Abstract
Background:
Human monoclonal antibodies that potently and broadly neutralize HIV-1 (bnMAbs) are under development to prevent and treat HIV-1 infection. We performed a phase 1 clinical trial to determine the safety, tolerability, and pharmacokinetic profile of the bnMAb VRC07–523LS, an engineered variant of VRC01 that targets the CD4 binding-site of the HIV-1 Env protein.
Methods:
This phase 1, open-label, dose-escalation clinical trial was done at the National Institutes of Health Clinical Center in Bethesda, MD, USA. Individuals were recruited from the greater Washington, DC, area by IRB-approved written and electronic media. We enrolled healthy, HIV-1-negative adults aged 18–50 years. Inclusion criteria were good general health, measured through clinical laboratory tests, medical history, and physical examination. Participants self-selected into one of seven open groups during enrollment without randomization. Four groups received a single intravenous dose of 1, 5, 20, or 40 mg/kg of VRC07–523LS, and one group received a single 5 mg/kg subcutaneous dose. Two groups received three doses of either 20 mg/kg intravenous VRC07–523LS, or 5 mg/kg subcutaneous VRC07–523LS at 12-week intervals. The primary outcome was the safety and tolerability of VRC07–523LS, assessed by dose, route, and number of administrations. This study is registered with ClinicalTrials.gov, NCT03015181.
Findings:
Between Feb 21, 2017, and September 13, 2017, we enrolled 26 participants, including 11 (42%) men and 15 (58%) women. Two (8%) participants withdrew from the study early: one participant in group 1 enrolled in the study but never received VRC07–523LS, and one participant in group 6 chose to withdraw after a single administration. One (4%) participant in group 7 received only one of the three scheduled administrations. 17 participants received intravenous administrations and 8 participants received subcutaneous administrations. Local and systemic reactogenicity were mild to moderate when reported. The most commonly reported symptoms following IV administration included malaise or myalgia in three participants (18%) and headache or chills in two participants (12%). Following SC administration, the most commonly reported symptoms included pain and tenderness in four participants (50%) and malaise or headache in three participants (38%). No serious adverse events or dose-limiting toxicities occurred.
Interpretations:
We found VRC07–523LS to be safe and well tolerated. These qualities make VRC07–523LS a strong and practical candidate for inclusion in HIV-1 prevention and therapeutic strategies. The results from this trial also indicate that an HIV-1 bnMAb engineered to improve pharmacokinetic properties and neutralization activity can be safe for clinical use.
Introduction
The advent of technology to rapidly select and isolate immunoglobulin genes from individual B cells, along with the discovery of individuals producing broad and potently neutralizing antibodies, has led to the identification of broadly neutralizing monoclonal antibodies (bnMAbs) for HIV-1. These bnMAbs bind to distinct sites on the HIV-1 envelope (Env) protein including the CD4-binding site, V1/V2 apex, glycan-V3 region, membrane proximal external region (MPER), and the interface between gp120 and gp41.1–4 Some of the bnMAbs are being manufactured for evaluation as interventions for HIV-1 prevention, treatment, and cure.1, 5, 6 Preclinical studies of nonhuman primates (NHPs) have shown that bnMAbs can both prevent infection in naïve animals and lower viral loads during chronic infection.5–7 This ability to temporarily reduce viral loads has been recapitulated in HIV-1 infected humans.8–13
VRC01 is a bnMAb targeting the CD4-binding site that neutralizes 80–90% of circulating HIV-1 strains.1, 7, 14, 15 It was one of the first bnMAbs for which the human safety and pharmacokinetic profiles were established in both HIV-1 infected and uninfected populations,8, 9, 16, 17 and has subsequently advanced into two randomized phase 2b efficacy trials to assess prevention of HIV-1 infection in high-risk populations (NCT02568215, NCT02716675). VRC01 was later modified with the “LS” mutation: a two amino acid substitution (M428L and N434S) in the Fc region of the antibody to create VRC01LS. This change increases binding affinity to the neonatal Fc receptor in a pH dependent manner, thereby prolonging the antibody’s serum persistence by enhancing IgG recirculation mechanisms.18 The LS mutation also leads to increased localization and persistence of antibodies in mucosal tissue in NHP studies, which may improve protection against HIV-1 infection.18 VRC01LS has displayed a strong safety profile in a clinical trial involving HIV-1 negative adults while maintaining its ability to neutralize virus after administration.19 The experiences with VRC01 and VRC01LS provided evidence that passive infusion of HIV-1 bnMAbs is safe, with proof that further optimization of bnMAbs can provide advantageous pharmacokinetic properties. In addition to enhanced serum persistence, increased breadth and potency of viral neutralization are also highly desirable traits, and efforts continue to discover improved bnMAbs to advance into product development for eventual licensure.
VRC07–523LS is a second generation bnMAb that is an engineered variant of a clonal relative of VRC01. This clonal relative, called VRC07, contains a four amino acid insertion in the heavy chain CDR3 and 16 additional amino acid mutations in the heavy chain variable domain compared to VRC01. VRC07 was then extensively engineered to improve potency, breadth, expression, and biophysical properties, and modified to include the LS mutation.7 The resulting bnMAb, VRC07–523LS, is approximately 5-fold more potent than VRC01 against clade B and C viruses and displays greater breadth, neutralizing 95% of viruses tested, and provides protection of NHPs in a SHIV challenge model at lower concentrations than VRC01LS.1, 7, 20 Based on preclinical neutralization and NHP protection data, the Vaccine Research Center (VRC), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), examined the safety, tolerability, and pharmacokinetic parameters of VRC07–523LS in healthy adults in this phase 1 clinical trial.
Methods
Study Design
We evaluated VRC07–523LS in an open-label, dose-escalation study in healthy HIV-1 negative adults (ClinicalTrials.gov NCT03015181). We conducted the study at the NIH Clinical Center by the VRC Clinical Trials Program in Bethesda, MD, USA. The NIAID Institutional Review Board reviewed and approved the clinical trial protocol.
Volunteers were recruited from the greater Washington, DC, region by IRB approved written and electronic media. We enrolled healthy, HIV-1 negative adults between the ages of 18 to 50 meeting the inclusion criteria of being in good general health through clinical laboratory tests, medical history, and physical examination. Participants agreed to adhere to reduced risk sexual behavior. Exclusion criteria included previous receipt of a licensed or investigational monoclonal antibody, body weight greater than 115 kg, history of a severe allergic reaction likely to recur on study, poorly controlled hypertension, or receipt of any investigational study agent within 28 days prior to enrollment. Volunteers provided written informed consent prior to participation.
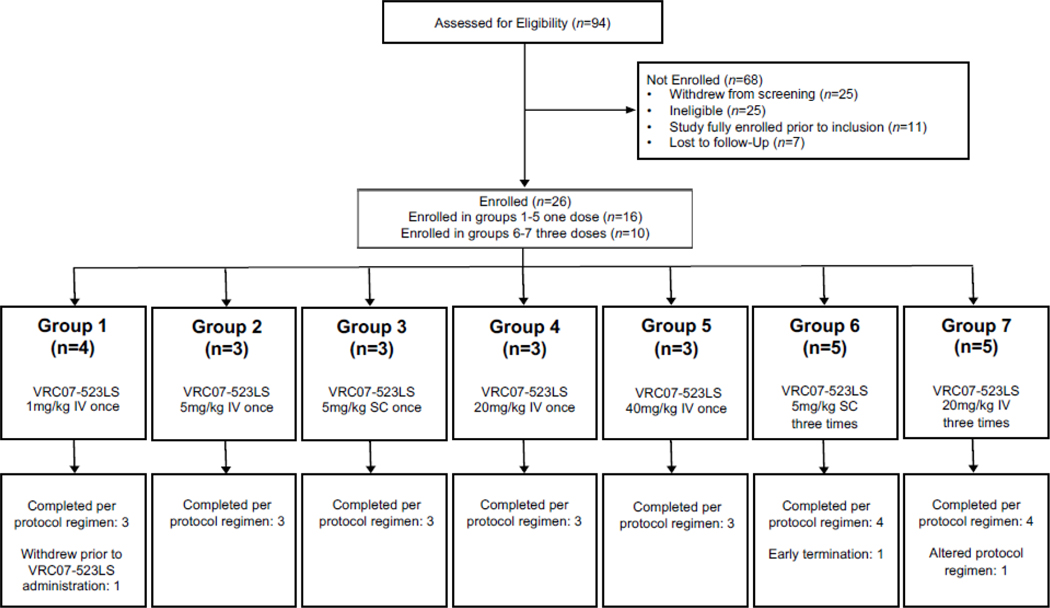
We based the dose groups on previous bnMAb trials (Figure 1).16, 19 Four groups received a single IV administration of 1, 5, 20, or 40 mg/kg of VRC07–523LS (Figure 1), and one group received a single 5 mg/kg SC administration. Two groups received three administrations at 12-week intervals: 20 mg/kg IV or 5 mg/kg SC. Interim safety reviews occurred prior to each dose-escalation. Volunteers self-selected into open groups during enrollment without randomization. Group sizes (3–5 individuals) were calculated to capture serious adverse events (SAEs) and were comparable with other bnMAb phase 1 trials.
Figure 1. CONSORT diagram for clinical trial VRC 605.
16 participants in groups 1–5 were enrolled to receive one dose of VRC07–523LS, and ten participants in groups 6–7 were enrolled to receive three doses of VRC07–523LS.
Procedures
VRC07–523LS (VRC-HIVMAB075–00-AB) was produced in a Chinese Hamster Ovary (CHO) DG44 cell line under current Good Manufacturing Practices (cGMP),21 at the VRC Pilot Plant operated under contract by the Vaccine Clinical Materials Program, Leidos Biomedical Research, Inc., Frederick, MD. Investigators identified the original VRC07 heavy chain by next-generation sequencing of antibody gene transcripts in the VRC01 donor and paired it with the VRC01 light chain.7 The allotype of the antibody was modified to GM1,17. Additional mutations that define the 523 designation include a G54H mutation in the heavy chain, and a deletion of the first two amino acids (glutamic acid and isoleucine) and a V3S mutation in the light chain.7 The LS mutation was introduced by site-directed mutagenesis as previously described.18
An NIH Clinical Center pharmacist individually prepared all administration doses. For intravenous (IV) administration, the calculated volume of the weight-based dose of VRC07–523LS was added to 100mL of 0·9% sodium chloride. Investigators administered IV infusions over 30-minutes using a volumetric pump, and administered subcutaneous (SC) weight-based doses into the abdomen by needle and syringe. The maximum SC volume was 2·5 mL per site. A study clinician monitored all product administrations, and obtained safety laboratory tests from participants prior to administration and throughout the study. Volunteers recorded solicited symptoms for three days after each administration and a clinician assessed the local site on the day of administration, the following day, and one week after. Clinicians recorded all adverse events (AEs) for 56 days after administration, and reported SAEs and new chronic medical conditions throughout the study. Clinicians coded AEs using the Medical Dictionary for Regulatory Activities (MedDRA) and graded severity using Version 2·0 of the DAIDS Table for Grading the severity of Adult and Pediatric Adverse Events. We followed participants for 24 weeks after the last VRC07–523LS administration.
We evaluated all participants for IgG1 GM (gamma marker) allotypes 1/a, 3/f, and 17/z. The GM3 and GM17 polymorphisms are located on the CH1 domain of the antibody, while GM1 is located on the CH3 domain. We used Sanger DNA sequencing for the determination of GM3 and GM17 allotypes using an ABI PRISM 3730xl DNA Analyzer. The CH1 region of γ1 chain was amplified using 5’ CCCCTGGCACCCTCCTCCAA 3’ and 5’ GCCCTGGACTGGGGCTGCAT 3’ primers.22 We determined the GM1 marker from serum samples by a standard hemagglutination-inhibition method.23
We quantified the VRC07–523LS serum concentrations as previously described using a Beckman Biomek-based automation platform to perform an anti-idiotype capture ELISA method.16 We analyzed threefold dilutions, from 1:100 to 1:218,700, in duplicate. We quantified sample concentrations using a linear regression of a standard curve of VRC07–523LS covering a range between 5–125 ng/mL.
We collected pharmacokinetic (PK) samples from participants for up to 24 weeks after the last dose. For the non-compartmental analysis, we calculated area under the concentration versus time curve (AUC) using the trapezoidal method following the first dose. We took the maximum serum concentration (Cmax), time to maximum serum concentration (Tmax), week four serum concentration (C28D), and week 12 serum concentration (C84D) from the observed data. We determined the terminal slope (λz) and associated half-life from the concentration time profile using Phoenix-WinNonlin 7·0 (Certara, Princeton). We calculated AUCinf as the sum of the observed AUC up to the final concentration (AUCobs) plus the AUC after the final concentration (AUCClast-inf) where AUCClast-inf was estimated as Clast/λz. Partial AUCs for 12 weeks after the first and third doses (AUC0–84D) were used to assess accumulation. We also fit PK data to a standard two-compartment model (ADVAN4, TRANS4) using the computer program NONMEM 7·3 (ICON, Dublin). We estimated the compartmental elimination half-life (t1/2β), clearance (CL), and apparent (CL/F) using the posthoc empiric Bayesian subroutine. Due to the small number of volunteers, we assumed population PK parameters to be proportional to body weight and we did not perform any formal covariate assessment of population PK parameters. We also performed simulations of serum concentrations over the course of 24 weeks after a single matched dose of VRC01,16, 17 VRC01LS,19 and VRC07–523LS based on a hypothetical efficacy trial study population.24 We graphed predicted coverage against clade C and multi-clade HIV-1 panels over time; and we predicted coverage for a virus if the geometric mean serum concentration at a specific time point was 100-fold greater than the viral in vitro inhibitory concentration 50% (IC50) or 80% (IC80) values.20, 25 We based the 100-fold estimate on the NHP SHIV-challenge model, where protection is achieved by CD4 binding-site bnMAbs if serum antibody concentrations are approximately 50 to 100-fold higher than the measured IC50 of the challenge virus.5 We considered viruses resistant if the IC50 or IC80 was greater than 10 mcg/mL.
We assessed the neutralization ability of serum samples using a single-round HIV-1 Env-pseudovirus infection of TZM-bl target cells as previously described.26 We tested sera against a multi-clade panel of 20 viral strains spanning a range of neuralization sensitivities for VRC07–523LS, VRC01LS, and VRC01. The panel included 2 viruses each from clade A (Q23·17, BG505_W6M_C2), clade B (PVO·04, THRO·18), clade D (57128·vrc15, 3016·v5 ·c45), and clade AE (CNE59, BJOX028000·10 ·3); and 12 from clade C (DU156·12, MW965·26, ZM53·12, 6322·v4 ·C1, CAP210, CAP382·2 ·00_D7·19, Ce704810053_2B7, 21369737_G11_F2, CT565_C7·48, 21197826_V1, 21561324_D3_B5, 21492713_B11_E3). We emphasized clade C as these viruses account for up to half of global HIV-1 infections.20 We included negative (MuLV, SIVMAC251·30) and positive controls in all assays. We tested a subset of human sera from this clinical trial and from matched doses from previous trials of VRC01 and VRC01LS in healthy adults.16, 19 The same VRC01 and VRC01LS samples from previous trials were used for the neutralization comparisons and PK comparisons. We displayed results as the dilution that produced 50% (ID50) or 80% neutralization (ID80) against the viruses tested.
Evaluation for the presence of anti-drug antibodies (ADA) followed a tiered approach based upon the Meso Scale Discovery (MSD) electrochemiluminescence (ECL) homogenous bridging assay, as previously described.16
Outcomes
The primary outcome examined the safety and tolerability of VRC07–523LS groups differing by dose, route, and number of administrations. The secondary objectives evaluated the pharmacokinetics of VRC07–523LS through 24 weeks after the last dose, and whether anti-drug antibodies are detected following administration. This study is registered with ClinicalTrials.gov, NCT03015181.
Role of the Funding Source
The VRC funded the study and its investigators had complete control over study design, data collection, data analysis, data interpretation, and writing of the report. The corresponding author had access to all the data in the studies and final responsibility for the decision to submit for publication.
Results
We enrolled 26 volunteers between February 21 and September 13, 2017 (Figure 1), including 11 (42%) males and 15 (58%) females (Table 1). The last participant visit for the trial occurred on July 10, 2018. Twenty-three (88%) volunteers received all VRC07–523LS administrations and completed follow-up visits. Two (8%) volunteers withdrew from the study early: one volunteer in group 1 enrolled in the study but never received VRC07–523LS and one in group 6 chose to withdraw after a single administration. One (4%) participant in group 7 received only one of the three scheduled administrations due to an unrelated intercurrent illness but otherwise remained active in the study.
Table 1.
Demographic characteristics of study participants
| Category | Sub-category | Group 1 (n=4) | Group 2 (n=3) | Group 3 (n=3) | Group 4 (n=3) | Group 5 (n=3) | Group 6 (n=5) | Group 7 (n=5) | Overall (n=26) |
|---|---|---|---|---|---|---|---|---|---|
| n (%) | |||||||||
| Gender | Male | 2 (50%) | 1 (33%) | 3 (100%) | 2 (67%) | 0 (0%) | 0 (0%) | 3 (60%) | 11 (42%) |
| Female | 2 (50%) | 2 (67%) | 0 (0%) | 1 (33%) | 3 (100%) | 5 (100%) | 2 (40%) | 15 (58%) | |
| Age (years) | 21–30 | 3 (75%) | 2 (67%) | 2 (67%) | 3 (100%) | 1 (33%) | 4 (80%) | 3 (60%) | 18 (69%) |
| 31–40 | 1 (25%) | 1 (33%) | 1 (33%) | 0 (0%) | 0 (0%) | 1 (20%) | 1 (20%) | 5 (19%) | |
| 41–50 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (67%) | 0 (0%) | 1 (20%) | 3 (12%) | |
| Mean (SD) | 26·5 (7·0) | 28·0 (9·5) | 30·0 (7·0) | 27·0 (2·6) | 40·0 (10·4) | 25·8 (4·1) | 30·0 (10·6) | 29·2 (8·1) | |
| Range | [22, 37] | [22, 39] | [25, 38] | [24, 29] | [28, 46] | [22, 32] | [22, 48] | [22, 48] | |
| Race | Asian | 1 (25%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (20%) | 1 (20%) | 3 (12%) |
| Black or African American | 0 (0%) | 1 (33%) | 0 (0%) | 0 (0%) | 1 (33%) | 0 (0%) | 1 (20%) | 3 (12%) | |
| White | 2 (50%) | 2 (67%) | 2 (67%) | 2 (67%) | 1 (33%) | 4 (80%) | 3 (60%) | 16 (62%) | |
| Multiracial | 1 (25%) | 0 (0%) | 1 (33%) | 1 (33%) | 1 (33%) | 0 (0%) | 0 (0%) | 4 (15%) | |
| Ethnicity | Non-Hispanic/Latino | 4 (100%) | 3 (100%) | 2 (67%) | 2 (67%) | 2 (67%) | 4 (80%) | 5 (100%) | 22 (85%) |
| Hispanic/Latino | 0 (0%) | 0 (0%) | 1 (33%) | 1 (33%) | 1 (33%) | 1 (20%) | 0 (0%) | 4 (15%) | |
| Mean Weight | kg (SD) | 75·8 (4·6) | 65·5 (17·3) | 89·4 (15·0) | 75·9 (11·0) | 67·2 (20·1) | 55·2 (7·1) | 76·1 (11·4) | 71·3 (14·9) |
| Education | High school graduate/GED | 1 (25%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (33%) | 0 (0%) | 0 (0%) | 2 (8%) |
| College/University | 2 (50%) | 2 (67%) | 2 (67%) | 2 (67%) | 2 (67%) | 4 (80%) | 4 (80%) | 18 (69%) | |
| Advanced degree | 1 (25%) | 1 (33%) | 1 (33%) | 1 (33%) | 0 (0%) | 1 (20%) | 1 (20%) | 6 (23%) | |
SD= standard deviation
A total of 41 administrations of VRC07–523LS occurred during the study. VRC07–523LS was safe and well tolerated, with no SAEs or dose-limiting toxicities. All reported local and systemic reactogenicity was mild to moderate in severity (Table 2). Investigators determined six AEs to be possibly related to VRC07–523LS administration. One participant in group 5 exhibited grade 1 dizziness on the day of infusion, and one in group 7 reported grade 1 abdominal pain the day after infusion. Two volunteers (one each in groups 5 and 7) displayed either grade 1 or grade 2 infusion reactions following each administration, with symptoms typical of those observed following receipt of monoclonal antibodies: fever, chills, myalgia, nausea, and headache.27 Sera collected from the group 7 participant after each infusion were analyzed in a post-hoc analysis and showed an elevation of serum cytokines including TNF-α, IL-6, and IL-13 (Appendix p·2). Importantly, the levels of histamine, tryptase, IFN-γ, sIL-2R, IL-1β, IL-2, IL-4, IL-5, IL-8, IL-10, IL-12, and IL-17 remained within normal levels, and the reaction grade decreased in this participant from grade 2 during the first two infusions to grade 1 during the final infusion. There were no symptoms of hypotension, urticaria, bronchospasm, or other symptoms of severe infusion reactions. Clinicians treated both volunteers symptomatically with acetaminophen and ibuprofen. All symptoms resolved within 8 hours without increased monitoring required.
Table 2.
Maximum local and systemic reactogenicity by group after administration of VRC07–523LS
| Symptom Intensity | IV Administration | SC Administration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 (1 mg/kg IV) | Group 2 (5 mg/kg IV) | Group 4 (20 mg/kg IV) | Group 5 (40 mg/kg IV) | Group 7 (20 mg/kg IV by repeat dosing) | Group 3 (5 mg/kg SC) | Group 6 (5 mg/kg SC by repeat dosing) | |||||
| (n=3) | (n=3) | (n=3) | (n=3) | (n=5) | (n=4) | (n=4) | (n=3) | (n=5) | (n=4) | (n=4) | |
| n (%) | |||||||||||
| LOCAL REACTOGENICITY | |||||||||||
| PAIN/TENDERNESSa | |||||||||||
| None | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | 5 (100%) | 4 (100%) | 4 (100%) | 2 (67%) | 2 (40%) | 1 (25%) | 2 (50%) |
| Mild | 0 (0.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (33%) | 3 (60%) | 3 (75%) | 2 (50%) |
| BRUISINGb | |||||||||||
| None | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | 4 (80%) | 4 (100%) | 4 (100%) | 3 (100%) | 5 (100%) | 4 (100%) | 4 (100%) |
| Mild | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (20%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| SWELLING | |||||||||||
| None | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | 5 (100%) | 4 (100%) | 4 (100%) | 3 (100%) | 5 (100%) | 4 (100%) | 4 (100%) |
| REDNESSc | |||||||||||
| None | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | 5 (100%) | 4 (100%) | 4 (100%) | 3 (100%) | 5 (100%) | 4 (100%) | 3 (75%) |
| Mild | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Moderate | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (25%) |
| PRURITUS | |||||||||||
| None | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | 5 (100%) | 4 (100%) | 4 (100%) | 3 (100%) | 5 (100%) | 4 (100%) | 4 (100%) |
| ANY LOCAL SYMPTOM | |||||||||||
| None | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | 4 (80%) | 4 (100%) | 4 (100%) | 2 (67%) | 2 (40%) | 1 (25%) | 1 (25%) |
| Mild | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (20%) | 0 (0%) | 0 (0%) | 1 (33%) | 3 (60%) | 3 (75%) | 2 (50%) |
| Moderate | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (25%) |
| SYSTEMIC REACTOGENICITY | |||||||||||
| MALAISEa | |||||||||||
| None | 3 (100%) | 3 (100%) | 3 (100%) | 2 (67%) | 3 (60%) | 3 (75%) | 3 (75%) | 3 (100%) | 2 (40%) | 4 (100%) | 4 (100%) |
| Mild | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (40%) | 1 (25%) | 1 (25%) | 0 (0%) | 3 (60%) | 0 (0%) | 0 (0%) |
| Moderate | 0 (0%) | 0 (0%) | 0 (0%) | 1 (33%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| MYALGIAa | |||||||||||
| None | 3 (100%) | 2 (67%) | 3 (100%) | 2 (67%) | 4 (80%) | 3(75%) | 3 (75%) | 3 (100%) | 3 (60%) | 4 (100%) | 4 (100%) |
| Mild | 0 (0%) | 1 (33%) | 0 (0%) | 1 (33%) | 1 (20%) | 1(25%) | 0 (0%) | 0 (0%) | 2 (40%) | 0 (0%) | 0 (0%) |
| Moderate | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0(0%) | 1 (25%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| HEADACHEa | |||||||||||
| None | 3 (100%) | 3 (100%) | 3 (100%) | 2 (67%) | 4 (80%) | 3 (75%) | 4 (100%) | 3 (100%) | 3 (60%) | 3 (75%) | 4 (100%) |
| Mild | 0 (0%) | 0 (0%) | 0 (0%) | 1 (33%) | 1 (20%) | 1 (25%) | 0 (0%) | 0 (0%) | 2 (40%) | 1 (25%) | 0 (0%) |
| CHILLSa | |||||||||||
| None | 3 (100%) | 3 (100%) | 3 (100%) | 2 (67%) | 4 (80%) | 3 (75%) | 3 (75%) | 3 (100%) | 4 (80%) | 4 (100%) | 4 (100%) |
| Mild | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (20%) | 0 (0%) | 0 (0%) |
| Moderate | 0 (0%) | 0 (0%) | 0 (0%) | 1 (33%) | 1 (20%) | 1 (25%) | 1 (25%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| NAUSEAd | |||||||||||
| None | 3 (100%) | 3 (100%) | 3 (100%) | 2 (67%) | 5 (100%) | 4 (100%) | 4 (100%) | 3 (100%) | 4 (80%) | 4 (100%) | 4 (100%) |
| Mild | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (20%) | 0 (0%) | 0 (0%) |
| Moderate | 0 (0%) | 0 (0%) | 0 (0%) | 1 (33%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (%) |
| TEMPERATUREe | |||||||||||
| None | 3 (100%) | 3 (100%) | 3 (100%) | 2 (67%) | 4 (80%) | 4 (100%) | 4 (100%) | 3 (100%) | 5 (100%) | 4 (100%) | 4 (100.%) |
| Mild | 0 (0%) | 0 (0%) | 0 (0%) | 1 (33%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Moderate | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (20%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| JOINT PAINa | |||||||||||
| None | 3 (100%) | 3 (100%) | 3 (100%) | 2 (67%) | 5 (100.0%) | 4 (100.0%) | 3 (75%) | 3 (100%) | 3 (60%) | 4 (100%) | 4 (100%) |
| Mild | 0 (0%) | 0 (0%) | 0 (0%) | 1 (33%) | 0 (0.0%) | 0 (0.0%) | 0 (0%) | 0 (0%) | 2 (40%) | 0 (0%) | 0 (0%) |
| Moderate | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0.0%) | 0 (0.0%) | 1 (25%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| ANY SYSTEMIC SYMPTOM | |||||||||||
| None | 3 (100%) | 2 (67%) | 3 (100%) | 2 (67%) | 3 (60%) | 3 (75%) | 3 (75%) | 3 (100%) | 1 (20%) | 3 (75%) | 4 (100%) |
| Mild | 0 (0%) | 1 (33%) | 0 (0%) | 0 (0%) | 1 (20%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (80%) | 1 (25%) | 0 (0%) |
| Moderate | 0 (0%) | 0 (0%) | 0 (0%) | 1 (33%) | 1 (20%) | 1 (25%) | 1 (25%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
There were no severe reactions throughout the trial. IV= intravenous, SC= subcutaneous
Symptoms of pain, malaise, myalgia, joint pain, headache, or chills causing no or minimal interference with usual social and/or function activities were graded as mild (grade 1), and moderate (grade 2) if subjects could still perform those activities but with greater than minimal interference.
Mild (grade 1) bruising indicated a maximum diameter of between 2.5 and 5 cm.
Moderate (grade 2) bruising indicated a maximum diameter of between 5 and 10 cm.
Mild (grade 1) nausea was transient or intermittent with no or minimal interference with oral intake, while moderate (grade 2) nausea was persistent resulting in decreased oral intake for 1–2 days.
Mild (grade 1) fever reached a maximum temperature between 38.0°C and 38.6°C, while moderate (grade 2) fever reached a maximum temperature between 38.6°C and 39.3°C.
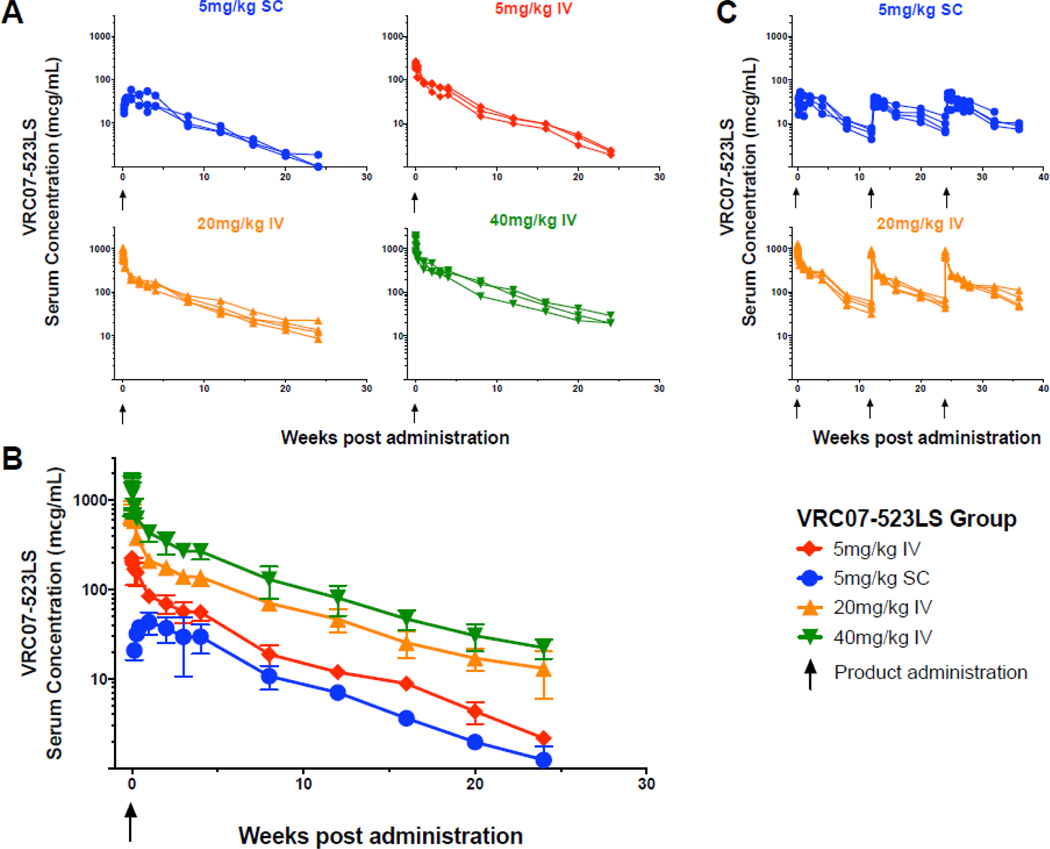
We analyzed the sera from all participants who received VRC07–523LS to determine the PK parameters of the antibody following both IV (17 participants) and SC (8 participants) administration. Maximum (CMAX) and 12-week (C84D) serum concentrations increased proportionally with IV doses from 5–40 mg/kg (Figure 2A, B, Table 3). The time after infusion where the maximum serum concentration (TMAX) was measured occurred within the first few hours after IV administration, and approximately 10 days following SC administration, similar to ranges previously observed for VRC01LS.19
Figure 2. Serum concentrations (mcg/mL) of VRC07–523LS.
(A) Serum concentrations (mcg/mL) by individual subjects and (B) group geometric mean titers with error bars indicating the standard deviation after a single administration of VRC07–523LS, or (C) by individual subject after repeat administrations at weeks 0, 12, and 24. Upward pointing arrows indicate VRC07–523LS administrations. One subject in group 7 (multiple infusions at 20 mg/kg IV) who only received a single dose of antibody was analyzed with group 4 (single infusion at 20 mg/kg IV) subjects.
Table 3.
Pharmacokinetic parameters after administration of VRC07–523LS
| Group and Dose | Maximum serum conc. CMAX [mcg/mL] | TMAX [days] | 4 week serum conc. C28D [mcg/mL] | 12 week serum conc. C84D [mcg/mL] | AUCa [mcg*d/mL] | CL [mL/day]b | t1/2β [days] | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose 1 | Dose 3c | Dose 1 | Dose 3c | Dose 1 | Dose 3c | |||||
| Mean (SD) | ||||||||||
| Single dose | ||||||||||
| 5 mg/kg SC (n=3) | 50 (11) | 10 (9·5) | 31 (11) | 7·1 (1·3) | 2,189 (309) | 226 (54) | 36 (6·7) | |||
| 1 mg/kg IV (n=3) | 47 (16) | 0·7 (0·5) | 14 (7·5) | 3·8 (0·7) | 1,381 (325) | 70 (20) | 48 (26) | |||
| 5 mg/kg IV (n=3) | 240 (35) | 0·04 (0·02) | 57 (11) | 12 (1·8) | 4,551 (904) | 78 (18) | 32 (1·1) | |||
| 20 mg/kg IV (n=4) | 869 (190) | 0·3 (0·4) | 148 (28) | 44 (14) | 13,748 (1,853) | 110 (19) | 45 (5·2) | |||
| 40 mg/kg IV (n=3) | 1,630 (644) | 0·04 (0·02) | 272 (52) | 85 (30) | 25,517 (5,097) | 105 (14) | 42 (5·1) | |||
| Multiple doses | ||||||||||
| 5 mg/kg SC (n=5) | 38 (17) | 37 (12) | 5·7 (5·0) | 25 (13) | 6·3 (1·5) | 9·8 (2·5) | 1,440 (563) | 1,671 (443) | 158 (34) | 31 (10) |
| 20 mg/kg IV (n=4) | 1,196 (74) | 799 (98) | 0·04 (0·02) | 242 (51) | 46 (13) | 71 (29) | 14,760 (2,646) | 13,573 (1,115) | 101 (15) | 27 (1·8) |
| Overall | ||||||||||
| SC groups (n=8) | 184 (52) | 33 (8·9) | ||||||||
| IV groups (n=17) | 94 (22) | 38 (12) | ||||||||
IV= intravenous, SC= subcutaneous
PK parameters shown for all subjects who received at least one administration of VRC07–523LS, and represent the first dose only PK except for t1/2β and CL (calculated from all doses). One subject in group 7 (multiple doses at 20mg/kg IV) who only received a single dose of antibody was analyzed with group 4 (single dose at 20mg/kg IV) subjects.
AUCinf shown for single dose groups, and AUC84D shown for repeat dose groups. AUCinf was taken as the sum of the observed AUC up to the final concentration (AUCobs) plus the AUC after the final concentration (AUCClast-inf) where AUCClast-inf was estimated as Clast/lz.
Value following SC administration represents CL/F.
Values calculated from 4 subjects per group.
In the IV groups, the elimination half-life (t1/2β) was 38±12 days with a clearance rate (CL) of 94±22 mL/day (Table 3). In the SC groups, the t1/2β was 33±8·9 days with a CL/F of 184±52 mL/day. The lower AUC following SC administration and difference between CL and CL/F indicate that the SC bioavailability is approximately 50%. An accumulation in 12-week trough serum concentration occurred following multiple administrations for both the IV and SC routes, with a 50–60% increase in C84D serum concentrations between the first and third dose, although CMAX and AUC0–84D remained consistent (Figure 2C, Table 3).
To compare the PK profile of VRC07–523LS to previously published results with bnMAbs VRC01 and VRC01LS, we compared the serum concentrations of VRC07–523LS in subjects who received a single dose of 20 mg/kg IV or 5 mg/kg SC to matching dose groups of VRC01LS and VRC01 from previous trials in healthy adults in a post hoc analysis (Appendix p·3).16, 19 For both dosing regimens, serum concentrations of VRC07–523LS remained consistently higher than levels previously observed for VRC01 but lower than levels previously observed for VRC01LS. At 12 weeks after a single administration of 20 mg/kg IV, we found VRC07–523LS serum concentrations to be 9·0-fold higher than VRC01 and 3·8-fold lower than VRC01LS. At 12 weeks after a single dose of 5 mg/kg SC, we found VRC07–523LS serum concentrations to be 8·2-fold higher than 2 doses of VRC01 (administered at weeks 0 and 4) and 3·5-fold lower than a single VRC01LS dose.
We also analyzed the IgG1 allotypes of each subject for possible influence on pharmacokinetics. These antigenic determinants are located on IgG heavy chains and can affect the PK of antibodies through differing FcRn affinities.28 No significant allotypic effects on the PK parameters of the participants were observed following VRC07–523LS administration in our small study population.
We further assessed trial participants for anti-drug antibody (ADA) development following VRC07–523LS administration (Appendix p·4). We tested samples at baseline and after the final administration of VRC07–523LS in all groups. We did not detect ADA in any sample after administration of VRC07–523LS.
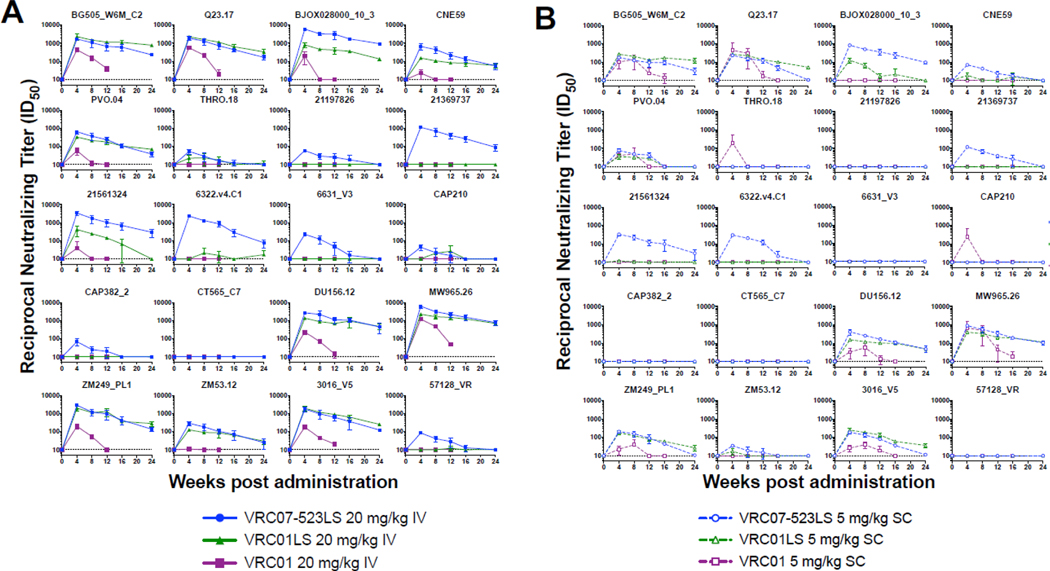
VRC07–523LS has displayed increased potency and breadth compared to VRC01 in multiple in vitro neutralization panels, particularly against clade C viruses.1, 7 To confirm that VRC07–523LS retained its enhanced neutralization characteristics following administration, we tested sera from the 20 mg/kg IV and 5 mg/kg SC dose groups over the first 24 weeks after administration against a panel of 20 genetically diverse HIV-1 Env-pseudoviruses (Appendix p·5). We chose the 5 mg/kg SC and 20 mg/kg IV groups for this analysis as these regimens are the most likely to move forward as therapeutic interventions. Overall, the VRC07–523LS retained its neutralization activity after administration (Appendix p·6). We also included human sera from previous trials containing matched doses of either VRC01 or VRC01LS for comparison in a post hoc analysis.16, 19 Sera containing VRC07–523LS displayed equivalent or greater neutralization than sera containing VRC01 or VRC01LS, even at time points where the absolute serum concentrations of VRC07–523LS were lower than VRC01LS (Figure 3, Appendix p·7–16). To assess the neutralization breadth of the human serum samples, we included several VRC01- and VRC01LS-resistant viruses in the panel (Appendix p·5). VRC07–523LS neutralized several of these resistant viruses, exemplified by pseudoviruses 21369737, 6322·v4 ·C1, and 57128_VR (Figure 3, Appendix p·7, 9–12). This increased neutralization was even maintained at week 12, when the serum concentrations of VRC07–523LS were lower than VRC01LS (Appendix p·13–16). Therefore, the enhanced neutralization capacity of VRC07–523LS observed in vitro remained following both IV and SC administration in our 20 virus panel.
Figure 3. Neutralization potency for up to 24 weeks after a single bnMAb administration using a single round of replication Env-pseudovirus assay.
Reciprocal neutralization titers (ID50) over time in subjects who received a single administration of (A) 20 mg/kg IV or (B) 5 mg/kg SC of VRC07–523LS, VRC01LS, or VRC01. Subjects who received VRC01 at 5 mg/kg SC received 2 doses of antibody: at weeks 0 and 4. n=3 for each dose group. Dotted line at 10 indicates limit of detection for the assay.
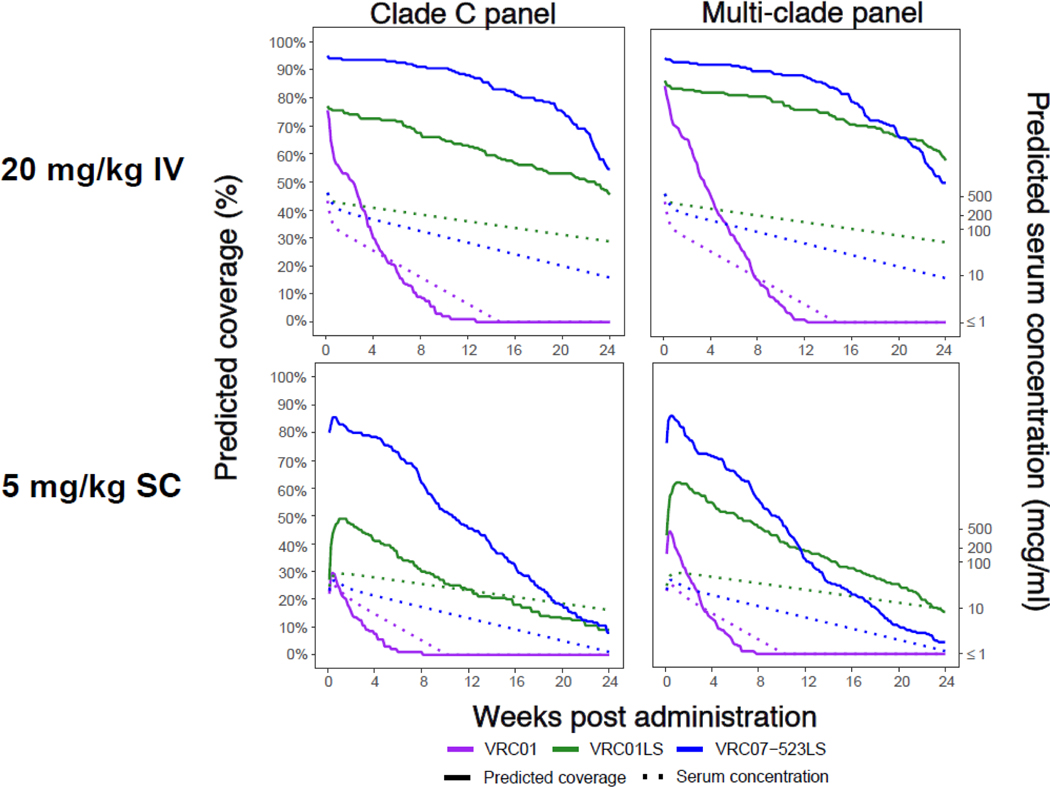
In order to extend the comparison between VRC07–523LS, VRC01LS, and VRC01, we performed PK modeling to predict serum concentration over time for each antibody.16, 19, 24 We then used the serum concentrations to predict neutralization coverage for each antibody over the first 24 weeks following a single administration in a post hoc analysis. We performed this modeling for two large panels of HIV-1 viruses that each contain approximately 200 HIV-1 strains: an acute/early clade C virus panel, and a diverse multi-clade virus panel (Figure 4, Appendix p·17).20, 25, 29 Notably, VRC07–523LS and VRC01LS, with extended half-lives, display substantially improved coverage over time compared to VRC01, highlighting the importance of antibody persistence. Overall, VRC07–523LS displayed greater predicted coverage compared to VRC01LS (Figure 4, Appendix p·17). We observed this pattern at all time points on the clade C panel. In the multi-clade panel, the superior coverage of VRC07–523LS remained through approximately 20 weeks after a single 20 mg/kg IV dose, and 12 weeks after a single 5 mg/kg SC dose. This loss of superiority in breadth of coverage for VRC07–523LS can also be observed in in the 5 mg/kg SC group on our 20 virus panel (BG505_W6M_C2, Q23.17, and 3016_V5) beginning around week 12 (Figure 3). These analyses indicate that VRC07–523LS would provide improved neutralization potency and breadth compared to VRC01LS, even with lower absolute serum concentrations.
Figure 4. Predicted coverage of VRC07–523LS, VRC01LS, and VRC01 for the first 24 weeks after a single administration based on known IC50 values.
Predicted coverages shown for either 20 mg/kg IV (top panels) or 5 mg/kg SC (bottom panels). The left panels display the percentage of viruses with predicted coverage based on a panel of acute/early clade C Env-pseudoviruses, while the right panels display the predicted coverage based on a panel of diverse multi-clade Env-pseudoviruses. Predicted coverage at a given concentration was defined as the % of viruses for which the IC50 value was 100-fold higher than the serum concentration, and a virus was considered resistant if the IC50 was greater than 10 mcg/mL. The solid lines indicate the predicted coverage for each bnMAb and the dotted lines reference the geometric mean of serum concentrations for hypothetical efficacy trial subjects in each dose group.
Discussion
VRC07–523LS is the first HIV-1 bnMAb engineered for improved breadth and potency that has been evaluated in humans. This phase 1 clinical trial provides evidence that VRC07–523LS has a favorable safety and pharmacokinetic profile and demonstrates key neutralization improvements over VRC01 and VRC01LS. There were no dose-limiting toxicities or SAEs during the trial, and local and systemic symptoms were mild to moderate in severity. Infusion reactions in two subjects showed typical self-limited symptoms requiring only minimal conservative intervention, and an investigation into their underlying etiology showed cytokine elevations commonly seen with infusion reactions from licensed monoclonal antibodies.30 VRC07–523LS displayed a serum half-life of 38 days after IV administration and 33 days after SC administration. Over three administrations, serum trough concentrations showed successive increases for both SC and IV routes with a trough serum concentration of 71 μg/mL after three doses of 20 mg/kg IV. Importantly, VRC07–523LS maintained its neutralizing activity through the course of the study, and no ADA was detected. One limitation of the current trial is the small number of subjects typical of phase 1 trials. VRC07–523LS has been advanced into additional human testing alone and in combination with other bnMAbs including 10E8VLS, PGT121, and PGDM1400 (NCT03565315, NCT03387150, NCT02840474, NCT02256631, NCT03721510, NCT03735849).
Absent a vaccine capable of providing robust active immunity against HIV-1, using bnMAbs to provide passive immunity to at-risk individuals could be an important public health measure, assuming such bnMAbs can prevent HIV-1 infection at achievable serum concentrations. Two ongoing randomized, placebo-controlled phase 2b studies are assessing the potential of an HIV-1 bnMAb to reduce the risk of HIV-1 infection, and additionally seeking to assess the serum concentration of antibody needed to provide protection against HIV-1 infection. These Antibody Mediated Prevention (AMP) trials are testing VRC01 infused IV every eight weeks at either 10 mg/kg IV or 30 mg/kg IV to men and transgender people who have sex with men and sexually active women. The AMP trials are being jointly conducted by the HIV prevention and vaccine trials networks (NCT02568215, NCT02716675). There is hope that results from the AMP trials will support the clinical development of next-generation bnMAbs with improved potency and breadth of neutralization, and extended serum persistence compared to VRC01.
Both VRC01LS and VRC07–523LS are examples of next-generation bnMAbs that demonstrate improved pharmacokinetic and neutralization characteristics compared to VRC01. The serum half-lives of these modified bnMAbs following IV administration are 38 days for VRC07–523LS and 71 days for VRC01LS in healthy adults compared to the 15 day half-life of VRC01.16, 19 While both VRC07–523LS and VRC01LS contain the same LS mutation, they display different PK parameters. Additional factors beyond FcRn binding affinity are also known to affect PK properties of monoclonal antibodies, including solubility, aggregation, off-target binding, and glycan-mediated clearance and tissue distribution.31 The choice of the parental bnMAb VRC07 or the structural engineering of VRC07–523LS may have affected these or other unknown factors contributing to its overall half-life. However, the results of this study indicate that the VRC07–523LS half-life, while shorter than VRC01LS, is sufficient to provide overall improved serum neutralization activity.
Of note, we performed the neutralization analyses for this trial using a well characterized Env pseudovirus assay which allows for accurate and reproducible data on diverse strains of HIV-1. This makes the results from the clinical samples more easily comparable to previously published in vitro panels with purified antibodies. Previous studies have demonstrated that clinical HIV-1 isolates derived from peripheral blood mononuclear cells (PBMCs) can be more difficult to neutralize than the related Env-pseudoviruses,32 which could affect the predicted coverage of these bnMAbs. Importantly, we used the same Env pseudovirus assay that will be used in the ongoing AMP trials to correlate potential clinical protection with serum neutralization. Results of those trials may provide an association between clinical protection and serum neutralization, giving the field a better understanding of how to assess coverage of neutralizing antibodies.
Ultimately, combination therapy with two or more bnMAbs will likely be more effective than a single bnMAb alone to potently neutralize more than 95% of diverse viral strains.1, 12 Thus, a major goal for bnMAb-based immunoprophylaxis is to find a combination of complementary bnMAbs with strong neutralization characteristics and extended serum persistence that could effectively be used to prevent HIV-1 infection. Additionally, improved antibody delivery methods to extend dosing intervals, such as using hyaluronidase to effectively increase the volume of subcutaneous administration,33 may improve the feasibility of bnMAbs for HIV-1 prevention and treatment. The results from this phase 1 clinical trial indicate that bnMAbs structurally engineered for improved pharmacokinetic properties and neutralization activity can be safe for clinical use, without evidence of increased ADA response, and support the notion that VRC07–523LS could be administered by both the IV and SC routes at extended intervals while maintaining serum levels capable of neutralizing the majority of HIV-1 viruses. VRC07–523LS should be evaluated further in HIV-1 infected individuals, and included in future combination trials.
Supplementary Material
Research in Context.
Evidence before this study
Monoclonal antibodies (mAbs) targeting the HIV-1 Env protein are under evaluation for HIV-1 treatment and prevention. On November 14, 2018, we searched PubMed using the terms “HIV-1” AND “monoclonal antibody” AND “clinical trial”. We excluded reviews, studies pertaining to HIV-1 antiretroviral or vaccine research, studies involving sera from HIV-1 chronically infected individuals, and other articles not directly reporting human clinical trial results involving the administration of human (or humanized) mAbs that target HIV-1 Env for either prevention or treatment of HIV-1 infection. We found 22 clinical trials in both healthy and HIV-infected adults that met these criteria. Ten of these trials involved the first generation of isolated human (or humanized) mAbs with limited breadth against diverse HIV-1 strains (neutralizing less than 40% of strains tested). These mAbs included 2F5, 4E10, 2G12, F105, hNM01, and KD-247. All were safe in humans and most had short elimination half-lives (ranging from 3–13 days, with 2G12 ranging from 14–22 days) and had limited anti-HIV-1 activity in viremic subjects. Combination treatments with these antibodies were also largely ineffective at significantly reducing viral loads in viremic individuals, likely due to the limited potency and breadth of these initial mAbs. The remaining 12 studies described clinical trials in humans involving more recently developed broadly neutralizing monoclonal antibodies (bnMAbs) displaying improved neutralization breadth and potency: VRC01, VRC01LS, 3BNC117, and 10–1074. These bnMAbs were well tolerated in the clinical trials without clear adverse safety concerns, demonstrated longer elimination half-lives (VRC01: 15 days, 3BNC117: 17 days, 10–1074: 24 days, and VRC01LS: 71 days), required less frequent administrations to maintain serum concentrations above a target threshold, and often resulted in viral suppression in viremic subjects. In studies involving analytic treatment interruption (ATI) of antiretroviral therapy, the bnMAbs extended the time to viral rebound by several weeks compared to control groups. Two of the bnMAbs, 3BNC117 and 10–1074, when used in combination resulted in significant viral suppression and extended time to viral rebound following ATI in some subjects.
Added value of this study
Our study evaluated the safety, tolerability, pharmacokinetics, and immunogenicity of VRC07–523LS, a bnMAb that targets the CD4 binding-site of the HIV-1 Env protein, in healthy adults. VRC07–523LS is a second generation bnMAb that is engineered for improved potency, breadth of reactivity, and pharmacokinetic properties. VRC07–523LS is variant of VRC07, which is a clonal relative of bnMAb VRC01. VRC07–523LS was well tolerated without anti-drug antibodies detected following both IV and SC administration, with an elimination half-life of 38 days for IV groups and 33 days for SC groups. Serum neutralizing capacity in samples from VRC07–523LS-treated subjects was superior to subjects treated with equivalent doses of either VRC01 or VRC01LS from previous trials.
Implications of all available evidence
Monoclonal antibodies against HIV-1 have the ability to neutralize HIV-1 in infected individuals while remaining safe and well tolerated. Further optimization of parental antibodies can lead to increased serum persistence with improved neutralization potency and breadth; characteristics which are retained in the serum of subjects following administration by either the IV or SC routes. VRC07–523LS is an example of an isolated bnMAb that demonstrates improved viral neutralization characteristics compared to previously evaluated bnMAbs, including VRC01 and VRC01LS. Future studies are necessary to evaluate VRC07–523LS for prevention and treatment in HIV-1 infected individuals, as well as in future combination trials.
Author List with Degrees and Full Professor details for:
Safety and pharmacokinetics of broadly neutralizing human monoclonal antibody VRC07–523LS in healthy adults: A phase 1 dose-escalation clinical trial
Martin R. Gaudinski, MD
Katherine V. Houser, PhD
Nicole A. Doria-Rose, PhD
Grace L. Chen, MD
Ro Shauna S. Rothwell, PhD
Nina Berkowitz, MPH
Pamela Costner, BSN
LaSonji A. Holman, MSN
Ingelise J. Gordon, RN
Cynthia S. Hendel, MSN
Florence Kaltovich, MS
Michelle Conan-Cibotti, PhD
Margarita Gomez Lorenzo, MD
Cristina Carter, MD
Sandra Sitar, MS
Kevin Carlton, MS
Jason Gall, PhD
Carolyn Laurencot, PhD
Bob C. Lin, BS
Robert T. Bailer, PhD
Adrian B. McDermott, PhD, Senior NIH Investigator
Sung-Youl Ko, PhD
Amarendra Pegu, PhD
Young D. Kwon, PhD
Peter D. Kwong, PhD, Senior NIH Investigator
Aryan M. Namboodiri, PhD
Janardan P. Pandey, PhD, Full Professor
Richard Schwartz, PhD
Frank Arnold, PhD
Zonghui Hu, PhD
Lily Zhang, MS
Yunda Huang, PhD
Richard A. Koup, MD, Senior NIH Investigator
Edmund V. Capparelli, PharmD, Full Professor
Barney S. Graham, MD, Senior NIH Investigator
John R. Mascola, MD, Senior NIH Investigator
Julie E. Ledgerwood, DO, Senior NIH Investigator
The VRC 605 study team for Pubmed
| First and Middle Name | Last Name |
|---|---|
| Floreliz | Mendoza |
| Laura | Novik |
| Kathy | Zephir |
| William | Whalen |
| Brenda | Larkin |
| Jamie | Saunders |
| Jennifer | Cunningham |
| Carol | Levinson |
| Xiaolin | Wang |
| Sarah | Plummer |
| Milalynn | Victorino |
| Abidemi | Ola |
| Catina | Boyd |
| Nilusha | Jayasinghe |
| Preeti | Apte |
| Cora Trelles | Cartagena |
| Renunda | Hicks |
| Pernell | Williams |
| Olga | Vasilenko |
| Galina | Yamshchikov |
| Maria | Burgos Florez |
| Iris | Pittman |
| Lucio | Gama |
| Joseph | Casazza |
| Hope | DeCederfelt |
| KC | Cheng |
| Judy | Stein |
Acknowledgements
We would like to thank the NIAID IRB, Michael Seaman and Carolyn Williamson for the clade C viruses, and the volunteers for their involvement with VRC 605 and furthering HIV-1 treatment and prevention research. Funding for this study was provided by the Intramural Research Program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health
VRC 605 Study Team
The VRC 605 study team not listed in the author line includes Floreliz Mendoza, Laura Novik, Kathy Zephir, William Whalen, Brenda Larkin, Jamie Saunders, Jennifer Cunningham, Carol Levinson, Xiaolin Wang, Sarah Plummer, Milalynn Victorino, Abidemi Ola, Catina Boyd, Nilusha Jayasinghe, Preeti Apte, Cora Trelles Cartagena, Renunda Hicks, Pernell Williams, Olga Vasilenko, Galina Yamshchikov, Maria Burgos Florez, Iris Pittman, Lucio Gama, Joseph Casazza, Hope DeCederfelt, KC Cheng, and Judy Stein.
Funding:
Intramural Research Program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health
Footnotes
Declaration of Interests
JRM, PDK, and YDK are listed on patents applications involving VRC07–523LS and YH reports a grant from NIAID U.S. Public Health Service during the conduct of the study. All other authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript.
Data Sharing Statement
Data generated in this study will be shared as de-identified data in www.ClinicalTrials.gov. Study protocol, statistical analysis plan, and informed consent form are available with the trial publication. Any additional data may be made available upon request to the corresponding author.
References
- 1.Wagh K, Bhattacharya T, Williamson C, Robles A, Bayne M, Garrity J, et al. Optimal Combinations of Broadly Neutralizing Antibodies for Prevention and Treatment of HIV-1 Clade C Infection. PLoS Pathog. 2016;12(3):e1005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCoy LE, Burton DR. Identification and specificity of broadly neutralizing antibodies against HIV. Immunol Rev. 2017;275(1):11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wibmer CK, Moore PL, Morris L. HIV broadly neutralizing antibody targets. Curr Opin HIV AIDS. 2015;10(3):135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwong PD, Mascola JR. HIV-1 Vaccines Based on Antibody Identification, B Cell Ontogeny, and Epitope Structure. Immunity. 2018;48(5):855–71. [DOI] [PubMed] [Google Scholar]
- 5.Pegu A, Hessell AJ, Mascola JR, Haigwood NL. Use of broadly neutralizing antibodies for HIV-1 prevention. Immunol Rev. 2017;275(1):296–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caskey M, Klein F, Nussenzweig MC. Broadly Neutralizing Antibodies for HIV-1 Prevention or Immunotherapy. N Engl J Med. 2016;375(21):2019–21. [DOI] [PubMed] [Google Scholar]
- 7.Rudicell RS, Kwon YD, Ko SY, Pegu A, Louder MK, Georgiev IS, et al. Enhanced potency of a broadly neutralizing HIV-1 antibody in vitro improves protection against lentiviral infection in vivo. J Virol. 2014;88(21):12669–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bar KJ, Sneller MC, Harrison LJ, Justement JS, Overton ET, Petrone ME, et al. Effect of HIV Antibody VRC01 on Viral Rebound after Treatment Interruption. N Engl J Med. 2016;375(21):2037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch RM, Boritz E, Coates EE, DeZure A, Madden P, Costner P, et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med. 2015;7(319):319ra206. [DOI] [PubMed] [Google Scholar]
- 10.Caskey M, Schoofs T, Gruell H, Settler A, Karagounis T, Kreider EF, et al. Antibody 10–1074 suppresses viremia in HIV-1-infected individuals. Nat Med. 2017;23(2):185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP Jr., Buckley N, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522(7557):487–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bar-On Y, Gruell H, Schoofs T, Pai JA, Nogueira L, Butler AL, et al. Safety and antiviral activity of combination HIV-1 broadly neutralizing antibodies in viremic individuals. Nat Med. 2018;24(11):1701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendoza P, Gruell H, Nogueira L, Pai JA, Butler AL, Millard K, et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature. 2018;561(7724):479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329(5993):856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010;329(5993):811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ledgerwood JE, Coates EE, Yamshchikov G, Saunders JG, Holman L, Enama ME, et al. Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin Exp Immunol. 2015;182(3):289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer KH, Seaton KE, Huang Y, Grunenberg N, Isaacs A, Allen M, et al. Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: Results of a phase 1 randomized trial. PLoS Med. 2017;14(11):e1002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko SY, Pegu A, Rudicell RS, Yang ZY, Joyce MG, Chen X, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514(7524):642–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaudinski MR, Coates EE, Houser KV, Chen GL, Yamshchikov G, Saunders JG, et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults. PLoS Med. 2018;15(1):e1002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hraber P, Rademeyer C, Williamson C, Seaman MS, Gottardo R, Tang H, et al. Panels of HIV-1 Subtype C Env Reference Strains for Standardized Neutralization Assessments. J Virol. 2017;91(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urlaub G, Kas E, Carothers AM, Chasin LA. Deletion of the diploid dihydrofolate reductase locus from cultured mammalian cells. Cell. 1983;33(2):405–12. [DOI] [PubMed] [Google Scholar]
- 22.Balbin M, Grubb A, Abrahamson M, Grubb R. Determination of allotypes G1m(f) and G1m(z) at the genomic level by subclass-specific amplification of DNA and use of allele-specific probes. Exp Clin Immunogenet. 1991;8(2):88–95. [PubMed] [Google Scholar]
- 23.Schanfield MS, van Loghem E. Human immunoglobulin allotypes. In: DM W, editor. Handbook of Experimental Immunology: Genetics and Molecular Immunology. 3. 4th ed. Boston: Blackwell Scientific; 1986. p. 94.1-.18. [Google Scholar]
- 24.Gilbert PB, Juraska M, deCamp AC, Karuna S, Edupuganti S, Mgodi N, et al. Basis and Statistical Design of the Passive HIV-1 Antibody Mediated Prevention (AMP) Test-of-Concept Efficacy Trials. Stat Commun Infect Dis. 2017;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong R, Xu K, Zhou T, Acharya P, Lemmin T, Liu K, et al. Fusion peptide of HIV-1 as a site of vulnerability to neutralizing antibody. Science. 2016;352(6287):828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarzotti-Kelsoe M, Bailer RT, Turk E, Lin CL, Bilska M, Greene KM, et al. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J Immunol Methods. 2014;409:131–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel SV, Khan DA. Adverse Reactions to Biologic Therapy. Immunol Allergy Clin North Am. 2017;37(2):397–412. [DOI] [PubMed] [Google Scholar]
- 28.Ternant D, Arnoult C, Pugniere M, Dhommee C, Drocourt D, Perouzel E, et al. IgG1 Allotypes Influence the Pharmacokinetics of Therapeutic Monoclonal Antibodies through FcRn Binding. J Immunol. 2016;196(2):607–13. [DOI] [PubMed] [Google Scholar]
- 29.Rademeyer C, Korber B, Seaman MS, Giorgi EE, Thebus R, Robles A, et al. Features of Recently Transmitted HIV-1 Clade C Viruses that Impact Antibody Recognition: Implications for Active and Passive Immunization. PLoS Pathog. 2016;12(7):e1005742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isabwe GAC, Garcia Neuer M, de Las Vecillas Sanchez L, Lynch DM, Marquis K, Castells M. Hypersensitivity reactions to therapeutic monoclonal antibodies: Phenotypes and endotypes. J Allergy Clin Immunol. 2018;142(1):159–70 e2. [DOI] [PubMed] [Google Scholar]
- 31.Liu L. Pharmacokinetics of monoclonal antibodies and Fc-fusion proteins. Protein Cell. 2018;9(1):15–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Louder MK, Sambor A, Chertova E, Hunte T, Barrett S, Ojong F, et al. HIV-1 envelope pseudotyped viral vectors and infectious molecular clones expressing the same envelope glycoprotein have a similar neutralization phenotype, but culture in peripheral blood mononuclear cells is associated with decreased neutralization sensitivity. Virology. 2005;339(2):226–38. [DOI] [PubMed] [Google Scholar]
- 33.Locke KW, Maneval DC, LaBarre MJ. ENHANZE((R)) drug delivery technology: a novel approach to subcutaneous administration using recombinant human hyaluronidase PH20. Drug Deliv. 2019;26(1):98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated in this study will be shared as de-identified data in www.ClinicalTrials.gov. Study protocol, statistical analysis plan, and informed consent form are available with the trial publication. Any additional data may be made available upon request to the corresponding author.