Abstract
Two copies of IS1675, a novel lactococcal insertion element from the IS4 family, are present on a 70-kb plasmid, where they frame the lantibiotic lacticin 481 operon. The whole structure could be a composite transposon designated Tn5721. This study shows that the lacticin 481 operon does not include any regulatory gene and provides a new example of a transposon-associated bacteriocin determinant. We identified five other IS1675 copies not associated with the lacticin 481 operon. The conservation of IS1675 flanking sequences suggested a 24-bp target site.
Insertion sequences (ISs) are small (<2.5-kb) phenotypically cryptic DNA elements that can insert into nonhomologous DNA sites. Their genetic organization is simple, since they contain only one or two open reading frames (ORFs) encoding a transposase (Tpase) (for a recent review, see reference 11). In the course of transposition, the Tpase interacts specifically with inverted repeats (IRs) located at the IS extremities (11) and with the target site, either directly or in conjunction with accessory proteins (3). Transposition of otherwise nonmobile DNA segments can be achieved when they are flanked by two copies of the same IS, forming a composite transposon. The genes carried by transposons usually confer an advantage to the host, such as antibiotic resistance, pathogenic function, or a catabolic pathway. Within lactic acid bacteria, ISs have often been found on large plasmids and are associated with industrially relevant traits (25). The complete sequences of pK214 and pMRC01 (29 and 60 kb, respectively) (5, 17) argue for an important role for ISs in the evolution of large lactococcal plasmids. Since many of them are conjugative, these plasmids can contribute to the horizontal transfer of transposons within bacteria. Although bacteriocin (antibacterial-peptide) production constitutes a clear advantage and although the genetic sequences responsible for it are frequently plasmid borne, only nisin was shown to be transposon encoded (10). A region of pMRC01 contains the operon for the bacteriocin lacticin 3147 framed by two IS946 copies, which led the authors to propose that this region corresponds to a composite transposon (5). In this study, we characterized IS1675, a novel IS found associated with the lacticin 481 operon. Lacticin 481 is a lantibiotic (18), i.e., a bacteriocin containing rare amino acids produced by posttranslational modifications of a prepeptide (10). Its operon is composed of six genes (Fig. 1A), encoding the prepeptide LctA, the enzyme LctM, putatively involved in the posttranslational modifications, and two ATP-binding cassette (ABC) transporters: LctT, responsible for the cleavage and excretion of the bacteriocin, and LctF/LctE/LctG, providing self-protection to the producer strain (23, 24). The lacticin 481 operon was localized on a conjugative plasmid from Lactococcus lactis strain CNRZ 481 and on pOS5, a 70-kb plasmid from L. lactis ADRIA 85LO30 (6, 18). This study shows that two IS1675 copies frame the lacticin 481 operon, suggesting that the latter is part of a composite transposon. We identified other insertion points of IS1675, and a putative consensus target site was deduced.
FIG. 1.
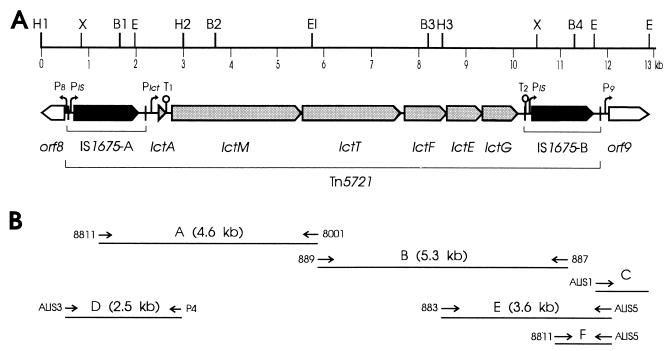
(A) Genetic organization and restriction map of Tn5721 and flanking regions. The boxes represent the ORFs, and their orientation shows the transcriptional direction. The lct genes constituting the lacticin 481 operon are grey. IS1675-A and -B are two identical copies of IS1675. Vertical bars, IRs delimiting IS1675; black boxes, IS1675 ORF; open boxes (orf8 and orf9), sequenced part of ORFs flanking Tn5721; Plct, PIS, P8, and P9, putative promoters; T1 and T2, putative Rho-independent terminator. The IS1675-A terminator is omitted because orf8 is divergent. BamHI, EcoRI, EcoRV, HindIII, and XbaI sites are abbreviated B, EI, E, H, and X, respectively, and are numbered when necessary. Some EcoRI, EcoRV, and XbaI sites are omitted. (B) Positions of the primers and of DNA fragments amplified by PCR. Arrows, primers (not to scale); lines A to F, PCR fragments.
DNA sequencing and analysis of IS1675.
The 3′ end of a putative Tpase gene was identified upstream of the lacticin 481 structural gene lctA from two strains: L. lactis CNRZ 481 and ADRIA 85LO30 (18, 24). To complete the sequence of the putative IS, we constructed pEF549 by subcloning into pBluescript SK (Stratagene) the 3-kb HindIII DNA fragment H1 to H2 (Fig. 1A) from λE23, which includes the 5′ end of the lct operon plus 10.9 kb of upstream DNA (23). Its nucleotide sequence was determined on both strands, extending by 2,112 bp the previously reported sequence (24). Sequence analysis revealed perfect 27-bp IRs, defining a possible 1,606-bp IS designated IS1675, the extremities of which are shown on Fig. 2A. Its GC content is 33.4%, intermediate to those of L. lactis chromosomal DNA (36 to 38%) and of L. lactis plasmids (29 to 32%) (5). The IRs frame a single ORF in the same orientation as the lct genes (Fig. 1A). This ORF potentially codes for a 439-residue protein showing similarities to Tpases (see below). A putative ribosome binding site (RBS) was found at the appropriate distance from the UUG initiation codon, and promoter-like sequences were identified between the left IR and the ORF (Fig. 2A). Whereas no putative terminator was identified downstream of the ORF, a Rho-independent terminator-like sequence was observed immediately downstream of the left IR.
FIG. 2.
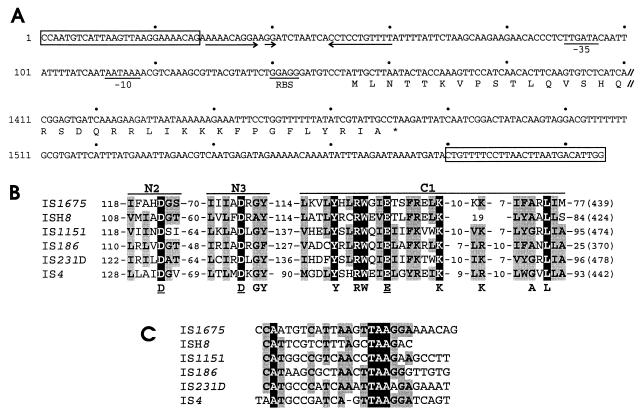
Characteristics of IS1675 and of its Tpase. (A) DNA sequences of the 5′ and 3′ ends of IS1675. //, separation between the two regions (nucleotides 1 to 200 and 1,411 to 1,606); boxes, IRs; arrows, putative Rho-independent terminator. The −35 and −10 sequences of a putative promoter and the RBS are indicated. The N- and C-terminal sequences of the encoded protein are given in the single-letter code. ∗, stop codon. Dots are placed every 20 bp. (B) Similarities between the IS1675-encoded protein and Tpases encoded by the IS4 family. Only region N2 and the most conserved parts of regions N3 and C1 are shown. Residues on a black background are conserved within all six proteins. Functionally related amino acids found at the same position in at least four proteins are on a grey background. The amino acids below the sequences form the DDE motif (underlined) and the signature motifs D-1-GY, Y-2-RW-2-E-6-K, and K-9/12-A-1-L. The numbers of residues preceding and following each region are given, and the total numbers of residues are in parentheses. The GenBank accession number of ISH8 is AF016485, the other accession numbers are in reference 22. (C) Sequence comparison of the left IRs from the same ISs as in panel B. Bases conserved in all six sequences are on a black background; bases conserved in four or five sequences are on a grey background.
IS1675 belongs to the IS4 family.
The IS1675-encoded protein showed up to 19% identity with Tpases such as those encoded by ISH8, IS1151, IS186, IS421, IS231, and IS4 (14, 22). These ISs all belong to the IS4 family, one of the most frequently encountered families of bacterial ISs (11). IS1675 presents the same overall structure as IS4-related elements: between 1.3 and 1.7 kb long, a single ORF encoding a protein of 370 to 480 amino acids, and IRs of around 25 bp. The Tpases encoded by the IS4 family display three conserved regions, N2, N3, and C1 (11, 22), which are conserved within the IS1675 protein (Fig. 2B). It contains in particular the three signature motifs of the N3 and C1 regions and the DDE motif. The last is highly conserved within Tpases from many ISs and retroviral integrases (11, 19, 22). The N3 and C1 regions are shared by the Tpases encoded by the IS4 and IS5 families, but these families were distinguished on the basis of the disposition of the N3 and C1 regions (22). Within the IS1675 protein, these regions are separated by 114 residues (Fig. 2B), which is characteristic of Tpases encoded by the IS4 family. The similarities between the Tpases encoded by the IS4 family are correlated with sequence conservations within the associated IRs (22), and most of the conserved bases are shared by the IS1675 IRs (Fig. 2C). IS1675 thus meets the criteria related to overall organization, to Tpase sequence, and to IR sequence to be included in the IS4 family. Mahillon and Chandler (11) listed 28 members of this family (isoforms not included) from gram-negative and -positive bacteria, mycobacteria, and halobacteria but none from gram-positive cocci. To our knowledge, IS1675 is the first lactococcal member of this family.
The lacticin 481 operon is included in a transposon-like structure.
The knowledge of the IS1675 sequence permitted us to identify, downstream of the lacticin 481 operon, a second IS1675 copy, the 5′ two-thirds of which was previously sequenced (24). We refer to the upstream and downstream copies as IS1675-A and IS1675-B, respectively (Fig. 1A). To complete the sequencing of IS1675-B, a 1.2-kb fragment containing the 3′ end of IS1675-B plus downstream DNA (Fig. 1B, fragment C) was amplified from plasmid pES2, which is a derivative of pOS5 from L. lactis ADRIA 85LO30 (6), by ligation-mediated PCR (LMPCR) as follows. The fragments of EcoRV-digested pES2 were ligated with SmaI/EcoRI-restricted pBluescript SK. After phenol extraction, the ligation products were digested with EcoRV to separate pES2 fragments ligated to each other. The resulting DNA was used as a template for PCR amplification with the primers ALIS1 and T3. The sequences and positions of the primers used in this study are given in Table 1. The partial sequencing of fragment C allowed the design of primer ALIS5, which was used to amplify the 3′ end of the lacticin 481 operon plus IS1675-B (Fig. 1B, fragment E), to verify the identity of the flanking sequence. The downstream half of IS1675-B was then amplified (Fig. 1B, fragment F) and sequenced, showing that IS1675-B is complete and 100% identical to IS1675-A. The lacticin 481 operon (lctAMTFEG) is thus flanked by two copies of IS1675 in the same orientation (Fig. 1A), lying 281 bp upstream of lctA and 147 bp downstream of lctG. This 11.5-kb structure could behave as a composite transposon, designated Tn5721, which could carry the bacteriocin operon. The transcript from the lacticin 481 operon enters the downstream IS since the putative transcriptional terminator T2 downstream of lctG (24) is internal to IS1675-B. Such a phenomenon increases the coherence of composite transposons by reducing the left-end activity of the downstream ISs, therefore favoring the mobility of transposons over individual ISs (11). In addition to ADRIA 85LO30 and CNRZ 481, a third L. lactis strain, SL2, was identified as a lacticin 481 producer (20). The expected DNA fragments A and B (Fig. 1B) were amplified from each strain, showing that the Tn5721 structure is conserved within these three strains.
TABLE 1.
PCR primers used in this study
| Primer | 5′–3′ sequencea | Positionb | Directionc |
|---|---|---|---|
| ALIS1 | CAACAGAATTCATTTATGAAATTAGAAC | IS1675 3′ end, nt 2099–2119 and 11750–11770 | + |
| ALIS2 | ATTTGATGAGACACTTG | IS1675 5′ end, nt 770–786 and 10421–10437 | − |
| ALIS3 | TGAACACCTACAACCCC | Upstream of IS1675-A, nt 527–543 | + |
| ALIS4 | GATAGACTTTGCCCTCC | IS1675 5′ end, nt 917–933 and 10568–10581 | − |
| ALIS5 | CCACATCCATTACTTCC | Downstream of IS1675-B, nt 12116–12132 | − |
| ALIS9 | TGAATTAGAAAAGCGCC | Upstream of IS1675-F | + |
| ALIS10 | GTACAATACCACAAGTC | Downstream of IS1675-F | − |
| P4 | AAAGCTTTACCTGTACT | lctM, nt 2986–3002 | − |
| 883 | ATCATGAACTCTGACTTAA | lctF, nt 8458–8476 | + |
| 887 | ATGCAAGAAATGATAATG | IS1675, nt 1526–1543 and 11177–11194 | − |
| 889 | CACTCATTGACCCTGCAA | lctT, nt 5858–5875 | + |
| 8001 | CTTTACCTATTGCAGGG | lctT, nt 5868–5884 | − |
| 8811 | TACGTGGCAGTGGACTT | IS1675, nt 1229–1245 and 10880–10896 | + |
| PEPO1 | CACCCGCTAAACTTCGT | pepO | + |
| PEPO3 | CTTAGTCGTCTTCGACG | Downstream of IS1675-C and -E | − |
| T3 | AATTAACCCTCACTAAAG | pBluescript primer | |
| SK | TCTAGAACTAGTGGATC | pBluescript primer |
Bases in boldface are not complementary to the target sequence.
Nucleotide (nt) numbers are relative to the sequence with GenBank accession no. U91581.
+, primers directed toward the 3′ end of the sequence; −, primers directed toward the 5′ end.
Tn5721 flanking sequences.
Upstream of IS1675-A, we identified an ORF in a divergent orientation compared to that of the lct and IS1675 genes (Fig. 1A, orf8). orf8 is separated from the left IR by 85 bp including a promoter-like sequence (TTGAAC-16 bp-TATAAT) and an RBS (AAGAGG). Additional single-strand sequencing indicated that orf8 codes for a 241-residue protein which did not show any significant similarity with known proteins. The 292 bp sequenced on both strands downstream of Tn5721 includes a putative promoter (TTGATA-19 bp-TATAAA) followed by an RBS (AGGAGC) and the first 35 codons of a gene in the same orientation as the lct and IS1675 genes. Additional single-strand sequencing showed that this gene contains more than 291 codons (Fig. 1A, orf9). The encoded protein showed similarities to ATP-binding proteins such as RecF proteins and ABC transporters. These similarities are limited to the region including Walker motif A (GSNGCGKTT in Orf9), which is characteristic of ATP-binding proteins (30). Hybridization and PCR experiments showed that Tn5721 is carried in all three lacticin 481 producer strains by a 70-kb plasmid, although they each exhibited a very distinct plasmid content (data not shown). Furthermore, DNA fragments D and E (Fig. 1B) from the three strains were amplified, showing that the Tn5721 flanking sequences in these strains are the same, which suggests that the 70-kb plasmids that they harbor are identical or related.
The presence of IS1675 on both sides of the lacticin 481 operon and the lack of similarity of Orf8 and Orf9 to regulatory proteins indicate that no specific regulator of lacticin 481 operon expression is encoded by an adjacent gene, unlike what is found for the other two known operons for lacticin 481-related lantibiotics. The streptococcin A-FF22 and mutacin II operons are similar to the lacticin 481 one since they contain counterparts of the lctAMTFEG genes. However, they are preceded by specific regulatory genes (scnK and scnR for streptococcin A-FF22, mutR for mutacin II), the products of which are essential for bacteriocin production (2, 12). The present results are in agreement with the observation that the introduction of lctAMTFEG into L. lactis IL1403 was sufficient to induce bacteriocin production (24). The expression of the lacticin 481 operon thus seems less tightly regulated than that of related operons. In that the production of lacticin 481 is stimulated up to fourfold by at least one environmental stress (27), it is likely that global regulators influence the expression of the lacticin 481 genes, as does the diacylglycerol kinase which is involved in stress resistance and stimulation of mutacin II operon transcription (1). Interestingly, Tpase genes are adjacent to the streptococcin A-FF22 and mutacin II gene clusters (2, 12), suggesting that the association between this bacteriocin family and ISs might be a general feature.
IS1675 can be found independently of the lacticin 481 operon.
Hybridization experiments showed that a 25-kb plasmid from L. lactis CNRZ 481 contains another IS1675 copy but does not include the lacticin 481 operon (data not shown). The IS1675 probe (the 1.2-kb fragment XbaI-EcoRV) also hybridized to one DNA fragment each from plasmid-free L. lactis MG1614 and LM0230 (7, 8), detecting 4.4- and 6.0-kb EcoRI fragments, respectively (data not shown). IS1675 is not a component of Tn5721 in these cases, since a single EcoRI fragment was detected per strain, whereas this enzyme cuts within the lacticin 481 operon. We also noted that the databases include two partial sequences of IS1675, one in pCI2001 from L. lactis NCDO 275 and the other downstream of the oppDFBCApepO operon from L. lactis SSL135 (accession no. AF179847 and L18760, including the 3′ 1,327 bp of IS1675 with a single mismatch and its 5′ 223 bp, respectively). The oppDFBCApepO operon is chromosomal and encodes an oligopeptide transport system and an endopeptidase (26).
Identification of the IS1675 flanking sequences.
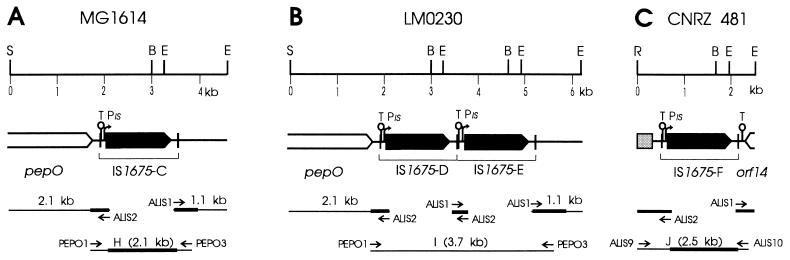
The sequences flanking IS1675 from L. lactis LM0230 and MG1614 chromosomal DNA were amplified by LMPCR as described above, using ScaI instead of EcoRV to obtain the upstream sequences. The primers were ALIS1 and T3 to amplify the downstream DNA, whereas two successive PCRs were needed to amplify the upstream sequences, with the primer couple T3 and 887 or T3 and ALIS4 and then SK and ALIS2. From both strains, the PCRs produced 2.1- and 1.1-kb fragments for the upstream and downstream regions, respectively (Fig. 3A and B). From LM0230, a second DNA fragment of 1.5 kb was obtained with ALIS1 and T3, suggesting the existence of a second copy of IS1675. As a single EcoRI restriction fragment had been detected with the IS1675 probe, the two IS copies seemed to be adjacent. A PCR using LM0230 DNA as the template and primers ALIS1 and ALIS2 yielded a 0.3-kb fragment (Fig. 3B), confirming the presence of two IS1675 copies in tandem, designated IS1675-D and -E. This fragment could not be amplified from MG1614, which therefore contains a single copy, IS1675-C. All the PCR fragments were sequenced from their IS extremities, revealing that IS1675-D and -E are separated by the 8-bp sequence GGTATACC. Such a dimer structure has been described for other ISs such as IS30 and IS21 (16, 21), the dimers of which are very active and unstable intermediates of transposition, as the junctions between the left and right IRs form a strong promoter expressing the Tpase. For IS1675, the IR junctions do not seem to create a strong promoter. The sequences upstream of IS1675-C and -D are identical to each other for at least 328 bp and nearly identical (about 99.5% identity over 376 and 328 bp, respectively) to the 3′ end of pepO and the sequence between pepO and IS1675 from L. lactis SSL135. The sequences downstream of IS1675-C and -E are identical for at least 345 bp and showed 83.8% identity over the first 204 bp with the noncoding sequence found between IS1675-A and lctA. However, neither lctA nor the characterized promoters of the lacticin operon are conserved downstream of IS1675-C and -E. The expected DNA fragments H and I could be amplified from L. lactis MG1614 and LM0230 chromosomal DNA (Fig. 3A and B). Single-strand sequencing of fragment H showed that IS1675-C is identical to IS1675-A and -B. DNA fragment H was also amplified from L. lactis CNRZ 481 total DNA, suggesting that this strain harbors a chromosomal IS1675 copy inserted downstream of pepO, in addition to its three plasmid-borne copies. This was confirmed by the sequencing of one flanking site. The location of IS1675 downstream of pepO at the same position in four strains suggests that this position is a hot spot of IS1675 insertion. Furthermore, IS1675 lies in the same orientation in each case, indicating an orientation preference for its insertion in this spot.
FIG. 3.
Genetic organization and restriction map of the IS1675-containing regions of chromosomal DNA from L. lactis MG1614 (A) and LM0230 (B) and of the 25-kb plasmid from L. lactis CNRZ 481 (C). IS1675 is represented as in Fig. 1A, and its four copies are noted IS1675-C to -F. Open boxes, endopeptidase gene pepO, extrapolated from our sequences and reference 26, and orf14; grey box (C), sequence almost identical to noncoding sequences from other lactococcal plasmids. BamHI, EcoRV, RsaI, and ScaI sites are abbreviated B, E, R, and S, respectively. Horizontal lines at the bottom, PCR-amplified fragments (portions of the PCR products we sequenced are thicker); arrows (not to scale), primers used.
The regions flanking IS1675-F from the 25-kb plasmid of L. lactis CNRZ 481 were sequenced after LMPCR amplification, which required restriction with RsaI and EcoRV to obtain the upstream and downstream regions, respectively. Expected DNA fragment J (Fig. 3C) was amplified with primers based on the two flanking sequences, confirming that the latter correspond to the same IS1675 copy. Whereas single-strand sequencing of fragment J showed that the IS1675-F copy is identical to IS1675-A, -B, and -C, its flanking sequences are distinct from those of the other IS1675 copies. No convincing ORF appeared within the 580 bp sequenced upstream of IS1675-F. The most upstream 300 bp proved to be almost identical to noncoding sequences found in the lactococcal plasmids pCI750, pND861, and pNZ4000 (4, 15, 29). The 3′ end of a putative ORF of more than 67 codons was identified 114 bp downstream of IS1675-F (Fig. 3C, orf14). The protein sequence deduced from orf14 showed 27% identity (52% similarity) with the C-terminal part of LciA, the protein responsible for the immunity to the nonlantibiotic bacteriocin lactococcin A (28), suggesting that IS1675 could be associated with another bacteriocin operon. The full sequencing of IS1675-A, -B, -C, and -F showed that they are identical. As they were found in distinct locations, their insertion could not occur by homologous recombination between the flanking sequences and target DNA but rather resulted from enzymatic transposition, giving strong indirect evidence that IS1675 is functional.
The IS1675 flanking sequences suggest a 24-bp target site.
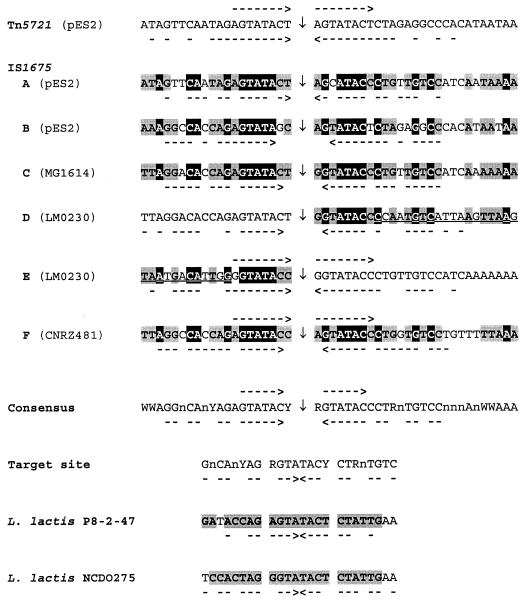
A strong sequence conservation appeared within the 20 bp preceding the left IR and the 27 bp downstream of the right IR of the five distinct IS1675 flanking sequences (Fig. 4). Among these 47 positions, 18 are invariable, 14 are conserved in all sequences but one, and 8 are shared by two bases. Part of the deduced consensus sequence is palindromic, as is the case for each insertion site (Fig. 4). Eight-base-pair direct repeats (DRs) were observed in IS1675-E and -F flanking sequences. The finding of 8-bp DRs on each side of Tn5721 (Fig. 4) but not in IS1675-A or -B flanking sequences indicates that the bacteriocin plasmid results from a Tn5721 insertion rather than from two individual IS1675 insertions, which argues indirectly in favor of Tn5721 functionality. We propose a 24-bp target site based on the symmetrical part of the consensus sequence and on the duplication of 8 bp upon insertion (Fig. 4). The sequence downstream of pepO from L. lactis P8-2-47 (13) is 96.6% identical to the L. lactis SSL135 sequence, but IS1675 is not present at this spot in P8-2-47, which thus contains an IS1675 target site-like sequence. pCI2000 and pCI2001 are two plasmids from L. lactis NCDO 275 and harbor closely related sequences (accession no. AF178424 and AF179847). A target sequence is found in pCI2000 since IS1675 is present in pCI2001 but not in the corresponding site of pCI2000. These two target sequences match our consensus and confirm that the central 8 bp of the target sequence is duplicated upon insertion (Fig. 4). The symmetry of the target site suggests its recognition by a multimer of Tpase binding directly or indirectly on both sides of the target (3). The latter is among the longest described, but all the bases of the sequence are not highly conserved, which could favor the occurrence of corresponding sites, as is the case for other ISs such as IS903 and IS30 (9, 16).
FIG. 4.
DNA sequences flanking IS1675 and Tn5721. The six copies of IS1675 identified in this study are noted A to F, as in Fig. 1 and 3, and their plasmids or strains of origin are in parentheses. Vertical arrows, insertion points of IS1675 or Tn5721; convergent arrows below sequences, palindromic bases; arrows in the same orientation above sequences, DRs; underlined bases, bases of IS1675 IRs (IS1675-D and -E are separated by 8 bp). The various sequences were compared with each other. The sequences upstream and downstream of Tn5721, upstream of IS1675-D, and downstream of IS1675-E were not included in the comparison since the first two are also the sequences upstream of IS1675-A and downstream of IS1675-B and the last two are identical to the sequences upstream and downstream of IS1675-C, respectively. The bases conserved in all sequences are on a black background. Positions where the same base is found in all sequences but one or is shared by two nucleotides are on a grey background. A consensus sequence, where R = A or G, W = A or T, Y = C or T, and n corresponds to an undefined base, has been deduced. A target site was deduced, the central 8 bp of which are duplicated upon insertion. Sequences from L. lactis P8-2-47 and of pCI2000 from L. lactis NCDO 275 (bases 2517 to 2540 and 87 to 110 of GenBank accession no. L04938 and AF178424 sequences, respectively) are shown, with the bases fitting the target site indicated by a grey background.
Nucleotide sequence accession number.
The new sequence data upstream and downstream of the lacticin 481 operon have been deposited with GenBank, updating the entry under accession no. U91581.
Acknowledgments
We are grateful to J.-C. Piard (INRA, Jouy-en-Josas, France) and to B. Mollet and A. C. Pittet (NESTEC Ltd., Nestlé Research Center, Lausanne, Switzerland) for providing us with strains. We thank P. W. Caufield (The University of Alabama at Birmingham, Birmingham) for sharing unpublished results and C. Rio for assistance in preparing figures.
REFERENCES
- 1.Chen P, Novak J, Qi F, Caufield P W. Diacylglycerol kinase is involved in regulation of expression of the lantibiotic mutacin II of Streptococcus mutans. J Bacteriol. 1998;180:167–170. doi: 10.1128/jb.180.1.167-170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen P, Qi F, Novak J, Caufield P W. The specific genes for lantibiotic mutacin II biosynthesis in Streptococcus mutans T8 are clustered and can be transferred en bloc. Appl Environ Microbiol. 1999;65:1356–1360. doi: 10.1128/aem.65.3.1356-1360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craig N L. Target site selection in transposition. Annu Rev Biochem. 1997;66:437–474. doi: 10.1146/annurev.biochem.66.1.437. [DOI] [PubMed] [Google Scholar]
- 4.Deng Y M, Liu C Q, Dunn N W. Genetic organization and functional analysis of a novel phage abortive infection system, AbiL, from Lactococcus lactis. J Biotechnol. 1999;67:135–149. doi: 10.1016/s0168-1656(98)00175-8. [DOI] [PubMed] [Google Scholar]
- 5.Dougherty B A, Hill C, Weidman J F, Richardson D R, Venter J C, Ross R P. Sequence and analysis of the 60 kb conjugative, bacteriocin-producing plasmid pMRC01 from Lactococcus lactis DPC3147. Mol Microbiol. 1998;29:1029–1038. doi: 10.1046/j.1365-2958.1998.00988.x. [DOI] [PubMed] [Google Scholar]
- 6.Dufour A, Thuault D, Boulliou A, Bourgeois C M, Le Pennec J-P. Plasmid-encoded determinants for bacteriocin production and immunity in a Lactococcus lactis strain and purification of the inhibitory peptide. J Gen Microbiol. 1991;137:2423–2429. doi: 10.1099/00221287-137-10-2423. [DOI] [PubMed] [Google Scholar]
- 7.Efstathiou J D, McKay L L. Inorganic salts resistance associated with a lactose-fermenting plasmid in Streptococcus lactis. J Bacteriol. 1977;130:257–265. doi: 10.1128/jb.130.1.257-265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gasson M J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu W Y, Derbyshire K M. Target choice and orientation preference of the insertion sequence IS903. J Bacteriol. 1998;180:3039–3048. doi: 10.1128/jb.180.12.3039-3048.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jack W R, Tagg J R, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahillon J, Chandler M. Insertion sequences. Microbiol Mol Biol Rev. 1998;62:725–774. doi: 10.1128/mmbr.62.3.725-774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLaughlin R E, Ferretti J J, Hynes W L. Nucleotide sequence of the streptococcin A-FF22 lantibiotic regulon: model for production of the lantibiotic SA-FF22 by strains of Streptococcus pyogenes. FEMS Microbiol Lett. 1999;175:171–177. doi: 10.1111/j.1574-6968.1999.tb13616.x. [DOI] [PubMed] [Google Scholar]
- 13.Mierau I, Tan P S T, Haandrikman A J, Kok J, Leenhouts K J, Konings W N, Venema G. Cloning and sequencing of the gene for a lactococcal endopeptidase, an enzyme with sequence similarity to mammalian enkephalinase. J Bacteriol. 1993;175:2087–2096. doi: 10.1128/jb.175.7.2087-2096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng W-L, Kothakota S, DasSarma S. Structure of the gas vesicle plasmid in Halobacterium halobium: inversion isomers, inverted repeats, and insertion sequences. J Bacteriol. 1991;173:1958–1964. doi: 10.1128/jb.173.6.1958-1964.1991. . (Erratum, 173:3933.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Connor L, Coffey A, Daly C, Fitzgerald G F. AbiG, a genotypically novel abortive infection mechanism encoded by plasmid pCI750 of Lactococcus lactis subsp. cremoris UC653. Appl Environ Microbiol. 1996;62:3075–3082. doi: 10.1128/aem.62.9.3075-3082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olasz F, Kiss J, Knig P, Buzs Z, Stalder R, Arber W. Target specificity of insertion element IS30. Mol Microbiol. 1998;28:691–704. doi: 10.1046/j.1365-2958.1998.00824.x. [DOI] [PubMed] [Google Scholar]
- 17.Perreten V, Schwarz F, Cresta L, Boeglin M, Dasen G, Teuber M. Antibiotic resistance spread in food. Nature. 1997;389:801–802. doi: 10.1038/39767. [DOI] [PubMed] [Google Scholar]
- 18.Piard J-C, Kuipers O P, Rollema H S, Desmazeaud M J, de Vos W M. Structure, organization, and expression of the lct gene for lacticin 481, a novel lantibiotic produced by Lactococcus lactis. J Biol Chem. 1993;268:16361–16368. [PubMed] [Google Scholar]
- 19.Polard P, Chandler M. Bacterial transposases and retroviral integrases. Mol Microbiol. 1995;15:13–23. doi: 10.1111/j.1365-2958.1995.tb02217.x. [DOI] [PubMed] [Google Scholar]
- 20.Pridmore D, Rekhif N, Pittet A C, Suri B, Mollet B. Variacin, a new lanthionine-containing bacteriocin produced by Micrococcus varians: comparison to the lacticin 481 of Lactococcus lactis. Appl Environ Microbiol. 1996;62:1799–1802. doi: 10.1128/aem.62.5.1799-1802.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reimmann C, Moore R, Little S, Savioz A, Willetts N S, Haas D. Genetic structure, function and regulation of the transposable element IS21. Mol Gen Genet. 1989;215:416–424. doi: 10.1007/BF00427038. [DOI] [PubMed] [Google Scholar]
- 22.Rezsöhazy R, Hallet B, Delcour J, Mahillon J. The IS4 family of insertion sequences: evidence for a conserved transposase motif. Mol Microbiol. 1993;9:1283–1295. doi: 10.1111/j.1365-2958.1993.tb01258.x. [DOI] [PubMed] [Google Scholar]
- 23.Rincé A, Dufour A, Le Pogam S, Thuault D, Bourgeois C M, Le Pennec J-P. Cloning, expression, and nucleotide sequence of genes involved in production of lactococcin DR, a bacteriocin from Lactococcus lactis subsp. lactis. Appl Environ Microbiol. 1994;60:1652–1657. doi: 10.1128/aem.60.5.1652-1657.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rincé A, Dufour A, Uguen P, Le Pennec J-P, Haras D. Characterization of the lacticin 481 operon: the Lactococcus lactis genes lctF, lctE, and lctG encode a putative ABC transporter involved in bacteriocin immunity. Appl Environ Microbiol. 1997;63:4252–4260. doi: 10.1128/aem.63.11.4252-4260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero D A, Klaenhammer T R. Transposable elements in lactococci: a review. J Dairy Sci. 1993;76:1–19. doi: 10.3168/jds.S0022-0302(93)77318-X. [DOI] [PubMed] [Google Scholar]
- 26.Tynkkynen S, Buist G, Kunji E, Kok J, Poolman B, Venema G, Haandrikman A. Genetic and biochemical characterization of the oligopeptide transport system of Lactococcus lactis. J Bacteriol. 1993;175:7523–7532. doi: 10.1128/jb.175.23.7523-7532.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uguen P, Hamelin J, Le Pennec J-P, Blanco C. Influence of osmolarity and the presence of an osmoprotectant on Lactococcus lactis growth and bacteriocin production. Appl Environ Microbiol. 1999;65:291–293. doi: 10.1128/aem.65.1.291-293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Belkum M J, Hayema B J, Jeeninga R E, Kok J, Venema G. Organization and nucleotide sequences of two lactococcal bacteriocin operons. Appl Environ Microbiol. 1991;57:492–498. doi: 10.1128/aem.57.2.492-498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Kranenburg R, Marugg J D, van Swam I I, Willem N J, de Vos W M. Molecular characterization of the plasmid-encoded eps gene cluster essential for exopolysaccharide biosynthesis in Lactococcus lactis. Mol Microbiol. 1997;24:387–397. doi: 10.1046/j.1365-2958.1997.3521720.x. [DOI] [PubMed] [Google Scholar]
- 30.Walker J E, Sarste M, Runswick M J, Gay N J. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]