Abstract
Importance:
Skin and dental lesions are common in tuberous sclerosis complex (TSC) and are a frequent concern for patients. Recognition of these lesions is imperative for early diagnosis given treatment advances that may improve patient outcomes.
Objective:
The 2012 International Tuberous Sclerosis Complex Consensus Conference was convened to update the last consensus statement in 1998. The purpose of this manuscript is to detail recommendations for the diagnosis, surveillance, and management of skin and dental lesions in TSC.
Evidence Review:
The TSC dermatology and dentistry subcommittee, one of 12 subcommittees, reviewed the relevant literature from 1997 to 2012.
Findings:
A consensus on skin and dental issues was achieved within the dermatology and dentistry subcommittee before recommendations were presented, discussed, and agreed upon in a group meeting of all subcommittees from June 14–15, 2012.
Conclusions and Relevance:
Skin and dental findings comprise 4 of 11 major features and 3 of 6 minor features in the diagnostic criteria. A definite diagnosis of TSC is defined as the presence of at least 2 major features or 1 major and 2 or more minor features; additionally, a pathological mutation in TSC1 or TSC2 is diagnostic. Skin and oral examinations should be performed annually and every 3–6 months, respectively. Intervention may be indicated for TSC skin or oral lesions that are bleeding, symptomatic, disfiguring, or negatively impacting function. Options presented include surgical excision, laser(s), or use of an mTOR inhibitor.
Introduction
Tuberous sclerosis complex (TSC) is a genetic disorder variably manifesting as cognitive impairment, seizures, and hamartomas of the brain, eyes, kidneys, heart, lungs, and skin1. The diagnosis and management of TSC has evolved since the time of the previous international TSC consensus meeting in 1998. The spectrum of clinical manifestations has been refined, aided by the ability to better define cohorts of patients by testing for mutations in the causative genes, TSC1 and TSC22. Treatment of patients has been transformed by the use of mTOR inhibitors (mTORi), drugs that target the signaling pathway activated in TSC tumors as a consequence of loss of function of the TSC1-TSC2 protein complex3–6. As a result of these advances, the Tuberous Sclerosis Alliance convened an international group of clinical experts and scientists to update the consensus documents of 19987,8. The goal of this manuscript is to provide additional detail of the dermatological and dental aspects of the updated diagnostic criteria9 and management recommendations10.
Methods
A subcommittee on Dermatology and Dentistry, one of twelve subcommittees, was composed of 7 dermatologists and 2 dentists with expertise in TSC. The subcommittee reviewed the relevant literature and arrived at a consensus opinion regarding dermatological and dental diagnostic criteria and treatment of TSC. These recommendations were presented to the entire group of 79 specialists from 14 countries for discussion and approval at the meeting in Washington D.C. from June 14–15, 2012. Final recommendations were incorporated into manuscripts reporting the updated diagnostic criteria9 and guidance for surveillance and treatment of TSC10. In anticipation of more detailed recommendations that would be published in disease focus areas10, the Dermatology and Dentistry Committee detailed these recommendations in the current manuscript, supported with updated literature since the last consensus statement. IRB approval was waived.
Diagnostic Criteria
Perhaps the most significant change in the diagnostic criteria is the addition of a genetic criterion (Table 1). The demonstration of a pathogenic mutation in TSC1 or TSC2 in normal tissue is now considered sufficient for diagnosis, independent of clinical manifestations9. The use of DNA testing as an independent criterion may facilitate early diagnosis of affected individuals who have not yet manifested sufficient clinical diagnostic features.
Table 1.
Revised Diagnostic Criteria for Tuberous Sclerosis Complex
| 1998 | 2012 |
|---|---|
| Genetic Criterion | |
| none | Pathogenic mutation in TSC1 or TSC2 |
| Major Features | |
| Facial angiofibromas or forehead plaque | Angiofibromas (≥3) or fibrous cephalic plaque |
| Hypomelanotic macules (≥3) | Hypomelanotic macules (≥3, at least 5mm diameter) |
| Non-traumatic ungual or periungual fibroma | Ungual fibromas (≥2) |
| Shagreen patch (connective tissue nevus) | Shagreen patch |
| Multiple retinal hamartomas | |
| Cortical tuber | Cortical dysplasia |
| Subependymal nodule | Subependymal nodules |
| Subependymal giant cell astrocytoma | Subependymal giant cell astrocytoma |
| Cardiac rhabdomyoma, single or multiple | Cardiac rhabdomyoma |
| Lymphangioleiomyomatosis | Lymphangioleiomyomatosis |
| Renal angiomyolipoma | Angiomyolipomas (≥2) |
| Minor Features | |
| Multiple randomly distributed pits in dental enamel | Dental enamel pits (≥3) |
| Gingival fibromas | Intraoral fibromas (≥2) |
| “Confetti” skin lesions | “Confetti” skin lesions |
| Non-renal hamartomas | Non-renal hamartomas |
| Multiple renal cysts | Multiple renal cysts |
| Retinal achromic patch | Retinal achromic patch |
| Hamartomatous rectal polyps | |
| Bone cysts | |
| Cerebral white matter migration lines |
Adapted from references 7 and 9. Letters in bold font indicate changes from the prior criteria.
Clinical diagnostic criteria continue to be important in diagnosis, as genetic testing may not identify a mutation in up to 25% of TSC patients and a normal gene test does not exclude TSC2. The updated clinical criteria are still divided into major and minor features (Table 1). A definite diagnosis is defined as the presence of at least 2 major features or 1 major and 2 or more minor features. The diagnosis of TSC is considered possible in the presence of 1 major or 2 or more minor features9.
The updated clinical criteria include a few noteworthy changes to the extracutaneous criteria. Cortical dysplasia replaces cortical tubers as a major feature to encompass both cortical tubers and cerebral white matter radial migration lines. While angiomyolipomas remain a major feature; anatomic location is no longer limited to the kidney and may include the liver or other organ systems. Hamartomatous rectal polyps and bone cysts were deleted as minor criteria due to lack of specificity9.
Dermatologic and Dental Criteria
The dermatologic and dental lesions used in the 1998 consensus are maintained in the updated criteria9, including hypomelanotic macules, angiofibromas, ungual fibromas, shagreen patch, “confetti” skin lesions, and dental pits (Table 1). These lesions are usually readily identified based on their appearance (Figure 1)11. The updated criteria incorporate several changes in terminology or number. The term forehead plaque is replaced with “fibrous cephalic plaque” because similar plaques may occur elsewhere on the face and scalp in patients with TSC. Similarly, oral fibromas are frequently gingival in TSC, but they may be observed at other intraoral sites including the buccal and labial mucosa as well as the tongue12. Therefore, the minor criterion of gingival fibromas is expanded to intraoral fibromas. Ungual fibroma is now recommended as a general term that encompasses periungual and subungual fibromas9.
Figure 1.
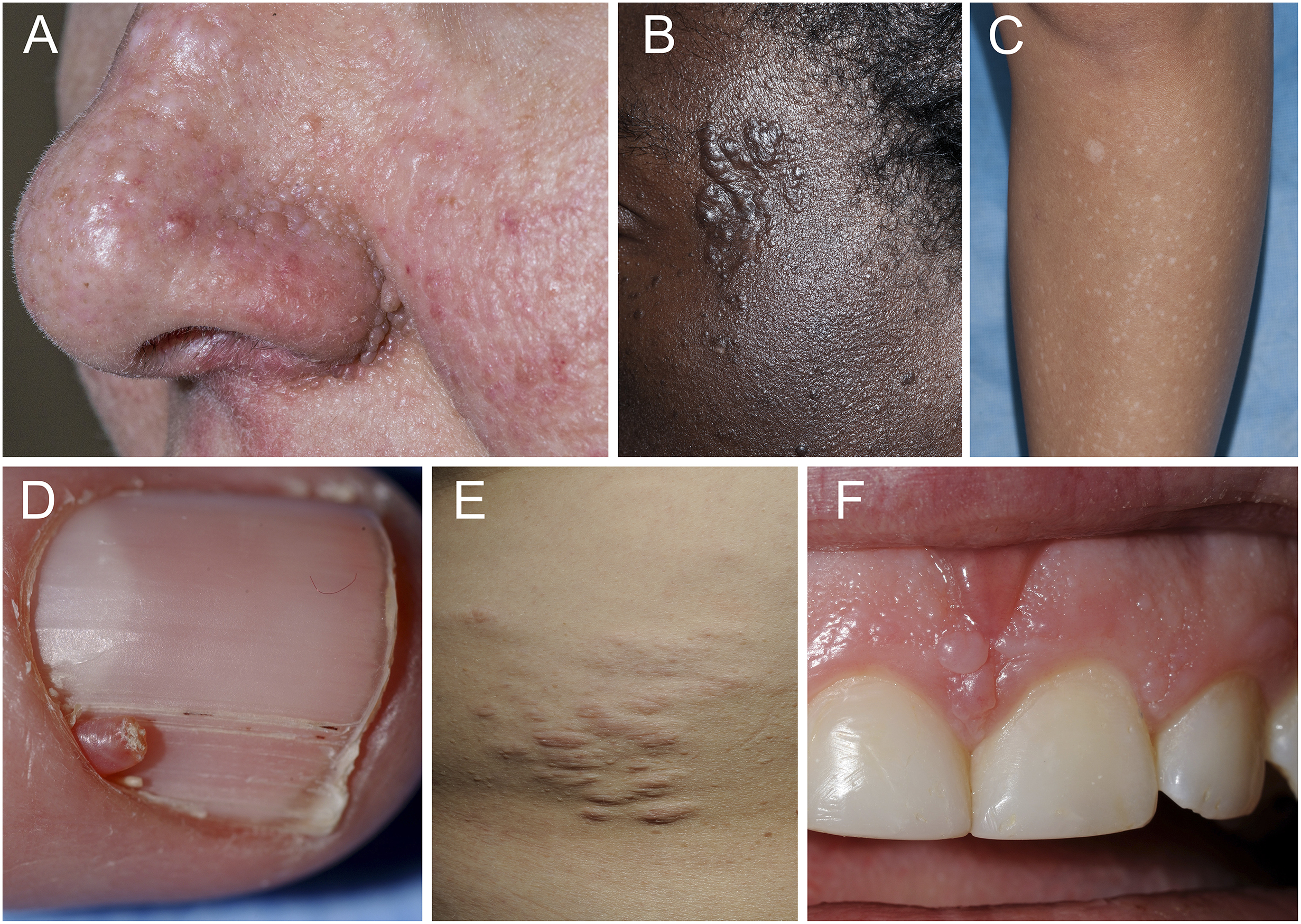
Representative skin and oral lesions in tuberous sclerosis complex. A. multiple facial angiofibromas, B. fibrous cephalic plaque, C. hypomelanotic macule and confetti-like hypopigmentation, D. ungual fibromas, E. shagreen patch, and F. gingival fibromas and dental pitting.
In the 1998 criteria, only hypomelanotic macules had a numerical requirement (3 or more). In the updated criteria, numerical requirements are added for several lesions, including angiofibromas (≥3), ungual fibromas (≥2), dental pits (≥3), and intraoral fibromas (≥2). The updated criteria also specify that hypomelanotic macules measure 5 mm or greater in largest diameter. Areas of poliosis may also be included in the count of hypomelanotic macules. Additional details about the rationale for these changes appear in the recent consensus document9, and herein we present recommendations of the dermatology and dental group regarding the application of these criteria and the presence of other TSC dermatological and dental lesions.
Application of the Dermatologic and Dental Criteria
Age-dependence:
TSC is a disease in which each lesion has a typical age of onset and periods for progression, stabilization, and in some cases, spontaneous resolution13,14. Hypomelanotic macules frequently present within the first few years of life, remain stable for decades, and become less apparent in late adulthood. Angiofibromas often start to appear at age 3–4, increase in number and size throughout the teenage years and become relatively stable in extent throughout adulthood. Ungual fibromas have the most tardive onset of cutaneous manifestations, as they typically arise during adolescence or occasionally adulthood14. In light of the age-related penetrance of skin lesions, clinicians should not discount the possibility of TSC based on the absence of skin lesions, especially in infants. Guidance to parents to seek medical evaluation if new lesions appear may be beneficial. A correct understanding of the natural history also helps prevent lesion misidentification. For example, hypopigmented macules that are expanding in size out of proportion to the child’s growth or acquired in adolescence or adulthood are not likely to be TSC-related. On the other hand, the diagnosis of angiofibromas should be considered for lesions mistaken for acne in children ages 1 to 7 years, or lesions which persist in the same location despite resolution of other acne lesions.
Sensitivity:
The clinical appearance of each TSC-related dermatologic or dental findings ranges from barely perceptible to conspicuous. Simple techniques may assist detection in subtle cases, including the use of Wood’s light to accentuate hypomelanotic macules, and the use of dental disclosing solution to enhance the appearance of dental pits on the otherwise smooth labial surface of the anterior teeth15. Clinicians may use these approaches to ensure a thorough evaluation.
The cumulative number of each lesion varies among patients with TSC but was a point of emphasis in the revised diagnostic criteria. TSC patients exhibiting a solitary lesion present a challenge for diagnosis as solitary lesions may present in the general population. For example, solitary ungual fibromas were found in 11%14 to 25%16 of TSC patients with ungual fibromas, but solitary lesions, known as acral or periungual fibrokeratomas, are also observed in the general population17. Hypopigmented macules numbered only one or two in 18%18 to 20%14 of TSC patients with this lesion type, but 1.6%19 to 4.7%20 of healthy children also have few hypopigmented macules. Solitary or small numbers of angiofibromas on the face, known as fibrous papules, is a relative common finding in adults. In a study of 62 patients with sporadic gastrinoma, fibrous papules were observed in 8% of adults21. Therefore, numerical requirements were added for several criteria to increase specificity for TSC. Although a patient presenting with only one type of TSC-related lesion that does not meet these numerical thresholds is unlikely to have TSC, it remains prudent to briefly screen for other TSC-related skin or dental findings. Additional positive findings should lead to further evaluation with consideration of imaging studies and genetic testing.
Specificity:
The emergence of multiple facial angiofibromas in a young child is nearly pathognomonic for TSC, but the differential diagnosis must be expanded when onset occurs in late adolescence or early adulthood. Multiple angiofibromas have been reported in adult patients with multiple endocrine neoplasia type I (MEN1)21,22. Compared to patients with TSC, angiofibromas in MEN1 are typically less numerous and less likely to cluster in the nasolabial folds. Patients with Birt-Hogg-Dubé (BHD) syndrome classically develop multiple facial fibrofolliculomas or trichodiscomas, but angiofibromas may also be observed, either as the only type of skin lesion or accompanied by the more pathognomonic skin lesions23,24. The possibility of TSC must be considered in adults as well as children, as the diagnosis may be missed earlier in life or TSC manifestations may not become penetrant until adulthood25.
A large shagreen patch is characteristic finding for TSC. In unusual cases, patients with TSC may exhibit multiple small, dome-shaped collagenomas without an associated shagreen patch. In patients presenting with multiple collagenomas in the absence of other features of TSC, the differential diagnosis should be expanded to include familial cutaneous collagenoma, eruptive collagenoma, MEN1, BHD syndrome, and, in the case of storiform collagenomas (sclerotic fibromas), Cowden syndrome26.
The utility of confetti lesions for TSC diagnosis in adults may be limited by the frequent occurrence of idiopathic guttate hypomelanosis, which bears a similar clinical appearance. In adults, accurate diagnosis of confetti lesions may be supported by congenital onset, asymmetric distribution, or occurrence in non-sun exposed areas.
Intraoral fibromas are a less specific finding in TSC and it is therefore a minor feature for diagnosis. Solitary fibroma is not uncommon in newborns and may also be associated with trauma. Acquired multiple fibromas on the gingiva is reported in those taking anti-epileptic medications such as phenytoin, where up to 50% patients taking the medication also develop gingival overgrowth27. The differential diagnosis of gingival fibromas includes gingival fibrous nodule, papilloma, focal epithelial hyperplasia, gingival cyst, and exostosis28. Therefore, late onset of papules on oral mucosa may require histologic confirmation. Although gingival papules are a relatively common finding in many hamartoma syndromes, including BHD, Cowden, and MEN1, careful examination may distinguish TSC by the additional findings of dental enamel pits or characteristic TSC skin findings.
Other TSC-related skin or dental lesions:
Patients with TSC may manifest skin or dental lesions that are not included in current diagnostic criteria. Folliculocystic and collagen hamartomas are large, rare tumors in TSC, but may be highly specific and may be added to the criteria pending confirmatory studies29. At present, their identification should prompt a thorough evaluation for other features of TSC. Additional TSC findings include maxillofacial intraosseous fibroblastic lesions30 and nail findings such as “red comets” and longitudinal grooves without a visible periungual fibroma16. Consideration of TSC should be given to those presenting with such lesions. Although many patients with TSC develop skin tags (molluscum fibrosum pendulum) or one to two café-au-lait macules31, these are common in the general population so they have little value in diagnosis of TSC.
Indications for biopsy:
Most patients with TSC do not require a biopsy for diagnosis. A biopsy may be required for skin lesions when there is uncertainty regarding the clinical diagnosis, particularly if internal manifestations of TSC are lacking or the diagnosis of TSC hinges on skin lesions for satisfying the criteria. The presence of multiple facial papules, in particular, can indicate the presence of alternative genodermatoses, including Cowden/PTEN macrocephaly hamartoma syndromes (tricholemmomas), Brook-Spiegler syndrome, (trichoepithelioma), or Birt-Hogg-Dubé syndrome (fibrofolliculoma/ trichodiscoma)32. In addition, other facial neoplasms such as basaloid follicular hamartoma, multiple discoid fibromas33, syringomas, dermal melanocytic nevi, even acne/rosacea may be mistaken for facial angiofibromas.
Recommendations for Surveillance and Management
The goal of the surveillance and management recommendations of the 2012 Consensus Conference was to provide evidence-based guidance regarding general supportive measures, treatments for each type of cutaneous lesion, indications for treatment, and possible risks, benefits and complications of specific interventions. The consensus document10 was necessarily brief in enumerating dermatological and dental recommendations as just one aspect of overall care of the patient with TSC, and additional explanation for these recommendations is provided below.
Detailed clinical dermatologic evaluation at the time of diagnosis is recommended for both pediatric and adult populations. Anticipatory guidance regarding expectations and potential treatments is advised. Parents should be advised to maintain good sun protection for their children. Hypomelanotic macules are prone to sunburn11 and the recent finding of UV “signature” mutations in angiofibromas suggests that sun exposure may contribute to the formation of facial angiofibromas34. Close surveillance and intervention are in general recommended for TSC-related skin lesions that are rapidly changing in size and/or number; causing functional interference, pain or bleeding; or inhibiting social interactions. Annual skin exam is recommended for children with TSC. Dermatologists may recommend more or less frequent referral to dermatology depending on manifestations in individual patients.
Many of the oral manifestations of TSC occur in young children; therefore, baseline oral evaluation is recommended as early as six month of age or at time of diagnosis. For the majority of TSC patients, regular exam every 6 months is recommended. Patients with special needs and difficulty maintaining oral hygiene may benefit from routine exam every 3 months. Because of the risk of bone cyst formation in the jaw, panoramic radiographic evaluation is recommended by age 6–7 years or earlier if asymmetry, asymptomatic swelling, or delayed or abnormal tooth eruption sequence is evident.
Treatment of Skin Lesions:
The management of TSC tumors has shifted dramatically with the introduction of mTORi such as sirolimus (rapamycin) and everolimus (RAD001). Sirolimus decreases the size of angiomyolipomas in patients with TSC, and everolimus is approved by the US Food and Drug Administration for the treatment of 1) renal angiomyolipomas that do not require immediate surgery in adults with TSC, and 2) subependymal giant cell astrocytomas (SEGA) that cannot be surgically resected in adults or children with TSC35. As described below, several large trials indicate that mTORi improve TSC skin lesions. The decision to pursue medical or surgical treatment options for TSC skin lesions should incorporate the overall clinical condition of the individual and the availability of equipment or physician expertise for carrying out specific procedures (Table 2). Current recommendations do not cover all potential treatments, patient scenarios or guarantee successful treatment, and are contingent upon emerging research advances.
Table 2.
Treatment options for skin, mucocutaneous and dental manifestations in patients with TSC
| Conditions | Treatment Consideration | Limitations |
|---|---|---|
| Facial Angiofibroma |
Topical mTOR inhibitor
Laser surgery
Surgical excision
|
|
| Cephalic Fibrous Plaque |
|
|
| Skin Tags |
|
|
| Hypo-pigmented Macules |
|
|
| Ungual Fibroma |
|
|
| Intraoral Fibroma |
|
|
| Dental Pits |
|
|
| Jaw Cysts |
|
|
A primary consideration in treatment for TSC skin lesions is whether the individual is currently or will soon be taking systemic therapy for extracutaneous manifestations, such as renal angiomyolipomas or SEGA. Many of these individuals may show improvement in their skin lesions while on systemic mTORi. In a phase 2 trial of oral sirolimus for renal angiomyolipomas, subjective improvement was noted in in 57% of 28 individuals with facial angiofibromas, 18% with hypomelanotic macules, 29% with shagreen patches, 29% with ungual fibromas, and 21% with forehead plaques5. In a randomized, placebo-controlled, phase 3 trial of everolimus for SEGA in children and adults with TSC, in which the response of at least one TSC skin lesion was assessed with the Physician’s Global Assessment of Clinical Condition scale, a partial response was observed in 42% of patients in the everolimus group and 11% of the placebo group (p=0.0004)4. The same scale was used to assess skin response in another double-blind, placebo-controlled, phase 3 trial of everolimus for angiomyolipomas. About 26% of 77 individuals treated with everolimus had partial or complete clearing, whereas no patients treated with placebo showed a response (p=0.0002)3. These results indicate the benefit of systemic mTORi in treatment of skin lesions associated with TSC. In those individuals with an inadequate skin response, concomitant use of a surgical approach may be considered with caution. Systemic therapy with mTORi is associated with incisional complications and delayed wound healing, particularly for major surgeries such as renal transplants36. For patients with no imminent indication for an mTORi, it may be prudent to evaluate for the presence of skin lesions deemed less likely to respond to conservative measures so that a surgical procedure can be performed prior to initiating systemic mTOR inhibition. For the majority of patients, the decision is still made on a case-by-case basis. Standard practice guidelines and stratified approaches are currently unavailable.
The use of oral sirolimus or everolimus specifically for the management of TSC skin lesions may be considered for individuals whose skin lesions pose serious medical risk, such as angiofibromas causing extensive recurrent bleeding or obstructed nasal breathing, in the context of a patient whose lesions are intractable to surgery or for whom surgical risks outweigh the risks of mTORi. Adverse events of systemic therapy with mTORi that occurred in more than 20% of patients included stomatitis, mouth ulceration, acne-like skin lesions, infections, hypertriglyceridemia, hypercholesterolemia, bone marrow suppression (anemia, mild neutropenia, leucopenia), proteinuria, and joint pain3–5. In most cases these adverse events are mild to moderate, but lethal complications such as non-infective pneumonitis have been noted in other studies37. When systemic medications are prescribed, it is not uncommon for adverse events to necessitate dose reduction or temporary interruption in treatment. Discontinuation of mTORi is associated with tumor regrowth6, so it is likely that patients will need long-term treatment to maintain benefit. However, there is limited data on the safety and tolerability of long-term treatment, or the potential for resistance to mTORi over time35.
In order to reduce the potential for adverse events associated with oral administration of mTORi, investigators have examined the use of topical sirolimus (rapamycin). Case reports and small case series have documented promising results in facial angiofibromas using topical sirolimus38–41. Overall these studies show decreased erythema and flattening of lesions with almost complete resolution in some individuals, especially children, minimal side effects such as skin irritation, and undetectable blood levels of sirolimus. However, there is no commercially available topical sirolimus on the market and it must be compounded by specialty pharmacy. At present there is no standard dose or formulation. Additionally, safety data are lacking for pediatric and long-term use.
TSC skin lesions have been treated using surgical approaches for decades11, and the effectiveness of these treatments is supported by recent case series42–45. Indications for surgical treatment of TSC skin lesions may include bleeding, irritation, pain, impaired function (such as vision, breathing, or mobility), and disfigurement. Immediate surgical intervention may be necessary in some patients, and for others the decision regarding timing for elective surgery may weigh the current rate of growth, the risk of recurrence, and the likelihood of scarring using different approaches. Younger children with flat angiofibromas may be appropriately treated using pulsed dye laser (PDL) near school age to reduce erythema11,42, whereas the treatment of larger angiofibromas using ablative lasers may be best performed in later adolescence at which time the rate of recurrence is lower43. PDL has little risk of hypo- or hyperpigmentation and scarring, but multiple treatments are usually required and responses are usually transient. PDL has also been combined with 5-aminolevulinic acid blue light photodynamic therapy44. Ablative approaches flatten the lesions but general anesthesia is often required and post-operative care can be difficult. Long-term complications include hypo- or hyperpigmentation, scarring, cobblestone skin texture, and regrowth or formation of new lesions. Such adverse events may be reduced by using a combination of ablative fractional resurfacing, vascular laser, and pinpoint electrosurgery.45 Other surgical approaches may also be appropriate such as shave excision, dermabrasion, electrosurgery or cryosurgery, depending on physician experience and equipment availability. Ungual fibromas and other TSC skin fibromas may be excised or treated with an ablative laser11.
Treatment of Intraoral Lesions:
Like hamartoma of the skin, early interventions are usually recommended for oral lesions that are symptomatic, rapidly changing in size and/or number, or causing functional compromise. As approximately 50% of children and adults with tuberous sclerosis have intellectual disability and behavioral issues, management of oral disease may be may require care by a dentist with advanced training46.
For dental pits, restorative treatment may be recommended if symptomatic, carious, or an esthetic concern exists. Gingival fibromas can exert local effects on dentition resulting in malocclusion or abnormal eruption. Symptomatic gingival papules or those interfering with oral hygiene should be removed via surgical excision or CO2 laser and electrosurgery47. Sustained efforts are needed to reduce gingival overgrowth and to delay or prevent recurrence of fibromas by improving oral hygiene as well as eliminating irritative factors48. Frequent clinical follow-up may be beneficial to ensure the early diagnosis of any possible lesions.
The potential for mTORi to improve oral fibromas in those with TSC has not been evaluated. Gingival overgrowth is much less of a problem in transplant recipients taking sirolimus than those on cyclosporine49 and periodontal inflammation is less common in transplant recipients taking everolimus compared to those treated with tacrolimus50. Studies are needed in this area as well as the safety of oral surgical procedures for those on systemic mTOR inhibition.
Maxillofacial intraosseus fibrous lesions and odontogenic tumors can be removed by surgical enucleation and curettage for patients who are symptomatic or at risk for localized bony destruction30.
Future trends
The introduction of mTORi for the treatment of TSC has shifted the management of cutaneous and systemic disease associated with TSC. Those receiving systemic treatment for internal tumors may require fewer treatments for skin lesions. Topical sirolimus for facial angiofibromas may lessen the need for surgical interventions, reducing the risks of surgery and permanent scarring. Large scale, placebo controlled trials are needed to evaluate the long-term safety and efficacy of these topical therapies.
Acknowledgement:
We thank Drs. Hope Northrup and Darcy Krueger for co-chairing the conference and providing input on the final manuscript. We thank Steven Roberds and Katie Smith of the TS Alliance for providing logistical support. We regret that we were unable to cite many original papers owing to space constraints.
Funding/Support:
The 2012 International TSC Clinical Consensus Conference was sponsored and organized by the Tuberous Sclerosis Alliance. The conference was supported in part by the Rothberg Institute for Childhood Diseases, Novartis Pharmaceuticals, Sandra and Brian O’Brien, and Questcor Pharmaceuticals. Photography was obtained with support of the Intramural Research Program, National Institutes of Health, National Heart, Lung, and Blood Institute.
Design and Conduct of study: No
Collection, management, analysis, and interpretation of data: No
Preparation, review, or approval of manuscript: No
Decision to submit manuscript for publication: No
Financial Disclosure: None reported.
References
- 1.Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372(9639):657–668. [DOI] [PubMed] [Google Scholar]
- 2.Au KS, Northrup H. Genotype-Phenotype Studies in TSC and Molecular Diagnostics. In: Kwiatkowski DJ, Whittemore VH, Thiele EA, eds. Tuberous Sclerosis Complex: Genes, Clinical Features, and Therapeutics. Weinheim, Germany: WILEY-VCH Verlag GmbH & Co. KGaA; 2010:61–84. [Google Scholar]
- 3.Bissler JJ, Kingswood JC, Radzikowska E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2013;381(9869):817–824. [DOI] [PubMed] [Google Scholar]
- 4.Franz DN, Belousova E, Sparagana S, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381(9861):125–132. [DOI] [PubMed] [Google Scholar]
- 5.Dabora SL, Franz DN, Ashwal S, et al. Multicenter Phase 2 Trial of Sirolimus for Tuberous Sclerosis: Kidney Angiomyolipomas and Other Tumors Regress and VEGF- D Levels Decrease. PLoS ONE. 2011;6(9):e23379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bissler JJ, McCormack FX, Young LR, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358(2):140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roach ES, Gomez MR, Northrup H. Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol. 1998;13(12):624–628. [DOI] [PubMed] [Google Scholar]
- 8.Roach ES, DiMario FJ, Kandt RS, Northrup H. Tuberous Sclerosis Consensus Conference: recommendations for diagnostic evaluation. National Tuberous Sclerosis Association. J Child Neurol. 1999;14(6):401–407. [DOI] [PubMed] [Google Scholar]
- 9.Northrup H, Krueger DA. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatr Neurol. 2013;49(4):243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krueger DA, Northrup H. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49(4):255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darling TN, Moss J, Mausner M. Dermatologic Manifestations of Tuberous Sclerosis Complex. In: Kwiatkowski DJ, Whittemore VH, Thiele EA, eds. Tuberous Sclerosis Complex: Genes, Clinical Features, and Therapeutics. Weinheim, Germany: WILEY-VCH Verlag GmbH & Co. KGaA; 2010:285–309. [Google Scholar]
- 12.Sparling JD, Hong CH, Brahim JS, Moss J, Darling TN. Oral findings in 58 adults with tuberous sclerosis complex. J Am Acad Dermatol. 2007;56(5):786–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jozwiak S, Schwartz RA, Janniger CK, Bielicka-Cymerman J. Usefulness of diagnostic criteria of tuberous sclerosis complex in pediatric patients. J Child Neurol. 2000;15(10):652–659. [DOI] [PubMed] [Google Scholar]
- 14.Wataya-Kaneda M, Tanaka M, Hamasaki T, Katayama I. Trends in the prevalence of tuberous sclerosis complex manifestations: an epidemiological study of 166 Japanese patients. PLoS ONE. 2013;8(5):e63910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mlynarczyk G Enamel pitting: a common symptom of tuberous sclerosis. Oral Surg Oral Med Oral Pathol. 1991;71(1):63–67. [DOI] [PubMed] [Google Scholar]
- 16.Aldrich CS, Hong CH, Groves L, Olsen C, Moss J, Darling TN. Acral lesions in tuberous sclerosis complex: insights into pathogenesis. J Am Acad Dermatol. 2010;63(2):244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlson RM, Lloyd KM, Campbell TE. Acquired periungual fibrokeratoma: a case report. Cutis. 2007;80(2):137–140. [PubMed] [Google Scholar]
- 18.Yates JR, Maclean C, Higgins JN, et al. The Tuberous Sclerosis 2000 Study: presentation, initial assessments and implications for diagnosis and management. Arch Dis Child. 2011;96(11):1020–1025. [DOI] [PubMed] [Google Scholar]
- 19.Karabiber H, Sasmaz S, Turanli G, Yakinci C. Prevalence of hypopigmented maculae and cafe-au-lait spots in idiopathic epileptic and healthy children. J Child Neurol. 2002;17(1):57–59. [DOI] [PubMed] [Google Scholar]
- 20.Vanderhooft SL, Francis JS, Pagon RA, Smith LT, Sybert VP. Prevalence of hypopigmented macules in a healthy population. J Pediatr. 1996;129(3):355–361. [DOI] [PubMed] [Google Scholar]
- 21.Asgharian B, Turner ML, Gibril F, Entsuah LK, Serrano J, Jensen RT. Cutaneous tumors in patients with multiple endocrine neoplasm type 1 (MEN1) and gastrinomas: prospective study of frequency and development of criteria with high sensitivity and specificity for MEN1. J Clin Endocrinol Metab. 2004;89(11):5328–5336. [DOI] [PubMed] [Google Scholar]
- 22.Darling TN, Skarulis MC, Steinberg SM, Marx SJ, Spiegel AM, Turner M. Multiple facial angiofibromas and collagenomas in patients with multiple endocrine neoplasia type 1. Arch Dermatol. 1997;133(7):853–857. [PubMed] [Google Scholar]
- 23.Schaffer JV, Gohara MA, McNiff JM, Aasi SZ, Dvoretzky I. Multiple facial angiofibromas: a cutaneous manifestation of Birt-Hogg-Dube syndrome. J Am Acad Dermatol. 2005;53(2 Suppl 1):S108–111. [DOI] [PubMed] [Google Scholar]
- 24.Toro JR, Wei MH, Glenn GM, et al. BHD mutations, clinical and molecular genetic investigations of Birt-Hogg-Dube syndrome: a new series of 50 families and a review of published reports. J Med Genet. 2008;45(6):321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seibert D, Hong CH, Takeuchi F, et al. Recognition of tuberous sclerosis in adult women: delayed presentation with life-threatening consequences. Ann Intern Med. 2011;154(12):806–813, W-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia Y, Darling TN. Rapidly growing collagenomas in multiple endocrine neoplasia type I. J Am Acad Dermatol. 2007;56(5):877–880. [DOI] [PubMed] [Google Scholar]
- 27.Kataoka M, Kido J, Shinohara Y, Nagata T. Drug-induced gingival overgrowth--a review. Biol Pharm Bull. 2005;28(10):1817–1821. [DOI] [PubMed] [Google Scholar]
- 28.Giunta JL. Gingival fibrous nodule. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88(4):451–454. [DOI] [PubMed] [Google Scholar]
- 29.Torrelo A, Hadj-Rabia S, Colmenero I, et al. Folliculocystic and collagen hamartoma of tuberous sclerosis complex. J Am Acad Dermatol. 2012;66(4):617–621. [DOI] [PubMed] [Google Scholar]
- 30.Barron RP, Kainulainen VT, Forrest CR, Krafchik B, Mock D, Sandor GK. Tuberous sclerosis: clinicopathologic features and review of the literature. J Craniomaxillofac Surg. 2002;30(6):361–366. [DOI] [PubMed] [Google Scholar]
- 31.Jozwiak S, Schwartz RA, Janniger CK, Michalowicz R, Chmielik J. Skin lesions in children with tuberous sclerosis complex: their prevalence, natural course, and diagnostic significance. Int J Dermatol. 1998;37(12):911–917. [DOI] [PubMed] [Google Scholar]
- 32.Vincent A, Farley M, Chan E, James WD. Birt-Hogg-Dube syndrome: a review of the literature and the differential diagnosis of firm facial papules. J Am Acad Dermatol. 2003;49(4):698–705. [DOI] [PubMed] [Google Scholar]
- 33.Starink TM, Houweling AC, van Doorn MB, et al. Familial multiple discoid fibromas: a look-alike of Birt-Hogg-Dube syndrome not linked to the FLCN locus. J Am Acad Dermatol. 2012;66(2):259 e251–259. [DOI] [PubMed] [Google Scholar]
- 34.Tyburczy ME, Wang JA, Li S, et al. Sun exposure causes somatic second-hit mutations and angiofibroma development in tuberous sclerosis complex. Hum Mol Genet. 2014;23(8):2023–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curatolo P, Moavero R. mTOR Inhibitors in Tuberous Sclerosis Complex. Current neuropharmacology. 2012;10(4):404–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nashan B, Citterio F. Wound healing complications and the use of mammalian target of rapamycin inhibitors in kidney transplantation: a critical review of the literature. Transplantation. 2012;94(6):547–561. [DOI] [PubMed] [Google Scholar]
- 37.Pallet N, Legendre C. Adverse events associated with mTOR inhibitors. Expert opinion on drug safety. 2013;12(2):177–186. [DOI] [PubMed] [Google Scholar]
- 38.Haemel AK, O’Brian AL, Teng JM. Topical rapamycin: a novel approach to facial angiofibromas in tuberous sclerosis. Arch Dermatol. 2010;146(7):715–718. [DOI] [PubMed] [Google Scholar]
- 39.Wataya-Kaneda M, Tanaka M, Nakamura A, Matsumoto S, Katayama I. A topical combination of rapamycin and tacrolimus for the treatment of angiofibroma due to tuberous sclerosis complex (TSC): a pilot study of nine Japanese patients with TSC of different disease severity. Br J Dermatol. 2011;165(4):912–916. [DOI] [PubMed] [Google Scholar]
- 40.Koenig MK, Hebert AA, Roberson J, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex: a double-blind, randomized, controlled trial to evaluate the safety and efficacy of topically applied rapamycin. Drugs in R&D. 2012;12(3):121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tu J, Foster RS, Bint LJ, Halbert AR. Topical rapamycin for angiofibromas in paediatric patients with tuberous sclerosis: Follow up of a pilot study and promising future directions. Australas J Dermatol. 2014;55(1):63–69. [DOI] [PubMed] [Google Scholar]
- 42.Papadavid E, Markey A, Bellaney G, Walker NP. Carbon dioxide and pulsed dye laser treatment of angiofibromas in 29 patients with tuberous sclerosis. Br J Dermatol. 2002;147(2):337–342. [DOI] [PubMed] [Google Scholar]
- 43.Belmar P, Boixeda P, Baniandres O, Fernandez-Lorente M, Arrazola JM. [Long-term follow up of angiofibromas treated with CO2 laser in 23 patients with tuberous sclerosis]. Actas dermo-sifiliograficas. 2005;96(8):498–503. [DOI] [PubMed] [Google Scholar]
- 44.Weinberger CH, Endrizzi B, Hook KP, Lee PK. Treatment of angiofibromas of tuberous sclerosis with 5-aminolevulinic acid blue light photodynamic therapy followed by immediate pulsed dye laser. Dermatol Surg. 2009;35(11):1849–1851. [DOI] [PubMed] [Google Scholar]
- 45.Weiss ET, Geronemus RG. New technique using combined pulsed dye laser and fractional resurfacing for treating facial angiofibromas in tuberous sclerosis. Lasers Surg Med. 2010;42(5):357–360. [DOI] [PubMed] [Google Scholar]
- 46.Guideline on management of dental patients with special health care needs. Pediatric dentistry. 2012;34(5):160–165. [PubMed] [Google Scholar]
- 47.Eisen DB, Fazel N. Treatment of gingival fibromas using CO2 laser and electrosurgery in a patient with tuberous sclerosis. Dermatol Online J. 2008;14(11):7. [PubMed] [Google Scholar]
- 48.Korol UB, Schoor R, Nanda V, Almas K, Phelan JA. Gingival enlargement as a manifestation of tuberous sclerosis: case report and periodontal management. J Periodontol. 2008;79(4):759–763. [DOI] [PubMed] [Google Scholar]
- 49.Cota LO, Aquino DR, Franco GC, Cortelli JR, Cortelli SC, Costa FO. Gingival overgrowth in subjects under immunosuppressive regimens based on cyclosporine, tacrolimus, or sirolimus. Journal of clinical periodontology. 2010;37(10):894–902. [DOI] [PubMed] [Google Scholar]
- 50.Pereira-Lopes O, Sampaio-Maia B, Sampaio S, et al. Periodontal inflammation in renal transplant recipients receiving everolimus or tacrolimus - preliminary results. Oral diseases. 2013;19(7):666–672. [DOI] [PubMed] [Google Scholar]


