Summary
Dolutegravir (DTG) is recommended for all people living with HIV because of its efficacy, high barrier to resistance, favourable safety and tolerance profile, and affordability. DTG has also been found to have the highest rates of viral suppression in pregnancy, therefore preventing perinatal HIV transmission. In view of these benefits, particularly for pregnant women, it is important to ask whether DTG is safe in pregnancy. DTG has been associated with metabolic complications including weight gain and rare events of hyperglycemia that could affect maternal, fetal, and postnatal health. Here we review the current clinical and experimentally-based literature on the implications of DTG usage for the pregnant female and for the developing embryo and fetus. Possible effects on folate status, energy metabolism, adipogenesis, and oxidative stress are considered. In many instances, insufficient data are available, pointing to the need for additional research in this important area of HIV treatment.
1. Introduction
Infection with the Human Immunodeficiency Virus (HIV) poses a severe disease burden, having claimed 36.3 million lives and currently affecting nearly 40 million people around the world.1 Reducing HIV viral load (VL) in people living with HIV (PLWH) to undetectable and therefore untransmissible (U=U) levels remains the most effective approach at reducing the incidence of HIV infection. The UNAIDS and WHO 95–95-95 goal aims for 95% of PLWH to be aware of their infection status, 95% of people diagnosed with HIV to receive treatment, and 95% of PWLH receiving treatment to have undetectable VLs.2 Perinatal transmission of HIV remains a serious concern for women of childbearing age living with HIV. The risk of transmission is highest at delivery and during breastfeeding, especially with detectable viremia in pregnancy, preterm delivery, and late initiation of treatment in pregnancy.3,4 Combination antiretroviral therapy (ART) remains the most reliable treatment option for HIV infection and has been shown to effectively suppress VL, prevent the development of AIDS, and minimize the risk of HIV transmission.3–5 Pregnant women initiating ART before conception had a 0·03% rate of vertical HIV transmission; the rate drops to 0·0% in the women that additionally had an undetectable VL at conception.4 As ART has transformed HIV infection into a manageable chronic illness, increased attention has been directed towards optimizing current regimens and understanding chronic HIV-related comorbidities associated with HIV infection and ART, with the goal of improving quality of life for PLWH over the long term.6
Dolutegravir (DTG)-containing regimens with varied nucleoside reverse-transcriptase inhibitor (NRTI) backbones have recently become the preferred regimens worldwide and are the WHO-recommended first-line therapy for all PLWH.3,5,7,8 DTG-based regimens are significantly more affordable compared to other first-line ART regimens, making them favourable in low-middle income countries.5 DTG also has a high barrier to HIV resistance as DTG-resistant mutations have been shown to reduce HIV fitness.9 Significantly lower rates of viral resistance have been reported with DTG use and DTG is successfully used as salvage treatment in virological failures.9,10 In clinical trials, DTG-based regimens had the same or improved efficacy as protease inhibitors (PIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), and other integrase strand transfer inhibitors (INSTIs).11–15
In the context of pregnancy, the Safety and Pharmacokinetics of Dolutegravir in Pregnant HIV Mothers and Their Neonates (DolPHIN-1,-2), and International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) network P2010 protocol (VESTED) randomized controlled trials (RCT), showed that DTG was associated with more rapid and effective viral suppression, compared to efavirenz (EFV), making DTG especially useful in pregnancy wherein rapid viral suppression is essential in preventing perinatal transmission.16–18 The Pediatric HIV/AIDS Cohort Study Surveillance and Monitoring for ART Toxicities (SMARTT) reported better viral suppression in women receiving DTG-based ART compared to non-DTG ART regimens, without any differences in fetal outcomes.19
DTG-based treatments are associated with lower toxicity, fewer drug-drug interactions than other ARV classes (PI and NNRTI), and a good tolerability profile, all of which improve the quality of life and regimen adherence among PLWH.11,20 However, DTG use has been associated with metabolic complications in non-pregnant adults, such as weight gain and rare events of hyperglycemia.21,22 In pregnancy, DTG appears to be generally well tolerated; however there are limited studies available that examine metabolic parameters or post-natal outcomes.16,17,23–26 Here we review available data on DTG safety in pregnancy, as well as clinical and experimentally-derived data of the metabolic effects of DTG. We further discuss how these DTG metabolic effects could impact fetal and maternal health.
2. Safety of DTG use in pregnancy
2.1. Clinical study findings on DTG and pregnancy and birth outcomes
Initial clinical surveillance studies, did not report an association between DTG-based ART and adverse birth outcomes (See Table 1 for a summary of studies).25,26 In the DolPHIN-2 open-label RCT, recruiting from South Africa and Uganda,, DTG was associated with greater pregnancy and postpartum (puerperium) adverse events compared to the EFV arm, however this was not replicated in other studies (Table 1).17,18,27 Analysis of data obtained in the Tsepamo study did not show differences in severe pregnancy outcomes, such as preterm birth, small for gestational age, or fetal demise, but an increased occurrence of maternal hypertension and increased intrapartum weight gain in women receiving a DTG versus EFV-based regimen was reported.28,29 Furthermore, DTG was associated with fewer severe adverse birth outcomes in women with lower BMI.28 In the VESTED trial DTG was associated with improved gestational weight gain and either similar or lower levels of adverse birth outcomes compared to EFV.17
Table 1:
Summary of clinical pregnancy studies including a DTG-based regimen
| Study Type, Location, Date | Regimen | N | Outcomes |
|---|---|---|---|
| Surveillance study, Botswana, Aug 2014 – May 201830 | DTG-based ART from conception | 426 | DTG from conception group had four cases (0·94%) of NTDs: encephalocele, myelomeningocele, iniencephaly, and anencephaly. NTD rates were similar between the HIV-negative (0·09%) and other ART groups (0·12%) |
| DTG-based ART started in pregnancy | 2812 | ||
| Other ART | 11,300 | ||
| HIV-negative | 66,057 | ||
| Surveillance study, Brazil, Jan 2015 – May 201834 | DTG-based ART | 382 | No increased risk for adverse peripartum outcomes associated with DTG exposure reported. 2/490 (0·18%) of NTDs in DTG treated women reported in study update Feb 2019. |
| EFV-based ART | 1,045 | ||
| Surveillance study, Botswana, Oct 2018 – Mar 201933 | DTG-based ART | 152 | One NTD in DTG from conception arm (0·66%). Two in HIV-negative pregnancies (0·09%). |
| Other ART | 544 | ||
| HIV-negative | 2328 | ||
| Surveillance study, United States, Puerto Rico, Swiss cohort, 2007 – Jan 202019 | DTG-based ART | 120 | DTG-based regimen was associated with a mildly higher risk of preterm births associated. 1 case of syndactyly, 2 cases of polydactyly in DTG group. |
| Atazanavir/r-based ART | 464 | ||
| Darunavir/r-based ART | 185 | ||
| Rilpivirine-based ART | 243 | ||
| RAL-based ART | 86 | ||
| Elvitegravir/c-based ART | 159 | ||
| Database analysis, Antiretroviral pregnancy registry, Jan 1989 – Jul 2022, APR 2022.35 | DTG-based ART | 1362 | The reported rates of congenital anomalies associated with DTG were 3·45% in APR, with one NTD case of anencephaly. |
| Surveillance study, Botswana, Aug 2014 – Aug 201625 | DTG-based ART | 1729 | No increased risk of adverse birth outcomes on DTG was reported. |
| EFV-based ART | 4593 | ||
| Surveillance study, Botswana, Aug 2014-Apr 202028 | DTG-based ART | 2,450* | Surveillance study showed that DTG regimen had the same or better peripartum outcomes as compared to other ART regimens in all maternal weight classes. |
| EFV-based ART | 7,459* | ||
| Other ART | 6,492* | ||
| NVP-based ART | 4,695* | ||
| LPV/r-based ART | 841* | ||
| Open-label RCT, South Africa, Uganda, Jan 2018 – Aug 201827 | TDF/FTC/DTG | 135 | DTG group showed slightly higher (24%) severe adverse events than EFV (18%) group. |
| TDF/XTC/EFV | 133 | ||
| Open-label RCT (72-week follow-up) South Africa, Uganda, Jan 2018 – Aug 201818 | TDF/FTC/DTG | 135 | Greater proportion of adverse pregnancy events were found in DTG (22%) than EFV (11%) arm. |
| TDF/FTC/EFV or 3TC | 133 | ||
| Open-label RCT, Zimbabwe, South Africa, Uganda, Brazil, Botswana, Tanzania, Thailand, United States, India, Jan 2018 – Feb 201917 | TDF/FTC/DTG | 204 | DTG group had lower rates of preterm birth (6%) compared to EFV group (12%). TAF/FTC/DTG had higher gestational weight gain (0·378kg/week) versus TDF/FTC/DTG (0·319kg/week) and TDF/FTC/EFV (0·291kg/week). |
| TAF/FTC/DTG | 201 | ||
| TDF/FTC/EFV | 200 | ||
| Surveillance study, Botswana, Aug 2014 – Mar 201929 | DTG (TDF/FTC 98.8%) | 621 | DTG group had 0·35 kg/week weight gain over 18–36 weeks gestation. EFV group had 0·31 and HIV-negative group had 0·44 kg/week weight gain. |
| EFV (TDF/FTC 99.8%) | 757 | ||
| HIV-negative | 11,280 | ||
| RCT, Botswana, Aug 2016 – May 201924 | Mothers receiving TDF/XTC/DTG | 182 | No difference in insulin sensitivity in exposed uninfected infants born to women taking DTG versus those taking EFV in pregnancy. |
| Mothers receiving TDF/XTC/DTG | 124 | ||
| Prospective surveillance study, Gaborone, Botswana, Aug 2016 – May 201923 | DTG-based ART | 197 | Lower rates of GDM were observed in DTG-treated (6·1%) vs EFV-treated (13·5%) women. Both rates were comparable to HIV-negative group (7·4%). |
| EFV-based ART | 126 | ||
| HIV-negative | 163 | ||
| Database analysis, France, 2012 – 2016100 | DTG-based ART | 49 | Higher birth defect rates in DTG arm at 4·1% versus RAL (1·3%) and Elvitegravir (1·4%). |
| RAL-based ART | 240 | ||
| Elvitegravir-based ART | 70 | ||
| Retrospective analysis, United States, 2015–2018101 | DTG-based ART | 66 | No side effects on DTG treatment were reported, with 2 cases of birth defects: a congenital heart abnormality and a nonimmune hydrops fetalis. |
| Retrospective analysis, Sweden, 2014 – Aug 2017102 | DTG-based ART | 36 | DTG-based regimen showed no difference in adverse pregnancy events from that of general population. |
Varied N for different outcomes
Abbreviations: ART, antiretroviral therapy; DTG, dolutegravir; EFV, efavirenz; FTC, emtricitabine; LPV, lopinavir; RAL, raltegravir; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; XTC, either 3TC (lamivudine) or FTC; /c, cobicistat booster; /r, ritonavir boosted.
2.2. Clinical study findings on DTG and NTDs
In 2018 the Tsepamo surveillance study in Botswana reported four newborns with NTDs among offspring of 426 women starting DTG at conception (0·94% [95%CI 0·37, 2·4]), compared to a 0·12% [0·07, 0·21] incidence with non-DTG based ART treatment and 0·09% [0·07, 0·12] incidence among offspring of women without HIV.30 A 2019 follow-up to the Tsepamo study reported a decrease from the initial report in NTDs prevalence amongst DTG treated pregnant women to 0·3% [0·13, 0·69] of 1683 deliveries.31 More anterior body wall defects (omphalocele and gastroschisis) were also reported in those receiving a DTG-based regimen from conception.31 In a further update in 2022, 10 NTDs were reported in 9,460 women taking a DTG-based regimen from conception, for a rate of 0·11% [0·06, 0·19], compared to 0.08% [0·04, 0·14] on EFV and 0.07% [0·05, 0·08] HIV-negative pregnancies. This brought the rate of NTDs in the DTG group to the same level that of other ARVs and women without HIV.32
Additional studies have reported on DTG and NTDs. Although none of these studies have the sample size of the Tsepamo study, all reported no significant differences in rates of congenital defects (including NTDs) between DTG and other ARVs.33–36 A summary of their findings can be found in Table 1.
Despite the disappearance of the initial NTD signal, the etiology of the increased rates of NTDs is unknown. The initial signal could have been a matter of chance due to small sample size. Alternatively, there may have been other risk factors present during the 2018–2019 years that had a combined effect with DTG on the emergence of NTDs, such as lower population folate levels or other environmental exposures. However, this remains speculative. In light of the most recent data from Botswana, DTG remains a preferred regimen for its superior efficacy in preventing HIV related mortality and transmission in women of childbearing potential.37,38
2.3. DTG effects in animal and in vitro reproductive studies
In pregnancy, DTG crosses the placenta and fetal exposure can be significant due to slow fetal metabolism of the drug.16,26 In reproductive toxicology studies performed in rats and rabbits, supratherapeutic DTG was not associated with fetotoxicity or higher risk for congenital defects (see Table 2).39 However, in a large fetotoxicity study performed in C57BL/6J mice fed a folate-sufficient diet, a small (was 0·47% (N=150 litters)) but significant increase in the incidence of NTDs was observed at the therapeutic DTG dose but surprisingly not at the supratherapeutic DTG dose (both delivered with therapeutic tenofovir (TDF)/emtricitabine (FTC) backbone).40 Mice receiving the therapeutic DTG regimen also had increased rates of microphthalmia, bleeding defects, and edema.40 Supratherapeutic DTG-only exposure from conception in C3H/HeJ mice resulted in one NTD (exencephaly) in 109 embryos from 17 litters, along with evidence of neuronal damage and neuroinflammation in the pups of DTG-treated dams (Table 2).41 DTG-exposure of rat embryos cultured through the period of neurogenesis did not demonstrate teratogenicity, although the design of the study was brought into question, particularly the sample size, DTG penetrance of the embryo, and potential for DTG metabolite teratogenicity (see Table 3).42,43 In a cell culture model, DTG has been shown to affect morphogenesis and survival of murine pluripotent and human embryonic stem cells, along with transcript changes of developmental regulator genes (Table 3).41,44,45 From these studies, it appears that DTG is essentially safe for use in human pregnancy although it may have the potential to affect some aspects of embryonic development. Although adverse developmental effects were observed in cell culture, they were rarer and milder in the in vivo models and largely absent in clinical studies, potentially due to compensation by whole organism homeostatic mechanisms.
Table 2:
Summary of in vivo reproductive studies with DTG
| Animal model, study | Regimen | Dosage (mg/kg) | Treatment start | N (Litters) | Results |
|---|---|---|---|---|---|
| C57BL/6J female mice40 | 1xDTG (DTG/FTC/TDF) equivalent to human therapeutic drug levels | 2·5/33·3/50 | GD 0.5 | 150 | Five cases of NTDs were observed in the therapeutic 1xDTG dosage only. Two had exencephaly, two had spina bifida, and one had potential anencephaly. |
| 5xDTG (DTG/FTC/TDF) reaching supratherapeutic levels | 12·5/33·3/50 | 111 | |||
| Control, water | - | 91 | |||
| C3H/HeJ female mice41 | DTG | 50 | GD 0·5 | 17 | Exencephaly in one fetus in DTG arm but N insufficient for statistical power. |
| Control, vehicle | - | 9 | |||
| Sprague-Dawley female rats39 | DTG | 5 | GD 6 | 22 | No significant differences in external abnormalities. Meningocele/absent eye bulge at 1000mg/kg dose but N insufficient for statistical power. |
| DTG | 50 | 21 | |||
| DTG | 100 | 27 | |||
| DTG | 300 | 27 | |||
| DTG | 1000 | 47 | |||
| Control | - | 49 | |||
| Japanese white female rabbits39 | DTG | 40 | GD 6 | 19 | No significant differences in external abnormalities observed. One cranioschisis at 40mg/kg dose but N insufficient for statistical power. |
| DTG | 100 | 3 | |||
| DTG | 200 | 18 | |||
| DTG | 300 | 5 | |||
| DTG | 1000 | 24 | |||
| Control | - | 24 |
Abbreviations: DTG, dolutegravir; FTC, emtricitabine; GD, gestational day; TDF, tenofovir disoproxil fumarate.
Table 3:
Summary of In vitro developmental toxicology studies with DTG
| Culture model, study | DTG dosage (μM)* | N | Results |
|---|---|---|---|
| Murine P19C5 pluripotent stem cells and human embryonic stem cells H944 | 0·25, 0·5, 1, 2, 4 | 46–48 aggregates per condition for morphogenesis | DTG was associated with impaired stem cell morphogenesis and changes to developmental regulator genes in a dose-dependent manner in both P19C5 and H9 cells. |
| CA1S human embryonic stem cells45 | 8·32 | 5 replicates | DTG was associated with reduced expression of pluripotency markers in CA1S cells. |
| H9 human embryonic stem cells45 | 8·32 | 6 replicates | DTG was associated with increased rates of apoptosis in H9 cells. |
| Sprague Dawley GD9 embryo culture42 | 12·6 | 16 embryos | DTG did not affect embryo lethality, visceral yolk sac, somite number, or embryo size. |
| 22·2 | 16 embryos | ||
| Zebrafish embryo culture49 | 100 | 2–4 experimental replicates | DTG was associated with developmental toxicity post-fertilization. |
Cmax for DTG in non-pregnant adults is reported as 701–1156 μM.103
3. DTG metabolic effects and implications for DTG safety in pregnancy
Inadequate nutrition and poor metabolic health increase the risk of adverse pregnancy outcomes and congenital defects, poor maternal health outcomes, and contribute to metabolic programming that increases the lifelong risk of poor metabolic health in neonates.46,47 Studies conducted to characterize DTG’s effects on metabolic pathways are summarized below, and implications for maternal–fetal health and avenues for future studies are discussed.
3.1. DTG-associated changes to folate metabolism
Interest in investigating the association between DTG and folate increased following the original report of a higher prevalence of NTDs.30 A comparison of serum folate levels in women participating in the ADVANCE trial found that folate levels increased in non-pregnant women taking DTG administered with TAF/FTC over 12 weeks, folate levels remained stable in women taking TDF/FTC/DTG, and decreased in women taking TDF/FTC/EFV.48 In the 26 women who became pregnant during the study, folate levels increased slightly in those taking TAF/FTC/DTG or TDF/FTC/DTG and decreased slightly in those taking TDF/FTC/EFV for 24 weeks, however, the pregnancy cohort was severely limited in sample size.48
Animal and in vitro studies examining the effects on dietary folate and folate transport indicate mild effects of DTG at therapeutic plasma levels (see Figure 1).49–51 DTG has been shown to be a partial antagonist of folate receptor 1 (FOLR1/FRα) in placental cell lines.49 In the same study folic acid supplementation was able to rescue early DTG-related (100 μM) toxicity in zebrafish.49 Data from the Zamek-Gliszczynski M et al. study support in vitro DTG antagonism of FOLR1, however, extrapolating quantified in vitro DTG inhibition to in vivo conditions, this effect was deemed not clinically relevant at therapeutic dosage of DTG.50 In DTG-treated placental explants and placenta cell lines, and in placentas isolated from DTG-treated mice, variable changes to the gene and protein expression of the folate transport and metabolism pathway were observed. DTG treatment of placental cell lines was associated with a modest reduction in expression of reduced folate carrier (RFC) and proton-coupled folate transporter (PCFT), along with a decrease in their transport function.51 In the Mohan H et al. fetotoxicity study, supratherapeutic DTG treatment was associated with lower rates of fetal anomalies than the therapeutic DTG dose, and concurrently higher levels of fetal folate (fetal folate levels in the therapeutic dose were similar to control), suggesting either potential compensation by increased folate uptake or biphasic effects of DTG on a system interacting with or affecting the folate pathway.40
Figure 1.
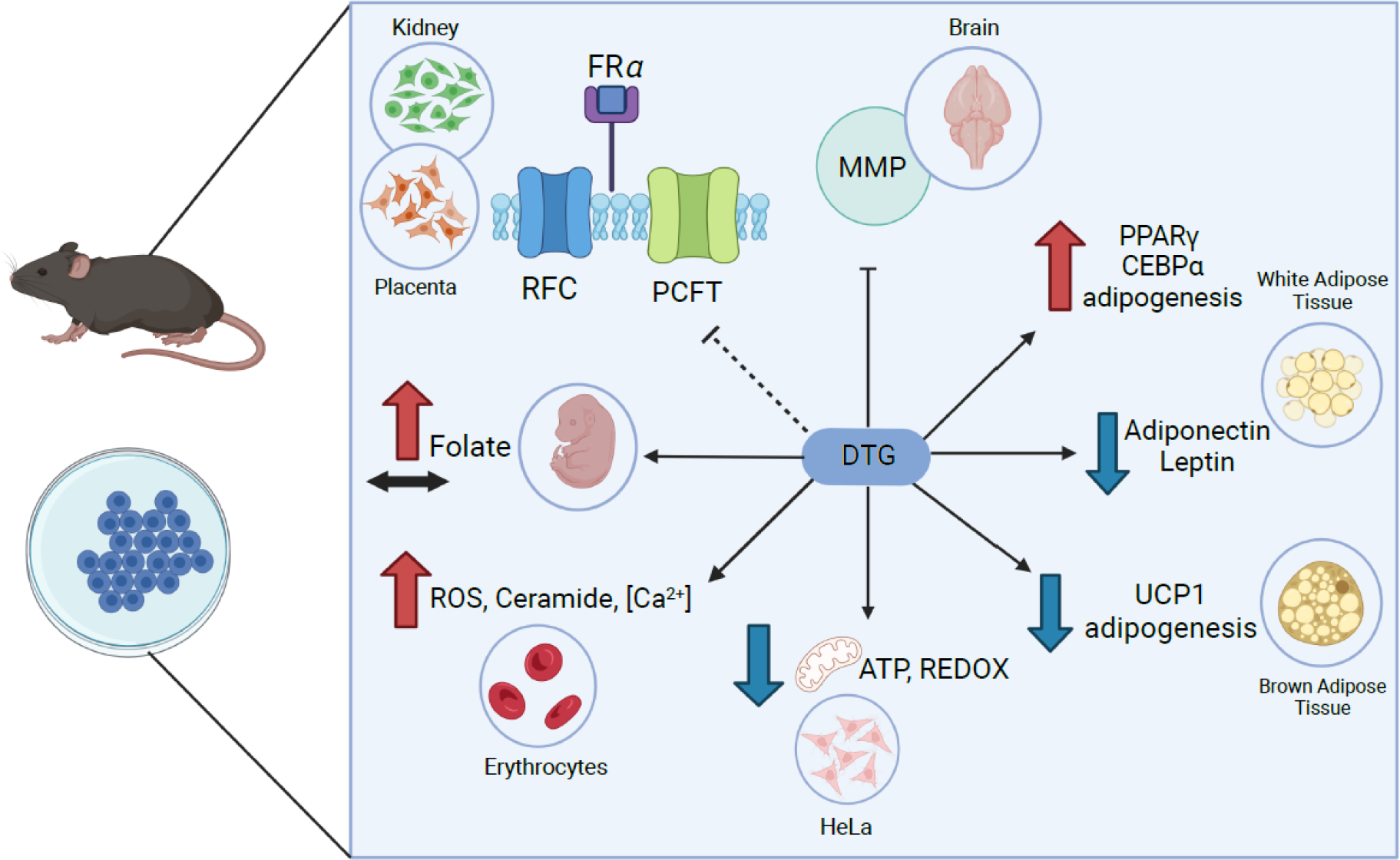
Summary of the observed effects associated with DTG from animal and in vitro studies. DTG effects on folate metabolism40,49–51, cellular energy homeostasis86,88, adipocyte function86,89,90,93, and matrix metalloproteinases41 are shown (anticlockwise from top left). Dotted line indicates variable effects reported. Red arrows indicated increase/higher; blue arrows indicate decrease/lower, black arrow indicates unchanged. FRa, folate receptor 1; RFC, reduced folate carrier; PCFT; proton-coupled folate transporter; MMP, matrix metalloproteinases; PPARγ, peroxisome proliferator-activated receptor gamma; CEBPα, CCAAT/enhancer-binding protein alpha; UCP1, uncoupling protein 1; ROS, reactive oxygen species.
It remains unclear whether the dysregulation of folate transport or metabolism by DTG has a clinical impact on human pregnancies. Neither clinical or animal data suggests that DTG reduces folate levels, but there is some evidence suggesting a diminished response to folate through FOLR1 inhibition and reduced folate transport across the placenta following DTG treatment. Therefore, folate insufficiency in pregnancy may exaggerate the effects of DTG and proper folate supplementation should have a protective effect against adverse events. Although fetal folate levels and active placental folate transport cannot be quantified in human pregnancies, studies examining the interaction of maternal folate levels and placental nutrient transporters in women receiving DTG-based ART may provide insight into the etiology of adverse perinatal events.
3.2. Metabolic effects of DTG use in clinical studies
Maternal obesity, hyperglycemia, prediabetes/type 2 diabetes mellitus (T2DM), and metabolic syndrome (defined as having three or more of increased waist circumference, blood pressure, plasma triglycerides, fasted blood glucose, and decreased HDL-cholesterol), increase the risk for adverse events in pregnancy and contribute to fetal metabolic programming towards increased risk for poor metabolic health.46,47
DTG has been associated with weight gain, rare new-onset hyperglycemia, and some studies report an increased risk for diabetes mellitus and metabolic syndrome, described in more detail below.21,22,52,53 However, few studies have addressed DTG metabolic effects in pregnancy, and results of clinical trials generally suggest improved pregnancy outcomes for DTG compared to other ARVs, primarily EFV.23,26 Larger-scale studies are needed to corroborate these results. In addition, studies comparing DTG metabolic effects in pregnancy to those of people without HIV are lacking. DTG-associated transient changes to fasted blood glucose in non-pregnant female mice have been reported.54 However animal studies investigating maternal glucose homeostasis in pregnancy are yet to be carried out.
3.2.1. DTG-associated weight gain
DTG-based regimens are associated with greater weight gain and in the long-term may contribute to other metabolic complications.21,53 In both naive and ART-experienced patients, DTG-based regimens are associated with greater weight gain than NNRTI-, PI-, and some other INSTI-based regimens.15,21,53,55–63 The degree of weight gain varies dramatically in relation to the backbone formulation, demographics, and baseline characteristics of the study participants, with TAF-containing NRTI backbones, female sex, older age, and black race being independently associated with greater risk for treatment-associated weight gain.15,21,58,64–66 In PLWH with advanced viremia and immune suppression (CD4+ T-cell count <200 cells/mm3), initiation of ART leads to weight gain as part of the ‘return-to-health’ phenomenon. Indeed, weight gain among treatment-naive individuals initiating DTG-based therapy is greater than in treatment-experienced PLWH switching to a DTG-based regimen.56,60,62,64,66–69 Furthermore, poor virological control, adverse events, and slower rate of viral suppression in PI and NNRTI drugs are often cited as reasons for smaller magnitude and rate of weight gain in comparison to DTG-based regimens. However, although some NNRTIs are associated with a slower rate of viral suppression in select studies, the INSTI elvitegravir (EVG) exhibits a similar viral suppression rate to DTG and is associated with similar weight gain to NNRTIs.59 Furthermore, increased weight gain continues to be an issue in the long-term, as shown in 5 year follow-up studies by Ando N et al. and Bourgi K et al.58,63 In most retrospective studies, the inclusion criteria include successful viral suppression and high CD4+ cell count prior to INSTI-treatment as a way to control for the return-to-health effect. Randomized ART-switch and double-blind RCTs corroborate the weight-gain effects of DTG.21,61
In pregnancy, women receiving DTG-based ART experienced greater intrapartum weight gain than EFV-based ART, however it was still below the recommended weekly weight for a healthy pregnancy.17,27–29 Sufficient weight gain during pregnancy reduces the risk of preterm birth, and small, and very small for gestational age neonates; DTG-based regimens therefore appear more favorable.70
3.2.2. DTG-associated hyperglycemia, T2DM, and metabolic syndrome
Currently, only short-term prospective, case-report, and cross-sectional study data exist on the effects of DTG on metabolic health, partly due to the recent implementation of DTG as a first-line treatment. There is also a significant degree of discrepancy between various studies, with some citing DTG-associated improvement to metabolic parameters71,72 while others report increased risk for T2DM, metabolic syndrome, and hyperglycemia.22,52 In an observational prospective study interrogating changes to insulin sensitivity and circulating lipids following a switch from ritonavir-boosted PI to a DTG-containing regimen in patients with stable virological control, DTG was associated with lower IL-6, triglycerides, LDL and total cholesterol, leptin, insulin, and HOMA-IR index.71 In ART-naive patients, initiating a DTG-based regimen was associated with lower rates of new-onset diabetes at 0·91%, in comparison to those starting a NNRTI- or a PI-containing regimen, 1·37% and 1·50% respectively.72 Hsu R et al. reported no increased risk of prediabetes or diabetes mellitus in ART naive and experienced patients on different INSTIs, however being on ART was associated with higher incidence of T2DM than the general population, 9–13 versus 6·7 per 1000 person-years.73 A cross-sectional study examining risk factors (including ART regimen, NRTI backbone, VL, BMI, sex, and lymphocyte count) for developing metabolic syndrome in PLWH receiving ART for ≥6 months in Zambia, reported that DTG-based regimens, compared to PI- and NNRTI-based therapy, were independently associated with doubling of the risk for metabolic syndrome.52 A national survey of HIV clinicians’ perspectives on DTG use for PLWH in Uganda in terms of tolerability and effectiveness, reported favorable outcomes for patients initiating or switching to DTG. However, it was noted that hyperglycemia, insomnia, and decreased libido were some of the side-effects associated with DTG treatment.74 Case reports of hyperglycemia following initiation of DTG have appeared throughout the literature, where hyperglycemia occurred in patients with normal BMI, experiencing weight gain or loss, and without prior history of insulin resistance.75–77 Discontinuation of INSTI-based therapy normalized glycemic control in the presented cases and the patients no longer needed anti-diabetic medication.75,78 A large-scale surveillance study performed in Uganda reported a greater incidence of new-onset hyperglycemia in PLWH switching to, or initiating, DTG-based regimens than in patients receiving non-DTG-based regimens, 0·47% vs. 0·03% respectively.22 Furthermore, no association of hyperglycemia with weight gain was observed, as in most cases of hyperglycemia the patients had lost weight.22 A caveat to the study was that a greater proportion of individuals in the DTG arm were male, older, and on ART for more than 5 years, all of which are risk factors for hyperglycemia.22 A recent study interrogating adverse-drug events in treatment-experienced and naive patients observed hyperglycemia with an incidence of 2·4% within 13 to 62 weeks of DTG-based regimen initiation.79 Furthermore, the SPRING-1, SPRING-2, SAILING, SINGLE, and FLAMINGO clinical trials, which assessed the efficacy of DTG, reported hyperglycemia among its adverse drug events; hyperglycemia also appears as an adverse drug event leading to DTG-discontinuation.79–84
To date, only one study reports on the incidence of gestational diabetes in patients receiving DTG-based treatment, wherein DTG-based ART was associated with a lower risk for gestational diabetes compared to EFV-based ART.23 No change to insulin sensitivity was observed in exposed uninfected infants born to women receiving a DTG- versus an EFV-based ART.24 In the follow-up to the IMPAACT 2010 VESTED study, no differences in maternal or fetal HbA1c between FTC/TDF/DTG, FTC/TAF/DTG, or FTC/TDF/EFV were found.85
Taken together, these studies show that DTG is associated with metabolic changes in non-pregnant adults. There remains a gap in knowledge of whether the observed effects in non-pregnant individuals are replicated in pregnancy and relevant to perinatal outcomes.
3.3. Animal and in vitro studies examining DTG-associated metabolic changes
DTG-associated weight gain and hyperglycemia observed in clinical studies may result from drug induced changes to energy homeostasis at the hypothalamic, tissue, and/or cellular levels. Animal models and in vitro studies using human samples have shown distinct alterations to adipose tissue function and insulin sensitivity, as well as changes to mitochondrial function and oxidative metabolism associated with DTG.86–90 Studies conducted to characterize DTG’s effects on these pathways are summarized below and shown in Figure 1.
3.3.1. DTG effect on the melanocortin system
DTG-associated weight gain and metabolic perturbations may be a symptom of a change to energy homeostasis regulation by the hypothalamus. Many hormones are involved in the regulation of satiety/hunger and energy expenditure and deviations from the physiological baseline in pregnancy may lead to fetal programming affecting metabolic health.
In the Tivicay (DTG) product monograph, DTG was shown to reduce alpha-melanocortin stimulating hormone (α-MSH) binding of melanocortin 4 receptor (MC4R) by 65% at the clinical DTG Cmax. This may shift the anorexigenic/orexigenic balance towards increased orexigenic neural tone, thereby increasing appetite and reducing post-prandial satiety resulting in increased food intake without altering energy expenditure – leading to weight gain.91 A potential role of MC4R in development has been sparsely documented, and has not yet been thoroughly studied.92 Analysis of MC4R binding by various INSTIs revealed a capacity for MC4R antagonism by bictegravir (BIC), cabotegravir (CAB), EVG, raltegravir (RAL), and DTG, with EC50 >100 fold beyond the unbound plasma Cmax for each individual drug.91 It would be ideal to conduct further studies examining DTG effects on hormones involved in regulation of satiety/hunger and energy expenditure, such as α-MSH, thyroid hormones, cortisol, and leptin.
3.3.2. DTG effects on adipocytes
Data from in vitro and animal studies suggest the DTG is associated with adipose tissue changes that could contribute to a mechanistic understanding of the clinically observed weight gain. White adipose tissue (WAT) has roles in both energy storage and endocrine signaling through adipokine secretion, whereas brown adipose tissue (BAT) contributes to energy consumption through oxidizing free fatty acids and generating non-shivering thermogenesis.89 White adipocytes secrete leptin, an anorexigenic pro-satiety peptide, and adiponectin, which improves insulin sensitivity. In perturbed metabolic states, such as insulin resistance, WAT tends towards hypertrophy and fibrosis, alongside plasma hyperlipidemia.87 Cold exposure, fasting, and beta-adrenergic stimulation promote BAT activation and WAT beiging, which are associated with better metabolic outcomes.89
Treatment with DTG has been shown to cause changes to adipose tissue composition, function, and signaling.87,89,90 In simian noninfected subcutaneous and visceral adipose tissue, TDF/FTC/DTG treatment induced adipose tissue fibrosis and hypertrophy, with increased mRNA expression of the adipogenic peroxisome proliferator-activated receptor gamma (PPAR-γ) and CCAAT/enhancer-binding protein alpha (CEBPα), and decreased mRNA expression of adiponectin.87 In obese PLWH, increased adipose tissue fibrosis was seen in those treated with INSTI-based rather than non-INSTI-based treatment.87 In cultured proliferating human adipocyte stem cells and mature adipocytes, standalone DTG treatment at Cmax was associated with mitochondrial dysfunction, increased fibrotic markers, lipid accumulation, and lipogenesis, and decreased leptin and adiponectin secretion, and insulin sensitivity.87 These findings were replicated by Pickering R et al., wherein DTG reduced leptin and adiponectin signaling in cultured subcutaneous adipocytes, while increasing pro-adipogenic and pro-fibrotic PPAR-γ and collagen-6 transcripts without altering total triacylglycerol (TAG) content in both subcutaneous and visceral cultured adipocytes.93 Long-term 2-year treatment with TDF/FTC/DTG of SIV-infected macaques was associated with a maintained pro-fibrotic, adipogenic phenotype of subcutaneous and visceral WAT.90 Interestingly, the emergent WAT phenotype of increased lipogenesis, decreased lipolysis, and insulin resistance seen in the SIV-infected macaques treated with DTG does not co-occur under healthy conditions or under the typical progression of obesity, T2DM, and metabolic syndrome.87 Characterization of oxidative BAT in cell culture and in vivo models with short-term (<2 weeks) DTG exposure demonstrates a reduction in thermogenesis, adipogenesis, BAT-specific markers, uncoupling protein 1 (UCP1) expression, and insulin sensitivity.89,90
If the DTG-associated adipose tissue changes discussed lead to clinically observable changes to circulating adipokines like leptin, body-composition, and whole-body energy expenditure, this may in part explain the weight gain. Therefore, it would be useful to monitor these parameters in patients receiving DTG-based ART. It will be also important to study DTG effects on leptin levels in the context of pregnancy, as leptin is produced by the placenta, and its production is altered in several pathologic conditions including preeclampsia and gestational diabetes.94,95
3.3.3. Oxidative stress and metal ion chelating associated with DTG exposure
At a cellular level, the decreases in BAT oxidative capacity and WAT insulin sensitivity may stem from altered cellular metabolism – initiating or resulting in oxidative stress. Oxidative stress in the context of pregnancy can negatively impact fetal development, and oxidative stress is common in many pathways leading to congenital defects.96
George JW et al. report a reduction to mitochondrial REDOX reactions and ATP production, alongside increased glycolysis, in HeLa cells after 24-hour DTG exposure.88 In erythrocytes, 48-hour DTG incubation increased reactive oxygen species (ROS) production, surface ceramide and phosphatidyl serine, and cytosolic [Ca2+], indicating cellular oxidative stress.86
DTG’s inhibitory action on the viral integrase is in part due to cation chelation, which is hypothesized to interfere with the host’s own enzymes.41,49,97 In the Bade AN et al. study, DTG was found to be a broad-spectrum MMP inhibitor by binding the Zn++ ion bound by this class of enzymes.41 MMPs have essential roles in neural crest migration, synapse development, axonal guidance, and angiogenesis in the embryo and contribute to uterine vascular remodeling by the cytotrophoblasts in the development of the placenta.41,98
The cation chelating property of DTG may extend to other metal-binding enzymes such as superoxide dismutases (Mn-, Zn-, and Cu-SOD), resulting in increased cellular ROS, although these effects have not yet been tested. Oxidative stress at the level of the placenta may result in lower fetal weight as reported in the Mohan H et al. study, however this effect was not observed clinically.40,99 To test whether these molecular effects have a systemic effect on development, experimental studies on placental function correlated to fetal outcomes ought to be conducted. The metal ion chelating property of DTG is an interesting mechanism to consider further, as it would affect a broad spectrum of pathways that could contribute to the variety of effects observed with DTG in in vitro and model studies. Further the degree of such insult would be modified by dietary factors and could explain clinically observed outcomes. Well-designed studies would be needed to assess this clinically.
4. Conclusions
The global HIV pandemic presents a severe healthcare burden, which can be successfully managed by ART. DTG-based ART is a preferred treatment option in both resource-rich and resource-limited settings because of its efficacy, high barrier to resistance, favourable safety and tolerance profile, and affordability. DTG-associated changes to maternal physiology such as weight change, hyperglycemia, and folate metabolism, along with changes to adipose tissue, oxidative stress, and potential interference with metal-binding enzymes may affect fetal development and influence metabolic health in the child. However, it remains unclear the degree to which the reported cellular changes impact physiology and whether targeting these pathways in treatment would improve the DTG-specific side effects observed clinically. Furthermore, despite increasing evidence of DTG-associated metabolic changes in non-pregnant adults, there have not been similar reports in pregnancy, and their connection to fetal development has not yet been studied. Studies investigating maternal metabolic health, such as weight and adipose change, plasma lipid profile, adipokine levels, glucose homeostasis correlating to pregnancy outcomes and long-term fetal health are warranted.
Specifically, addressing the following questions would provide great insight: Does DTG affect maternal metabolic health? Do maternal metabolic health changes resulting from DTG treatment affect pregnancy outcomes and fetal metabolic health? How does maternal nutritional status interact with DTG in influencing birth outcomes? In clinical practice, it is pertinent to increase focus on monitoring maternal health and metabolic alterations occurring as a result of DTG treatment. Further, given the scale at which ART is being used in pregnancy it is important that systematic monitoring of adverse events and pregnancy/birth outcomes is implemented, as even small changes in risk have the potential to translate into many pregnancies and babies affected. In the absence of a mechanistic understanding, adequate nutrition and folic acid supplementation should be encouraged.
Search strategy and selection criteria.
References for this review were identified through PubMed searches, authors’ general knowledge of the field, and research papers from presenting authors at HIV conferences. Only papers written in English were included. PubMed searches were the following. Search 1: “dolutegravir.tw” AND (“pregnan*.ti” OR “conception.ti”). Search 2: “dolutegravir.tw” AND (“hyperglyc*.ti” OR “diabet*.ti”). Search was performed for all papers up to October 2022.
Funding statement
This work was supported by funds from the Eunice Kennedy Shriver National Institutes of Child Health & Human Development of the National Institutes of Health, award # R01HD104553. The funder played no role in the writing of the manuscript or the decision to submit it for publication.
Footnotes
Conflict of Interest Statement
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pandey A, Galvani AP. The global burden of HIV and prospects for control. Lancet HIV. 2019; 6: e809–11. [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS. Understanding Fast-Track Targets. Accelerating action to end the AIDS epidemic by 2030. UNAIDS; 2015. [Google Scholar]
- 3.Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission. Recommendations for the Use of Antiretroviral Drugs During Pregnancy and Interventions to Reduce Perinatal HIV Transmission in the United States [Internet]. Department of Health and Human Services; Available from: https://clinicalinfo.hiv.gov/en/guidelines/perinatal. [Google Scholar]
- 4.Sibiude J, Le Chenadec J, Mandelbrot L, Hoctin A, Dollfus C, Faye A, et al. Update of Perinatal Human Immunodeficiency Virus Type 1 Transmission in France: Zero Transmission for 5482 Mothers on Continuous Antiretroviral Therapy From Conception and With Undetectable Viral Load at Delivery. Clin Infect Dis. 2023; 76: e590–8. [DOI] [PubMed] [Google Scholar]
- 5.Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva, Switzerland: World Health Organization; 2021. [PubMed] [Google Scholar]
- 6.Kumar S, Samaras K. The Impact of Weight Gain During HIV Treatment on Risk of Pre-diabetes, Diabetes Mellitus, Cardiovascular Disease, and Mortality. Front Endocrinol. 2018; 9: 705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. [Internet]. 2021. Available from: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv. [Google Scholar]
- 8.Ryom L, De Miguel R, Cotter AG, Podlekareva D, Beguelin C, Waalewijn H, et al. Major revision version 11.0 of the European AIDS Clinical Society Guidelines 2021. HIV Med. 2022; 23: 849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llibre JM, Pulido F, García F, García Deltoro M, Blanco JL, Delgado R. Genetic barrier to resistance for dolutegravir. AIDS Rev. 2015; 17: 56–64. [PubMed] [Google Scholar]
- 10.Kouamou V, Manasa J, Katzenstein D, McGregor AM, Ndhlovu CE, Makadzange AT. Drug resistance and optimizing dolutegravir regimens for adolescents and young adults failing antiretroviral therapy. AIDS. 2019; 33: 1729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanters S, Vitoria M, Doherty M, Socias ME, Ford N, Forrest JI, et al. Comparative efficacy and safety of first-line antiretroviral therapy for the treatment of HIV infection: a systematic review and network meta-analysis. Lancet HIV. 2016; 3: e510–20. [DOI] [PubMed] [Google Scholar]
- 12.NAMSAL ANRS 12313 Study Group, Kouanfack C, Mpoudi-Etame M, Omgba Bassega P, Eymard-Duvernay S, Leroy S, et al. Dolutegravir-Based or Low-Dose Efavirenz-Based Regimen for the Treatment of HIV-1. N Engl J Med. 2019; 381: 816–26. [DOI] [PubMed] [Google Scholar]
- 13.Venter WDF, Sokhela S, Simmons B, Moorhouse M, Fairlie L, Mashabane N, et al. Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): week 96 results from a randomised, phase 3, non-inferiority trial. Lancet HIV. 2020; 7: e666–76. [DOI] [PubMed] [Google Scholar]
- 14.Calmy A, Tovar Sanchez T, Kouanfack C, Mpoudi-Etame M, Leroy S, Perrineau S, et al. Dolutegravir-based and low-dose efavirenz-based regimen for the initial treatment of HIV-1 infection (NAMSAL): week 96 results from a two-group, multicentre, randomised, open label, phase 3 non-inferiority trial in Cameroon. Lancet HIV. 2020; 7: e677–87. [DOI] [PubMed] [Google Scholar]
- 15.Venter WDF, Moorhouse M, Sokhela S, Fairlie L, Mashabane N, Masenya M, et al. Dolutegravir plus Two Different Prodrugs of Tenofovir to Treat HIV. N Engl J Med. 2019; 381: 803–15. [DOI] [PubMed] [Google Scholar]
- 16.Waitt C, Orrell C, Walimbwa S, Singh Y, Kintu K, Simmons B, et al. Safety and pharmacokinetics of dolutegravir in pregnant mothers with HIV infection and their neonates: A randomised trial (DolPHIN-1 study). PLoS Med. 2019;16:e1002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lockman S, Brummel SS, Ziemba L, Stranix-Chibanda L, McCarthy K, Coletti A, et al. Efficacy and safety of dolutegravir with emtricitabine and tenofovir alafenamide fumarate or tenofovir disoproxil fumarate, and efavirenz, emtricitabine, and tenofovir disoproxil fumarate HIV antiretroviral therapy regimens started in pregnancy (IMPAACT 2010/VESTED): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet. 2021; 397: 1276–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kintu K, Malaba TR, Nakibuka J, Papamichael C, Colbers A, Byrne K, et al. Dolutegravir versus efavirenz in women starting HIV therapy in late pregnancy (DolPHIN-2): an open-label, randomised controlled trial. Lancet HIV. 2020;7: e332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel K, Huo Y, Jao J, Powis KM, Williams PL, Kacanek D, et al. Dolutegravir in Pregnancy as Compared with Current HIV Regimens in the United States. N Engl J Med. 2022; 387: 799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kandel CE, Walmsley SL. Dolutegravir - a review of the pharmacology, efficacy, and safety in the treatment of HIV. Drug Des Devel Ther. 2015; 9: 3547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sax PE, Erlandson KM, Lake JE, Mccomsey GA, Orkin C, Esser S, et al. Weight Gain Following Initiation of Antiretroviral Therapy: Risk Factors in Randomized Comparative Clinical Trials. Clin Infect Dis. 2020; 71: 1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamorde M, Atwiine M, Owarwo NC, Ddungu A, Laker EO, Mubiru F, et al. Dolutegravir-associated hyperglycaemia in patients with HIV. Lancet HIV. 2020; 7: e461–2. [DOI] [PubMed] [Google Scholar]
- 23.Mmasa KN, Powis K, Sun S, Makhema J, Mmalane M, Kgole S, et al. Gestational diabetes in women living with HIV in Botswana: lower rates with dolutegravir- than with efavirenz-based antiretroviral therapy. HIV Med. 2021; 22: 715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jao J, Sun S, Bonner LB, Legbedze J, Mmasa KN, Makhema J, et al. Lower Insulin Sensitivity in Newborns With In Utero HIV and Antiretroviral Exposure Who Are Uninfected in Botswana. J Infect Dis. 2022; 226: 2002–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zash R, Jacobson DL, Diseko M, Mayondi G, Mmalane M, Essex M, et al. Comparative safety of dolutegravir-based or efavirenz-based antiretroviral treatment started during pregnancy in Botswana: an observational study. Lancet Glob Health. 2018; 6: e804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill A, Clayden P, Thorne C, Christie R, Zash R. Safety and pharmacokinetics of dolutegravir in HIV-positive pregnant women: a systematic review. J Virus Erad. 2018; 4: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malaba TR, Nakatudde I, Kintu K, Colbers A, Chen T, Reynolds H, et al. 72 weeks post-partum follow-up of dolutegravir versus efavirenz initiated in late pregnancy (DolPHIN-2): an open-label, randomised controlled study. Lancet HIV. 2022; 9: e534–43. [DOI] [PubMed] [Google Scholar]
- 28.Zash R, Caniglia EC, Diseko M, Mayondi G, Mabuta J, Luckett R, et al. Maternal weight and birth outcomes among women on antiretroviral treatment from conception in a birth surveillance study in Botswana. J Int AIDS Soc. 2021; 24: e25763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caniglia EC, Shapiro R, Diseko M, Wylie BJ, Zera C, Davey S, et al. Weight gain during pregnancy among women initiating dolutegravir in Botswana. EClinicalMedicine. 2020; 29–30: 100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zash R, Makhema J, Shapiro RL. Neural-Tube Defects with Dolutegravir Treatment from the Time of Conception. N Engl J Med. 2018; 379: 979–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zash R, Holmes L, Diseko M, Jacobson DL, Brummel S, Mayondi G, et al. Neural-Tube Defects and Antiretroviral Treatment Regimens in Botswana. N Engl J Med. 2019; 381: 827–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zash R, Holmes LB, Diseko M, Jacobson DL, Mayondi G, Mabuta J, et al. Update on neural tube defects with antiretroviral exposure in the Tsepamo Study, Botswana. In 2022. Available from: https://programme.aids2022.org/Abstract/Abstract/?abstractid=12759
- 33.Raesima MM, Ogbuabo CM, Thomas V, Forhan SE, Gokatweng G, Dintwa E, et al. Dolutegravir Use at Conception - Additional Surveillance Data from Botswana. N Engl J Med. 2019; 381: 885–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira GFM, Kim A, Jalil EM, Fernandes Fonseca F, Shepherd BE, Veloso VG, et al. Dolutegravir and pregnancy outcomes in women on antiretroviral therapy in Brazil: a retrospective national cohort study. Lancet HIV. 2021; 8: e33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral Pregnancy Registry Interim Report for 1 January 1989 through 31 July 2022. [Internet]. Wilmington, NC: Registry Coordinating Center; 2022. Available from: www.APRegistry.com [Google Scholar]
- 36.Money D, Lee T, O’Brien C, Brophy J, Bitnun A, Kakkar F, et al. Congenital anomalies following antenatal exposure to dolutegravir: a Canadian surveillance study. BJOG Int J Obstet Gynaecol. 2019;126:1338–45. [DOI] [PubMed] [Google Scholar]
- 37.Phillips AN, Venter F, Havlir D, Pozniak A, Kuritzkes D, Wensing A, et al. Risks and benefits of dolutegravir-based antiretroviral drug regimens in sub-Saharan Africa: a modelling study. Lancet HIV. 2019;6:e116–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dugdale CM, Ciaranello AL, Bekker LG, Stern ME, Myer L, Wood R, et al. Risks and Benefits of Dolutegravir- and Efavirenz-Based Strategies for South African Women With HIV of Child-Bearing Potential: A Modeling Study. Ann Intern Med. 2019; 170: 614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanislaus DJ, Posobiec LM, Laffan SB, Solomon HM, Ziejewski MK, Romach EH. Absence of developmental and reproductive toxicity in animals exposed to dolutegravir. Birth Defects Res. 2020; 112: 245–61. [DOI] [PubMed] [Google Scholar]
- 40.Mohan H, Lenis MG, Laurette EY, Tejada O, Sanghvi T, Leung KY, et al. Dolutegravir in pregnant mice is associated with increased rates of fetal defects at therapeutic but not at supratherapeutic levels. EBioMedicine. 2021; 63: 103167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bade AN, McMillan JM, Liu Y, Edagwa BJ, Gendelman HE. Dolutegravir Inhibition of Matrix Metalloproteinases Affects Mouse Neurodevelopment. Mol Neurobiol. 2021; 58: 5703–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Posobiec LM, Chapman SP, Murzyn SF, Rendemonti JE, Stanislaus DJ, Romach EH. No developmental toxicity observed with dolutegravir in rat whole embryo culture. Birth Defects Res. 2021; 113: 1190–7. [DOI] [PubMed] [Google Scholar]
- 43.Copp AJ, Greene NDE, Jao J, Zash R, Mohan H, Dontsova V, et al. Dolutegravir and rat whole embryo culture. Birth Defects Res. 2022; 114: 23–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirkwood-Johnson L, Katayama N, Marikawa Y. Dolutegravir Impairs Stem Cell-Based 3D Morphogenesis Models in a Manner Dependent on Dose and Timing of Exposure: An Implication for Its Developmental Toxicity. Toxicol Sci Off J Soc Toxicol. 2021; 184: 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith MSR, Mohan H, Ajaykumar A, Hsieh AYY, Martineau L, Patel R, et al. Second-Generation Human Immunodeficiency Virus Integrase Inhibitors Induce Differentiation Dysregulation and Exert Toxic Effects in Human Embryonic Stem Cell and Mouse Models. J Infect Dis. 2022; 226: 1992–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.King JC. Maternal obesity, metabolism, and pregnancy outcomes. Annu Rev Nutr. 2006; 26: 271–91. [DOI] [PubMed] [Google Scholar]
- 47.Stothard KJ, Tennant PWG, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009; 301: 636–50. [DOI] [PubMed] [Google Scholar]
- 48.Chandiwana NC, Chersich M, Venter WDF, Akpomiemie G, Hill A, Simmons B, et al. Unexpected interactions between dolutegravir and folate: randomized trial evidence from South Africa. AIDS. 2021; 35: 205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cabrera RM, Souder JP, Steele JW, Yeo L, Tukeman G, Gorelick DA, et al. The antagonism of folate receptor by dolutegravir: developmental toxicity reduction by supplemental folic acid. AIDS. 2019; 33: 1967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zamek-Gliszczynski MJ, Zhang X, Mudunuru J, Du Y, Chen JL, Taskar KS, et al. Clinical Extrapolation of the Effects of Dolutegravir and Other HIV Integrase Inhibitors on Folate Transport Pathways. Drug Metab Dispos Biol Fate Chem. 2019; 47: 890–8. [DOI] [PubMed] [Google Scholar]
- 51.Gilmore JC, Hoque MT, Dai W, Mohan H, Dunk C, Serghides L, et al. Interaction between dolutegravir and folate transporters and receptor in human and rodent placenta. EBioMedicine. 2022; 75: 103771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamooya BM, Mulenga LB, Masenga SK, Fwemba I, Chirwa L, Siwingwa M, et al. Metabolic syndrome in Zambian adults with human immunodeficiency virus on antiretroviral therapy: Prevalence and associated factors. Medicine (Baltimore). 2021; 100: e25236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCann K, Shah S, Hindley L, Hill A, Qavi A, Simmons B, et al. Implications of weight gain with newer anti-retrovirals: 10-year predictions of cardiovascular disease and diabetes. AIDS. 2021; 35: 1657–65. [DOI] [PubMed] [Google Scholar]
- 54.Dontsova V, Mohan H, Dunk CE, Jao J, Greene NDE, Copp AJ, et al. Effect of Dolutegravir on Glucose Homeostasis in Female Mice. In 2022. Available from: https://seatoskymeeting.eventsair.com/QuickEventWebsitePortal/31st-annual-canadian-conference-on-hivaids-research-cahr-2022/cahr-2022-virtual-agenda/Agenda/AgendaItemDetail?id=4275e976-13d1-227f-8f54-39fefc85e70d
- 55.Griesel R, Kawuma AN, Wasmann R, Sokhela S, Akpomiemie G, Venter WDF, et al. Concentration-response relationships of dolutegravir and efavirenz with weight change after starting antiretroviral therapy. Br J Clin Pharmacol. 2022; 88: 883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Esber AL, Chang D, Iroezindu M, Bahemana E, Kibuuka H, Owuoth J, et al. Weight gain during the dolutegravir transition in the African Cohort Study. J Int AIDS Soc. 2022; 25: e25899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Norwood J, Turner M, Bofill C, Rebeiro P, Shepherd B, Bebawy S, et al. Brief Report: Weight Gain in Persons With HIV Switched From Efavirenz-Based to Integrase Strand Transfer Inhibitor-Based Regimens. J Acquir Immune Defic Syndr. 2017; 76: 527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bourgi K, Jenkins CA, Rebeiro PF, Palella F, Moore RD, Altoff KN, et al. Weight gain among treatment-naïve persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J Int AIDS Soc. 2020; 23: e25484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bourgi K, Rebeiro PF, Turner M, Castilho JL, Hulgan T, Raffanti SP, et al. Greater Weight Gain in Treatment-naive Persons Starting Dolutegravir-based Antiretroviral Therapy. Clin Infect Dis. 2020; 70: 1267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calza L, Colangeli V, Borderi M, Bon I, Borioni A, Volpato F, et al. Weight gain in antiretroviral therapy-naive HIV-1-infected patients starting a regimen including an integrase strand transfer inhibitor or darunavir/ritonavir. Infection. 2020; 48: 213–21. [DOI] [PubMed] [Google Scholar]
- 61.Ibrahim F, Samarawickrama A, Hamzah L, Vincent R, Gilleece Y, Waters L, et al. Bone mineral density, kidney function, weight gain and insulin resistance in women who switch from TDF/FTC/NNRTI to ABC/3TC/DTG. HIV Med. 2021; 22: 83–91. [DOI] [PubMed] [Google Scholar]
- 62.Ruderman SA, Crane HM, Nance RM, Whitney BM, Harding BN, Mayer KH, et al. Brief Report: Weight Gain Following ART Initiation in ART-Naïve People Living With HIV in the Current Treatment Era. J Acquir Immune Defic Syndr. 2021; 86: 339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ando N, Nishijima T, Mizushima D, Inaba Y, Kawasaki Y, Kikuchi Y, et al. Long-term weight gain after initiating combination antiretroviral therapy in treatment-naïve Asian people living with human immunodeficiency virus. Int J Infect Dis. 2021; 110: 21–8. [DOI] [PubMed] [Google Scholar]
- 64.Taramasso L, Bonfanti P, Ricci E, Orofino G, Squillace N, Menzaghi B, et al. Factors Associated With Weight Gain in People Treated With Dolutegravir. Open Forum Infect Dis. 2020; 7: ofaa195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taramasso L, Ricci E, Menzaghi B, Orofino G, Passerini S, Madeddu G, et al. Weight Gain: A Possible Side Effect of All Antiretrovirals. Open Forum Infect Dis. 2017; 4: ofx239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lake JE, Wu K, Bares SH, Debroy P, Godfrey C, Koethe JR, et al. Risk Factors for Weight Gain Following Switch to Integrase Inhibitor-Based Antiretroviral Therapy. Clin Infect Dis. 2020; 71: e471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rizzardo S, Lanzafame M, Lattuada E, Luise D, Vincenzi M, Tacconelli E, et al. Dolutegravir monotherapy and body weight gain in antiretroviral naïve patients. AIDS. 2019; 33: 1673–4. [DOI] [PubMed] [Google Scholar]
- 68.Goldberg RN, Kania AT, Michienzi SM, Patel M, Badowski ME. Weight Gain in Incarcerated Individuals Living With HIV After Switching to Integrase Strand Inhibitor-Based Therapy. J Int Assoc Provid AIDS Care. 2021; 20: 2325958221996860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Calza L, Borderi M, Colangeli V, Miani T, Nuti B, Bon I, et al. Weight gain in treatment-naive HIV-1 infected patients starting abacavir/lamivudine/dolutegravir or tenofovir alafenamide/emtricitabine/bictegravir. AIDS. 2022; 36: 153–5. [DOI] [PubMed] [Google Scholar]
- 70.Hoffman RM, Ziemba L, Chinula L, João E, Stringer JSA, Fairlie L, et al. Antepartum weight gain and adverse pregnancy outcomes: a mediation analysis. In 2023. Available from: https://www.croiconference.org/wpcontent/uploads/sites/2/posters/2023/Weight_mediation_Analysis_Poster_12Feb23_Final-133209583712785646.pdf
- 71.Calza L, Colangeli V, Borderi M, Coladonato S, Tazza B, Bon I, et al. Improvement in insulin sensitivity and serum leptin concentration after the switch from a ritonavir-boosted PI to raltegravir or dolutegravir in non-diabetic HIV-infected patients. J Antimicrob Chemother. 2019; 74: 731–8. [DOI] [PubMed] [Google Scholar]
- 72.Ursenbach A, Max V, Maurel M, Bani-Sadr F, Gagneux-Brunon A, Garraffo R, et al. Incidence of diabetes in HIV-infected patients treated with first-line integrase strand transfer inhibitors: a French multicentre retrospective study. J Antimicrob Chemother. 2020; 75: 3344–8. [DOI] [PubMed] [Google Scholar]
- 73.Hsu R, Brunet L, Fusco JS, Mounzer K, Vannappagari V, Henegar CE, et al. Incident type 2 diabetes mellitus after initiation of common HIV antiretroviral drugs. AIDS. 2021; 35: 81–90. [DOI] [PubMed] [Google Scholar]
- 74.Zakumumpa H, Kiguba R, Ndagije HB, Ategeka G, Ssanyu JN, Kitutu FE. Patient experiences of sexual dysfunction after transition to dolutegravir-based HIV treatment in mid-Western Uganda: a qualitative study. BMC Infect Dis. 2022; 22: 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McLaughlin M, Walsh S, Galvin S. Dolutegravir-induced hyperglycaemia in a patient living with HIV. J Antimicrob Chemother. 2018; 73: 258–60. [DOI] [PubMed] [Google Scholar]
- 76.Hailu W, Tesfaye T, Tadesse A. Hyperglycemia After Dolutegravir-Based Antiretroviral Therapy. Int Med Case Rep J. 2021; 14: 503–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hirigo AT, Gutema S, Eifa A, Ketema W. Experience of dolutegravir-based antiretroviral treatment and risks of diabetes mellitus. SAGE Open Med Case Rep. 2022; 10: 2050313X221079444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fong PS, Flynn DM, Evans CD, Korthuis PT. Integrase strand transfer inhibitor-associated diabetes mellitus: A case report. Int J STD AIDS. 2017; 28: 626–8. [DOI] [PubMed] [Google Scholar]
- 79.Namulindwa A, Wasswa JH, Muyindike W, Tamukong R, Oloro J. Prevalence and factors associated with adverse drug events among patients on dolutegravir-based regimen at the Immune Suppression Syndrome Clinic of Mbarara Regional Referral Hospital, Uganda: a mixed design study. AIDS Res Ther. 2022; 19: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Lunzen J, Maggiolo F, Arribas JR, Rakhmanova A, Yeni P, Young B, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis. 2012; 12: 111–8. [DOI] [PubMed] [Google Scholar]
- 81.Cahn P, Pozniak AL, Mingrone H, Shuldyakov A, Brites C, Andrade-Villanueva JF, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet. 2013; 382: 700–8. [DOI] [PubMed] [Google Scholar]
- 82.Walmsley SL, Antela A, Clumeck N, Duiculescu D, Eberhard A, Gutiérrez F, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013; 369: 1807–18. [DOI] [PubMed] [Google Scholar]
- 83.Raffi F, Jaeger H, Quiros-Roldan E, Albrecht H, Belonosova E, Gatell JM, et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2013; 13: 927–35. [DOI] [PubMed] [Google Scholar]
- 84.Clotet B, Feinberg J, van Lunzen J, Khuong-Josses MA, Antinori A, Dumitru I, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet. 2014; 383: 2222–31. [DOI] [PubMed] [Google Scholar]
- 85.Chinula L, Goldberg E, McCathy K, Krotje C, Knowles K, Mathad J, et al. Pregnancy Hemoglobin A1c and glucose with DTG vs EFV, TDF vs TAF: IMPAACT 2010. In 2022. Available from: https://www.croiconference.org/abstract/pregnancy-hemoglobin-a1c-and-glucose-with-dtg-vs-efv-tdf-vs-taf-impaact-2010/
- 86.Al Mamun Bhuyan A, Signoretto E, Bissinger R, Lang F. Enhanced Eryptosis Following Exposure to Dolutegravir. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2016; 39: 639–50. [DOI] [PubMed] [Google Scholar]
- 87.Gorwood J, Bourgeois C, Pourcher V, Pourcher G, Charlotte F, Mantecon M, et al. The Integrase Inhibitors Dolutegravir and Raltegravir Exert Proadipogenic and Profibrotic Effects and Induce Insulin Resistance in Human/Simian Adipose Tissue and Human Adipocytes. Clin Infect Dis. 2020; 71: e549–60. [DOI] [PubMed] [Google Scholar]
- 88.George JW, Mattingly JE, Roland NJ, Small CM, Lamberty BG, Fox HS, et al. Physiologically Relevant Concentrations of Dolutegravir, Emtricitabine, and Efavirenz Induce Distinct Metabolic Alterations in HeLa Epithelial and BV2 Microglial Cells. Front Immunol. 2021; 12: 639378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jung I, Tu-Sekine B, Jin S, Anokye-Danso F, Ahima RS, Brown TT, et al. Dolutegravir Suppresses Thermogenesis via Disrupting UCP1 Expression and Mitochondrial Function in Brown/Beige Adipocytes in preclinical models. J Infect Dis. 2022; jiac175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ngono Ayissi K, Gorwood J, Le Pelletier L, Bourgeois C, Beaupère C, Auclair M, et al. Inhibition of Adipose Tissue Beiging by HIV Integrase Inhibitors, Dolutegravir and Bictegravir, Is Associated with Adipocyte Hypertrophy, Hypoxia, Elevated Fibrosis, and Insulin Resistance in Simian Adipose Tissue and Human Adipocytes. Cells. 2022; 11: 1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McMahon C, Trevaskis JL, Carter C, Holsapple K, White K, Das M, et al. Lack of an association between clinical INSTI-related body weight gain and direct interference with MC4 receptor (MC4R), a key central regulator of body weight. PloS One. 2020; 15: e0229617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu H, Zhang H, Fang Y, Yang H, Chen Y, Zhang C, et al. Activation of the Melanocortin-4 receptor signaling by α-MSH stimulates nerve-dependent mouse digit regeneration. Cell Regen. 2021; 10: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pickering R, Asundi A, Lin N. In vitro model to assess antiretroviral therapy on adipocyte biology [CROI Abstract 514]. vCROI 2021 Abstr. Eb. 2021; : 196. [Google Scholar]
- 94.Pérez-Pérez A, Toro A, Vilariño-García T, Maymó J, Guadix P, Dueñas JL, et al. Leptin action in normal and pathological pregnancies. J Cell Mol Med. 2018; 22: 716–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sagawa N, Yura S, Itoh H, Kakui K, Takemura M, Nuamah MA, et al. Possible role of placental leptin in pregnancy: a review. Endocrine. 2002; 19: 65–71. [DOI] [PubMed] [Google Scholar]
- 96.Wells PG, McCallum GP, Chen CS, Henderson JT, Lee CJJ, Perstin J, et al. Oxidative stress in developmental origins of disease: teratogenesis, neurodevelopmental deficits, and cancer. Toxicol Sci. 2009; 108 :4–18. [DOI] [PubMed] [Google Scholar]
- 97.Barreca ML, Iraci N, De Luca L, Chimirri A. Induced-fit docking approach provides insight into the binding mode and mechanism of action of HIV-1 integrase inhibitors. ChemMedChem. 2009; 4: 1446–56. [DOI] [PubMed] [Google Scholar]
- 98.Majali-Martinez A, Hiden U, Ghaffari-Tabrizi-Wizsy N, Lang U, Desoye G, Dieber-Rotheneder M. Placental membrane-type metalloproteinases (MT-MMPs): Key players in pregnancy. Cell Adhes Migr. 2016; 10: 136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Poston L, Raijmakers MTM. Trophoblast oxidative stress, antioxidants and pregnancy outcome--a review. Placenta. 2004; 25 Suppl A: S72–78. [DOI] [PubMed] [Google Scholar]
- 100.Chouchana L, Beeker N, Treluyer JM. Is There a Safety Signal for Dolutegravir and Integrase Inhibitors During Pregnancy? J Acquir Immune Defic Syndr. 2019; 81: 481–6. [DOI] [PubMed] [Google Scholar]
- 101.Grayhack C, Sheth A, Kirby O, Davis J, Sibliss K, Nkwihoreze H, et al. Evaluating outcomes of mother-infant pairs using dolutegravir for HIV treatment during pregnancy. AIDS. 2018; 32: 2017–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bornhede R, Soeria-Atmadja S, Westling K, Pettersson K, Navér L. Dolutegravir in pregnancy-effects on HIV-positive women and their infants. Eur J Clin Microbiol Infect Dis. 2018; 37: 495–500. [DOI] [PubMed] [Google Scholar]
- 103.Kala S, Watson B, Zhang JG, Papp E, Guzman Lenis M, Dennehy M, et al. Improving the clinical relevance of a mouse pregnancy model of antiretroviral toxicity; a pharmacokinetic dosing-optimization study of current HIV antiretroviral regimens. Antiviral Res. 2018; 159: 45–54. [DOI] [PubMed] [Google Scholar]


