Abstract
Many transcription factors activate by directly interacting with RNA polymerase (RNAP). The C terminus of the RNAP α subunit (αCTD) is a common target of activators. We used both random mutagenesis and alanine scanning to identify αCTD residues that are crucial for MetR-dependent activation of metE and metH. We found that these residues localize to two distinct faces of the αCTD. The first is a complex surface consisting of residues important for α-DNA interactions, activation of both genes (residues 263, 293, and 320), and activation of either metE only (residues 260, 276, 302, 306, 309, and 322) or metH only (residues 258, 264, 290, 294, and 295). The second is a distinct cluster of residues important for metE activation only (residues 285, 289, 313, and 314). We propose that a difference in the location of the MetR binding site for activation at these two promoters accounts for the differences in the residues of α required for MetR-dependent activation. We have designed an in vitro reconstitution-purification protocol that allows us to specifically orient wild-type or mutant α subunits to either the β-associated or the β′-associated position within RNAP (comprising α2, β, β′, and ς subunits). In vitro transcriptions using oriented α RNAP indicate that a single αCTD on either the β- or the β′-associated α subunit is sufficient for MetR activation of metE, while MetR interacts preferentially with the αCTD on the β-associated α subunit at metH. We propose that the different αCTD requirements at these two promoters are due to a combination of the difference in the location of the activation site and limits on the rotational flexibility of the αCTD.
In Escherichia coli and Salmonella enterica serovar Typhimurium, the final step in methionine biosynthesis is the transfer of a methyl group to homocysteine to form methionine. The reaction can be catalyzed by either of two transmethylases encoded by metE or metH (12). The metH gene product is dependent on vitamin B12 as a cofactor, whereas the metE gene product is not.
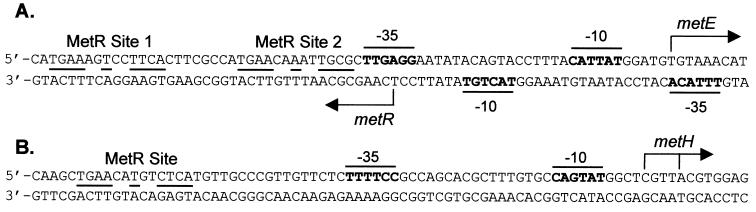
Transcription of a number of genes in the methionine biosynthetic pathway, including metE and metH, is dependent on the MetR activator protein; MetR is a dimer in solution (25) and binds to the consensus site 5′-TGAANNTNNTTCA-3′ (49). Using homocysteine as a coactivator, MetR stimulates expression of the metE promoter up to 200-fold (48). MetR protects two adjacent sites, from bp −26 to −49 (site 2) and from bp −50 to −73 (site 1) upstream of the Salmonella serovar Typhimurium metE transcription start site, from DNase I cleavage (Fig. 1). Within each DNase I-protected region are perfect (site 1) or near-perfect (site 2) matches to the MetR consensus binding site sequence centered at −63 and −42 for sites 1 and 2, respectively (49, 54). Although both sites are necessary for activation of metE, genetic data suggest that site 2 is the activation site; MetR at the high-affinity site 1 appears to promote the homocysteine-dependent filling by a second MetR dimer at the lower-affinity site 2 (54). The Salmonella serovar Typhimurium metH gene is activated up to 19-fold by the presence of MetR (5); however, homocysteine, in contrast to its role at the metE promoter, decreases this activation 3-fold, probably by an indirect effect of lowering MetR levels (46, 48). Genetic and biochemical analyses indicate that there is a single MetR dimer binding site at metH as well as two alternative start sites separated by 3 intervening bp (Fig. 1) (5, 47); however, regardless of which start site is used, the center of the activation site at metH (either at −57 or at −61) is clearly different from the center of the activation site of metE at −42. Because of the difference in the number of MetR binding sites and the location of the activation site at these promoters, it is possible that MetR may use different mechanisms to activate these two promoters.
FIG. 1.
The metE-metR and metH promoters of Salmonella serovar Typhimurium. Promoter −10 and −35 sequences are boldfaced; those for metE and metH are overlined, and those for metR are underlined. MetR binding sequences are lined between the DNA strands. Transcription start sites are marked with arrows. The metE and metH transcription start sites were determined by primer extension (unpublished data). (A) metE-metR promoter region; (B) metH promoter region.
Activation at many promoters results from interactions between an activator protein and RNA polymerase (RNAP) (reviewed in reference 14). The specific subunit of RNAP (α2, β, β′, or ς) that is contacted by an activator appears to depend at least in part on the location of the activator binding site relative to the RNAP binding site. For many activator proteins that bind upstream of the promoter, such as cyclic AMP receptor protein (CRP), which binds at the lacP1 promoter, the major contact site on RNAP is the C terminus of the α dimer subunits (αCTD). The α-CRP interaction increases the affinity of RNAP for the lac promoter, which, in turn, increases transcription (reviewed in reference 8). Recruitment may be a common mechanism for activators that bind upstream of promoters; it has been shown that activation can occur when the αCTD is replaced with a heterologous protein domain capable of interacting with a specific partner protein bound upstream of the promoter (7). However, the αCTD is not the exclusive target of activator proteins, and activation may also be mediated by RNAP-activator interactions that influence steps of transcription initiation subsequent to RNAP binding. At promoters where the activator binds to sites adjacent to or overlapping the promoter elements, contacts with the N terminus of α (e.g., CRP at gal) and with ς70 (e.g., λ cI at PRM) have been reported (23, 31). The phage N4 single-stranded DNA binding protein activates N4 late promoters through an interaction with the β′ subunit, and the ς54-dependent activator C4-dicarboxylic acid transport protein D of Rhizobium meliloti interacts in solution with both the β subunit and ς54 (22, 26).
In this report, we describe experiments aimed at identifying and understanding the role of activator-RNAP interactions in MetR-dependent activation of metE and metH. It has previously been shown that removal of the entire αCTD eliminates activation by MetR at both metE and metH in vitro (15a). Here we describe the effects of various point mutations in the αCTD on the activation by MetR at these two promoters in vivo and in vitro. In addition, we describe a protocol for the reconstitution of RNAP containing oriented α subunits. We have used the oriented α RNAPs to show that MetR-dependent activation at metE and metH have different requirements for the location of wild-type α subunits within the RNAP enzyme complex in order for activation by MetR to occur, suggesting that the mechanisms of activation by MetR differ at these two promoters.
MATERIALS AND METHODS
Bacterial strains, plasmids, and phages.
The strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| GS162 | E. coli K-12 ΔlacU169 | This laboratory |
| GS244 | GS162 derivative ΔmetR::Mu | This laboratory |
| GS972 | GS162 derivative carrying chromosomal operator constitutive mutation in metR promoter | This laboratory |
| GS1040 | GS162 derivative carrying chromosomal E261K rpoA allele | This laboratory |
| GS1106 | GS162 derivative carrying chromosomal rpoA112 allele | This study |
| BL21(DE3) | E. coli λDE3, which encodes T7 RNAP from the lacUV5 promoter | Novagen |
| Plasmids | ||
| pREIIα (and derivatives) | ori-pBR322; lppP-′lacPUV5-rpoA (and derivatives carrying N268D, L270H, alanine substitutions at residues 275 to 301 and 303 to 329, or N-terminal Strep tag) | 3, 10, 18, 53; This study |
| pHTf1α (and derivatives) | ori-pBR322; ori-f1; lppP-′lacPUV5-rpoA (and derivatives carrying alanine substitutions at residues 255 to 271, 273, or 302) | 10, 42 |
| pHTT7f1-NHα (and derivatives) | ori-pBR322; ori-f1; φ10P-rpoA with N-terminal His6 tag (and derivatives carrying R265A, N268D, L270H, or G296A substitutions, or ΔCTD) | 41; this study |
| pT7-Strepα (and derivatives) | ori-pBR322; ori-f1; φ10P-rpoA with N-terminal Strep tag (and derivatives carrying an R45A substitution, ΔCTD, or an R45A substitution plus ΔCTD) | This study |
| pMKSe2 | ori-pBR322; lacP-rpoB | 38 |
| pT7β′ | ori-pBR322; φ10P-rpoC | 56 |
| pRLG593 | ori-pBR322; lacPUV5 | 35 |
| pGS395 | ori-pDF41; tacP-metR | This laboratory |
Plasmid pREII-Strepα was constructed using site-directed mutagenesis to insert the sequence 5′-GCT TGG AGC CAC CCG CAG TTC GAA AAA GGT GCT-3′ (encoding the Strep-tag II amino acid sequence WSHPQFEK [37] flanked by an alanine on the 5′ end and a glycine and an alanine on the 3′ end) between codons 1 and 2 of the rpoA gene in plasmid pREIIα. For production of Strep-tagged α protein, plasmid pT7-Strepα was constructed by replacement of the XbaI-BamHI fragment of pHTT7f1-NHα with the corresponding fragment from pREII-Strepα. This plasmid did not overproduce the Strep-tagged α as well as plasmid pHTT7f1-NHα overproduces the His6-tagged α (data not shown); however, we could purify enough Strep-tagged α for reconstitutions of oriented α RNAP. Plasmid pT7-Strep(R45A)α was constructed by site-directed mutagenesis to convert codon 45 of rpoA from a CGT (arginine) to a GCT (alanine) codon in plasmid pT7-Strepα. Plasmid pHTT7f1-NH(ΔCTD)α was constructed by site-directed mutagenesis to convert codon 257 of the rpoA gene in plasmid pHTT7f1-NHα from GTT (valine) to TAA (stop) and create a BamHI restriction site following the new stop codon. Plasmid pT7-Strep(R45A/ΔCTD)α was constructed by replacement of the HindIII-BamHI fragment of pT7-Strep(R45A)α with the corresponding fragment from pHTT7f1-NH(ΔCTD)α. The plasmids used to express the His6-tagged N268D, L270H, and G296A α subunits were generated by replacing the HindIII-BamHI fragments of pHTT7f1-NHα with the corresponding fragments from pREII268Dα, pREII270Hα, and pREII296Aα. The His6-tagged R265A derivative was generated by replacing the HindIII-BamHI fragment of pHTT7f1-NHα with the HindIII-BstYI fragment of pHTf1265Aα. The constructs were confirmed by DNA sequencing.
The λTElac1 phage, a λgt2 derivative containing a metE-lacZ translational fusion, is a temperature-resistant derivative of λElac1, which has been previously described (33). The λHlac phage, also a λgt2 derivative but carrying a metH-lacZ translational fusion, has been described previously (47).
Media and growth conditions.
Tryptone broth (TB), Luria-Bertani broth (LB), and lactose tetrazolium agar were prepared as described previously (27). Glucose minimal medium was Vogel and Bonner minimal salts (51) supplemented with 0.4% glucose. Minimal medium was also supplemented with phenylalanine (50 μg/ml) and thiamine (1 μg/ml), since most of the strains carry the pheA905 and thi markers. Strains carrying α plasmids were maintained in medium supplemented with ampicillin at 100 μg/ml; however, transformants used for the production of RNAP subunits were supplemented with ampicillin at 200 μg/ml. Strains carrying pGS395 were maintained in medium supplemented with kanamycin at 20 μg/ml.
Lysogens were grown at 37°C, except for λHlac lysogens, which were grown at 30°C, since the λHlac phage carries the λcI857, mutation resulting in a temperature-sensitive λcI repressor (32).
β-Galactosidase enzyme assays.
For all strains except those carrying the rpoA112 allele, cells were grown in TB to an optical density at 600 nm (OD600) of approximately 0.5 and then placed on ice for 20 min. The rpoA112 strain GS1106 was grown as 2- by 2-cm patches on LB agar overnight at the desired temperature; prior to being assayed, the cells were scraped from the plate, resuspended in 1× Vogel and Bonner minimal salts to an OD600 of approximately 0.5, and then placed on ice for 20 min. β-Galactosidase enzyme activity was assayed using the chloroform-sodium dodecyl sulfate (SDS) lysis procedure (27). Assays were performed at least twice, with the activity of each sample determined in triplicate.
Mutant isolation. (i) Selection for mutations that affect metE expression.
Random mutagenesis of the 1-kb XbaI-BamHI rpoA fragment from pREIIα was performed as described previously (42). The PCR-mutagenized rpoA gene was cloned into pREIIα in place of the wild-type XbaI-BamHI fragment. The selection strain, GS972λTElac1, forms white colonies on lactose tetrazolium agar when transformed with pREIIα. Transformants of GS972λTElac1 with decreased expression of the metE-lacZ fusion were identified as red colonies on lactose tetrazolium agar. The mutations were further mapped to the αCTD by subcloning the HindIII-BamHI fragment (codons 230 to 329) from the mutant plasmids into pREIIα.
(ii) Screen for mutations that affect metE and/or metH expression.
Plasmids from an alanine substitution library of the αCTD (residues 255 to 329) were individually transformed into GS162λTElac1 and GS972λHlac. As a preliminary screen, a single colony of each transformant was grown for 3 h (OD600 of approximately 0.5) in TB and assayed for β-galactosidase activity. Transformants that showed decreases in either metE-lacZ or metH-lacZ expression were selected for further study. Eight of the alanine substitutions increased metE expression in the preliminary screen (maximum metE-lacZ expression was 2.3-fold higher than that in wild-type α). However, these mutants were not included in this study.
Preparation of core RNAP containing α homodimers.
The His6-tagged α subunits were prepared under denaturing conditions as previously described (40) with the following modifications: cells were lysed by sonication in buffer B (6 M guanidine-HCl, 20 mM Tris-HCl [pH 7.9], 500 mM NaCl, 5 mM imidazole) instead of a nondenaturing buffer, and the ammonium sulfate precipitation step was eliminated. Inclusion bodies containing β and β′ were prepared as described previously (40). Reconstitutions were performed as described previously (40) with the following exceptions: reconstitution of ς70 was not performed at the same time as the core RNAP reconstitution, and activation of the RNAP following dialysis was performed in the absence of ς70. Following activation, the core RNAP mixture was cleared by centrifugation and the reconstituted RNAP was purified by Ni2+ ion affinity chromatography as described previously (40). The resulting sample was concentrated to approximately 50 μl with a Microcon YM-100 concentrator (Millipore, Bedford, Mass.), mixed with an equal volume of glycerol, and stored at −20°C.
Preparation of Strep-tagged α.
E. coli strain BL21(DE3) transformed with pT7-Strepα (or derivatives) was shaken at 37°C in 100 ml of LB plus ampicillin (200 μg/ml) to an OD600 of approximately 0.5, induced by the addition of isopropyl-β-d-thiogalactoside (IPTG) to 1 mM, and shaken an additional 6 to 15 h at 22°C. The cells were harvested by centrifugation (4,500 × g; 12 min at 4°C) and resuspended in 1.5 ml of buffer W (100 mM Tris-HCl [pH 8.0]–1 mM EDTA) at 4°C. Cells were lysed by sonication, and the lysate was cleared by centrifugation (16,000 × g; 15 min at 4°C). The supernatant was treated with 7 μl of a 1-mg/ml avidin solution (prepared in buffer W) for 30 min at 4°C and cleared by centrifugation (16,000 × g; 15 min at 4°C). The extract was adsorbed to a 1-ml StrepTactin column (Genosys Biotechnologies, Woodlands, Tex.) preequilibrated with buffer W, washed five times with 1 ml of buffer W, and eluted six times with 0.5 ml of buffer E (buffer W with 2.5 mM desthiobiotin). The bulk of the protein eluted in one fraction; the yield was 100 μg of protein as determined by the Bradford assay (4), and the purity was >50% as determined by SDS-polyacrylamide gel electrophoresis (PAGE). The contaminating proteins appeared, based on size, to be the other RNAP subunits; however, native, non-tagged α is distinguishable from the Strep-tagged α on our SDS–15% PAGE gels, and we could not detect any contaminating non-tagged α by staining with Coomassie brilliant blue R.
Preparation of core RNAP containing oriented α subunits.
β, β′, and His6-tagged and Strep-tagged α subunits were prepared as described above. For each reconstitution, 30 μg of each α species was added to make 60 μg of total α. Reconstitution and a first partial purification of core RNAP by Ni2+ ion affinity chromatography based on the His6 tag were performed as described above for the purification of core RNAP containing α homodimers. The eluate from the Ni2+-nitrilotriacetic acid resin (Qiagen Inc., Valencia, Calif.) following this first purification was then applied to a StrepTactin column (bed volume, 1 ml). Adsorption, washes, and elution of the StrepTactin column were performed as described above for the purification of Strep-tagged α. The RNAP typically eluted in one or two fractions that were concentrated as described above for the purification of core RNAP containing α homodimers. The yield was typically 5 to 10 μg, which is much lower than what is recovered from the single purification step for homodimeric RNAP (40); however, these yields were sufficient for several transcription experiments. The concentrated sample contained no traces of native, non-tagged α, as determined by Coomassie brilliant blue R staining of an SDS–15% PAGE gel (data not shown).
In vitro transcription assays.
Aliquots of reconstituted core RNAP were incubated in the presence of a 1.5 molar excess of purified ς70 subunit to ensure the formation of RNAP holoenzyme for use in single-round runoff transcription assays in the presence of heparin as previously described (16). The activity of each reconstituted RNAP was normalized based on the amounts of lacUV5 and RNA-I transcripts produced when the supercoiled plasmid pRLG593 was used as the template (35). Based on these initial experiments, the enzymes were used at the concentrations indicated in Fig. 3A and Fig. 4A to test the effect of RNAP containing mutant homodimers or oriented heterodimers of α on metE and metH transcription in the absence or presence of MetR (final dimer concentration, 135 and 270 nM). Template DNA containing both the metE and metR promoters and the metH template DNA were added simultaneously to reaction mixtures when the RNAPs containing homodimers of α were tested. In transcriptions with the oriented α RNAP, the metE-metR and metH templates were tested separately. Following electrophoresis, the gels were dried and analyzed by autoradiography. When quantitated, the bands corresponding to the metE and metH transcripts were analyzed with a Packard InstantImager (Meriden, Conn.). For reporting, the quantitated amount of metE and metH transcript produced by each RNAP was normalized to the sum of the lacUV5 and RNA-I transcripts generated from the supercoiled template pRLG593 (35) by the same RNAP. Results for α homodimer RNAP are averages from two transcription experiments. Oriented α RNAP results are averages from two transcription experiments for metE and three transcriptions for metH.
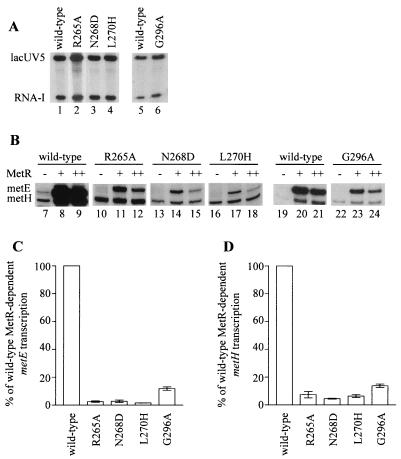
FIG. 3.
In vitro transcriptions using RNAP with αCTD substitutions. The RNAP is designated by the α subunits in the holoenzyme. (A) Transcripts from single-round transcription experiments with the supercoiled plasmid pRLG593 as a template to equalize the activity of each RNAP. Indicated are the positions of the αCTD-independent transcript initiated at the lacUV5 promoter and the plasmid-derived RNA-I transcript. The activity of the G296A RNAP was determined in a separate experiment, so it is shown with the wild-type RNAP from the same experiment. RNAPs were used at the following concentrations in these experiments: 20 nM wild-type, R265A, and N268D RNAPs, 60 nM L270H RNAP, and 50 nM G296A RNAP. (B) Transcripts from single-round transcription experiments using a linear template carrying the metE-metR control region as well as a linear template with the metH promoter in the absence (−) and the presence of 135 nM (+) or 270 nM (++) MetR dimer. The transcripts initiated at the metE and metH promoters are indicated. (C and D) Effects of αCTD substitutions on metE (C) and metH (D) activation. The metE transcript produced by each RNAP in the presence of 135 nM MetR and the metH transcript produced by each RNAP in the presence of 270 nM MetR from two independent in vitro transcription experiments were quantitated and normalized to the amounts of lacUV5 and RNA-I transcripts produced by the same RNAP. The normalized amount of transcript produced by each mutant RNAP is reported as a percentage of the expression by wild-type RNAP (± 1 standard deviation).
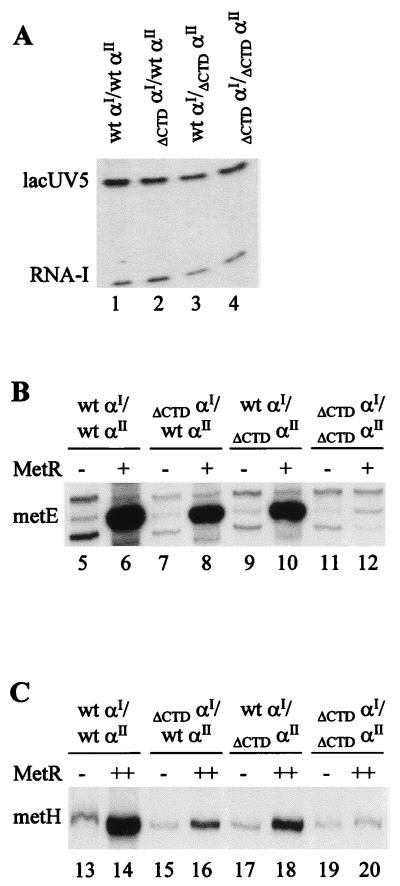
FIG. 4.
In vitro transcriptions using RNAP with oriented α subunits. The RNAP is designated by the α subunits in the holoenzyme. (A) Transcripts from single-round transcription experiments with the supercoiled plasmid pRLG593 as a template to equalize the activity of each RNAP. Indicated are the positions of the αCTD-independent transcripts initiated at the lacUV5 promoter and the plasmid-derived RNA-I transcript. The RNAPs were used at the following concentrations in these experiments: 10 nM wt αI–wt αII and ΔCTDαI–wt αII RNAPs, and 13 nM wt αI–ΔCTDαII and ΔCTDαI–ΔCTDαII RNAPs. (B) Transcripts from single-round transcription experiments using a linear template carrying the metE-metR control region in the absence (−) and presence (+) of 135 nM MetR. The transcript initiated at the metE promoter is indicated. (C) Transcripts from single-round transcription experiments using a linear template carrying the metH promoter in the absence (−) and presence (++) of 270 nM MetR. The transcript initiated at the metH promoter is indicated.
RESULTS
Selection for α mutations that decrease metE expression.
A plasmid-borne rpoA gene was randomly mutagenized for use in a genetic screen that would identify colonies with decreased metE expression. The strain used for the selection, GS972, constitutively expresses MetR. The overexpression of MetR was necessary because the rich medium used for the selection (lactose tetrazolium agar) would repress the wild-type metR promoter. A λ lysogen of GS972 carrying a metE-lacZ fusion, GS972λTElac1, when transformed with plasmid pREIIα expressing wild-type rpoA, produces white colonies on lactose tetrazolium agar. Although the chromosomal rpoA gene is present in this and all of the transformants used in this study, it has been shown by Western blotting that the α proteins expressed from the high-copy-number plasmids are the predominant α species in the cell (42). Screening of three independent plasmid pools carrying PCR-mutagenized rpoA genes yielded two transformants that formed red colonies on lactose tetrazolium agar, indicating decreased metE-lacZ expression. The metE-down phenotype of one of the mutants was shown to be plasmid associated and could be localized to the αCTD by subcloning of the rpoA gene. Sequencing of this mutant revealed a single-base-pair substitution that resulted in a change from asparagine to aspartic acid at amino acid 268 (N268Dα). For the second mutant, a plasmid association test revealed that the metE-down phenotype was unstable in GS972λTElac1, and although subcloning showed that the phenotype was associated with the αCTD, the subclones were also unstable in GS972λTElac1. Parallel subcloning of this mutant in GS162λTElac1, the wild-type parent of GS972 carrying the metE-lacZ fusion, eventually gave rise to a stable phenotype by β-galactosidase assays (data not shown). Sequencing of this stable mutant revealed a single-base-pair substitution that resulted in a change from leucine to histidine at amino acid 270 (L270Hα).
To quantitate the effects of these αCTD mutations on metE expression, GS162λTElac1 was transformed with plasmids expressing either wild-type α or one of the mutant α alleles. These transformants were grown in TB, and β-galactosidase levels were determined. As expected, the N268D and L270H substitutions strongly decreased metE-lacZ expression (Table 2). The metE-down phenotype associated with these mutations was lost if the mutant alleles were expressed in GS244λTElac1, a metR strain, suggesting that these αCTD mutations disrupt MetR-dependent expression of metE instead of causing a general defect in transcription (Table 2).
TABLE 2.
Effects of point mutations at amino acids 268 and 270 of the α subunit on metE-lacZ and metH-lacZ expression
| Plasmid | Plasmid rpoA allele | Expression in the indicated straina of:
|
|||||
|---|---|---|---|---|---|---|---|
|
metE-lacZ
|
metH-lacZ
|
||||||
| GS162 (MetR+) | GS244 (MetR−) | GS972 (MetR+++) | GS162(MetR+) | GS244 (MetR−) | GS972 (MetR+++) | ||
| pREIIα | Wild type | 487 | 53 | 5,046 | 29 | 13 | 143 |
| pREII-N268Dα | N268D | 56 | 56 | 1,857 | 32 | 15 | 53 |
| pREII-L270Hα | L270H | 96 | 48 | 5,051 | 25 | 11 | 122 |
Lysogens were grown in TB to mid-log phase. Results (β-galactosidase activity) are reported as Miller units (27). Standard deviations between assays varied by less than 12% of the reported means.
Following multiple manipulations, including plasmid preparation and subcloning, the N268D and L270H rpoA alleles were assayed in the retransformed lysogen GS972λTElac1. The N268D substitution decreased metE expression only threefold, compared to a ninefold decrease in GS162λTElac1. Surprisingly, however, the L270H phenotype was completely suppressed (Table 2), so it is unclear how the L270H mutant was isolated as a red colony on lactose tetrazolium plates. We speculate that the original isolate, which exhibited a stronger phenotype on lactose tetrazolium plates than the N268D mutant, may have carried a second mutation (or an alternate substitution at amino acid 270) that was lost because of the detrimental effect it had on overall cellular gene expression. We further speculate that the second mutation was in the αCTD because the phenotype transferred, although unstably, with the αCTD subclones.
The N268D and L270H mutations were also tested for their effects on metH-lacZ expression. The same three strains used to test the effects on metE-lacZ expression were lysogenized with a phage carrying a metH-lacZ fusion. In GS162λHlac, neither α mutation caused a decrease in metH-lacZ expression. However, in GS972λHlac, the N268D substitution caused nearly a threefold decrease in metH-lacZ expression while the L270H substitution yielded essentially wild-type levels (Table 2). The effect of the N268D substitution on metH expression is MetR dependent, because the phenotype was lost in a metR background (Table 2).
Screen for alanine substitutions in the αCTD that alter metE and/or metH expression.
To more efficiently identify residues in the αCTD important for activation of metE and/or metH, we switched to screening an alanine substitution plasmid library of the αCTD (residues 255 to 329). Lysogens GS162λTElac1 and GS972λHlac were transformed with the plasmid library to assess the effect of each alanine substitution on both metE-lacZ and metH-lacZ expression. Transformants that showed decreases in either metE-lacZ or metH-lacZ expression in a preliminary screen were selected for further study.
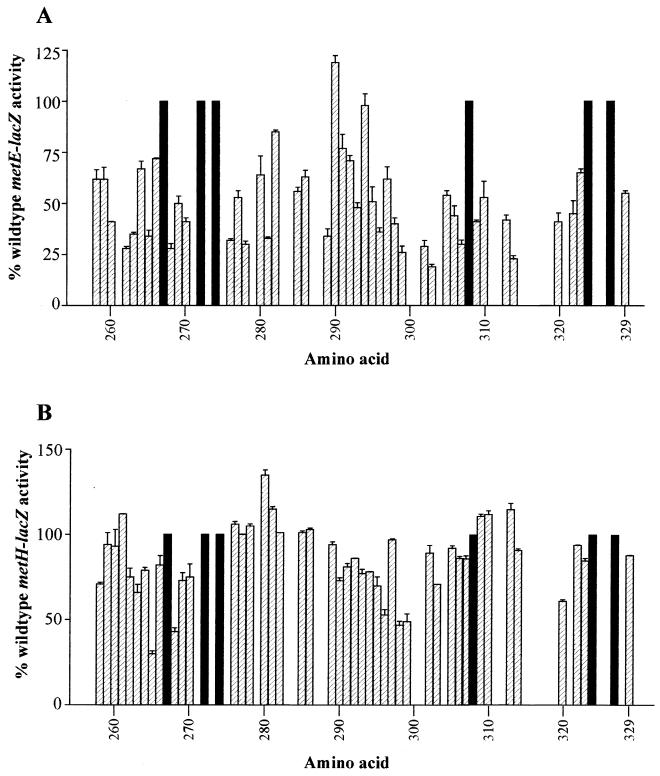
Many of the alanine substitutions had modest effects on metE expression (Fig. 2A). Among the residues that, when changed to alanine, cause at least a twofold decrease in metE expression are the previously characterized DNA binding residues of α: L262, R265, N268, C269, G296, K298, and S299 (10, 17, 28). Consistent with the selection using PCR mutagenesis, L270 was also identified as important for metE expression in this screen. In addition, alanine substitutions at residues L260, T263, H276, I278, L281, L289, P293, E302, I303, V306, L307, S309, S313, L314, N320, and P322 all cause at least twofold decreases in metE-lacZ expression, with changes at E302, I303, and L314 being more severe than changes at most of the DNA binding residues. The alanine substitution mutants that showed a metE-down phenotype in GS162λTElac1 were also tested in GS244λTElac1 to determine the effects of the alanine substitutions on basal metE expression. With the exception of I303A, the alanine substitutions caused at most a 1.3-fold down effect on metE-lacZ expression in the absence of MetR (data not shown). These results suggest that the metE-down phenotypes caused by these alanine substitutions are MetR dependent. The I303A substitution caused a twofold reduction in basal metE-lacZ levels (data not shown). Since this substitution caused a fivefold reduction in MetR-dependent metE expression (Fig. 2A), the I303A substitution appears to have an effect on both basal and activated transcription of metE. Based on the solution structure of the αCTD (10, 17), all but five of these residues (L270, I278, L281, I303, and L307) are surface exposed; therefore, these residues could contact MetR for activation.
FIG. 2.
Alanine scan of αCTD. Plasmids expressing rpoA with single alanine substitutions at each residue in the CTD (residues 255 to 329) were assayed for effects on chromosomal metE-lacZ (A) and metH-lacZ (B) expression. Results are expressed as percentages of the activity in cells carrying plasmids expressing the wild-type rpoA gene. Residues 267, 272, 274, 308, 324, and 327 (filled bars) are alanines in the wild-type protein. Residues with no data did not decrease expression of either gene in an initial screen.
Far fewer αCTD alanine substitutions cause at least twofold decreases in metH expression (Fig. 2B). Those that do, R265, N268, K298, and S299, are all DNA binding residues of α. However, based on in vitro results, using a twofold decrease in expression as a cutoff for determining whether a residue is important for metH expression may be too stringent (see below). Alanine substitutions at D258, L262, T263, V264, C269, L270, L290, P293, N294, L295, G296, I303, and N320 reproducibly cause 1.3- to 1.9-fold decreases in metH expression. These 17 alanine substitution mutants were also tested in GS244λHlac to determine the effect of the loss of MetR on the phenotype (data not shown). The metH-down phenotype of all of these mutants was lost in the metR background, suggesting that these alanine substitutions cause decreases in MetR-dependent expression of metH. All but two of these residues (L270 and I303) are surface exposed (10, 17); therefore, these residues could contact MetR for activation.
In vitro analysis of αCTD mutants.
Many of the αCTD mutations show some MetR-dependent phenotype in vivo, especially at metE. However, some of these phenotypes may be due to indirect effects, e.g., altering levels of the activator or levels of the enzymes involved in homocysteine metabolism. To determine the direct effect of αCTD mutations on MetR-dependent expression, we reconstituted RNAP in vitro in the presence of either His6-tagged wild-type or mutant α subunits for use in an in vitro transcription system.
Four α mutants were tested in vitro: three substitutions in α DNA binding residues (R265A, N268D, and G296A) and L270H, since this substitution affects metE but not metH expression in vivo. The activity of each reconstituted RNAP was normalized based on its ability to make the αCTD-independent lacUV5 and RNA-I transcripts from plasmid pRLG593 (Fig. 3A) (34).
In a purified transcription system containing template DNA, homocysteine, ribonucleoside triphosphates, and wild-type RNAP, both metE and metH expression were dependent on the addition of MetR (Fig. 3B, lanes 7 to 9). However, when the mutant RNAPs were used, all four α mutations caused severe decreases in MetR-dependent metE expression (Fig. 3B [compare lane 8 to lanes 11, 14, and 17, and compare lane 20 to lane 23] and 3C). Quantification of metE levels in the absence of MetR indicates that all four of these mutations in the αCTD caused less than 2.5-fold decreases in basal metE expression (Fig. 3B, lanes 7, 10, 13, 16, 19, and 22); however, this quantification is subject to large errors because the metE basal levels are so low. As indicated above, the in vivo metE-down phenotype of most of the αCTD substitutions, including R265A, N268D, L270H, and G296A, was lost in the absence of MetR, suggesting that these mutations primarily affect activated metE transcription.
The effects of the αCTD mutations on metH were also tested in the same transcription reactions. All four α mutations resulted in severe defects in MetR-dependent activation of metH expression (Fig. 3B [compare lane 9 to lanes 12, 15, and 18, and compare lane 21 to lane 24] and 3D). The L270H substitution had only a minor effect on metH expression in vivo (Table 2); however, in the in vitro system, the effect of this mutation on metH expression was as severe as those of the other point mutations (Fig. 3D). As is the case for metE, quantification of the basal metH levels is subject to large errors; however, if we measure these levels, none of the substitutions caused more than a 1.6-fold decrease in basal metH expression. These in vitro results are consistent with our in vivo analysis of basal metH levels, where we found that none of the αCTD substitutions that affected metH levels in the presence of MetR had any effect on metH levels in the absence of MetR.
In vivo, the metE-down phenotypes of all four of these mutations could be at least partially suppressed by the high constitutive MetR levels in GS972 (Table 2 and data not shown). This is not the case in vitro, however. As more MetR was added to reaction mixtures (270 nM dimer) with wild-type RNAP, the amount of metE transcript decreased (Fig. 3B; compare lanes 8 and 9). The mutant RNAPs also produced less metE transcript in the presence of 270 nM MetR dimer than with 135 nM MetR (Fig. 3B; compare lanes 11 and 12, 14 and 15, 17 and 18, and 23 and 24). These results suggest that the suppression seen in vivo is due to the presence of chromosomally derived wild-type α that is incorporated into RNAP.
A wild-type αCTD in the β′-associated α subunit is sufficient for activation of metE in vivo.
The in vivo and in vitro experiments show significant differences in the magnitudes of the effects of the mutant αCTDs on activation, the effect of the L270H substitution on metH expression, and the suppression of the metE phenotypes by high concentrations of MetR. We considered the possibility that these differences were due to the nature of the mutant RNAPs used in vivo and in vitro. In vivo, even though the mutant rpoA genes expressed from high-copy-number plasmids should be the predominant form of α in RNAP, the chromosomal rpoA is intact, so wild-type α can also be incorporated into RNAP either as homodimers of wild-type α or heterodimers of wild-type and mutant α. In contrast, the in vitro purification and reconstitution protocol produces wild-type or mutant RNAP having α dimers of homogeneous subunits. In an attempt to reproduce in vivo the severe loss of MetR activation observed in vitro, we constructed a derivative of the wild-type GS162λTElac1 lysogen, GS1106λTElac1, in which the chromosomal copy of rpoA was replaced with the rpoA112 allele encoding an α subunit reported to have a temperature-sensitive lethal defect for assembly of core polymerase (20). Strains carrying rpoA112 on the chromosome cannot grow at the nonpermissive temperature (42°C) unless an alternative functional rpoA gene is also present, such as a plasmid-borne rpoA gene. However, certain αCTD substitution mutations are unable to complement the rpoA112 allele at 42°C, including R265A, N268A and G296A (10). The L270Hα allele can complement rpoA112, so the effect of L270Hα on metE-lacZ was examined in the rpoA112 background. Since expression of the metR promoter is severely reduced at high temperatures (data not shown), the cells used for the assay also carried plasmid pGS395, which constitutively expresses MetR from a heterologous promoter.
As expected based on the results in GS972λTElac1, when tested in the rpoA112 background at a permissive temperature (37°C) that allows the RpoA112 α protein to participate normally in the assembly of RNAP, the L270H substitution did not affect metE-lacZ expression (Table 3). Surprisingly, when tested at the nonpermissive temperature (42°C) that inhibits normal assembly by the RpoA112 α protein, the cells expressing the L270Hα did not mimic the severe loss of MetR activation predicted from the in vitro results if all of the cellular RNAP at 42°C had incorporated homogeneous L270Hα dimers; instead, these cells expressed metE-lacZ as well as did the wild type (Table 3).
TABLE 3.
Effect of the L270Hα mutation on metE-lacZ expression in an rpoA112 background
| Lysogena | Plasmid | Plasmid rpoA allele | β-Galactosidase activityb at:
|
|
|---|---|---|---|---|
| 37°C | 42°C | |||
| GS1106λTElac1 | pREIIα | Wild type | 12,016 | 7,724 |
| GS1106λTElac1 | pREII-L270Hα | L270H | 17,652 | 7,767 |
A λTElac1 lysogen carrying plasmid pGS395 (which overexpresses MetR) was transformed with the indicated rpoA-expressing plasmid.
Units of β-galactosidase activity from cultures grown in TB are reported as Miller units (27). Standard deviations between assays varied by less than 14% of the reported means.
The rpoA112 assembly defect at the nonpermissive temperature is due to an arginine-to-cysteine substitution at position 45 of α (R45Cα) (15). An alanine substitution at the same α position (R45Aα) can still dimerize but can no longer interact with the β subunit even at 37°C to assemble the α2β precursor required for core polymerase formation (21). However, mixtures of R45Aα and wild-type α can form α heterodimers in vitro that successfully interact with the β subunit via the wild-type moiety and can thus form core polymerase having oriented α subunits: wild-type α contacting the β subunit (αI) and R45Aα contacting the β′ subunit (αII) (29). If the consequence of the rpoA112 mutation (R45Cα) at the nonpermissive temperature is the same as that of the R45Aα substitution, then cultures of the rpoA112 lysogen GS1106λTElac1 expressing the plasmid-encoded L270Hα and grown at 42°C should produce a fraction of RNAP that contains L270Hα homodimers, as well as some RNAP that contains mixed L270HαI–R45CαII heterodimers. The homodimeric L270Hα RNAP should be equivalent to the purified His6-tagged L270Hα RNAP used in the in vitro transcription experiments and would thus contribute very little to metE-lacZ expression (Fig. 3C). According to this hypothesis, the nearly wild-type level of expression of metE-lacZ in the rpoA112 background cell must be due principally to the heterodimeric L270HαI–R45CαII RNAP. Furthermore, these results would predict that MetR activation of metE is insensitive to the residue at position 270 of αI provided that the wild-type residue is present on αII.
The equivalent in vivo experiment to address the effect of oriented α RNAP on metH expression is technically not possible. The rpoA112 allele requires a temperature of at least 42°C for the orienting phenotype; unfortunately, the metH promoter is itself temperature sensitive and shows virtually no expression at 42°C (data not shown).
Orientation requirements of a wild-type αCTD within RNAP for MetR-dependent activation of metE and metH.
Since the results above suggest that a point mutation in the CTD of αI does not interfere with MetR-dependent activation of metE, we wanted to test whether an RNAP with a mutation in the CTD of αII (and a wild-type αI) would also activate metE, i.e., whether a single wild-type αCTD is sufficient for activation at metE. We also wanted to know what the requirements for the orientation of α were for activation at the metH promoter, since we were not able to test the effect of the L270HαI–R45CαII RNAP on metH in vivo due to the temperature sensitivity of the metH promoter. Although β-orienting substitutions (L48A, K86A, and V173A) in α analogous to the β′-orienting R45A substitution have been described (21), simple in vivo assays with such mutants are not possible because conditional, chromosomal versions of the β-orienting mutants are presently not available. We therefore designed an in vitro reconstitution-purification protocol to purify RNAP containing oriented α subunits. This protocol is an extension of the scheme developed by Tang et al. (40), which involves purification of RNAP from an in vitro reconstitution mixture by Ni2+ ion affinity chromatography based on a His6-tagged α. We included in the reconstitution mixture a second source of α that carries an alternate affinity tag, allowing sequential purification of RNAP based on both tags, ensuring that the final RNAP incorporates an α dimer composed of two differently tagged monomers. Furthermore, by specifically including the R45A substitution in one of the two differently tagged α monomers, we can direct this monomer to the αII position, and, by default, direct the other monomer to the αI position. Thus, αCTD mutations can be incorporated into either the αI- or αII-specific monomers to test the orientation-dependent effects of the αCTD mutations in vitro. We constructed a plasmid, pREII-Strepα, that expresses an α containing the Strep-tag II sequence (Strep tag) between codons 1 and 2 of rpoA, the same location as the N-terminal His6 tag in pHTT7f1-NHα (see Materials and Methods). It has been previously shown that incorporation of a His6 tag into the N terminus of α does not impair enzyme function (41). To determine whether the Strep-tagged α is also functional, we tested for the ability of Strep-tagged α to complement a known rpoA mutant in vivo. The mutant strain GS1040 carries the chromosomal E261K rpoA allele, which prevents growth on glucose minimal medium (16). Transformants of GS1040 carrying the pREII-Strepα plasmid exhibit growth on glucose minimal medium indistinguishable from that of the wild-type strain (GS162) or from that of transformants of GS1040 carrying pREIIα, suggesting that Strep-tagged α is incorporated into RNAP and that the Strep tag does not interfere with function.
Since neither an N-terminal His6 tag nor a Strep tag interferes with RNAP assembly or function, purified His6- and Strep-tagged α were added simultaneously to the reconstitution mixture. Incorporation of the R45A substitution into the Strep tag α construct ensures that this monomer could be incorporated into RNAP only at the αII position. Following renaturation-reconstitution, the RNAP was purified in two steps, first based on Ni2+ ion affinity of the His6-tagged α subunit, then based on StrepTactin affinity of the Strep-tagged α subunit, generating a single population of RNAP containing oriented α subunits. The C-terminal domains of these rpoA plasmids can be altered to generate a set of αCTD mutant RNAPs with oriented α subunits. For this analysis, we chose to use a version of α in which the final 73 amino acids were deleted (ΔCTDα) instead of the L270H mutant α, because the effect of substitutions at L270 may be indirect (see Discussion).
Using this reconstitution and purification protocol, we generated four different RNAP species to test the positional requirements of the αCTD for MetR-dependent activation in vitro. The first RNAP contained α subunits with both αCTDs intact (wt αI–wt αII); in the second RNAP, the final 73 amino acids of α were deleted from both α subunits (ΔCTDαI–ΔCTDαII); in the third and fourth RNAP species, the αCTD was deleted from either the β-associated αI (ΔCTDαI–wt αII) or the β′-associated αII (wt αI–ΔCTDαII). The activity of each RNAP was normalized to the wild-type enzyme using αCTD-independent promoters (Fig. 4A), and the effect of deleting each αCTD on metE and metH expression was examined. As expected from previous studies (15a), MetR-dependent activation of metE transcription seen with the wild-type RNAP was almost completely lost when both αCTDs were deleted (Fig. 4B; compare lanes 6 and 12). However, both single-αCTD RNAP derivatives, ΔCTDαI–wt αII and wt αI–ΔCTDαII, responded to MetR-mediated activation nearly as well (50% ± 2% and 70% ± 2%, respectively) as the wild-type RNAP (Fig. 4B, lanes 6, 8, and 10). These results indicate that a single wild-type αCTD in either the αI or αII position of RNAP is sufficient for MetR-dependent activation of metE.
The oriented α RNAPs were also tested for their effects on MetR-dependent expression of metH in vitro. Consistent with previous results (15a), the ΔCTDαI–ΔCTDαII RNAP derivative did not show any appreciable increase in metH transcription in the presence of MetR (Fig. 4C; compare lanes 13 and 14 to lanes 19 and 20). Consistent with its activity at the metE promoter, the RNAP derivative having a deletion of the αCTD only at αII responded to MetR activation of metH 47% ± 3% as well as the wild-type RNAP (Fig. 4C, lanes 14 and 18). In contrast, the RNAP derivative having a deletion of the αCTD only at αI responded poorly to MetR activation of metH, showing only 21% ± 3% of the levels seen with the wild-type RNAP (Fig. 4C, lanes 14 and 16). These results indicate that MetR exhibits a more stringent requirement for a functional CTD on the β-associated αI subunit than for the CTD of αII for activation of metH.
DISCUSSION
Amino acids in the αCTD important for MetR activation of metE.
Through random PCR mutagenesis of rpoA we have identified two residues, N268 and L270, that are important for MetR-dependent activation of metE. Residue N268 has previously been characterized as a DNA binding residue of α (10, 17, 28). The N268D substitution has also been shown to affect CRP- and OxyR-dependent activation of the lacP1 and katG promoters, respectively (44, 57). An alanine substitution at position 268 not only disrupts activation at promoters containing an UP element (10, 28), where the αCTD contacts the DNA directly, but also has been found to decrease activation at nearly every native promoter tested that requires the αCTD for activation (for examples, see reference 30).
Amino acid L270 is not an α-DNA interaction residue; however, an L270A substitution has been shown to disrupt CRP-dependent activation at lacP1 and TyrR-mediated activation of mtr to nearly the same extent as substitutions in α-DNA binding residues (e.g., R265A) (28, 55). In addition, an L270P substitution in α disrupts CRP activation of lacP1 and CysB activation of adi (39, 57). As previously suggested by Murakami et al. (28), the effect of a mutation at 270 may not define a point of contact between α and activator proteins but instead may be indirect. Amino acid 270 is located in helix 1 of the αCTD, which also contains three of the DNA binding residues (R265, N268, and C269) (10, 17); therefore, substitutions at 270 may disrupt the structure of helix 1, thereby altering a portion of the α-DNA binding surface.
The N268D and L270H substitutions are interesting in that the metE phenotypes of these mutations can be partially to completely suppressed by high levels of the activator in vivo. The phenomenon of activator overexpression suppressing an rpoA phenotype is not novel. In Salmonella serovar Typhimurium, if the FNR homologue OxrA is overexpressed, the phenotype of the rpoA8 mutation (G311Rα) is partially suppressed (24). However, suppression of the N268D and L270H phenotypes is not observed in the in vitro transcriptions where purified RNAP containing N268Dα or L270Hα is used (Fig. 3B), suggesting that when MetR levels are high in vivo, wild-type α RNAP will be preferentially recruited to metE.
Screening of an αCTD alanine substitution library identified additional residues that are important for metE activation by MetR. Most of the surface-exposed residues identified in this screen cluster to a complex face of α that includes residues important for activation of both metE and metH (Fig. 5). Residues L262, R265, N268, C269, G296, K298, and S299, which have previously been identified as DNA binding residues of α (10, 17, 28), localize to this complex face. It is possible that these residues define an interaction surface on α for contact with MetR because they affect MetR-dependent activation of metE in vivo; however, we favor the alternate hypothesis previously proposed for these residues in CRP-, Mor-, and Ogr-dependent activation (1, 3, 10, 36, 53): L262, R265, N268, C269, G296, K298, and S299 are involved in nonspecific protein-DNA interactions that stabilize the activator-α interaction.
FIG. 5.
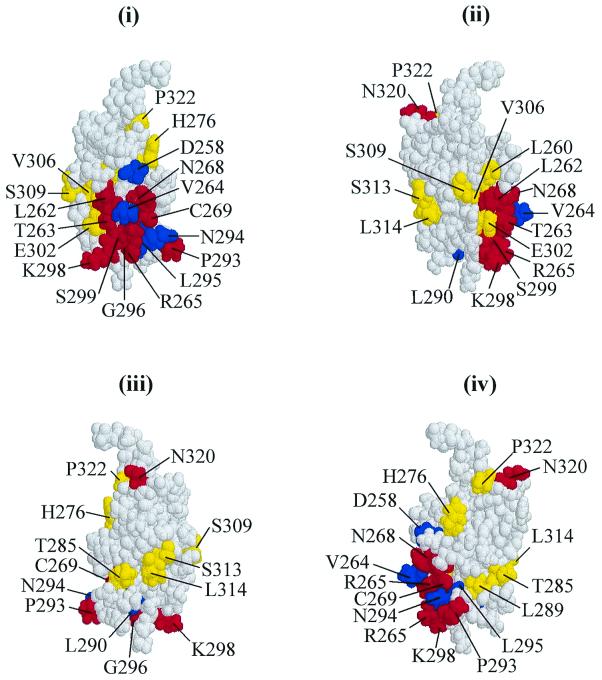
The solution structure of the αCTD (17), showing the positions of residues identified as important for MetR-dependent activation at metE and metH in vivo and, in some cases, verified in vitro. Red, residues important for both metE and metH expression; yellow, residues found to be important for MetR-dependent expression of metE only; blue, residues found to be important for MetR-dependent expression of metH only. Views (i) to (iv) are related by 90° rotations about the vertical axis.
Residues L260, T263, H276, P293, E302, V306, S309, N320, and P322 also localize to this complex face of α, with H276, N320, and P322 forming an outlying extension of the face and the others situated near the DNA binding residues (Fig. 5). Since the phenotype caused by alanine substitutions at these positions is MetR dependent and because many of these residues are situated near the α-DNA binding residues, these residues could be stabilizing the interaction of α with DNA after being positioned by MetR. Alternatively, these residues could form a protein-protein interaction surface for contacting MetR. It has been postulated that if indeed the αCTD is involved in both nonspecific DNA interactions and specific protein-protein interactions, then mutations in the residues involved in the protein-protein interactions should result in a stronger phenotype than mutations in the DNA binding residues (1). Despite the fact that the alanine substitution experiments were performed in the presence of a wild-type, chromosomal copy of rpoA, several of these substitutions do indeed exhibit stronger metE phenotypes than do substitutions in the α-DNA binding residues (Fig. 2A). In addition, a change from isoleucine to alanine at residue 303, which is buried within α but behind residues E302, V306, and S309, causes the most drastic reduction in MetR-dependent activation of metE (Fig. 2A), presumably by disrupting a MetR interaction surface of α. In contrast, a change from leucine to alanine at 270, which we propose disrupts the α-DNA interaction by altering helix 1 of the αCTD, has a more modest effect on MetR-dependent metE expression (Fig. 2A). Although we favor a model where these residues interact with MetR for activation, we have not ruled out the possibility that substitutions at these residues may cause a metE-down phenotype due to some indirect effect, e.g., altering levels of MetR or expression of homocysteine biosynthetic enzymes in vivo.
The other surface-exposed residues of the αCTD that caused decreases in metE activation when changed to alanine, L289, S313, and L314, do not localize to the complex face of α. However, these residues, along with T285 (which causes nearly a twofold decrease in metE activation in vivo when changed to alanine [Fig. 2A]), form a discrete patch on the face of the αCTD opposite the complex face (Fig. 5). These residues lie within the 20- by 10-Å surface of the αCTD that has been shown to be important for CRP activation of the synthetic CC(−41.5) promoter and is proposed to be the surface used for an αCTD-CRP interaction (36). The CC(−41.5) synthetic promoter has an activator binding site in essentially the same location (−41.5) as the activation site for MetR on the metE promoter (−42). Therefore, these residues could define a third MetR contact patch on the αCTD. The analogy between metE and CC(−41.5) is not quite straightforward because of the second, upstream MetR binding site (site 1) at metE that is located in the position thought to be contacted by the αCTD at CC(−41.5); however, since the rotational geometry of the proteins at these promoters is not known, this does not necessarily present a steric problem.
At the well-studied promoter CC(−41.5), αCTD-CRP and αCTD-DNA interactions increase the binding of RNAP to the promoter but have no effect on the isomerization to open complex. CRP uses additional interactions with residues in the αNTD to facilitate the closed-to-open-complex isomerization (reviewed in reference 4a). Using CC(−41.5) as a model for metE, we propose that RNAP is recruited to metE through specific interactions between MetR and residues T285, L289, S313, and L314 of the αCTD. These residues lie within the same 20- by 10-Å surface of α that contains the residues important for the recruitment of CRP to CC(−41.5) (4a, 36). The MetR-RNAP interaction may then be stabilized by αCTD-DNA interactions involving residues L262, R265, N268, C269, G296, K298, and S299, which are properly positioned to contact DNA upon interaction between the αCTD and MetR. The αCTD-DNA contacts made at other promoters where the activator binds overlapping the −35 sequence can be observed as an extension of the footprint upstream of the promoter in the presence of wild-type RNAP but not with RNAP carrying α subunits with CTD deletions (for an example, see reference 2), suggesting that the αCTD reaches over the activator to contact the DNA. At metE, however, an upstream extension of the MetR footprint is not observed with wild-type RNAP (15a); therefore, we favor a model where the αCTD interacts with DNA within the MetR footprint, probably by binding to a face of the helix different from that where MetR binds. This second, upstream MetR binding site at metE may be the reason why we identified additional residues within the complex face of α that are important for MetR-dependent activation. Residues L260, T263, H276, P293, E302, V306, S309, N320, and P322 may also be used to stabilize the MetR-RNAP interaction. We did not identify any residues in the αNTD that were important for MetR-dependent activation; however, αNTD substitutions did not, in general, cause strong decreases in CRP-dependent activation at CC(−41.5) (31), so our initial selection for PCR-mutagenized rpoA genes that decreased metE expression may not have been sensitive enough to detect αNTD mutants. Alternatively, residues L260, T263, H276, P293, E302, V306, S309, N320, and P322 of the αCTD may replace the αNTD-activator interaction used at CC(−41.5) to facilitate the closed-to-open-complex isomerization.
Amino acids in the αCTD important for MetR activation of metH.
In the initial selection for α mutations that alter MetR-dependent activation, we were able to identify only one substitution, N268D, which had a significant effect on metH expression. Interestingly, we were able to detect a metH phenotype only for the rpoA alleles tested in GS972, whereas rpoA mutations that affected metE expression were best detected in GS162 (Table 2). We speculate that a metH-down phenotype is observed only in GS972 because GS162 does not produce enough MetR to fully activate metH, and a metH-down phenotype is apparent only when there is sufficient MetR available.
In the screen of the αCTD alanine substitution library, only α-DNA interaction residues (R265, N268, K298, and S299) were found to significantly disrupt metH activation if changed to alanine (Fig. 2B). We confirmed the metH phenotypes of R265 and N268 substitutions in vitro as well as identifying another α-DNA interaction residue, G296, as important for metH activation (Fig. 3D). Therefore, five of the residues previously identified as important for α-DNA interactions are also important for metH activation: R265, N268, G296, K298, and S299 (Fig. 5). We were also able to show that mutations with a slight phenotype in vivo, e.g., L270H, could indeed cause a significant phenotype in vitro (Fig. 3D). Therefore, we consider mutations that cause less than a twofold down phenotype for metH expression in vivo to be potentially necessary for MetR activation of metH. By these less stringent criteria, a number of other surface-exposed αCTD residues, including D258, L262, T263, V264, C269, L290, P293, N294, L295, G296, and N320, also disrupt metH activation if changed to alanine. Several of these residues have also previously been shown to be important for activator-dependent transcription at other promoters: D258 is important for CRP, TyrR, and bacteriophage Mu Mor protein activation of lac, mtr, and Pm, respectively (1, 42, 55); V264 mutations can suppress a positive control mutant of OmpR to partially restore activation at ompF (19); L290 is necessary for P2 Ogr activation at P4 late promoters (53); N294 is required for activation at katG by OxyR and UP element activation of rrnBp1 (10, 44); and L295 mutations disrupt UP element activation of rrnBp1 (10).
All of the residues identified as crucial for metH activation are located within the complex face of α (Fig. 5). While some of the residues in this complex face are important for activation of both metE and metH, others are important for metH activation only (Fig. 5). Substitutions in residues D258, V264, L290, N294, and L295 all affect metH but not metE expression. Since a number of the metH-specific residues have been shown to be important for activator-dependent expression in other systems, we propose that residues D258, T263, V264, L290, P293, N294, L295, and N320 contact MetR for activation at metH. Alternatively, D258 may interact with the ς70 subunit, a role previously proposed for this residue in CRP-dependent activation at lac (4a). Furthermore, we propose that the MetR-αCTD interaction is stabilized by interactions between residues L262, R265, N268, C269, G296, K298, and S299 and metH promoter DNA.
The metE and metH promoters differ not only in the number of MetR binding sites but also in the locations of the activation sites. However, a simple comparison of the locations of the active sites relative to the transcriptional start sites is problematic because both S1 nuclease mapping (47) and primer extension (data not shown) show that metH has two transcription start sites separated by 3 intervening bp that appear to be used with equal efficiency in the absence and in the presence of MetR; thus, it is likely that both transcripts depend on the same Pribnow box. Because of the dual start sites at metH, we use the 3′-most T base of the Pribnow box, which is highly conserved in most promoters (13), as a reference point to determine the relative locations of the MetR sites at the metE and metH promoters. The center of the MetR activation site (site 2) at metE is 36 bp upstream of this reference point, while the center of the single MetR site at metH is 52 bp upstream of this point (Fig. 1); therefore, the MetR site at metH is 16 bp, or one and one-half helical turns, further upstream than the metE promoter. This means that MetR binds to opposite helical faces of DNA at these promoters. We propose that the differences in the αCTD residues that are important for MetR activation result from the differences in the locations of the MetR activation sites at metE and metH.
Differential orientation requirements for wild-type αCTD within RNAP for activation at metE and metH.
Our experiments with RNAP containing oriented α subunits indicate that the CTD of either α subunit is capable of making the interactions necessary for MetR-dependent activation at metE. This interchangeability of the αCTD was also observed for UP element subsite recognition at rrnBp1 by Estrem et al. (9). It has also been reported that the αCTD functions interchangeably for CRP activation at lac and CC(−41.5) (4a). In contrast, MetR activation at metH has a more stringent requirement for an intact αCTD on the β-associated αI; the CTD of the β′-associated αII substitutes very poorly for the αI CTD. The metH promoter is similar to the lacP1 promoter in that the activators bind well upstream of the −35 sequence in both cases; however, using the 3′ T base of the Pribnow box as a reference, CRP binds to a site centered 54.5 bp upstream (6), while MetR binding at metH is centered 52 bp upstream (5). This means that CRP binds one-quarter of a helical turn further upstream at lac than MetR binds at metH. We speculate that the restriction on which of the two αCTDs is capable of activation at metH is due to limits on the rotational flexibility of the αCTD with respect to the rest of RNAP. It has previously been shown that the α linker confers considerable two-dimensional flexibility on the αCTD, allowing it to reach long distances from the core promoter to interact with activator proteins, as long as the helical phasing of the activator is maintained (11, 50, 52). Phasing experiments with FNR have shown that changes of as little as 1 or 2 bp from a position favorable for FNR activation can destroy FNR-dependent activation (52). From these results, we speculate that the αCTD has considerable flexibility such that it can stretch to reach distant activators but lacks a rotational flexibility that would allow it to both reach for activators and wrap around the DNA to contact activators that bind to a helical face of the DNA different from that where RNAP binds. This restricted rotational flexibility could also limit the mobility of each αCTD such that an activator that binds “off to the side” of the DNA (relative to the plane set by RNAP) would be able to contact one αCTD but the other could not substitute if the critical αCTD was mutated or deleted. This model would predict that other αCTD-dependent activators that bind “off to the side” may display an α-specificity, as was seen for MetR at metH. One example might be the OxyR-dependent promoter katG, which has previously been shown to require the αCTD (43). OxyR at katG binds 47 bp upstream of the 3′ T of the Pribnow box (45), meaning that it also binds “off to the side” of the DNA but it binds to the side opposite MetR at metH; therefore, we would predict that if OxyR at katG does show an αCTD specificity, it may require that the αCTD on the β′-associated αII be intact. Furthermore, the limited rotational flexibility of the αCTD proposed in this model would predict that αCTD-dependent activators that bind to the opposite helical face of the DNA relative to RNAP would need to bind in such a way that the activating region of the activator protein would be wrapped around the DNA and would thus be accessible to the αCTD. Such a mechanism has the potential to lead to an α-specificity which could be examined in vitro by using oriented α RNAPs.
ACKNOWLEDGMENTS
We thank Robert Landick for purified ς70 protein, and Tamas Gaal, Richard Gourse, and Richard Ebright for plasmids.
This work was supported by a Carver Medical Research Initiative Grant. P.S.F. is supported by a NIH Predoctoral Training Grant in Biotechnology (GM08365) and The University of Iowa Center for Biocatalysis and Bioprocessing.
REFERENCES
- 1.Artsimovitch I, Murakami K, Ishihama A, Howe M M. Transcription activation by the bacteriophage Mu Mor protein requires the C-terminal regions of both α and ς70 subunits of Escherichia coli RNA polymerase. J Biol Chem. 1996;271:32343–32348. doi: 10.1074/jbc.271.50.32343. [DOI] [PubMed] [Google Scholar]
- 2.Attey A, Belyaeva T, Savery N, Hoggett J, Fujita N, Ishihama A, Busby S. Interactions between the cyclic AMP receptor protein and the alpha subunit of RNA polymerase at the Escherichia coli galactose operon P1 promoter. Nucleic Acids Res. 1994;22:4375–4380. doi: 10.1093/nar/22.21.4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blatter E E, Ross W, Tang H, Gourse R L, Ebright R H. Domain organization of RNA polymerase α subunit: C-terminal 85 amino acids constitute a domain capable of dimerization and DNA binding. Cell. 1994;78:889–896. doi: 10.1016/s0092-8674(94)90682-3. [DOI] [PubMed] [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 4a.Busby S, Ebright R H. Transcription activation by catabolite activator protein (CAP) J Mol Biol. 1999;293:199–213. doi: 10.1006/jmbi.1999.3161. [DOI] [PubMed] [Google Scholar]
- 5.Byerly K A, Urbanowski M L, Stauffer G V. The MetR binding site in the Salmonella typhimurium metH gene: DNA sequence constraints on activation. J Bacteriol. 1991;173:3547–3553. doi: 10.1128/jb.173.11.3547-3553.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickson R C, Abelson J, Barnes W M, Reznikoff W S. Genetic regulation: the lac control region. Science. 1975;187:27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- 7.Dove S L, Joung J K, Hochschild A. Activation of prokaryotic transcription through arbitrary protein-protein contacts. Nature. 1997;386:627–630. doi: 10.1038/386627a0. [DOI] [PubMed] [Google Scholar]
- 8.Ebright R H, Busby S. The Escherichia coli RNA polymerase α subunit: structure and function. Curr Opin Genet Dev. 1995;5:197–203. doi: 10.1016/0959-437x(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 9.Estrem S T, Ross W, Gaal T, Chen Z W S, Niu W, Ebright R H, Gourse R L. Bacterial promoter architecture: subsite structure of UP elements and interactions with the carboxy-terminal domain of the RNA polymerase α subunit. Genes Dev. 1999;13:2134–2147. doi: 10.1101/gad.13.16.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaal T, Ross W, Blatter E E, Tang H, Jia X, Krishnan V V, Assa-Munt N, Ebright R H, Gourse R L. DNA-binding determinants of the α subunit of RNA polymerase: novel DNA-binding domain architecture. Genes Dev. 1996;10:16–26. doi: 10.1101/gad.10.1.16. [DOI] [PubMed] [Google Scholar]
- 11.Gaston K, Bell A, Kolb A, Buc H, Busby S. Stringent spacing requirements for transcription activation by CRP. Cell. 1990;62:733–743. doi: 10.1016/0092-8674(90)90118-x. [DOI] [PubMed] [Google Scholar]
- 12.Greene R C. Biosynthesis of methionine. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: American Society for Microbiology; 1996. pp. 542–560. [Google Scholar]
- 13.Harley C B, Reynolds R P. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987;15:2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hochschild A, Dove S L. Protein-protein contacts that activate and repress prokaryotic transcription. Cell. 1998;92:597–600. doi: 10.1016/s0092-8674(00)81126-5. [DOI] [PubMed] [Google Scholar]
- 15.Igarashi K, Fujita N, Ishihama A. Sequence analysis of two temperature-sensitive mutations in the alpha subunit gene (rpoA) of Escherichia coli RNA polymerase. Nucleic Acids Res. 1990;18:5945–5948. doi: 10.1093/nar/18.20.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Jafri S. Mutations in the α subunit of RNA polymerase and their effects on metE expression in Escherichia coli. Ph.D. thesis. Iowa City: University of Iowa; 1996. [Google Scholar]
- 16.Jafri S, Urbanowski M L, Stauffer G V. The glutamic acid residue at amino acid 261 of the α subunit is a determinant of the intrinsic efficiency of RNA polymerase at the metE core promoter in Escherichia coli. J Bacteriol. 1996;178:6810–6816. doi: 10.1128/jb.178.23.6810-6816.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeon Y H, Negishi T, Shirakawa M, Yamazaki T, Fujita N, Ishihama A, Kyogoku Y. Solution structure of the activator contact domain of the RNA polymerase α subunit. Science. 1995;270:1495–1497. doi: 10.1126/science.270.5241.1495. [DOI] [PubMed] [Google Scholar]
- 18.Kainz M, Gourse R L. The C-terminal domain of the alpha subunit of Escherichia coli RNA polymerase is required for efficient rho-dependent transcription termination. J Mol Biol. 1998;284:1379–1390. doi: 10.1006/jmbi.1998.2272. [DOI] [PubMed] [Google Scholar]
- 19.Kato N, Aiba H, Mizuno T. Suppressor mutations in α-subunit of RNA polymerase for a mutant of the positive regulator, OmpR, in Escherichia coli. FEMS Microbiol Lett. 1996;139:175–180. doi: 10.1111/j.1574-6968.1996.tb08199.x. [DOI] [PubMed] [Google Scholar]
- 20.Kawakami K, Ishihama A. Defective assembly of ribonucleic acid polymerase subunits in a temperature-sensitive α-subunit mutant of Escherichia coli. Biochemistry. 1980;19:3491–3495. doi: 10.1021/bi00556a013. [DOI] [PubMed] [Google Scholar]
- 21.Kimura M, Ishihama A. Functional map of the alpha subunit of Escherichia coli RNA polymerase: amino acid substitution within the amino-terminal assembly domain. J Mol Biol. 1995;254:342–349. doi: 10.1006/jmbi.1995.0621. [DOI] [PubMed] [Google Scholar]
- 22.Lee J H, Hoover T R. Protein crosslinking studies suggest that Rhizobium meliloti C4-dicarboxylic acid transport protein D, a ς54-dependent transcriptional activator, interacts with ς54 and the β subunit of RNA polymerase. Proc Natl Acad Sci USA. 1995;92:9702–9706. doi: 10.1073/pnas.92.21.9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li M, Moyle H, Susskind M M. Target of the transcriptional activation function of phage λ cI protein. Science. 1994;263:75–77. doi: 10.1126/science.8272867. [DOI] [PubMed] [Google Scholar]
- 24.Lombardo M-J, Bagga D, Miller C G. Mutations in rpoA affect expression of anaerobically regulated genes in Salmonella typhimurium. J Bacteriol. 1991;173:7511–7518. doi: 10.1128/jb.173.23.7511-7518.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maxon M E, Wigboldus J, Brot N, Weissbach H. Structure-function studies on Escherichia coli MetR protein, a putative prokaryotic leucine zipper. Proc Natl Acad Sci USA. 1990;87:7076–7079. doi: 10.1073/pnas.87.18.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller A, Wood D, Ebright R H, Rothman-Denes L B. RNA polymerase β′ subunit: a target of DNA binding-independent activation. Science. 1997;275:1655–1657. doi: 10.1126/science.275.5306.1655. [DOI] [PubMed] [Google Scholar]
- 27.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 28.Murakami K, Fujita N, Ishihama A. Transcription factor recognition surface on the RNA polymerase α subunit is involved in contact with the DNA enhancer element. EMBO J. 1996;15:4358–4367. [PMC free article] [PubMed] [Google Scholar]
- 29.Murakami K, Kimura M, Owens J T, Meares C F, Ishihama A. The two α subunits of Escherichia coli RNA polymerase are asymmetrically arranged and contact different halves of the DNA upstream element. Proc Natl Acad Sci USA. 1997;94:1709–1714. doi: 10.1073/pnas.94.5.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nègre D, Oudot C, Prost J-F, Murakami K, Ishihama A, Cozzone A J, Cortay J-C. FruR-mediated transcriptional activation at the ppsA promoter of Escherichia coli. J Mol Biol. 1998;276:355–365. doi: 10.1006/jmbi.1997.1548. [DOI] [PubMed] [Google Scholar]
- 31.Niu W, Kim Y, Tau G, Heyduk T, Ebright R H. Transcription activation at class II CAP-dependent promoters: two interactions between CAP and RNA polymerase. Cell. 1996;87:1123–1134. doi: 10.1016/s0092-8674(00)81806-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panasenko S M, Cameron J R, Davis R W, Lehmen I R. Five-hundred-fold overproduction of DNA ligase after induction of a hybrid lambda lysogen constructed in vitro. Science. 1977;196:188–189. doi: 10.1126/science.322281. [DOI] [PubMed] [Google Scholar]
- 33.Plamann L S, Urbanowski M L, Stauffer G V. Salmonella typhimurium metE operator-constitutive mutations. Gene. 1988;73:201–208. doi: 10.1016/0378-1119(88)90326-5. [DOI] [PubMed] [Google Scholar]
- 34.Ross W, Gosink K K, Salomon J, Igarashi K, Zou C, Ishihama A, Severinov K, Gourse R L. A third recognition element in bacterial promoters: DNA binding by the α subunit of RNA polymerase. Science. 1993;262:1407–1413. doi: 10.1126/science.8248780. [DOI] [PubMed] [Google Scholar]
- 35.Ross W, Thompson J F, Newlands J T, Gourse R L. E. coli Fis protein activates ribosomal RNA transcription in vitro and in vivo. EMBO J. 1990;9:3733–3742. doi: 10.1002/j.1460-2075.1990.tb07586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savery N J, Lloyd G S, Kainz M, Gaal T, Ross W, Ebright R H, Gourse R L, Busby S J W. Transcription activation at class II CRP-dependent promoters: identification of determinants in the C-terminal domain of the RNA polymerase α subunit. EMBO J. 1998;17:3439–3447. doi: 10.1093/emboj/17.12.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt T G M, Koepke J, Frank R, Skerra A. Molecular interaction between the Strep-tag affinity peptide and its cognate target, streptavidin. J Mol Biol. 1996;255:753–766. doi: 10.1006/jmbi.1996.0061. [DOI] [PubMed] [Google Scholar]
- 38.Severinov K, Soushko M, Goldfarb A, Nikiforov V. Rifampicin region revisited: new rifampicin-resistant and streptolydigin-resistant mutants in the β-subunit of Escherichia coli RNA polymerase. J Biol Chem. 1993;268:14820–14825. [PubMed] [Google Scholar]
- 39.Shi X, Bennett G N. Effects of rpoA and cysB mutations on acid induction of biodegradative arginine decarboxylase in Escherichia coli. J Bacteriol. 1994;176:7017–7023. doi: 10.1128/jb.176.22.7017-7023.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang H, Kim Y, Severnov K, Goldfarb A, Ebright R H. Escherichia coli RNA polymerase holoenzyme: rapid reconstitution from recombinant α, β, β′, and ς subunits. Methods Enzymol. 1996;273:130–134. doi: 10.1016/s0076-6879(96)73012-4. [DOI] [PubMed] [Google Scholar]
- 41.Tang H, Severinov K, Goldfarb A, Ebright R H. Rapid RNA polymerase genetics: one-day, no-column preparation of reconstituted recombinant Escherichia coli RNA polymerase. Proc Natl Acad Sci USA. 1995;92:4902–4906. doi: 10.1073/pnas.92.11.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang H, Severinov K, Goldfarb A, Fenyo D, Chait B, Ebright R H. Location, structure, and function of the target of a transcriptional activator protein. Genes Dev. 1994;8:3058–3067. doi: 10.1101/gad.8.24.3058. [DOI] [PubMed] [Google Scholar]
- 43.Tao K, Fujita N, Ishihama A. Involvement of the RNA polymerase α subunit C-terminal region in co-operative interaction and transcriptional activation with OxyR protein. Mol Microbiol. 1993;7:859–864. doi: 10.1111/j.1365-2958.1993.tb01176.x. [DOI] [PubMed] [Google Scholar]
- 44.Tao K, Zou C, Fujita N, Ishihama A. Mapping of the OxyR protein contact site in the C-terminal region of RNA polymerase α subunit. J Bacteriol. 1995;177:6740–6744. doi: 10.1128/jb.177.23.6740-6744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toledano M B, Kullik I, Trinh F, Baird P T, Schneider T D, Storz G. Redox-dependent shift of OxyR-DNA contacts along an extended DNA-binding site: a mechanism for differential promoter selection. Cell. 1994;78:897–909. doi: 10.1016/s0092-8674(94)90702-1. [DOI] [PubMed] [Google Scholar]
- 46.Urbanowski M L, Stauffer G V. Regulation of the metR gene of Salmonella typhimurium. J Bacteriol. 1987;169:5841–5844. doi: 10.1128/jb.169.12.5841-5844.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urbanowski M L, Stauffer G V. The control region of the metH gene of Salmonella typhimurium LT2: an atypical met promoter. Gene. 1988;73:193–200. doi: 10.1016/0378-1119(88)90325-3. [DOI] [PubMed] [Google Scholar]
- 48.Urbanowski M L, Stauffer G V. Role of homocysteine in metR-mediated activation of the metE and metH genes in Salmonella typhimurium and Escherichia coli. J Bacteriol. 1989;171:3277–3281. doi: 10.1128/jb.171.6.3277-3281.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urbanowski M L, Stauffer G V. Genetic and biochemical analysis of the MetR activator-binding site in the metE metR control region of Salmonella typhimurium. J Bacteriol. 1989;171:5620–5629. doi: 10.1128/jb.171.10.5620-5629.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ushida C, Aiba H. Helical phase dependent action of CRP: effect of the distance between the CRP site and the −35 region on promoter activity. Nucleic Acids Res. 1990;18:6325–6330. doi: 10.1093/nar/18.21.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vogel H J, Bonner D M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956;218:97–106. [PubMed] [Google Scholar]
- 52.Wing H J, Williams S M, Busby S J W. Spacing requirements for transcription activation by Escherichia coli FNR protein. J Bacteriol. 1995;177:6704–6710. doi: 10.1128/jb.177.23.6704-6710.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wood L F, Tszine N Y, Christie G E. Activation of P2 late transcription by P2 Ogr protein requires a discrete contact site on the C terminus of the α subunit of Escherichia coli RNA polymerase. J Mol Biol. 1997;274:1–7. doi: 10.1006/jmbi.1997.1390. [DOI] [PubMed] [Google Scholar]
- 54.Wu W-F, Urbanowski M L, Stauffer G V. Characterization of a second MetR-binding site in the metE metR regulatory region of Salmonella typhimurium. J Bacteriol. 1995;177:1834–1839. doi: 10.1128/jb.177.7.1834-1839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang J, Murakami K, Camakaris H, Fujita N, Ishihama A, Pittard A J. Amino acid residues in the α-subunit C-terminal domain of Escherichia coli RNA polymerase involved in activation of transcription from the mtr promoter. J Bacteriol. 1997;179:6187–6191. doi: 10.1128/jb.179.19.6187-6191.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zalenskaya K, Lee J, Gujuluva C N, Shin Y K, Slutsky M, Goldfarb A. Recombinant RNA polymerase: inducible overexpression, purification and assembly of Escherichia coli rpo gene products. Gene. 1990;89:7–12. doi: 10.1016/0378-1119(90)90199-2. [DOI] [PubMed] [Google Scholar]
- 57.Zou C, Fujita N, Igarashi K, Ishihama A. Mapping the cAMP receptor protein contact site on the α subunit of Escherichia coli RNA polymerase. Mol Microbiol. 1992;6:2599–2605. doi: 10.1111/j.1365-2958.1992.tb01437.x. [DOI] [PubMed] [Google Scholar]