Abstract
Background
To date, there are no studies regarding the Mrp 8/14 in predicting the occurrence of acute respiratory distress syndrome (ARDS) induced by sepsis. Thus, the objective of this study was to investigate the expression of Myeloid-related proteins 8 and 14 (Mrp 8/14) and its role in ARDS induced by sepsis.
Methods
A total of 168 septic patients were enrolled in the observational study. The baseline information and clinical outcomes were obtained retrospectively. Serum Mrp 8/14 level was determined by enzyme linked immunosorbent assay (ELISA). The patients were categorized into sepsis and ARDS group based on whether they developed ARDS during the intensive care unit (ICU) hospitalization.
Results
There was significant difference in the level of Mrp 8/14 between the sepsis group and ARDS groups (P < 0.05). Mrp 8/14 correlated positively with procalcitonin (PCT), interleukin-6 (IL-6), acute physiology and chronic health evaluation II (APACHE II) score, sequential organ failure assessment (SOFA) score on day 1, mechanical ventilation time, length of ICU stay and hospitalization expenses in ICU (all P < 0.05). Logistic regression analysis showed Mrp 8/14 was the independent factor for forecasting the occurrence of sepsis- induced ARDS (P < 0.05). The areas under receiver operating characteristic curves for Mrp 8/14 were higher than that of PCT, APACHE II score and SOFA score on day 1 (P < 0.05).
Conclusion
The serum Mrp 8/14 level at admission may be a potential marker for predicting the occurrence of ARDS induced by sepsis. Early detection of serum Mrp 8/14 could help clinicians to identify and evaluate the severity of ARDS induced by sepsis.
Keywords: sepsis, acute respiratory distress syndrome, Mrp 8/14, inflammation, intensive care unit
Introduction
Sepsis, one of the most prevalent complications in the intensive care unit (ICU), refers to a life-threatening organ dysfunction caused by a dysregulated host response to infection.1,2 The lung, being the primary organ for gas exchange and a reservoir for pathogens in the body, is often the initial target organ susceptible to damage during sepsis and the compromised exchange of oxygen and nutrients in the pulmonary micro-circulation is considered the most dreaded complication of septic lung damage. Serious lung injury may lead to acute respiratory distress syndrome (ARDS) and ultimately respiratory failure.3 As a consequence, the occurrence of ARDS is frequently considered a fatal consequence of sepsis, with sepsis accounting for approximately 32% of its etiology.4 Alarmingly, despite significant advancements in the diagnosis of ARDS, there is still an upward trend in the prevalence of ARDS associated with sepsis. The mortality rate of sepsis-related ARDS is approximately 40%, and in certain countries, it can even reach up to 50%.5,6 Additionally, although the majority of individuals who have survived ARDS regain normal or near-normal pulmonary function, a significant number continues to experience functional limitations attributed to muscle weakness, deconditioning, or psychological consequences resulting from the severity of their illness.7,8 Therefore, the expeditious identification of ARDS induced by sepsis is an essential prerequisite for improving patients’ survival.
Currently, the diagnosis of ARDS primarily relies on the oxygenation defect, such as PaO2/FiO2 ratio, which is derived predominantly from clinical data according to the definition provided by Berlin.9 The PaO2/FiO2 ratio, however, is highly dependent on the ventilator settings, and the presence of pulmonary edema or dysfunction in the pulmonary vasculature can also have a detrimental impact. The current challenge lies in the absence of sensitive biomarkers that can serve as quantitative indicators, thus impeding accurate identification and prediction of ARDS occurrence and progression in septic patients.10,11 As a result, it is of significant importance to explore reliable biomarkers that are easily detectable in the early diagnosis and progression of ARDS induced by sepsis.
It is well established that the neutrophil activation and excessive inflammatory responses play a crucial role in the progression of sepsis-induced lung injury and ARDS.12,13 The protein myeloid-related proteins 8 and 14 (Mrp 8/14), also known as calprotectin or S100 calcium-binding protein A8 and A9 (S100 A8/A9), is a leukocyte calgranulin that constitutes 40% of the cytosolic protein content in neutrophils and is present at lower levels in monocytes.14 It has been reported that S100A9 expression was significantly increased in the lungs of lipopolysaccharide (LPS)-treated mice and inhibition of S100A9 could effectively attenuate the inflammatory responses and mitigate LPS-induced lung injury,15,16 indicating that S100A9 is closely related to lung injury induced by sepsis. Furthermore, recent studies have also suggested that S100A9 is associated with corona-virus disease 2019 (COVID-19)-induced pneumonia and ARDS,17,18 suggesting that S100A9 may also exert a significant impact on lung injury induced by COVID-19. However, whether serum Mrp 8/14 could be used as a potential marker for predicting the occurrence of ARDS induced by sepsis has not been previously investigated. In addition, there is currently a lack of clinical research investigating the potential correlation between serum Mrp 8/14 levels and the severity of ARDS.
Therefore, the aim of our exploratory study was to investigate whether neutrophil-derived plasma markers, Mrp 8/14, can serve as a reliable biomarker for early detection of sepsis-associated ARDS, thereby enabling timely intervention by clinicians and ultimately enhancing the overall quality of life.
Materials and Methods
Study Population
This single-center, retrospective cohort study was reviewed and approved by the Institutional Review Board of Nanjing First Hospital, Nanjing Medical University (IRB no: KY20201102-03), and all procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki. Patients admitted to ICU of Nanjing First Hospital, Nanjing Medical University from August 2021 to July 2023, were enrolled. All participants were over 18 years old and signed informed consent; the patients were in the ICU for a duration exceeding over 24 hours. The patients diagnosed with sepsis were screened at ICU admission based on the International Consensus on the Definition of Sepsis and Septic Shock 3.0 published in 2016.19 The diagnosis of ARDS patients was confirmed by evaluating their clinical characteristics, conducting arterial blood gas analysis, analyzing pathogenic factors and reviewing radiographic information by two bedside ICU attending clinicians based on the Berlin definition.9 The specific criteria were as follows: (1) an acute onset of new or worsening respiratory symptoms within 1 week and incubation for mechanical ventilation support; (2) PaO2/FiO2 ratio (arterial oxygen/fraction of inspired oxygen)≤300 mmHg; (3) bilateral opacity on chest radiograph. Patients were excluded from the study if they met the following exclusion criteria: (1) patients with less than 24 h stay or more than 30 days in ICU; (2) patients with malignancies, immunodeficiency or pregnancy; (3) patients with history of asthma, chronic obstructive pulmonary disease or interstitial lung disease; (4) age <18 years; (5) The patients with ARDS identified before ICU admission, for whom the timing of ARDS diagnosis was uncertain. The enrolled septic patients were categorized into sepsis group and ARDS group based on whether they developed ARDS during their stay in the ICU.
Clinical Data
After enrolment, the following baseline information was collected from the electronic medical records (EMR), including demographic data and laboratory indicators: age, sex, sequential organ failure assessment (SOFA) score (ICU admission day one), acute physiology and chronic health evaluation II (APACHE II) score within the first 24 hours, duration of mechanical ventilation, length of ICU stay, length of hospital stay, renal replacement therapy percentage, ICU hospitalization expenses and mortality. All patients’ blood samples were collected on the first day of ICU admission, prior to the occurrence of ARDS, for detection of the following laboratory indicators: white blood cell count (WBC), neutrophil count, procalcitonin (PCT) level, C-reactive protein (CRP) level, interleukin-6 (IL-6), platelet, albumin, creatinine and blood urea nitrogen (Bun).
Mrp 8/14 Assay
The peripheral venous blood was collected from all enrolled patients on the first day of ICU admission, prior to the onset of ARDS, for detection of Mrp 8/14. All blood samples were allowed to clot for 2 hours at room temperature before centrifugation (20 min, 2000rpm, 4°C) and stored at −80 °C until analyzed. Mrp 8/14 was measured using an enzyme linked immunosorbent assay (ELISA) with Human S100 Calcium-Binding Protein A8/A9 Complex (Mrp 8/14) ELISA Kit on serum samples (catalogue number. CSB-E12149h) (cusabio, China). All procedures were executed in accordance with the manufacturer’s instructions. The ELISA assay demonstrated an acceptable range of inter-assay variability (coefficient of variability < 10%). The blood samples were collected within 24 hours following admission to the ICU.
Statistical Analysis
All data were analyzed using SPSS 22.0. Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test. Continuous data were expressed as means ± standard deviation (SD) if they met the normal distribution, and the independent-sample t-test was used for inter-group comparison. Non-normally distributed continuous data were expressed as the median and interquartile range (IQR). The Mann–Whitney U-test was used for comparison. Qualitative/categorical variables were compared between groups using the chi-square test or Fisher’s exact test, and the results are shown as numbers and percentages. Spearman correlation analysis was used for correlation analysis. Univariate analysis and multiple logistic regression analysis were conducted to determine independent risk factors for ARDS occurrence. The ORs and corresponding 95% confidence interval (CI) were calculated for all variables. The diagnostic value was determined by receiver operating characteristic (ROC) curve analysis. A P value of <0.05 was considered statistically significant.
Results
Baseline of Patients’ Characteristics
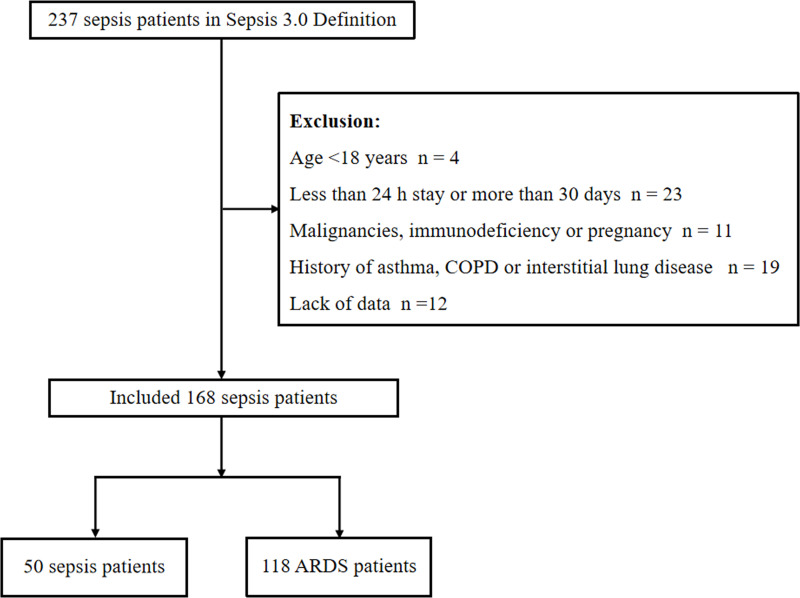
There were 237 patients who met the sepsis definition by Sepsis-3. A total of 69 patients were excluded according to the following criteria: lack of data (n = 12), age <18 years (n = 4), less than 24 h stay or more than 30 days (n = 23), malignancies, immunodeficiency or pregnancy (n = 11) and history of asthma, COPD or interstitial lung disease (n = 19). One hundred and sixty-eight patients met the inclusion criteria, including 118 patients in ARDS group and 50 patients in sepsis group (Figure 1). The comorbid conditions of septic patients enrolled in the study, such as coronary heart disease (30.36%, 51/168), hypertension (60.10%, 106/168), diabetes mellitus (50.60%, 85/168) and cerebrovascular disease (33.33%, 56/168), were documented. The sites of infection in septic patients leading to ICU admission such as respiratory infection (50.60%, 85/168), urinary tract infection (11.31%, 19/168), bloodstream infection (9.52%, 16/168), abdominal cavity infection (20.83%, 35/168) and so on (7.74%, 13/168) were recorded. The median age in the sepsis group was 69.28 ± 9.39 years, with 48.00% being men, and the ARDS group was 71.01 ± 8.69 years, with 62.71% being men. Compared to sepsis group, the patients in ARDS group had a higher APACHE II score, SOFA score on day 1, PCT and IL-6 level (P < 0.05, Table 1). In addition, no significant difference was found in other baseline characteristics between the two groups (P > 0.05, Table 1).
Figure 1.
Flow chart of the study patients to illustrate study screening and recruitment.
Abbreviations: COPD, chronic obstructive pulmonary disease; ARDS, Acute respiratory distress syndrome.
Table 1.
Baseline and Clinical Characteristics of the Study Population
| Patients Characteristics | Sepsis (n = 50) | ARDS (n = 118) | χ2/Z/t | P |
|---|---|---|---|---|
| Sex [male, n (%)] | 24 (48.00) | 74 (62.71) | 3.127 | 0.077 |
| Age, years, mean ± SD | 69.28 ± 9.39 | 71.01 ± 8.69 | −1.151 | 0.327 |
| Infection sites leading to ICU admission, n(%) | 3.307 | 0.508 | ||
| Respiratory infection | 20 (40.00) | 65 (55.08) | 3.197 | 0.074 |
| Urinary tract infection | 7 (14.00) | 12 (10.17) | 0.514 | 0.474 |
| Bloodstream infections | 6 (12.00) | 10 (8.47) | 0.180 | 0.674 |
| Abdominal cavity infection | 12 (24.00) | 23 (19.49) | 0.433 | 0.511 |
| Others | 5 (10.00) | 8 (6.78) | 0.159 | 0.690 |
| Past medical history, n (%) | 3.042 | 0.385 | ||
| Coronary heart disease | 16 (32.00) | 35 (29.66) | 0.091 | 0.763 |
| Hypertension | 26 (52.00) | 80 (67.80) | 3.763 | 0.052 |
| Diabetes mellitus | 29 (58.00) | 56 (47.46) | 1.561 | 0.211 |
| Cerebrovascular disease | 20 (40.00) | 36 (30.51) | 1.424 | 0.233 |
| Laboratory indicators | ||||
| WBC, x 109/L, mean ± SD | 15.18 ± 7.25 | 13.38 ± 6.06 | 1.655 | 0.100 |
| Neutrophil, x 109/L, mean ± SD | 12.94 ± 6.40 | 11.06 ± 6.30 | 1.762 | 0.080 |
| CRP, mg/L, mean ± SD | 132.24 ± 82.31 | 118.04 ± 80.05 | 1.043 | 0.299 |
| PCT, ng/mL, median (IQR) | 12.60 (2.38,24.68) | 23.36 (8.34, 44.77) | −2.545 | 0.011 |
| IL-6, pg/mL, median (IQR) | 129.41 (42.05, 1888.24) | 1827.28 (210.70, 9904.21) | −3.556 | 0.000 |
| Platelet, x109 /L, mean ± SD | 163.56 ± 104.94 | 160.88 ± 90.40 | −0.034 | 0.515 |
| Albumin, g/L, mean ± SD | 30.93 ± 5.24 | 30.06 ± 4.71 | 0.511 | 0.977 |
| Creatinine, umol/L, median (IQR) | 157.95 (94.80, 231.43) | 123.95 (77.08, 227.73) | −1.431 | 0.152 |
| Bun, mmol/L, median (IQR) | 14.03 (10.29, 20.30) | 13.48 (7.31, 23.25) | −1.003 | 0.316 |
| APACHE II score, median (IQR) | 21.00 (17.00, 26.00) | 27.00 (19.00,32.00) | −3.755 | 0.000 |
| SOFA score on day 1, median (IQR) | 7.00 (5.00, 10.00) | 11.00 (8.00, 15.00) | −5.193 | 0.000 |
| Calcium, mmol/L, median (IQR) | 1.14 (1.06, 1.26) | 1.15 (1.07,1.23) | −0.049 | 0.961 |
| Sodium, mmol/L, mean ± SD | 140.83 ± 6.86 | 138.88 ± 8.73 | 0.886 | 0.435 |
| Mrp 8/14, ug/mL, median (IQR) | 3.94 (2.06, 10.77) | 15.61 (9.67,24.34) | −6.697 | 0.000 |
Notes: P value below 0.05 indicate statistical significance.
Abbreviations: APACHE II score, Acute physiology and chronic health evaluation II score; WBC, White blood cell; CRP, C-reactive protein; PCT, Procalcitonin; SOFA score, Sequential Organ Failure Assessment score; IL-6, Interleukin-6; Bun, Blood urea nitrogen; Mrp 8/14, Myeloid-related proteins 8 and 14; ICU, Intensive care unit; IQR, Inter quartile range; SD, Standard deviation.
Level of Serum Mrp 8/14 in ICU Sepsis and ARDS Patients
The expression of serum Mrp 8/14 was detected in all enrolled subjects, including patients with sepsis and ARDS induced by sepsis. The mean level of serum Mrp 8/14 in ARDS group was 15.61 (9.67, 24.34)ug/mL, which was significantly exceeded the mean level of Mrp 8/14 in sepsis group at 3.94 (2.06, 10.77)ug/mL (P = 0.000) (Table 1).
Comparison of Clinical Outcomes
As illustrated in Table 2, the clinical outcome of patients with sepsis or ARDS was assessed based on the following parameters, including the mechanical ventilation rate, duration of mechanical ventilation, renal replacement therapy, shock rate, length of ICU stay, hospitalization expenses in ICU and ICU mortality. We found that mechanical ventilation rate, duration of mechanical ventilation, length of ICU stay, hospitalization expenses in ICU and ICU mortality were higher in ARDS group than that in sepsis group [χ2 = 20.885, P = 0.000; 3.00 (5.00, 8.00) vs 2.00 (0.00, 6.00), P = 0.000; 10.00 (6.00, 15.00) vs 7.00 (5.00, 11.00), P = 0.005; 8.47 (6.93, 14.30) vs 5.77 (4.69, 8.10), P = 0.000; χ2 = 5.721, P = 0.017)]. However, there were no significant differences in renal replacement therapy and shock rate between the two groups (all P > 0.05).
Table 2.
Clinical Outcomes of the Two Groups
| Outcomes | Sepsis (n = 50) | ARDS (n = 118) | χ2/Z | P |
|---|---|---|---|---|
| Renal replacement therapy, n (%) | 5 (10.00) | 27 (22.88) | 3.779 | 0.052 |
| Shock rate, n (%) | 37 (74.00) | 87 (73.73) | 0.001 | 0.971 |
| Mechanical ventilation rate, n (%) | 29 (58.00) | 105 (88.98) | 20.885 | 0.000 |
| Duration of Mechanical ventilation, days, median (IQR) | 2.00 (0.00–6.00) | 3.00 (5.00, 8.00) | −3.507 | 0.000 |
| Length of ICU stay, days, median (IQR) | 7.00 (5.00, 11.00) | 10.00 (6.00, 15.00) | −2.791 | 0.005 |
| Hospitalization expenses in ICU, median (IQR), (×10,000 yuan) | 5.77 (4.69, 8.10) | 8.47 (6.93, 14.30) | −4.279 | 0.000 |
| ICU mortality, n (%) | 37 (74.00) | 64 (54.24) | 5.721 | 0.017 |
Notes: P value below 0.05 indicate statistical significance.
Abbreviations: ICU, Intensive care unit; ARDS, Acute respiratory distress syndrome; IQR, Inter quartile range.
Mrp 8/14 Correlation with Laboratory Parameters of Infection and PaO2/FiO2
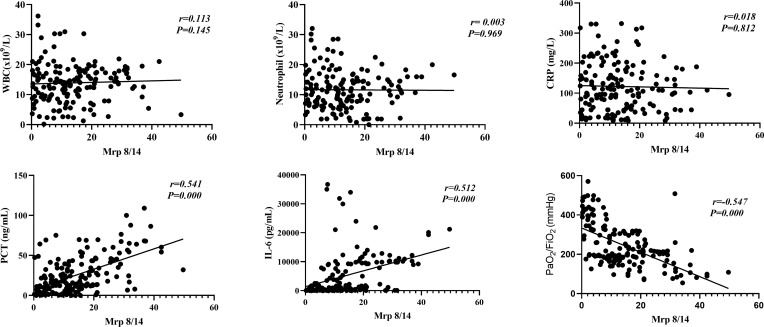
We further analyzed the association between serum Mrp 8/14 level and infection-related laboratory parameters, including WBC, neutrophil, CRP, PCT and IL-6. Mrp 8/14 displayed a positive correlation with the serum level of PCT and IL-6 (Spearman correlation coefficient 0.541 and 0.512, respectively, all P = 0.000) but no correlation with WBC, neutrophil and CRP (Spearman correlation coefficient 0.113, 0.003 and 0.018, respectively, all P > 0.05). In addition, the Mrp 8/14 exhibited a significant negative correlation with PaO2/FiO2 (Spearman correlation coefficient −0.547, P = 0.000) (more details seen in Figure 2).
Figure 2.
Mrp 8/14 correlation with infectious laboratory parameters and PaO2/FiO2. Mrp 8/14 has a positive correlation with the serum level of PCT and IL-6 (Spearman correlation coefficient 0.541 and 0.512 respectively, all P = 0.000) and has a negative correlation with PaO2/FiO2 (Spearman correlation coefficient −0.547, P = 0.000).
Abbreviations: PCT, Procalcitonin; IL-6, Interleukin-6; WBC, White blood cell; CRP, C-reactive protein; Mrp 8/14, Myeloid-related proteins 8 and 14; ARDS, Acute respiratory distress syndrome. P value below 0.05 indicates statistical significance.
Mrp 8/14 Correlation with Severity and Prognosis
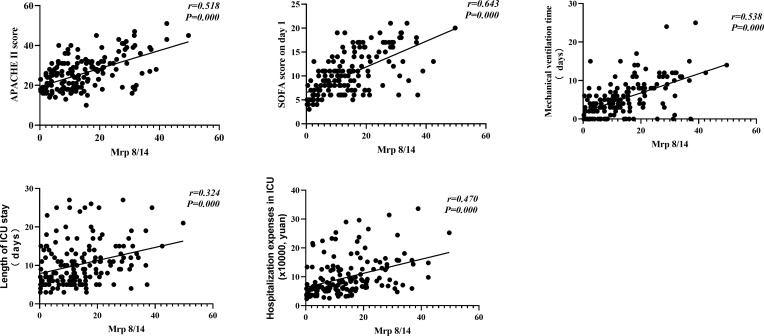
There was a positive correlation between serum Mrp 8/14 level and the APACHE II score, SOFA score on day 1, mechanical ventilation time, length of ICU stay and hospitalization expenses in ICU (Spearman correlation coefficient 0.518, 0.643, 0.538, 0.324, and 0.470, respectively, all P = 0.000) (more details depicted in Figure 3).
Figure 3.
Mrp 8/14 correlation with severity and prognosis of sepsis-induced ARDS. Mrp 8/14 has a positive correlation with the APACHE II score, SOFA score on day 1, mechanical ventilation time, length of ICU stay and hospitalization expenses in ICU (Spearman correlation coefficient 0.518, 0.643, 0.538, 0.324 and 0.470 respectively, all P = 0.000).
Abbreviations: APACHE II, Acute physiology and chronic health evaluation II score; ICU, intensive care unit; SOFA, Sequential Organ Failure Assessment; Mrp 8/14, Myeloid-related proteins 8 and 14; ARDS, Acute respiratory distress syndrome. P value below 0.05 indicates statistical significance.
Factors Associated with the Occurrence of ARDS in Patients with Sepsis
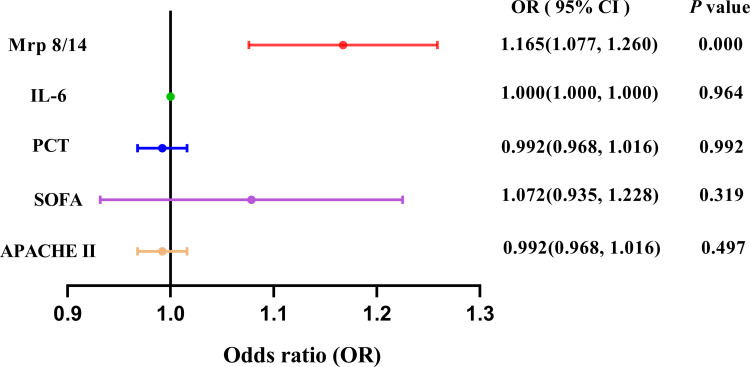
The Forest plot of logistic regression analysis showed Mrp 8/14 was the independent factor for forecasting the occurrence of ARDS induced by sepsis during ICU stay [OR = 1.165, 95% CI = (1.077, 1.260), P = 0.000], as shown in Figure 4.
Figure 4.
Forest plot of logistic regression analysis for ARDS in patients with sepsis. Adjusted for age, gender, baseline APACHE II score and SOFA score. The logistic regression analysis showed Mrp 8/14 was the independent factor for forecasting the occurrence of ARDS induced by sepsis [OR = 1.165, 95% CI = (1.077, 1.260), P = 0.000].
Abbreviations: APACHE II, Acute physiology and chronic health evaluation II score; ICU, Intensive care unit; SOFA, Sequential Organ Failure Assessment; Mrp 8/14, Myeloid-related proteins 8 and 14; ARDS, Acute respiratory distress syndrome; IL-6, Interleukin-6; PCT, Procalcitonin; OR, odds ratio; CI, confidence interval. P value below 0.05 indicates statistical significance.
Predictive Value of Mrp 8/14 for the Occurrence of ARDS in Patients with Sepsis
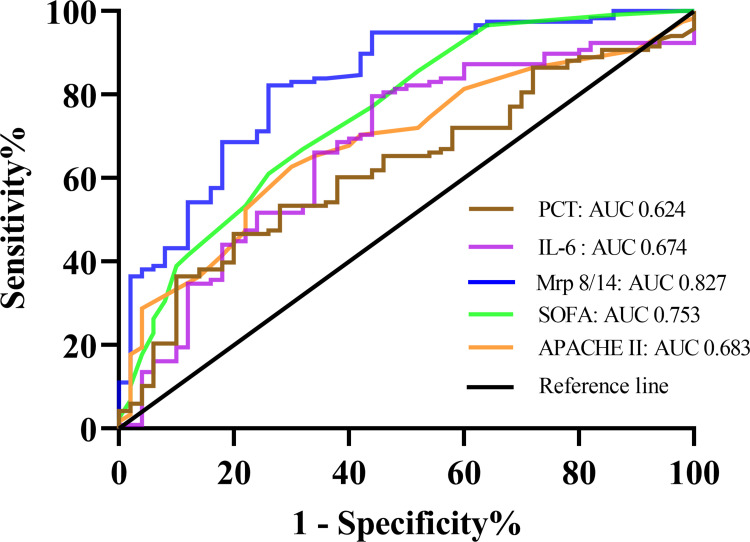
To investigate the potential applicability of Mrp 8/14 as a predictive marker for the occurrence of ARDS in septic patients during ICU stay, the ROC analysis was conducted using clinical and laboratory indicators. As documented in Figure 5 and Table 3, among the SOFA score on day 1, APACHE II score, PCT, IL-6 and Mrp 8/14, AUC for Mrp 8/14 (AUC 0.827, 95% CI 0.758–0.896, P = 0.000) was higher than the AUC for PCT (AUC 0.624, 95% CI 0.536–0.713, P = 0.011), IL-6 (AUC 0.674, 95% CI 0.583–0.764, P = 0.000), APACHE II score (AUC 0.683, 95% CI 0.600–0.767, P = 0.000) and SOFA score on day 1 (AUC 0.753, 95% CI 0.671–0.835, P = 0.000).
Figure 5.
Receiver operating characteristic (ROC) curve analysis of Mrp 8/14, APACHE II score, SOFA score, PCT and IL-6 for predicting the occurrence of ARDS in septic patients.
Abbreviations: APACHE II score, Acute physiology and chronic health evaluation II score; SOFA score, Sequential Organ Failure Assessment score; Mrp 8/14, Myeloid-related proteins 8 and 14; PCT, Procalcitonin; IL-6, Interleukin-6; ARDS, Acute respiratory distress syndrome; OR, odds ratio; CI, confidence interval. P value below 0.05 indicates statistical significance.
Table 3.
The Performance of Mrp 8/14 Upon Admission for Predicting the Occurrence of ARDS in Septic Patients
| Indicators | Cut-off | AUC (95% CI) | Sensitivity | Specificity | Youden | P |
|---|---|---|---|---|---|---|
| Mrp 8/14 | 11.23 | 0.827 (0.758, 0.896) | 0.686 | 0.820 | 0.506 | 0.000 |
| SOFA | 6.50 | 0.753 (0.671, 0.835) | 0.856 | 0.480 | 0.336 | 0.000 |
| APACHE II | 24.50 | 0.683 (0.600, 0.767) | 0.627 | 0.700 | 0.327 | 0.000 |
| PCT | 25.51 | 0.624 (0.536, 0.713) | 0.466 | 0.800 | 0.266 | 0.011 |
| IL-6 | 169.90 | 0.674 (0.583, 0.764) | 0.800 | 0.560 | 0.357 | 0.000 |
Notes: P value below 0.05 indicate statistical significance.
Abbreviations: APACHE II score, Acute physiology and chronic health evaluation II score; SOFA score, Sequential Organ Failure Assessment score; PCT, Procalcitonin; IL-6, Interleukin-6; Mrp 8/14, Myeloid-related proteins 8 and 14; ARDS, Acute respiratory distress syndrome; AUC, Area under the curve; CI, Confidence interval.
Discussion
In this retrospective cohort study, we initially observed differential expression of serum Mrp 8/14 in peripheral blood samples from patients with sepsis and sepsis-induced ARDS, suggesting that Mrp 8/14 is involved in the pathophysiologic process of sepsis-induced ARDS. Furthermore, this study represents the first clinical investigation into the correlation between serum Mrp 8/14 levels and sepsis-induced ARDS. Importantly, our study also demonstrates that serum Mrp 8/14 levels correlate significantly with the severity and prognosis of patients with ARDS induced by sepsis and can predict the occurrence of ARDS in septic patients.
Studies have demonstrated that neutrophils are considered as the primary inflammatory cells in the pathogenesis of lung injury induced by LPS20,21 due to their rapid activation and recruitment into the injury sites following exposure to injury stimuli.22 Mrp 8/14, a protein linked to inflammatory responses, is released abundantly and actively participates in the recruitment of leukocytes and cytokine secretion upon infection, thereby exacerbating inflammatory responses and tissue damage.23–25 In our study, we performed a preliminary experiment exploration and observed a clear detectable difference in the expression level of Mrp 8/14 between septic patients and ARDS patients, which is consistent with the previous study that serum Mrp 8/14 was significantly higher in ARDS and mechanical ventilation patients.18 However, the higher expression level of Mrp 8/14 observed in our study may be attributed to the inclusion of ARDS patients with not only viral infections but also disease-causing microorganisms such as bacteria compared to the previous one.18 In addition, as is seen in Table 2 and Figure 3, we found that the clinical outcomes of the patients in ARDS group were worse than that of those in sepsis group. Further analysis shows that serum Mrp 8/14 levels exhibit a significant positive correlation with the APACHE II score, SOFA score on day 1, length of ICU stay, mechanical ventilation time and hospitalization expenses in ICU, suggesting that Mrp 8/14 may serve as an independent serum marker that accurately reflects the severity and prognosis of ARDS induced by sepsis.
As an acute-phase reactant protein, CRP is extensively utilized and investigated as a biomarker.26,27 PCT is one of the most extensively researched biomarkers and enables early diagnosis and effective therapy management of sepsis.28,29 IL-6 plays a crucial role as a mediator in the acute phase of response to inflammation during sepsis, and its clinical significance has been evaluated in various septic conditions through multiple studies.30,31 We also observed that the levels of PCT, IL-6, and CRP are commonly utilized in clinical practice to evaluate the severity of infection in patients. Therefore, we conducted an integrated analysis of clinical infectious laboratory indicators, revealing a significant elevation in PCT and IL-6 levels among patients with ARDS compared to those with sepsis.
To further validate the relationship between serum Mrp 8/14 with infection, we conducted a comparative analysis of serum Mrp 8/14 expression with clinical biomarkers associated with infection parameter, such as WBC count, neutrophil count, IL-6 levels, PCT levels and CRP levels. The findings demonstrated a close correlation between serum Mrp 8/14 levels and both PCT (Spearman correlation coefficient = 0.541, P = 0.000) and IL-6 (Spearman correlation coefficient = 0.512, P = 0.000). In conjunction with our findings, it is reasonable to deduce a significant correlation between serum Mrp 8/14 levels and infection. However, the present study did not observe a direct correlation between serum Mrp 8/14 levels and WBC and neutrophil. In fact, the existing study has revealed that the diagnostic value of WBC and neutrophil levels in identifying infection and sepsis remains limited due to its susceptibility to various non-infectious factors when administered with corticosteroids, catecholamines and critical illness stress32 and may even markedly decrease in the context of severe infection, particularly if the patients have compromised immune function.33 Additionally, we also observed that CRP, not PCT, had no correlation with the serum Mrp 8/14, which may be attributed that the subjects in present study included partial trauma patients which experience various degrees of stress that elicits an inflammatory response and ultimately causes an elevation in PCT concentrations.34 Simultaneously, the finding was consistent with the study that PCT exhibits superior sensitivity compared to CRP in distinguishing between bacterial and non-bacterial infections.35
The multivariable analysis indicated that the occurrence of ARDS in septic patients admitted to ICU was closely associated with the detection of serum Mrp 8/14 on the first day. ROC analysis demonstrated that serum Mrp 8/14, as a single indicator, exhibited a higher specificity of 82.0% compared to PCT, IL-6, APACHE II and SOFA score. In clinical practice, serum Mrp 8/14 might provide more information about the prediction of ARDS in patients with sepsis quickly and objectively. In addition, the present study also showed that the IL-6 and PCT had the similar prediction value of ARDS in septic patients, which is in line with the previous study that IL-6 has diagnostic value that is comparable, but not superior, to PCT in patients with sepsis and septic shock.30,36
It is worth noting that the diagnosis of ARDS patients included in the present study was still confirmed based on the Berlin definition9 rather than the latest New Global Definition.37 It is known that the New Global Definition is recognized as an extension of the Berlin definition of ARDS, aiming primarily to enhance the diagnostic feasibility of ARDS, particularly in resource-limited settings. However, as an tertiary teaching hospital, the diagnosis of ARDS patients admitted to the ICU of our hospital primarily relies on the clinical presentation, PaO2/FiO2 ratio and identification of bilateral opacity on chest radiograph, which still persists in the New Global Definition. Therefore, the latest diagnostic criteria for ARDS did not affect the results of the present study based on the Berlin definition.
To the best of our knowledge, this study represents the first attempt to assess serum Mrp 8/14 as a potential biomarker for predicting the development of sepsis-induced ARDS. However, we could not ignore that there were several limitations in the present study. First, the present study was limited by its single-center design, which may introduce several inherent biases. Given the constraints imposed by the sample size, it is important to interpret the findings with caution and consider them as preliminary conclusions. Therefore, further investigations involving larger sample sizes and multiple centers are warranted to validate our results. Second, a portion of the septic patients enrolled in our institution had been transferred with prior management for sepsis, including administration of antibiotics, fluids, or vasopressors, which could potentially impact the progression from sepsis to ARDS. Furthermore, due to the inability to continuously monitor PaO2/FiO2 ratio and chest imaging in septic patients, we could not obtain the specific time information on the occurrence of ARDS. Therefore, the relationship between the collection time of Mrp 8/14 samples and the development of ARDS was not analyzed, despite the fact that Mrp8/14 blood samples were collected on the first day of ICU admission, prior to the occurrence of ARDS. Finally, the clinical findings could not provide direct evidence demonstrating the involvement of serum Mrp 8/14 in the regulation of sepsis-induced ARDS, and the molecular mechanisms regarding the serum Mrp 8/14 in the development of ARDS induced by sepsis should be further explored.
In conclusion, the data presented in this study demonstrate a good performance of serum Mrp 8/14 in predicting the occurrence of ARDS induced by sepsis. In addition, the presence of serum Mrp 8/14 may serve as an indicator for both the severity of sepsis-induced ARDS and the presence of infection. Of course, the present study has given some insight into the issue, and further multicentric investigations with a larger cohort will be needed to corroborate these findings. On all accounts, the serum Mrp 8/14 level of septic patients upon admission to the ICU deserves increased attention.
Funding Statement
This work was supported by Nanjing Medical Science and Technique Development Foundation Project (YKK22115, YKK20114).
Abbreviations
APACHE II score, Acute physiology and chronic health evaluation II score; WBC, White blood cell; PCT, Procalcitonin; SOFA score, Sequential Organ Failure Assessment score; IL-6, Interleukin-6; Bun, Blood urea nitrogen; Mrp 8/14, Myeloid-related proteins 8 and 14; ARDS, Acute respiratory distress syndrome; COPD, Chronic obstructive pulmonary disease; S100 A8/A9, Calprotectin or S100 calcium-binding protein A8 and A9; LPS, Lipopolysaccharide; COVID-19, Corona-virus disease 2019.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cecconi M, Evans L, Levy M, et al. Sepsis and septic shock. Lancet. 2018;392(10141):75–87. doi: 10.1016/S0140-6736(18)30696-2 [DOI] [PubMed] [Google Scholar]
- 2.Hollenberg SM, Singer M. Pathophysiology of sepsis-induced cardiomyopathy. Nat Rev Cardiol. 2021;18(6):424–434. doi: 10.1038/s41569-020-00492-2 [DOI] [PubMed] [Google Scholar]
- 3.Hwang JS, Kim KH, Park J, et al. Glucosamine improves survival in a mouse model of sepsis and attenuates sepsis-induced lung injury and inflammation. J Biol Chem. 2019;294(2):608–622. doi: 10.1074/jbc.RA118.004638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu Q, Hao C, Tang S. From sepsis to acute respiratory distress syndrome (ARDS): emerging preventive strategies based on molecular and genetic researches. Biosci Rep. 2020;40(5):BSR20200830. doi: 10.1042/BSR20200830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- 6.Auriemma CL, Zhuo H, Delucchi K, et al. Acute respiratory distress syndrome-attributable mortality in critically ill patients with sepsis. Intensive Care Med. 2020;46(6):1222–1231. doi: 10.1007/s00134-020-06010-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herridge MS, Tansey CM, Matté A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802 [DOI] [PubMed] [Google Scholar]
- 8.Cheung AM, Tansey CM, Tomlinson G, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174(5):538–544. doi: 10.1164/rccm.200505-693OC [DOI] [PubMed] [Google Scholar]
- 9.Ranieri VM, Rubenfeld GD, Taylor Thompson B, et al.; ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 10.De Freitas Caires N, Gaudet A, Portier L, et al. Endocan, sepsis, pneumonia, and acute respiratory distress syndrome. Crit Care. 2018;22(1):280. doi: 10.1186/s13054-018-2222-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toner P, McAuley DF, Shyamsundar M. Aspirin as a potential treatment in sepsis or acute respiratory distress syndrome. Crit Care. 2015;19:374. doi: 10.1186/s13054-015-1091-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asaduzzaman M, Zhang S, Lavasani S, et al. LFA-1 and MAC-1 mediate pulmonary recruitment of neutrophils and tissue damage in abdominal sepsis. Shock. 2008;30(3):254–259. doi: 10.1097/shk.0b013e318162c567 [DOI] [PubMed] [Google Scholar]
- 13.Zhang S, Rahman M, Zhang S, et al. Simvastatin antagonizes CD40L secretion, CXC chemokine formation, and pulmonary infiltration of neutrophils in abdominal sepsis. J Leukoc Biol. 2011;89(5):735–742. doi: 10.1189/jlb.0510279 [DOI] [PubMed] [Google Scholar]
- 14.Vogl T, Gharibyan AL, Morozova-Roche LA. Pro-inflammatory S100A8 and S100A9 proteins: self-assembly into multifunctional native and amyloid complexes. Int J Mol Sci. 2012;13(3):2893–2917. doi: 10.3390/ijms13032893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding Z, Du F, Averitt VRG, et al. Targeting S100A9 Reduces Neutrophil Recruitment, Inflammation and Lung Damage in Abdominal Sepsis. Int J Mol Sci. 2021;22(23):12923. doi: 10.3390/ijms222312923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao B, Lu R, Chen J, et al. S100A9 blockade prevents lipopolysaccharide-induced lung injury via suppressing the NLRP3 pathway. Respir Res. 2021;22(1):45. doi: 10.1186/s12931-021-01641-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee A, Nahm CH, Lee JS, et al. Assessment of antiphospholipid antibodies and calprotectin as biomarkers for discriminating mild from severe COVID-19. J Clin Lab Anal. 2021;35(11):e24004. doi: 10.1002/jcla.24004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kassianidis G, Siampanos A, Poulakou G, et al. Calprotectin and Imbalances between acute-phase mediators are associated with critical illness in COVID-19. Int J Mol Sci. 2022;23(9):4894. doi: 10.3390/ijms23094894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6 [DOI] [PubMed] [Google Scholar]
- 20.Steck P, Ritzmann F, Honecker A, et al. Interleukin 17 receptor E (IL-17RE) and IL-17C mediate the recruitment of neutrophils during acute streptococcus pneumoniae Pneumonia. Infect Immun. 2019;87(11):e00329–19. doi: 10.1128/IAI.00329-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang P, Hou Y, Zhang W, et al. Pseudoginsenoside-F11 attenuates lipopolysaccharide-induced acute lung injury by suppressing neutrophil infiltration and accelerating neutrophil clearance. Inflammation. 2019;42(5):1857–1868. doi: 10.1007/s10753-019-01047-5 [DOI] [PubMed] [Google Scholar]
- 22.Kim ND, Luster AD. The role of tissue resident cells in neutrophil recruitment. Trends Immunol. 2015;36(9):547–555. doi: 10.1016/j.it.2015.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schenten V, Plançon S, Jung N, et al. Secretion of the Phosphorylated Form of S100A9 from neutrophils is essential for the proinflammatory functions of extracellular Mrp 8/14. Front Immunol. 2018;9:447. doi: 10.3389/fimmu.2018.00447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovačić M, Mitrović-Ajtić O, Beleslin-čokić B, et al. TLR4 and RAGE conversely mediate pro-inflammatory S100A8/9-mediated inhibition of proliferation-linked signaling in myeloproliferative neoplasms. Cell Oncol. 2018;41(5):541–553. doi: 10.1007/s13402-018-0392-6 [DOI] [PubMed] [Google Scholar]
- 25.Revenstorff J, Ludwig N, Hilger A, et al. Role of Mrp 8/14 in platelet-neutrophil complex formation during acute inflammation. Cells. 2022;11(23):3944. doi: 10.3390/cells11233944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierrakos C, Velissaris D, Bisdorff M, et al. Biomarkers of sepsis: time for a reappraisal. Crit Care. 2020;24(1):287. doi: 10.1186/s13054-020-02993-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McFadyen JD, Zeller J, Potempa LA, et al. C-reactive protein and its structural isoforms: an evolutionary conserved marker and central player in inflammatory diseases and beyond. Subcell Biochem. 2020;94:499–520. doi: 10.1007/978-3-030-41769-7_20 [DOI] [PubMed] [Google Scholar]
- 28.Wacker C, Prkno A, Brunkhorst FM, et al. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(5):426–435. doi: 10.1016/S1473-3099(12)70323-7 [DOI] [PubMed] [Google Scholar]
- 29.Klouche K, Cristol JP, Devin J, et al. Diagnostic and prognostic value of soluble CD14 subtype (Presepsin) for sepsis and community-acquired pneumonia in ICU patients. Ann Intensive Care. 2016;6(1):59. doi: 10.1186/s13613-016-0160-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma L, Zhang H, Yin YL, et al. Role of interleukin-6 to differentiate sepsis from non-infectious systemic inflammatory response syndrome. Cytokine. 2016;88:126–135. doi: 10.1016/j.cyto.2016.08.033 [DOI] [PubMed] [Google Scholar]
- 31.Takahashi W, Nakada TA, Yazaki M, et al. Interleukin-6 levels act as a diagnostic marker for infection and a prognostic marker in patients with organ dysfunction in intensive care units. Shock. 2016;46(3):254–260. doi: 10.1097/SHK.0000000000000616 [DOI] [PubMed] [Google Scholar]
- 32.Zhang D, Tang Z, Huang H, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574(7779):575–580. doi: 10.1038/s41586-019-1678-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray CK, Hoffmaster RM, Schmit DR, et al. Evaluation of white blood cell count, neutrophil percentage, and elevated temperature as predictors of bloodstream infection in burn patients. Arch Surg. 2007;142(7):639–642. doi: 10.1001/archsurg.142.7.639 [DOI] [PubMed] [Google Scholar]
- 34.AlRawahi AN, AlHinai FA, Doig CJ, et al. The prognostic value of serum procalcitonin measurements in critically injured patients: a systematic review. Crit Care. 2019;23(1):390. doi: 10.1186/s13054-019-2669-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon L, Gauvin F, Amre DK, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39(2):206–217. doi: 10.1086/421997 [DOI] [PubMed] [Google Scholar]
- 36.Hamed S, Behnes M, Pauly D, et al. Diagnostic value of Pentraxin-3 in patients with sepsis and septic shock in accordance with latest sepsis-3 definitions. BMC Infect Dis. 2017;17(1):554. doi: 10.1186/s12879-017-2606-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthay MA, Arabi Y, Arroliga AC, et al. A new global definition of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2023. doi: 10.1164/rccm.202303-0558WS [DOI] [PMC free article] [PubMed] [Google Scholar]