Abstract
Neurodegenerative diseases represent one of the utmost imperative well-being health issues and apprehensions due to their escalating incidence of mortality. Natural derivatives are more efficacious in various preclinical models of neurodegenerative illnesses. These natural compounds include phytoconstituents in herbs, vegetables, fruits, nuts, and marine and freshwater flora, with remarkable efficacy in mitigating neurodegeneration and enhancing cognitive abilities in preclinical models. According to the latest research, the therapeutic activity of natural substances can be increased by adding phytoconstituents in nanocarriers such as nanoparticles, nanogels, and nanostructured lipid carriers. They can enhance the stability and specificity of the bioactive compounds to a more considerable extent. Nanotechnology can also provide targeting, enhancing their specificity to the respective site of action. In light of these findings, this article discusses the biological and therapeutic potential of natural products and their bioactive derivatives to exert neuroprotective effects and some clinical studies assessing their translational potential to treat neurodegenerative disorders.
Graphical Abstract
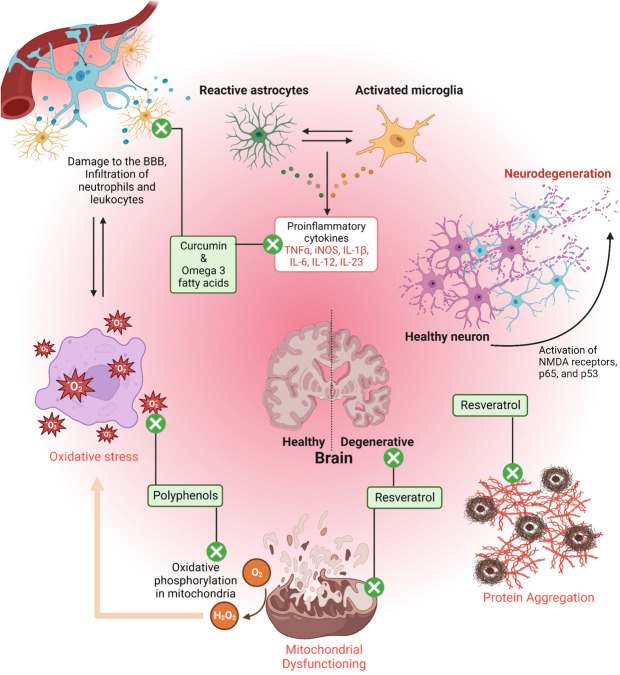
Common mechanisms, therapeutic targets, and molecular pathogenesis of neurodegeneration. It is focused on the biological and therapeutic potential of natural products and their bioactive derivatives to exert a neuroprotective effect on the pathologies of neurodegenerative diseases.
Keywords: Natural products, Neurodegenerative diseases, Neuroinflammation, Oxidative stress, Nanotechnology
Introduction
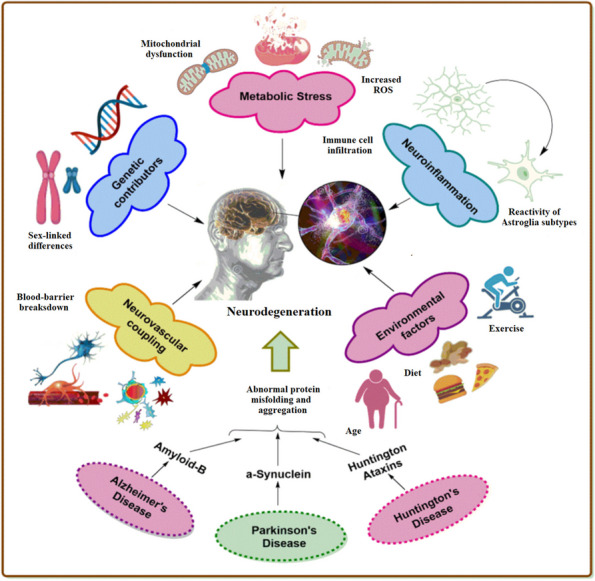
Neurodegenerative diseases (NDs), comprising a diverse array of disorders, are typified by the progressive degeneration of both the structural and functional components of either the central nervous system (CNS) or peripheral nervous system (PNS). Among the most prevalent of these maladies are Alzheimer's disease (AD), Parkinson's disease (PD), and spinal cord injury, which typically afflict individuals beyond the age of 60 years [57]. These debilitating conditions engender a prodigious burden on individuals and society, as the progressive loss of structural features and functions marks them. However, the root causes of several NDs remain obscure within the current healthcare system [41]. These NDs often present with a range of biological phenomena, including neuroinflammation, oxidative stress, cognitive decline, the accumulation of neurofibrillary tangles (NFTs), abnormal deposition of amyloid-β peptide (Aβ), diminution, or inadequate amalgamation of neurotransmitters and abnormal ubiquitination are linked to the progression of NDs [43]. Nevertheless, the role of aging in NDs is crucial, given their irreversible nature, the attendant social and economic burdens, and the paucity of efficacious therapeutic interventions [9].
Acute neurodegeneration is a clinical condition characterized by rapid damage resulting from abrupt insult or traumatic events, i.e., strokes, traumatic brain injuries, head injuries, ischemic brain damage, subarachnoid, or cerebral hemorrhage. Conversely, chronic neurodegeneration represents a protracted ailment in which neurons undergo a neurodegenerative process that typically commences gradually and exacerbates progressively due to various aspects, ultimately causing the irreversible devastation of specific neuron populations. Chronic neurodegenerative disorders comprise a variety of conditions, including ADs, PDs, Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS) [42]. Other neurological disorders, such as spinal muscular atrophy, Cockayne's syndrome, Coffin-lowry syndrome, Triple-A syndrome, and Rett syndrome, also fall within the purview of chronic NDs [35], described in Fig. 1 and summarized in Table 1.
Fig. 1.
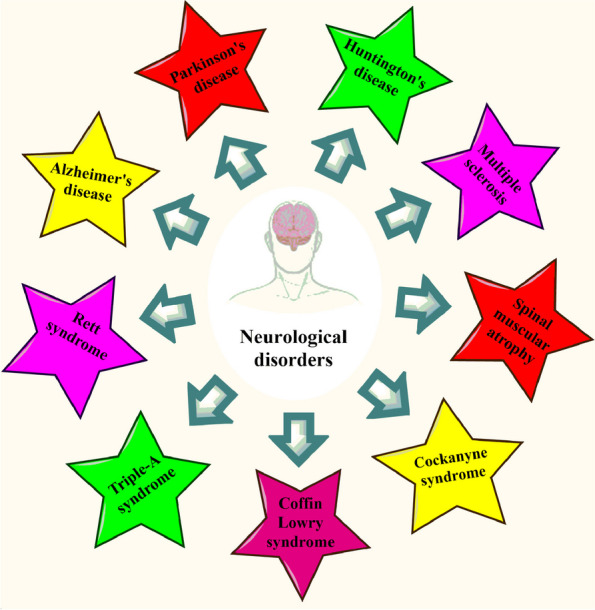
Neurological disorders
Table 1.
Neurological diseases: pathogenesis, genetic basis, disease mechanism and manifestations
| Neurodegenerative disease | Pathogenesis | Genetic basis | Disease mechanism | Manifestations |
|---|---|---|---|---|
| Multiple sclerosis | Autoimmune disorder | Multifactorial with contributions from genetic and environmental factors | Plaques/lesions formation in the spinal cord and brain, Demyelination of nerve fibers in CNS | Impaired motor function, sensory deficits, cognitive impairment |
| Spinal muscular atrophy | Neuromuscular disorder | Autosomal recessive inheritance with a mutation in the SMN1 gene | In the spinal cord, loss of neurons exist | Muscle weakness and atrophy, respiratory difficulties |
| Cockayne syndrome | Progeria syndrome | Autosomal recessive inheritance with mutations in the ERCC6 or ERCC8 gene | Impaired DNA repair mechanisms | Growth failure, premature aging, photosensitivity, neurological abnormalities |
| Coffin-Lowry syndrome | Intellectual disability disorder | X-linked dominant inheritance with a mutation in the RPS6KA3 gene | Defective signaling pathways in the brain | Intellectual disability, facial dysmorphism, skeletal abnormalities |
| Triple-A syndrome (Allgrove syndrome) | Endocrine disorder | Autosomal recessive inheritance with a mutation in the AAAS gene | Dysfunction of the adrenal gland and autonomic nervous system | Esophageal achalasia, adrenal insufficiency, lacrimal, neurological abnormalities |
| Rett syndrome | Neurodevelopmental disorder | X-linked dominant inheritance with MECP2 gene mutation | MECP2 gene mutation | Loss of acquired motor and language skills, intellectual disability, breathing irregularities, seizures |
Alzheimer's disease (AD) is a chronic and progressive neurodegenerative disorder that predominantly distorts the human brain's vast cerebral cortex and hippocampus regions. The disease is characterized by a range of symptoms, including mental and memory impairments, cognitive decline, and personality changes, and it predominantly affects the elderly population, especially in patients above 65 years [36]. It is distinguished by two key neuropathological features i.e., (i) intracellular accretion of hyperphosphorylated tau- proteins, which form NFTs in the brain, and (ii) extracellular development and deposition of amyloid-beta (Aβ) plaques [3].
Parkinson's disease (PD) is another most common NDs that significantly impair the eminence of life and dependency and increase the menace of premature death in affected individuals [11, 16]. This disease is instigated via substantial damage to dopaminergic nigrostriatal neurons, leading to reduced motor function and induced symptoms i.e., bradykinesia, resting tremor, postural imbalance, and muscular rigidity. PD are distinguished by the accretion of protein aggregates, Lewy neurites, and Lewy bodies, primarily composed of aggregated and misfolded forms of pre-synaptic protein α-synuclein [42].
Amyotrophic lateral sclerosis (ALS) is a devastating ND characterized by substantial degeneration and demise of both lower and upper motor neurons, ultimately failing the respiratory system and causing paralysis, leading to death. Despite extensive research, the underlying mechanisms of ALS remain unknown. However, various aspects, including oxidative stress, autoimmune response, impaired axonal transport, excitotoxicity, genetic factors, neurofilament aggregation, and mitochondrial dysfunction, have been considered as potential contributors to the development and progression of ALS [61]. The complex interplay of these factors leads to the liberal loss of motor neurons, ultimately leading to the debilitating symptoms of ALS.
On the other hand, Huntington’s disease (HD) is pathologically characterized by excessive dopaminergic potential and reduced functioning of gamma-aminobutyric acid (GABA) in basal ganglia and clinically characterized via cognitive deficits, atypical movements, and psychiatric disturbances [19]. This disorder is prompted by a trinucleotide repeat expansion of the CAG (nucleotide’s cytosine, adenine, and guanine) sequence in the Huntingtin (HTT) gene, that exists on the short arm of chromosome-4 [28].
Material and methods
In this comprehensive review, we meticulously investigated the role of natural products in managing neurodegenerative diseases. Our methodology involved an exhaustive literature search, systematic data extraction, and critical analysis of relevant studies. Emphasizing transparency and rigor, we adhered to ethical guidelines to ensure the integrity of this review on natural interventions for neurodegenerative diseases.
Our search strategy encompassed databases such as PubMed and Scopus, employing keywords related to natural products and neurodegenerative diseases. Rigorous study selection involved predefined inclusion and exclusion criteria, ensuring relevance and quality. Transparent data extraction methods were applied, systematically capturing key findings to facilitate a robust analysis in our review on natural products for neurodegenerative diseases.
Mechanism and therapeutic targets of neurodegenerative disorders
The presence of protein aggregates, oxidative stress, and inflammation within CNS marks neurodegenerative disorders. Various biological progressions have been allied to these disorders, including neurotransmitter depletion or insufficient synthesis, abnormal ubiquitination, and oxidative stress [40]. Neurodegenerative disorders are complex and multifactorial, and their underlying mechanisms are intricate. These disorders share common characteristics such as inflammation, mitochondrial deficits, abnormal cellular transport and protein deposition, excitotoxicity, intracellular Ca2+ overload, and unrestrained reactive oxygen species (ROS) generation. These characteristics imply the existence of converging neurodegeneration pathways, highlighting the significance of these pathways as communal markers for intervention approaches [7, 12].
Large protein aggregates within the brain, extracellular space, or neurons are among the most prominent features associated with NDs. These protein aggregates are called amyloid plaques. According to genetic evidence, one of the significant drivers of NDs is the alteration of the initially native and soluble proteins into the protein aggregates and their antecedent oligomers [26]. The common mechanisms, therapeutic targets [58], and molecular pathogenesis [41] of neurodegeneration are revealed in these articles.
Role of naturally derived products and their metabolites in neurodegenerative diseases
Traditional medicines are crucial in fulfilling the primary healthcare requirements of developing nations, serving as a cornerstone for maintaining good health [49]. It has been reported that natural derivatives are a significant source of bioactive compounds and an imperative source of drug leads [6, 22]. In fact, according to a study, at least one-third of the drugs available in the market have their origins or were derived from different natural resources [49]. Therefore, natural derivatives continue to be extensively researched for their therapeutic potential in modern medicine. Using natural derivatives in research studies has proven to be an efficacious methodology for discovering novel, innovative, and physiologically active medicaments [47]. Natural herbs have been used to treat several ailments and improve human health and well-being for thousands of years [38].
Recently, research on natural products and their bioactive compounds as excellent therapeutic and biological agents for NDs has substantially increased. The promising potential of natural compounds in preventing and treating NDs has been widely acknowledged. However, there are some clinical concerns regarding their use, primarily due to insufficient scientific evidence supporting their efficiency and patient safety [39].
The significance of plant-based natural derivatives is evident because many of the medications currently employed to treat NDs are derived from plants. For instance, opioids alkaloids, and anticholinesterases i.e., neostigmine, physostigmine, and galantamine are derived from plants [22]. The neuroprotective characteristics of naturally derived compounds and their metabolites have been studied and reported in the literature for treating NDs. Table 2 and Fig. 2 summarizes the wide-ranging therapeutic effects of various naturally derived compounds and their metabolites in combating NDs [48, 56].
Table 2.
Naturally derived compounds and their metabolites with neuroprotective potential in treating NDs (Pre-clinical approaches)
| S. No. | Plant Source | Major phytoconstituents | Neuroprotective activities | Model Used | References |
|---|---|---|---|---|---|
| 1 | Blueberries (Vaccinium angustifolium) | Polyphenols | Reduces the ROS levels in the brain and also helps in the activation of cellular stress pathways in the brain | In vitro Neurodegenerative cell Model | [27] |
| 2 | Capsicum annuum | Capsicum | Prohibits the neurodegeneration in the hippocampus, cerebral cortex, and substantial nigra by diminishing the brain 5-lipoxygenase activity, subdues the intensification of nitric oxide levels and brain malondialdehyde, restores the glutathione (GSH) level, and cholinesterase activity | In vitro model /Retinone intoxication mice model | (Abdel-Salam et al. 2018) [1] |
| 3 | Curcuma longa | Curcuminoids (Turmeric) | Improvement in the motor functions and behavioral properties, overturns the iNOS and GFAP (Glial fibrillary acidic protein) expressions and abridges the total nitrite generation and proinflammatory cytokines in the striatum | In vitro cell model/MPTP (1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine) model | (Ojha et al. 2012, Hishikawa et al. 2012) [18, 34] |
| 4 | Dioscorea nipponica | Diosgenin | Protects against neuroinflammation by inhibition of NF-κB, MAPK, ERK, JNK, and p38 pathways | In vitro cell line studies on RAW 264 cells | (Hirai et al. 2010) [17] |
| 5 | Sesamum indicum | Sesame oil | It significantly improved the learning and memory impairments, restored the elevated level of AChE and Aβ overexpression, and mitigate the oxidative stress status in the brain | In vitro Rat model of AD | (Mohamed et al. 2021) [32] |
| 6 | Vitis vinifera | Reserveratrol | Inhibits the amalgamation and liberation of pro-inflammatory mediators, constraints iNOS, NF-κB, COX-II, and AP-1, and promotion of IL-10 | In vitro cell line studies/ 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrasodium bromide (MTT) assay in BV2 microglia cells | (Song et al. 2014) [51] |
| 7 | Nicotiana tabacum | Osmotin | Reduction of Aβ accretion and expression of BACE-1, ameliorates memory impairment, prevents Aβ-induced neurotoxic effects of neuronal- HT22 cells, and reverses synaptic deficits | In vitro Y Maze test | (Ali et al. 2015) [2] |
| 8 | Coptis chinensis | Berberine | Triggers the regulations of AKT/GSK-3β/Nrf2, persuades the secretion of NGF and BDNF, and inhibition of COX-II, iNOS, TNF-α, NF-κB, and IL-1β | In vitro cell line studies/MTT assay in BV2 microglia cells | (Lee et al. 2012; Jia et al. 2012) [20, 23] |
| 9 | Morus alba | Quercetin | Inhibits COX-II, GSK-3β, 5-LOX enzymes, and NF-κB activation, and intricates in the free radical scavenging | In vitro animal model/MTTP (1,2,3,6-tetrahydropyridine) induced neurodegeneration | (Pany et al. 2014) [37] |
| 10 | Vitis vinifera | Polyphenols | Abridges iNOS, PARP, and TNF-α expression and level of nitro-tyrosine, and subdues Bcl-2 and caspase-3 expressions | Mice model of autoimmune encephalomyelitis | (Giacoppo et al. 2015) [14] |
| 11 | Zingiber officinale | 6-shogaol (Ginger) | Persuades the secretion of NGF, BDNF, and GDNF, inhibition of iNOS, IL-1β, TNF-α, p38, NF-κB, Bax, PGE2, NO, and ROS, and upsurges Bcl-2 levels | Primary Cell culture | (Ha et al. 2012) [15] |
| 12 | Ginkgo biloba | Ginkgolide B | Overwhelms PI3K/Akt and NF-κB pathways, upregulation of expression of anti-apoptotic proteins, and reduces LDH, ROS and caspase3 | Primary Cell culture/ MTT assay | (Nabavi et al. 2015; Xiao et al. 2010) [8, 62] |
| 13 | Panax ginseng | Ginsenoside Rg3 | Triggers the cAMP/MAPK and Trk-mediated neurogenesis, inhibition of NF-κB, TNF-α, iNOS, and IL-1β | MTT Assay with BV2 microglial cell lines | (Joo et al. 2008) [21] |
Fig. 2.
Neurodegenerative pathways and the role of bioactives in the prevention of neurodegeration
Clinical studies on the translational prospectives of natural derivatives to treat neurogenerative disorders
Clinical trials are currently underway to develop and test a wide range of interventions for NDs. These interventions encompass a broad spectrum of therapeutic approaches, including cognitive enhancement, anti-amyloid and anti-tau interventions, anti-neuroinflammation interventions, neuroprotection, and neurotransmitter modification, in relieving behavioral psychological symptoms. A range of natural compounds have shown promise in clinical trials, and ongoing investigations are focused on elucidating their mechanisms of action and potential therapeutic benefits, as depicted in Table 3. Table 4 further details clinical trials and human evaluation doses for various phytochemicals demonstrating neuroprotective effects.
Table 3.
Clinical trials on phytochemicals/phytoconstituents employed in the management of NDs
| S.No. | Phytoconstituents | Mechanism of action | NCT number | Sponsor | Status |
|---|---|---|---|---|---|
| 1 | Ginkgo biloba | Antioxidant activity and anti-amyloid aggregation | NCT03090516 | Nanjing Medical University | Recruiting |
| 2 | Guanfacine | Alpha-2A-adrenoceptor agonist, an effective 5-HT2B receptor agonist | NCT03116126 | Imperial College London | Recruiting |
| 3 | Coconut oil | Attenuation in the expression of ADP-ribosylation factor-1 protein | NCT01883648 | University of South Florida | Terminated |
| 4 | Caffeine | Antagonizes the adenosine receptors and improves the motor system and also has an impact on Levodopa serum concentrations | NCT01738178 | Research Institute of the McGill University Health Centre | Recruitment- Completed |
| 5 | Huperzine-A | Cholinesterase inhibitor, also decreases the levels of soluble and insoluble beta-amylase levels in AD | NCT00083590 | - | Recruitment- Completed |
Table 4.
Clinical trials and human evaluation doses for various phytochemicals as neuroprotective
| S. No. | Phytochemicals | Mechanism of action | Dose | Clinical Trial Data | References |
|---|---|---|---|---|---|
| 1 | Edaravone (Trade Name: Radicava) | Free radical scavenger | 3 mg/kg, (two times a day for 14 days) | The compound showed great potential in the management of ischemic stroke and now has been sanctioned for the treatment of the same in Japan in 2001 | (Watanabe et al. 2018) [59] |
| 2 | Dl-3-n-butylphthalide | A multi-target drug exerts its actions via antioxidant, anti-apoptosis, and anti-inflammation, and also protects the mitochondria | 40 mg/kg-200 mg/kg | The compound was found to be effective in the management of Ischemic stroke and was sanctioned for the treatment of the same in China in 2002 | (Liao et al. 2018) [25] |
| 3 | Baicailein from Scutellaria baicalensis | A multi-target drug exerts its actions via antioxidants, anti-apoptosis, and inflammation and also protects the mitochondria. Also inhibits LOX/p38/cPLA2 pathway, and overwhelms the NF-κB activation | 24 mg/kg (i.v) dose | Phase I clinical trials, single-center, randomized, placebo-controlled, double-blind, single dose-escalation, healthy male and females volunteers were used | (Li et al. 2014) [24] |
| 4 | Scutellarin (scutellarein-7-O-glucuronide) from Erigeron breviscapus | Acts by suppressing microglial activation and inflammation | 30–40 ml/day for 8–12 days was found to be safe and effective along with Dengzhanxixin | Great potential for its clinical use. Recently, an injection of Dengzhanxixin is approved for the management of ischemic shock (approval number Z53021569) in China. Scutellarin is the main component present in this injection | (Wang et al. 2018) [55] |
| 5 | Naringenin | It inhibits NF-κB, lessers the inflammation, decreases the BBB dysfunction, and enhances Nrf2-mediated anti-oxidation | 120 (mg/kg) i.v for 15 min | Phase 1 clinical trials NCT0358255, recruiting | (Nouri et al. 2019) [33] |
A glimpse of recent patents granted or filed on phytoconstituents for their neuroprotective action
The brain is undoubtedly one of the most sensitive and crucial organs in the human body, and any damage inflicted upon it can have catastrophic consequences. However, recent research investigations have revealed that numerous phytoconstituents hold promise in reversing brain damage and preventing further harm. Several compounds have been studied extensively for their neuroprotective actions in the past years, with many receiving patents. The potential of these phytoconstituents lies in their ability to mitigate the damage triggered by inflammation, oxidative stress, and other factors contributing to neurodegeneration. By protecting and repairing damaged neurons and improving overall brain function, these compounds offer a novel and promising approach to treating a wide range of NDs. Furthermore, their use could help to discourse the unmet medical needs in this field, which have remained largely unfulfilled due to the limited effectiveness and significant side effects associated with existing treatments. In this way, the discovery of neuroprotective phytoconstituents represents a significant breakthrough in neurology and holds enormous promise for improving the eminence of life of those affected by NDs.
Table 5 represents the data of patents of neuroprotective agents along with their therapeutic receptors.
Table 5.
Patented data of various neuroprotective phytoconstituents
| S. No. | Name of the compound | Therapeutic action | Details of Patent |
|---|---|---|---|
| 1 | Rhizoma coptidis (Coptis chinensis, Radix scutellariae, Cortex phellodendri in 3:2:2:3 dry weight | Used in the treatment of stroke, ADs, and dementia |
US patent No US9375457B2 |
| 2 | Cannabinoids such as cannabidivarin, cannabichromene, and cannabidivarin acid | Used and approved for the treatment of ADs |
US patent No US10258580B2 |
| 3 | Limonoids | Used for the prophylaxis and treatment of neurodegeneration |
US patent No US9289412B2 |
| 4 | Elazi tannins | Used for the treatment of delirium, dementia, learning, and attention deficit disorder (ADD) |
Japanese Patent No JP6935331B2 |
| 5 | Novobiocin analogs | Used for the treatment of beta-amyloid disorder, and is most preferably ADs |
US patent No US7960353B2 |
| 6 | Cardiac glycosides | Used for the treatment of ADs, HDs, or stroke |
Australia Patent No AU2016262784B2 |
Role of nanotechnology in the drug formulation and development of phytochemicals
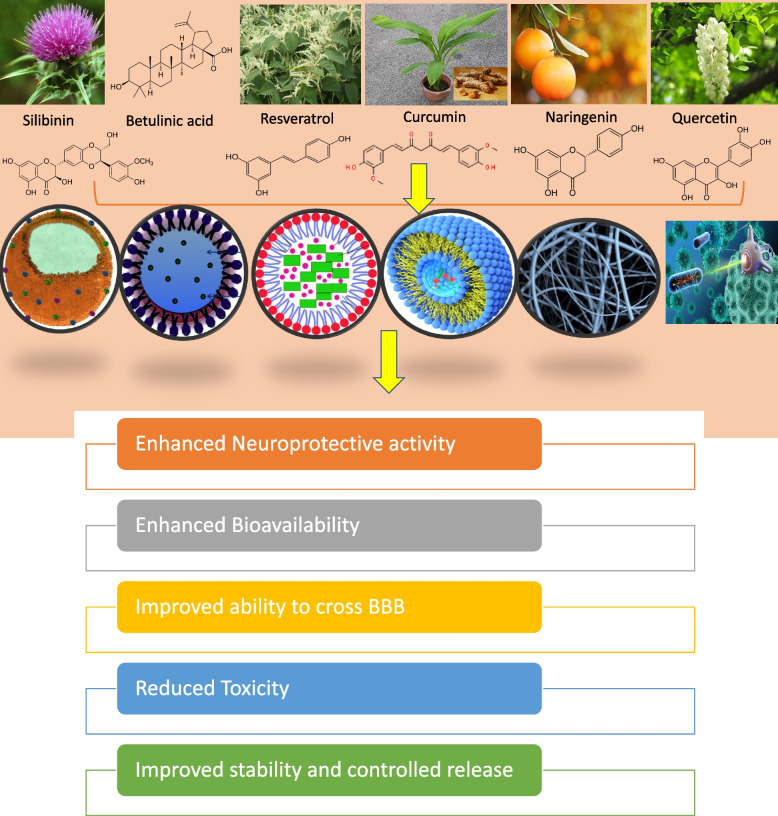
Phytoconstituents display various therapeutic functions, including anticancer, antioxidant, and neuroprotection properties. However, their efficacies are often limited by issues related to solubility and bioavailability. The scale-up issue from laboratory to commercialization has hindered the application of natural compounds in the pharmaceutical industry, primarily due to solubility and bioavailability concerns when administered in conventional forms. To overcome these limitations, nanotechnology has emerged as a potential solution. Specifically, nanosponges, nanoemulsions, nanogels, nano micelles, and nanoparticles are innovative drug delivery systems based on nanotechnology that can improve the solubility and specificity of naturally derived bioactive compounds [31, 44].
Nanotechnology-based drug delivery methods can potentially enhance the specificity of natural bioactive compounds by precisely targeting their site of action. This targeted approach can effectively prevent receptor-specific diseases, such as breast cancer, by targeting HER receptors with increased efficacy. Furthermore, researchers are currently investigating brain targeting and the target of neurological receptors for diagnosing, preventing, and managing NDs. Thus, the application of nanotechnology-based drug delivery can facilitate the utilization of neuroprotective phytoconstituents in the pharmaceutical industry and potentially revolutionize the treatment of various diseases. Indeed, one of the most significant advantages of using nanotechnology-based drug delivery systems is the ability to minimize the side effects of various drugs. This is primarily due to the improved bioavailability, which reduces both the dose and dose-related toxicity. With the help of nanotechnology, drugs can be delivered more precisely to the envisioned site of action, and diminishing their effects on healthy tissues. Additionally, the use of nanocarriers can help to protect drugs from premature degradation or elimination, further enhancing their therapeutic potential [4, 5, 62].
Nanotechnology has proven to be a favorable solution in enhancing the efficacy of herbal compounds. Although synthetic and semisynthetic compounds also face the issue of limited bioavailability, their problems are related to poor solubility, efficacy, and bioavailability, which can be resolved by creating salt forms or derivatives. However, this is not a feasible solution for herbal compounds. Nanotechnology, on the other hand, offers a more effective approach to addressing these issues. Nanotechnology offers several methods to enhance the efficacy of poorly bioavailable compounds. The bioavailability of the compounds can be improved by formulating nanoformulations such as solid lipid nanoparticles, nanocrystals, and nanosponges. These nanoformulations prevent their first-pass metabolism and degradation of these compounds while aiding in their targeting of specific sites of action. As a result, the bioavailability and efficacy of the drugs can be enhanced significantly.
Furthermore, using lipids as drug carriers has emerged as a promising area of research. Lipids can protect fragile drugs from degradation, and incorporating these components into lipid carriers can facilitate safe delivery to their targets while preventing metabolic degradation. Another approach for enhancing efficacy is hydrogels, which can stabilize the bioactivity and improve their delivery to specific targets. These strategies offer great potential for developing more effective and efficient treatments for various diseases. Some of the nanotechnology-based delivery methods for phytoconstituents are mentioned in Table 6 and Fig. 3. These mentioned compounds were selected based on their neuroprotective data available on various data bases [29, 46].
Table 6.
Nanotechnology-based phytochemicals used for the treatment of NDs
| Sr. No. | Phytoconstituents | Drug Delivery System | Combating disease | References |
|---|---|---|---|---|
| 1 | Resveratrol | Nanostructured lipid carriers, and solid lipid nanoparticles (SLNPs) | Treatment of ADs | (Fonseca-Santos et al. 2015) [13] |
| 2 | Curcumin | PLGA based nanoparticles | Treatment of ADs | (Yavarpour-Bali et al. 2019) [63] |
| 3 | Naringenin | Nanoemulsions | To combat PDs and treatment of ADs | (Nouri et al. 2019) [33] |
| 4 | Quercetin | PLGA nanoparticles, nanoencapsulation | To combat PDs and treatment of ADs | (Enteshari Najafabadi et al. 2018) [10] |
| 5 | Epigallocatechin-3 gallate | Selenium nanoparticles coated with Tet-1 peptide |
Increase neuronal alpha-secretase, Increased oral bioavailability |
(Singh et al. 2015) [50] |
| 6 | Ferulic acid | SLPNs | Antioxidant action | [52] |
| 7 | Huperzine-A | Lactoferrin-conjugated N-trimethylated chitosan nanoparticles | Increased mucoadhesion | (Wen et al. 2017) [60] |
Fig. 3.
Few phytoconstituents and various nanoformulations are used nowadays
Challenges, limitations and endorsements for future research
Natural herbs, either in their whole form or as extracts, are widely recognized for their potential neuroprotective properties against various NDs. Although numerous preclinical investigations have established the efficiency of these herbs for treating neurodegeneration, there has been a significant gap in successfully translating these findings from research to commercialization. While preclinical evidence is abundant, clinical testing remains limited. As a result, the potential of natural herbs as a viable treatment option for neurodegenerative disease remains largely unexplored. Using natural products for neuroprotection faces various challenges related to their physicochemical stability, solubility, metabolism, crossing the blood–brain barrier (BBB), and therapeutic efficacy. Even though several natural substances, such as Resveratrol, turmeric, and apigenin, have been shown to possess multiple neuroprotective properties, their efficacy is hampered by poor stability, solubility, and bioavailability.
Addressing the complexities of natural products in neurodegenerative disease management, challenges include the need for standardized methodologies, rigorous clinical trials, and understanding intricate molecular mechanisms. Limitations encompass variability in bioavailability and inconsistent study designs. Future research should prioritize large-scale, well-controlled trials, exploring synergistic effects of natural compounds. Endorsements for advanced technologies, such as omics approaches, could unravel novel therapeutic targets. Additionally, interdisciplinary collaboration between researchers, clinicians, and industry partners is essential for advancing the field. Overcoming these challenges and embracing innovative strategies will pave the way for more efficacious natural product-based interventions in neurodegenerative disease treatment [30, 45].
Furthermore, the BBB poses a significant obstacle for these substances, preventing them from crossing the bloodstream to the brain. However, nanotechnology and nanocarriers have the potential to improve their solubility, bioavailability, and stability. The use of encased nanocarriers to deliver natural compounds has shown significant improvements in their bioavailability and stability. Several types of nanocarriers, such as nanosuspension, nano gels, nano micelles, and nanostructured lipid carriers, have been formulated to deliver phytoconstituents. These nanocarriers help in the phytoconstituents entrapment and considerably improve their stability, as demonstrated by recent research [39, 44, 53, 54].
Conclusion
Preclinical studies have provided compelling evidence of the therapeutic potential of phytoconstituents as neuroprotectors. The documented bioactivities of natural substances, such as scavenging of reactive oxygen species, antioxidant action, antiproliferative activity, and antibacterial and anticancer properties, along with their neuroprotective effects, are well established. Several natural substances, including luteolin, hesperidin, resveratrol, and genistein have demonstrated efficacy against neurodegeneration. However, their therapeutic potential is limited by solubility, stability, and efficacy issues that impede their clinical translation. Recent studies have shown that natural substances can be made more therapeutically effective by incorporating them into nanocarriers, such as nanogels, nanoparticles, and nanostructured lipid carriers. This strategy can potentially overcome natural substances' limitations and significantly improve bioactive compounds' stability, solubility, and specificity, thereby enhancing their therapeutic activity.
Acknowledgements
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number ISP23-101.
Abbreviations
- 5-HT2B
5-Hydroxytryptamine receptor 2B
- 5-LOX
Arachidonate 5-lipoxygenase
- AAAS
Achalasia–Addisonianism–Alacrima syndrome
- Aβ
Amyloid-β peptide
- AChE
Acetylcholine
- AD
Alzheimer's disease
- AKT
Ak strain transforming
- ALS
Amyotrophic lateral sclerosis
- AP-1
Activating protein-1
- BACE-1
Beta-site amyloid precursor protein cleaving enzyme 1
- BBB
Blood-brain barrier
- Bcl-2
B-cell lymphoma 2
- BDNF
Brain-derived neurotrophic factor
- CAG
Nucleotide’s cytosine, adenine, and guanine
- cAMP
Cyclic adenosine monophosphate.
- CNS
Central nervous system
- COX-II
Cyclooxygenase II
- cPLA2
Cytosolic phospholipases A2
- ERCC6
ERCC Excision Repair 6, Chromatin Remodeling Factor
- ERCC8
ERCC excision repair 8, CSA ubiquitin ligase complex subunit
- ERK
Extracellular signal-regulated kinase
- GABA
Gamma-aminobutyric acid
- GDNF
Glial cell line-derived neurotrophic factor
- GFAP
Glial fibrillary acidic protein
- GSH
Glutathione level
- GSK-3β
Glycogen synthase kinase-3 beta
- HD
Huntington’s disease
- HT22
Immortalized mouse hippocampal neuronal cell line
- HTT
Huntingtin gene
- iNOS
Inducible nitric oxide synthase
- JNK
Jun N-terminal kinase
- IL-10
Interleukin 10
- IL-1β
Interleukin-1 beta
- LDH
Lactate dehydrogenase
- MAPK
Mitogen-activated protein kinase
- MECP2
Methyl-CpG Binding Protein 2
- MTT
3-[4,5-Dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide assay
- NDs
Neurodegenerative diseases
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NFTs
Neurofibrillary tangles
- NGF
Nerve growth factor
- NO
Nitric oxide
- Nrf2
Nuclear factor erythroid 2–related factor 2
- PARP
Poly (ADP-ribose) polymerases
- PD
Parkinson's disease
- PGE2
Prostaglandin E2
- PI3K
Phosphoinositide 3-kinase
- PNS
Peripheral nervous system
- ROS
Reactive oxygen species
- RPS6KA3
Ribosomal Protein S6 Kinase A3
- SLNPs
Solid lipid nanoparticles
- SMN1
Survival of motor neuron 1
- TNF-α
Tumor necrosis factor alpha
Authors’ contributions
Rajat Goyal, Conceptualization, Investigation, Resources, Writing—Original Draft. Pooja Mittal, Conceptualization, Investigation, Resources, Writing—Original Draft. Rupesh K. Gautam, Investigation, Writing—Original Draft. Mohammad Amjad Kamal, Resources, Writing—Original Draft. Asma Perveen, Resources, Writing—Original Draft. Vandana Garg, Resources, Writing—Original Draft. Athanasios Alexiou, Supervision, Writing—Review & Editing. Muhammad Saboor, Funding acquisition, Writing—Review & Editing. Shafiul Haque, Funding acquisition, Writing—Review & Editing. Aisha Farhana, Writing—Review & Editing. Marios Papadakis, Funding acquisition, Writing—Review & Editing. Ghulam Md Ashraf, Supervision, Writing—Review & Editing. The authors provided their final approval of all content and submission for publication.
Funding
Open Access funding enabled and organized by Projekt DEAL. Deputyship for Research & Innovation, Ministry of Education, Saudi Arabia - Project Number ISP23-101. Open Access funding enabled and organized by Project DEAL. This work was supported by the University of Witten-Herdecke Germany.
Availability of data and materials
All the available data are included in the manuscript. No new data was generated.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rajat Goyal and Pooja Mittal contributed equally to this work.
Contributor Information
Rupesh K. Gautam, Email: rupeshgautammmu@gmail.com
Marios Papadakis, Email: marios_papadakis@yahoo.gr, Email: drmariospapadakis@gmail.com.
Ghulam Md Ashraf, Email: ashraf.gm@gmail.com.
References
- 1.Abdel-Salam, O.M., Sleem, A.A., Youness, E.R., Yassen, N.N., Shaffie, N., El-Toumy, S.A. Capsicum protects against rotenone-induced toxicity in mice brain via reduced oxidative stress and 5-lipoxygenase activation. J. Pharm. Pharmacol. Res. 2018;2(3), 60–77. 10.26502/jppr.0011/.
- 2.Ali T, Yoon GH, Shah SA, Lee HY, Kim MO. Osmotin attenuates amyloid beta-induced memory impairment, tau phosphorylation and neurodegeneration in the mouse hippocampus. Sci Rep. 2015;5(1):1–17. doi: 10.1038/srep11708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apostolova LG. Alzheimer disease. Contin. Lifelong Learn. Neurol. 2016;22(2):419. doi: 10.1212/CON.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharti K, Mittal P, Mishra B. Formulation and characterization of fast dissolving oral films containing buspirone hydrochloride nanoparticles using design of experiment. J Drug Deliv Sci Technol. 2019;49:420–432. doi: 10.1016/j.jddst.2018.12.013. [DOI] [Google Scholar]
- 5.Bonde GV, Yadav SK, Chauhan S, Mittal P, Ajmal G, Thokala S, Mishra B. Lapatinib nano-delivery systems: a promising future for breast cancer treatment. Expert Opin Drug Deliv. 2018;15(5):495–507. doi: 10.1080/17425247.2018.1449832. [DOI] [PubMed] [Google Scholar]
- 6.Chopra H, Bibi S, Goyal R, Gautam RK, Trivedi R, Upadhyay TK, Mujahid MH, Shah MA, Haris M, Khot KB, Gopan G. Chemopreventive Potential of Dietary Nanonutraceuticals for Prostate Cancer: An Extensive Review. Front Oncol. 2022;12:925379. doi: 10.3389/fonc.2022.925379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dadhania VP, Trivedi PP, Vikram A, Tripathi DN. Nutraceuticals against neurodegeneration: a mechanistic insight. Curr Neuropharmacol. 2016;14(6):627–640. doi: 10.2174/1570159x14666160104142223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daglia M, Braidy N, Rosa Loizzo M, Tundis R, Fazel Nabavi S. Neuroprotective effects of ginkgolide B against ischemic stroke: A review of current literature. Curr Top Med Chem. 2015;15(21):2222–2232. doi: 10.2174/1568026615666150610142647. [DOI] [PubMed] [Google Scholar]
- 9.Di Paolo M, Papi L, Gori F, Turillazzi E. Natural products in neurodegenerative diseases: A great promise but an ethical challenge. Int J Mol Sci. 2019;20(20):5170. doi: 10.3390/ijms20205170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enteshari Najafabadi R, Kazemipour N, Esmaeili A, Beheshti S, Nazifi S. Using superparamagnetic iron oxide nanoparticles to enhance bioavailability of quercetin in the intact rat brain. BMC Pharmacol Toxicol. 2018;19(1):1–12. doi: 10.1186/s40360-018-0249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Essa, M.M., Braidy, N., Bridge, W., Subash, S., Manivasagam, T., Vijayan, R.K., Guillemin, G.J. Review of natural products on Parkinson’s disease pathology. J. Aging Res. Clin. Pract. 2014;3(1):1–8. https://www.jarlife.net/614-review-of-natural-products-on-parkinsons-disease-pathology.htm.
- 12.Fachel FNS, Schuh RS, Veras KS, Bassani VL, Koester LS, Henriques AT, Braganhol E, Teixeira HF. An overview of the neuroprotective potential of rosmarinic acid and its association with nanotechnology-based delivery systems: A novel approach to treating neurodegenerative disorders. Neurochem Int. 2019;122:47–58. doi: 10.1016/j.neuint.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Fonseca-Santos B, Gremião MPD, Chorilli M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int J Nanomed. 2015;10:4981. doi: 10.1016/j.neuint.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giacoppo S, Galuppo M, Lombardo GE, Ulaszewska MM, Mattivi F, Bramanti P, Navarra M. Neuroprotective effects of a polyphenolic white grape juice extract in a mouse model of experimental autoimmune encephalomyelitis. Fitoterapia. 2015;103:171–186. doi: 10.1016/j.fitote.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Ha SK, Moon E, Ju MS, Kim DH, Ryu JH, Oh MS, Kim SY. 6-Shogaol, a ginger product, modulates neuroinflammation: a new approach to neuroprotection. Neuropharmacology. 2012;63(2):211–223. doi: 10.1016/j.neuropharm.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Harris JR. Protein aggregation and fibrillogenesis in cerebral and systemic amyloid disease (Vol. 65). Springer Science & Business Media; 2012.
- 17.Hirai S, Uemura T, Mizoguchi N, Lee JY, Taketani K, Nakano Y, Kawada T. Diosgenin attenuates inflammatory changes in the interaction between adipocytes and macrophages. Mol Nutr Food Res. 2010;54(6):797–804. doi: 10.1002/mnfr.200900208. [DOI] [PubMed] [Google Scholar]
- 18.Hishikawa N, Takahashi Y, Amakusa Y, Tanno Y, Tuji Y, Niwa H, Krishna UK. Effects of turmeric on Alzheimer's disease with behavioral and psychological symptoms of dementia. Ayu. 2012;33(4):499. doi: 10.4103/0974-8520.110524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu YT, Chang YG, Chern Y. Insights into GABA Aergic system alteration in Huntington's disease. Royal Soc Open Biol. 2018;8(12):180165. doi: 10.1098/rsob.180165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia L, Liu J, Song Z, Pan X, Chen L, Cui X, Wang M. Berberine suppresses amyloid-beta-induced inflammatory response in microglia by inhibiting nuclear factor-kappa-B and mitogen-activated protein kinase signalling pathways. J Pharm Pharmacol. 2012;64(10):1510–1521. doi: 10.1111/j.2042-7158.2012.01529.x. [DOI] [PubMed] [Google Scholar]
- 21.Joo SS, Yoo YM, Ahn BW, Nam SY, Kim YB, Hwang KW, Lee DI. Prevention of inflammation-mediated neurotoxicity by Rg3 and its role in microglial activation. Biol Pharm Bull. 2008;31(7):1392–1396. doi: 10.1248/bpb.31.1392. [DOI] [PubMed] [Google Scholar]
- 22.Karim, N., Abdelhalim, H., Gavande, N., Khan, I., Khan, H. Natural products as an emerging therapeutic alternative in the treatment of neurological disorders. Evid. Based Complementary Altern. Med. 2018:3056847. 10.1155/2018/3056847. [DOI] [PMC free article] [PubMed]
- 23.Lee B, Sur B, Shim I, Lee H, Hahm DH. Phellodendron amurense and its major alkaloid compound, berberine ameliorates scopolamine-induced neuronal impairment and memory dysfunction in rats. Korean J Physiol Pharmacol. 2012;16(2):79–89. doi: 10.4196/kjpp.2012.16.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li M, Shi A, Pang H, Xue W, Li Y, Cao G, Yan B, Dong F, Li K, Xiao W, He G, Du G, Hu X. Safety, tolerability, and pharmacokinetics of a single ascending dose of baicalein chewable tablets in healthy subjects. J Ethnopharmacol. 2014;156:210–215. doi: 10.1016/j.jep.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 25.Liao D, Xiang D, Dang R, Xu P, Wang J, Han W, Fu Y, Yao D, Cao L, Jiang P. Neuroprotective Effects of dl-3-n-Butylphthalide against Doxorubicin-Induced Neuroinflammation, Oxidative Stress, Endoplasmic Reticulum Stress, and Behavioral Changes. Oxid Med Cell Longev. 2018;2018:13. Article ID 9125601. 10.1155/2018/9125601. [DOI] [PMC free article] [PubMed]
- 26.Limbocker R, Errico S, Barbut D, Knowles TP, Vendruscolo M, Chiti F, Zasloff M. Squalamine and trodusquemine: two natural products for neurodegenerative diseases, from physical chemistry to the clinic. Nat Prod Rep. 2022 doi: 10.1039/D1NP00042J. [DOI] [PubMed] [Google Scholar]
- 27.Ma H, Johnson SL, Liu W, DaSilva NA, Meschwitz S, Dain JA, Seeram NP. Evaluation of polyphenol anthocyanin-enriched extracts of blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry for free radical scavenging, reactive carbonyl species trapping, anti-glycation, anti-β-amyloid aggregation, and microglial neuroprotective effects. Int J Mol Sci. 2018;19(2):461. [DOI] [PMC free article] [PubMed]
- 28.MacDonald ME, Ambrose CM, Duyao MP, Myers RH, Lin C, Srinidhi L, Harper PS. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72(6):971–983. doi: 10.1016/0092-8674(93)90585-E. [DOI] [PubMed] [Google Scholar]
- 29.Makkar R, Behl T, Bungau S, Zengin G, Mehta V, Kumar A, ..., Oancea R. Nutraceuticals in neurological disorders. Int J Mol Sci. 2020;21(12):4424. [DOI] [PMC free article] [PubMed]
- 30.Makkar R, Behl T, Bungau S, Zengin G, Mehta V, Kumar A, Uddin MS, Ashraf GM, Abdel-Daim MM, Arora S. Nutraceuticals in neurological disorders. Int J Mol Sci. 2020;21(12):4424. doi: 10.3390/ijms21124424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mittal P, Vardhan H, Ajmal G, Bonde GV, Kapoor R, Mittal A, Mishra B. Formulation, optimization, hemocompatibility and pharmacokinetic evaluation of PLGA nanoparticles containing paclitaxel. Drug Dev Ind Pharm. 2019;45(3):365–378. doi: 10.1080/03639045.2018.1542706. [DOI] [PubMed] [Google Scholar]
- 32.Mohamed EA, Ahmed HI, Zaky HS, Badr AM. Sesame oil mitigates memory impairment, oxidative stress, and neurodegeneration in a rat model of Alzheimer's disease. A pivotal role of NF-κB/p38MAPK/BDNF/PPAR-γ pathways. J Ethnopharmacol. 2012;1:113468. doi: 10.1016/j.jep.2020.113468. [DOI] [PubMed] [Google Scholar]
- 33.Nouri Z, Fakhri S, El-Senduny FF, Sanadgol N, Abd-ElGhani GE, Farzaei MH, Chen J-T. On the neuroprotective effects of naringenin: Pharmacological targets, signaling pathways, molecular mechanisms, and clinical perspective. Biomolecules. 2019;9(11):690. doi: 10.3390/biom9110690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ojha, R.P., Rastogi, M., Devi, B.P., Agrawal, A., Dubey, G.P. Neuroprotective effect of curcuminoids against inflammation-mediated dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. J. Neuroimmune Pharmacol. 2012;7(3):609–618. 10.1007/s11481-012-9363-2. [DOI] [PubMed]
- 35.Ovechkina VS, Zakian SM, Medvedev SP, Valetdinova KR. Genetically Encoded Fluorescent Biosensors for Biomedical Applications. Biomedicines. 2021;9(11):1528. doi: 10.3390/biomedicines9111528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandey SN, Rangra NK, Singh S, Arora S, Gupta V. Evolving Role of Natural Products from Traditional Medicinal Herbs in the Treatment of Alzheimer’s Disease. ACS Chem Neurosci. 2021;12(15):2718–2728. doi: 10.1021/acschemneuro.1c00206. [DOI] [PubMed] [Google Scholar]
- 37.Pany S, Pal A, Sahu PK. Neuroprotective effect of quercetin in neurotoxicity induced rats: role of neuroinflammation in neurodegeneration. Asian J Pharm Clin Res. 2014;7(4):152–156. [Google Scholar]
- 38.Patel SS, Udayabanu M. Effect of natural products on diabetes associated neurological disorders. Rev Neurosci. 2017;28(3):271–293. doi: 10.1515/revneuro-2016-0038. [DOI] [PubMed] [Google Scholar]
- 39.Rahman M, Bajgai J, Fadriquela A, Sharma S, Trinh TT, Akter R, Jeong YJ, Goh SH, Kim C-S, Lee K-J. Therapeutic potential of natural products in treating neurodegenerative disorders and their future prospects and challenges. Molecules. 2021;26(17):5327. doi: 10.3390/molecules26175327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasool, M., Malik, A., Qureshi, M.S., Manan, A., Pushparaj, P.N., Asif, M., Sheikh, I.A. Recent updates in the treatment of neurodegenerative disorders using natural compounds. Evid. Based Complementary Altern. Med. 2014:979730. 10.1155/2014/979730. [DOI] [PMC free article] [PubMed]
- 41.Ratheesh G, Tian L, Venugopal JR, Ezhilarasu H, Sadiq A, Fan TP, Ramakrishna S. Role of medicinal plants in neurodegenerative diseases. Biomanuf Rev. 2017;2(1):1–16. doi: 10.1007/s40898-017-0004-7. [DOI] [Google Scholar]
- 42.Sairazi, N.S.M., Sirajudeen, K.N.S. Natural products and their bioactive compounds: neuroprotective potentials against neurodegenerative diseases. Evid. Based Complementary Altern. Med. 2020:6565396. 10.1155/2020/6565396. [DOI] [PMC free article] [PubMed]
- 43.Shal B, Ding W, Ali H, Kim YS, Khan S. Anti-neuroinflammatory potential of natural products in attenuation of Alzheimer's disease. Front Pharmacol. 2018;9:548. doi: 10.3389/fphar.2018.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh J, Mittal P, Vasant Bonde G, Ajmal G, Mishra B. Design, optimization, characterization and in-vivo evaluation of Quercetin enveloped Soluplus®/P407 micelles in diabetes treatment. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S546–S555. doi: 10.1080/21691401.2018.1501379. [DOI] [PubMed] [Google Scholar]
- 45.Singh S, Singh TG. Role of nuclear factor kappa B (NF-κB) signalling in neurodegenerative diseases: an mechanistic approach. Curr Neuropharmacol. 2020;18(10):918–935. doi: 10.2174/1570159X18666200207120949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh S, Singh TG. Role of nuclear factor kappa B (NF-κB) signalling in neurodegenerative diseases: an mechanistic approach. Curr Neuropharmacol. 2020;18(10):918–35. [DOI] [PMC free article] [PubMed]
- 47.Singla, R.K., Sai, C.S., Chopra, H., Behzad, S., Bansal, H., Goyal, R., Shen, B. Natural Products for the Management of Castration-Resistant Prostate Cancer: Special Focus on Nanoparticles Based Studies. Front. Cell Dev. Biol. 2012;9. 10.3389/fcell.2021.745177. [DOI] [PMC free article] [PubMed]
- 48.Singla, R.K., Wang, X., Gundamaraju, R., Joon, S., Tsagkaris, C., Behzad, S., Khan, J., Gautam, R., Goyal, R., Rakmai, J., Dubey, A.K. Natural products derived from medicinal plants and microbes might act as a game-changer in breast cancer: a comprehensive review of preclinical and clinical studies. Crit. Rev. Food Sci. Nutr. 2022:1–45. 10.1080/10408398.2022.2097196. [DOI] [PubMed]
- 49.Singla RK, Behzad S, Khan J, Tsagkaris C, Gautam RK, Goyal R, Chopra H, Shen B. Natural Kinase Inhibitors for the Treatment and Management of Endometrial/Uterine Cancer: Preclinical to Clinical Studies. Front Pharmacol. 2022;13:801733. doi: 10.3389/fphar.2022.801733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh, N.A., Mandal, A. K.A., Khan, Z. A. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr. J. 2015;15(1), 1–17. 10.1186/s12937-016-0179-4. [DOI] [PMC free article] [PubMed]
- 51.Song J, Cheon SY, Jung W, Lee WT, Lee JE. Resveratrol promotes the expression of interleukin-10 and brain-derived neurotrophic factor in microglia under hypoxia. Int J Mol Sci. 2014;15:15512–15529. doi: 10.3390/ijms150915512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thapliyal S, Singh T, Handu S, Bisht M, Kumari P, Arya P, ..., Gandham R. A review on potential footprints of ferulic acid for treatment of neurological disorders. Neurochem Res. 2021;46:1043–57. [DOI] [PubMed]
- 53.Vardhan H, Mittal P, Adena SKR, Mishra B. Long-circulating polyhydroxybutyrate-co-hydroxyvalerate nanoparticles for tumor targeted docetaxel delivery: Formulation, optimization and in vitro characterization. Eur J Pharm Sci. 2017;99:85–94. [DOI] [PubMed]
- 54.Vardhan H, Mittal P, Adena SKR, Upadhyay M, Mishra B. Development of long-circulating docetaxel loaded poly (3-hydroxybutyrate-co-3-hydroxyvalerate) nanoparticles: optimization, pharmacokinetic, cytotoxicity and in vivo assessments. Int J Biol Macromol. 2017;103:791–801. [DOI] [PubMed]
- 55.Wang, Liping; Ma, Qiang. Clinical benefits and pharmacology of Scutellarin: A comprehensive review. Pharmacology & Therapeutics, 2018:S0163725818300809–. 10.1016/j.pharmthera.2018.05.006. [DOI] [PubMed]
- 56.Wang R, Tang XC. Neuroprotective effects of huperzine A. Neurosignals. 2005;14(1-2):71–82. doi: 10.1159/000085387. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z, He C, Shi JS. Natural products for the treatment of neurodegenerative diseases. Curr Med Chem. 2020;27(34):5790–5828. doi: 10.2174/0929867326666190527120614. [DOI] [PubMed] [Google Scholar]
- 58.Wareham LK, Liddelow SA, Temple S, Benowitz LI, Di Polo A, Wellington C, Calkins DJ. Solving neurodegeneration: common mechanisms and strategies for new treatments. Mol Neurodegener. 2022;17(1):1–29. doi: 10.1186/s13024-022-00524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watanabe K, Tanaka M, Yuki S, Hirai M, Yamamoto Y. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis?. J Clin Biochem Nutr. 2018;62(1):20–38. [DOI] [PMC free article] [PubMed]
- 60.Wen MM, El-Salamouni NS, El-Refaie WM, Hazzah HA, Ali MM, Tosi G, Farid RM, Blanco-Prieto MJ, Billa N, Hanafy AS. Nanotechnology-based drug delivery systems for Alzheimer's disease management: Technical, industrial, and clinical challenges. J Control Release. 2017;245:95–107. doi: 10.1016/j.jconrel.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 61.Wijesekera LC, Nigel Leigh P. Amyotrophic lateral sclerosis. Orphanet J Rare Dis. 2009;4(1):1–22. doi: 10.1186/1750-1172-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao Q, Wang C, Li J, Hou Q, Li J, Ma J, Wang Z. Ginkgolide B protects hippocampal neurons from apoptosis induced by beta-amyloid 25–35 partly via up-regulation of brain-derived neurotrophic factor. Eur J Pharmacol. 2010;647(1–3):48–54. doi: 10.1016/j.ejphar.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 63.Yavarpour-Bali H, Ghasemi-Kasman M, Pirzadeh M. Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. Int J Nanomed. 2019;14:4449. doi: 10.2147/IJN.S208332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the available data are included in the manuscript. No new data was generated.