Abstract
We have developed a method, using laser, optical tweezers and direct microscopic analysis of reproductive potential and membrane integrity, to assess single-cell viability in a stationary-phase Escherichia coli population. It is demonstrated here that a reduction in cell integrity, determined by using the fluorescent nucleic acid stain propidium iodide, correlated well with a reduction in cell proliferating potential during the stationary-phase period studied. Moreover, the same cells that exhibited reduced integrity were found to be the ones that failed to divide upon nutrient addition. A small but significant number of the intact cells (496 of 7,466 [6.6%]) failed to replicate. In other words, we did not find evidence for the existence of a large population of intact but nonculturable cells during the stationary-phase period studied but it is clear that reproductive ability can be lost prior to the loss of membrane integrity. In addition, about 1% of the stationary-phase cells were able to divide only once upon nutrient addition, and in a few cases, only one of the two cells produced by division was able to divide a second time, indicating that localized cell deterioration, inherited by only one of the daughters, may occur. The usefulness of the optical trapping methodology in elucidating the mechanisms involved in stationary-phase-induced bacterial death and population heterogeneity is discussed.
Most attempts to define life emphasize three major things necessary for something to qualify as a living organism: (i) it must be a physically contained entity partly insulated from the surrounding environment; (ii) it must have an autocatalytic metabolic system; and (iii) it must demonstrate the properties of multiplication, variation, and heredity (e.g., see reference 10). In bacteriology, the last of these aspects (i.e., the capacity for self-replication and colony formation on nutrient agar plates) is usually used for operational reasons in experimental determination of cellular life and death (10). In most cases, assessing colony-forming ability is a reliable and sufficient method for determination of the live/dead fraction of a bacterial population. However, the method is, in principle, indirect and it has been argued that the failure of a bacterial cell to reproduce on standard nutrient agar plates may not mean that the cell was dead at the time of sampling; the cell could be temporarily but reversibly nonculturable or the culturing conditions could be suboptimal (10). For example, the apparent die-off during stationary phase of Escherichia coli cells lacking the regulator OxyR is caused by a diminished ability of these cells to reproduce on culture plates unless plating is performed anaerobically or on culture plates containing catalase (3). In addition, it is known that under some conditions bacteria may lose their capacity to form colonies while remaining physically intact and metabolically active (see, e.g., references 9 and 12). Such observations have led to the not-so-surprising conclusion that bacteria can lose their reproductive ability while remaining intact and metabolically functional as individuals; this type of cell has been referred to as viable but nonculturable (VBNC) (see, e.g., references 9 and 12). The only reason for calling nonculturable (or more correctly, not cultured) cells viable is, as far as we understand, that this state is reversible (e.g., see reference 2). However, reports on true resuscitation of VBNC cells are very rare (but see references 7, 8, 13, and 14) and the usefulness and semantics of the VBNC concept have been questioned (1, 2).
Another problem is that assessments of metabolic activity, such as the often-used assays for respiration and uptake of substrates, are not sufficient to distinguish live and dead cells, firstly because the activity of the cells may be below the threshold for detection and secondly because cells that have irreversibly lost reproductive ability may well exhibit detectable metabolic activity (reviewed in reference 2). For example, E. coli minicells, which lack chromosomal DNA, would, by the criterion normally used for detecting VBNC cells (9), be defined as viable! Also, a caveat of VBNC assessment is that it relies on comparisons between one method that is retrospective and involves observations made at the population level (CFU enumeration) and another that is direct (microscopic) and involves observations made at the individual level (metabolic activity measurements such as respiration assays).
To overcome these obstacles, we wanted to develop a method that would allow two independent methods for viability assessment (one of which should be an assay of replicative ability) to be applied on the same individual cells analyzed under the microscope. To do this, we needed to sort, trap, and assign coordinates to a large number of cells that subsequently could be subjected to viability assays. This was achieved by designing a suitable optical tweezers methodology and using it in combination with fluorescence microscopy. With this system at hand we determined the viability of nongrowing, stationary-phase E. coli cells in two different ways. First, we used a fluorescent kit (LIVE/DEAD BacLight) to score for membrane integrity, and second, the same cells were analyzed under the microscope with respect to their ability to reproduce upon addition of fresh nutrients. We report here on the results of this analysis, the details of the technology, and its potential usefulness in stationary-phase research as well as in single cell genetics and isolation of mutants.
MATERIALS AND METHODS
Bacteria and culturing conditions.
E. coli cells were grown aerobically in complex medium (nutrient broth) at 37°C. Samples were withdrawn during exponential phase and during stationary phase for optical trapping and viability assessments. The AF1000 (F− araD139 Δ(argF-lac)U169 rpsL150 flbB5301 deoC1 ptsF25 rbsR) strain of E. coli was used since this strain lacks flagella which would otherwise increase the movement of the bacteria and make optical sorting more difficult. The AF1000 strain is MC4100 in which the relA1 allele has been crossed out and replaced by relA+.
Microscopic equipment.
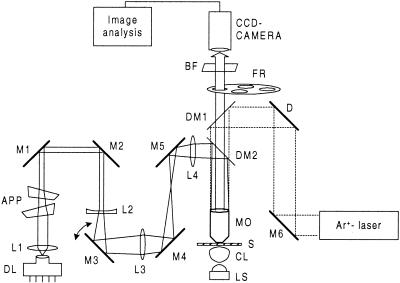
Today commercial microscopes that have optical tweezers integrated with epifluorescent capabilities are available. The disadvantage of this kind of system is that the fluorescence analysis cannot be performed while the optical trap is in use, and the optical trap is often fixed. One of the goals of our design was that it should be possible not only to move the optical trap but also to use the optical tweezers and the fluorescence analysis function simultaneously. A condenser lens with a high numerical aperture was placed under the microscope table along with an illumination source (Fig. 1). The specimen in the sample was viewed through a high-numerical-aperture 100× oil immersion microscope objective and imaged by a charge-coupled device (CCD) camera (Fig. 1).
FIG. 1.
Optical trapping and fluorescent microscopy setup. The Ar+ laser serves to excite the dye used in the sample, while the diode laser, which works in the infrared region, serves as the trapping beam. By tilting mirror M3, the optical trap can be moved in the trapping plane. Since both the trapping beam and the beam used for excitation are directed down through the microscope objective, only the bacteria within the field of view are excited. DL, diode laser; APP, anamorphic prism pair; M1 to M6, mirrors; L1 to L4, lenses; DM1 to DM2, dichroic mirrors; MO, microscope objective; S, sample; CL, condenser lens; LS, light source; D, diffuser; FR, filter revolver; BF, blocking filter; CCD, charge-coupled device.
Fluorescence microscope.
To excite the dyes used in the sample, the 488-nm line of an Ar+ laser was used. The laser beam was reflected by a diffuser onto a dichroic mirror (DM1) with a cutoff frequency of 495 nm and directed down through the microscope objective (Fig. 1). In this way we excited only the bacteria within the field of view. This also made it possible to use the microscope with or without fluorescence. To view the result from the fluorescence analysis separately, two interference filers were mounted in a filter revolver (Fig. 1). A blocking filter was placed in front of the CCD camera to avoid any loss in contrast caused by interference of the reflected Ar+ laser light (Fig. 1).
Optical tweezers methodology and optical trapping.
To trap the bacteria in the sample, a mirror (DM2) with a cutoff frequency of 700 nm was placed to reflect the 836-nm trapping beam from the diode laser into the microscope objective (Fig. 1) using an output power of 50 mW (measured between the mirror DM2 and the microscope objective [Fig. 1]). The negative lens L2 expanded the beam so that the optical trap would be in the object plane of the microscope objective. Lenses L3 and L4 worked together as an imaging telescope, and by tilting M3 it was possible to make small adjustments of the trap in the trapping plane (4). An anamorphic prism pair was used to reshape the beam from the laser diode from an elliptical to a more circular shape. Both dichroic mirrors were designed to allow the fluorescence to pass right through.
Direct measurement of cell viability.
The fluorescent LIVE/DEAD BacLight bacterial viability kit (Molecular Probes, Inc.) consists of two dyes: the green fluorescent nucleic acid stain SYTO 9, which stains the nucleic acids of both living and dead bacteria, and the traditional red fluorescent nucleic acid stain propidium iodide, which does not enter bacteria that have intact cell membranes and thus only stains bacteria that have damaged and leaky membranes. When properly mixed into a bacterial suspension, live bacteria will fluoresce in green whereas dead bacteria will fluoresce in red. The two solid-phase components of the LIVE/DEAD BacLight kit (L-13152) were mixed together in 5 ml of distilled H2O. A diluted bacterial suspension and the LIVE/DEAD BacLight solution were then mixed together in equal volumes and incubated in the dark for 30 min. For the frozen solution of LIVE/DEAD BacLight (L-7012), 1.5 μl each of the two components (3.34 mM SYTO 9 and 20 mM propidium iodide (PI), both in anhydrous dimethyl sulfoxide) were mixed together with 1 ml of bacterial suspension (4.5 × 109 cells/ml). The solution was mixed thoroughly and incubated for 30 min. The rest of the solution was used to make a series of dilutions to determine the number of CFU per milliliter and to confirm the optical density with our reference. A small sample of the LIVE/DEAD BacLight bacterial solution was then placed on a Thoma counting chamber. The counting chamber consisted of two sets of grids, each containing 256 squares divided by small lines; each square was 0.015 by 0.015 mm. The counting chamber was placed under the microscope objective. With the help of the optical tweezers, bacteria were moved and placed in ordered arrays of 500 cells per experimental cycle on the glass surface of the counting chamber. Nonflagellated E. coli cells were efficiently trapped and positioned by this method. The position of each bacterium was recorded, and, by using the green or red fluorescence signals after LIVE/DEAD BacLight staining, each bacterium was marked as damaged or intact. The reproductive ability of the same individuals was then analyzed by determining their ability to divide upon addition of fresh nutrients (fresh complex medium was added to the cell suspension in a 200/1 ratio). To qualify as viable, we required the individual cell to go through at least two divisions. The fresh medium was allowed to enter the chamber by capillary forces, and this did not affect the positioning of cells in the array. The total time required from sampling of the bacteria until a cell array had been formed took about 15 min. Typically, the cells were allowed 3 h to respond to the added medium and the viability reading and recording required an additional 30 min. Thus, collecting viability data for 500 cells requires about 4 h at the present manual operation of optical trapping.
RESULTS
Trapping laser light and LIVE/DEAD BacLight dyes do not damage bacteria.
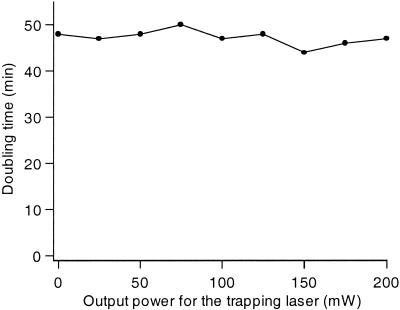
If a bacterium trapped by the optical tweezers were to absorb energy at a level corresponding to the wavelength of the trapping beam it would very quickly be damaged. It was therefore of utmost importance to first elucidate the effects of the trapping laser beam on the bacterial cell. Thus, we elucidated the effects of changing the output power of the trapping diode laser on the bacterial viability and doubling time studied directly under the microscope. As seen in Fig. 2, the doubling time of the bacteria remained constant when the output power of the infrared trapping laser was changed. In fact, an output power of 2 W does not affect the plating efficiency of the E. coli cells used (not shown). In this work, we subsequently used an output power of 50 mW in the experiments. In addition, we determined the doubling time as a function of output power for the trapping beam also in the presence of both fluorescent dyes and we concluded that neither the laser nor the dyes affected the bacteria to the extent that the doubling time was reduced (Table 1).
FIG. 2.
Doubling time of E. coli bacteria at room temperature as a function of the output power of the trapping diode laser. The bacteria were held with the optical trap in the nutrient broth (NB) complex medium. For each data point, 1,000 cells were analyzed. The doubling time is an average for four generations studied directly under the microscope.
TABLE 1.
Analysis of reproductive ability and doubling time after staining with fluorescent dyes and/or optical trapping with exciting laser lighta
| Fluorescent dye | Exciting laser light | No. (%) of bacteria able to divide | Doubling time (min) |
|---|---|---|---|
| None | No | 100 | 48 |
| None | Yes | 93 (± 5) | 47 |
| PI + SYTO 9 | No | 90 (± 5) | 52 |
| PI + SYTO 9 | Yes | 97 (± ) | 49 |
The nontreated cell culture was used as a control and was assigned the value 100% viability. For each data point, 1,000 cells were analyzed. Doubling time was measured for four generations of optically trapped cells. Values in parentheses are standard deviations.
Comparison of plate counts and direct viability assessment in exponential-phase and in stationary-phase cultures.
To determine cell viability we wanted to apply two independent methods applied directly on individual single cells studied under the microscope. One method made use of the commercially available LIVE/DEAD BacLight kit which includes stains to determine whether cells that appear to be intact under standard light or phase-contrast microscopes in fact have damaged and leaky membranes. The other viability assessment selected was a direct analysis of reproductive ability on the single-cell level. This is, in principle, a new version of the microscopic slide-culture technique (11), but with the help of laser tweezers we were able to keep the cells in broth conditions and avoid the use of surface growth to score for reproductive ability. In addition, the laser technology allowed us to trap and organize the cells in certain patterns that could be analyzed for extended periods of time. By using the two methods of viability assessment, we first established that the methods gave results similar to those obtained with the standard CFU assessment for a culture growing in exponential phase. In this phase, the CFU and total cell counts are close to identical and there is no statistically significant evidence for a fraction of dead cells in a exponentially growing culture of the E. coli AF1000 strain. Similarly, no cells were found to have lost membrane integrity when analyzed using the LIVE/DEAD BacLight methodology and we were thus unable to find false positives during the exponential phase of growth (1,000 cells were examined in each of four replicates; thus, we can only say that the fraction of nonviable cells is below 0.1%). Gallant and Palmer (5) found that a small fraction (0.5% of the total number of cells) of an exponentially growing E. coli culture failed to produce colonies on nutrient agar plates.
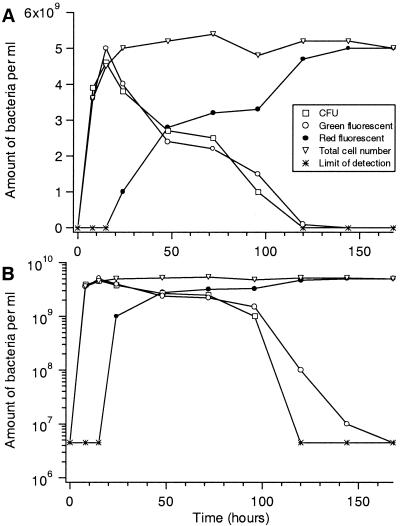
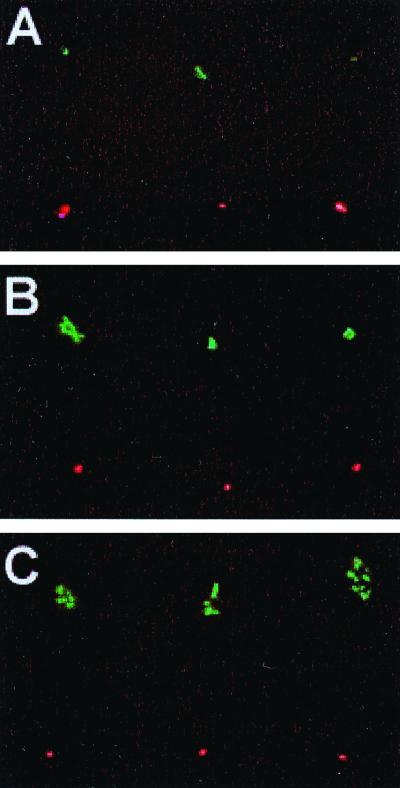
Next, we applied the viability assays on a stationary-phase culture at times of growth arrest, when the CFU counts and total counts clearly differed. During the stationary phase of an E. coli population viability, as determined by CFU measurements, is gradually lost. However, as determined by phase-contrast microscopy, 100% of the original cell population remains intact for extended periods of time. Thus, we wanted first to determine whether standard CFU counts differed significantly from results obtained by direct microscopic determination of live bacteria using the LIVE/DEAD BacLight dyes. As shown in Fig. 3, the direct microscopic assessment of viability gave values nearly identical to those obtained by standard CFU determination throughout the experiment, and both methods indicated that about 45% of the cell population was nonviable after about 38 h in stationary phase (38 h after the end of increase in culture optical density), whereas very few cells were intact (green) and able to form colonies after about 90 h (Fig. 3). Next we determined whether the cells that were judged to be intact were the same cells that retained an ability to reproduce. For a cell to qualify as viable, we required that it produce at least four cells during the incubation, and an example of how the cells appeared under the microscope is shown in Fig. 4. For this figure, cells from stationary phase were trapped by the laser and sorted into patterns under the microscope. The live/dead fraction was determined using the LIVE/DEAD BacLight kit dyes, and the cells' ability to respond to nutrient addition by initiating cell divisions was subsequently determined. This analysis could in principle distinguish among six theoretical categories of bacteria: (i) cells that fluoresced in green (intact) and divided more than once on the counting chamber, (ii) cells that fluoresced in green and divided only once on the counting chamber, (iii) cells that fluoresced in green and did not divide on the counting chamber, (iv) cells that fluoresced in red (leaky) and divided more than once on the counting chamber, (v) cells that fluoresced in red and divided once on the counting chamber, and (vi) cells that fluoresced in red and did not divide on the counting chamber. The results are summarized in Table 2, and as seen in this table, we could not detect any cells belonging to the fourth and fifth categories, indicating that the LIVE/DEAD BacLight assay is reliable during the conditions used, since no false positives could be detected. Moreover, the same cells that exhibited reduced integrity (red) were found to be the ones that failed to divide upon nutrient addition (Fig. 4; Table 2). Also, we did not find evidence for a large population of intact but nonculturable cells during the stationary-phase period studied. Among the stationary-phase cells that were recorded as intact (green), 6.6% (496 of 7,466 cells) were unable to divide and 1% divided only once (Table 2). This fraction of cells (5.4% of total population) is thus potentially nonculturable but metabolically active, demonstrating that reproductive ability may be lost prior to the collapse of membrane potential. In addition, in a few cases only one of the two cells produced by division was able to go through a subsequent second division.
FIG. 3.
Number of bacteria (in billions) per milliliter as a function of time during growth and stationary phase in NB complex medium. The same data is plotted in a linear (A) and semilog (B) scale for comparison. Cell numbers were analyzed using the two different techniques (CFU counting versus fluorescent response and total cell counts). In the figure, total counts, CFU counts, the number of green fluorescent bacteria, and the number of red fluorescent bacteria are depicted. The detection limit was 0.1% of the total number of cells at the time points shown. The sum of the counts of green and red fluorescent bacteria corresponded to total counts at all time points analyzed.
FIG. 4.
Bacterial viability studied under the microscope. The bacteria were placed in a lattice using the optical tweezers. Bacteria stained fluorescent green (SYTO 9) have intact membranes, whereas bacteria stained red (PI) have damaged membranes that allow PI to enter. Since the binding affinity of PI is higher than that of SYTO 9 for binding to the DNA, bacteria with a damaged membrane will stain fluorescent red. Pictures were taken 0 min (A), 50 min (B), and 3 h (C) after the addition of fresh medium. After 50 min, the first cell divisions of bacteria stained green were visible, and after 3 h such divisions were obvious. None of the bacteria stained fluorescent red were found to be able to divide in any the experiments so far carried out.
TABLE 2.
Analysis of reproductive ability and cell integrity by direct microscopic measurement of cell division upon nutrient addition and staining with LIVE/DEAD BacLight dyesa
| Categories of stationary-phase cells | No. of fluorescing cellsb | % of total no. of cells |
|---|---|---|
| Bacteria that fluoresced in green and: | ||
| Divided more than once on the counting chamber | 6,897 | 65.2 |
| Divided once on the counting chamber | 73 | 0.7 |
| Did not divide on the counting chamber | 496 | 4.7 |
| Total green fluorescence | 7,466 | 70.6 |
| Bacteria that fluoresced in red and: | ||
| Divided on the counting chamber | 0 | 0 |
| Did not divide on the counting chamber | 3,112 | 29.4 |
| Total red fluorescence | 3,112 | 29.4 |
Data are for cells in stationary phase for 38 h.
Total number of cells that fluoresced = 10,758.
DISCUSSION
In this paper, we report on a new approach for direct microscopic assessment of bacterial viability using optical trapping and cell sorting. We argued that the two assays used in concert will, if working properly, give good and true indications of cell viability. The microscopic assessment of reproductive ability essentially elucidates the same properties as plate counts but was performed directly at the level of individual cells in suspension. However, like standard plate count techniques, it does not answer whether the loss of reproductive ability is temporary or irreversible. The LIVE/DEAD BacLight assay, on the other hand, is designed to distinguish between cells with an intact membrane(s) (green) and cells with leaky membranes (red). The presence of nucleic acids in the cells is a prerequisite for staining using the BacLight system, and ghost cells will therefore not be detected. Cells stained red by propidium iodide should exhibit a collapsed membrane potential and be unable to both replicate and perform homeostatic metabolism. Moreover, the loss of membrane potential should be irreversible and it would be hard to argue against calling such cells dead. However, we did not know how accurate the commercial LIVE/DEAD BacLight (Molecular Probes, Inc.) dyes worked in our system and whether the propidium iodide could give false positives. To approach these questions we performed a series of experiments using fluorescent dyes and compared the results from the fluorescence-based technique with those from the streak-plate technique and direct microscopic measurements of the reproductive ability. In order to allow measurements of integrity and reproductive ability to be performed on the same individual cells, we developed a system of optical laser trapping in combination with fluorescence microscopy.
Our conclusion from the studies reported here is that the LIVE/DEAD BacLight assay accurately reports on the viability of the growing and stationary-phase E. coli culture analyzed. For the 10,000 cells analyzed, we found no propidium iodide-positive ones that were able to perform cell division upon nutrient addition. In addition, no propidium iodide-positive cells were detected in the exponential phase of growth. Also, we did not find evidence for a large population of intact but nonculturable cells during the stationary phase period studied but it is clear that reproductive ability can be lost prior to the loss of membrane integrity. We do not know whether the failure of the bacteria in this group to perform division is reversible or not but we noted that for 15 cells the color changed from fluorescent green to red during the experiment, indicating that at least a fraction of these cells are moribund. Based on the results, the stationary-phase population analyzed can be divided into two major categories and one minor category, as follows: live bacteria (65%; green with dividing capacity), dead bacteria (29.6%; red and nondividing), and intact but nonreplicative (5.4% green and nondividing). These numbers matched the data obtained by standard plate counts (Results). Using the optical tweezers, we are now setting up a rapid and automated technology to isolate and concentrate cells from these individual categories for further analysis, using proteomics and microarray determination of transcription patterns. Thus, we can approach the question of population heterogeneity in gene expression and correlate this to the categories identified above. In addition, we think the technique lends itself nicely to further analysis of bacteria reported to readily enter a nonculturable state.
The laser trapping methodology can be used also for manual isolation of mutants at the level of single cells rather than colonies. For example, the optical traps used here in combination with fluorescence microscopy can be used in the analysis of single-cell gene expression (with fluorescent reporter systems). By using a gene fusion-reporter system that is normally activated by, say, a critical quorum of bacteria, we can screen for mutants that fail to elicit such quorum response in dense populations studied directly under the microscope and subsequently isolate this mutant cell from the population by laser trapping and transfer to a growth medium. Finally, using optical trapping we are setting up single-cell chemostat operations with the aim of elucidating whether bacteria, in contrast to eukaryotic cells, including unicellular yeast cells, really lack the type of limitation in their reproductive potential described by Hayflick and Moorehead (i.e., the loss of proliferative capacity with successive cell divisions) (6). This assumption has not been put through a close experimental scrutiny.
ACKNOWLEDGMENTS
We thank Anne Farewell for useful discussions throughout this work.
This work was supported by grants from the Swedish Research Council for Engineering Sciences (TFR) to T.N. and D.H., by a grant from the Swedish Natural Science Research Council (NFR) to T.N., and by a grant from the Carl Tryggers Foundation for Scientific Research to D.H.
REFERENCES
- 1.Barer M R. Viable but non-culturable and dormant bacteria: time to resolve an oxymoron and a misnomer? J Med Microbiol. 1997;46:629–631. doi: 10.1099/00222615-46-8-629. [DOI] [PubMed] [Google Scholar]
- 2.Barer M R, Gribbon L T, Harwood C R, Nwoguh C E. The viable but non-culturable hypothesis and medical bacteriology. Rev Med Microbiol. 1993;4:183–191. [Google Scholar]
- 3.Dukan S, Nyström T. Oxidative stress defence and deterioration of growth-arrested Escherichia coli cells. J Biol Chem. 1999;274:26027–26032. doi: 10.1074/jbc.274.37.26027. [DOI] [PubMed] [Google Scholar]
- 4.Fällman E, Axner O. Design for fully steerable dual-trap optical tweezers. Appl Opt. 1997;36:2107–2113. doi: 10.1364/ao.36.002107. [DOI] [PubMed] [Google Scholar]
- 5.Gallant J, Palmer L. Error propagation in viable cells. Mech Ageing Dev. 1979;10:27–38. doi: 10.1016/0047-6374(79)90068-x. [DOI] [PubMed] [Google Scholar]
- 6.Hayflick L, Moorehead P S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 7.Kaprelyants A S, Mukamolova G V, Kell D B. Estimation of the dormant Micrococcus luteus cells by penicillin lysis and by resuscitation in cell-free spent culture medium at high dilution. FEMS Microbiol Lett. 1994;115:347–352. [Google Scholar]
- 8.Mukamolova G V, Kaprelyants A S, Young D I, Young M, Kell D B. A bacterial cytokine. Proc Natl Acad Sci USA. 1998;95:8916–8921. doi: 10.1073/pnas.95.15.8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliver J D. Formation of viable but nonculturable cells. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. pp. 239–272. [Google Scholar]
- 10.Postgate J R. A microbial way of death. New Sci. 1989;20:43–47. [Google Scholar]
- 11.Postgate J R, Crumpton J E, Hunter J R. The measurement of bacterial viabilities by slide culture. J Gen Microbiol. 1961;24:15–24. doi: 10.1099/00221287-24-1-15. [DOI] [PubMed] [Google Scholar]
- 12.Roszak D B, Colwell R R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987;51:365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roth W G, Leckie M P, Dietzler D N. Restoration of colony-forming activity in osmotically stressed Escherichia coli by betaine. Appl Environ Microbiol. 1988;54:3142–3146. doi: 10.1128/aem.54.12.3142-3146.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Votyakova T V, Keprelyants A S, Kell D B. Influence of viable cells on the resuscitation of dormant cells in Micrococcus luteus cultures held in an extended stationary phase: the population effect. Appl Environ Microbiol. 1994;60:3284–3291. doi: 10.1128/aem.60.9.3284-3291.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]