harousseau J-L, Leyvraz S, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108(10):3289–3294. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 14.Lokhorst HM, van der Holt B, Zweegman S, et al. A randomized phase 3 study on the effect of thalidomide combined with adriamycin, dexamethasone, and high-dose melphalan, followed by thalidomide maintenance in patients with multiple myeloma. Blood. 2010;115(6):1113–1120. doi: 10.1182/blood-2009-05-222539. [DOI] [PubMed] [Google Scholar]
- 15.Maiolino A, Hungria VTM, Garnica M, et al. Thalidomide plus dexamethasone as a maintenance therapy after autologous hematopoietic stem cell transplantation improves progression-free survival in multiple myeloma. Am J Hematol. 2012;87(10):948–952. doi: 10.1002/ajh.23274. [DOI] [PubMed] [Google Scholar]
- 16.Stewart AK, Trudel S, Bahlis NJ, et al. A randomized phase 3 trial of thalidomide and prednisone as maintenance therapy after ASCT in patients with MM with a quality-of-life assessment: the National Cancer Institute of Canada Clinicals Trials Group Myeloma 10 Trial. Blood. 2013;121(9):1517–1523. doi: 10.1182/blood-2012-09-451872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan GJ, Davies FE, Gregory WM, et al. Long-term follow-up of MRC Myeloma IX trial: Survival outcomes with bisphosphonate and thalidomide treatment. Clin Cancer Res. 2013;19(21):6030–6038. doi: 10.1158/1078-0432.CCR-12-3211. [DOI] [PubMed] [Google Scholar]
- 18.Morgan GJ, Gregory WM, Davies FE, et al. The role of maintenance thalidomide therapy in multiple myeloma: MRC Myeloma IX results and meta-analysis. Blood. 2012;119(1):7–15. doi: 10.1182/blood-2011-06-357038. [DOI] [PubMed] [Google Scholar]
- 19.Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide Maintenance after Stem-Cell Transplantation for Multiple Myeloma. New England Journal of Medicine. 2012;366(19):1782–1791. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen D, Frezza M, Schmitt S, Kanwar J, Dou QP. Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives. Curr Cancer Drug Targets. 2011;11(3):239–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonneveld P, Schmidt-Wolf IGH, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J Clin Oncol. 2012;30(24):2946–2955. doi: 10.1200/JCO.2011.39.6820. [DOI] [PubMed] [Google Scholar]
- 23.Rosiñol L, Oriol A, Teruel AI, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012;120(8):1589–1596. doi: 10.1182/blood-2012-02-408922. [DOI] [PubMed] [Google Scholar]
- 24.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. The Lancet Oncology. 2014;15(12):e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 25.Shank BR, Brown VT, Schwartz RN. Multiple myeloma maintenance therapy: a review of the pharmacologic treatment. J Oncol Pharm Pract. 2015;21(1):36–51. doi: 10.1177/1078155213514468. [DOI] [PubMed] [Google Scholar]
- 26.Nathwani N, Larsen JT, Kapoor P. Consolidation and Maintenance Therapies for Newly Diagnosed Multiple Myeloma in the Era of Novel Agents. Curr Hematol Malig Rep. 2016;11(2):127–136. doi: 10.1007/s11899-016-0310-9. [DOI] [PubMed] [Google Scholar]
- 27.Paper: Bortezomib Induction and Maintenance in Patients with Newly Diagnosed Multiple Myeloma: Long-Term Follow-up of the HOVON-65/GMMG-HD4 Trial. https://ash.confex.com/ash/2015/webprogram/Paper79113.html. Accessed May 26, 2016.
- 28.Al-Mansour Z, Ramanathan M, Al-Mansour Z, Ramanathan M. Post- Autologous (ASCT) Stem Cell Transplant Therapy in Multiple Myeloma, Post-Autologous (ASCT) Stem Cell Transplant Therapy in Multiple Myeloma. Advances in Hematology, Advances in Hematology. 2014;2014, 2014:e652395. doi: 10.1155/2014/652395, 10.1155/2014/652395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azab AK, Muz B, Ghazarian R, Ou M, Luderer M, Kusdono H. Spotlight on ixazomib: potential in the treatment of multiple myeloma. Drug Design, Development and Therapy. January 2016:217. doi: 10.2147/DDDT.S93602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paper: Phase II Study of the Combination of Ixazomib with Lenalidomide As Maintenance Therapy Following Autologous Stem Cell Transplant in Patients with Multiple Myeloma. https://ash.confex.com/ash/2015/webprogram/Paper83552.html. Accessed May 26, 2016.
- 31.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. The Lancet Oncology. 2014;15(12):e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y-C, Szmania S, van Rhee F. Profile of elotuzumab and its potential in the treatment of multiple myeloma. Blood Lymphat Cancer. 2014;2014(4):15–27. doi: 10.2147/BLCTT.S49780. [DOI] [PMC free article] [PubMed] [Google Scholar]