Abstract
Purpose of Review
Type 2 diabetes is common, burdensome, and preventable. Landmark trials such as the Diabetes Prevention Program (DPP) demonstrated that resource–intensive lifestyle support interventions resulting in modest weight loss via healthy diet changes and physical activity can lower the rate of diabetes development by 58%. We performed a review of efforts to translate and implement DPP-like programs throughout the USA to identify challenges and opportunities for improvement.
Recent Findings
For more than a decade, multiple stakeholders have worked to translate evidence-based principles of diabetes prevention to reach 84 million Americans with prediabetes. DPP-like programs have been delivered by over 1500 organizations, reaching almost 300,000 people, but this number represents less than 1% of the target population. Research has uncovered large gaps in efforts to diagnose, raise awareness, and provide access to DPP-like programs for adults with prediabetes, requiring further stakeholder engagement and coordination to resolve.
Summary
Efforts to address prevailing gaps in diabetes prevention must address distinct and sometimes conflicting priorities and concerns of stakeholders. Our review recommends several areas of further research and action to improve type 2 diabetes prevention on a population scale.
Keywords: Prediabetes, Type 2 diabetes, Diabetes prevention, Dissemination of evidence, Implementation challenges
Introduction
More than 30 million US adults have type 2 diabetes [1], exacting a large health and economic burden for individuals who have the condition, their caregivers, and society. This burden is preventable. Major randomized trials provide strong evidence that intensive lifestyle interventions promoting healthy diet, physical activity, and modest weight loss reduce the risk of developing type 2 diabetes [2–5]. The US Diabetes Prevention Program (DPP) demonstrated that an intensive lifestyle intervention lowers the development of type 2 diabetes by 58% among adults who have elevated blood glucose concentrations just below the threshold used for a diagnosis of diabetes. Individuals with elevated blood glucose have since been labeled as having “prediabetes,” and are being targeted for prevention efforts.
Despite having blood glucose concentrations below a threshold for diabetes treatment, some people experience direct harm from prediabetes, developing micro- and macrovascular complications even before the onset of type 2 diabetes [6, 7]. In addition, up to 10% of people with prediabetes will develop type 2 diabetes each year [2, 8], which then requires more intensive medication management, self-monitoring of blood glucose, and lifestyle modifications. Despite these risks, however, prediabetes is asymptomatic, making it a problem that most individuals do not know they have. The CDC estimates that about 84.1 million Americans have prediabetes, but only about 12% identified through screening report that they previously had been made aware of their risk status [9•].
The evidence that prediabetes is common, harmful, treatable, and a “silent threat” to most people has elevated it as a public health priority. The public health community has taken considerable action to raise awareness, recommend population screening to identify individuals with prediabetes, and support efforts to provide access to evidence-based intensive lifestyle interventions. Today, more than 1500 organizations are delivering high-fidelity, DPP-like programs throughout the USA, but total participation has reached only about 0.4% of the 65 million Americans with prediabetes who are overweight or obese [10, 11••]. The purpose of this narrative review is to summarize evidence regarding challenges faced during implementation and scaling of diabetes prevention interventions, as well as to highlight gaps in this evidence and discuss potential opportunities to improve population prevention efforts.
Organizing Framework for the Review
Ongoing efforts to translate and implement evidence-based diabetes prevention approaches involve numerous stakeholder groups, each with distinct yet complementary goals that underlie specific implementation challenges and opportunities. Our literature summary is organized according to key implementation priorities and concerns of different stakeholder groups (Table 1), with discussion of implications and opportunities for future work in the text that follows.
Table 1.
Examples of the implementation priorities and concerns of different stakeholder groups
| Public health stakeholders |
|
| DPP providers |
|
| Third-party payers |
|
| Healthcare providers |
|
| At-risk individuals |
|
Priorities and Concerns for Public Health Stakeholders
The public health sector, led by the US Centers for Disease Control and Prevention (CDC), has assumed a pivotal role in organizing other stakeholders and empowering them with resources designed to support the implementation of evidence-based prediabetes detection and DPP delivery efforts on a national scale. CDC has played a key role in promoting pre-diabetes screening and diagnosis recommendations, which have evolved greatly since 2003. The DPP trial only enrolled participants with both elevated fasting glucose and impaired glucose tolerance (IGT), defined by plasma glucose levels of 140–199 mg/dL 2 h after a 75-g oral glucose challenge. This requirement identified a population with a very high rate of diabetes development, about 11% per year [2]. Adopting these same criteria for population-based diabetes prevention efforts following the DPP presented challenges, because oral glucose tolerance tests (OGTTs) are not performed routinely in most US clinical practice settings.
Recognizing this, the CDC aligned with recommendations of the American Diabetes Association (ADA), which in 2003 expanded its criteria for defining prediabetes to include individuals with fasting plasma glucose (FPG) levels as low as 100 mg/dL. ADA made this recommendation based on analysis of longitudinal studies suggesting that the use of this lower cutoff would maximize the area under the ROC curve for predicting progression to diabetes [13]. ADA also argued that if the OGTT was not performed, retaining the previous (higher) FPG range of 110–125 mg/dL would miss 56% of people with IGT who have FPG levels below 110 mg/dL [14]. In 2010, ADA added hemoglobin A1c 5.7–6.4% as a third criterion for diagnosis of prediabetes [14]. Though these recommendations achieved the goals of improving the feasibility and sensitivity of prediabetes case finding in real-world clinical practice settings, they also introduced two major challenges for public health action: (1) a doubling of the US target population for prevention efforts and (2) assigning a diagnosis of prediabetes to many people who are at lower risk for diabetes development than participants in the DPP trial. Whereas, the rate of diabetes development in the control group of the DPP trial was about 11% per year [2], the average rate of progression among all 84.1 million Americans who meet the current definition of prediabetes today is likely closer to 1 to 2% per year [1, 8].
If the target population for DPP-like interventions has a lower probability of developing diabetes without receiving treatment, then the number of people who must be treated to prevent one case of type 2 diabetes will be higher than in the DPP trial. The number of people needing treatment is important when considering policies that may transfer the costs of a DPP-like intervention from the individual participant to a health insurance provider, employer, or public program. When the budget of these third-party payers is limited, it is possible that some higher risk individuals may be less likely to receive the DPP as attention and resources are focused partly on lower risk individuals. More research is needed to define the best range of glycemic test results for which intensive lifestyle interventions have the most favorable number needed to treat and cost-effectiveness. It also is important to explore whether it is of value to offer all people with prediabetes the same DPP-like interventions or whether more intensive and costly interventions should be reserved for higher risk sub-groups. Also, elevated blood glucose is a sign rather than a cause of prediabetes [7], so strategies to identify higher risk or “higher benefitting” groups need not be defined by glucose or A1c testing. Further research is needed on other biomarkers that may be useful in routine clinical practice to guide targeting of patients for DPP interventions.
CDC also has played a central role supporting the development of a large delivery network for DPP-like programs. These efforts have focused on preserving fidelity to evidence-based intervention components, while reducing costs for program providers, participants, and third-party payers. Though it is likely that most overweight or obese adults could benefit from healthy eating, physical activity, and modest weight loss, research has shown that highly intensive forms of support are needed to achieve success, and providing this support imposes an incremental cost for each person who receives treatment [15]. For example, the DPP’s one-on-one coaching format involved about 25 contact hours per person per year, and it cost approximately $1400 per person in 2003 [16]. Because many individuals may be unable to pay such a fee, much attention has been placed on whether adaptations to the DPP’s format can lower intervention costs without diminishing efficacy.
Research on DPP-like programs has demonstrated that weight loss efficacy is greatest for lifestyle interventions that focus on both healthy dietary behaviors and physical activity, while incorporating behavioral approaches like goal setting, self-monitoring, self-regulation, stimulus control, and problem solving [17]. According to systematic reviews, DPP-like interventions are more effective when they involve at least 8 to 26 h of intervention contact time delivered during 12 to 26 sessions that are offered over the first 12 to 18 months [18]. This level of contact helps to define a minimum necessary “dose” of intervention exposure. Moreover, the facilitator or “coach” adds a critical element of “supportive accountability” [19]. Accountability toward a behavior exists when a person has the expectation that he/she may be called upon to justify his/her actions or inactions to another person [19]. Establishing accountability requires real or perceived interactions with another human (i.e., a coach) that can occur in person, by telephone, or via an electronic channel. Though attempts should be made to preserve fidelity to essential components, there also is a pressing need to diversify formats and delivery channels of DPP-like programs to engage different segments of our population in ways that are attractive, fun, accessible, and culturally salient.
In DPP, about 43% of intervention costs were attributable to individual visits with interventionists [16]. One primary strategy used by stakeholders to reduce costs has been conversion to a group delivery format, which could eliminate about 71% of total intervention costs [16]. Other strategies to lower costs include using lower cost or non-profit delivery organizations, such as YMCAs, or “virtual” coaching via the internet [20]. A large body of research demonstrates the effectiveness of group-based DPP adaptations delivered in diverse community settings [20]. Additional studies have developed and tested alternate delivery formats, such as via telephone, web-based programs, and smartphone applications [21]. Taken together, this significant body of work has reported weight loss and other cardiometabolic improvements that are only slightly less than in the DPP trial [20, 22, 23]. Informed by this research, the CDC established the National Diabetes Prevention Program (NDPP) in 2010 to provide infrastructure that would support delivery of evidence-based DPP-like programs on a national scale [10].
The NDPP promotes large-scale diabetes prevention efforts in the following ways: (1) training individuals to deliver the program, (2) supporting a network of DPP delivery sites, (3) recognizing organizations that implement the program with fidelity and effectiveness, and (4) recommending marketing approaches to increase program participation by high-risk individuals [24]. By providing an assurance of program fidelity, NDPP aims to encourage participant referrals from healthcare providers who typically are skeptical of recommending resources outside the health system. In addition, an assurance of effectiveness also encourages third-party payment of DPP services by a participant’s health insurer or employer. The work of these additional stakeholders is summarized further below.
By supporting DPP workforce development and other forms of capacity building for community program providers, the NDPP has assembled a network of 1520 DPP suppliers that are registered with the NDPP certification program, with approximately one-third having full recognition status [10]. Recognized programs are in place in all 50 states, and roughly 1 in 4 of all US counties [25, 26]. However, a 2017 CDC study reported that counties with the greatest socioeconomic disadvantages and the highest incidence of diabetes were less likely to have NDPP-recognized programs [25], and a more recent analysis found that only 14.7% of rural counties (compared with 47.8% of urban counties) had access to at least one NDPP program (p < 0.001) [26]. As adoption of NDPP continues to expand, further research must examine NDPP access at the local and individual level. It also will be important to differentiate whether participation levels that remain lower than expected are a result of limited program availability, a lack of interest in the formats that are available, or other barriers such as low awareness.
Priorities and Concerns for DPP Providers
Small businesses and many community organizations succeed by providing goods and services that are in high demand by individuals. Some community organizations, such as YMCAs, became involved in DPP implementation because the health and well-being of people and families is central to their mission [27]. Unlike many other programs that YMCAs traditionally offer, a decision to offer DPP did not begin because community residents or members asked for it [28]; the “business case” was initially less clear. Tax exempt community organizations such as YMCAs have limited capital to invest in administrative and IT infrastructure, workforce development, or legal steps needed for delivery of DPP, particularly if the implementation requirements are very different from their usual business practices. NDPP recognition and now the Medicare Diabetes Prevention Program (MDPP), which enables federal payment for select NDPP providers that deliver services to Medicare beneficiaries, have raised the bar considerably, adding requirements such as local health system partnerships to identify eligible participants, new data collection and reporting requirements, HIPAA considerations, legal agreements, and fraud and abuse protections [29]. Though NDPP offers support for meeting DPP provider recognition requirements, the added steps required to seek reimbursement from Medicare and some other third-party payers may simply be insurmountable for many smaller organizations. This likely has limited the adoption and reach of the MDPP, with only 153 organizations currently operating as suppliers in 639 locations in 40 states nationally [29].
Participant-level eligibility criteria required by NDPP also present additional challenges for providers. Full recognition as an NDPP provider requires that at least 35% of participants have either a blood test indicating prediabetes or have a history of gestational diabetes mellitus [12]. The remaining participants may be eligible with a high score on the CDC/ADA Prediabetes Risk Test [30]. Although these criteria enable recruiting participants through multiple channels, they limit who can enroll, and therefore may force some interested consumers away from the program. NDPP’s recognition criteria also limit the extent to which local delivery organizations can modify the intervention content and formats. Although these policies are in place to retain fidelity to the DPP, they may also constrain providers from designing unique program elements that could make interventions more engaging or culturally salient for the populations they serve. Efforts to expand NDPP and MDPP capacity nationally must continually seek to balance the public health sector’s goal of preserving program fidelity with a service organization’s goal to provide a program that is in high demand by a large population of eligible individuals. Research is still needed to validate the efficacy of novel delivery channels and curriculum adaptations that allow local providers to introduce consumer-driven changes to the program, with the goals of increasing DPP participation without diminishing effectiveness.
Priorities and Concerns for Third-Party Payers
The last 10 years have witnessed the advent of third-party payments for DPP delivery. The DPP has been shown to improve health-related quality of life [31], diminish absences from work [16], enable some people to reduce their need for medications to treat high blood pressure or for new onset diabetes [32], and possibly lower future healthcare expenditures [15, 33, 34]. US employers purchase healthcare for their employees, which drives their interest in providing resources and services that can improve both the health and productivity of their workforce. Employers are also interested in providing access to wellness benefits, which has become common in the marketplace [35]. In this context, many employers have considered paying for access to DPP-like programs on behalf of eligible employees.
Health insurance payers are a second stakeholder group with interest in providing payment on behalf of participants. Health insurers generally have interest in paying for services that are effective and provide value, but they must also be affordable within existing budget constraints. Insurance plans and programs operate under fixed sources of financing, such as monthly premium payments from individuals and their employers or tax revenues that are allocated for use by public insurance programs. Adopting a health payer policy to reimburse DPP providers for participation by health plan enrollees would generally require that premiums be increased to provide added funding or that the costs spent on the DPP today are considered budget neutral because participation somehow leads to better health and, subsequently, lower healthcare expenditures on other health issues, such as a new case of diabetes. This funding challenge often results in health plans offering the DPP to only those individuals who are at the highest risk for developing diabetes, a group for whom costs are anticipated to increase much more if the DPP is not provided. Another strategy has been to negotiate with DPP providers to accept payments only for individuals who achieve a minimum threshold of classes attended or weight loss achieved (i.e., only pay if the program appears to be working) [27, 29].
As one example, YMCA of the USA partnered with UnitedHealth Group, CDC, and others in 2010 to implement an adapted version of the DPP intensive lifestyle intervention nationally, with the support of payments from employers and private health insurers [27, 36]. UnitedHealth contracted with numerous employers to provide access to the DPP, which was offered by local YMCAs to any employee with prediabetes. Most eligible employees were identified via blood testing performed during work–health screening events, and UnitedHealth coordinated with YMCAs to enroll high-risk individuals in local DPP programs. UnitedHealth then provided YMCA with escalating payments based on participant attendance and weight loss outcomes. Overall, the screening approach, which was outside of healthcare delivery, identified only about 10% of the target population [36]. However, 39% of those identified with prediabetes attended the DPP, and mean weight loss from the first to last visit was 7.5 pounds (3.4%) [36]. The payment scheme limited YMCA’s charges to UnitedHealth to an average of only $212 per person. Paying this additional cost did not increase net health expenditures for UnitedHealth over the first 2 years [36]. Similar payment approaches are now being used by many other third-party payers nationally, including Medicare [29], but the worksite-based screening approach proved to be too costly and inefficient, causing UnitedHealth to discontinue this strategy. Today, insurance coverage for NDPP providers represents a sustainable and important action for expanding access nationally, and alternative forms of participant engagement, such as via healthcare-based screening and referral, have become more of an area of focus.
Priorities and Concerns for Healthcare Providers
The US Preventive Services Task Force (USPSTF) is an independent panel of experts that develops national practice recommendations for performance of clinical preventive services. Evidence given a grade of “A” or “B” by USPSTF is considered sufficiently strong to recommend that a preventive service be performed routinely. In 2014, USPSTF issued a grade “B” recommendation that healthcare providers offer intensive lifestyle interventions to overweight or obese adults with cardiovascular risk conditions, including prediabetes [37]. This recommendation highlighted the DPP as an example of the type of program that healthcare providers should offer. In 2015, the USPSTF issued another “B” recommendation that overweight or obese adults aged 40 to 70 undergo screening for prediabetes and diabetes [38]. The Affordable Care Act (ACA) directs commercial health plans, Medicare, and Medicaid programs to provide coverage for any USPSTF “A” or “B” recommendation, with no cost-sharing imposed upon eligible patients [39]. Conceptually, this should alleviate concerns by healthcare providers about recommending services that might otherwise impose costs for their patients. However, this action does not address the workflow challenges imposed on busy healthcare teams by introducing a new preventive service [40]. Despite strong guidance from USPSTF and others, most people living with prediabetes today continue to have low awareness, with only about 12% of American adults with screen-detected prediabetes reporting that they have been told by a healthcare provider about their high-risk status [9•].
Health systems concerned with implementing routine prediabetes screening, risk communication, and NDPP referrals would benefit from understanding if lack of patient awareness is a result of low performance of screening tests or a failure to communicate effectively with patients when a test result indicates prediabetes. Past research does suggest that many healthcare providers have limited knowledge about assessing patients’ diabetes risk. A survey of 155 primary care providers found that only 6% identified all risk factors that should prompt screening for diabetes [41]. That said, analysis of electronic health records suggest that as many as 75% of primary care patients with risk factors have completed a test that could be used to determine their prediabetes status [42, 43]. However, only about half of those with tests that are abnormal have evidence that a diagnosis of prediabetes has been made [42]. Missed opportunities to diagnose prediabetes routinely could result from knowledge gaps or unreliable workflows for ensuring that test results are interpreted and acted upon. Less is known about whether some patients are told of prediabetes but that the risk or treatment information is not communicated in ways that are understandable or actionable. More research is needed to demonstrate how best to perform screening routinely, to document prediabetes consistently, and to effectively communicate to patients what may happen to them with and without their participation in DPP-like intervention programs.
Finally, when healthcare providers do identify and inform patients of prediabetes, it is also recommended that they provide referrals to available intervention options. Overall, healthcare providers’ awareness of DPP-like programs remains limited. A national survey of 1256 primary care providers found that 38% were aware of DPP-based interventions and only 23% had made a referral [44]. Even when aware, primary care providers also may deprioritize prediabetes as less urgent than many other health conditions [45].
Some current research is evaluating whether other members of the primary care team can effectively counsel patients about prediabetes and its management. One study tested a shared decision-making intervention led by primary care pharmacists, which involved reviewing a web-based decision aid with prediabetic patients for approximately 40 min [46]. Those who received the intervention had higher uptake of DPP-based programs compared with propensity-matched controls (23.4% vs. 0.4%, P < 0.001) [46]. Overall, our understanding of healthcare provider knowledge, attitudes, and beliefs toward prediabetes remains limited, and more research is needed to guide how electronic health record tools and different members of healthcare teams can be leveraged to routinely offer prediabetes treatment options, to assist interested patients in navigating DPP access and third-party payment, and to coordinate with DPP providers to ensure ongoing engagement.
Priorities and Concerns for Individuals
The CDC recently reported that all organizations recognized by NDPP had collectively reached 297,065 eligible adults through January 2019 [47]. These numbers are encouraging and show great progress in reaching many individuals with DPP-like programs. However, more than half of participants may qualify without a blood test, so participation may be lower among people with confirmed prediabetes. At best, this number constitutes only about 0.4% US adults with prediabetes, demonstrating the need for greater reach of the program nationally. Also, a majority of attendees have been women (75.3%) and non-Hispanic white (64.6%) [47], so there is a need to increase the engagement of other high-risk groups. For example, Hispanics constitute only 9.0% of NDPP participants to date [47], while comprising over 18% of the US population. Non-Hispanic blacks constitute 11.9% of NDPP participants to date [47], which is closer this group’s representation overall in the US adult population (13.4%). Among these underserved groups, efforts to increase access to the DPP, as well as rates of session attendance and completion, are urgently needed.
As reviewed above, population-based studies have demonstrated that only about 11.8% of the 84.1 million US adults with prediabetes (about 33.9% of the adult population) report being told by a healthcare provider of their high-risk status [9•]. However, if being aware was sufficient to achieve participation in the DPP, we would anticipate that about 4% (11.8% × 33.9%) of the population would be participating. Conversely, recent estimates suggest that only about 0.1% of the US non-pregnant adult population reports participating in a DPP-like program [11••]. This suggests a 40-fold gap between being made aware and participating in a DPP-like program. It is important to understand if the gap results from a deficit of available NDPP-like programs, low awareness of programs that are available, or limited interest in programs or the ways in which they are being delivered. In the same recent analysis, only 4.9% of adults who were aware of their prediabetes reported having been referred by their healthcare provider to a DPP-like program, and 39.6% of those who were referred reported taking part in such a DPP-like program [11••]. Moreover, “taking part” may not imply full participation or weight loss success. Even in the DPP trial, only 50% of lifestyle intervention participants met the weight loss goal after about 6 months [2]. In community implementation studies, this number has been closer to 30% [48]. For NDPP-recognized programs nationally, CDC has reported that 39.7% of participants attended ≥ 17 sessions, 31.4% achieved ≥ 5% weight loss, and 45.0% completed ≥ 150 min per week of physical activity [47]. An earlier analysis of NDPP participants found that male sex and older age (≥ 65 years old) were associated with achieving 5% weight loss [49], and that racial/ethnic minority participants had significantly lower odds of achieving 5% weight loss [49]. These “gaps” leading to lower than desired participation are illustrated in Fig. 1.
Fig. 1.
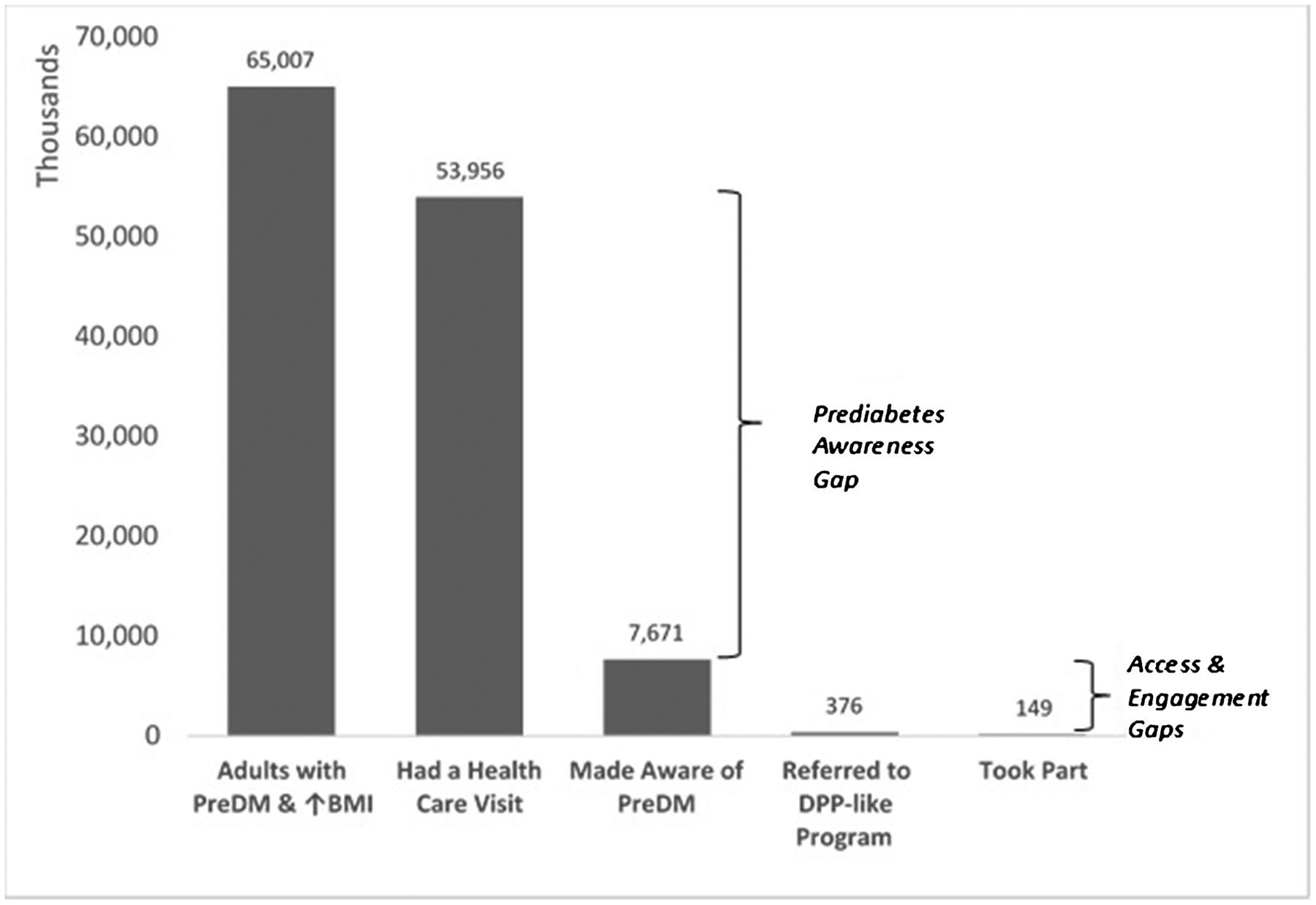
Nature of gaps leading to low overall participation in DPP-like programs nationally. (Figure created with data from references [9, 11])
Efforts to address participation gaps have centered on expanding program availability and healthcare provider referrals. It is possible that increasing local or eHealth program availability may itself increase referrals by making NDPP locations more visible to the healthcare community. Alternatively, some providers may only make referrals to patients whom they believe will attend. If true, then simply increasing referrals of “less interested” patients may not translate into as dramatic an effect on participation as might be expected. As efforts intensify to improve healthcare-to-NDPP referrals, more research is needed to guide how best to provide referrals in ways that most engage patients to attend a DPP-like intervention.
Another prevailing gap is our knowledge of the perceptions of individuals who have risk factors for prediabetes, as well as how to shift those perceptions to generate greater population-level demand for screening tests, referrals, and a variety of program designs that appeal to different segments of our society. Patients who ask their healthcare providers for recommended diagnostic or screening tests are significantly more likely to receive an order for that request [50, 51]. Similarly, patients who already understand the value of preventive services are more likely to accept and complete them when they are offered [52]. Little is currently known about whether and why some patients ask about prediabetes or to complete testing, while others do not. If the onus of detection rests purely on healthcare providers and not on people with risk factors, then closing the awareness gap may continue to proceed very slowly.
Promoting knowledge and awareness of prediabetes outside the clinical setting may also increase population-level demand for DPP-like programs. National stakeholders have collaborated to develop television advertisements aimed at educating the public about prediabetes [53]; however, to our knowledge, these efforts have not been evaluated. Successes from prior media campaigns focused on tobacco, human immunodeficiency virus, and other conditions may provide guidance for future public awareness efforts targeting prediabetes [54, 55]. Media campaigns targeting obesity have generally been less successful, highlighting challenges to promoting weight loss in this way [56]. Given the paucity of work in this area, there is a great opportunity for research focused on promoting public awareness about prediabetes and DPP-based programs through traditional media and social media.
Though the efforts of multiple stakeholder groups highlighted above may continue to improve prediabetes awareness and engagement in DPP-like programs, progress will remain limited if stakeholders assume that most people who are informed and referred will make a “rational” decision to participate in a DPP-like program when it is offered to them. On the contrary, health behavioral research indicates otherwise [57–59]. Paying people to participate also appears ineffective; one recent study found that offering financial incentives for DPP participation or outcomes can improve attendance but not weight loss success [60].
Because of the non-rational nature of many behavioral choices, recent work has attempted to map the “consumer journey” by which individuals become aware and informed of prediabetes and ultimately engage in DPP-like programs to achieve successful behavioral change [61]. This journey and its outcomes are influenced by knowledge, attitudes, and beliefs of individuals, shaped also by information and actions of multiple external influences and stakeholders. The net effect of these different influences on an individual’s actions is not always predictable. For example, many individuals who have previously been unsuccessful losing weight may develop “learned helplessness,” or a belief that they have little control over a making a successful change; other patients suffer over-confidence in their own ability to make meaningful lifestyle changes, causing both groups to resist engaging in a DPP-like program [61•]. Social influence and identity is also likely important, as many high-risk individuals live in neighborhoods or work environments where other individuals and groups may not be exhibiting healthy behaviors. In these contexts, any attempt by one individual to eat differently or exercise more may cause them to feel excluded or even stigmatized by others. Efforts to change social norms or shift key individuals to exhibit healthier behaviors as desirable or exciting could flip the direction of social influence and may have larger effects than individual efforts alone. This area of enhancing consumer engagement in DPP offerings using behavioral economic strategies is an important area for further research.
Conclusions
Now, 17 years following publication of the DPP, stakeholders spanning broad reaches of the public health, healthcare, community, commercial, health insurance, and consumer sectors are working together and in parallel to transfer the benefits of type 2 diabetes prevention to a very large and diverse population who are at high risk. Over 1500 NDPP providers have engaged almost 300,000 Americans in high-fidelity programs throughout the USA, but this level of reach represents only a small fraction of the population being targeted. Research has uncovered large gaps between opportunistic test results that are already available for as many as three quarters of high-risk individuals who see primary care providers, with only about 1 in 9 who report having been informed that they are at high risk. Moreover, only 1 in 20 who are aware have been referred to evidence-based DPP-like programs, and 40% of those referred have taken part. Addressing these gaps with research and further stakeholder coordination is essential. However, those efforts must address the distinct and sometimes conflicting priorities and concerns of different stakeholder groups. Including consumer perspectives more in the design of public health, health system, and commercial sector approaches is essential to success. Further research and innovation is needed, particularly in promising areas such as the use of behavioral economic approaches that address common individual barriers to action, as well as novel approaches to shift social norms and influence in ways that accelerate engagement in DPP-like programs and the adoption of behaviors that are known to provide the strongest opportunity currently known for type 2 diabetes prevention at a population scale.
Footnotes
Conflict of Interest Ronald T. Ackermann and Matthew J. O’Brien each declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.U.S. Centers for Diseaese Control and Prevention. National Diabetes Statistical Report Atlanta, GA, USA: Department of Health and Human Services; Centers for Disease Control and Prevention; 2017. [Available from: https://www.cdc.gov/diabetes/data/statistics/statistics-report.html.] [Google Scholar]
- 2.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–44. [DOI] [PubMed] [Google Scholar]
- 4.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49(2):289–97. [DOI] [PubMed] [Google Scholar]
- 5.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344(18):1343–50. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol 2010;55(13):1310–7. [DOI] [PubMed] [Google Scholar]
- 7.Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warren B, Pankow JS, Matsushita K, Punjabi NM, Daya NR, Grams M, et al. Comparative prognostic performance of definitions of prediabetes: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol 2017;5(1):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.•.Gopalan A, Lorincz IS, Wirtalla C, Marcus SC, Long JA. Awareness of Prediabetes and Engagement in Diabetes Risk-Reducing Behaviors. Am J Prev Med 2015;49(4):512–9 [DOI] [PubMed] [Google Scholar]; Findings from this study uncover the large prevailing gap in prediabetes awareness in the USA.
- 10.U.S. Centers for Diseaese Control and Prevention. National Diabetes Prevention Program Atlanta, GA: Centers for Disease Control and Prevention; 2019. [updated August 10, 2019. Available from: https://www.cdc.gov/diabetes/prevention/index.html.] [Google Scholar]
- 11.••.Ali MK, McKeever Bullard K, Imperatore G, Benoit SR, Rolka DB, Albright AL, et al. Reach and use of diabetes prevention services in the United States, 2016–2017. JAMA Netw Open. 2019;2(5):e193160. [DOI] [PMC free article] [PubMed] [Google Scholar]; Findings from this study begin to highlight the relative contributions of different barriers to participation in DPP-like programs, including limited healthcare-based detection, risk communication, and referral to DPP-like programs.
- 12.U.S. Centers for Diseaese Control and Prevention. Centers for Disease Control and Prevention Diabetes Prevention Recognition Program: Standards and Operating Procedures Atlanta, GA, USA: U.S. Centers for Disease Control and Prevention; 2018. [Available from: https://www.cdc.gov/diabetes/prevention/pdf/dprp-standards.pdf.] [Google Scholar]
- 13.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–7. [DOI] [PubMed] [Google Scholar]
- 14.James C, Bullard KM, Rolka DB, Geiss LS, Williams DE, Cowie CC, et al. Implications of alternative definitions of prediabetes for prevalence in U.S. adults. Diabetes Care. 2011;34(2):387–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li R, Qu S, Zhang P, Chattopadhyay S, Gregg EW, Albright A, et al. Economic evaluation of combined diet and physical activity promotion programs to prevent type 2 diabetes among persons at increased risk: a systematic review for the Community Preventive Services Task Force. Ann Intern Med 2015;163(6):452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herman WH, Brandle M, Zhang P, Williamson DF, Matulik MJ, Ratner RE, et al. Costs associated with the primary prevention of type 2 diabetes mellitus in the diabetes prevention program. Diabetes Care. 2003;26(1):36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venditti EM, Kramer MK. Necessary components for lifestyle modification interventions to reduce diabetes risk. Curr Diab Rep 2012;12(2):138–46. [DOI] [PubMed] [Google Scholar]
- 18.Leblanc ES, O’Connor E, Whitlock EP, Patnode CD, Kapka T. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2011;155(7):434–47. [DOI] [PubMed] [Google Scholar]
- 19.Mohr DC, Cuijpers P, Lehman K. Supportive accountability: a model for providing human support to enhance adherence to eHealth interventions. J Med Internet Res 2011;13(1):e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood). 2012;31(1):67–75. [DOI] [PubMed] [Google Scholar]
- 21.Tice JA, Chapman R, Shore KK, Seidner M, Ollendorf DA, Weissberg J, et al. Diabetes prevention programs: effectiveness and value. Boston: Institute for Clinical and Economic Review, (2016). [Available from: https://icer-review.org/wpcontent/uploads/2016/05/CTAF_DPP_Draft_Evidence_Report_050916-1.pdf.] [Google Scholar]
- 22.Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care. 2014;37(4): 922–33. [DOI] [PubMed] [Google Scholar]
- 23.Mudaliar U, Zabetian A, Goodman M, Echouffo-Tcheugui JB, Albright AL, Gregg EW, et al. Cardiometabolic risk factor changes observed in diabetes prevention programs in US settings: a systematic review and meta-analysis. PLoS Med 2016;13(7):e1002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albright AL, Gregg EW. Preventing type 2 diabetes in communities across the U.S.: the National Diabetes Prevention Program. Am J Prev Med 2013;44(4 Suppl 4):S346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jayapaul-Philip B, Dai S, Kirtland K, Haslam A, Nhim K. Availability of the National Diabetes Prevention Program in United States Counties, March 2017. Prev Chronic Dis 2018;15: E109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ariel-Donges AH, Gordon EL, Dixon BN, Eastman AJ, Bauman V, Ross KM, et al. Rural/urban disparities in access to the National Diabetes Prevention Program. Transl Behav Med 2019. Jun 22. 10.1093/tbm/ibz098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vojta D, Koehler TB, Longjohn M, Lever JA, Caputo NF. A coordinated national model for diabetes prevention: linking health systems to an evidence-based community program. Am J Prev Med 2013;44(4 Suppl 4):S301–6. [DOI] [PubMed] [Google Scholar]
- 28.Ackermann RT, Marrero DG. Adapting the Diabetes Prevention Program lifestyle intervention for delivery in the community: the YMCA model. Diabetes Educ 2007;33(1):69 74–5, 7–8. [DOI] [PubMed] [Google Scholar]
- 29.(CMS) CfMMS. Medicare Diabetes Prevention Program (MDPP) Expanded Model Baltimore, MD, USA: US Department of Health and Human Services; Centers for Medicare & Medicaid Services, ; CMS.gov; 2019. [Available from: https://innovation.cms.gov/initiatives/medicare-diabetes-prevention-program/.] [Google Scholar]
- 30.U.S. Centers for Diseaese Control and Prevention. Prediabetes Risk Test Atlanta, GA, USA: U.S. Centers for Disease Control and Prevention; 2019. [Available from: https://www.cdc.gov/prediabetes/takethetest/.] [Google Scholar]
- 31.Florez H, Pan Q, Ackermann RT, Marrero DG, Barrett-Connor E, Delahanty L, et al. Impact of lifestyle intervention and metformin on health-related quality of life: the diabetes prevention program randomized trial. J Gen Intern Med 2012;27(12):1594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratner R, Goldberg R, Haffner S, Marcovina S, Orchard T, Fowler S, et al. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the diabetes prevention program. Diabetes Care. 2005;28(4):888–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herman WH, Hoerger TJ, Brandle M, Hicks K, Sorensen S, Zhang P, et al. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med 2005;142(5):323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhuo X, Zhang P, Gregg EW, Barker L, Hoerger TJ, Tony P-C, et al. A nationwide community-based lifestyle program could delay or prevent type 2 diabetes cases and save $5.7 billion in 25 years. Health Aff (Millwood). 2012;31(1):50–60. [DOI] [PubMed] [Google Scholar]
- 35.Claxton G, Rae M, Damico A, Young G, McDermott D, Whitmore H, et al. Employer health benefits: 2019 annual survey. San Francisco: The Henry J. Kaiser Family Foundation, (2019). [Available from: https://www.kff.org/health-costs/report/2019-employer-health-benefits-survey/.] [Google Scholar]
- 36.Ackermann RT, Kang R, Cooper AJ, Liss DT, Holmes AM, Moran M, et al. Effect on health care expenditures during nationwide implementation of the Diabetes Prevention Program as a health insurance benefit. Diabetes Care. 2019;42(9):1776–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LeFevre ML. Force USPST. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2014;161(8):587–93. [DOI] [PubMed] [Google Scholar]
- 38.Siu AL. Screening for abnormal blood glucose and type 2 diabetes mellitus: U.S. Preventive Services Task Force Recommendation Statement. Annals of internal medicine. 2015;163(11):861–8. [DOI] [PubMed] [Google Scholar]
- 39.Patient Protection and Affordable Care Act, (2010). [Available from: http://www.ncsl.org/documents/health/ppaca-consolidated.pdf.]
- 40.Yarnall KS, Pollak KI, Ostbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? Am J Public Health. 2003;93(4):635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tseng E, Greer RC, O’Rourke P, Yeh HC, Mcguire MM, Albright AL, et al. National survey of primary care physicians’ knowledge, practices, and perceptions of prediabetes. J Gen Intern Med 2019: 34(11):2475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keck JW, Thomas AR, Hieronymus L, Roper KL. Prediabetes knowledge, attitudes, and practices at an academic family medicine practice. J Am Board Fam Med 2019;32(4):505–12. [DOI] [PubMed] [Google Scholar]
- 43.O’Brien MJ, Lee JY, Carnethon MR, Ackermann RT, Vargas MC, Hamilton A, et al. Detecting dysglycemia using the 2015 United States Preventive Services Task Force screening criteria: a cohort analysis of community health center patients. PLoS Med 2016;13(7):e1002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nhim K, Khan T, Gruss SM, Wozniak G, Kirley K, Schumacher P, et al. Primary care providers’ prediabetes screening, testing, and referral behaviors. Am J Prev Med 2018;55(2):e39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kandula NR, Moran MR, Tang JW, O’Brien MJ. Preventing diabetes in primary care: providers’ perspectives about diagnosing and treating prediabetes. Clin Diabetes. 2018;36(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moin T, Duru OK, Turk N, Chon JS, Frosch DL, Martin JM, et al. Effectiveness of shared decision-making for diabetes prevention: 12-month results from the Prediabetes Informed Decision and Education (PRIDE) Trial. J Gen Intern Med 2019;34(11):2652–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gruss SM, Nhim K, Gregg E, Bell M, Luman E, Albright A. Public health approaches to type 2 diabetes prevention: the US National Diabetes Prevention Program and Beyond. Curr Diab Rep 2019;19(9):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ackermann RT, Liss DT, Finch EA, Schmidt KK, Hays LM, Marrero DG, et al. A randomized comparative effectiveness trial for preventing type 2 diabetes. Am J Public Health. 2015;105(11): 2328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ely EK, Gruss SM, Luman ET, Gregg EW, Ali MK, Nhim K, et al. A national effort to prevent type 2 diabetes: participant-level evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care. 2017;40(10):1331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fenton JJ, Franks P, Feldman MD, Jerant A, Henry SG, Paterniti DA, et al. Impact of patient requests on provider-perceived visit difficulty in primary care. J Gen Intern Med 2015;30(2):214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kravitz RL, Bell RA, Azari R, Krupat E, Kelly-Reif S, Thom D. Request fulfillment in office practice: antecedents and relationship to outcomes. Med Care. 2002;40(1):38–51. [DOI] [PubMed] [Google Scholar]
- 52.Bednarczyk RA, Chamberlain A, Mathewson K, Salmon DA, Omer SB. Practice-, provider-, and patient-level interventions to improve preventive care: development of the P3 model. Prev Med Rep 2018;11:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.AdCouncil. Type 2 Diabetes Prevention New York, NY, USA: The Advertising Council, Inc.; 2019. [Available from: https://www.adcouncil.org/Our-Campaigns/Health/Type-2-Diabetes-Prevention.] [Google Scholar]
- 54.LaCroix JM, Snyder LB, Huedo-Medina TB, Johnson BT. Effectiveness of mass media interventions for HIV prevention, 1986–2013: a meta-analysis. J Acquir Immune Defic Syndr 2014;66(Suppl 3):S329–40. [DOI] [PubMed] [Google Scholar]
- 55.Niederdeppe J, Fiore MC, Baker TB, Smith SS. Smoking-cessation media campaigns and their effectiveness among socioeconomically advantaged and disadvantaged populations. Am J Public Health. 2008;98(5):916–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walls HL, Peeters A, Proietto J, McNeil JJ. Public health campaigns and obesity - a critique. BMC Public Health. 2011;11:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burns RJ, Donovan AS, Ackermann RT, Finch EA, Rothman AJ, Jeffery RW. A theoretically grounded systematic review of material incentives for weight loss: implications for interventions. Ann Behav Med 2012;44(3):375–88. [DOI] [PubMed] [Google Scholar]
- 58.Shaw PA, Yancy WS Jr, Wesby L, Ulrich V, Troxel AB, Huffman D, et al. The design and conduct of Keep It Off: An online randomized trial of financial incentives for weight-loss maintenance. Clin Trials. 2017;14(1):29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yancy WS Jr, Shaw PA, Wesby L, Hilbert V, Yang L, Zhu J, et al. Financial incentive strategies for maintenance of weight loss: results from an internet-based randomized controlled trial. Nutr Diabetes. 2018;8(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.VanEpps EM, Troxel AB, Villamil E, Saulsgiver KA, Zhu J, Chin JY, et al. Effect of process- and outcome-based financial incentives on weight loss among prediabetic New York Medicaid patients: a randomized clinical trial. Am J Health Promot 2019;33(3):372–80. [DOI] [PubMed] [Google Scholar]
- 61.•.Soler RE, Proia K, Jackson MC, Lanza A, Klein C, Leifer J, et al. Nudging to change: using behavioral economics theory to move people and their health care partners toward effective type 2 diabetes prevention. Diabetes Spectr 2018;31(4):310–9 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study proposes a framework for mapping important behavioral biases and principles of behavioral economics to the area of diabetes prevention.


