Abstract
Bacimethrin is an analog of the 4-amino-5-hydroxymethyl-2-methylpyrimidine (HMP) moiety of thiamine and inhibits the growth of Salmonella enterica serovar Typhimurium on a defined medium. Two classes of mutants that had increased bacimethrin resistance were isolated and characterized. Results showed that overexpression of the thi operon or specific lesions in thiD resulted in a bacimethrin-resistant phenotype. Phenotypic analyses of the thiD mutants suggested that they had a specific defect in one of the two kinase activities associated with this gene product and, further, that ThiD and not PdxK was primarily responsible for salvage of HMP from the medium.
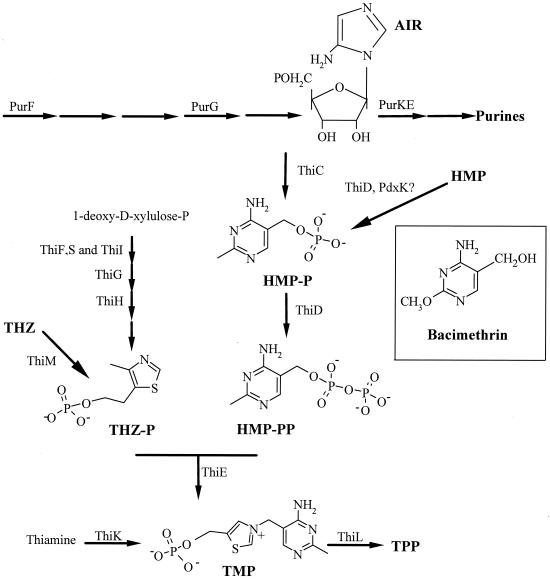
Thiamine is synthesized de novo by many bacteria. Although the general outline of the thiamine biosynthetic pathway in enteric microorganisms is known, the individual reactions in this pathway are not yet fully understood. Thiamine pyrophosphate (TPP), the biologically active cofactor, is generated by the condensation of two independently synthesized intermediates, 4-methyl-5-(β-hydroxyethyl)thiazole (THZ) phosphate and 4-amino-5-hydroxymethyl-2-methylpyrimidine (HMP) pyrophosphate. In vivo labeling studies demonstrated that the carbon atoms in HMP were derived from the purine intermediate aminoimidazole ribonucleotide (AIR), although the biochemical details of this conversion have not been elucidated (2). Mutations causing an unconditional requirement for exogenous HMP or thiamine map to a single gene, thiC. In vivo labeling also determined the metabolic precursors to the THZ moiety, and several genes have been implicated in this synthesis (2, 18). Our current understanding of the synthesis and salvage of TPP is presented in Fig. 1.
FIG. 1.
Biosynthetic and salvage pathways for thiamine. The biosynthetic and salvage pathways for thiamine in S. enterica, including gene products where known, is represented. The structure of bacimethrin is shown in the inset. TMP, thiamine monophosphate. Other terms are as defined in the text.
While the low cellular requirement for thiamine has complicated the detailed analysis of its biosynthetic pathway, this characteristic has made thiamine synthesis an attractive model system for dissecting the integration of metabolic pathways (25). Characterization of mutations that perturbed, but did not eliminate, thiamine synthesis has resulted in the identification of a number of loci required for optimal synthesis of this vitamin and has increased our understanding of the integration of thiamine biosynthesis with central cellular processes (1, 5, 6, 8, 10–12, 24b, 25, 27).
Use of metabolic inhibitors, or analogs, has provided insight in the studies of a number of metabolic pathways, including histidine (17), folate (3), and branched-chain amino acids (19–21). Bacimethrin is a thiamine antagonist produced by gram-positive bacteria, including Streptomyces albus (7) and Bacillus megaterium (29) (Fig. 1). Previous reports showed that the inhibitory effect of bacimethrin on defined medium could be overcome by exogenous addition of thiamine or the HMP moiety (7).
Wild-type Salmonella enterica serovar Typhimurium strain LT2 was unable to grow on solid minimal medium in the presence of 130 nM bacimethrin, and resistant mutants arose spontaneously at a frequency of ∼10−7 (data not shown). Growth of the wild-type strain was restored by the addition of either HMP or thiamine, indicating a specific antagonism for the pyrimidine moiety of thiamine (7). Mutations allowing the growth of LT2 in the presence of 130 nM bacimethrin were isolated, either spontaneously or following localized chemical mutagenesis (15), in the thi loci at 90 min (thiCEFSGH) and 46 min (thiMD).
Increased transcription of the thiCEFSGH operon results in bacimethrin resistance.
Two bacimethrin-resistant mutants with causative lesions at 90 min on the chromosome were characterized to determine if expression of the thiCEFSGH operon was altered. A DNA fragment containing the 400 bp upstream of the thiC initiating codon was PCR amplified from wild-type strain LT2 and bacimethrin-resistant strains DM4078 (thi-1132) and DM4081 (thi-1133). The resulting fragments were ligated in front of the promoterless lacZ gene in pKC1 (4), generating plasmids pThi, pThi4, and pThi8, respectively. Strains containing these plasmids, grown on medium in the presence or absence of thiamine, were assayed for β-galactosidase activity and the results are shown in Table 1. Two points could be made from these data: (i) transcription from the mutant constructs was three- to fourfold increased compared to the wild-type construct, and (ii) in both the wild-type and mutant constructs, exogenous thiamine repressed transcription. These results suggested that the causative mutations affected primarily promoter strength rather than regulation of the operon.
TABLE 1.
thi promoter region from bacimethrin-resistant mutants directs increased expressiona
| Plasmid | β-Galactosidase activityb ± SD
|
Fold repression | |
|---|---|---|---|
| Without thiamine | With thiamine | ||
| pThi | 2,402 ± 219 | 756 ± 31 | 3.2 |
| pThi4 | 8,947 ± 1,303 | 1,685 ± 353 | 5.3 |
| pThi8 | 8,933 ± 1,825 | 2,036 ± 515 | 4.4 |
Derivatives of LT2 containing the indicated plasmid were grown overnight in minimal medium containing chloramphenicol and subcultured into minimal medium with or without thiamine. Subcultures were grown to 0.3 to 0.4 A650 before being assayed. β-Galactosidase activity was assayed as previously described (9, 24), except that volumes were reduced 10-fold, and absorbance was read in a microtiter plate by a Spectramax Plus (Molecular Devices).
Activities are reported as nanomoles of o-nitrophenyl-β-d-galactopyranosidase hydrolyzed/minute/A650 ± 1 standard deviation of the mean.
To confirm that increased expression of the thiCEFSGH operon was responsible for the bacimethrin-resistant phenotype of these strains, a multicopy plasmid carrying the entire thiCEFSGH operon from Escherichia coli was electroporated into strain LT2. This plasmid, pVJS717 (30), and not the parent plasmid (pGEM) allowed growth in the presence of 130 nM bacimethrin. Since bacimethrin is structurally similar to HMP, ThiC and ThiE were potential targets for inhibition. To address whether overexpression of thiC or thiE alone was sufficient to cause bacimethrin resistance, multicopy plasmids containing either thiC (pThiC) or thiE (pThiE) were generated, with expression of the thi gene from the lacZ promoter of pSU19 (22). Neither strain DM5717 (LT2/pThiE) nor strain DM5654 (LT2/pThiC) was able to grow on solid medium in the presence of 130 nM bacimethrin. These results suggested either that expression of the respective genes from the lacZ promoter in these constructs was not high enough or that overexpression of more than one gene was required to generate the bacimethrin-resistant phenotype.
Mutations in thiD can result in a bacimethrin-resistant phenotype.
Two bacimethrin-resistant strains whose lesions mapped to 46 min on the S. enterica chromosome were examined in detail (Table 2). Single gene clones of the mutant alleles were generated by PCR amplifying thiD from the chromosome of DM2983 (thiD1126) and DM4147 (thiD1125) and ligating it into pSU19, resulting in plasmids pThiD1126 and pThiD1125, respectively. Each plasmid was electroporated into a strain carrying a null mutation in thiD (DM456). Both of the resulting strains (DM5556 and DM5557), but not a strain with a plasmid carrying the wild-type thiD gene, were able to grow on solid minimal medium in the presence of 130 nM bacimethrin. These results demonstrated that the bacimethrin-resistant mutations in strains DM2983 and DM4147 were alleles of thiD. Sequence analyses of plasmids pThiD1126 and pThiD1125 identified a single base change in each ThiD coding sequence that would result in the amino acid changes P186Q and N15S, respectively.
TABLE 2.
Phenotype of bacimethrin-resistant mutants
| Strain | Genotype | Zone of inhibition (cm) with bacimethrina:
|
|
|---|---|---|---|
| 0.13 | 1.3 | ||
| LT2 | Wild type | 2.4 | 3.4 |
| DM2983 | thiD1126 | 0 | 0.2 |
| DM4147 | thiD1125 | 0 | 0.5 |
The diameter of the zone of inhibition was measured when 1 or 10 μl, containing 0.13 or 1.3 pmol of bacimethrin, respectively, was spotted onto a soft agar overlay of cells on minimal medium.
The thiD gene product has been shown to catalyze two kinase reactions relevant to thiamine biosynthesis (2, 24a). Since both the original bacimethrin-resistant thiD mutants and the plasmid-containing strains were prototrophic, we concluded that the HMP-P kinase activity of ThiD required for de novo thiamine synthesis was present.
Increased bacimethrin resistance could theoretically result from mutant forms of ThiD with increased catalytic activity or increased substrate specificity. In both of these scenarios, the mutant thiD would be dominant to wild type. However, when plasmids pThiD1126 and pThiD1125 were electroporated into LT2, the resulting strains remained sensitive to bacimethrin, indicating that the mutant alleles of thiD were recessive. We reasoned that loss of the HMP kinase, but not the HMP-P kinase activity of ThiD, would eliminate the initial phosphorylation of bacimethrin, preventing it from interfering with thiamine synthesis (T. Begley, personal communication). This scenario predicted that the bacimethrin-resistant thiD mutants would be defective in the incorporation of exogenous HMP to thiamine. To address this, de novo HMP synthesis was blocked in strains containing thiD1125, thiD1126, and the wild-type strain LT2 by introducing a purG insertion mutation (Fig. 1). The minimal concentration of HMP required for growth of the resulting strains was determined. As shown by the data in Table 3, each of the strains containing a bacimethrin-resistant allele of thiD required an increased amount of HMP (compared to the purG mutant) for growth. Specifically, the thiD1125-containing strain required 100-fold more HMP than the purG single mutant. It was noted that the mutant most resistant to bacimethrin (DM2983, containing thiD1126) did not contain the allele that caused the most dramatic increase in HMP requirement (thiD1125 in DM5570). There are a number of formal possibilities to explain this apparent inconsistency, including different affinities for HMP compared to bacimethrin.
TABLE 3.
Bacimethrin-resistant mutants have increased requirement for exogenous HMP
| Strain | Genotype | Diam of growth (cm) after addition of HMPa:
|
|||||
|---|---|---|---|---|---|---|---|
| 100 | 50 | 10 | 5 | 1 | 0.5 | ||
| DM40 | purG | 3.2 | 3.0 | 2.3 | 2.0 | 0.8 | 0.7b |
| DM5571 | purG thiD1126 | 2.7 | 2.1 | 1.2 | 1 | NG | NG |
| DM5570 | purG thiD1125 | 1.6 | 0.9 | NG | NG | NG | NG |
Diameter of growth zone resulting from the addition of the indicated amount of HMP (in picomoles) to a soft agar overlay of cells on minimal medium containing 0.4 mM adenine. NG, no growth.
Strain DM40 (purG) failed to grow with 0.1 pmol of HMP.
These results strongly suggested that thiD alleles resulting in bacimethrin resistance were specifically defective in the HMP kinase activity. If confirmed by in vitro kinetic analyses of the mutant proteins, this model provides a positive selection for thiD mutants defective in one of the two kinase activities and supports the prediction that the active sites for the two phosphorylation reactions would be quite different (26).
A role for PdxK in HMP salvage?
The prediction that the phenotypes shown in Tables 2 and 3 were caused by lack of HMP kinase activity caused us to consider the role of PdxK in HMP salvage. The pdxK gene was identified by the role of its gene product in the salvage of pyridoxine in the synthesis of pyridoxal 5′-phosphate synthesis (30). The in vitro analysis of PdxK demonstrated that it phosphorylated HMP with the same general kinetic parameters as it had for pyridoxine (26). Further, the Km for HMP of PdxK was ∼5-fold lower, and the kcat/Km ratio was 10-fold higher than the same parameters of ThiD, suggesting that in vivo PdxK would play a major role in HMP salvage (26, 30). The identification of mutations in thiD that resulted in a significantly increased requirement for HMP in strains carrying a wild-type pdxK locus suggested that if PdxK plays a role in HMP salvage, it is a minor one.
Sensitivity to bacimethrin reflects flux through the HMP branch of the thiamine biosynthetic pathway.
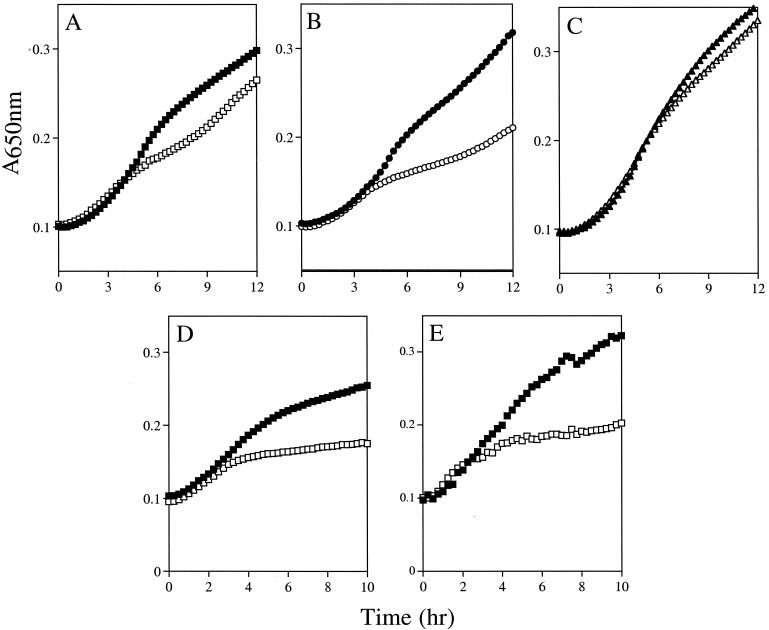
From the above results it was formally possible that bacimethrin uptake was inhibited by HMP and that its target was at a different metabolic step. A correlation of endogenous HMP synthesis and bacimethrin resistance would eliminate this possibility. Strains not auxotrophic for thiamine but likely to have increased or decreased HMP synthesis were assessed for bacimethrin resistance. The growth data from some of these analyses are presented in Fig. 2 and indicated that the sensitivity of a strain to bacimethrin correlated with the anticipated flux through the HMP biosynthetic pathway. The growth of wild-type strain LT2 in liquid minimal medium was not significantly affected by 516 nM bacimethrin (Fig. 2A). The difference in effective concentrations on a solid medium versus a liquid medium was noted but was not pursued since the strain differences were qualitatively the same in the two media.
FIG. 2.
Phenotypes of representative strains. Strains were grown aerobically in a microtiter plate on glucose minimal medium, and the absorbance was monitored at 650 nm on a Spectramax plate reader (Molecular Devices, Sunnyvale, Calif.). In panels B and C adenine was included at 0.4 mM. All panels compare growth on minimal medium (closed symbols) with growth in the presence of 516 nM bacimethrin (open symbols). The strains include LT2 (A and B), purE (C), panE (D), and apbC (E).
Exogenous addition of purines to the growth medium has been shown to reduce flux through the biosynthetic pathway ∼90%, due to the combined transcriptional and allosteric regulatory effects of purines on the pathway (14, 16, 23, 31). This flux reduction does not generate a requirement for thiamine in a wild-type strain (J. Zilles and D. M. Downs, submitted for publication). When purines (e.g., adenine) were included in the growth medium, wild-type LT2 was more sensitive to bacimethrin (Fig. 2B). A purE mutant, blocked immediately after the purine-thiamine branch point, was not sensitive to bacimethrin under the same conditions (Fig. 2C) (25).
This analysis was extended to mutants with a less-characterized effect on HMP synthesis. Mutations in panE reduce internal coenzyme A pools 10-fold, which results in a thiamine requirement only if flux through the purine pathway is also reduced (11). When panE mutants are grown on minimal medium, no growth defect is measurable, and internal metabolite pools of TPP are wild type (11). However, as shown in Fig. 2D, the panE mutant was sensitive to bacimethrin. A similar response was found with an apbC mutant that is thought to be defective in HMP synthesis (Fig. 2E) (13, 24b). Taken together, these results suggest that bacimethrin sensitivity can provide a qualitative measure of flux through the HMP biosynthetic pathway.
Conclusions.
Results from this study have increased our understanding of the antagonistic effects of bacimethrin in S. enterica by identifying two distinct lesions resulting in resistance to this analog. We showed that increased expression of the thi operon or specific lesions in thiD allow cells to grow in the presence of increased bacimethrin. Phenotypic analysis of the thiD mutants suggested that the mutant proteins were defective in one of the two kinase activities associated with this protein. Analyses of these mutants suggested that the HMP-kinase activity of PdxK was the primary route for HMP salvage (26).
Acknowledgments
We thank T. Begley for providing us with bacimethrin and for helpful discussion and S. Weber for technical assistance. Plasmid pVJS717 was obtained from V. Stewart.
This work was supported by competitive grant MCB9723830 from the National Science Foundation and a Shaw Scientists Award from the Milwaukee Foundation. J.L.Z. was supported by a National Science Foundation Graduate Fellowship and a Wisconsin Alumni Research Foundation Annual Fellowship.
REFERENCES
- 1.Beck B J, Connolly L E, de las Penas A, Downs D M. Evidence that rseC, a gene in the rpoE cluster, has a role in thiamine synthesis in Salmonella typhimurium. J Bacteriol. 1997;179:6504–6508. doi: 10.1128/jb.179.20.6504-6508.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Begley T P, Downs D M, Ealick S, McLafferty F, van Loon D, Taylor S, Chiu H, Kinsland C, Reddick J, Xi J, Campobasso N. Thiamin synthesis in prokaryotes. Arch Microbiol. 1999;171:293–300. doi: 10.1007/s002030050713. [DOI] [PubMed] [Google Scholar]
- 3.Brown G M. The biosynthesis of folic acid. Inhibition by sulfonamides. J Biol Chem. 1962;237:536–540. [PubMed] [Google Scholar]
- 4.Cho K O, Yanofsky C. Development of a trpE promoter-strength measuring system and its use in comparison of the trpEDCA, trpR and aroH promoters. J Mol Biol. 1988;204:41–50. doi: 10.1016/0022-2836(88)90597-9. [DOI] [PubMed] [Google Scholar]
- 5.Christian T, Downs D M. Defects in pyruvate kinase cause a conditional increase of thiamine synthesis in Salmonella typhimurium. Can J Microbiol. 1999;45:565–572. [PubMed] [Google Scholar]
- 6.Claas K, Weber S, Downs D M. Lesions in the nuo operon, encoding NADH dehydrogenase complex I, prevent PurF-independent thiamine synthesis and reduce flux through the oxidative pentose phosphate pathway in Salmonella enterica serovar Typhimurium. J Bacteriol. 2000;182:228–232. doi: 10.1128/jb.182.1.228-232.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drautz H, Messerer W, Zahner H. Bacimethrin isolated from Streptomyces albus identification, derivatives, synthesis and biological properties. J Antibiot. 1987;40:1431–1439. doi: 10.7164/antibiotics.40.1431. [DOI] [PubMed] [Google Scholar]
- 8.Enos-Berlage J L, Downs D M. Involvement of the oxidative pentose phosphate pathway in thiamine biosynthesis in Salmonella typhimurium. J Bacteriol. 1996;178:1476–1479. doi: 10.1128/jb.178.5.1476-1479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escalante-Semerena J C, Roth J R. Regulation of cobalamin biosynthetic operons in Salmonella typhimurium. J Bacteriol. 1987;169:2251–2258. doi: 10.1128/jb.169.5.2251-2258.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frodyma M, Downs D M. The panE gene, encoding ketopantoate reductase, maps at 10 minutes and is allelic to apbA in Salmonella typhimurium. J Bacteriol. 1998;180:4757–4759. doi: 10.1128/jb.180.17.4757-4759.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frodyma M, Rubio A, Downs D M. Reduced flux through the purine biosynthetic pathway results in an increased requirement for coenzyme A in thiamine synthesis in Salmonella enterica serovar Typhimurium. J Bacteriol. 2000;182:236–240. doi: 10.1128/jb.182.1.236-240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frodyma M E, Downs D M. ApbA, the ketopantoate reductase enzyme of Salmonella typhimurium is required for the synthesis of thiamine via the alternative pyrimidine biosynthetic pathway. J Biol Chem. 1998;273:5572–5576. doi: 10.1074/jbc.273.10.5572. [DOI] [PubMed] [Google Scholar]
- 13.Gralnick J, Webb E, Beck B, Downs D. Lesions in gshA (encoding γ-l-glutamyl-l-cystein synthetase) prevent aerobic synthesis of thiamine in Salmonella enterica serovar Typhimurium LT2. J Bacteriol. 2000;182:5180–5187. doi: 10.1128/jb.182.18.5180-5187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He B, Shiau A, Choi K Y, Zalkin H, Smith J M. Genes of the Escherichia coli pur regulon are negatively controlled by a repressor-operator interaction. J Bacteriol. 1990;172:4555–4562. doi: 10.1128/jb.172.8.4555-4562.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong J S, Ames B N. Localized mutagenesis of any specific small region of the bacterial chromosome. Proc Natl Acad Sci USA. 1971;68:3158–3162. doi: 10.1073/pnas.68.12.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houlberg U, Jensen K F. Role of hypoxanthine and guanine in regulation of Salmonella typhimurium pur gene expression. J Bacteriol. 1983;153:837–845. doi: 10.1128/jb.153.2.837-845.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston H M, Roth J R. Genetic analysis of the histidine operon control region of Salmonella typhimurium. J Mol Biol. 1981;145:713–734. doi: 10.1016/0022-2836(81)90311-9. [DOI] [PubMed] [Google Scholar]
- 18.Kambampati R, Lauhon C T. IscS is a sulfurtransferase for the in vitro biosynthesis of 4-thiouridine in Escherichia coli tRNA. Biochemistry. 1999;38:16561–16568. doi: 10.1021/bi991119r. [DOI] [PubMed] [Google Scholar]
- 19.LaRossa R A, Van Dyk T K. Leaky pantothenate and thiamin mutations of Salmonella typhimurium conferring sulphometuron methyl sensitivity. J Gen Microbiol. 1989;135:2209–2222. doi: 10.1099/00221287-135-8-2209. [DOI] [PubMed] [Google Scholar]
- 20.LaRossa R A, Van Dyk T K. Utilization of sulfometuron methyl, an acetolactate synthase inhibitor, in molecular biological and metabolic studies of plants and microbes. Methods Enzymol. 1988;166:97–107. doi: 10.1016/s0076-6879(88)66015-0. [DOI] [PubMed] [Google Scholar]
- 21.LaRossa R A, Van Dyk T K, Smulski D R. Toxic accumulation of α-ketobutyrate caused by inhibition of the branched-chain amino acid biosynthetic enzyme acetolactate synthase in Salmonella typhimurium. J Bacteriol. 1987;169:1372–1378. doi: 10.1128/jb.169.4.1372-1378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez E, Bartolome B, de la Cruz F. pACYC184-derived cloning vectors containing the multiple cloning site and lacAa reporter gene of pUC8/9 and pUC18/19 plasmids. Gene. 1988;68:159–162. doi: 10.1016/0378-1119(88)90608-7. [DOI] [PubMed] [Google Scholar]
- 23.Messenger L J, Zalkin H. Glutamine phosphoribosyl pyrophosphate amidotransferase from Escherichia coli. J Biol Chem. 1979;254:3382–3392. [PubMed] [Google Scholar]
- 24.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 24a.Petersen L A, Downs D M. Identification of an operon in Salmonella typhimurium involved in thiamine biosynthesis. J Bacteriol. 1997;179:4894–4900. doi: 10.1128/jb.179.15.4894-4900.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24b.Petersen L A, Downs D M. Mutations in apbC (mrp) prevent function of the alternative pyrimidine biosynthetic pathway in Salmonella typhimurium. J Bacteriol. 1996;178:5676–5682. doi: 10.1128/jb.178.19.5676-5682.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen L A, Enos-Berlage J E, Downs D M. Genetic analysis of metabolic crosstalk and its impact on thiamine synthesis in Salmonella typhimurium. Genetics. 1996;143:37–44. doi: 10.1093/genetics/143.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddick J J, Kinslan C, Nicewonger R, Christian T, Downs D M, Winkler M E, Begley T P. Overexpression, purification and characterization of two phyrimidne kinase involved in the biosynthesis of thiamin; 4-amino-5-hydroxymethyl-2-methylpyrimidine kinase and 4-amino-5-hydroxymethyl-2-methylpyrimidine phosphate kinase. Tetrahedron. 1998;54:15983–15991. [Google Scholar]
- 27.Skovran E, Downs D M. Phenotypic characterization of mutants defective in the isc locus of Salmonella enterica serovar Typhimurium: implications for thiamine synthesis. J Bacteriol. 2000;182:3896–3903. doi: 10.1128/jb.182.14.3896-3903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka F, Takeuchi S, Tanaka H, Yonehara H, Umezawa H, Sumiki Y. Bacimethrin, a new antibiotic produced by B. megatherium. J Antibiot Ser. 1961;14:161–162. [Google Scholar]
- 29.Vander Horn P B, Backstrom A D, Stewart V, Begley T P. Structural genes for thiamine biosynthetic enzymes (thiCEFGH) in Escherichia coli K-12. J Bacteriol. 1993;175:982–992. doi: 10.1128/jb.175.4.982-992.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Shao G, Winkler M E. Identification of the pdxK gene that encodes pyridoxine (vitamin B6) kinase in Escherichia coli K-12. FEMS Microbiol Lett. 1996;141:89–95. doi: 10.1111/j.1574-6968.1996.tb08368.x. [DOI] [PubMed] [Google Scholar]
- 31.Zalkin H, Nygaard P. Biosynthesis of purine nucleotides. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. Vol. 1. Washington, D.C.: ASM Press; 1996. [Google Scholar]