Abstract
Infection with Junín virus (JUNV) is currently being effectively managed in the endemic region using a combination of targeted vaccination and plasma therapy. However, the long-term sustainability of plasma therapy is unclear and similar resources are not available for other New World arenaviruses. As a result, there has been renewed interest regarding the potential of drug-based therapies. To facilitate work on this issue, we present the establishment and subsequent optimization of a JUNV minigenome system to a degree suitable for high-throughput miniaturization, thereby providing a screening platform focused solely on factors affecting RNA synthesis. Using this tool, we conducted a limited drug library screen and identified AVN-944, a non-competitive inosine monophosphate dehydrogenase (IMPDH) inhibitor, as an inhibitor of arenavirus RNA synthesis. We further developed a transcription and replication competent virus-like particle (trVLP) system based on these minigenomes and used it to screen siRNAs against IMPDH, verifying its role in supporting arenavirus RNA synthesis. The antiviral effect of AVN-944, as well as siRNA inhibition, on JUNV RNA synthesis supports that, despite playing only a minor role in the activity of ribavirin, exclusive IMPDH inhibitors may indeed have significant therapeutic potential for use against New World arenaviruses. Finally, we confirmed that AVN-944 is also active against arenavirus infection in cell culture, supporting the suitability of arenavirus lifecycle modelling systems as tools for the screening and identification, as well as the mechanistic characterization, of novel antiviral compounds.
Keywords: Life cycle modelling systems, Arenavirus, Inosine monophosphate dehydrogenase (IMPDH), AVN-944
1. Introduction
Members of the Arenaviridae family cause several important human diseases, including Lassa fever (LF) in Africa, and multiple viral hemorrhagic fevers (VHFs) endemic to South America. In particular, Junín virus (JUNV) continues to pose a significant public health risk in Argentina, while the closely related Machupo virus (MACV) and Guanarito virus (GTOV) are ongoing problems in Bolivia and Venezuela, respectively (Aguilar et al., 2009; Fulhorst et al., 2008). Other highly pathogenic New World arenaviruses have so far demonstrated little public health significance, but remain a concern particularly due to agricultural intensification (Zimmerer, 2013).
Pathogenic South American arenaviruses cause clinically similar forms of VHF with case fatality rates of 20–30% in untreated patients, and research is restricted to biosafety level 4 (BSL4) laboratories, although for JUNV a safe and effective vaccine (i.e. Candid#1) as well as effective post-exposure convalescent plasma therapy have been developed (Enria et al., 2008). However, inconsistent funding for vaccine production, declining numbers of convalescent plasma donors and the spreading endemic region of JUNV remain causes for concern. Further, plasma therapy carries the risk of developing a late neurological syndrome of unknown etiology, which affects about 10% of patients (Enria et al., 1985; Maiztegui et al., 1979). No disease management strategies are available for other VHF-causing South American arenaviruses. Thus, the identification of additional therapeutic options remains an important priority. In particular, there has been renewed interest regarding the potential of small molecule inhibitors of arenavirus infection, especially RNA synthesis inhibitors such as ribavirin and T-705 (Gowen et al., 2013, 2017; Mendenhall et al., 2011a, 2011b; Moreno et al., 2011; Olschlager et al., 2011; Safronetz et al., 2015; Salazar et al., 2012), either alone or in combination where they have exhibited in vivo synergistic effects (Oestereich et al., 2016; Westover et al., 2016). Further, there is on-going interest in developing systems to identify novel antiviral agents, including with the aid of high-throughput screening approaches (Pasquato and Kunz, 2016). The basis for many such screening systems is minigenomes, i.e. viral genome analogues with one or more viral gene(s) replaced by a reporter gene, which allow monitoring of viral RNA synthesis and screening for inhibitors that specifically target this process. Such systems can also be extended into transcription and replication competent virus-like particle (trVLP) systems by including the components for budding, which leads to the production of minigenome-containing particles that can “infect” new cells expressing NP and L, to allow further replication of the incoming minigenomes. Such trVLP systems can thus be used to identify inhibitors of nearly any step in infection. Minigenome systems have been previously reported for both JUNV and its apathogenic relative Tacaribe virus (TCRV) (Albarino et al., 2009; Emonet et al., 2011; Lopez et al., 2001); however, such systems are frequently hampered by low dynamic range and/or contain unsuitable reporters, which can limit their utility.
In this study we describe a JUNV luciferase-based minigenome system and its optimization for high-throughput screening in a 96 well format. We also develop a trVLP system in this format for confirmatory work and demonstrate the utility of these systems for screening drug libraries and siRNA activity, respectively. Based on the findings, we identify AVN-944 as a novel inhibitor of New World arenavirus infection, and confirm that its target protein, IMPDH, is a pro-viral factor for JUNV RNA synthesis. Consistent with the broad spectrum activity of other IMPDH inhibitors against a range of arenaviruses, we could verify the activity AVN-944 using the related BSL2 virus TCRV. The assay and approaches in this study thus constitute the basis for a completely BSL2 pipeline for high throughput screening and validation of drugs with anti-arenaviral activity and/or siRNAs targeting host factors involved in the virus lifecycle.
2. Materials and methods
2.1. Cell culture
BHK-21 (CCLV-RIE0179), HEK293T (CCLV-RIE1018) and HeLa (CCLV-RIE0082) were grown in MEM with Hanks’ Salts or Earle’s Salts (1:1 mixture) and 1x non-essential amino acids. HEK293 (CCLV-RIE0197) and Vero76 (CCLV-RIE0228) were grown in DMEM with Earle’s Salts. A549 (CCLV-RIE1035) and HuH-7 (CCLV-RIE1079) were grown in Ham’s F12 and Iscove’s Modified Dulbecco’s Medium (1:1 mixture). BSR-T7/5 cells (kindly provided by Stefan Finke, Friedrich-Loeffler-Institut, Germany; CCLV-RIE0583 (Buchholz et al., 1999)) were grown in MEM Glasgow with 1 mg/ml Geneticin added every other passage. Cell lines were supplemented with 2 mM L-glutamine (Q), penicillin (100 U/mL)/streptomycin (100 μg/mL; PS), and 10% fetal calf serum (FCS), except BSR-T7/5 cells, for which 10% newborn calf serum (NCS) was used. Cells were cultured at 37 °C with 5% CO2.
2.2. Cloning
The JUNV minigenome was based on the S segment from the Romero strain of JUNV (GenBank Accession: AY619641) cloned in cRNA orientation between the T7 promoter and HDV ribozyme/T7 terminator elements of the previously described vector pAmp (Groseth et al., 2012). The viral open reading frames (ORFs) encoding NP and GPC were excised and reporter ORFs introduced into the GPC locus, generating either green fluorescent protein (GFP) or NanoLuciferase (nLuc) expressing minigenome versions (Fig. 1A). Variants were generated that incorporated promoter-less decoy eGFP ORFs cloned in both possible orientations downstream of the T7 terminator (site #1), between the T7 terminator and HDV ribozyme (site #2), or at both sites (Fig. 2A). pCAGGS-Cyp was generated based on the Cypridina reporter from pCLuc-Basic 2 (NEB). Primer sequences and cloning strategies are available upon request.
Fig. 1. Establishment of Junín virus (JUNV) minigenome systems.
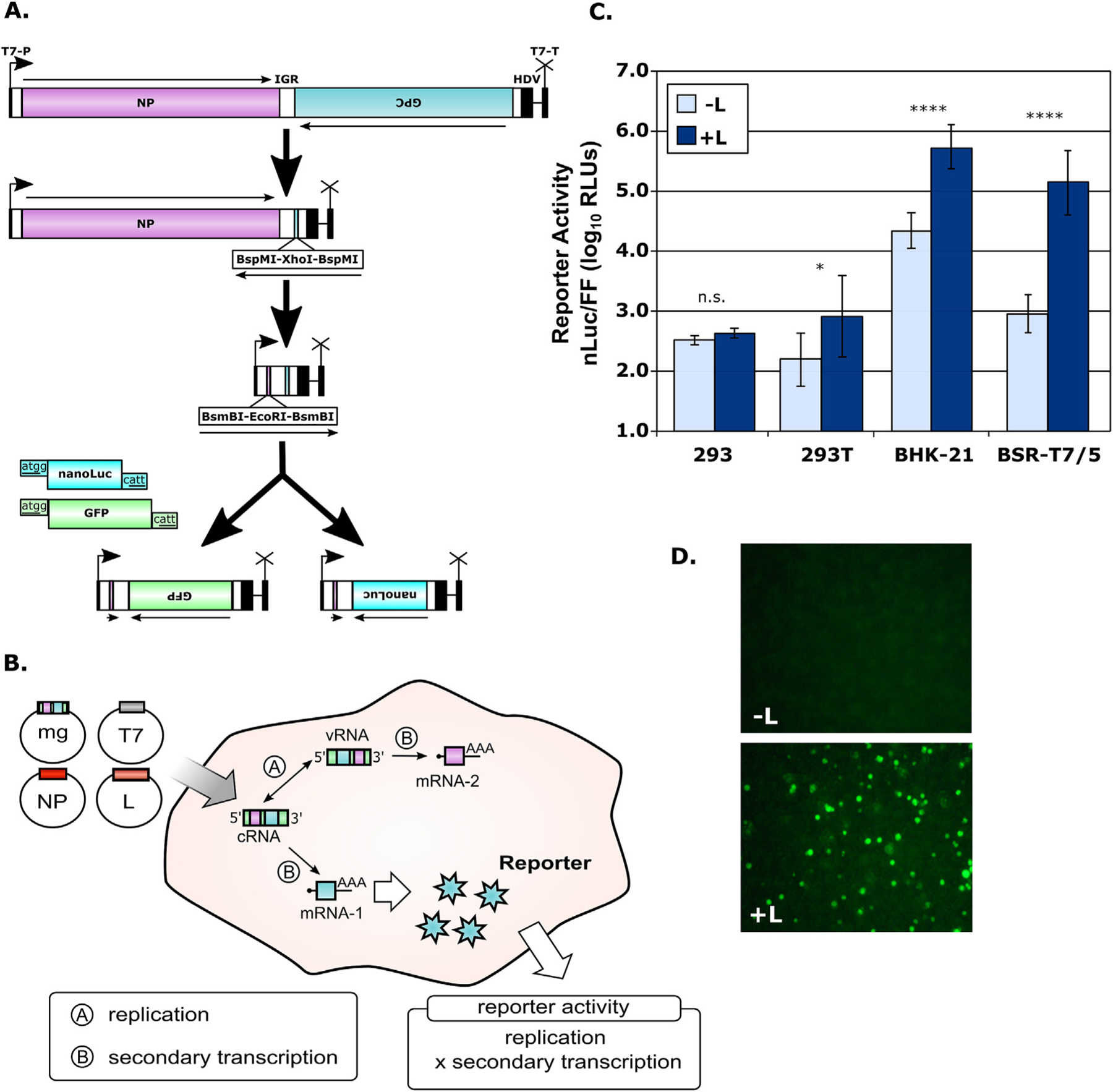
(A) Schematic diagram of JUNV minigenome plasmid construction. Minigenomes were based on the S-segment of the JUNV (strain Romero) cloned in cRNA orientation into the pAmp expression vector, which contains a T7 promoter, as well as a Hepatitis Delta Virus (HDV) ribozyme and T7 terminator elements, as indicated. The open reading frames encoding the NP and GP were then excised leaving a unique cloning cassette in each location. Subsequently, either NanoLuciferase (nLuc) or green-fluorescent protein (GFP) was inserted in place of the GPC gene to generate the final minigenome constructs. (B) Schematic diagram of the JUNV minigenome system. Transfection of the minigenomes described in (A), along with pCAGGS constructs encoding the T7 polymerase, JUNV nucleoprotein (NP) and polymerase (L), leads to transcription of these three proteins by RNA polymerase II and subsequent translation. T7 then directs transcription of the minigenome RNA, which is autocatalytically processed by HDV ribozyme cleavage to generate a construct containing authentic JUNV leader and trailer sequences. This genome analogue can then be encapsidated by NP and transcribed and replicated by L. The transcribed minigenome mRNA from the GPC gene encodes an assayable reporter protein, in this case either nLuc or GFP, and the production of this reporter reflects the cumulative viral RNA synthesis (i.e. transcription and replication) taking place in these cells. (C) JUNV minigenome assay for detection of NanoLuciferase expression. The indicated cell lines were transfected as described in (B) with a monocistronic JUNV minigenome encoding nLuc as well as support plasmids, either with or without JUNV L. A control plasmid pCAGGS-Firefly (FF) was also transfected. Cells were harvested 48 h later and measured for both nLuc (viral RNA synthesis) and FF (host cell RNA synthesis) activity. The means and standard deviations of normalized reporter levels (nLuc/FF) are shown and represent data from three independent experiments. The results of a one-way ANOVA analysis to compare sample pairs with and without L are indicated. (D) JUNV minigenome assay for detection of GFP expression. Cells were transfected as described in (C) but using a GFP-expressing minigenome and omitting the pCAGGS-FF control plasmid. Samples were prepared either without (top panel) or with (bottom panel) JUNV L and fluorescence was examined after 48 h.
Fig. 2. Minigenome optimization, scale-up, and comparison of intracellular and secreted reporter luciferase measurement.

(A) Schematic overview of decoy open reading frame (ORF) location and orientation. Constructs based on the NanoLuciferase (nLuc)-expressing minigenome were generated that encoded an additional promoter-less green fluorescent protein (GFP) in either a forward (5′-3’; right, R) or reverse (3′-5’; left, L) orientation at one or both of two insertion sites located either after the T7 terminator (site 1) or between the HDV ribozyme and the T7 terminator elements (site 2). (B) Reporter activity of decoy ORF-containing minigenome constructs in 6-well format. The decoy ORF-encoding minigenome plasmids described in (A), or the original unmodified construct (−) were transfected into BSR-T7/5 cells along with support plasmids and either with or without JUNV L. A control plasmid, either pCAGGS-Firefly (FF) or pCAGGSCypridina (Cyp), was also transfected. Cells lysates (left panel) were measured for nLuc (viral RNA synthesis) and FF (host cell RNA synthesis), while supernatants (right panel) were measured 48 h later for nLuc (viral RNA synthesis) and Cyp (host cell RNA synthesis) expression. The means and standard deviations of normalized reporter levels [nLuc/FF (lysates) or nLuc/Cyp (supernatants)] are shown and represent data from three independent experiments. Dynamic range [DR: < 2 (red)/2–3 (orange)/> 3 (green)], separation band [SB: < 1(red)/1–2(orange)/> 2(green)] and Z factor [Z’: < 0.2(red)/.2–0.5(orange)/> 0.5(green)] are shown as measures of assay performance. (C) Reporter activity of decoy ORF-containing minigenome constructs in 96-well format. Transfected plasmids amounts were reduced uniformly to account for the smaller surface areas in a 96-well plate, but experiments were otherwise performed and analyzed as described in (B). The means and standard deviations shown represent data for 12 biological replicates from 2 independent experiments.
2.3. Establishment and optimization of a JUNV minigenome assay
BHK-21, BSR-T7/5, HEK293 or HEK293T cells were seeded in 6-, 12-, or 96-well plates one day before transfection for 30–50% confluence. Transfection was performed using TransIT-LT1 (Mirus) with complex formation in OptiMEM using 3 μl TransIT-LT1/μg DNA. Each sample received the following previously described plasmids: pCAGGS-NP, pCAGGS-T7 (Groseth et al., 2010; Watt et al., 2014), and pCAGGS-FF (encoding Firefly luciferase as a normalization control (Watt et al., 2014)), as well as a JUNV minigenome plasmid, as indicated. pCAGGS-Cyp was substituted for pCAGGS-FF when examining secreted luciferase activity. Samples were prepared with or without pCAGGS-L, using empty pCAGGS to equalize transfected plasmid mass. Construct amounts are indicated in Suppl. Table 1. At 24 h post-transfection (p.t.), the medium on 6- or 12-well plates was replaced with fresh 5% FCS-containing medium. GFP-expression was imaged in living cells at 48 h p.t. using an Axiovert 40-C microscope (Zeiss) and a Ds-F1 camera (Nikon). For nLuc measurement, cells were harvested at 48 h p.t. by replacing the medium with 250 μl (6-well) or 200 μl (12-well) GloLysis Buffer (Promega), incubating samples on a rocking platform for 10–15 min at room temperature (RT), and clearing lysates at 10,000 × g for 3 min. For 96-well plates, 100 μl GloLysis buffer was used and samples were incubated at RT for 10 min. Luciferase activity in lysates was measured using the NanoGlo and BrightGlo luciferase assay systems (Promega). Where pCAGGS-Cyp was used, cell supernatants were collected, spun at 10,000 × g for 5 min and assayed for reporter activity using the NanoGlo (Promega) and Biolux Cypridina (NEB) assay systems. Measurements were performed using either a GloMax-Multi Microplate Reader (Promega; optimization experiments and drug library screening) or an Infinite F200 PRO microplate reader (Tecan; all other experiments).
2.4. Drug screening
Screening was performed using a previously published library of 34 drugs (Suppl Table 2) (Nelson et al., 2017) plus Ribavirin (Sigma). BHK21 cells (in 96-well plates) were incubated with 10 μM, 1 μM, or 0.1 μM (final concentration) of each drug for 2 h, after which cells were additionally transfected for minigenome assay with the optimized 1L2L decoy-containing minigenome (section 2.3). For AVN-944 (Adooq Bioscience) and Ribavirin, additional concentrations were analyzed as indicated. Additionally, cytotoxicity was analyzed using CellTiter-Glo reagent (Promega), which measures cellular ATP concentration, according to the manufacturer’s instructions.
2.5. Establishment of JUNV transcription/replication competent virus-like particle (trVLP) assay for analysis of siRNA-mediated IMPDH inhibition
To generate trVLPs, transfections were performed in BSR-T7/5 (p0) cells in 6-well or 12-well formats as described for the minigenome assay (section 2.3). After 24 h these cells were further transfected using the previously described constructs pCAGGS-Z and pCAGGS-GPC (Groseth et al., 2010) (Suppl. Table 3). On the next day target Huh7 (p1) cells were seeded for 30–50% confluence in 96-well plates, and after 24 h transfected with pCAGGS-NP and pCAGGS-L (Suppl. Table 3). After a further 24 h trVLP-producing p0 cells were lysed and assayed for nLuc and FF activity (section 2.3), while supernatants were centrifuged at 800 × g for 5 min, and p1 target cells were infected with 100 μl of trVLPs. After 48 h p1 cells were lysed and assayed for nLuc activity only (section 2.3).
Alternatively, p1 cells were transfected with SilencerSelect siRNAs (ThermoFisher) validated for inosine monophosphate dehydrogenase, isoform 2 (IMPDH2; Suppl. Fig. 1) knock-down, SilencerSelect Negative Control No. 2 siRNA (ThermoFisher), or AllStars Hs Cell Death control siRNA (Qiagen) by incubating 2 pmol lyophilized siRNA in 96-well plates with 50 μl OptiMEM and 0.2 μl Lipofectamine RNAiMax (ThermoFisher) for 30 min, and then adding 5000 Huh7 cells in 50 μl growth medium. After 24 h these cells were further processed as described above for p1 cells.
2.6. Validation of AVN-944 antiviral activity during TCRV infection
To test AVN-944 and Ribavirin activity during in vitro virus infection, Vero76 cells were infected with TCRV for 1 h at an MOI of 0.01 and afterwards AVN-944, Ribavirin or DMSO was added to the cells, as indicated. After 48 h supernatants were titrated by TCID50 and cells were analyzed for cytotoxicity using CellTiter-Glo reagent (section 2.4). TCID50 assays were performed on Vero76 cells in a 96-well format with 3 replicates per sample and dilution. Titers were calculated using the Spearman-Kärber method.
2.7. Statistical analysis and evaluation of assay metrics
Data analysis included multiple biological replicates (i.e. replicates of a sample within an experiment) from multiple independent experiments, as indicated for the individual assays. One-way ANOVA was performed in GraphPad Prism v6 (GraphPad). Significance cut-offs: * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; **** p ≤ 0.0001. Post hoc tests were performed using Dunnett’s (comparison to a control) and/or Tukey’s (multiple pairwise comparisons) test. Post hoc test selection of did not affect the conclusions of the experiments. Assay performance was evaluated using a combination of dynamic range (DR; difference between the mean of the positive control and the mean of the negative control), separation band (SB; difference between the mean of the positive control – 3 standard deviations and the mean of the negative control + 3 standard deviations) and Z factor (Z’; ratio of SB to DR).
3. Results
3.1. Minigenome assay establishment and optimization for high-throughput formats
Initially, both GFP-based and luciferase-based JUNV minigenomes were developed and assessed in 6-well and 12-well format (Fig. 1A). Following transfection of baby hamster kidney cells (BHK21 or BSR-T7/5) cells (Fig. 1B) we saw high levels of reporter activity with both constructs (Fig. 1C and D). In contrast, without the polymerase, activity was markedly lower (i.e. 1.5–2 logs lower for nLuc constructs). Interestingly, little or no activity was observed using 293 or 293T cells, although these cells are often favored for minigenome assays with other virus families due to their transfectability.
We next miniaturized the assay for 96-well format, including streamlining the protocol to eliminate the medium exchange at 24 h p.t., rocking during lysis and centrifugation of lysates prior to measurement. These changes did not affect performance, but reduced the workload considerably. However, while the values for assay metrics (DR, SB and Z′) were similar in 6-well and 96-well formats (Fig. 2B and C, left-most column pairs), they remained suboptimal for further miniaturization. In particular, background (-L) was high compared to other systems we have developed for non-segmented viruses. Therefore, cryptic promoter activity from the vector, potentially resulting in viral RNA synthesis-independent mRNA expression, was considered as a potential issue. To address this possibility, decoy ORFs encoding GFP were introduced in either orientation [i.e. R (5′-3′) or L (3′-5′)] immediately downstream of the T7 terminator (site 1) and/or between the HDV ribozyme and T7 terminator (site 2; Fig. 2A) in an attempt to distance our minigenome-encoded reporter ORF from any such element (s) and/or provide an irrelevant alternate upstream ORF as a target for translation on any such spuriously produced mRNAs, thereby reducing the potential for non-specific background reporter production. While positive signals remained unchanged, background was reduced > 10-fold, with the best results obtained using two decoy ORFs (Fig. 2B and C, left panels), resulting in DR values ≥ 3 log, SB values ≥ 2 logs and a Z′ of > 0.5 (a commonly applied threshold to evaluate the feasibility of further assay miniaturization).
As an alternative to measuring luciferase activity in cell lysates we also sought to exploit the non-specific secretion of nLuc, together with a second secreted control luciferase (i.e. Cyp), for direct measurement in supernatants. While this assay performed adequately for manual data analysis (Fig. 2B and C, right panels), increased variability in replicate samples negatively affected the metrics for these assays, making them suboptimal for further miniaturization to truly high-throughput formats with automated analysis.
3.2. Application of the minigenome assay for small-molecule antiviral compound screening
We then used the minigenome assay to screen a panel of 34 potentially anti-viral small molecules (Nelson et al., 2017) (Supplemental Table 2; Fig. 3A) in addition to Ribavirin, which served as a positive control. Among these compounds, only AVN-944 (Compound #26) and Ribavirin (RIB) showed marked inhibition of viral RNA synthesis (nLuc activity) over and above the effect on host cell RNA synthesis (FF activity) (Fig. 3B). Additional testing showed that at 1.6 μM AVN-944 reduces minigenome reporter activity to background levels without pronounced cytotoxicity, as judged by intracellular ATP levels (Fig. 3C). This strongly suggests that the changes in host cell RNA synthesis observed with AVN-944 in the minigenome-based screen (Fig. 3B) are due to specific effects of this drug, and in particular are consistent with the known mechanism of action for AVN-944 as a selective, noncompetitive inhibitor of IMPDH - the rate-limiting enzyme in cellular de novo GTP synthesis. Similar results were achieved using Ribavirin at 400 μM (Fig. 3C).
Fig. 3. Antiviral compound screening using the JUNV minigenome system.

(A) Schematic of the antiviral compound screening workflow. BSR-T7/5 cells were treated with a small set of 34 drugs, in addition to Ribavirin (RIB) as a control, for 2 h. Subsequently, these cells were transfected with the optimized 1L2L decoy-containing JUNV nLuc-expressing minigenome and helper plasmids in the continued presence of drug. The control plasmid pCAGGS-Firefly (FF) was also transfected. Cell lysates were measured 48 h later for nLuc (viral RNA synthesis) and FF (host cell RNA synthesis). (B) Drug library screening results. Each compound to be tested was applied in three different concentrations (0.1 μM, 1 μM, and 10 μM) according to the workflow described in (A) with 0.02%, 0.2% and 2% DMSO used as controls. The effects of treatment on viral RNA synthesis (nLuc) and cellular RNA synthesis (FF) are shown separately as means and standard deviations representing data from six biological replicates from two independent experiments. (C) Analysis of minigenome inhibition at additional AVN-944 and Ribavirin concentrations. BSR-T7/5 cells were treated with the indicated concentrations of AVN-944 or Ribavirin, DMSO alone, or left untreated for 2 h. Subsequently, these cells were transfected with the optimized 1L2L decoy-containing JUNV nLuc-expressing minigenome and helper plasmids. Untreated controls were run either with or without JUNV L, as indicated. Cell lysates were measured 48 h later for nLuc activity (viral RNA synthesis, left panel), FF activity (cellular RNA synthesis; right panel). Cell viability was also measured based on cellular ATP levels (both panels). Means and standard deviations shown represent at least 4 biological replicates from at least 2 independent experiments. Results of a one-way ANOVA to compare each sample to the DMSO control are indicated when statistically significant. Comparison of AVN-944 (1.6 μM) and Ribavirin (400 μM) treatments are also indicated.
3.3. Development of a JUNV trVLP system and evaluation of siRNA-based knock-down of IMPDH
In order to confirm the role of IMPDH in supporting arenavirus RNA synthesis, we further established a JUNV trVLP system (Fig. 4A). This system advances the minigenome system by providing the matrix protein (Z) and the glycoprotein (GPC) to facilitate generation of minigenome-containing virus-like particles (from p0 cells), which can then be delivered to target (p1) cells primed with NP and L, where they can replicate, producing reporter activity that can be used to assess “infection” with these trVLPs. As expected, efficient production of trVLPs was dependent on the matrix protein and glycoprotein, with Huh7 cells providing the best results as p1 target cells (Fig. 4B). Using this platform we performed knock-down experiments (Fig. 4C) using commercially available siRNAs against human IMPDH2, both of which markedly reduced RNA synthesis in JUNV trVLP infected cells (Fig. 4D), supporting a role for IMPDH in facilitating efficient arenavirus RNA synthesis.
Fig. 4. Development of a JUNV trVLP assay and testing of siRNA-mediated IMPDH knock-down.

(A) Schematic diagram of the JUNV trVLP system. Transfection of producer cells (p0 cells) with the optimized 1L2L decoy-containing JUNV nLuc-expressing minigenome and helper plasmids encoding the T7 polymerase, JUNV nucleoprotein (NP) and polymerase (L) leads to transcription of these three proteins by RNA polymerase II and subsequent translation. T7 then directs initial transcription of the minigenome RNA, which is autocatalytically processed by HDV ribozyme cleavage to generate a construct containing authentic JUNV leader and trailer sequences. This genome analogue can then be encapsidated by NP and transcribed and replicated by L. The transcribed mRNA from the GPC gene encodes the assayable nLuc reporter protein. The production of reporter in these p0 cells reflects the cumulative RNA synthesis (i.e. transcription and replication) taking place in these cells. An additional second transfection of plasmids encoding the JUNV glycoprotein precursor (GPC) and matrix protein (Z) results in inhibition of further RNA synthesis and budding of transcription and replication competent virus-like particles (trVLPs) that have a structure analogous to actual virus particles, but instead contain only a minigenome as their genetic material. These particles can be used to infect new target cells (p1 cells), which have been pretransfected with pCAGGS-NP and pCAGGS-L, and thus are able to further transcribe and replicate the viral minigenome. As such, if used in drug/siRNA treatment experiments, reporter activity in these p1 cells reflects the cumulative effects of treatment on RNA synthesis (in p0 and p1 cells), trVLP production and trVLP entry. Alternatively, if only the p1 cells are treated, the effects reflect changes in trVLP entry and subsequent p1 cell RNA synthesis only. (B) JUNV trVLP assay for detection of nLuc expression. BSR-T7/5 cells (p0 cells) were transfected as described in (A) either with (+L) or without JUNV L (−L). A control plasmid pCAGGSFirefly (FF) was also transfected in all cells. After 24 h cells were further transfected with pCAGGS-Z and pCAGGS-GPC (+Z, +GP), or with pCAGGS as a control (−Z, −GP). These p0 cells were harvested 48 h later and measured for both nLuc (viral RNA synthesis) and FF (host cell RNA synthesis) activity. In addition, the supernatants from these p0 cells were transferred onto the indicated cell lines, which had been pre-transfected with pCAGGS-NP and pCAGGS-L (p1 cells) as described in (A). After 48 h the p1 cells were harvested and measured for nLuc (viral RNA synthesis). The means and standard deviations of normalized reporter levels (nLuc/FF, p0 cells) or nLuc activity alone (p1 cells) are shown and represent data from two independent experiments. Results of a one-way ANOVA to compare p0 samples or p1 samples either with or without Z and GP among the different cell types tested are shown. (C) Schematic of the siRNA analysis workflow. Huh7 cells were first transfected with siRNAs for 24 h, after which they were further transfected with pCAGGS-NP and pCAGGS-L helper plasmids. After 24 h the cells were infected with trVLPs containing supernatants produced as described in (A) and (B). After an additional 48 h cell lysates were measured for nLuc (viral RNA synthesis) activity. (D) Cells were transfected with 2 pmol of commercially available validated siRNAs against IMPDH2 (Supplemental Fig. 1), or positive and negative control siRNAs, according to the workflow described in (A). The effects of siRNA treatments on viral RNA synthesis (nLuc) in p1 cells are shown as means and standard deviations of three independent experiments. Results of a one-way ANOVA to compare each sample to the untreated control are shown.
3.4. Inhibition of virus infection by AVN-944
To confirm the effects of AVN-944 on arenavirus infection in vitro, we treated Vero76 cells with increasing amounts of AVN-944 immediately after infection with TCRV. At 48 h we could see a clear decrease in virus titers (> 90%) when using drug concentrations at or above 7.5 μM. At this concentration cytotoxicity remained minimal (Fig. 5A), consistent with observations in Huh7 cells (Fig. 3C), although at higher concentrations AVN-944 showed increasing cytotoxicity (Fig. 5A). However, in comparison to Ribavirin, AVN-944 showed superior activity at much lower doses (7.5 μM vs. 400 μM) with less cytotoxicity (Fig. 5B).
Fig. 5. Validation of AVN-944 efficacy during in vitro infection with Tacaribe virus.
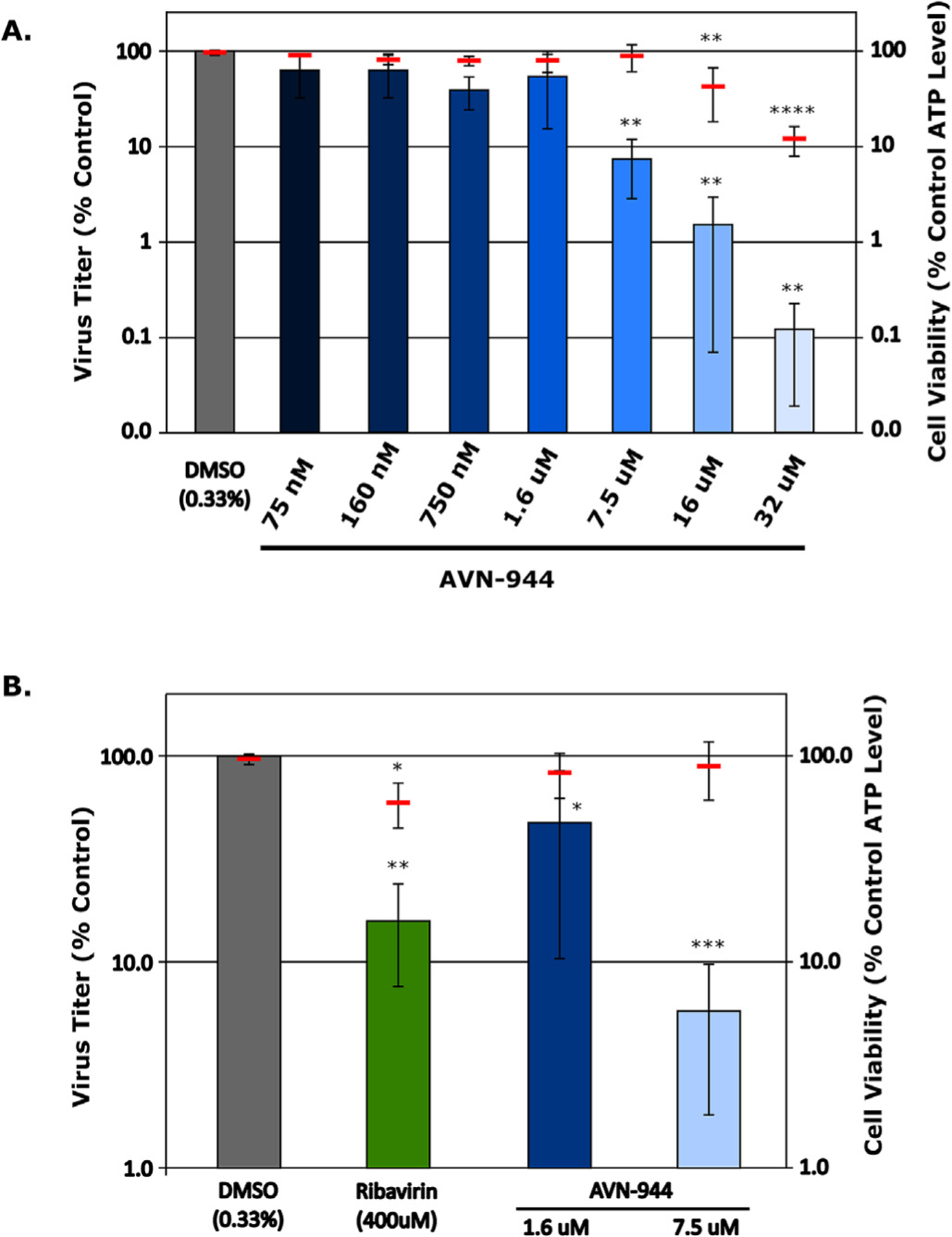
(A) Assessment of AVN-944 inhibition at various doses. Vero76 cells were infected with Tacaribe virus for 1 h at an MOI of 0.01 after which they were treated with medium containing increasing doses of AVN-944, as indicated, or DMSO (as a control). After 48 h supernatants were harvested for titer determination by TCID50 and cells were analyzed for cytotoxicity based on cellular ATP levels. Titers were calculated using the Spearman-Kärber method and data are shown as the means and standard deviations of 9 biological replicates from 3 independent experiments. Results of a one-way ANOVA to compare each sample to the DMSO control are shown when statistically significant (B) Comparison of AVN-944 efficacy and cytotoxicity to Ribavirin. Vero76 cells were infected as described in (A) before being treated with DMSO (0.33%), AVN-944 (1.6 μM or 7.5 μM) or Ribavirin (400 μM), as indicated. After 48 h supernatants were harvested for titer determination by TCID50 and cells were analyzed for cytotoxicity, also as described in (A). Data are shown as the means and standard deviations of at least 12 biological replicates from at least 4 independent experiments. Results of a one-way ANOVA to compare each sample to the DMSO control are shown when statistically significant.
4. Discussion
Recent advances in life cycle modelling for various virus families have resulted in a variety of tools for screening and analysis of antiviral compounds. In particular, these systems provide a major advantage for BSL4 pathogens by allowing work to be safely performed under BSL1 or BSL2 conditions, depending on local regulations (reviewed in (Hoenen et al., 2011)), facilitating access to a variety of expertise and specialized equipment including high-throughput screening facilities. However, to realize their full potential, these systems need adequate optimization. Indeed, strong positive signals, low background, and low sample-to-sample variability become very important in successfully transitioning these assays to increasingly smaller formats. In this study we have explored various parameters for the optimization of JUNV life cycle modelling systems for application to drug library and siRNA screening in a high-throughput compatible format. Importantly, while we have initially approached the development of these assays with this comparatively well-studied virus species, we expect the developed approach will also provide solutions relevant to generating similar resources for other virus systems, especially for closely related pathogens such as Machupo virus and Guanarito virus, for which similar reverse genetics-based resources are largely lacking.
One of the great strengths of the minigenome system is its simplicity, modelling only viral RNA synthesis. This makes it an outstanding tool for screening studies focused solely on inhibitors of these processes or for studying mechanism of action. However, the use of T7 to generate initial naked RNA transcripts, which can potentially serve as an innate immune stimulus, the need for these RNAs to be illegitimately encapsidated by NP to form functional nucleocapsids, and the plasmid-based overexpression of the RNP components constitute a rather artificial context (reviewed in Hoenen and Feldmann, 2014). Still for approaches focused solely on examining RNA synthesis, minigenome assays remains the method of choice, given their specificity and robustness, especially for initial screening work. In contrast, trVLP assays provide a much more authentic platform for screening, while still preserving the safety element inherent to all lifecycle modelling systems. Here the only artificial aspect of the system is the overexpression of RNP components in p1 cells. However, these systems are more time-consuming and complex regarding the experimental approach and the resources required. This often makes them less ideal for larger-scale experiments, unless specific requirements warrant their use – for instance 1) a need to eliminate unencapsidated RNA (e.g. for immunological analyses), 2) where an analysis of steps not modelled in the minigenome assay, i.e. budding and entry, is desired, or 3) where their use may provide a specific technical advantage, such as allowing the use of different cell types. As such, in many cases the minigenome system makes an ideal platform for initial analysis and screening, with follow-up analysis using the trVLP assay and/or authentic virus as needed. Indeed, the consistency of the results obtained with these different experimental approaches contributes to a growing body of evidence that lifecycle modelling systems are indeed a valuable first-line screening tools with significant predictive power (McCarthy et al., 2016; Nelson et al., 2016, 2017).
In this study, as a proof-of-concept, we screened a small panel of compounds (Nelson et al., 2017) in addition to ribavirin, a known inhibitor of arenavirus RNA synthesis, for antiviral activity using our optimized minigenome system. This led to the identification of AVN-944 (Fig. 3), which is a specific, noncompetitive inhibitor of IMPDH, the enzyme catalyzing the rate-limiting step of de novo GTP synthesis (Hamilton et al., 2009). The importance of IMPDH for the arenavirus lifecycle was also supported by siRNA knock down, which negatively affected reporter production in target cells of a trVLP assay (Fig. 4). Given the critical role of IMPDH in maintaining intracellular GTP levels, it is unsurprisingly required for both DNA and RNA synthesis, but also contributes to many other host cell processes, including GTP-dependent enzyme activity, capping of mRNAs, microtubule dynamics, etc.
Interestingly, Ribavirin, which is the current standard of care for LASV treatment (McCormick et al., 1986) and is also an inhibitor of IMPDH, was recently reported not to act primarily in a fashion dependent on the role of IMPDH in GTP synthesis (Olschlager et al., 2011), but rather through one or more of its other known mechanisms of action. However, our data support a growing body of data suggesting that also exclusive IMPDH inhibitors have antiviral activity against arenaviruses (Sepulveda et al., 2012; Tong et al., 2018). Interestingly, a recent proteomic study indicated an interaction between the arenavirus Z protein and IMPDH2 (Ziegler et al., 2018). However, those data suggested an impact of IMPDH on virus budding, via an as yet undefined mechanism (Ziegler et al., 2018), while our data clearly indicate a more classical impact of IMPDH inhibition on RNA synthesis. Importantly, our data also suggest that the role of IMPDH is not exclusive to JUNV, which was the basis for our screening platforms, but is important for other New World arenaviruses as well (i.e. TCRV, Fig. 5). Consistent with our approach to develop a fully BSL2-oriented set of tools for our analysis it is certainly beneficial to have a widely available BSL2 proxy virus such as TCRV available for confirmatory screening. However, one does have to take into consideration that this approach may not be successful with virus species-specific intervention approaches. In these cases, follow-up work with Candid #1 may be an option (for JUNV only and depending on local regulatory classification), or involvement of a BSL4 partner laboratory may be necessary. However, for investigating compounds anticipated to have a broad activity, or common host factors, the systems outlined here provide an exclusively BSL2 analysis approach from initial screening through to confirmation in the virus context.
The availability of a growing number of arenavirus inhibitors with distinct mechanisms of action raises the possibility of improving upon the standard of treatment for arenaviruses using synergistic drug combinations. In particular, synergistic benefit of IMPDH inhibitor/nucleoside analogue combinations has been shown to occur in several viral contexts (Borroto-Esoda et al., 2004; Margolis et al., 1999; Neyts et al., 1998), and also recent data from LASV animal models combining Ribavirin with T705 have been quite promising (Oestereich et al., 2016). In this context GTP depletion by Ribavirin, although not its main mechanism of action (Olschlager et al., 2011), has been suggested to potentially favor incorporation of other nucleoside analogues, i.e. T705. If this is indeed the case, it suggests additional room for optimization may exist based on the use of more potent IMPDH inhibitors, such as AVN-944, or the structurally related VX-148 and Merimepodib (Hedstrom, 2009; Markland et al., 2000), which has also recently been demonstrated to have anti-arenaviral activity in vitro (Tong et al., 2018). Such a strategy is aided by the fact that numerous IMPDH inhibitors have already been extensively characterized and tested clinically to various extents, or in some cases even licensed, either as immunosuppressive therapies (e.g. for organ transplantation) or as potential cancer therapies. Indeed, AVN-944 was originally developed and underwent Phase I and II clinical trials for the treatment of various hematological malignancies (summarized in (Petrelli et al., 2013)), where good tolerance was observed in patients (Hamilton et al., 2009; Ishitsuka et al., 2005). Thus, while further testing is clearly still required to establish the therapeutic potential of IMPDH inhibitors, including as part of synergistic therapies, this gene target appears to present a promising candidate for both Old and New World arenaviruses.
While the potential benefit is clear for arenavirus infections where no standard therapy is available, even where an effective monotherapy is available, as is currently the case with neutralizing antibody therapy for management of JUNV infection in Argentina, novel combination approaches may still provide significant benefits, and might aid in reducing/preventing the development of late neurological syndrome following passive antibody therapy alone. In this regard, the identification and testing of novel antiviral drugs and synergistic drug combinations is clearly an important avenue towards improving anti-arenaviral therapy and it is expected that the availability of the described systems will fuel such efforts in the future, including among labs without access to BSL4 facilities.
5. Conclusions
Here we report the optimization of JUNV minigenome and trVLP systems for use in smaller high-throughput formats suitable for various practical applications, including the screening of drug and siRNA libraries. Using a minigenome assay-based screen, we identified a novel inhibitor of New World arenavirus RNA synthesis, AVN-944, which is known to inhibit IMPDH. We confirmed a role of IMPDH in the JUNV lifecycle using a trVLP-based assay to examine the effects of siRNA-knockdown of this gene. These findings were further supported by the effect of AVN-944 on in vitro growth of TCRV. Overall, our data highlight the predictive power of lifecycle modelling systems for the identification of novel antiviral compounds, as well as their utility in mechanism of action studies, and specifically demonstrate an important role of IMPDH in New World arenavirus RNA synthesis.
Supplementary Material
Acknowledgements
The authors thank Stefan Finke for providing the BSR-T7/5 cells and the Friedrich-Loeffler-Institut Cell Bank for their assistance in supplying the other cell lines used in this work. This work was funded by the Friedrich-Loeffler-Institut and by the Division of Intramural Research, NIAID, NIH. The funding sources had no involvement in the study design, the collection, analysis or interpretation of data, in the writing of the report, or in the decision to submit the article for publication. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the NIH.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.antiviral.2018.07.009.
References
- Aguilar PV, Camargo W, Vargas J, Guevara C, Roca Y, Felices V, Laguna-Torres VA, Tesh R, Ksiazek TG, Kochel TJ, 2009. Reemergence of Bolivian hemorrhagic fever, 2007–2008. Emerg. Infect. Dis 15, 1526–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albarino CG, Bergeron E, Erickson BR, Khristova ML, Rollin PE, Nichol ST, 2009. Efficient reverse genetics generation of infectious junin viruses differing in glycoprotein processing. J. Virol 83, 5606–5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroto-Esoda K, Myrick F, Feng J, Jeffrey J, Furman P, 2004. In vitro combination of amdoxovir and the inosine monophosphate dehydrogenase inhibitors mycophenolic acid and ribavirin demonstrates potent activity against wild-type and drug-resistant variants of human immunodeficiency virus type 1. Antimicrob. Agents Chemother 48, 4387–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz UJ, Finke S, Conzelmann KK, 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol 73, 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonet SF, Seregin AV, Yun NE, Poussard AL, Walker AG, de la Torre JC, Paessler S, 2011. Rescue from cloned cDNAs and in vivo characterization of recombinant pathogenic Romero and live-attenuated Candid #1 strains of Junin virus, the causative agent of Argentine hemorrhagic fever disease. J. Virol 85, 1473–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enria DA, Briggiler AM, Sanchez Z, 2008. Treatment of Argentine hemorrhagic fever. Antivir. Res 78, 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enria DA, de Damilano AJ, Briggiler AM, Ambrosio AM, Fernandez NJ, Feuillade MR, Maiztegui JI, 1985. Late neurologic syndrome in patients with Argentinian hemorrhagic fever treated with immune plasma. Medicina (B Aires) 45, 615–620. [PubMed] [Google Scholar]
- Fulhorst CF, Cajimat MN, Milazzo ML, Paredes H, de Manzione NM, Salas RA, Rollin PE, Ksiazek TG, 2008. Genetic diversity between and within the arenavirus species indigenous to western Venezuela. Virology 378, 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen BB, Juelich TL, Sefing EJ, Brasel T, Smith JK, Zhang L, Tigabu B, Hill TE, Yun T, Pietzsch C, Furuta Y, Freiberg AN, 2013. Favipiravir (T-705) inhibits Junin virus infection and reduces mortality in a Guinea pig model of Argentine hemorrhagic fever. PLoS Neglected Trop. Dis 7, e2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen BB, Westover JB, Sefing EJ, Van Wettere AJ, Bailey KW, Wandersee L, Komeno T, Furuta Y, 2017. Enhanced protection against experimental Junin virus infection through the use of a modified favipiravir loading dose strategy. Antivir. Res 145, 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groseth A, Marzi A, Hoenen T, Herwig A, Gardner D, Becker S, Ebihara H, Feldmann H, 2012. The Ebola virus glycoprotein contributes to but is not sufficient for virulence in vivo. PLoS Pathog. 8, e1002847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groseth A, Wolff S, Strecker T, Hoenen T, Becker S, 2010. Efficient budding of the tacaribe virus matrix protein z requires the nucleoprotein. J. Virol 84, 3603–3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JM, Harding MW, Genna T, Bol DK, 2009. A phase I dose-ranging study of the pharmacokinetics, pharmacodynamics, safety, and tolerability of AVN944, an IMPDH inhibitor, in healthy male volunteers. J. Clin. Pharmacol 49, 30–38. [DOI] [PubMed] [Google Scholar]
- Hedstrom L, 2009. IMP dehydrogenase: structure, mechanism, and inhibition. Chem. Rev 109, 2903–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenen T, Feldmann H, 2014. Reverse genetics systems as tools for the development of novel therapies against filoviruses. Expert Rev. Anti Infect. Ther 12, 1253–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenen T, Groseth A, de Kok-Mercado F, Kuhn JH, Wahl-Jensen V, 2011. Minigenomes, transcription and replication competent virus-like particles and beyond: reverse genetics systems for filoviruses and other negative stranded hemorrhagic fever viruses. Antivir. Res 91, 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitsuka K, Hideshima T, Hamasaki M, Raje N, Kumar S, Podar K, Le Gouill S, Shiraishi N, Yasui H, Roccaro AM, Tai YZ, Chauhan D, Fram R, Tamura K, Jain J, Anderson KC, 2005. Novel inosine monophosphate dehydrogenase inhibitor VX-944 induces apoptosis in multiple myeloma cells primarily via caspase-independent AIF/Endo G pathway. Oncogene 24, 5888–5896. [DOI] [PubMed] [Google Scholar]
- Lopez N, Jacamo R, Franze-Fernandez MT, 2001. Transcription and RNA replication of tacaribe virus genome and antigenome analogs require N and L proteins: Z protein is an inhibitor of these processes. J. Virol 75, 12241–12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiztegui JI, Fernandez NJ, de Damilano AJ, 1979. Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet 2, 1216–1217. [DOI] [PubMed] [Google Scholar]
- Margolis D, Heredia A, Gaywee J, Oldach D, Drusano G, Redfield R, 1999. Abacavir and mycophenolic acid, an inhibitor of inosine monophosphate dehydrogenase, have profound and synergistic anti-HIV activity. J. Acquir. Immune Defic. Syndr 21, 362–370. [PubMed] [Google Scholar]
- Markland W, McQuaid TJ, Jain J, Kwong AD, 2000. Broad-spectrum antiviral activity of the IMP dehydrogenase inhibitor VX-497: a comparison with ribavirin and demonstration of antiviral additivity with alpha interferon. Antimicrob. Agents Chemother 44, 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy SD, Majchrzak-Kita B, Racine T, Kozlowski HN, Baker DP, Hoenen T, Kobinger GP, Fish EN, Branch DR, 2016. A rapid screening assay identifies monotherapy with interferon-ss and combination therapies with nucleoside analogs as effective inhibitors of ebola virus. PLoS Neglected Trop. Dis 10, e0004364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick JB, King IJ, Webb PA, Scribner CL, Craven RB, Johnson KM, Elliott LH, Belmont-Williams R, 1986. Lassa fever. Effective therapy with ribavirin. N. Engl. J. Med 314, 20–26. [DOI] [PubMed] [Google Scholar]
- Mendenhall M, Russell A, Juelich T, Messina EL, Smee DF, Freiberg AN, Holbrook MR, Furuta Y, de la Torre JC, Nunberg JH, Gowen BB, 2011a. T-705 (favipiravir) inhibition of arenavirus replication in cell culture. Antimicrob. Agents Chemother 55, 782–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall M, Russell A, Smee DF, Hall JO, Skirpstunas R, Furuta Y, Gowen BB, 2011b. Effective oral favipiravir (T-705) therapy initiated after the onset of clinical disease in a model of arenavirus hemorrhagic fever. PLoS Neglected Trop. Dis 5, e1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno H, Gallego I, Sevilla N, de la Torre JC, Domingo E, Martin V, 2011. Ribavirin can be mutagenic for arenaviruses. J. Virol 85, 7246–7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EA, Barnes AB, Wiehle RD, Fontenot GK, Hoenen T, White JM, 2016. Clomiphene and its isomers block ebola virus particle entry and infection with similar potency: potential therapeutic implications. Viruses 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EA, Dyall J, Hoenen T, Barnes AB, Zhou H, Liang JY, Michelotti J, Dewey WH, DeWald LE, Bennett RS, Morris PJ, Guha R, Klumpp-Thomas C, McKnight C, Chen YC, Xu X, Wang A, Hughes E, Martin S, Thomas C, Jahrling PB, Hensley LE, Olinger GG Jr., White JM, 2017. The phosphatidylinositol-3-phosphate 5-kinase inhibitor apilimod blocks filoviral entry and infection. PLoS Neglected Trop. Dis 11, e0005540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyts J, Andrei G, De Clercq E, 1998. The novel immunosuppressive agent mycophenolate mofetil markedly potentiates the antiherpesvirus activities of acyclovir, ganciclovir, and penciclovir in vitro and in vivo. Antimicrob. Agents Chemother 42, 216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestereich L, Rieger T, Ludtke A, Ruibal P, Wurr S, Pallasch E, Bockholt S, Krasemann S, Munoz-Fontela C, Gunther S, 2016. Efficacy of favipiravir alone and in combination with ribavirin in a lethal, immunocompetent mouse model of Lassa fever. J. Infect. Dis 213, 934–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olschlager S, Neyts J, Gunther S, 2011. Depletion of GTP pool is not the predominant mechanism by which ribavirin exerts its antiviral effect on Lassa virus. Antivir. Res 91, 89–93. [DOI] [PubMed] [Google Scholar]
- Pasquato A, Kunz S, 2016. Novel drug discovery approaches for treating arenavirus infections. Expet Opin. Drug Discov 11, 383–393. [DOI] [PubMed] [Google Scholar]
- Petrelli R, Vita P, Torquati I, Felczak K, Wilson DJ, Franchetti P, Cappellacci L, 2013. Novel inhibitors of inosine monophosphate dehydrogenase in patent literature of the last decade. Recent Pat. Anti-Cancer Drug Discov 8, 103–125. [PubMed] [Google Scholar]
- Safronetz D, Rosenke K, Westover JB, Martellaro C, Okumura A, Furuta Y, Geisbert J, Saturday G, Komeno T, Geisbert TW, Feldmann H, Gowen BB, 2015. The broad-spectrum antiviral favipiravir protects Guinea pigs from lethal Lassa virus infection post-disease onset. Sci. Rep 5, 14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar M, Yun NE, Poussard AL, Smith JN, Smith JK, Kolokoltsova OA, Patterson MJ, Linde J, Paessler S, 2012. Effect of ribavirin on junin virus infection in Guinea pigs. Zoonoses Publ. Health 59, 278–285. [DOI] [PubMed] [Google Scholar]
- Sepulveda CS, Garcia CC, Fascio ML, D’Accorso NB, Docampo Palacios ML, Pellon RF, Damonte EB, 2012. Inhibition of Junin virus RNA synthesis by an antiviral acridone derivative. Antivir. Res 93, 16–22. [DOI] [PubMed] [Google Scholar]
- Tong X, Smith J, Bukreyeva N, Koma T, Manning JT, Kalkeri R, Kwong AD, Paessler S, 2018. Merimepodib, an IMPDH inhibitor, suppresses replication of Zika virus and other emerging viral pathogens. Antivir. Res 149, 34–40. [DOI] [PubMed] [Google Scholar]
- Watt A, Moukambi F, Banadyga L, Groseth A, Callison J, Herwig A, Ebihara H, Feldmann H, Hoenen T, 2014. A novel life cycle modeling system for Ebola virus shows a genome length-dependent role of VP24 in virus infectivity. J. Virol 88, 10511–10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westover JB, Sefing EJ, Bailey KW, Van Wettere AJ, Jung KH, Dagley A, Wandersee L, Downs B, Smee DF, Furuta Y, Bray M, Gowen BB, 2016. Low-dose ribavirin potentiates the antiviral activity of favipiravir against hemorrhagic fever viruses. Antivir. Res 126, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler CM, Eisenhauer P, Kelly JA, Dang LN, Beganovic V, Bruce EA, King BR, Shirley DJ, Weir ME, Ballif BA, Botten J, 2018. A proteomic survey of Junin virus interactions with human proteins reveals host factors required for arenavirus replication. J. Virol 92 e01565–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerer KS, 2013. The compatibility of agricultural intensification in a global hotspot of smallholder agrobiodiversity (Bolivia). Proc. Natl. Acad. Sci. U. S. A 110, 2769–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


