Abstract
After a few minutes of germination, nucleoids in the great majority of spores of Bacillus subtilis and Bacillus megaterium were ring shaped. The major spore DNA binding proteins, the α/β-type small, acid-soluble proteins (SASP), colocalized to these nucleoid rings early in spore germination, as did the B. megaterium homolog of the major B. subtilis chromosomal protein HBsu. The percentage of ring-shaped nucleoids was decreased in germinated spores with lower levels of α/β-type SASP. As spore outgrowth proceeded, the ring-shaped nucleoids disappeared and the nucleoid became more compact. This change took place after degradation of most of the spores' pool of major α/β-type SASP and was delayed when α/β-type SASP degradation was delayed. Later in spore outgrowth, the shape of the nucleoid reverted to the diffuse lobular shape seen in growing cells.
During the process of sporulation in Bacillus subtilis the sporulating cell is divided into two unequally sized compartments, the larger mother cell and the smaller forespore. Each compartment of the sporulating cell ultimately contains a complete chromosome, but the patterns of transcription from the genomes in these two compartments are quite different (8). The gross structures of the nucleoids in the two compartments are also very different. After formation of the septum separating the mother cell and forespore, the forespore nucleoid appears extremely compact, while the nucleoid in the mother cell retains the diffuse lobular appearance of the nucleoid in growing cells (19). The cause of the forespore nucleoid condensation is not clear but may be in part a reflection of the small size of the forespore compartment. Several hours later, the forespore nucleoid decondenses slightly and takes on the appearance of a ring-shaped or doughnut-like structure. This change is due to the binding of forespore DNA by a group of small, acid-soluble proteins (SASP) of the α/β type, which have been localized on the ring-shaped nucleoid (14). These proteins are the products of a multigene family that is expressed only in the forespore just prior to the conversion of the forespore nucleoid to a ring-shaped structure. There are two major α/β-type SASP in B. subtilis, termed SASP-α and -β, as well as two minor proteins of this type (21, 22). Deletion of the genes encoding SASP-α and -β (termed sspA and -B, respectively) results in spores (termed α− β−) lacking ∼85% of total α/β-type SASP. The α− β− spores are much more sensitive than are wild-type spores to a variety of treatments, including heat and UV radiation, and extensive work has shown that α/β-type SASP saturate the spore chromosome and protect spore DNA from many types of damage (21, 22). During sporulation of the α− β− strain, the forespore nucleoid condenses normally but does not assume the ring-shaped structure seen in wild-type forespores (14). These findings indicate that α/β-type SASP are essential for formation of the ring-shaped nucleoid structure. However, the precise function of this nucleoid structure is not clear, since sporulation of α− β− strains appears relatively normal, although not completely so (21, 23; B. Setlow, K. A. McGinnis, and P. Setlow, unpublished data). In addition to α/β-type SASP, the major chromosomal protein in growing B. subtilis cells, HBsu, has also been localized to the ring-shaped forespore nucleoid (17).
Although the structure of the forespore nucleoid has been studied to some degree, much less is known about the structure of the nucleoid early in spore germination. One study presented a compelling electron micrograph of a germinating spore, suggesting that the nucleoid is ring shaped in this stage of development as well (15, 16). However, this structure has not been studied in detail and there has not been any assessment of the contribution of α/β-type SASP to the nucleoid structure in the germinated spore. Their contribution is of special interest since α/β-type SASP are degraded early in spore germination, and thus the nucleoid structure should revert to that found in growing cells. In addition, analysis of the nucleoid structure early in spore germination and comparison with that in the forespore may give further insight into the nucleoid structure in the dormant spore, since it has been impossible to directly assess the structure of the nucleoid in the dormant spore, which is relatively impermeable to the fixatives and stains used in microscopy. In this work we report the analysis of the structures of the nucleoids of germinating spores, with or without various α/β-type SASP, and analyze the locations of α/β-type SASP and the Bacillus megaterium homolog of HBsu in the germinating spore. These analyses were carried out using both B. subtilis, for which strains lacking gpr as well as strains containing and lacking a variety of α/β-type SASP genes are available, and B. megaterium, for which a gpr mutant is available (9, 10, 18, 24). The major reason for the use of B. megaterium was that the larger size of its spores relative to those of B. subtilis greatly simplifies microscopy and, in particular, localization of proteins by immunofluorescence microscopy.
MATERIALS AND METHODS
Bacterial strains used and preparation of spores.
The bacterial strains used in this work are listed in Table 1. B. megaterium strains are derivatives of strain QMB1551; B. subtilis strains are derivatives of strain PS832. B. megaterium strains were sporulated in supplemented nutrient broth (5) at 30°C, and B. subtilis strains were sporulated in 2× SG medium (12) at 37°C. Spores were purified and stored as described previously (5, 12). Antibiotics were added to media at the following concentrations: 3 μg/ml for chloramphenicol and 10 μg/ml for kanamycin.
TABLE 1.
Bacterial strains
| Strain | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| B. megaterium | ||
| QMB1551 | Wild type | Hillel Levinson |
| PS1551 | gpr::cam Cmr GPR− | 18 |
| B. subtilis | ||
| PS223 | ΔsspB::cam β− Cmr | 10 |
| PS260 | ΔsspA::cam α− Cmr | 10 |
| PS578 | ΔsspA ΔsspB [pUB110] α− β− Kmr | 9 |
| PS832 | Trp+ revertant of wild-type strain 168 | Laboratory stock |
| PS1029 | gpr::cam Cmr GPR− | 18 |
| PS1450 | ΔsspA ΔsspB [pSspCwt] α− β− SspCwt Cmr Kmr | 24 |
| PS1465 | ΔsspA ΔsspB [pSspCwt] α− β− SspCAla Cmr Kmr | 24 |
Cm, chloramphenicol; Km, kanamycin.
Spore germination and SASP extraction and analysis.
Spores (1 to 5 mg [dry weight]/ml) in water were heat shocked for 15 min at 60°C (B. megaterium) or 30 min at 70°C (B. subtilis). After being cooled in ice, spores were germinated at 0.13 mg [dry weight]/ml at either 30°C (B. megaterium) or 37°C (B. subtilis) in Tris-Spizizen's minimal medium (3) supplemented with 10 mM l-alanine. In one experiment this medium was supplemented with either 10 mM KCN or 50 μg of chloramphenicol per ml.
For measurements of SASP levels, samples (65 ml) were harvested by centrifugation at various times during spore germination and outgrowth and the pellet was lyophilized. The dry spores were disrupted in a dental amalgamator (Wig-L-Bug) with glass beads (100 mg) as the abrasive, SASP were extracted with cold 3% acetic acid, and the supernatant fluid was dialyzed and lyophilized as described previously (12). The dry residue was dissolved in a small volume of 8 M urea and subjected to polyacrylamide gel electrophoresis at low pH, and the gel was stained with Coomassie blue as described previously (12).
Fixation, staining, and microscopy of germinated and outgrowing spores.
For fixation and DNA staining, samples (250 μl) of germinating spores were collected at various times and mixed with 250 μl of fixative solution (3% [wt/vol] paraformaldehyde and 0.4% [vol/vol] glutaraldehyde in HEPES-buffered saline [pH 7.05] [per liter, 16 g of NaCl, 0.74 g of KCl, 0.27 g of Na2 HPO4 · 2H2O, 2 g of dextrose, 10 g of HEPES]). After 15 min at room temperature, fixation was continued on ice for 50 min. The samples were then washed twice by centrifugation with phosphate-buffered saline (PBS) (10 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4 [pH 7.4]) and resuspended in 100 μl of GTE (5 mM glucose, 25 mM Tris-HCl, 10 mM EDTA [pH 8.0]). Aliquots (10 μl) of this suspension were mixed with 10 μl of 2-μg/ml 4′,6′-diamino-2-phenylindole (DAPI) and held at room temperature for 15 min before being loaded on poly-l-lysine-coated multiwell slides (ICN Biomedicals). Slides were washed twice with PBS, mounted using a Slow Fade Anti Fade kit (Molecular Probes), and stored at 4°C.
For fixation and immunostaining of SASP and the HBsu homolog in B. megaterium, samples of germinating spores were collected and fixed as described previously (6, 14, 17) but freshly prepared lysozyme (3 mg/ml) in GTE was added and incubation was for 15 min at room temperature before cells were distributed in the wells of multiwell slides. Some of these cells were stained with guinea pig antiserum against HBsu and rabbit antiserum against α/β-type SASP, the guinea pig immunoglobulin G (IgG) was detected with fluorescein isothiocyanate (FITC)-labeled secondary antibody, the rabbit IgG was detected with biotinylated goat anti-rabbit IgG and then with indocyanine (Cy3)-conjugated streptavidin, and the cells were mounted as described previously (18). Other cells were stained with 10 μl of 2-μg/ml DAPI or 2.4 nM quinolinium, 1,1′-[1-3-propanediylbis [(dimethylimino) - 3,1 - propanediyl] ]bis [ 4 - [ ( 3 - methyl - 2 (3H) - benzoxazolylidene)methyl]]-tetraiodide (YOYO) (Molecular Probes, Eugene, Oreg.), to visualize DNA. After 15 min at room temperature, slides were washed twice with PBS and mounted as described above.
Slides were viewed on a Zeiss (Thornwood, N.Y.) Axiovert 100 microscope using either a 63×, 1.4 numerical aperture oil immersion Plan apochromat lens or a 63×, 1.25 numerical aperture oil immersion Plan neofluar lens and a 1.6× optivar lens and appropriate filter blocks. Images were collected using a Roper Scientific (Tuscon, Ariz.) PXL-cooled charge-coupled device camera and processed using Adobe Photoshop version 5.0. Measurements of spore, nucleoid, or SASP ring dimensions were by pixel counting using SCION software downloaded from the National Institutes of Health. Confocal microscopy was done using a Zeiss LSM410 confocal microscope.
RESULTS
Appearance of nucleoids in germinated spores.
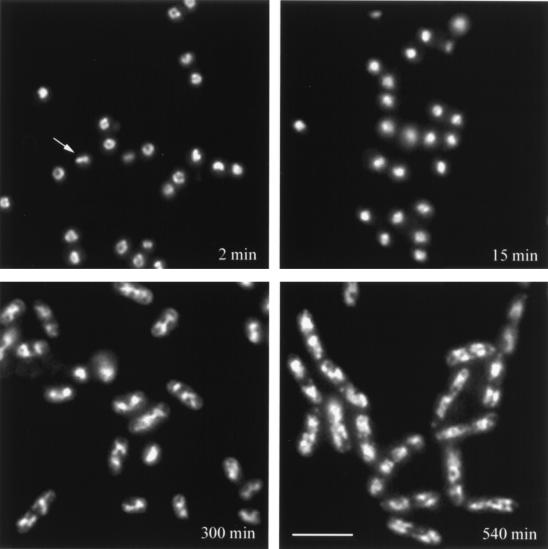
Examination of spores of either B. megaterium or B. subtilis in the first minute of germination after staining with DAPI revealed that the majority of nucleoids in the germinated spores were ring shaped (Fig. 1; Table 2; and data not shown). While all the spores had not yet initiated germination by 2 min, only the nucleoids of germinated spores were stained by DAPI. Analysis of DAPI-stained germinated spores by confocal fluorescence microscopy further revealed that the nucleoid is indeed ring shaped and not a hollow sphere (data not shown). It was somewhat surprising that we observed such a high percentage of ring-shaped nucleoids in germinated spores, as depending on the precise orientation of the nucleoid in the microscope field, not only rings but also sharp lines of DAPI staining should be observed. While some of the latter structures were seen (Fig. 1), the percentage was very low. Possibly the spores, which are not perfect spheres, affix to microscope slides in nonrandom orientations, which increases the percentage of spores which show ring-shaped nucleoids in a microscope field. Analysis of the nucleoid rings in germinated spores indicated that these were generally spherical (Fig. 1) and that the average diameters of the nucleoids were similar in both B. megaterium and B. subtilis spores, even though the germinated B. megaterium spores themselves had almost twice the diameter of the germinated B. subtilis spores (Table 3). Note that the treatment conditions of the samples in Fig. 1 and the upper half of Table 3 are the same but different from those used in the lower half of Table 3.
FIG. 1.
Analysis of nucleoid shape during germination and outgrowth of wild-type spores. Wild-type spores of B. megaterium were germinated; samples were taken at the times indicated, fixed, and stained with DAPI; and nucleoids were visualized by fluorescence microscopy as described in Materials and Methods. The arrow points to a bar-shaped nucleoid. The scale bar is 5 μm.
TABLE 2.
Percentages of germinated spores with ring-shaped nucleoidsa
| Strainb | Germinated spores with ring-shaped nucleoids (%) |
|---|---|
| B. megateriumQMB1551 (wt) | 86 |
| B. megaterium PS1551 (gpr) | 65 |
| B. subtilis PS832 (wt) | 72 |
| B. subtilis PS1029 (gpr) | 85 |
| B. subtilis PS260 (α−) | 22 |
| B. subtilis PS223 (β−) | 14 |
| B. subtilis PS578 (α− β−) | 3 |
| B. subtilis PS1450 (α− β− pSspCwt) | 58 |
| B. subtilis PS1465 (α− β− pSspCAla) | 22 |
Spores of various strains were germinated for 2 min, fixed, and stained with DAPI as described in Materials and Methods. The presence of ring-shaped nucleoids was scored in random fields of a fluorescence microscope; 300 spores containing nucleoids stained with DAPI were counted for each strain.
wt, wild type.
TABLE 3.
Dimensions of germinated spores, their nucleoids, and α/β-type SASP rings
| Treatment | Spores, nucleoids, or rings | Diameter (μm)a in:
|
|
|---|---|---|---|
| B. subtilis | B. megaterium | ||
| Stained without lysozyme treatment | Sporesb | 1.3 ± 0.3 | 2.1 ± 0.4 |
| Ring-shaped nucleoidsb | 1.3 ± 0.1 | 1.2 ± 0.1 | |
| Condensed nucleoids, wt sporesc | 0.8 ± 0.1 | 0.8 ± 0.1 | |
| Condensed nucleoids, α− β− sporesd | 0.8 ± 0.1 | Not tested | |
| Stained after lysozyme treatment | Ring shaped nucleoids, DAPI stainede | 1.5 ± 0.2 | 1.6 ± 0.2 |
| Ring shaped nucleoids, YOYO stainede | Not done | 1.4 ± 0.1 | |
| α/β-type SASP ringse | 1.4 ± 0.2 | 1.5 ± 0.2 | |
| HBsu homolog rings | Not done | 1.5 ± 0.2 | |
Values are average diameters ± maximum variations.
Wild-type spores were germinated for 5 min, fixed, DAPI stained, and examined by fluorescence microscopy or under bright-field microscopy. Values given are from ≥40 spores with ring-shaped nucleoids that appeared to be parallel to the plane of focus.
Wild-type (wt) spores were germinated for 20 min and treated as described in footnote b above, and the sizes of non-ring-shaped nucleoids were determined. Values given are from measurements of ∼60 spores.
Spores of strain PS578 (α− β−) were germinated for 5 min, fixed, stained, and analyzed as described in footnote b above. Values given are from measurements of ≥40 spores which did not contain ring-shaped nucleoids.
Wild-type spores were germinated for 5 min, fixed, lysozyme treated, and then stained for DNA with DAPI or YOYO or treated with antiserum against α/β-type SASP or HBsu, and the primary antibody was detected as described in Materials and Methods. The values given are from measurements of ∼60 spores with DNA or α/β-type SASP rings and of 30 spores with HBsu homolog rings.
Previous work has shown that formation of the ring-shaped nucleoid in the developing forespore is dependent on the synthesis of α/β-type SASP (14), so it was of obvious interest to analyze the nucleoid structure in germinated spores of B. subtilis strains with various α/β-type SASP contents. As expected, the frequency of ring-shaped nucleoids was greatly reduced in germinated α− β− spores, which lack the two major α/β-type SASP, although a few such ring-shaped nucleoids were observed (Table 2). Germinated spores lacking either SASP-α or SASP-β (α− and β− spores, respectively) exhibited an intermediate level of ring-shaped nucleoids, while germinated α− β− spores with a plasmid overexpressing a normally minor α/β-type SASP (termed SspCwt) to the level of SASP-α plus -β in wild-type spores (21, 22, 24) had levels of ring-shaped nucleoids close to that in wild-type spores. However, when the α/β-type SASP overexpressed in α− β− spores to levels similar to those of SASP-α plus -β in wild-type spores was SspCAla, a variant of SspCwt that binds both poorly and relatively nonproductively to DNA (24; C. S. Hayes and P. Setlow, unpublished data), the level of ring-shaped nucleoids in germinated spores was only about one-third of that in wild-type spores (Table 2), although this value was significantly higher than that in α− β− spores.
Changes in nucleoid structure during spore germination and outgrowth.
Analysis of the nucleoid appearance throughout spore germination and outgrowth revealed that with both B. megaterium and B. subtilis the nucleoid rings disappeared relatively rapidly, with very few ring-shaped nucleoids remaining 20 to 60 min after the initiation of spore germination (Fig. 1 and 2). For these two species, >95% of the spores had initiated spore germination after ∼30 min (data not shown). When the ring-shaped nucleoids present in the first minute of germination disappeared, the nucleoids became slightly smaller and more condensed (Fig. 1; Table 3). The size and appearance of these more condensed nucleoids were essentially identical to those of the nucleoids in the great majority of α− β− spores immediately after the initiation of spore germination (Table 3 and data not shown). There should of course be intermediates in the conversion of the ring-shaped nucleoids to the more compact forms, but we did not observe any such intermediates, possibly because the identification of such structures is beyond the resolution of our microscopy. The condensed nucleoids present in germinated spores were also similar to those in developing forespores prior to the synthesis of α/β-type SASP (19). Eventually as outgrowth of wild-type spores proceeded, the nucleoid shape changed once again to the more diffuse lobular appearance of the vegetative cell nucleoid (Fig. 1). In the medium used in this experiment, wild-type B. megaterium spores underwent their first cell division at ∼250 to ∼300 min, as determined by septal staining with wheat germ agglutinin coupled to Oregon Green (data not shown). Analyses using B. megaterium further showed that in spores germinating as described in Materials and Methods but with KCN to block ATP production or chloramphenicol to block protein synthesis, the nucleoid rings disappeared at the same rate as in spores germinating without these inhibitors (data not shown).
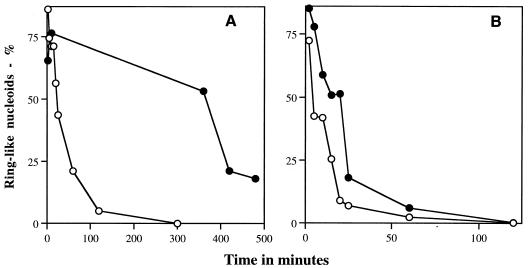
FIG. 2.
Percentage of ring-shaped nucleoids in germinating spores of B. megaterium (A) and B. subtilis (B). Spores were germinated, fixed, stained, and examined by microscopy for ring-shaped nucleoids as described in Materials and Methods. From 220 to 1,000 stained nucleoids were examined at each time point. The symbols used and the spores analyzed were as follows: in panel A, ○, B. megaterium QMB1551 (wild type), and ●, B. megaterium PS1551 (gpr); and in panel B, ○, B. subtilis PS832 (wild-type), and ●, B. subtilis PS1029 (gpr).
Analysis of levels of major α/β-type SASP showed that in B. megaterium and B. subtilis spores, these proteins were gone (≥95%) by 15 min and 40 min after the initiation of germination, respectively (data not shown), similar to what has been found previously (17). Thus, the nucleoid rings can persist for at least some time without high levels of major α/β-type SASP. When nucleoids were examined in germinated gpr spores which lack the major protease initiating SASP degradation during spore germination (18), the nucleoid rings persisted longer after the initiation of spore germination than in wild-type spores (Fig. 1 and 2A); this was particularly striking with the B. megaterium gpr spores. Note that B. megaterium gpr spores do not undergo their first cell division until >500 min after the start of spore germination (data not shown). Analysis of the levels of major α/β-type SASP in germinated gpr spores showed that these proteins persisted at significant levels at least 150 min after the initiation of germination of B. megaterium gpr spores but that they were largely, if not completely, gone from B. subtilis gpr spores after 60 to 80 min (reference 18 and data not shown). Presumably B. subtilis spores have higher levels than B. megaterium spores of proteases other than the gpr gene product that can degrade α/β-type SASP during spore germination.
Analysis of possible nucleoid-associated proteins in germinated spores.
Analyses using a number of techniques have shown that α/β-type SASP are associated with the nucleoid in developing forespores, dormant spores, and spores early in germination (4, 14, 18, 20). Thus, it was of obvious interest to examine the distribution of α/β-type SASP in germinated spores. Not surprisingly, immunostaining of α/β-type SASP early in spore germination gave ring-shaped structures with both B. megaterium and B. subtilis gpr and wild-type spores (Fig. 3 and data not shown). These ring-shaped structures were absent in α− β− B. subtilis spores early in germination (data not shown). Previous work has shown that the α/β-type SASP rings colocalize with DNA rings in the developing forespore (14). However, we were unable to effectively stain germinated spore DNA when the samples had been immunostained for α/β-type SASP, even when a number of fluorescent DNA stains were used. The reason for this failure is not clear; possibly the antibody reaction with α/β-type SASP alters the DNA sufficiently to preclude DNA staining. However, the size of the α/β-type SASP rings in germinated spores was identical to that of the nucleoid rings (Table 3), consistent with the colocalization of α/β-type SASP and DNA in the germinated spore, as occurs in the developing forespore and the dormant spore (4, 14, 20).
FIG. 3.
Localization of α/β-type SASP in germinated gpr spores by immunofluorescence microscopy. Spores of B. megaterium (PS1551 gpr) or B. subtilis (PS1029 gpr) were germinated for 10 min, fixed, treated, immunostained for α/β-type SASP, and visualized by fluorescence microscopy as described in Materials and Methods. The scale bar is 1 μm.
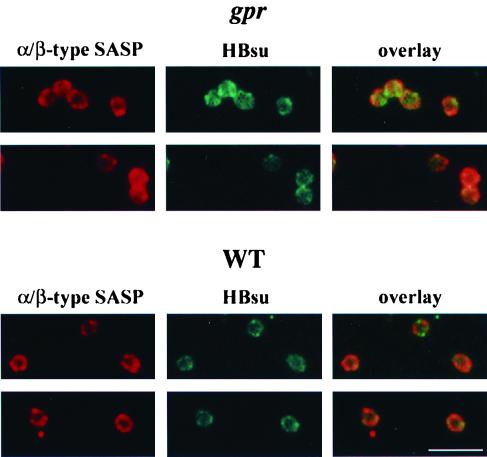
To provide further evidence for the colocalization of α/β-type SASP and DNA in germinated spores, we also examined the location of the HBsu homolog in germinated spores of B. megaterium. HBsu is the major protein on the nucleoid in vegetative cells of B. subtilis, where it covers ∼5% of the DNA (8, 11, 17). HBsu also colocalizes with α/β-type SASP in the developing forespore and is almost certainly on dormant spore DNA, where again it is present in sufficient amounts to cover ∼5% of the genome and likely modulates some of the effects of α/β-type SASP on DNA properties (17). Immunostaining of either wild-type or gpr germinated spores of B. megaterium for its HBsu homolog again revealed ring-shaped structures (Fig. 4). As expected, these rings largely colocalized with α/β-type SASP rings (Fig. 4) and the HBsu homolog rings were also the same size as the α/β-type SASP rings (Table 3), suggesting that α/β-type SASP and the HBsu homolog are together on the ring-shaped nucleoid early in the germination of wild-type B. megaterium spores.
FIG. 4.
Localization of α/β-type SASP and the HBsu homolog in germinated B. megaterium spores by immunofluorescence microscopy. Wild-type (WT) and gpr (PS1551) spores of B. megaterium were germinated for 2 and 10 min, respectively, fixed, treated, immunostained for both α/β-type SASP and HBsu, and examined by fluorescence microscopy as described in Materials and Methods. The panels labeled “α/β-type SASP” show the Cy3 images, the panels labeled “HBsu” show the FITC images, and the panels labeled “overlay” show the merged images from the Cy3 and FITC images. The scale bar is 5 μm.
DISCUSSION
The work reported in this communication allows a number of conclusions. First, the nucleoid has a ring-shaped structure early in spore germination. The nucleoid structure in the dormant spore has not been determined because of the impermeability of the dormant spore core to fixatives and stains. Consequently, the demonstration that germinating spores contain a ring-shaped nucleoid, as well as previous work indicating that developing forespores contain ring-shaped nucleoids (14), strongly suggests that dormant spores also contain ring-shaped nucleoids. Second, both α/β-type SASP and HBsu homologs are on the ring-shaped nucleoid of the germinating spore. Again, this has not been demonstrated for the dormant spore, whose core is refractory to immunolocalization techniques. Since α/β-type SASP and HBsu and its homologs are on the ring-shaped nucleoid in developing forespores (4, 14, 17), our new findings strongly suggest that both types of protein are also on the nucleoid in the dormant spore. Third, the size of the ring-shaped nucleoids is the same in germinated spores of both B. megaterium and B. subtilis. Given the significantly larger diameter of germinated B. megaterium spores, this observation indicates that the size of the ring-shaped nucleoid is not determined directly by the cell's diameter. However, the precise mechanisms determining the size as well as the shape of the ring-shaped nucleoid in germinated spores and developing forespores are not clear. Fourth, high levels of major α/β-type SASP are required for maximal levels of ring-shaped nucleoids, as was suggested previously based on analyses of ring-shaped nucleoids in developing forespores (14). However, we also observed a low level of ring-shaped nucleoids in germinated α− β− spores and ring-shaped nucleoids persisted in germinated wild-type spores well after the great majority of α/β-type SASP had been degraded. These observations suggest that high levels of major α/β-type SASP are not essential for the formation and maintenance of at least some ring-shaped nucleoids. There are a number of minor α/β-type SASP in spores (21, 22), and some of these bind more tightly to DNA than do the major α/β-type SASP (C. S. Hayes and P. Setlow, unpublished data). It is possible that in some α− β− cells there are enough of these minor α/β-type SASP to trigger formation of ring-shaped nucleoids in developing forespores, and presumably this process persists in germinated spores. Interestingly, a small percentage of Escherichia coli cells have also been reported to contain ring-shaped nucleoids under some conditions (13).
Some minor α/β-type SASP, especially ones that bind tightly to DNA, may also be degraded much more slowly by GPR during spore germination than the major α/β-type SASP (C. S. Hayes and P. Setlow, unpublished data), and again it may be these minor proteins which cause the retention of the ring-shaped nucleoid structure after the great majority of the major α/β-type SASP have been degraded. Alternatively, it may be that degradation of a small amount of major α/β-type SASP is extremely slow, perhaps because a fraction of these proteins is bound very tightly to particular regions of the chromosome; again, this small amount of residual α/β-type SASP may be sufficient to preserve the structure of the ring-shaped nucleoid. Any of these scenarios or even some combination of them would then explain the long lag between the degradation of most of the major α/β-type SASP during spore germination and the disappearance of ring-shaped nucleoids. This lag is seen most dramatically with spores of B. megaterium, as >50% of germinated spores retained ring-shaped nucleoids 25 min after the start of spore germination, when ≥95% of all α/β-type SASP are gone. However, α/β-type SASP degradation does appear to be essential for the ultimate loss of ring-shaped nucleoids during spore germination, as this loss was greatly slowed during germination of gpr spores but was not affected by inhibition of ATP production or protein synthesis. Thus, neither transcription nor translation is needed for loss of ring-shaped nucleoids during spore germination.
Previous work has shown that levels of total major α/β-type SASP are decreased somewhat in spores lacking the gene for either SASP-α or -β (10), and presumably this is the reason for the decrease in the percentages of germinated spores of α− and β− strains with ring-shaped nucleoids. Overproduction of SspCwt is sufficient to saturate the spore chromosome with this protein, and this largely reverses the phenotypic effects of the deletion of genes coding for SASP-α and -β (24). Since SspCwt binds to spore DNA and has the same effects on DNA properties in vivo as major α/β-type SASP (21, 22, 24), it is not surprising that germinated α− β− spores with pSspCwt have near wild-type levels of ring-shaped nucleoids. However, it was somewhat surprising that overexpression of SspCAla resulted in a significant amount of ring-shaped nucleoids in germinated α− β− spores. This SspC variant has an Ala-for-Gly substitution in a residue that is conserved in α/β-type SASP, and SspCAla does not restore UV and heat resistance to α− β− spores and does not cause the change in DNA structure and properties caused by binding of SspCwt or other wild-type α/β-type SASP (21, 22, 24). However, SspCAla does bind to DNA (C. S. Hayes and P. Setlow, unpublished data), and possibly this is sufficient to promote formation of some ring-shaped nucleoids.
While high levels of α/β-type SASP are clearly essential for maximal formation of ring-shaped nucleoids in spores, if not for their maintenance, it seems likely that other proteins may also be involved in the formation of these structures. Two such proteins are HBsu and its B. megaterium homolog, which are associated with the ring-shaped nucleoids in developing forespores (17) and germinated spores as shown in this work. Unfortunately, HBsu is an essential protein in B. subtilis (11), so its role in the formation of ring-shaped nucleoids in spores cannot be easily tested. Additional candidates for a role in formation of ring-shaped nucleoids in spores are minor SASP which are not related to α/β-type SASP (1). These proteins are present in spores at levels lower than those of α/β-type SASP but still at quite substantial levels. While these minor SASP have not been shown to be DNA binding proteins, many are basic proteins and at least two have some as yet unknown role in spore outgrowth (1, 2). Interestingly, loss of either of these two proteins slows outgrowth of wild-type but not α− β− spores, as might be expected if these minor proteins altered the spore's nucleoid structure. It will be of interest to analyze spore nucleoid structures in strains lacking some of these minor SASP.
While knowing the constituents in and the structure of the ring-shaped spore nucleoid is clearly of interest, the function of this unusual nucleoid structure is of even more interest. At present no specific function can be ascribed to this structure, although it is not absolutely essential for either sporulation or spore germination and outgrowth, as α− β− strains sporulate and α− β− spores germinate and grow out. However, the time for return of α− β− spores to vegetative growth is significantly longer than that for wild-type spores (9). Some of this time difference may be due to the lack of production of amino acids through degradation of α/β-type SASP in α− β− spores, but even in very rich media, α− β− spores take longer to grow out than wild-type spores (9). Possibly the ring-shaped nucleoid structure somehow facilitates optimal transcription during spore germination and outgrowth. If this is the case, one might also expect some defect in sporulation efficiency or kinetics in α− β− strains. While sporulation of α− β− strains is qualitatively similar to that of wild-type strains (9), recent work has indeed suggested that there may be some differences between the sporulation of wild-type and that of α− β− strains (23; B. Setlow, K. A. McGinnis, and P. Setlow, unpublished data). Consequently, it might be worthwhile to analyze sporulation of α− β− strains quantitatively, as it is difficult to imagine that such a structure as the ring-shaped nucleoids of spores has no effect on gene expression. This work is in progress.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institutes of Health (GM19698).
Microscopy was performed at the Center for Biomedical Imaging Technology at the University of Connecticut Health Center, except for analysis of the colocalization of the α/β-type SASP and the HBsu homolog, which required the microscope of Timothy Hla. We are grateful to Lotte Pedersen and Jason Kirk for assistance and advice and to members of the Setlow lab for suggestions.
REFERENCES
- 1.Bagyan I, Setlow B, Setlow P. New small, acid-soluble proteins unique to spores of Bacillus subtilis: identification of the coding genes and regulation and function of two of these genes. J Bacteriol. 1998;180:6704–6712. doi: 10.1128/jb.180.24.6704-6712.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabrera-Hernandez A, Setlow P. Analysis of the regulation and function of five genes encoding small, acid-soluble spore proteins of Bacillus subtilis. Gene. 2000;248:169–181. doi: 10.1016/s0378-1119(00)00125-6. [DOI] [PubMed] [Google Scholar]
- 3.Cutting S M, Vander Horn P B. Genetic analysis. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, United Kingdom: John Wiley and Sons; 1990. pp. 27–74. [Google Scholar]
- 4.Francesconi S C, MacAlister T J, Setlow B, Setlow P. Immunoelectron microscopic localization of small, acid-soluble spore proteins in sporulating cells of Bacillus subtilis. J Bacteriol. 1988;170:5963–5967. doi: 10.1128/jb.170.12.5963-5967.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldrick S, Setlow P. Expression of a Bacillus megaterium sporulation specific gene in Bacillus subtilis. J Bacteriol. 1983;155:1459–1462. doi: 10.1128/jb.155.3.1459-1462.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harry E J, Pogliano K, Losick R. Use of immunofluorescence to visualize cell-specific gene expression during sporulation in Bacillus subtilis. J Bacteriol. 1995;177:3386–3393. doi: 10.1128/jb.177.12.3386-3393.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohler P, Marahiel M A. Association of the histone-like protein HBsu with the nucleoid of Bacillus subtilis. J Bacteriol. 1997;179:2060–2064. doi: 10.1128/jb.179.6.2060-2064.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin P A, Losick R. Asymmetric division and cell fate during sporulation in Bacillus subtilis. In: Brun Y V, Shimkets L J, editors. Prokaryotic development. Washington, D.C.: American Society for Microbiology; 1999. pp. 167–190. [Google Scholar]
- 9.Mason J M, Setlow P. Evidence for an essential role for small, acid-soluble, spore proteins in the resistance of Bacillus subtilis spores to ultraviolet light. J Bacteriol. 1986;167:174–178. doi: 10.1128/jb.167.1.174-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mason J M, Setlow P. Different small, acid-soluble proteins of the α/β-type have interchangeable roles in the heat and ultraviolet radiation resistance of Bacillus subtilis spores. J Bacteriol. 1987;169:3633–3637. doi: 10.1128/jb.169.8.3633-3637.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Micka B, Marahiel M A. The DNA binding protein HBsu is essential for normal growth and development in Bacillus subtilis. Biochimie. 1997;74:641–650. doi: 10.1016/0300-9084(92)90136-3. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson W L, Setlow P. Sporulation, germination and outgrowth. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, United Kingdom: John Wiley and Sons; 1990. pp. 391–450. [Google Scholar]
- 13.Niki H, Yoshihara Y, Hiraga S. Dynamic organization of chromosomal DNA in Escherichia coli. Genes Dev. 2000;14:212–223. [PMC free article] [PubMed] [Google Scholar]
- 14.Pogliano K, Harry E, Losick R. Visualization of the subcellular location of sporulation proteins in Bacillus subtilis using immunofluorescence microscopy. Mol Microbiol. 1995;18:459–470. doi: 10.1111/j.1365-2958.1995.mmi_18030459.x. [DOI] [PubMed] [Google Scholar]
- 15.Robinow, C., and E. Kellenberger. 1994. The bacterial nucleoid revisited. 58:211–232. [DOI] [PMC free article] [PubMed]
- 16.Robinow C F. Spore structure as revealed by thin sections. J Bacteriol. 1953;66:300–311. doi: 10.1128/jb.66.3.300-311.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross M A, Setlow P. The Bacillus subtilis HBsu protein modifies the effects of α/β-type small, acid-soluble spore proteins on DNA. J Bacteriol. 2000;182:1942–1948. doi: 10.1128/jb.182.7.1942-1948.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez-Salas J-L, Santiago-Lara M L, Setlow B, Sussman M D, Setlow P. Properties of mutants of Bacillus megaterium and Bacillus subtilis which lack the protease that degrades small, acid-soluble proteins during spore germination. J Bacteriol. 1992;174:807–814. doi: 10.1128/jb.174.3.807-814.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Setlow B, Magill N, Febbroriello P, Nakhimovsky L, Koppel D E, Setlow P. Condensation of the forespore nucleoid early in sporulation of Bacillus species. J Bacteriol. 1991;173:6270–6278. doi: 10.1128/jb.173.19.6270-6278.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Setlow B, Setlow P. Localization of low molecular weight basic proteins in Bacillus megaterium spores by irradiation with ultraviolet light. J Bacteriol. 1979;139:486–494. doi: 10.1128/jb.139.2.486-494.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Setlow P. Small acid-soluble, spore proteins of Bacillus species: structure, synthesis, genetics, function and degradation. Annu Rev Microbiol. 1988;42:319–338. doi: 10.1146/annurev.mi.42.100188.001535. [DOI] [PubMed] [Google Scholar]
- 22.Setlow P. Resistance of bacterial spores. In: Storz G, Hengge-Aronis R, editors. Bacterial stress responses. Washington, D.C.: American Society for Microbiology; 2000. pp. 217–230. [Google Scholar]
- 23.Tennen R, Setlow B, Davis K L, Loshon C A, Setlow P. Mechanisms of killing of spores of Bacillus subtilis by iodine, glutaraldehyde and nitrous acid. J Appl Microbiol. 2000;89:1–10. doi: 10.1046/j.1365-2672.2000.01114.x. [DOI] [PubMed] [Google Scholar]
- 24.Tovar-Rojo F, Setlow P. Analysis of the effects of mutant small, acid-soluble spore proteins from Bacillus subtilis on DNA in vivo and in vitro. J Bacteriol. 1991;173:4827–4835. doi: 10.1128/jb.173.15.4827-4835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]