Abstract
The erector spinae plane block (ESPB) is a novel fascial planar block technique, which is used to reduce postoperative pain in several surgical procedures, including breast, thoracic, spine and hip surgery. Due to its recognizable anatomy and low complication rate, the application of ESPB has been significantly increased. However, it is rarely used in clinical practice for postoperative analgesia after posterior lumbar spine surgery, while the choice of adjuvant drugs, block levels and drug doses remain controversial. Based on the current literature review, ropivacaine and dexmedetomidine could be considered as the best available drug combination. The present review aimed to analyze the currently available clinical evidence and summarize the benefits and challenges of ESPB in spinal surgery, thus providing novel insights into the application of ESPB in the postoperative management of posterior lumbar surgery.
Keywords: ESPB, posterior lumbar surgery, nerve block, analgesia
1. Introduction
Patients undergoing posterior lumbar spine surgery commonly experience severe postoperative pain due to skin and paraspinal tissue dissection and bone tissue removal. In a study comparing the intensity of postoperative pain on the first day after surgery, in 179 different surgical procedures across a wide range of surgical specialties, lumbar spinal fusion surgery ranked second in terms of pain intensity (1,2). Inadequate control of acute pain can potentially lead to the development of chronic pain, which can significantly affect patients' recovery and postoperative quality of life (3).
The most common postoperative pain management approaches for posterior lumbar spine surgery include oral or intramuscular opioid medications, patient-controlled intravenous analgesia, epidural analgesia and continuous wound infiltration. These pain management strategies are commonly associated with the significant administration of opioid medications, which can interfere with normal gastrointestinal motility, increase the incidence of respiratory complications and prolong hospital stay (4). Strategies for promoting rapid postoperative recovery typically include the use of regional anesthetic techniques, which can reduce the use of opioid medications (5).
There are several analgesic options for postoperative lumbar spine surgery, including traditional local wound infiltration anesthesia, erector spinae plane block (ESPB) and thoracolumbar interfacial plane (TLIP) block (6). Local wound infiltration anesthesia is characterized by the infusion of anesthetics at the incision site, thus reducing pain via blocking peripheral nerve endings. This is a simple and safe analgesic option that relieves postoperative pain and reduces the use of opioids. However, this option can be applied only in a few hours after surgery and has a limited range of blockade (7). Since lumbar spine surgery requires stripping the paraspinal muscles and removing part of the vertebrae, analgesia is mainly aimed at blocking the dorsal branch of the spinal nerves that innervate the spine and paraspinal tissues (8). ESPB functions via blocking the dorsal branch of the spinal nerves, which is considered as the best way to alleviate pain after lumbar spine surgery. In addition, it is applied far away from the incision site, thus reducing the risk of poor incision healing and infection. The aforementioned finding was verified by a previous randomized controlled trial, where ESPB and local wound infiltration were performed with the same dose of anesthetics in lumbar spine surgery. Therefore, lower postoperative numerical rating scale scores, decreased postoperative opioid consumption and shorter hospital stay were recorded in the ESPB group, thus further supporting the beneficial effect of ESPB on controlling postoperative pain and reducing opioid consumption (9).
TLIP, originally proposed by Hand et al (10), blocks the posterior branch of the spinal nerve and its branches across the paraspinal muscles via the injection of anesthetic drugs into the fascial plane between the longest and the multifidus muscle. This method was later modified via injecting anesthetic drugs into the fascial plane between the longest and the iliopsoas muscle away from the midline (11). TLIP is an intermuscular fascial plane block, which is prone to deforming the lumbar spinal structures, thus interfering with the recognition of the injection site and the diffusion of local anesthetic (LA) drugs (12). ESPB can inject anesthetics deep into the muscle, which in turn can spread over the fatty interosseous compartment to the cranial and caudal sides, thus allowing for a wider range of block and avoiding the drug from being washed out during surgery. Therefore, it mainly focusses on lumbar spine postoperative analgesia and can therefore improve the duration and quality of analgesia (13). A study based on the efficacy and safety of ESPB and TLIP in spinal surgery demonstrated that ESPB exhibited lower pain scores and reduced opioid consumption in the short-term postoperative period and improved analgesic effects compared with TLIP, thus effectively relieving lumbar postoperative pain (14).
In summary, analgesia after lumbar spine surgery is currently a thorny problem for surgeons, yet there is no perfect solution. The ESPB is a new fascial plane block technique that is simple to perform, has a low learning curve, achieves effective analgesia in the acute postoperative period, and has fewer complications, making it a highly desirable postoperative analgesic solution for lumbar spine. Moreover, it has significant advantages over other analgesic protocols such as local wound infiltration anesthesia and TLIP. Secondly, the application of ESPB in postoperative analgesia after lumbar spine surgery is relatively rare (15), and there are still more controversies about adjuvant drugs, block level selection and drug dosage. There are fewer reviews that comprehensively summarize adjuvant drugs, block level selection and drug dosage. Finally, previous and recent studies were summarized and the currently more academically accepted ESPB-based lumbar postoperative analgesia regimen was proposed. Therefore, the present review may be important for further in-depth clinical or basic research.
2. Origin and mechanism of ESPB
ESPB, a relatively novel regional anesthesia technique introduced by Forero et al (16) in 2016, involves the injection of a LA into the fascial plane between the transverse process (TP) and the erector spinae muscle (Fig. 1). LAs can diffuse through the fascial plane into a cranio-caudal and medial-lateral direction, thus resulting in blockade of the posterior branches of the spinal nerves around the erector spinae muscle, eventually promoting analgesia. Currently, the particular mechanisms underlying the effects of ESPB are not fully understood. The prevailing hypothesis is based on the idea that LAs diffuse cranially and caudally through the thoracolumbar fascia, thus resulting in multiple areas of sensory block (17). Several studies have suggested that the mechanism involves the spread of LAs into the paravertebral space, intervertebral foramina and extradural space, thereby reaching the dorsal branches of the spinal nerves and the communicating branches of the sympathetic chain, eventually causing blockade (18). Another potential mechanism is based on the systemic absorption of Las (19).
Figure 1.
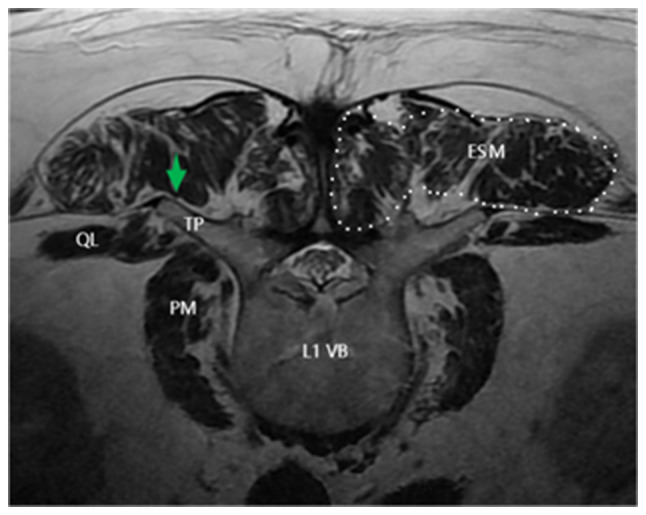
Cross sectional magnetic resonance imaging of the L1 VB. The blocking site, namely the fascia compartment of the erector spinal muscle, is indicated by an arrow. L1 VB, lumbar 1 vertebral body; ESM, erectoral spine muscle; TP, transverse process; QL, quadratus lumborum; PM, psoas major.
3. Ultrasound-guided ESPB
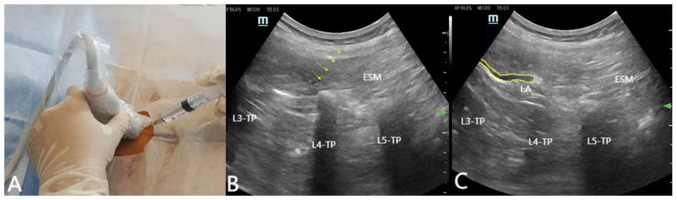
Ultrasound-guided ESPB allows clinicians and patients to avoid ionizing radiation and is recommended in particular populations, such as obstetrics or pediatrics (20). The aforementioned method allows for more precise targeting and real-time monitoring of the extent of drug diffusion during injection. Following the induction of general anesthesia, the patient is placed in the prone position and bilateral ESPB is performed prior the surgical incision. The surgical procedure is shown in Fig. 2. An ultrasound probe is first used to locate the sacrum in the sagittal plane and then the probe is moved cephalad to find the corresponding lumbar vertebral level. A high-frequency linear probe is positioned into the parasagittal midline to identify the spinous processes. The probe is then moved laterally to visualize the tip of the TP and the erector spinae muscles ~4-6 cm lateral to the spinous process. Τhe needle is subsequently tilted to the side of the head and is pulled back slightly when it touches the transverse protuberance. To verify and open the correct plane, a saline hydro-dissection is conducted. After the correct position of the needle is verified, repeated aspiration is performed. Ιf no blood is drawn, then 20-30 ml of LA solution is injected (21,22). Although TLIF and PLIF surgery in the posterior lumbar can expose muscles to the articular process joints or the base of the TP, surgeons can more accurately and directly inject drugs into the fascia plane between the TP and the erector spinae muscles before wound suturing. However, ultrasound guided ESPB has the following advantages: Firstly, an intact deep fascial plane is necessary for improved diffusion of the anesthetic solution craniolaterally and caudolaterally to achieve greater analgesia. Secondly, ESPB is time-delayed, and in order to prevent the anesthetic effects of ESPB from being untimely in the postoperative period, it is usually necessary to perform the block earlier. Finally, lumbar spine surgery is becoming increasingly inclined to be minimally invasive, and for most procedures that do not require lumbar muscle exposure, the advantages of preoperative ESPB are even more pronounced.
Figure 2.
(A) Ultrasound-guided ESPB at L4 site; the patient is female (68 years old; weight, 75 kg; height, 155 cm). (B) Ultrasound image showing the trajectory of the needle in the L4 plane during the ESPB process (arrow). (C) The area within the yellow line indicates the deep diffusion of the local anesthetic into the erector spinal muscle. ESPB, erector spinae plane block; L4, lumbar 4.
4. Selection of anesthetics and adjuvants for ESPB
Selection of LA drugs
ESPB has been extensively used in the postoperative period in several surgical procedures, including breast, thoracic, abdominal and spinal surgery. Its effectiveness has been confirmed (15). However, the analgesic effect of a single block is usually temporary, lasting no more than 24 h. The difference in block time mainly depends on the type of LA used and the amount of injection (23). Currently, the most commonly used anesthetics for ESPB include ropivacaine and bupivacaine, at concentrations of 0.2-0.5% (24). The analgesic mechanisms of ropivacaine and bupivacaine have been previously elucidated in both basic and clinical studies. Therefore, a study reported that both drugs could preferentially block c-, A-delta and A-beta fibers, thus inhibiting pain transmission and exerting analgesic effects (25). Compared with lidocaine, ropivacaine and bupivacaine are characterized by higher drug dissociation and constant lipid solubility, which is beneficial for diffusion within the nerve and ion channel blockade (26), eventually resulting in a faster onset of action and improved analgesic efficacy. Ropivacaine and bupivacaine are medium- to long-acting LAs and their analgesic effect lasts for ~8-12 h. Therefore, simply increasing their concentration or dosage cannot significantly extend their analgesic effect (27,28). However, severe pain after posterior lumbar surgery commonly occurs on the first day after surgery. Since single block alone is not sufficient to reduce postoperative pain and opioid consumption, high doses of opioids are required to relief patients' pain within the first day after surgery. Therefore, extending the duration of a single ESPB for pain relief is of great importance.
Adjuvant
The placement of a catheter for nerve block can prolong postoperative analgesia. However, catheter placement can increase operation time, cost and the risk of infection and neurological complications (29). To overcome this shortcoming, various adjuvants, such as epinephrine (30), sufentanil (31), midazolam (32), clonidine (33) and butorphanol (34) have been used in combination with anesthetics to prolong analgesia, however, with limited success. A previous study identified dexmedetomidine and dexamethasone as clinically effective adjuvants (35).
Dexmedetomidine, an alpha-adrenergic receptor agonist, is a highly selective drug with anti-anxiety, sedative and analgesic properties (36). It is currently receiving increasing attention as an adjunct to regional anesthesia. Previous studies also revealed that the application of dexmedetomidine combined with LAs in nerve and fascia blocks could accelerate block onset, prolong block duration, enhance analgesic effects and significantly reduce opioid consumption (37,38). The locking mechanism could be as follows: Dexmedetomidine is an imidazole derivative with a prolonged analgesic action (39). It has been reported that it can promote vasoconstriction, delay the absorption of LAs and prolong the effect of Las (40). Another study suggested that dexmedetomidine could block hyperpolarized sodium and potassium ion currents, thus attenuating acute LA-induced peripheral inflammation (41). Additionally, the combination of dexmedetomidine with ropivacaine in ESPB could prolong sensory blockade for 18-24 h, thus significantly prolonging postoperative analgesia and sleep duration, ultimately resulting in rapid recovery (21). A previous meta-analysis indicated that low dose of dexmedetomidine, as an adjunct to ESPB, exerted a beneficial effect on postoperative analgesia and reduction of nausea, without increasing the risk of arrhythmias and hypotension. Different doses of dexmedetomidine exhibited different effects on prolonging analgesia, with dexmedetomidine at doses of 1 and 0.5 µg/kg prolonging analgesia by ~11 and 4.86 h, respectively (42). Although increasing evidence has suggested that dexmedetomidine, as an adjunct to LA, can prolong analgesia, further subgroup studies are needed to determine the optimal drug doses and its associated complications.
It has been verified that dexamethasone, a highly potent and long-lasting glucocorticoid, has analgesic and anti-inflammatory effects (43). The combination of dexamethasone with LAs cannot only shorten the onset of sensory and motor blockade, but also prolong the duration of analgesia or sensory block. Its prolonged analgesic effect could be due to the capacity of dexamethasone to inhibit potassium channels through glucocorticoid receptors, thus reducing the activity of pain sensory C-fibers (44). Dexamethasone is a steroid that promotes vasoconstriction and reduces the absorption of Las (45). Once absorbed into the blood vessels, dexamethasone has a systemic anti-inflammatory effect. A study suggested that co-administration of dexamethasone with LAs (ropivacaine or bupivacaine) could notably prolong nerve blockade. Although bupivacaine demonstrated a longer duration of blockade compared with ropivacaine, its combined effect with dexamethasone resulted in an almost identical analgesia at 22 h (1.9-fold for ropivacaine and 1.5-fold for bupivacaine) (28). The aforementioned findings suggested that prolongation of analgesia could be associated with the co-administration of dexamethasone. However, further dose-related studies are needed to improve elucidation of the optimal balance between drug doses, efficacy and side effects, particularly in low-dose studies.
5. Injection plane and amount
There is an ongoing debate regarding the efficacy, injection site and dosage of ESPB. Due to the relatively larger size and thicker fascia surrounding the lumbar erector spinae muscles, lumbar ESP injections have limited cranial and caudal diffusion compared with the thoracic ones. In lumbar posterior surgery, the lower thoracic ESPB can only extend to the L2-L3 level. Therefore, the analgesic efficacy of high-level injections remains unconfirmed (46). A study investigating ESPB at different levels, including the thoracic, lumbar and responsible vertebral levels, indicated that performing ESPB at the respective vertebral incision level could be the most appropriate approach compared with the fixed lumbar or thoracic levels (15). However, the majority of studies do not provide a precise description of the injection site. In another cadaveric study investigating drug spread in the lumbar erector spinae plane, 20 ml solution was injected into 12 erector spinae planes of six cadavers. The selected injection site was at the midpoint between the spinous and TP. Finally, two samples showed anterior spread beyond the TP (47). However, in another experimental study conducted on five cadavers with a total of nine erector spinae muscle regions, injection of 20 ml solution exhibited no anterior spread in all samples. However, the selected injection site was at the tip of the TP (48). The aforementioned results indicated that different injection sites in the same plane could result in varying range of solution spread, with injections closer to the midline and paravertebral space being more likely to penetrate the paravertebral space or the lumbar plexus, thus leading to lower limb motor weakness (46).
In addition to the injection site, the effectiveness of blockade varies with the volume of anesthetic injected. Studies on anesthetic injection volumes tended to favor larger anesthetic volumes, with the majority of them reporting a volume of 20-30 ml per side as the appropriate anesthetic volume after spinal surgery (25). A cadaveric study revealed that injecting 20 ml dye on one side resulted in limited spread only to the posterior margin of the TP. However, injection with 30 or 40 ml of dye promoted its spread to the anterior TP area, the paravertebral space of the lumbar spine and part of the lumbar plexus (49). The aforementioned finding suggested that ESPB exhibited volume-dependent diffusion. However, the aforementioned cadaveric study had some limitations, since dye diffusion in the living human body could be different, with particular anatomical features affecting propagation. In addition, there could be a variation in the permeability and spreading potential of LAs compared with dye solutions.
Data on the association between injection sites and volumes and the diffusion of LAs are listed in Table I. These data could be used as a reference for the level of ESPB in posterior lumbar surgery. According to Table I, a volume of ~4-6 ml LA is typically required to cover a single lumbar vertebral body. The most common blocks are at the L3 and L4 levels. Therefore, at the aforementioned levels, 20 ml of LA can cover almost the entire lumbar vertebral level and diffuse to the L2-S1 level, respectively. Therefore, increasing the volume of anesthetic can extend the diffusion of the LA to cover more vertebral levels.
Table I.
Relationship between local anesthetic volume and diffusion level.
| (Refs.) | Year | Experimental sample type | Local anesthetic | Capacity (one side) | Blocking site | Diffusion level | Capacity/number of segments |
|---|---|---|---|---|---|---|---|
| Tulgar and Senturk (50) | 2018 | Patient | Bupivacaine 0.25% + lidocaine 0.3% | 30 ml | L4 | T12-L4 | 6 |
| Chung and Kim (51) | 2018 | Patient | 5 ml 2% lidocaine and 15 ml contrast medium | 20 ml | L4 | L2-S1 | 4 |
| De Lara González et al (47) | 2019 | Corpse | Contrast medium | 20 ml | L4 | L2-L5 | 5 |
| Mantuani et al (52) | 2019 | Patient | 20 ml of 1% lidocaine containing adrenaline | 20 ml | L1 | T10-L2 | 4 |
| Celik et al (53) | 2019 | Patient | 40 ml injection, including 20 ml bupivacaine, 10 ml lidocaine, 8.6 ml physiological saline, 40 mg/ml methylprednisolone, 1 ml, and 0.4 ml contrast agent | 40 ml | L4 | L1-S4 | 4.4 |
| Ahiskalioglu et al (54) | 2020 | Patient | 20 ml 0.5% bupivacaine, 10 ml 2% lidocaine, and 10 ml physiological saline | 20 ml | L4 | L2-L5 | 5 |
| Breidenbach et al (55) | 2023 | Corpse | 1 ml methylene blue and 19 ml 0.25% bupivacaine | 20 ml | L4 | L2-S1 | 4 |
| Zhang et al (56) | 2021 | Patient | 20 ml 0.4% ropivacaine | 20 ml | L3 | L1-L5 | 4 |
| Yi-Han et al (21) | 2022 | Patient | 20 ml 0.375% ropivacaine and 20 ml dexmedetomidine | 20 ml | L3 | L1-L5 | 4 |
In summary, the current literature review indicated that ropivacaine and dexmedetomidine could be the best currently available drug combination for posterior lumbar surgery. Therefore, the combination of ropivacaine at a concentration of 0.375% with 1 µg/kg dexmedetomidine, with 20 ml of the drug injected in the plane of the lumbar surgical site, is considered as the optimal analgesic regimen for posterior lumbar surgery (21,42,57,58).
6. Complications
ESPB is characterized by a low complication rate, including local infection, nerve and vessel injury, hematoma formation, LA toxicity reactions, block failure, as well as specific spinal surgery-related complications, such as lower limb motor and sensory impairment (55,59). However, it has been reported that when lower limb motor and sensory impairments occur after ESPB, neurologic recovery typically follows a distal-to-proximal pattern. Therefore, impairments are gradually improved from the periphery to the center, with sensory recovery preceding motor one. This pattern is commonly observed ~90 min after patient awakening from general anesthesia (60).
7. Limitations and drawbacks
Since ESPB delays postoperative analgesia in the lumbar spine and it often takes ~1.5 h to achieve maximal analgesic efficacy, it is commonly administered prior anesthesia (61). Ultrasound-guided ESPB often requires additional preparation space, time and sedation (62). Accurate ultrasound localization in obese patients and severely degenerated spinal structures remains a challenge (20). However, obesity and severe spinal degeneration are common in lumbar fusion surgery.
8. Conclusions
In posterior lumbar surgery, ESPB can alleviate postoperative pain, reduce opioid consumption and shorten hospital stay, thus making it a safe and effective approach. By adjusting the choice of LA and the level of blockade, maximum pain relief can be achieved, thus providing postoperative comfort and improving sleep in patients undergoing lumbar surgery, eventually enhancing their satisfaction. Ropivacaine and dexmedetomidine could be considered as the best currently available drug combination. The current review aimed to help spine surgeons to safely incorporate these blockade techniques into the clinical practice, thus accelerating patient recovery.
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was supported by Jingzhou First People's Hospital Doctoral Research Initiation Fund Project (grant no. 2022DIF01), the Dean's Fund of Southern University of Science and Technology Hospital (grant no. 2022-A2) and Nanshan Health System Science and Technology Major Project (grant no. NSZD2023065).
Availability of data and materials
Not applicable.
Authors' contributions
KD completed the writing of the first draft of the manuscript. KH and GFW proposed ideas, analyzed the data and perfected the manuscript. All authors read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Goel VK, Chandramohan M, Murugan C, Shetty AP, Subramanian B, Kanna RM, Rajasekaran S. Clinical efficacy of ultrasound guided bilateral erector spinae block for single-level lumbar fusion surgery: A prospective, randomized, case-control study. Spine J. 2021;21:1873–1880. doi: 10.1016/j.spinee.2021.06.015. [DOI] [PubMed] [Google Scholar]
- 2.van den Broek RJC, van de Geer R, Schepel NC, Liu WY, Bouwman RA, Versyck B. Evaluation of adding the Erector spinae plane block to standard anesthetic care in patients undergoing posterior lumbar interbody fusion surgery. Sci Rep. 2021;11(7631) doi: 10.1038/s41598-021-87374-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu L, Wang M, Wang X, Wang Y, Chen L, Li J. Changes of opioid consumption after lumbar fusion using ultrasound-guided lumbar erector spinae plane block: A Randomized controlled trial. Pain Physician. 2021;24:E161–E168. [PubMed] [Google Scholar]
- 4.Jin Y, Zhao S, Cai J, Blessing M, Zhao X, Tan H, Li J. Erector spinae plane block for perioperative pain control and short-term outcomes in lumbar laminoplasty: A randomized clinical trial. J Pain Res. 2021;14:2717–2727. doi: 10.2147/JPR.S321514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan M, Law LS, Gan TJ. Optimizing pain management to facilitate Enhanced Recovery After Surgery pathways. Can J Anaesth. 2015;62:203–218. doi: 10.1007/s12630-014-0275-x. [DOI] [PubMed] [Google Scholar]
- 6.Hong B, Baek S, Kang H, Oh C, Jo Y, Lee S, Park S. Regional analgesia techniques for lumbar spine surgery: A frequentist network meta-analysis. Int J Surg. 2023;109:1728–1741. doi: 10.1097/JS9.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kjaergaard M, Moiniche S, Olsen KS. Wound infiltration with local anesthetics for post-operative pain relief in lumbar spine surgery: A systematic review. Acta Anaesthesiol Scand. 2012;56:282–290. doi: 10.1111/j.1399-6576.2011.02629.x. [DOI] [PubMed] [Google Scholar]
- 8.Kraiwattanapong C, Arnuntasupakul V, Kantawan R, Woratanarat P, Keorochana G, Langsanam N. Effect of multimodal drugs infiltration on postoperative pain in split laminectomy of lumbar spine: A randomized controlled trial. Spine (Phila Pa 1976) 2020;45:1687–1695. doi: 10.1097/BRS.0000000000003679. [DOI] [PubMed] [Google Scholar]
- 9.Vergari A, Frassanito L, DI Muro M, Nestorini R, Chierichini A, Rossi M, DI Stasio E. Bilateral lumbar ultrasound-guided erector spinae plane block versus local anesthetic infiltration for perioperative analgesia in lumbar spine surgery: A randomized controlled trial. Minerva Anestesiol. 2022;88:465–471. doi: 10.23736/S0375-9393.22.15950-X. [DOI] [PubMed] [Google Scholar]
- 10.Hand WR, Taylor JM, Harvey NR, Epperson TI, Gunselman RJ, Bolin ED, Whiteley J. Thoracolumbar interfascial plane (TLIP) block: A pilot study in volunteers. Can J Anaesth. 2015;62:1196–1200. doi: 10.1007/s12630-015-0431-y. [DOI] [PubMed] [Google Scholar]
- 11.Bilge A, Basaran B. Postoperative quality of recovery with erector spinae plane block or thoracolumbar interfascial plane block after major spinal surgery: A randomized controlled trial. Eur Spine J. 2024;33:68–76. doi: 10.1007/s00586-023-07998-6. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Wu Y, Dou L, Chen K, Liu Y, Li Y. Comparison of two ultrasound-guided plane blocks for pain and postoperative opioid requirement in lumbar spine fusion surgery: A prospective, randomized, and controlled clinical trial. Pain Ther. 2021;10:1331–1341. doi: 10.1007/s40122-021-00295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tantri AR, Rahmi R, Marsaban AHM, Satoto D, Rahyussalim AJ, Sukmono RB. Comparison of postoperative IL-6 and IL-10 levels following Erector Spinae Plane Block (ESPB) and classical Thoracolumbar Interfascial Plane (TLIP) block in a posterior lumbar decompression and stabilization procedure: A randomized controlled trial. BMC Anesthesiol. 2023;23(13) doi: 10.1186/s12871-023-01973-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng Q, Meng B, Yang S, Ban Z, Zhang Y, Hu M, Zhao W, Wu H, Tao Y, Zhang L. Efficacy and safety of erector spinae plane block versus thoracolumbar interfascial plane block in patients undergoing spine surgery: A systematic review and meta-analysis. Clin J Pain. 2024;40:114–123. doi: 10.1097/AJP.0000000000001177. [DOI] [PubMed] [Google Scholar]
- 15.Oh SK, Lim BG, Won YJ, Lee DK, Kim SS. Analgesic efficacy of erector spinae plane block in lumbar spine surgery: A systematic review and meta-analysis. J Clin Anesth. 2022;78(110647) doi: 10.1016/j.jclinane.2022.110647. [DOI] [PubMed] [Google Scholar]
- 16.Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: A novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41:621–627. doi: 10.1097/AAP.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 17.Oezel L, Hughes AP, Arzani A, Okano I, Amini DA, Moser M, Sama AA, Cammisa FP, Soffin EM. Surgeon-Placed erector spinae plane catheters for multilevel lumbar spine fusion: Technique and outcomes compared with single-shot blocks. Int J Spine Surg. 2022;16:697–705. doi: 10.14444/8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karaca O, Pinar HU. Is high dose lumbar erector spinae plane block safe? Journal of clinical anesthesia. 2020;62(109721) doi: 10.1016/j.jclinane.2020.109721. [DOI] [PubMed] [Google Scholar]
- 19.Viderman D, Aubakirova M, Umbetzhanov Y, Kulkaeva G, Shalekenov SB, Abdildin YG. Ultrasound-Guided erector spinae plane block in thoracolumbar spinal surgery: A systematic review and meta-analysis. Front Med (Lausanne) 2022;9(932101) doi: 10.3389/fmed.2022.932101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim E, Alshoubi A. Fluoroscopic-guided erector spinae plane block for spine surgery. Saudi J Anaesth. 2022;16:229–231. doi: 10.4103/sja.sja_694_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi-Han W, Rong T, Jun L, Min W, Yan Z, Yi L, Jie-Ting L, Sheng-Hui H. Dexmedetomidine combined with ropivacaine for erector spinae plane block after posterior lumbar spine surgery: A randomized controlled trial. BMC Musculoskelet Disord. 2022;23(235) doi: 10.1186/s12891-022-05198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yorukoglu HU, Icli D, Aksu C, Cesur S, Kus A, Gurkan Y. Erector spinae block for postoperative pain management in lumbar disc hernia repair. J Anesth. 2021;35:420–425. doi: 10.1007/s00540-021-02920-0. [DOI] [PubMed] [Google Scholar]
- 23.Finnerty DT, Buggy DJ. Efficacy of the erector spinae plane (ESP) block for quality of recovery in posterior thoraco-lumbar spinal decompression surgery: Study protocol for a randomised controlled trial. Trials. 2021;22(150) doi: 10.1186/s13063-021-05101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao X, Zhu T, Wang L, Zhou H, Zhang Y. Efficacy of postoperative analgesia by erector spinal plane block after lumbar surgery: A systematic review and meta-analysis of randomized controlled trials. Comput Math Methods Med. 2022;2022(3264142) doi: 10.1155/2022/3264142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chin KJ, El-Boghdadly K. Mechanisms of action of the erector spinae plane (ESP) block: A narrative review. Can J Anaesth. 2021;68:387–408. doi: 10.1007/s12630-020-01875-2. [DOI] [PubMed] [Google Scholar]
- 26.Simpson D, Curran MP, Oldfield V, Keating GM. Ropivacaine: A review of its use in regional anaesthesia and acute pain management. Drugs. 2005;65:2675–2717. doi: 10.2165/00003495-200565180-00013. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Li H, Wei S, Zhang G, Ni C, Sun L, Zheng H. Dexmedetomidine added to ropivacaine for ultrasound-guided erector spinae plane block prolongs analgesia duration and reduces perioperative opioid consumption after thoracotomy: A randomized, controlled clinical study. Clin J Pain. 2021;38:8–14. doi: 10.1097/AJP.0000000000000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cummings KC III, Napierkowski DE, Parra-Sanchez I, Kurz A, Dalton JE, Brems JJ, Sessler DI. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth. 2011;107:446–453. doi: 10.1093/bja/aer159. [DOI] [PubMed] [Google Scholar]
- 29.Ilfeld BM. Continuous peripheral nerve blocks: An update of the published evidence and comparison with novel, alternative analgesic modalities. Anesth Analg. 2017;124:308–335. doi: 10.1213/ANE.0000000000001581. [DOI] [PubMed] [Google Scholar]
- 30.Zanfini BA, Biancone M, Famele M, Catarci S, Lavalle R, Frassanito L, Piersanti A, Olivieri C, Lanzone A, Draisci R, Draisci G. Comparison of ropivacaine plasma concentration after posterior Quadratus Lumborum Block in Cesarean Section with ropivacaine with epinephrine vs. plane. Minerva Anestesiol. 2021;87:979–986. doi: 10.23736/S0375-9393.21.15354-4. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Xu M. Comparison of ropivacaine combined with sufentanil for epidural anesthesia and spinal-epidural anesthesia in labor analgesia. BMC Anesthesiol. 2020;20(1) doi: 10.1186/s12871-019-0855-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rekhi BK, Kaur T, Arora D, Dugg P. Comparison of intravenous dexmedetomidine with midazolam in prolonging spinal anaesthesia with ropivacaine. J Clin Diagn Res. 2017;11:UC01–UC04. doi: 10.7860/JCDR/2017/23874.9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saied NN, Gupta RK, Saffour L, Helwani MA. Dexamethasone and clonidine, but not epinephrine, prolong duration of ropivacaine brachial plexus blocks, cross-sectional analysis in outpatient surgery setting. Pain Med. 2017;18:2013–2026. doi: 10.1093/pm/pnw198. [DOI] [PubMed] [Google Scholar]
- 34.Babu S, Gupta BK, Gautam GK. A Comparative study for post operative analgesia in the emergency laparotomies: Thoracic epidural ropivacaine with nalbuphine and ropivacaine with butorphanol. Anesth Essays Res. 2017;11:155–159. doi: 10.4103/0259-1162.186593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albrecht E, Vorobeichik L, Jacot-Guillarmod A, Fournier N, Abdallah FW. Dexamethasone is superior to dexmedetomidine as a perineural adjunct for supraclavicular brachial plexus block: Systematic review and indirect meta-analysis. Anesth Analg. 2019;128:543–554. doi: 10.1213/ANE.0000000000003860. [DOI] [PubMed] [Google Scholar]
- 36.Bajwa S, Kulshrestha A. Dexmedetomidine: An adjuvant making large inroads into clinical practice. Ann Med Health Sci Res. 2013;3:475–483. doi: 10.4103/2141-9248.122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vorobeichik L, Brull R, Abdallah FW. Evidence basis for using perineural dexmedetomidine to enhance the quality of brachial plexus nerve blocks: A systematic review and meta-analysis of randomized controlled trials. Br J Anaesth. 2017;118:167–181. doi: 10.1093/bja/aew411. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Ran G, Chen X, Xie C, Wang J, Liu X, Lu Y, Fang W. The effect of ultrasound-guided erector spinae plane block combined with dexmedetomidine on postoperative analgesia in patients undergoing modified radical mastectomy: A randomized controlled trial. Pain Ther. 2021;10:475–484. doi: 10.1007/s40122-020-00234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Zhang S, Wang C, Huang Y, Wu H, Zhao G, Wang T. Real-time evaluation of the independent analgesic efficacy of dexmedetomidine. BMC Anesthesiol. 2023;23(68) doi: 10.1186/s12871-023-02022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guensch DP, Terbeck S, Gerber D, Erdoes G. Local vasoconstriction following ropivacaine/dexmedetomidine parasternal block in a neonate. Paediatr Anaesth. 2023;33:1108–1109. doi: 10.1111/pan.14744. [DOI] [PubMed] [Google Scholar]
- 41.Shi T, Zhao J, Long K, Gao M, Chen F, Chen X, Zhang Y, Huang B, Shao D, Yang C, et al. Cationic mesoporous silica nanoparticles alleviate osteoarthritis by targeting multiple inflammatory mediators. Biomaterials. 2023;303(122366) doi: 10.1016/j.biomaterials.2023.122366. [DOI] [PubMed] [Google Scholar]
- 42.Yu L, Shen X, Liu H. The effect and safety of dexmedetomidine as an adjuvant to local anesthetics in erector spinae plane block: A systematic review and meta-analysis of randomized controlled trials. BMC Anesthesiol. 2023;23(61) doi: 10.1186/s12871-023-02019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Albrecht E, Kern C, Kirkham KR. A systematic review and meta-analysis of perineural dexamethasone for peripheral nerve blocks. Anaesthesia. 2015;70:71–83. doi: 10.1111/anae.12823. [DOI] [PubMed] [Google Scholar]
- 44.Desai N, Albrecht E. Local anaesthetic adjuncts for peripheral nerve blockade. Curr Opin Anaesthesiol. 2023;36:533–540. doi: 10.1097/ACO.0000000000001272. [DOI] [PubMed] [Google Scholar]
- 45.Rommen C, Leopold CS, Lippold BC. Do local anesthetics have an influence on the percutaneous penetration of a model corticosteroid? An in vivo study using the vasoconstrictor assay. Eur J Pharm Sci. 1999;9:227–234. doi: 10.1016/s0928-0987(99)00060-3. [DOI] [PubMed] [Google Scholar]
- 46.Elsharkawy H, Bajracharya GR, El-Boghdadly K, Drake RL, Mariano ER. Comparing two posterior quadratus lumborum block approaches with low thoracic erector spinae plane block: An anatomic study. Reg Anesth Pain Med. 2019;(rapm-2018-100147) doi: 10.1136/rapm-2018-100147. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 47.De Lara Gonzalez SJ, Pomes J, Prats-Galino A, Gracia J, Martinez-Camacho A, Sala-Blanch X. Anatomical description of anaesthetic spread after deep erector spinae block at L-4. Rev Esp Anestesiol Reanim (Engl Ed) 2019;66:409–416. doi: 10.1016/j.redar.2019.07.001. (In English, Spanish) [DOI] [PubMed] [Google Scholar]
- 48.Harbell MW, Seamans DP, Koyyalamudi V, Kraus MB, Craner RC, Langley NR. Evaluating the extent of lumbar erector spinae plane block: An anatomical study. Reg Anesth Pain Med. 2020;45:640–644. doi: 10.1136/rapm-2020-101523. [DOI] [PubMed] [Google Scholar]
- 49.Azevedo AS, Silva VTG, Xavier AL, da Silva LFF, Hojaij FC, Ashmawi HA, Vieira JE, Fernandes HS. Comparison of different injection volumes on spread of lumbar erector spinae plane block: An anatomical study. J Clin Anesth. 2021;72(110268) doi: 10.1016/j.jclinane.2021.110268. [DOI] [PubMed] [Google Scholar]
- 50.Tulgar S, Senturk O. Ultrasound guided Erector Spinae Plane block at L-4 transverse process level provides effective postoperative analgesia for total hip arthroplasty. J Clin Anesth. 2018;44(68) doi: 10.1016/j.jclinane.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Chung K, Kim ED. Continuous erector spinae plane block at the lower lumbar level in a lower extremity complex regional pain syndrome patient. J Clin Anesth. 2018;48:30–31. doi: 10.1016/j.jclinane.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 52.Mantuani D, Luftig J, Herring A, Dreyfuss A, Nagdev A. A novel technique to reduce reliance on opioids for analgesia from acute appendicitis: The ultrasound-guided erector spinae plane block. Clin Pract Cases Emerg Med. 2019;3:248–251. doi: 10.5811/cpcem.2019.4.42117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Celik M, Tulgar S, Ahiskalioglu A, Alper F. doi: 10.1136/rapm-2019-100514. Is high volume lumbar erector spinae plane block an alternative to transforaminal epidural injection? Evaluation with MRI. Reg Anesth Pain Med: Apr 16, 2019 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 54.Ahiskalioglu A, Tulgar S, Celik M, Ozer Z, Alici HA, Aydin ME. Lumbar erector spinae plane block as a main anesthetic method for hip surgery in high risk elderly patients: Initial experience with a magnetic resonance imaging. Eurasian J Med. 2020;52:16–20. doi: 10.5152/eurasianjmed.2020.19224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Breidenbach KA, Wahezi SE, Kim SY, Koushik SS, Gritsenko K, Shaparin N, Kaye AD, Viswanath O, Wu H, Kim JH. Contrast spread after erector spinae plane block at the fourth lumbar vertebrae: A cadaveric study. Pain Ther. 2023;12:241–249. doi: 10.1007/s40122-022-00453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Q, Wu Y, Ren F, Zhang X, Feng Y. Bilateral ultrasound-guided erector spinae plane block in patients undergoing lumbar spinal fusion: A randomized controlled trial. J Clin Anesth. 2021;68(110090) doi: 10.1016/j.jclinane.2020.110090. [DOI] [PubMed] [Google Scholar]
- 57.Liu H, Zhu J, Wen J, Fu Q. Ultrasound-guided erector spinae plane block for postoperative short-term outcomes in lumbar spine surgery: A meta-analysis and systematic review. Medicine (Baltimore) 2023;102(e32981) doi: 10.1097/MD.0000000000032981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao Z, Xiao Y, Wang Q, Li Y. Comparison of dexmedetomidine and dexamethasone as adjuvant for ropivacaine in ultrasound-guided erector spinae plane block for video-assisted thoracoscopic lobectomy surgery: A randomized, double-blind, placebo-controlled trial. Ann Transl Med. 2019;7(668) doi: 10.21037/atm.2019.10.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Z, Zhu RL, Yue L, Li X, Ma JH, Kong H, Li CD, Zhang H, Wang DX. Bilateral ultrasound-guided erector spinae plane block versus wound infiltration for postoperative analgesia in lumbar spinal fusion surgery: A randomized controlled trial. Eur Spine J. 2023;32:301–312. doi: 10.1007/s00586-022-07453-y. [DOI] [PubMed] [Google Scholar]
- 60.Guna Pratheep K, Sonawane K, Rajasekaran S, Shetty AP, Subramanian BJ, Kanna RM. Transient paraplegia in lumbar spine surgery-a potential complication following erector spinae plane block. Eur Spine J. 2022;31:3719–3723. doi: 10.1007/s00586-021-07059-w. [DOI] [PubMed] [Google Scholar]
- 61.Chung WC, Kuo YJ, Chan SM, Hou JD, Lin TH, Lin JA. Onset time of lumbar erector spinae plane block compared with its thoracic counterpart: Case reports. Healthcare (Basel) 2023;11(1158) doi: 10.3390/healthcare11081158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yesiltas S, Abdallah A, Uysal O, Yilmaz S, Cinar I, Karaaslan K. The efficacy of intraoperative freehand erector spinae plane block in lumbar spondylolisthesis: A randomized controlled study. Spine (Phila Pa 1976) 2021;46:E902–E910. doi: 10.1097/BRS.0000000000003966. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.