Abstract
Background:
MicroRNAs (miRNAs) represent a subset of small noncoding RNAs and carry tremendous potential for regulating gene expression at the post-transcriptional level. They play pivotal roles in distinct cellular mechanisms including inhibition of bacterial, parasitic, and viral infections via immune response pathways. Intriguingly, pathogens have developed strategies to manipulate the host’s miRNA profile, fostering environments conducive to successful infection. Therefore, changes in an arthropod host’s miRNA profile in response to pathogen invasion could be critical in understanding host-pathogen dynamics. Additionally, this area of study could provide insights into discovering new targets for disease control and prevention. The main objective of the present study is to investigate the functional role of differentially expressed miRNAs upon Ehrlichia chaffeensis, a tick-borne pathogen, infection in tick vector, Amblyomma americanum.
Methods:
Small RNA libraries from uninfected and E. chaffeensis-infected Am. americanum midgut and salivary gland tissues were prepared using the Illumina Truseq kit. Small RNA sequencing data was analyzed using miRDeep2 and sRNAtoolbox to identify novel and known miRNAs. The differentially expressed miRNAs were validated using a quantitative PCR assay. Furthermore, a miRNA inhibitor approach was used to determine the functional role of selected miRNA candidates.
Results:
The sequencing of small RNA libraries generated >147 million raw reads in all four libraries and identified a total of >250 miRNAs across the four libraries. We identified 23 and 14 differentially expressed miRNAs in salivary glands, and midgut tissues infected with E. chaffeensis, respectively. Three differentially expressed miRNAs (miR-87, miR-750, and miR-275) were further characterized to determine their roles in pathogen infection. Inhibition of target miRNAs significantly decreased the E. chaffeensis load in tick tissues, which warrants more in-depth mechanistic studies.
Conclusions:
The current study identified known and novel miRNAs and suggests that interfering with these miRNAs may impact the vectorial capacity of ticks to harbor Ehrlichia. This study identified several new miRNAs for future analysis of their functions in tick biology and tick-pathogen interaction studies.
Keywords: Amblyomma americanum, Ehrlichia chaffeensis, small RNA Sequencing, microRNAs, miRNA inhibitors
Introduction
MicroRNAs (miRNAs) are non-coding RNAs with a size ranging from 18–25 nucleotides and play a significant role in post-transcriptional gene regulation (Bartel, 2009; Bartel and Chen, 2004). Latest studies have revealed the significance of miRNAs in arthropod immunity and host-pathogen interactions (Momen-Heravi and Bala, 2018; Miesen et al., 2016). In animals, miRNAs regulate post-transcriptional gene expression by binding to the 3’-untranslated region (3’-UTR), but there are also instances where the miRNA binds to the 5’-untranslated regions (5’-UTR), or coding regions. Perfect complementarity of 2–8 nucleotides at the 5’ end of the miRNA (seed region) is necessary for miRNA regulation, and the remaining sequence of miRNA might carry mismatches or bulges (Bartel, 2009; Rigoutsos, 2009; Schnall-Levin et al. 2010). The miRNA is transcribed as a primary miRNA transcript and processed by Drosha and Pasha into a pre-miRNA. The pre-miRNA is exported to the cytoplasm and processed by Dicer into a mature miRNA, which is then loaded into the microRNA-induced silencing complex (miRISC) and targets the complementary mRNA for degradation (Asgari, 2018, Flynt et al., 2010). Small non-coding RNAs (SncRNAs), including miRNAs, have shown tremendous potential in gene regulation at the post-transcriptional level in animals, plants, and arthropods, including ticks (Bartel, 2004; Carrington et al., 2003; Lai, 2015; Griffiths-Jones et al., 2008). Although more than 800 tick species are present worldwide, ticks are underrepresented in available miRNA resources. Databases such as miRbase have 49 Ixodes scapularis miRNAs and 24 Rhipicephalus microplus miRNAs, while MirGeneDB 2.1 contains 64 Ixodes scapularis miRNAs (Fromm et al., 2022).
The lone-star tick (Amblyomma Americanum) is an aggressive human-biting tick species, a known vector of numerous disease-causing agents, including Ehrlichia chaffeensis, E. ewingii, heartland virus, Bourbon virus, Francisella tularensis, Borrelia lonestari (Sanchez-Vicente and Tokarz, 2023). Lone star tick bites are also known to cause a food allergy, Alpha-Gal Syndrome (AGS), or red meat allergy (Commins et al., 2011; Crispell et al., 2019; Sharma and Karim 2022; Sharma et al., 2024). Am. americanum ticks are prevalent in the southern United States and have expanded their geographic range to the northeastern United States, and Canada (Stafford et al., 2018; Nelder et al., 2019). E. chaffeensis, a tick-borne Gram-negative obligatory intracellular bacterium, causes a severe flu-like febrile disease called human monocytic ehrlichiosis (HME), a prevalent life-threatening disease (Adams et al., 2017). Ehrlichiosis is an underreported tick-borne disease, and the pathogen infection of E. chaffeensis within the tick vector is a black box, and dynamics of vectorial capacity are largely unknown.
Given the contribution of miRNAs in numerous cellular processes, including development, immunity, and pathogen response in arthropods, the functional characterization of tick miRNAs in tick biology, and host-pathogen interactions remains to be investigated (Alvarez-Garcia et al., 2005; Saldana et al., 2017). Several omics studies have characterized the time-dependent, tissues-dependent blood-meal and pathogen-induced differential gene expression in variety of tick species (Karim et al., 2011; Karim and Ribeiro 2015; Guizzo et al., 2022; Adegoke et al., 2023; 2024; Anderson et al., 2008; Villar et al., 2015; Antunes et al., 2019; Popara et al., 2015; Bartel, 2004). However, studies addressing the role of pathogens in differentially modulating tick’s small RNAs are limited (Kumar et al., 2022; Artigas-Jerónimo et al., 2019; Hermance et al., 2019; Ramasamy et al., 2020). A handful of studies have investigated how miRNAs regulate the tick-pathogen interaction. These studies have shown that tick miRNAs promote the transmission of Anaplasma phagocytophilum and Powassan virus, thereby facilitating infection establishment in Ixodes scapularis (Artigas-Jerónimo et al., 2019; Hermance et al., 2019; Ramasamy et al., 2020). An elegant study demonstrated the tick miRNA-mediated regulation of vertebrate host gene expression at the tick-host interface (Hackenberg et al., 2017). However, regarding miRNA-mediated gene expression, the lone star tick (Am. americanum) is an underrepresented tick species, and there is an urgent need to investigate how E. chaffeensis utilizes tick miRNAs to promote its survival and persistence within the tick vector. This knowledge gap is of great concern, especially considering the increasing threat of the lone star tick to public health significance.
Understanding the molecular interactions between tick vectors and E. chaffeensis, and the characterization of differentially regulated tick miRNAs are needed to develop new approaches to combat tick-borne infections. Identification of new and novel miRNAs by using new sequencing platforms opened up a new avenue of research (Kumar et al., 2022; Artigas-Jerónimo et al., 2019; Luo et al., 2022). In this work, a small RNA sequencing approach was utilized to identify miRNAs induced by E. chaffeensis in A. americanum tissues, and differentially expressed miRNAs were functionally characterized using a miRNA inhibitor approach.
Materials and Methods
Ethics statement
All animal experiments were performed in strict accordance with the recommendations in the NIH Guide for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee of the University of Southern Mississippi approved the protocol for blood feeding of field-collected ticks (protocol # 15101501.3).
Ticks and tissue dissections
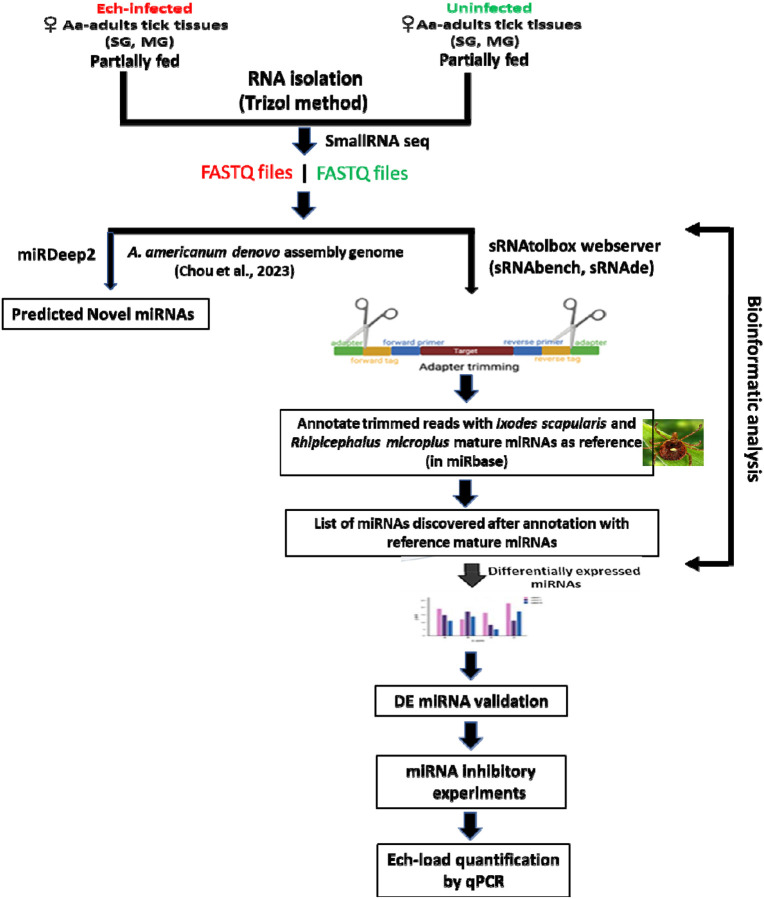
Adult Am. americanum ticks, both infected and uninfected were prepared as described (Karim et al., 2012). Briefly, lab-grown E. chaffeensis (Arkansas strain) was microinjected in engorged nymphs and 1x DMEM media was injected as a control cohort for uninfected ticks (Karim et al., 2012; Budachetri et al., 2020:2022; Adegoke et al., 2024a; 2024). The injected engorged nymphs were kept in sterile vials with a piece of filter paper. The engorged nymphs molted into either unfed male or female adult ticks within 2 months. The ticks were maintained under standard conditions as outlined by Patrick and Hair (1975). A qPCR assay was used to determine the infection level of E. chaffeensis in freshly molted ticks (Dunphy et al., 2014). Uninfected and E. chaffeensis adult ticks were infested on a rabbit for blood-feeding (see the experimental design Figure 1). Partially blood-fed uninfected and pathogen-infected female ticks were removed from the rabbit, and tick tissues were dissected as described earlier (Karim et al., 2012), and dissected tissues were directly stored in Trizol (Life Technologies, Carlsbad, CA, USA). Samples were kept at −80°C until use.
Figure 1.
Schematic workflow of microRNA study in lone star tick tissues.
RNA extraction and small RNA sequencing
RNA was extracted using Trizol RNA extraction methods from ten pooled tick tissue samples (uninfected, E. chaffeensis-infected midguts, and salivary glands). The integrity of the extracted RNA was determined using the standard spectrophotometric method as described earlier (Kumar et al., 2022). Small RNA library synthesis and small RNA sequencing were outsourced to the University of Mississippi Medical Center (UMMC) Molecular and Genomics Core laboratory. Briefly, four small RNA libraries (uninfected midguts, E. chaffeensis infected midguts, uninfected salivary glands, and E. chaffeensis infected salivary glands) were prepared using the Illumina Truseq Kit according to the manufacturer’s guidelines. Short adapter oligonucleotides were ligated to each end of the small RNAs. Following this, a cDNA copy was made with reverse transcriptase, and polymerase chain reaction (PCR) was utilized to incorporate sample-specific barcodes and Illumina sequencing adapters. The final concentration of all Next Generation Sequencing (NGS) libraries was determined with a Qubit fluorometric assay and a DNA 1000 high-sensitivity chip on an Agilent 2100 Bioanalyzer was used to assess the DNA fragment size for each library followed a purification step by polyacrylamide gelelectrophoresis. The sample libraries were pooled and sequenced on an Illumina Next Seq 500 (single end 36 bases) using TruSeq SBS kit v3 (Illumina) according to the manufacturer’s protocols.
Data Analysis
There are currently two computational strategies available for miRNA profiling: 1) the genome-based strategy, which maps small RNA-seq reads to a reference genome and evaluates sequences that generate the characteristic hairpin structure of miRNA precursors (Bortolomeazzi et al., 2019), and 2) the machine-learning-based strategy, where biogenesis features of sequences are extracted based on the available miRNA sequences in microRNA databases such as miRBase (Kozomara et al., 2014) and from the analysis of miRNA duplex structures (Vitsios et al., 2017). During the analysis of this data, the Amblyomma americanum genome sequence was not available. Therefore, microRNA data was analyzed by the smallRNAtoolbox webserver (Aparicio-Puerta et al., 2022). Recently, Chou et al., (2023) sequenced the genome of the Am. americanum assembled the long-read sequenced genome and provided a file of a partially annotated genome. The miRDeep2 software package version 2.0.0.8 (Friedländer et al., 2012) was used to predict the novel and known miRNAs in all tick tissue samples using the partially annotated genome of Am. americanum (Supplementary Table S1–5). sRNAtoolbox algorithm has not been optimized for novel microRNA discovery or may have limitations in accurately identifying and characterizing novel microRNAs.Since the genome of the tick Am. americanum has not been fully annotated for noncoding RNAs such as rRNAs, tRNAs, snRNAs, snoRNAs, etc., the percentage abundance of these non-coding RNAs in our data has not been determined.
Bioinformatics
As mentioned above, our small RNA data were analyzed by using a smallRNAtoolbox webserver (Aparicio-Puerta et al., 2022). Briefly, after an initial sequencing quality control step in FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc), preprocessing, mapping, and annotation were mainly conducted in sRNAbench (Aparicio-Puerta et al., 2022) with customized scripts as necessary. Simply, the obtained sequence reads were 36 nucleotides (nt) in length, but many small RNAs were between 27 and 33 nt. When forcing the detection of at least 10 nt of adapter as a typically used minimum length, only RNA molecules of up to 26 nt can be resolved (read length plus minimum adapter length). Therefore, to detect all small RNAs <36 nucleotides, we implemented iterative adapter detection and trimming. First, the adapter was detected in the whole read, and, if not found, it was then searched using iteratively shorter minimum adapter lengths at the 3′ end. After adapter trimming, the reads collapsed into unique reads followed by read count assignment, i.e., counting the number of times that each unique read was sequenced.
Differential expression and normalization
Our experimental design resulted in several possible comparisons: (i) uninfected versus E.chaffeensis infected salivary glands (SGs), (ii) uninfected versus E. chaffeensis infected midgut (MGs), (iii) uninfected MGs versus uninfected SGs, and (iv) E. chaffeensis-infected MGs versus E. chaffeensis-infected SGs. An edgeR tool in the sRNAtool box was used to determine differential miRNAs expression between uninfected and E. chaffeensis-infected tick tissues (Robinson et al. 2010). Briefly, using the differential expression module of sRNAtoolbox (i.e. sRNAde), we generated an expression matrix with the raw read counts for input into edgeR to obtain differential miRNA expression. The edgeR normalizes the data using the trimmed mean of M-svalues (TMM) method. We also generated an expression matrix with reads per million (RPM)-normalized expression values using the “single assignment” procedure in sRNAbench. As a result, each read mapping multiple times was only assigned once to the miRNA with the highest expression and only affected reads mapping to several different reference sequences, i.e., normally miRNA sequences from the same family. The RPM values were obtained by dividing the read count of a given miRNA by the total number of reads mapped to the miRNA library.
Validation of differentially expressed miRNAs
All miRNAs that were differentially expressed in small RNA sequencing data were validated by qRT-PCR in Am. americanum tick tissues. Mir-X miRNA qRT-PCR TB Green kit (Takara BIO, San Jose, CA, USA) was used for cDNA synthesis and miRNA expression analysis. This kit includes the Mir-X miRNA First-Strand Synthesis Kit, which transforms RNA into complementary DNA (cDNA) and enables the quantification of particular miRNA sequences through real-time PCR. Briefly, utilizing a single-tube method, RNA molecules undergo polyadenylation and reverse transcription by the action of poly(A) polymerase and SMART® MMLV Reverse Transcriptase, both components of the mRQ Enzyme Mix are supplied with the kit. Subsequently, real-time qPCR is conducted using the TB Green Advantage® qPCR Premix and mRQ 3’ Primer, in combination with miRNA-specific 5’ primers, to quantify specific miRNA expression. Primers used are listed in supplementary Table S6. Conditions used for qRT-PCR were initial denaturation of 95° C for 10 mins, then 40 cycles of 95° C for 5 secs, and 60° C for 20 secs.
MicroRNA inhibition assay
We selected three microRNAs, including Aam-miR-87, Aam-miR-750, and Aam-miR-275 for further characterization. A miRNA inhibitor approach works by sterically blocking specific miRNA functions using an oligonucleotide that complements the mature miRNA target (Lennox et al., 2013). These inhibitors were designed and synthesized by Integrated DNA Technologies (IDT, Coralville, IA), which also synthesized non-target negative controls alongside the specific inhibitors. Three groups of E. chaffeensis-infected ticks were injected with miRNA inhibitors for each selected miRNA (Aam-miR-87, Aam-miR-750, and Aam-miR-275), while a fourth group was injected with non-target negative controls. Each group contained 25 female ticks. Using a published study (Ramasamy et al. 2020), we selected a 1.05 nanomoles dose for this assay. Briefly, 1.05 nanomoles of each microRNA inhibitor and negative control were injected into each female tick within their respective groups. The injected ticks were allowed a 48-hour recovery period along with non-treated males (25 females/15 males) in a laboratory incubator, maintained at a temperature range of 23 ± 2°C with a humidity level of 95%, and under a light cycle of 14 hours of light and 10 hours of darkness. After the recovery period of 48 hrs, all groups of ticks were infested on a sheep in separate stockinet cells to blood feed. The partially blood-fed ticks were removed from the sheep, and tick tissues (MGs, and SGs) were dissected within 4 hrs for downstream analysis. RNA was extracted from each tissue using the Trizol method as described previously (Kumar et al., 2022). In a qPCR assay, the E. chaffeensis infection in individual tick tissues was assessed using disulfide bond formation (dsb) gene primers (Dunphy et al., 2014). The level of miRNA inhibition was determined by using QRT-PCR primers designed by the miRprimer2 algorithm (Busk, 2014), in conjunction with the Mir-X™ miRNA qRT-PCR TB Green® Kit from TAKARA (San Jose, CA, USA). After confirming miRNA inhibition in tick tissues, the relative E. chaffeensis load compared to the non-target negative control was quantified in the respective tick tissues.
Results and Discussion
Profile characteristics of small RNA libraries
The small RNA sequencing yielded a total of >147 million raw reads, comprised of >32 million from uninfected midgut, >32million from the E. chaffeensis-infected midgut, >40million from uninfected salivary glands, and >41 million from the E. chaffeensis-infected salivary glands. Following the adapter trimming process and subsequent removal of short reads (those ≤20 nucleotides (nt) in length), the small RNA reads left for downstream analysis were >41 million from E. chaffeensis-infected samples and >36 million from uninfected samples. The distribution of read length presents an indication of the types of small RNAs present in both E. chaffeensis-infected and uninfected tick tissues (midgut and salivary gland). Sequencing stats including raw reads, adapter cleaned reads, reads in analysis, quality filter reads have been provided in supplementary Table S7, while processing stats of reads (in percentage) and raw miRNA summary (number of detected miRNAs and its precursors) have been provided correspondingly as supplementary Table S8 and S9. Both types of samples, E. chaffeensis-infected and uninfected, exhibited two main peaks at 22 nt (representing miRNAs/siRNAs) and 29 nt (representing piRNAs) (Figure 2). Notably, PIWI-interacting RNAs (piRNAs) constitute a class of small RNAs, which usually vary in size from 26 to 31 nucleotides (Iwasaki et al., 2015; Santos et al., 2023). The piRNAs associate with PIWI proteins, which belong to the Argonaute family of proteins and are active in the testes of mammals. These RNAs play a crucial role in germ cell and stem cell development in invertebrates. One of their primary functions is the silencing of transposable elements (TEs) to protect genomic integrity (Aravin et al., 2008; Brennecke et al., 2007; Brennecke et al., 2008).piRNAs are passed down from the female germline to progeny, ensuring the stability of the genome across generations. When males harboring a specific family of transposon elements (TEs) mate with females devoid of these elements, the females lack the necessary complementary piRNAs to defend their genome resulting in an overabundance of transposons. Such proliferation could potentially lead to sterility, a situation termed hybrid dysgenesis (Erwin et al., 2015). This concept of hybrid dysgenesis holds potential for tick control.
Figure 2.
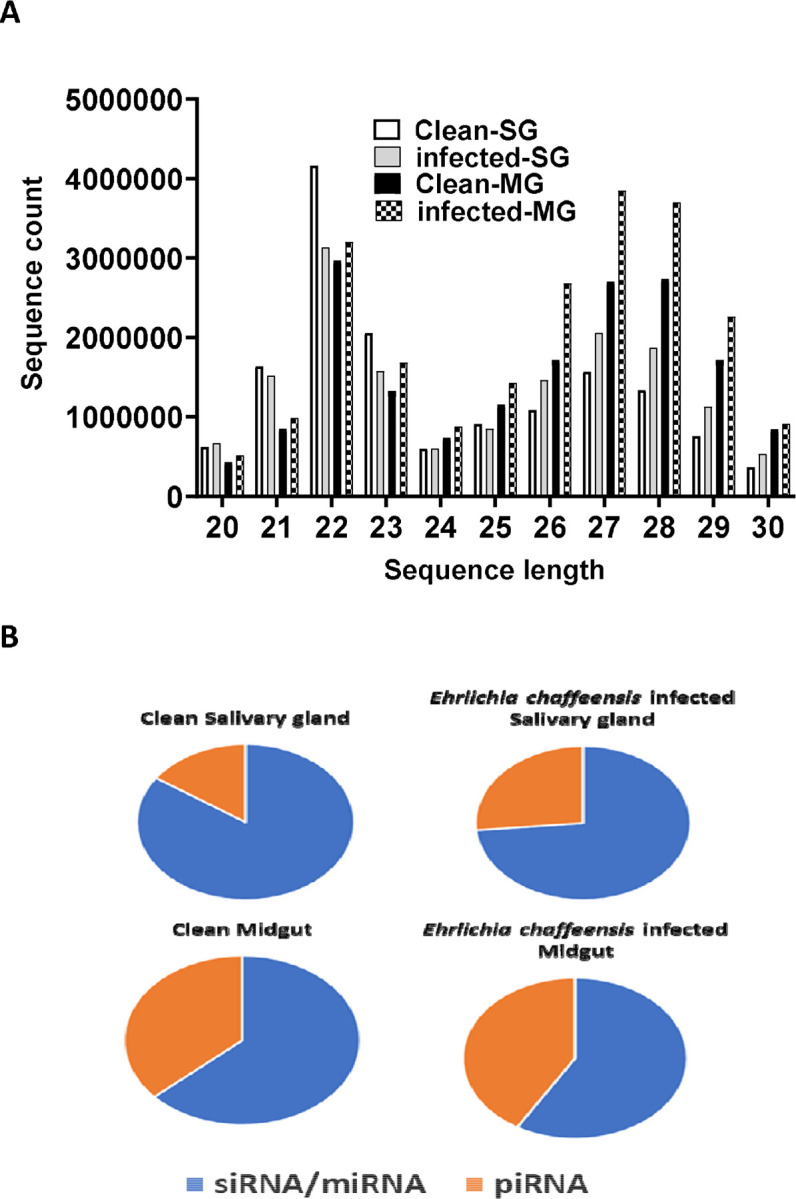
A. Small RNA sequence length distribution in uninfected (clean) and Ehrlichia chaffeensis-infected tick tissues (SG, MG). MicroRNAs are twenty-two (22) nucleotides in length. B. Pie-chart distribution of small RNA reads. There are mainly two characteristic peaks of tick small RNAs (miRNAs/siRNAs-22 nt, piRNAs-29 nt). Aa-Amblyomma americanum, PF-Partially fed (2 days), SG- Salivary glands, MG- Midgut, nt-nucleotide. Ech – E. chaffeensis infected.
In silico mapping of E. chaffeensis infected small RNA sequences to E. chaffeensisArkansas strain genome
Upon performing in silico mapping of small RNA reads infected with E. chaffeensis to the genome of the E. chaffeensis Arkansas strain (GCF_000013145.1_ASM1314v1_genomic.fna), we detected 1,413 E. chaffeensis sequences in the midgut out of a total of 2,492,851 reads. In addition, we found 3,185 E. chaffeensis sequences in the salivary glands from a total of 19,103,069 reads. From these results, it appears that our samples were infected with E. chaffeensis, and the infection level was low, a hallmark of Ehrlichia infection within the tick vector (Kennedy and Marshall, 2021).
Differentially expressed microRNAs in E. chaffeensis infected tick salivary glands
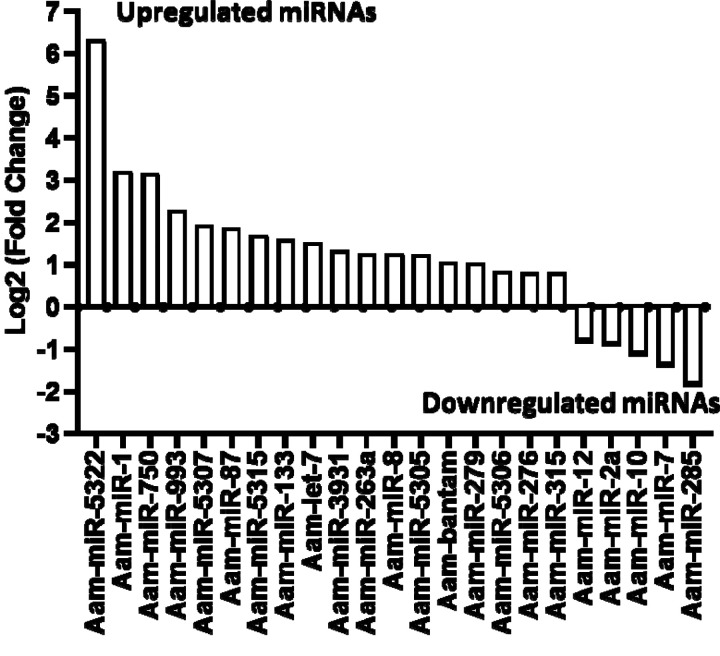
Our small RNA sequencing analysis identified 360 microRNAs including known and predicted ones in uninfected and E. chaffeensis-infected tick tissues (Supplementary data Table S1). The list of differentially expressed miRNAs in E. chaffeensis-infected salivary glands (SGs) includes 18 upregulated miRNAs, Aam-miR-5322, Aam-miR-1, Aam-miR-750, Aam-miR-993, Aam-miR-5307, Aam-miR-87, Aam-miR-5315, Aam-miR-133, Aam-let-7, Aam-miR-3931, Aam-miR-263a, Aam-miR-8, Aam-miR-5305, Aam-bantam, Aam-miR-279, Aam-miR-5306, Aam-miR-276, Aam-miR-315) and 5 downregulated miRNAs Aam-miR-12, Aam-miR-2a, Aam-miR-10, Aam-miR-7, Aam-miR-285 (Figure 3).
Figure 3.
In silico differential expression of predicted microRNAs in Ehrlichiachaffeensisinfected partially fed salivary gland relative to partially fed clean salivary glands. EdgeR was used for differential expression analysis. miRNAs with a Log2 fold-change expression > |0.8|, p-value < 0.05 were considered significantly differentially expressed.
Differentially expressed microRNAs in E. chaffeensis infected tick midgut
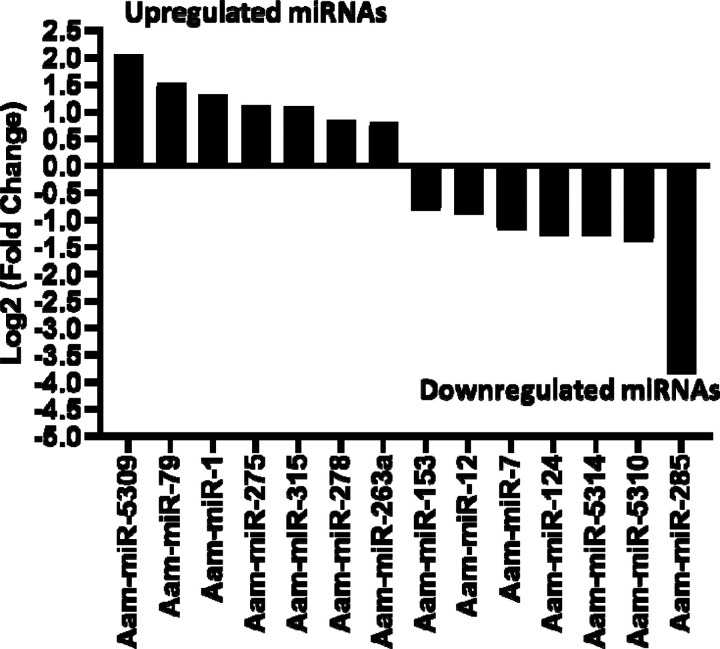
Likewise, in the E. chaffeensis infected tick midgut (MG), seven miRNAs were upregulated in Ehrlichia infection, Aam-miR-5309, Aam-miR-79, Aam-miR-1, Aam-miR-275, Aam-miR-315, Aam-miR-278, Aam-miR-263a and 7 downregulated miRNAs, Aam-miR-153, Aam-miR-12, Aam-miR-7, Aam-miR-124, Aam-miR-5314, Aam-miR-5310, Aam-miR-285 (Figure 4). Heat map representation of DE miRNAs (Supplementary Figure S1–S2) has also been provided as supplementary information). All these miRNAs are listed in miRbase (22.1) and have also been identified in Ixodes scapularis or Rhipicephalus microplus tick species. It is necessary to investigate the roles of these differentially expressed miRNAs in E. chaffeensis-infected tick tissues. It is crucial to gain a deeper understanding through these differentially expressed miRNAs of how E. chaffeensis manipulates the expression of tick microRNAs to ensure its survival, persistence, and transmission. This knowledge holds immense potential in the development of innovative tools to effectively block the transmission of tick-borne pathogens.
Figure 4.
In silico differential expression of predicted microRNAs in Ehrlichiachaffeensis infected partially fed midgut relative to the partially fed clean midgut. EdgeR was used for differential expression analysis. miRNAs with a Log2 fold-change expression > |0.8|, p-value < 0.05 were considered significantly differentially expressed.
In this study, we found miR-1 upregulated in E. chaffeensis infected tick tissues, i.e. SG, and MG in comparison to uninfected ones. It is noteworthy that miR-1 often exhibits increased expression during pathogen infections. miR-1 belongs to a conserved family, which includes miR-7 and miR-34, and is conserved across various organisms such as fruit flies, shrimps, and humans (Takane et al. 2010). It participates in analogous pathways in these organisms, including development and apoptosis, and its upregulation has also been identified during stressful conditions (Huang et al., 2012). In mosquitoes, miR-1 is similarly upregulated during Plasmodium infection (Huang and Zhang, 2012), and facilitates West Nile Virus infection (Hussain et al., 2012). For Bombyx mori, the Nucleo-polyhedrosis virus (NPV) releases miR-1 inside the host to regulate its target RAN (exportin 5, co-factor), a player in miRNA biogenesis responsible for transporting pre-miRNA from the nucleus to the cytoplasm (Singh et al., 2012). Moreover, during Listeria infection in macrophages, miR-1 stimulates IFN-γ-dependent activation of the innate immune response (Xu et al., 2019). Based on these findings, we speculate that miR-1 could be upregulated in E. chaffeensis-infected tick tissues to trigger an immune response against E. chaffeensis.
The upregulation of miR-87 in Ehrlichia-infected SGs points out its putative role in ticks’ innate immune response. Earlier work on other arthropods, including Manduca sexta and Aedes albopictus hint at its potential role in interfering with innate immunity, particularly IMD and toll receptor signaling pathways (Zhang et al., 2014; Liu et al., 2015; Avila-Bonilla et al., 2017). Its in silico predicted targets in Aedes albopictus include TOLL pathway signaling Ser/Thr. Kinase, Toll-like receptor TOLL 1A, Class A Scavenger receptor with Se-Protease domain, and Galectin (Liu et al., 2015), as well as putative TLR 5b (Avila-Bonilla et al., 2017). In Manduca sexta, its predicted target is FADD, an adaptor protein involved in DISC formation (Zhang et al., 2014). Based on the published studies, we hypothesize that Ehrlichia infection differentially regulates the miR-87 to inhibit the Toll pathway for its survival in the tick vector.
The upregulation of miR-bantam in Ehrlichia-infected SGs also suggests its putative role in the pathogen infection of E. chaffeensis. Interestingly, miR-bantam, a conserved microRNA, exhibits high expression levels in insects. For example, it is highly expressed in Drosophila and participates in several key cellular processes such as cell proliferation, apoptosis, development, and the circadian rhythm. In Drosophila, miR-bantam serves two major roles: inhibiting apoptosis by down-regulating the apoptotic gene hid (Brennecke et al., 2003) and promoting cell proliferation by targeting genes like mad (Robins et al., 2005). The inhibition of apoptosis might act as a survival mechanism for E.chaffeensis within tick salivary gland cells. Our hypothesis warrants in-depth future studies to determine the role of miR-bantam in the vectorial capacity of the tick vector.
Another miRNA candidate, mir-79 was also significantly upregulated Ehrlichia infected MG. Earlier studies have described its role in various pathways, including immunity, cell differentiation, neurogenesis, and apoptosis. It has also been implicated in cancer and disease caused by viral infection (Artigas-Jerónimo et al., 2019; Yuva-Aydemir et al., 2011; Seddiki et al., 2013; Pedersen et al., 2013; Ouyang et al., 2015; Dong et al., 2017). It is known that mir-79 disrupts the JNK pathway by targeting its component genes pvr (CG8222) and puc (CG7850) (Fullaondo and Lee., 2012). The JNK pathway is an immune response pathway against Gram-negative bacterial pathogens (Bond and Foley, 2009). In the current study, mir-79 is upregulated in the E. chaffeensis-infected midgut. Similarly, in ticks infected with Anaplasma phagocytophilum (a Gram-negative bacterial pathogen), mir-79 was found upregulated, thereby facilitating infection by targeting the Roundabout protein 2 pathway (Robo2) (Artigas-Jerónimo et al., 2019). The upregulation of mir-79 could potentially be a mechanism used by E. chaffeensis to evade the tick’s immune system.
Our in-silico data reveals the downregulation of miR-5310 in the midgut of E. chaffeensis-infected ticks. MiR-5310, a tick-specific miRNA (Barrero et al., 2011), may play a role in tick feeding, as it was shown to be downregulated in Rhipicephalus microplus tick larvae exposed to host odor without being allowed to feed (Barrero et al., 2011). Another study showed its downregulation in Anaplasma phagocytophilum-infected nymphs compared to unfed, uninfected nymphs (Ramaswamy et al., 2020). Previous research has also shown the modulation of signaling events upon A. phagocytophilum infection of ticks (Khanal et al., 2018; Neelakanta et al., 2010; Sultana et al., 2010; Taank et al., 2017; Turck et al., 2019; Ramaswamy et al., 2020). Thus, in the case of the E. chaffeensis-infected tick midgut in the present study, miR-5310’s speculated role might be to modulate signaling events to protect E. chaffeensis. Its potential role could also be in tick feeding, as shown by its downregulation in Rhipicephalus microplus tick larvae exposed to host odor without being allowed to feed. In our data, miR-133 is upregulated in the salivary glands of E. chaffeensis-infected ticks. According to a recent study, the infection of ticks with the pathogen Anaplasmaphagocytophilum results in the downregulation of tick microRNA-133 (miR-133), leading to the induction of the Ixodes scapularis organic anion transporting polypeptide (isoatp4056) gene expression, which is critical for the pathogen’s survival within the vector and its transmission to the vertebrate host (Ramaswamy et al., 2020). Therefore, the upregulation of miR-133 in E. chaffeensis-infected tick salivary glands in our study might suggest the downregulation of organic anion transporting polypeptide (isoatp4056) gene expression, potentially inhibiting E. chaffeensis survival and transmission. This is a hypothetical explanation for the observed miR-133 upregulation, and further investigation is necessary for confirmation.
Let-7, an evolutionarily conserved microRNA in bilateral animals, plays a role in developmental regulation, such as molting and metamorphosis in arthropods, and can disrupt innate immunity by targeting the antimicrobial peptide diptericin (Carrington and Ambros, 2003; Hertel et al., 2012; Pasquinelli et al., 2000; Ling et al., 2014; Garbuzov and Tatar, 2010). Recent studies have also suggested its role in the molting of Hyalomma asiaticum ticks by targeting the ecdysteroid receptor (ECR), a part of the 20E signaling pathway (Sempere et al., 2003; Wu et al., 2019). In this study, let-7’s upregulation in tick salivary glands implies a possible role in targeting the antimicrobial peptide diptericin, potentially allowing E. chaffeensis to evade innate immunity. This may represent a mechanism for E. chaffeensis’s survival and successful transmission in tick salivary glands, but further investigation is required. It should be noted that diptericin inhibits Gram-negative bacteria by disrupting membrane integrity.
In this study, miR-275 is upregulated in the midgut of E. chaffeensis-infected ticks. MiR-275 directly targets and positively regulates the sarco/endoplasmic reticulum Ca2+ adenosine triphosphatase (SERCA), an active player in transporting Ca2+ from the cytosol to the sarco/endoplasmic reticulum (ER) in mosquito guts (Zhao et al., 2017). The transportation of Ca++ from the cytoplasm to the ER is required for the spreading process of Ehrlichia canis (Alves et al., 2014), suggesting that E. chaffeensis may modulate tick machinery via upregulation of miR-275. However, a follow-up study is necessary for confirmation. It’s also worth noting that miR-275 was found to be crucial for blood digestion and egg development in the mosquito Aedes aegypti (Bryant et al. 2010).
In our data set, miR-750 is upregulated in E. chaffeensis-infected tick salivary glands. Past studies have suggested its role in innate immunity, hormone signaling, and stress response (Rebijith et al., 2016; Nunes et al., 2013; Kanoksinwuttipong et al., 2022; Queiroz et al., 2020). A recent study indicated that upregulated miR-750 suppresses its target, the sarcoplasmic calcium-binding protein (Scp), and inhibits apoptosis, thus contributing to pathogen propagation (Kanoksinwuttipong et al., 2022). Given these previous studies, the possible role of miR-750 in inhibiting apoptosis and promoting E. chaffeensis propagation in tick salivary glands can be speculated. This could represent a mechanism by which E. chaffeensis avoids cellular apoptosis and propagates for effective transmission in the tick salivary glands. The roles of all the differentially expressed miRNAs mentioned above are listed in Table 1, along with other necessary details.
Table 1.
List of differentially expressed microRNAs detected in Ehrlichia chaffeensis infected tick tissues (salivary gland, midgut) and their putative roles
| microRNA | Role of ortholog microRNA | Target genes | Reference |
|---|---|---|---|
| miR-1 | Stress response, immunity, development, facilitating infection | HSP60, HSP70, GATA4, RAN (exportin) | Huang et al., 2012; Huang and Zhang, 2012, Hussain et al., 2012; Xu et al., 2019, Singh et al., 2012; Liu et al., 2017] |
| miR-79 | Innate immunity, differentiation, apoptosis | Roundabout protein 2 pathway (robo2), DRAPER, HP2 (Hemolymph protease), P38, pvr, puc | Fullaondo and Lee., 2012; Bond and Foley, 2009; Artigas-Jeronimo et al., 2019; Yuva-Aydemir et al., 2011; Seddiki et al., 2013; Pedersen et al., 2013; Ouyang et al., 2015; Dong et al., 2017 |
| let-7 | Developmental regulation such as molting and metamorphosis in arthropods, disrupts innate immunity | Antimicrobial peptide diptericin | Pasquinelli et al., 2000; Ling et al., 2014; Garbuzov and Tatar, 2010; Sempere et al., 2003;Wu et al., 2019 |
| miR-133 | Anaplasmaphagocytophilum survival inside ticks, transmission | Organic anion transporting polypeptide (isoatp4056) gene | Ramaswamy et al., 2020 |
| miR-bantam | Proliferation, apoptosis inhibition, development, circadian rhythm | hid, mad | Brennecke et al., 2003; Robins et al., 2005 |
| miR-87 | Pathogen survival (disruption of Toll and IMD pathway) | Serine/Threnine Kinase, Toll 1A, Putative TLR 5b, FADD | Zhang et al., 2014; Liu et al., 2015; Avila-Bonilla et al., 2017 |
| miR-5310 | Pathogen survival by modulating signaling pathways, Feeding behavior | Barrero et al., 2011; Khanal et al., 2018; Neelakanta et al., 2010; Sultana et al., 2010; Taank et al., 2017; Turck et al., 2019; Ramaswamy et al., 2020 | |
| miR-275 | Active transport of Ca2+ from cytosol to the endoplasmic reticulum, blood digestion, egg development | Zhao et al., 2017; Alves et al., 2014; Bryant et al., 2010 | |
| miR-750 | innate immunity, hormone signaling and stress response, apoptosis inhibition | Sarcoplasmic calcium binding protein (Scp) | Rebijith et al., 2016; Nunes et al., 2013; Kanoksinwuttipong et al., 2022; Queiroz et al., 2020 |
| miR-153 | Development, immune response | Wu et al., 2013 | |
| miR-5307 | Propagation of Powassan virus inside vero cells | Hermance et al., 2017 | |
| miR-8 | Development, Reproduction | SWIM | Bryant et al., 2010; Feng et al., 2018; Lucas et al.,2015 |
| miR-12 | critical for persistence of wolbachia | DNA replication licensing (MCM6), monocarboxylate transporter (MCT1) | Osei-Amo et al., 2012 |
| miR-263a | Development | Hu et al., 2015 | |
miR-8 is upregulated in E. chaffeensis-infected tick salivary glands. Its role in innate immunity is unknown so far, but our results have indicated its possible role against Ech-infection, but further work is required for validation. It is a conserved miRNA, and previous studies have shown its role in development and reproduction. Its expression was found up-regulated in Aedes aegypti during the pupation stage, with its highest expression levels observed in the mid-pupal period. Previous work by Bryant et al. (2010) revealed the upregulation of miR-8 in the fat body of blood-fed female mosquitoes, suggesting a potential regulatory function in the reproduction of Ae. aegypti. In contrast to Ae. aegypti, miR-8 shows abundant expression at different developmental stages of Anopheles stephensi (Feng et al., 2018) and is equally expressed in uninfected and infected Ae. albopictus saliva following CHIKV infection (Maharaj et al., 2015).
miR-263a was also found upregulated in E. chaffeensis infected tick salivary glands indicating its possible role in either innate immunity or transmission, which needs to be revealed by further work. Previous studies have shown its role in development. It was found highly expressed in uninfected and infected Ae. aegypti saliva (Maharaj et.al. 2015), and is amongst the most highly expressed miRNAs across developmental stages in many mosquito species (Hu et.al.,2015).
miR-12 was found downregulated in E. chaffeensis infected tick salivary glands indicating its possible role in activating immune pathways, and its downregulation might have a probable effect on Ehrlichia survival, and therefore successful transmission. Further work is required to validate this hypothesis. Although its immunological role is unknown in previous studies. Although previous work has shown its role in affecting Wolbachia density in mosquitoes (Osei-Amo et.al. 2012). The preferential expression of miR-12 in Anopheles gambiae occurs in the thorax of both males and females, predominantly in midguts and twice as much in their heads (Winter F et.al.,2007), and its targets are DNA replication licensing factor (MCM6) and monocarboxylate transporter (MCT1) genes, as validated in A.aegypti, by which it affects Wolbachia density in host cells (Osei-Amo et.al. 2012). Our in silico data indicates that miR-279 is upregulated in the salivary glands of ticks infected with Ehrlichia chaffeensis, suggesting it may have a role in the pathogen’s survival or its transmission. These hypotheses, still in the realm of speculation, warrant further study to be substantiated. Additionally, a recent study has proposed that miR-279 might influence the resistance to the insecticide deltamethrin. It does this by regulating the expression of its target gene CYP325BB1, which codes for the enzyme cytochrome P450 325bb1, in the mosquito species Culex pipiens pallens (Li et al., 2021). Given that the functions of microRNAs are conserved, this research work might offer substantial insights into the mechanisms driving acaricide resistance, which would be essential in developing new and effective strategies for tick control in the future.
Validation of in silico differentially expressed microRNAs by qRT-PCR
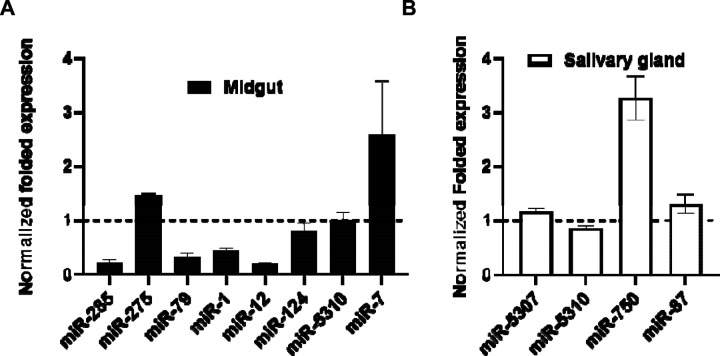
The expression levels of differentially expressed miRNAs were validated using qRT-PCR assays on E. chaffeensis-infected and uninfected tick tissues (Figure 5). The qRT-PCR patterns of the differentially expressed miRNAs were consistent with the next-generation sequencing (NGS) results for the majority of evaluated miRNAs. However, inconsistencies between the NGS and qRT-PCR data patterns were detected. These discrepancies may arise from the different methodologies used to quantify miRNA expression (Saldana et al., 2017).
Figure 5.
qPCR validation of differentially expressed miRNAs (in silico)in Ehrlichia chaffeensis infected and partially fed tick tissues. A) Midgut B) Salivary glands. Expression of miRNAs was normalized with clean and partially fed tick tissues (indicated as 1 on the y-axis). Statistical significance for qRT-PCR-based differential expression was determined by the 2-tailed Student’s t-test where * is p<0.05. At least three biological replicates were used in each of the experiments.
miRNA inhibition in tick tissues reduced E. chaffeensis load
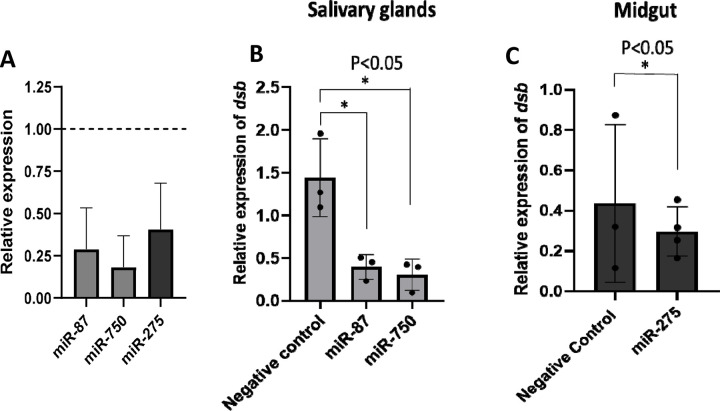
Three differentially expressed miRNAs Aam-miR-87, Aam-miR-750, and Aam-miR-275 were selected for the miRNA inhibition assay in tick tissues based on their putative role in tick immune responses (Table 1). As depicted in figures 2 and 3, Aam-miR-87 and Aam-miR-750 were found to be upregulated in the salivary glands of Ehrlichia chaffeensis-infected ticks, while Aam-miR-275 was upregulated in the midgut of ticks infected with Ehrlichia chaffeensis. The results of our miRNA inhibitory experiments revealed that suppressing these microRNAs individually reduced the E. chaffeensis load in tick tissues suggesting a role for miRNAs in tick immunity (Figure 6), although further validation is necessary. Subsequent studies will explore the targets of these microRNAs to gain a deeper understanding of their importance in pathways essential for the survival of E. chaffeensis. Additional functional studies will examine how miRNAs and their specific targets impact pathways that affect tick vector competence. Further investigation is also needed to explore additional differentially expressed miRNAs identified in this study, which could potentially offer valuable insights for preventing E. chaffeensis infection.
Figure 6.
microRNA inhibition reduces Ehrlichia chaffeensis load in tick tissues. A) microRNA inhibition in tick tissues (MG and SG). miR-87 and miR-750 were inhibited by ~75% and ~80% in tick salivary glands while miR-275 was inhibited by ~63% in tick midgutExpression of microRNAs in negative control tick tissues were given a normalized fold expression value of 1, as represented by the dashed line. Tick microRNA inhibition resulted in E. chaffeensis load reduction (dsb gene) in B) tick salivary glands and C) midguts. At least three biological replicates were used in each of the experiments. Statistically significant change (P<0.05) is indicated by an asterisk (*).
Limitations and conclusion
This study highlights the differential expression of miRNAs in E. chaffeensis-infected tick tissues, which could significantly influence the survival, colonization, and transmission of E. chaffeensis. Additionally, these miRNAs may play a role in the tick’s immune response against the pathogen. Selected microRNAs miR-87, miR-750, and miR-275 have shown promising results against E. chaffeensis survival or colonization in ticks. Further investigation of other differentially expressed miRNAs through miRNA inhibitory experiments is needed to explore these aspects. These tick-specific and E. chaffeensis specific differentially expressed miRNAs could provide potent avenues for treating or inhibiting E. chaffeensis.
Here it is noteworthy to mention that analyzing microRNA sequencing data (from Illumina i.e. short reads) within the context of a long-read sequenced genome may pose challenges in read mapping, alignment, and data integration due to the differences in read lengths and sequencing technologies. Aligning short Illumina reads to a long-read sequenced genome could result in decreased mapping efficiency, especially in regions with structural variations or repetitive elements. This may impact the accuracy of microRNA expression profiling and annotation. While long-read sequences may better capture genomic heterogeneity and structural variations compared to short reads, and provide valuable insights into genome architecture, they may also introduce challenges in accurately identifying and characterizing microRNAs, particularly in regions of high complexity or variation. Long-read sequencing technologies may have significantly higher error rates, which can pose challenges in accurately reconstructing the genome, mapping microRNA sequences, and distinguishing true variations from sequencing errors.
Supplementary Material
Acknowledgment
This work utilized the Magnolia High-Performance Computing at USM (http://magnolia.usm.edu).
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was principally supported by the NIH NIAID Awards #R15AI167013, #R21AI175885, and # R01AI163857. We thank the UMMC Molecular and Genomics facility, supported by the NIHNIGMS (#P20GM103476 & P20GM144041). The funders played no role in the study design, data collection, analysis, publication, decision, or manuscript preparation.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was principally supported by the NIH NIAID Awards #R15AI167013, #R21AI175885, and # R01AI163857. We thank the UMMC Molecular and Genomics facility, supported by the NIHNIGMS (#P20GM103476 & P20GM144041). The funders played no role in the study design, data collection, analysis, publication, decision, or manuscript preparation.
Footnotes
Ethics statement
The animal study was reviewed and approved by the University of Southern Mississippi’s Institutional Animal Care and Use Committee (IACUC protocols # 15101501.3 and 17101206.2). The study was conducted in accordance with the local legislation and institutional requirements.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Data availability statement
The raw small RNA sequences were deposited into the NCBI Sequence Read Archive (SRA) repository under the BioProject ID PRJNA992656.
References
- Adams D. A., Thomas K. R., Jajosky R. A., Foster L., Baroi G., Sharp P., et al. (2017). Summary of Notifiable Infectious Diseases and Conditions - United States, 2015. MMWR Morb Mortal Wkly Rep. 64, 1–143. doi: 10.15585/mmwr.mm6453a1 [DOI] [PubMed] [Google Scholar]
- Adegoke A, Hanson J, Smith R, Karim S. Ehrlichia chaffeensis co-opts phagocytic hemocytes for systemic dissemination in the Lone Star tick, Amblyomma americanum. Preprint. bioRxiv. 2023;2023.08.17.553720. Published 2023 Aug 20. doi: 10.1101/2023.08.17.553720 [DOI] [PMC free article] [PubMed]
- Adegoke A, Ribeiro JMC, Smith RC, Karim S. Tick innate immune responses to hematophagy and Ehrlichia infection at single-cell resolution. Front Immunol. 2024;14:1305976. Published 2024 Jan 11. doi: 10.3389/fimmu.2023.1305976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzov Alina& Tatar Marc (2010) Hormonal regulation of Drosophila microRNA let-7 and miR-125 that target innate immunity, Fly, 4:4, 306–311, DOI: 10.4161/fly.4.4.13008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Garcia I, Miska EA: MicroRNA functions in animal development and human disease. Development 2005, 132(21):4653–4662. [DOI] [PubMed] [Google Scholar]
- Alves RN, Levenhagen MA, Levenhagen MM, Rieck SE, Labruna MB, Beletti ME. The spreading process of Ehrlichiacanis in macrophages is dependent on actin cytoskeleton, calcium and iron influx and lysosomal evasion. Vet Microbiol. 2014;168(2–4):442–446. doi: 10.1016/j.vetmic.2013.11.030 [DOI] [PubMed] [Google Scholar]
- Anderson JM, Sonenshine DE, Valenzuela JG. Exploring the mialome of ticks: an annotated catalogue of midgut transcripts from the hard tick, Dermacentor variabilis (Acari: Ixodidae). BMC Genomics. 2008; 9:552. 10.1186/1471-2164-9-552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes S, Couto J, Ferrolho J, Sanches GS, Merino Charrez JO, De la Cruz Hernandez N, et al. Transcriptome and Proteome Response of Rhipicephalus annulatus Tick Vector to Babesia bigemina Infection. Frontiers in Physiology. 2019; 10:318. 10.3389/fphys.2019.00318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio-Puerta E., Gómez-Martín C., Giannoukakos S., Medina J. M., Scheepbouwer C., García-Moreno A., Carmona-Saez P., Fromm B., Pegtel M., Keller A., Marchal J. A., & Hackenberg M. (2022). sRNAbench and sRNAtoolbox 2022 update: accurate miRNA and sncRNA profiling for model and non-model organisms. Nucleic acids research, 50(W1), W710–W717. Advance online publication. 10.1093/nar/gkac363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravin AA, Hannon GJ, Brennecke J (2007) The Piwi-piRNA pathway provides an adaptive defense in the transposon arms race. Science 318: 761–764. [DOI] [PubMed] [Google Scholar]
- Artigas-Jerónimo S, Alberdi P, Villar Rayo M, et al. Anaplasma phagocytophilum modifies tick cell microRNA expression and upregulates isc-mir-79 to facilitate infection by targeting the Roundabout protein 2 pathway. Sci Rep. 2019. doi: 10.1038/s41598-019-45658-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari S: Chapter Two - microRNAs as Regulators of Insect Host–Pathogen Interactions and Immunity. In: Advances in Insect Physiology. Edited by Smagghe G, vol. 55: Academic Press; 2018: 19–45. [Google Scholar]
- Avila-Bonilla R.G.; Yocupicio-Monroy M.; Marchat L.A.; De Nova-Ocampo M.A.; Del Angel R.M.; Salas-Benito J.S. Analysis of the miRNA profile in C6/36 cells persistently infected with dengue virus type 2. Virus Res. 2017, 232, 139–151. [DOI] [PubMed] [Google Scholar]
- Barrero RA, Keeble-Gagnère G, Zhang B, et al. Evolutionary conserved microRNAs are ubiquitously expressed compared to tick-specific miRNAs in the cattle tick Rhipicephalus (Boophilus) microplus. BMC Genomics. 2011. doi: 10.1186/1471-2164-12-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [DOI] [PubMed] [Google Scholar]
- Bartel D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell. 2009, 136, 215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D.P.; Chen C.Z. Micromanagers of gene expression: The Potentially Widespread Influence of Metazoan MicroRNAs. Nat. Rev. Genet. 2004, 5, 396–400. [DOI] [PubMed] [Google Scholar]
- Bond D, Foley E. A quantitative RNAi screen for JNK modifiers identifies Pvr as a novel regulator of Drosophila immune signaling. PLoSPathog. 2009;5(11):e1000655. doi: 10.1371/journal.ppat.1000655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolomeazzi M, Gaffo E, Bortoluzzi S. A survey of software tools for microRNA discovery and characterization using RNA-seq. Brief Bioinformatics. 2019. 21;20(3):918–30.Google Scholar [DOI] [PubMed] [Google Scholar]
- Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, et al. (2007) Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 128: 1089–1103. [DOI] [PubMed] [Google Scholar]
- Brennecke J, Malone CD, Aravin AA, Sachidanandam R, Stark A, et al. (2008) An Epigenetic Role for Maternally Inherited piRNAs in Transposon Silencing. Science 322: 1387–1392. doi: 10.1126/science.1165171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke J., Hipfner D. R., Stark A., Russell R. B., and Cohen S. M. (2003). Bantam Encodes a Developmentally Regulated microRNA That Controls Cell Proliferation and Regulates the Proapoptotic Gene Hid in Drosophila. Cell 113, 25–36. doi: 10.1016/s0092-8674(03)00231-9 [DOI] [PubMed] [Google Scholar]
- Bryant B, Macdonald W, Raikhel AS. microRNA miR-275 is indispensable for blood digestion and egg development in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2010;107(52):22391–22398. doi: 10.1073/pnas.1016230107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budachetri K., Teymournejad O., Lin M., Yan Q., Mestres-Villanueva M., Brock G. N., et al. (2020). An Entry-Triggering Protein of Ehrlichia Is a New Vaccine Candidate Against Tick-Borne Human Monocytic Ehrlichiosis. mBio 11, 1–13. doi: 10.1128/mBio.00895-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budachetri K, Lin M, Chien RC, Zhang W, Brock GN, Rikihisa Y. Efficacy and Immune Correlates of OMP-1B and VirB2–4 Vaccines for Protection of Dogs from Tick Transmission of Ehrlichia chaffeensis. mBio. 2022;13(6):e0214022. doi: 10.1128/mbio.02140-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busk PK. A tool for design of primers for microRNA-specific quantitative RT-qPCR. BMC Bioinformatics. 2014;15:29. Published 2014 Jan 28. doi: 10.1186/1471-2105-15-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J.C.; Ambros V. Role of microRNAs in plant and animal development. Science 2003, 301, 336–338. [DOI] [PubMed] [Google Scholar]
- Chou S, Poskanzer KE, Rollins M, Thuy-Boun PS. (2023). De novo assembly of a long-read Amblyomma americanum tick genome. 10.57844/arcadia-9b6j-q683 [DOI]
- Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, Kocan KM, Fahy JV, Nganga LW, Ronmark E, Cooper PJ, Platts-Mills TA. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α−1,3-galactose. J Allergy Clin Immunol. 2011. May;127(5):1286–93.e6. doi: 10.1016/j.jaci.2011.02.019. Epub 2011 Mar 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispell G, Commins SP, Archer-Hartman SA, et al. Discovery of Alpha-Gal-Containing Antigens in North American Tick Species Believed to Induce Red Meat Allergy. Front Immunol. 2019;10:1056. Published 2019 May 17. doi: 10.3389/fimmu.2019.01056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C., Sun X., Guan Z., Zhang M. & Duan M. Modulation of infuenza A virus replication by microRNA-9 through targeting MCPIP1. J. Med. Virol. 89, 41–48 (2017). [DOI] [PubMed] [Google Scholar]
- Dunphy P. S., Luo T., & McBride J. W. (2014). Ehrlichia chaffeensis exploits host SUMOylation pathways to mediate effector-host interactions and promote intracellular survival. Infection and immunity, 82(10), 4154–4168. 10.1128/IAI.01984-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin A. A., Galdos M. A., Wickersheim M. L., Harrison C. C., Marr K. D., Colicchio J. M., & Blumenstiel J. P. (2015). piRNAs Are Associated with Diverse Transgenerational Effects on Gene and Transposon Expression in a Hybrid Dysgenic Syndrome of D. virilis. PLoS genetics, 11(8), e1005332. 10.1371/journal.pgen.1005332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Zhou S, Wang J, Hu W (2018) microRNA profiles and functions in mosquitoes. PLOS Neglected Tropical Diseases 12(5): e0006463. 10.1371/journal.pntd.0006463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynt AS, Greimann JC, Chung WJ, Lima CD, Lai EC: MicroRNA biogenesis via splicing and exosome-mediated trimming in Drosophila. Mol Cell 2010, 38(6):900–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedländer MR, Mackowiak SD, Li N, Chen W, Rajewsky N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012;40(1):37–52. doi: 10.1093/nar/gkr688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm B., Høye E., Domanska D., Zhong X., Aparicio-Puerta E., Ovchinnikov V., Umu S. U., Chabot P. J., Kang W., Aslanzadeh M., Tarbier M., MármolSánchez E., Urgese G., Johansen M., Hovig E., Hackenberg M., Friedländer M. R., & Peterson K. J. (2022). MirGeneDB 2.1: toward a complete sampling of all major animal phyla. Nucleic acids research, 50(D1), D204–D210. 10.1093/nar/gkab1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullaondo A, Lee SY. Identification of putative miRNA involved in Drosophila melanogaster immune response. Dev Comp Immunol. 2012;36(2):267–273. doi: 10.1016/j.dci.2011.03.034 [DOI] [PubMed] [Google Scholar]
- Garbuzov A, Tatar M. Hormonal regulation of Drosophila microRNA let-7 and miR-125 that target innate immunity. Fly (Austin). 2010. Oct-Dec;4(4):306–11. doi: 10.4161/fly.4.4.13008. Epub 2010 Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Research. 2008; 36(Database issue):D154–8. 10.1093/nar/gkm952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guizzo MG, Dolezelikova K, Neupane S, Frantova H, Hrbatova A, Pafco B, Fiorotti J, Kopacek P, Zurek L. Characterization and manipulation of the bacterial community in the midgut of Ixodes ricinus. Parasit Vectors. 2022. Jul 9;15(1):248. doi: 10.1186/s13071-022-05362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenberg M, Langenberger D, Schwarz A, Erhart J, Kotsyfakis M. In silico target network analysis of de novo-discovered, tick saliva-specific microRNAs reveals important combinatorial effects in their interference with vertebrate host physiology. RNA. 2017;23(8):1259–1269. doi: 10.1261/rna.061168.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermance M.E.; Widen S.G.; Wood T.G.; Thangamani S. Ixodes scapularis salivary gland microRNAs are differentially expressed during Powassan virus transmission. Sci. Rep. 2019, 9, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel J, Bartschat S, Wintsche A, Otto C, Stadler PF. Evolution of the let-7 microRNA family. RNA Biol. 2012;9:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Criscione F, Liang S, Tu Z. MicroRNAs of two medically important mosquito species: Aedes aegypti and Anopheles stephensi. Insect Mol Biol. 2015;24(2):240–252. doi: 10.1111/imb.12152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T.; Xu D.; Zhang X. Characterization of host microRNAs that respond to DNA virus infection in a crustacean. BMC Genomics 2012, 13, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T.; Zhang X. Functional analysis of a crustacean microRNA in host-virus interactions. J. Virol. 2012, 86, 12997–13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M.; Torres S.; Schnettler E.; Funk A.; Grundhoff A.; Pijlman G.P.; Khromykh A.; Asgari S. West Nile virus encodes a microRNA-like small RNA in the 30 untranslated region which up-regulates GATA4 mRNA and facilitates virus replication in mosquito cells. Nucleic Acids Res. 2012, 40, 2210–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki Y. W., Siomi M. C., &Siomi H. (2015). PIWI-Interacting RNA: Its Biogenesis and Functions. Annual review of biochemistry, 84, 405–433. 10.1146/annurev-biochem-060614-034258 [DOI] [PubMed] [Google Scholar]
- Kanoksinwuttipong N, Jaree P, Somboonwiwat K. Shrimp pmo-miR-750 regulates the expression of sarcoplasmic calcium-binding protein facilitating virus infection in Penaeus monodon. Fish Shellfish Immunol. 2022. Oct;129:74–84. doi: 10.1016/j.fsi.2022.08.046. Epub 2022 Aug 23. [DOI] [PubMed] [Google Scholar]
- Karim S, Singh P, Ribeiro JM. A deep insight into the sialotranscriptome of the gulf coast tick, Amblyomma maculatum. PLoS One. 2011;6(12):e28525. doi: 10.1371/journal.pone.0028525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S, Ribeiro JM. An Insight into the Sialome of the Lone Star Tick, Amblyomma americanum, with a Glimpse on Its Time Dependent Gene Expression. PLoS One. 2015;10(7):e0131292. Published 2015 Jul 1. doi: 10.1371/journal.pone.0131292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S, Browning R, Ali L, Truhett R. Laboratory-Infected Ehrlichiachaffeensis Female Adult Amblyommaamericanum Salivary Glands Reveal Differential Gene Expression, Journal of Medical Entomology, Volume 49, Issue 3, 1 May 2012, Pages 547–554, 10.1603/ME11214 [DOI] [PubMed] [Google Scholar]
- Karim S.; Essenberg R.C.; Dillwith J.W.; Tucker J.S.; Bowman A.S.; Sauer J.R. Identification of SNARE and cell trafficking regulatory proteins in the salivary glands of the lone star tick, Amblyommaamericanum (L.). Insect Biochem. Mol. Biol. 2002, 32, 1711–1721. [DOI] [PubMed] [Google Scholar]
- Kennedy AC; BCE1, Marshall E. Lone Star Ticks (Amblyomma americanum)∷ An Emerging Threat in Delaware. Dela J Public Health. 2021;7(1):66–71. Published 2021 Jan 21. doi: 10.32481/djph.2021.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanal S, Sultana H, Catravas JD, Carlyon JA, Neelakanta G. Anaplasmaphagocytophilum infection modulates expression of megakaryocyte cell cycle genes through phosphatidylinositol-3-kinase signaling. PloS One. 2017; 12(8):e0182898. 10.1371/journal.pone.0182898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014. Jan 1;42(Database issue):D68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D.; Embers M.; Mather T.N.; Karim S. Is selenoprotein K required for Borrelia burgdorferi infection within the tick vector Ixodes scapularis? Parasites Vectors 2019, 12, 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Downs LP, Embers M, Flynt AS, Karim S. Identification of microRNAs in the Lyme Disease Vector Ixodes scapularis. Int J Mol Sci. 2022;23(10):5565. Published 2022 May 16. doi: 10.3390/ijms23105565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai EC. Two decades of miRNA biology: lessons and challenges. RNA. 2015; 21(4):675–7. 10.1261/rna.051193.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox KA, Owczarzy R, Thomas DM, Walder JA, Behlke MA. Improved Performance of Anti-miRNA Oligonucleotides Using a Novel Non-Nucleotide Modifier. Mol Ther Nucleic Acids. 2013;2(8):e117. Published 2013 Aug 27. doi: 10.1038/mtna.2013.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Hu S., Zhang H., Yin H., Wang H., Zhou D., Sun Y., Ma L., Shen B., & Zhu C. (2021). MiR-279–3p regulates deltamethrin resistance through CYP325BB1 in Culex pipiens pallens. Parasites & vectors, 14(1), 528. 10.1186/s13071-021-05033-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Ge X, Li Z, Zeng B, Xu J, Aslam AFM, et al. MicroRNA Let-7 regulates molting and metamorphosis in the silkworm, Bombyx mori. Insect Biochem Mol Biol. 2014;53:13–21. [DOI] [PubMed] [Google Scholar]
- Liu W, Hao Z, Huang L, et al. Comparative expression profile of microRNAs in Anopheles anthropophagus midgut after blood-feeding and Plasmodium infection. Parasites and Vectors. 2017. doi: 10.1186/s13071-017-2027-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Zhou Y.; Wu J.; Zheng P.; Li Y.; Zheng X.; Puthiyakunnon S.; Tu Z.; Chen X.-G. The expression profile of Aedes albopictus miRNAs is altered by dengue virus serotype-2 infection. Cell Biosci. 2015, 5, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas KJ, Roy S, Ha J, Gervaise AL, Kokoza VA, Raikhel AS. MicroRNA-8 targets the Wingless signaling pathway in the female mosquito fat body to regulate reproductive processes. Proc Natl Acad Sci U S A. 2015;112(5):1440–1445. doi: 10.1073/pnas.1424408112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Peng L, Shan W, Sun M, Luo L, Liang W. Machine learning in the development of targeting microRNAs in human disease. Front Genet. 2023;13:1088189. Published 2023 Jan 4. doi: 10.3389/fgene.2022.1088189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharaj PD, Widen SG, Huang J, Wood TG, Thangamani S. Discovery of mosquito saliva microRNAs during CHIKV infection. PLoS Negl Trop Dis. 2015;9(1):e0003386. Published 2015 Jan 22. doi: 10.1371/journal.pntd.0003386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesen P, Ivens A, Buck AH, van Rij RP: Small RNA Profiling in Dengue Virus 2-Infected Aedes Mosquito Cells Reveals Viral piRNAs and Novel Host miRNAs. PLoSNegl Trop Dis 2016, 10(2):e0004452. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momen-Heravi F, Bala S: miRNA regulation of innate immunity. J Leukoc Biol 2018. [DOI] [PubMed] [Google Scholar]
- Neelakanta G, Sultana H, Fish D, Anderson JF, Fikrig E. Anaplasmaphagocytophilum induces Ixodes scapularis ticks to express an antifreeze glycoprotein gene that enhances their survival in the cold. J Clin Invest. 2010. Sep;120(9):3179–90. doi: 10.1172/JCI42868. Epub 2010 Aug 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelder MP, Russell CB, Clow KM, et al. Occurrence and distribution of Ambylomma americanum as determined by passive surveillance in Ontario, Canada (1999–2016). Ticks Tick Borne Dis. 2019;10(1):146–155. doi: 10.1016/j.ttbdis.2018.10.001 [DOI] [PubMed] [Google Scholar]
- Nunes FMF, Ihle KE, Mutti NS, Simões ZLP, Amdam GV. The gene vitellogenin affects microRNA regulation in honey bee (Apis mellifera) fat body and brain. J. Exp. Biol. 2013; 216: 3724–3732. doi: 10.1242/jeb.089243 [DOI] [PubMed] [Google Scholar]
- Osei-Amo S, Hussain M, O’Neill SL, Asgari S. Wolbachia-induced aae-miR-12 miRNA negatively regulates the expression of MCT1 and MCM6 genes in Wolbachia-infected mosquito cell line. PLoS One. 2012;7(11):e50049. doi: 10.1371/journal.pone.0050049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W., Wang Y. S., Du X. N., Liu H. J. & Zhang H. B. gga-miR-9* inhibits IFN production in antiviral innate immunity by targeting interferon regulatory factor 2 to promote IBDV replication. Vet. Microbiol. 178, 41–49 (2015). [DOI] [PubMed] [Google Scholar]
- Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408:86–9. [DOI] [PubMed] [Google Scholar]
- Patrick C.D.; Hair J.A. Laboratory rearing procedures and equipment for multi-host ticks (Acarina: Ixodidae). J. Med. Entomol. 1975, 12, 389–390. [DOI] [PubMed] [Google Scholar]
- Pedersen M. E. et al. An epidermal microRNA regulates neuronal migration through control of the cellular glycosylation state. Science. 341, 1404–1408 (2013). [DOI] [PubMed] [Google Scholar]
- Popara M, Villar M, de la Fuente J. Proteomics characterization of tick-host-pathogen interactions. Methods Mol Biol. 2015; 1247:513–27. 10.1007/978-1-4939-2004-4_34 [DOI] [PubMed] [Google Scholar]
- Queiroz FR, Portilho LG, Jeremias WJ, Babá ÉH, do Amaral LR, Silva LM, Coelho PMZ, Caldeira RL, Gomes MS. Deep sequencing of small RNAs reveals the repertoire of miRNAs and piRNAs in Biomphalaria glabrata. Mem Inst Oswaldo Cruz. 2020. Jul 1;115:e190498. doi: 10.1590/0074-02760190498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy E.; Taank V.; Anderson J.F.; Sultana H.; Neelakanta G. Repression of tick microRNA-133 induces organic anion transporting polypeptide expression critical for Anaplasmaphagocytophilum survival in the vector and transmission to the vertebrate host. PLoS Genet. 2020, 16, e1008856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebijith KB, Asokan R, Hande HR, Krishna Kumar NK. The First Report of miRNAs from a Thysanopteran Insect, Thrips palmiKarny Using High-Throughput Sequencing. PLoS One. 2016. Sep 29;11(9):e0163635. doi: 10.1371/journal.pone.0163635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigoutsos I: New tricks for animal microRNAS: targeting of amino acid coding regions at conserved and nonconserved sites. Cancer Res 2009, 69(8):3245–3248. [DOI] [PubMed] [Google Scholar]
- Robins H, Li Y, Padgett RW. Incorporating structure to predict microRNA targets. Proc Natl Acad Sci U S A. 2005. Mar 15;102(11):4006–9. doi: 10.1073/pnas.0500775102. Epub 2005 Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldaña M.; Etebari K.; Hart C.E.; Widen S.G.; Wood T.G.; Thangamani S.; Asgari S.; Hughes G.L. Zika virus alters the microRNA expression profile and elicits an RNAi response in Aedes aegypti mosquitoes. PLoSNegl. Trop. Dis. 2017, 11, e0005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vicente S, Tokarz R. Tick-Borne Co-Infections: Challenges in Molecular and Serologic Diagnoses. Pathogens. 2023;12(11):1371. Published 2023 Nov 20. doi: 10.3390/pathogens12111371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos D, Feng M, Kolliopoulou A, Taning CNT, Sun J, Swevers L. What Are the Functional Roles of Piwi Proteins and piRNAs in Insects? Insects. 2023. Feb 14;14(2):187. doi: 10.3390/insects14020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnall-Levin M, Zhao Y, Perrimon N, Berger B: Conserved microRNA targeting in Drosophila is as widespread in coding regions as in 3’UTRs. Proc Natl Acad Sci U S A 2010, 107(36):15751–15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddiki N. et al. Te microRNA-9/B-lymphocyte-induced maturation protein-1/IL-2 axis is diferentially regulated in progressive HIV infection. Eur. J. Immunol. 43, 510–520 (2013). [DOI] [PubMed] [Google Scholar]
- Sempere LF, Sokol NS, Dubrovsky EB, Berger EM, Ambros V. Temporal regulation of microRNA expression in Drosophila melanogaster mediated by hormonal signals and broad-Complex gene activity. Dev Biol. 2003;259:9–18. [DOI] [PubMed] [Google Scholar]
- Sharma SR, Karim S. Tick Saliva and the Alpha-Gal Syndrome: Finding a Needle in a Haystack. Front Cell Infect Microbiol. 2021;11:680264. Published 2021 Jul 20. doi: 10.3389/fcimb.2021.680264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SR, Choudhary SK, Vorobiov J, Commins SP, Karim S. Tick bite-induced Alpha-Gal Syndrome and Immunologic Responses in an Alpha-Gal Deficient Murine Model. Preprint. bioRxiv. 2023;2023.11.09.566281. Published 2023 Nov 13. doi: 10.1101/2023.11.09.566281 [DOI] [PMC free article] [PubMed]
- Singh CP, Singh J, Nagaraju J. A Baculovirus-Encoded MicroRNA (miRNA) Suppresses Its Host miRNA Biogenesis by Regulating the Exportin-5 Cofactor Ran. J Virol. 2012. doi: 10.1128/jvi.00064-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford KC 3rd, Molaei G, Little EAH, Paddock CD, Karpathy SE, Labonte AM. Distribution and Establishment of the Lone Star Tick in Connecticut and Implications for Range Expansion and Public Health. J Med Entomol. 2018;55(6):1561–1568. doi: 10.1093/jme/tjy115 [DOI] [PubMed] [Google Scholar]
- Sultana H, Neelakanta G, Kantor FS, Malawista SE, Fish D, Montgomery RR, et al. Anaplasmaphagocytophilum induces actin phosphorylation to selectively regulate gene transcription in Ixodes scapularis ticks. The Journal of Experimental Medicine. 2010; 207(8):1727–43. 10.1084/jem.20100276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taank V, Dutta S, Dasgupta A, Steeves TK, Fish D, Anderson JF, et al. Human rickettsial pathogen modulates arthropod organic anion transporting polypeptide and tryptophan pathway for its survival in ticks. Scientific Reports. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takane K., Fujishima K., Watanabe Y., Sato A., Saito N., Tomita M., & Kanai A. (2010). Computational prediction and experimental validation of evolutionarily conserved microRNA target genes in bilaterian animals. BMC genomics, 11, 101. 10.1186/1471-2164-11-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turck JW, Taank V, Neelakanta G, Sultana H. Ixodes scapularis Src tyrosine kinase facilitates Anaplasmaphagocytophilum survival in its arthropod vector. Ticks and Tick-Borne Diseases. 2019; 10 (4):838–47. 10.1016/j.ttbdis.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar M, Ayllon N, Alberdi P, Moreno A, Moreno M, Tobes R, et al. Integrated Metabolomics, Transcriptomics and Proteomics Identifies Metabolic Pathways Affected by Anaplasmaphagocytophilum Infection in Tick Cells. Molecular & Cellular Proteomics: MCP. 2015; 14(12):3154–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitsios DM, Kentepozidou E, Quintais L, Benito-Gutiérrez E, van Dongen S, Davis MP, et al. Mirnovo: genome-free prediction of microRNAs from small RNA sequencing data and single-cells using decision forests. Nucleic Acids Res. 2017. Dec 1;45(21):e177–e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter F, Edaye S, Hüttenhofer A, Brunel C. Anopheles gambiae miRNAs as actors of defence reaction against Plasmodium invasion. Nucleic Acids Res. 2007;35(20):6953–6962. doi: 10.1093/nar/gkm686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Luo J, Chen Z, et al. MicroRNA let-7 regulates the expression of ecdysteroid receptor (ECR) in Hyalommaasiaticum (Acari: Ixodidae) ticks. Parasit Vectors. 2019;12(1):235. Published 2019 May 15. doi: 10.1186/s13071-019-3488-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H.; Jiang Y.; Xu X.; Su X.; Liu Y.; Ma Y.; Zhao Y.; Shen Z.; Huang B.; Cau X. Inducible degradation of lncRNA Sros1 promotes IFN-gamma-mediated activation of innate immune responses by stabilizing Stat1 mRNA. Nat. Immunol. 2019, 20, 1621–1630. [DOI] [PubMed] [Google Scholar]
- Yuva-Aydemir Y., Simkin A., Gascon E. & Gao F. B. MicroRNA-9: functional evolution of a conserved small regulatory RNA. RNA Biol. 8, 557–564 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Zheng Y.; Jagadeeswaran G.; Ren R.; Sunkar R.; Jiang H. Identification of conserved and novel microRNAs in Manduca sexta and their possible roles in the expression regulation of immunity-related genes. Insect. Biochem. Mol. Biol. 2014, 47, 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Lucas KJ, Saha TT, Ha J, Ling L, Kokoza VA, et al. (2017) MicroRNA-275 targets sarco/endoplasmic reticulum Ca2+ adenosine triphosphatase (SERCA) to control key functions in the mosquito gut. PLoS Genet 13(8): e1006943. 10.1371/journal.pgen.1006943 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw small RNA sequences were deposited into the NCBI Sequence Read Archive (SRA) repository under the BioProject ID PRJNA992656.