Abstract
Alcohol consumption leads to neuroinflammation and blood–brain barrier (BBB) damage, resulting in neurological impairment. We previously demonstrated that ethanol-induced disruption of barrier function in human brain endothelial cells was associated with mitochondrial injury, increased ATP and extracellular vesicle (EV) release, and purinergic receptor P2X7R activation. Therefore, we aimed to evaluate the effect of P2X7r blockade on peripheral and neuro-inflammation in EtOH-exposed mice.
In a chronic intermittent ethanol (CIE)-exposed mouse model, P2X7R was inhibited by two different methods: Brilliant Blue G (BBG) or gene knockout. We assessed blood ethanol concentration (BEC), plasma P2X7R and P-gp, number of extra-cellular vesicles (EV), serum ATP and EV-ATP levels. Brain microvessel gene expression and EV mtDNA copy numbers were measured by RT2 PCR array and digital PCR, respectively.
A RT2 PCR array of brain microvessels revealed significant upregulation of proinflammatory genes involved in apoptosis, vasodilation, and platelet activation in CIE-exposed animals, which were decreased 15–50-fold in BBG-treated CIE-exposed animals. Plasma P-gp levels and serum P2X7R shedding were significantly increased in CIE-exposed animals. Pharmacological or genetic suppression of P2X7R decreased P2X7R shedding to levels equivalent to those in control group. The increase in EV number and EV-ATP content in the CIE-exposed mice was significantly reduced by P2X7R inhibition. CIE mice showed augmented EV-mtDNA copy numbers which were reduced in EVs after P2X7R inhibition or receptor knockout.
These observations suggested that P2X7R signaling plays a critical role in ethanol-induced brain injury. Increased eATP, EV-ATP, EV numbers, and EV-mtDNA copy numbers highlight a new mechanism of brain injury during alcohol exposure via P2X7R and biomarkers of such damage. In this study, for the first time, we report the in vivo involvement of P2X7R signaling in CIE-induced brain injury.
Keywords: CIE, Blood-brain barrier, ATP, P2X7R, Extracellular vesicles
Introduction
Alcohol abuse and its detrimental effects on the central nervous system (CNS) have long been recognized as significant public health concerns. Excessive alcohol consumption is listed as one of the leading risk factors for population health, disease, disability, and death worldwide. A recent report, “World Alcohol and Health Situation,” from the World Health Organization indicated that more than 3 million deaths attributed to alcohol consumption correspond to one death every 10 seconds [1].
Several studies have reported that excessive alcohol consumption causes damage to various organs [2, 3, 4]. The key mechanisms underlying alcohol-induced neurotoxicity involve neuroinflammation and blood-brain barrier (BBB) disruption, which contribute to neuronal damage and dysfunction [3, 5]. Loss of BBB function is associated with increased permeability and downregulated expression of key proteins in the BBB [6, 7]. Disruption of BBB integrity leads to the infiltration of peripheral immune cells, cytokine release, and subsequent neuroinflammatory responses, exacerbating neuronal injury [8, 9].
Purinergic receptor-mediated signaling is essential in the CNS for maintaining physiological neural cell function and has emerged as a crucial modulator of neuroinflammation and BBB function [10, 11]. Among purinergic receptors, the purinergic receptor P2X7 (P2X7R) is involved in inflammatory processes and cell death cascades [12]. Additionally, its association with BBB disruption is of interest [13]. Adverse cellular conditions, such as stress and cellular damage, lead to an increase in extracellular ATP (eATP) concentrations, which act as damage-associated molecular patterns (DAMPs); supraphysiologic ATP concentrations are responsible for P2X7R activation. In vitro chronic alcohol exposure of human macrophages results in the activation of the P2X7R-mediated Nod-like receptor pyrin domain containing 3 (NLRP3) inflammasome pathway, which causes the secretion of interleukin 1 beta (IL-1β) [14]. Moreover, ethanol (EtOH)-dependent P2X7R overactivation causes alcohol-induced BBB damage with increased levels of the proinflammatory cytokines IL-1β, tumor necrosis factor alpha (TNF-α), and interleukin-6 (IL-6) in mice [15, 16]. In a series of investigations, our laboratory revealed the effects of EtOH exposure on brain microvascular endothelial cells (BMVECs) and revealed a compelling link between substance exposure and dysregulation of purinergic signaling pathways. EtOH-exposed BMVECs showed the mito-stress and enhanced eATP release, which were prevented by P2X7R antagonist [17–19].
Extracellular ATP stimulates P2X7R, which triggers extracellular vesicle (EV) shedding [20, 21]. EVs are cargo-carrying cell-derived vesicles which can communicate between originating and recipient cells. Several reports have stated the changes in cargo composition based on host cell health status [22–24]. P2X7R activation can change EV proteome and may be involved in the propagation of inflammation. EVs carry cytokines, various mRNAs, lipids, and ATP molecules [22, 23, 25]. Chronic EtOH exposure increases levels of proinflammatory molecules in EVs [25–27]. Studies have also detected the presence of mitochondrial DNA (mtDNA) fragments with DAMP-like properties in EVs isolated after chronic EtOH exposure [27, 28].
Several studies have reported the undeniable role of P2X7R signaling in BBB injury in vitro [18, 29–33]. However, the precise mechanisms underlying P2X7R-mediated effects in alcohol-induced neuroinflammation in vivo remain incompletely understood. In this study, we hypothesize that blocking of P2X7r signaling either by administration of BBG (P2X7r blocker) or P2X7r genetic deletion (P2X7r−/−) will reduce neuroinflammation and BBB injury in chronic EtOH-exposed mice.
In this study, we found that pharmacologic or genetic inhibition of P2X7R significantly decreased the levels of upregulated brain proinflammatory cytokines, circulating P2X7R, serum ATP levels, EVs, EV-ATP, and EV-mtDNA fragments in a mouse model of chronic intermittent exposure (CIE) to EtOH. Furthermore, the genes involved in apoptosis, vasodilation, and platelet activation, which were significantly upregulated in the brain microvessels of alcohol-exposed mice, were downregulated in CIE-exposed mice treated with the P2X7R inhibitor.
Materials and Methods
Animals
Wild-type and P2X7r−/− C57BL/6 (B6.129P2-P2rx7tm1Gab/J, stock no. 005576) mice (male, 16–17 weeks old) were obtained from Jackson Laboratories, Bar Harbor, ME. To achieve statistical significance, 5–15 mice in each experimental group were used. In the pharmacologically P2X7R-inhibited cohort, 7 mice were used in the air control group, 5 in the BBG-treated CIE-unexposed, 7 in the CIE-exposed, and 8 in the BBG-treated-CIE-exposed group. For P2X7R knockout cohort, 8 mice were used in the wild-type air control group, 6 in the P2X7r−/− CIE-unexposed, 15 in the wild-type CIE-exposed, and 15 in the P2X7r−/− CIE-exposed group. The mice were housed five per cage with food and water available ad libitum (12-h light-dark cycle). All in vivo experiments were approved by the Temple University Institutional Animal Care and Use Committee in accordance with guidelines based on the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals and the Animal Research: Reporting in Vivo Experiments (ARRIVE) guidelines (www.nc3rs.org.uk/arrive-guidelines; accessed on March 19, 2022).
CIE and BBG injections
A mouse model of CIE exposure was developed as described previously [34–36] with the following modifications. All the mice in the CIE-exposed groups were exposed to continuous ethanol vapor for 16 h, followed by 8 h in room air each day for four days a week (1 cycle; Fig. 1A). The exposure cycle was repeated three times. Before placing the mice in ethanol vapor, an intraperitoneal (i.p.) injection of an alcohol dehydrogenase inhibitor, pyrazole (P56607–5G; Merck, USA, 85 mg/kg), and a loading dose of 1.0 g/kg ethanol (32801; Decon labs Inc.) (20% w/v) in 0.9% saline were given to initiate and maintain stable ethanol intoxication in the CIE-exposed mice [37]. The mice in the air control group were injected with 85 mg/kg pyrazole in saline. To deliver ethanol vapor, 190-proof ethanol was volatilized, mixed with fresh air at a rate of 10 L/min, and then pumped into the ethanol inhalation chamber. At the end of every cycle, a 2 mL air sample was drawn through a port in the chamber door to measure the amount of ethanol present in the chamber. Mice from the BBG- and BBG-CIE-exposed groups were injected i.p. with 45 mg/kg mouse body weight BBG ([ab120389; Abcam] in 100 μL of 0.9% saline) to inhibit P2X7R in vivo.
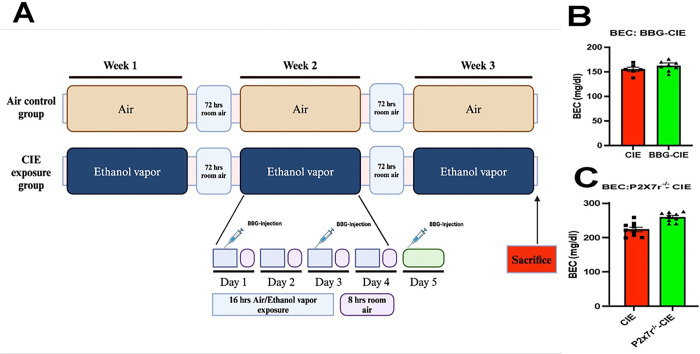
Figure 1. Schematic of the experiment and blood ethanol concentrations (BECs).
(A) CIE exposure paradigm. Created with BioRender.com (B and C) BECs were assessed to ensure that pathophysiologically relevant ethanol levels were obtained at the end of the experiment. The mean BECs were 154.98 ± 10.70 mg/dl, 161 ± 10.19 mg/dL, 223.59 ± 20.15 mg/dL, and 259.73 ± 13.73 mg/dL in the CIE, BBG-CIE, CIE and CIE-P2X7r−/− groups, respectively.
Blood ethanol concentrations
At the end of the experiment, blood ethanol concentrations (BECs) were measured [38]. Blood samples were collected in 0.5 M EDTA (pH 8) through the submandibular vein punch immediately after removal of the mice from the ethanol vapor chamber. The isolated serum samples were subjected to a spectrophotometric enzymatic assay (ECET-100TM Ethanol Assay Kit; BioAssay Systems, San Francisco, USA).
Brain Microvessel Isolation
Mouse brain microvessels were isolated using an earlier published protocol with some modifications. [19, 39, 40]. In brief, the mice were perfused with saline, and the brains were harvested. All the following steps were carried out on ice. Following a wash in PBS and removal of the cerebellum, meninges, and large superficial blood vessels, the right hemisphere of the brain was homogenized in 1 mL of ice-cold Hank’s balanced salt solution (HBSS) using a Dounce homogenizer (357538; Grienger, Philadelphia, USA) (0.25 mm clearance). Overall, the resulting homogenate was centrifuged at 1000 × g for 10 minutes, and the pellet was resuspended in ~ 5 mL of cold 17.5% dextran and centrifuged for 15 minutes at 4400 × g at 4°C. Using a cut tip, the supernatant containing the myelin layer was removed, and the remaining pellet was resuspended in ~ 5 mL of HBSS containing 1% BSA. After the suspension was broken up using a 10 mL pipet in a Petri dish, it was passed through a 100 μm mesh nylon filter. The collected filtrate was passed through a 40 μm mesh nylon filter. The microvessels retained in the filter were collected by inverting the filter and rinsing it with 3 mL of HBSS containing 1% BSA. Finally, after centrifugation, the microvessels were collected and stored at −80°C for further processing.
Gene expression profiling (qRT–PCR)
cDNA was synthesized from 300 ng of total RNA from microvessels using a High-Capacity cDNA Reverse Transcription Kit according to the manufacturer’s instructions. The synthesized cDNA samples were stored at −20°C for later use. Real-time PCR was carried out by using a QuantStudio™ 3 Real-Time PCR System (Thermo Fisher Scientific; Waltham, USA).
qRT–PCR was performed by using the Qiagen Mouse Endothelial Cell Biology RT2 Profiler PCR Array (PAMM-015Z) in combination with RT2 SYBR® Green qPCR Mastermix (Qiagen, USA) according to the manufacturer’s recommendations [41, 42].
Serum proinflammatory markers
Multiplex detection of serum proinflammatory markers was performed using the V-PLEX Proinflammatory Panel 1 Mouse Kit (MSD) (Cat No: K15048D-1; Meso Scale Discovery, Rockville, USA) according to the manufacturer’s instructions [43]. The assay allowed for the measurement of keratinocyte chemoattractant (KC)/human growth-regulated oncogene (GRO) (KC/GRO), TNF-α, interferon gamma (IFN-γ), IL-1β, interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-5 (IL-5), IL-6, interleukin-10 (IL-10), and interleukin-12 p70 (IL12p70). Data from V-PLEX Meso Scale experiments were analyzed based on standard curves included in the respective assays using MSD Discovery Workbench software (DISCOVERY WORKBENCH version 4.0.13).
Mouse glycoprotein (P-gp) and P2X7R levels
Plasma P-glycoprotein (P-gp) levels were determined using a kit-based protocol according to the manufacturer’s instructions (MBS450526, MyBioSource, San Diego, USA). P2X7R ELISA was performed using a mouse purinergic P2X7r ELISA kit (Cat. No. E12339m-American Research Products, Waltham, MA, USA) with some modifications [17]. Circulating P2X7R levels were detected in serum samples collected at the end of harvest. The absorbance was measured at 450 nm using a microplate reader (SpectraMax® M5).
Plasma EV isolation and nanoparticle tracking analysis
EVs from plasma samples were isolated according to a kit-based protocol (cat. no. 4484450; Invitrogen, USA) [44]. Nanoparticle tracking analysis (NTA) of isolated EVs was performed using the NanoSight NS300 system fixed with a 488 nm laser (Malvern Technologies, Malvern, UK). Briefly, EV samples were diluted (1:500) in 1 mL of particle-free Milli-Q water (Milliporesigma, Burlington, USA) and injected into the NanoSight chamber using a 1 mL BD slip-tip syringe (Cat. No. 309659, Franklin Lakes, USA). Prior to running the samples, the machine was calibrated using 100 nm latex beads from Malvern (Cat. No. NTA4088). The data were analyzed by NTA 3.3.104 software [17, 45].
ATP detection in serum and EVs
Extracellular ATP levels in serum samples and EV suspensions were measured using the Luminescent ATP Detection Assay Kit from Abcam (Cat. No. ab113849, Cambridge, UK) in accordance with the manufacturer’s instructions with a few modifications. The EV suspension was subjected to sonication to lyse and then centrifuged at 10,000 rpm for 5 min [17]. Serum samples (35 μL) or EV supernatants (50 μL) were added to a Corning® black clear bottom 96-well plate (Cat. No. 3603, Corning, USA) along with the standards. A total of 50 μL of detergent was added to each well and incubated for 5 min at 600 rpm on an orbital shaker. Then, 50 μL of substrate was added to all the wells, followed by shaking at 600 rpm. The plates were covered and incubated in the dark for 10 min. Finally, luminescence was measured on an Infinite® 200 M PRO (Tecan Austria GmbH).
EV DNA isolation
To remove any DNA affixed to the EV surface, 100 μL of the EV suspension was treated with 10 U of DNase (LGC Biosearch Technologies, Cat. No. DB0715K, Hoddesdon, UK) for 20 min at 37°C. DNase activity was stopped by the addition of 10 μL of 10X DNase stop solution. Following further dilution with 100 μL of nuclease-free water (NFW), the resultant EV suspension was lysed at room temperature using 20 μL of proteinase K (Cat. no. 4485229, Thermo Fisher Scientific; Waltham, USA). The DNeasy® Blood & Tissue Kit from Qiagen (Cat. no. 69506, Hilden, DE) was used to isolate DNA from this EV suspension [17, 46, 47].
EV mtDNA Quantification by Digital PCR
The isolated EV-DNA was diluted to a working concentration of 1 ng/μL with NFW. Mitochondrial gene-specific Taqman™ probes for ATP8 [mt-ATP8] (Cat. no. 4331182 Mm04225236_g1), NADH dehydrogenase 2 [mt-ND2] (Cat. no. 4331182 Mm04225288_s1), cytochrome c oxidase subunit II [mt-COX2] (Cat. no. 4331182 Mm03294838_g1), and 16S ribosomal RNA [mt-RNR2] (Cat. no. 4331182 Mm04260181_s1) were used in this experiment [47] (Thermo Fisher Scientific; Waltham, USA). PCRs were performed using 2 μL of 5X Absolute Q™ DNA Digital PCR Master Mix (Cat. no. A52490), 2 μL of EV-DNA template (2 ng), 0.5 μL of FAM-Taqman™ probe, and 5.5 μL of NFW. A total of 9 μL of the above reaction mixture was loaded onto a QantStudioTMMAP16 Digital PCR plate (Cat. no. 10246917). Following the addition of 15 μL of QuantStudioTM Absolute QTM Isolation Buffer (Cat. no. A52730) to each sample, the wells were sealed using gaskets that were provided with the dPCR plates. The PCR for mtDNA dPCR was as follows: 10 min at 96°C, followed by 40 cycles of 5 s at 96°C and 15 s at 60°C. The QuantStudio™ Absolute Q Digital PCR System and QuantStudio dPCR software were used for DNA amplification, and the number of microchambers with successful mtDNA amplification was counted.
Statistical analysis
The results are expressed as the mean ± SEM. The significance between the groups was assessed using Student’s t test. Multiple group comparisons were performed by one-way analysis of variance (ANOVA) with Tukey’s post hoc test [19]. Statistical analyses were performed utilizing Prism v10.2.1 (339) software (GraphPad Software Inc., La Jolla, CA). p ≤ 0.05 was considered to indicate statistical significance.
Results
BECs
Mice were exposed to ethanol vapors 4 days per week (16 h/day) to ensure that pathophysiologically relevant BECs were generated and maintained throughout the experiment. The observed BECs were 154.98 ± 10.70 mg/dL, 161 ± 10.19 mg/dL, 223.59 ± 20.15 mg/dL, and 259.73 ± 13.73 mg/dL in the CIE, BBG-CIE, CIE, and CIE-P2X7r−/− groups, respectively (Fig. 1B and C).
Effect of alcohol and P2X7R inhibition on gene expression in brain microvessels
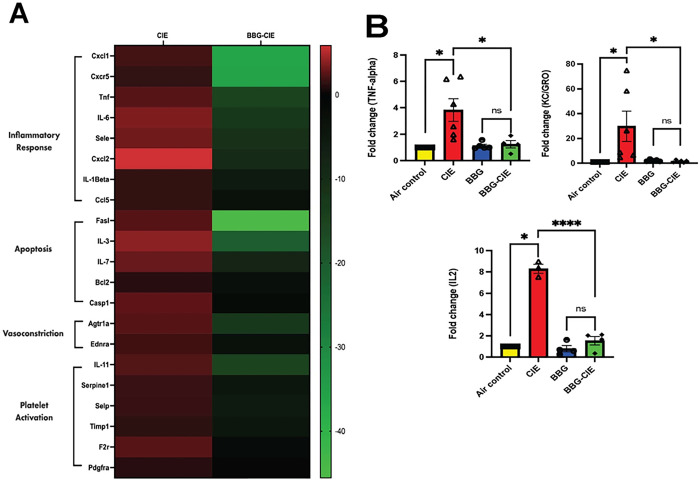
Several studies have used brain microvessels to study the BBB and inflammation in vitro [48, 49]. CIE exposure significantly upregulated the expression profile of genes associated with inflammation (cxcl1, il1b, cxcr5, tnf, il6, sele, cxcl2, and ccl2), apoptosis (fasl, il3, bcl2, casp1, and il7), vasodilation (ednra and agtr1a), and platelet activation (serpine1, selp, timp1, il11, f2r, and pdgfra) in the brain microvessels of CIE-exposed animals. P2X7R inhibition significantly downregulated the CIE-induced neuroinflammatory response by 12–50-fold in the BBG-treated CIE-exposed group (Fig. 2A and Table 1).
Figure 2. P2X7R inhibition downregulated the increase in the expression of genes involved in inflammation, apoptosis, vasodilation, and platelet activation in brain microvessels and serum cytokine levels in BBG-treated CIE-exposed animals.
(A) Heatmap shows the upregulation of genes involved in inflammation, apoptosis, vasodilation, and platelet activation in the brain microvessels of CIE animals. BBG treatment led to significant downregulation of these genes. (B) Cytokine levels after CIE exposure were analyzed by MSD ELISA. The levels of the proinflammatory cytokines TNF-a, KC/GRO, and IL-2 were significantly lower in BBG-treated CIE-exposed animals than in CIE-exposed animals only. A two-tailed t test was used for the statistical analyses. The values are presented as the mean ± SEM;n = 3–7; *p <0.05, and **** p < 0.0001 compared with CIE-exposed mice.
Table 1. Fold changes in the expression of genes involved in inflammation, apoptosis, vasodilation, and platelet activation in the brain microvessels of CIE-exposed animals treated with or without BBG.
The observed values are normalized against the air control group.
| Fold regulation | |||
|---|---|---|---|
| Gene | Gene | CIE-Exposed | BBG-CIE |
| Nppb | Natriuretic peptide type B | 3.04 | −51.23 |
| Pig | Plasminogen | 1.25 | −48.29 |
| Fasl | Fas ligand (TNF superfamily, member 6) | 2.48 | −45.54 |
| Cxcl1 | Chemokine (C-X-C motif) ligand 1 | 2.00 | −36.23 |
| Cxcr5 | Chemokine (C-X-C motif) receptor 5 | 1.45 | −35.94 |
| Mmp1a | Matrix metallopeptidase 1a (interstitial collagenase) | 6.25 | −25.97 |
| Il3 | Interleukin 3 | 3.90 | −20.45 |
| Il11 | Interleukin 11 | 2.33 | −15.05 |
| Tnf | Tumor necrosis factor | 2.49 | −15.18 |
| Agtrla | Angiotensin II receptor, type 1a | 2.46 | −12.56 |
| Il6 | Interleukin 6 | 3.59 | −12.50 |
| Sele | Selectin, endothelial cell | 3.16 | −11.05 |
| Cxcl2 | Chemokine (C-X-C motif) ligand 2 | 5.86 | −9.42 |
| Ccl2 | Chemokine (C-C motif) ligand 2 | 2.72 | −8.66 |
| Il7 | Interleukin 7 | 2.97 | −7.64 |
| Tymp | Thymidine phosphorylase | 1.85 | −4.82 |
| Serpine1 | Serine (or cysteine) peptidase inhibitor, clade E, member 1 | 1.67 | −4.54 |
| Pgf | Placental growth factor | 1.67 | −2.86 |
| Selp | Selectin, platelet | 1.62 | −5.52 |
| Il1b | Interleukin 1 beta | 1.49 | −5.49 |
| Ccl5 | Chemokine (C-C motif) ligand 5 | 1.46 | −3.08 |
| Tmp1 | Tissue inhibitor of metalloproteinase 1 | 1.28 | −4.75 |
| Bcl2 | B-cell leukemia/lymphoma 2 | 1.16 | −2.75 |
| Ednra | Endothelin receptor type A | 1.88 | −2.96 |
| Casp1 | Caspase 1 | 2.69 | −1.54 |
| Mmp9 | Matrix metallopeptidase 9 | 1.58 | −1.59 |
| Tfpi | Tissue factor pathway inhibitor | 2.57 | −1.12 |
| Kit | Kit oncogene | 1.46 | −1.66 |
| Angpt1 | Angiopoietin 1 | 1.45 | −1.39 |
| F2r | Coagulation factor II (thrombin) receptor | 2.49 | −1.85 |
| Plau | Plasminogen activator, urokinase | 1.51 | −1.08 |
| Pdgfra | Platelet derived growth factor receptor, alpha polypeptide | 1.16 | −1.09 |
Modulation of serum cytokine levels by P2X7R inhibition
Analysis of serum cytokine levels using MSD ELISA revealed a significant increase (2–30-fold) in proinflammatory cytokine levels in CIE-exposed animals. A notable reduction in the serum levels of proinflammatory cytokines was observed after P2X7R suppression by BBG in CIE-exposed animals. Significant decreases in TNF-α, KC/GRO, and IL-2 levels were detected in BBG-treated CIE-exposed animals compared with CIE-exposed controls (Fig. 2B). Although not reaching statistical significance, the IL-1β, IFN-γ, and IL-5 levels also exhibited a decreasing trend in BBG-treated animals. A greater level of IL-10 was detected in BBG-treated CIE-exposed animals than in CIE-exposed animals (data not shown).
P2X7R levels are increased in CIE mice
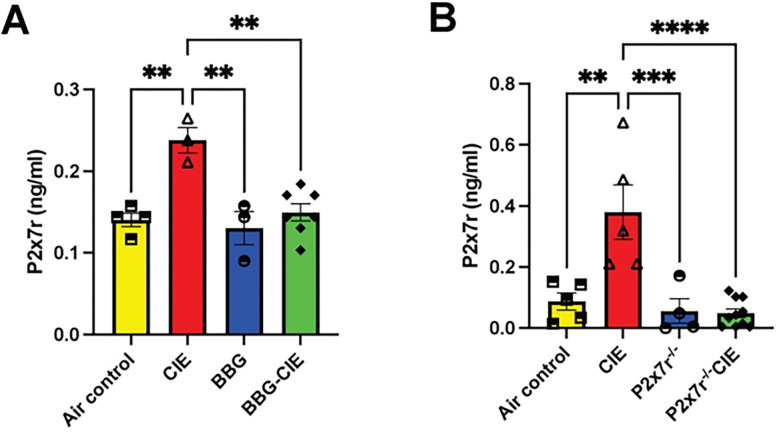
P2X7R shedding has been implicated in chronic inflammation and neurodegenerative diseases (Jiang et al., 2022; Savio et al., 2018; Wang et al., 2017). Earlier in the in vitro study, we found enhanced P2X7R shedding after EtOH exposure [18]. In vivo CIE exposure increased serum P2X7R levels by 2–4-fold compared with the air control group. BBG treatment or P2X7R knockout significantly reduced P2X7R shedding in CIE-exposed mice (Fig. 3A and B).
Figure 3. Serum levels of P2X7R upregulated by CIE were suppressed by receptor inhibition.
A) BBG treatment and B) P2X7R−/− resulted in significantly less P2X7R shedding in the serum than that in the serum of the CIE-exposed mice. One-way ANOVA followed by Tukey’s post hoc test was used for the statistical analyses; ** p ≤ 0.01, ***p ≤ 0.001, **** p < 0.0001 compared with CIE-exposed mice as controls. (n = 3–7, mean ± SEM).
CIE-upregulated P-glycoprotein (P-gp) was not reduced by P2X7R inhibition or genetic knockout
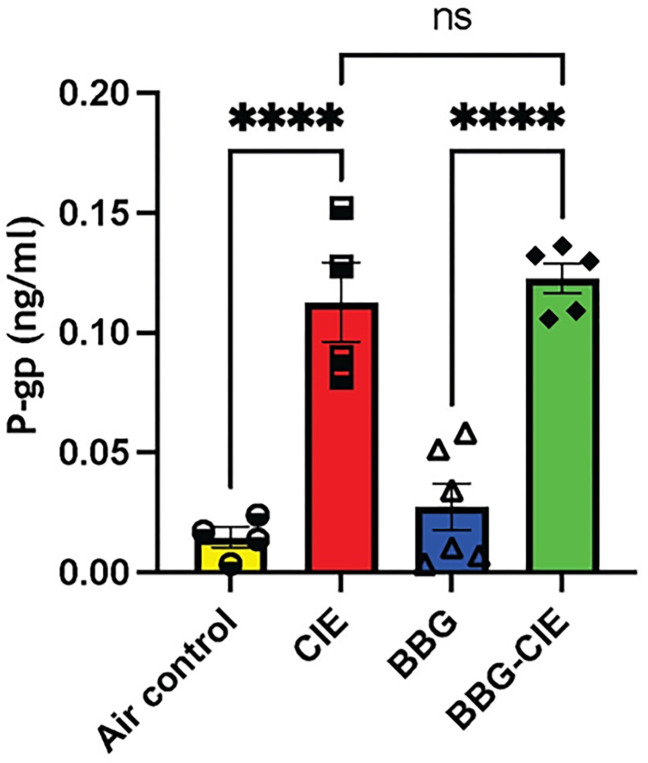
P-gp, an ATP-binding cassette subfamily B member 1 (ABCB1), plays a crucial role in BBB function and is involved in the efflux of toxic compounds back to the bloodstream [50]. It is only expressed on brain endothelium; therefore, it increase in blood indicate BBB injury. Plasma P-gp levels were significantly upregulated in CIE-exposed mice as compared to air-control mice. BBG treatment did not alter P-gp levels in CIE-exposed animals (Fig. 4).
Figure 4. CIE exposure resulted in increased blood levels of P-glycoprotein (P-gp).
The levels of P-gp after CIE exposure were analyzed by ELISA. Compared with those in air-control mice,plasma P-gp levels in CIE-exposed mice were significantly greater. Treatment with BBG had no effect on P-gp levels in BBG-treated CIE-exposed animals. One-way ANOVA followed by Tukey’s post hoc test was used for the statistical analyses. n = 5–7, mean ± SEM and ****p < 0.0001.
The increase in serum ATP and EV-ATP levels induced by CIE was reversed by pharmacologic or genetic P2X7R inhibition
P2X7R overactivation increases ATP concentrations [51]. CIE exposure increased serum ATP levels by 2–6-fold in mice. BBG-treated CIE-exposed mice and P2X7R−/− CIE-exposed mice exhibited significantly lower serum ATP levels than CIE-exposed mice (*p < 0.05, **p < 0.01, and ***p < 0.0001) (Fig. 5A and B).
Figure 5. Serum ATP levels in CIE exposed animals were normalized by pharmacologic or genetic P2X7R inhibition.
Serum ATP levels were lower in (A) BBG-treated CIE-exposed and B) P2X7R−/− CIE-exposed mice than in CIE-exposed mice. One-way ANOVA followed by Tukey’s post hoc test was used for the statistical analyses. N = 5–7, mean ± SEM and *p ≤ 0.05, **p < 0.01, and ***p < 0.0001; ns= nonsignificant. A two-tailed t test was used for the statistical analyses.
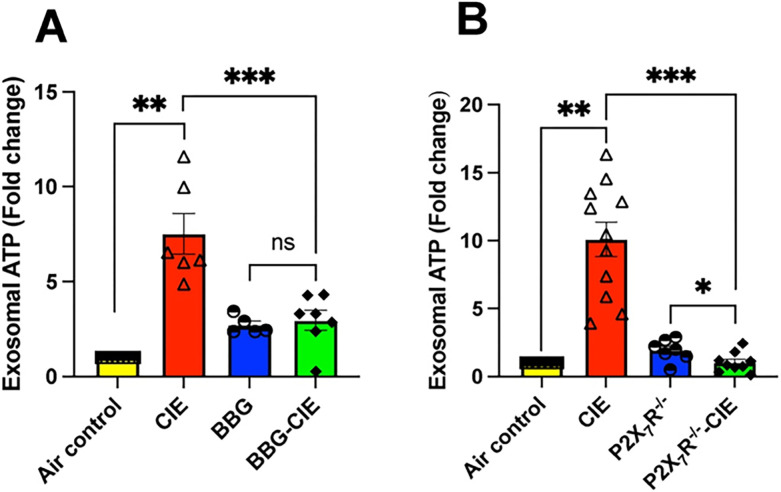
Serum EV-ATP levels were upregulated 7–10-fold in CIE-exposed animals as compared to air control group. Serum EV-ATP levels were significantly downregulated in BBG-treated CIE-exposed mice and CIE-exposed P2X7R−/− mice when compared to in CIE-exposed mice (*p < 0.05, **p < 0.01 and ***p < 0.001) (Fig. 6A and B).
Figure 6. EV ATP content increased in CIE animals were diminished by pharmacologic or genetic P2X7R inhibition.
The serum EV-ATP levels upregulated 7–10-fold by CIE were reduced in (A) BBG-treated CIE-exposed and (B) P2X7R−/− CIE-exposed mice. One-way ANOVA followed by Tukey’s post hoc test was used for the statistical analyses. n = 5–7, mean ± SEM and *p £ 0.05, **p < 0.01 and ***p < 0.001, ns= nonsignificant; two-tailed t test was used for the statistical analyses.
Increased EV numbers in CIE-exposed animals were reduced after P2X7R inhibition
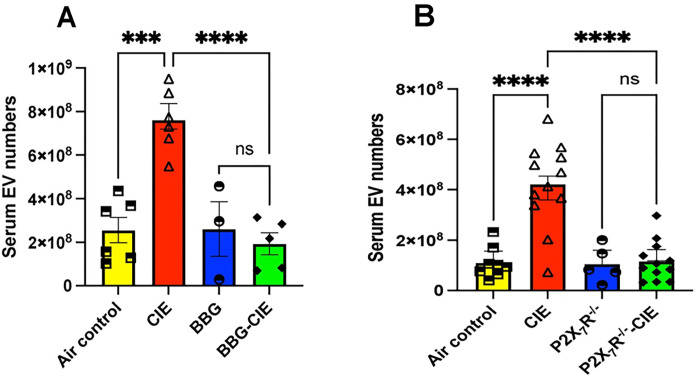
EVs play critical roles in intercellular communication and can transport various bioactive molecules [52]. Here, we investigated the impact of P2X7R inhibition followed during CIE exposure on EV generation and found a 2–4-fold increase in the number of EVs (Fig. 7A and B). We found a significant reduction (p < 0.0001) in the number of EVs in BBG-treated CIE-exposed and CIE-exposed P2X7R−/− mice compared to their respective CIE-exposed controls.
Figure 7. Genetic and pharmacologic P2X7R inhibition reduced EV numbers in CIE exposed mice.
EV numbers were significantly lower in (A) BBG+CIE-exposed and (B) P2X7R−/− CIE-exposed mice than in the respective CIE-exposed mice. One-way ANOVA followed by Tukey’s post hoc test was used for the statistical analyses; ***p < 0.001, **** p < 0.0001, and ns= nonsignificant (n = 5–7, mean ± SEM).
Genetic and pharmacologic P2X7R inhibition downregulated mtDNA copy numbers in EVs
We evaluated the three genes (mt-ND2, mt-ATP8, mt-Cox2) having crucial role mitochondrial respiration and other one (mt-RNR2) part of mitochondrial ribosome. We utilized digital PCR to quantify the copy numbers of mtDNA present in EVs. CIE-exposed mice showed significantly higher mtDNA level in EVs than air-control group which was significantly reduced in the EVs from BBG-treated-CIE-exposed mice. Copy numbers of mt-ND2 and mt-ATP8 were significantly lower in EVs from CIE-exposed P2X7R−/− mice than in EVs isolated from CIE-exposed mice alone (Fig. 8A and B).
Figure 8. Genetic and pharmacologic P2X7R inhibition downregulated mitochondrial gene expression enhanced by CIE.
Digital PCR was used to quantify the copy numbers of mtDNA present in EVs, and bar graphs represent the copy number of genes per microliter of DNA in the experimental groups. (A) P2X7R inhibition or (B) P2X7R−/− knockout reduces mtDNA copy numbers in the EVs isolated from BBG-treated CIE exposed animals and CIE-exposed P2X7R−/− compared to that of EVs from CIE-exposed animals. One-way ANOVA followed by Tukey’s post hoc test were used for the statistical analyses. mean ± SEM, * p ≤ 0.01, **p ≤ 0.001, **** p < 0.0001, and ns= nonsignificant. (n = 3–8)
Discussion
The present study sought to understand the role of P2X7R signaling in neuroinflammatory responses and BBB damage caused by CIE exposure in mice. We report, for the first time, that pharmacological blocking of P2X7R and genetic knockout of P2X7r may diminish CIE-induced BBB injury in vivo.
Chronic alcohol consumption alters the peripheral immune profile, signaling peripheral organs and brain microglia and astrocytes to release pro-inflammatory cytokines [53]. Upon CIE exposure, we noted significant increase in the serum levels of TNF-α, KC/GRO, IL-2, IL-1β, IFN-γ, and IL-5. Notably, increased levels of these cytokines in periphery are associated with alcohol use disorder in humans [54]. IFN-γ acts as potent inducer of TNF-α during neuroinflammation [55, 56]. The increased concentration of KC/GRO has been reported at the time of BBB damage [57]. Alcohol intoxication induced plasma IL-1β and IL-2 in rhesus macaques [58]. In the present study, levels of these cytokines were alleviated by pharmacological blockage and genetic knockout of P2X7R suggesting its crucial role in CIE-induced neuroinflammation (Fig. 2B). Similarly, Asatryan and collogues reported overactivation of the P2X7R and increased mRNA expression of proinflammatory cytokines IL-1B, TNF-α, and IL-6 in the mouse model of chronic EtOH exposure combined with high-fat diet [16, 59].
Studies have shown that BBB dysfunction amplifies neuroinflammation [9]. Brain microvessels serve as an excellent ex vivo model to study the BBB [48, 49, 60, 61]. Targeted blockade of P2X7R serves as a potential path to combat neuroinflammation [62–66]. In experimental autoimmune encephalomyelitis, BBG-dependent P2X7R antagonism resulted in decreased BBB damage with normalized levels of PDGFβR and claudin-5 and pro-inflammatory cytokines, IL-1β, IL-6, and TNF-α [67, 68]. Elevated CCL2 production via MCP-1/CCR2-mediated pathway was followed by P2X7R activation in brain [69]. P2X7R is involved in the caspase-1 activation leading to increased IL-1β and TNF-α levels, which causing apoptosis [70]. We found that BBG-induced P2X7R blockade resulted in a 2–50-fold downregulation of genes associated with inflammation (cxcl1, il1b, cxcr5, tnf, il6, sele, cxcl2, and ccl2), apoptosis (fasl, il3, bcl2, casp1, and il7), vasodilation (ednra and agtr1a), and platelet activation (serpine1, selp, timp1, il11, f2r, and pdgfra) (Table 1, Fig. 2A). These changes in gene expression in BBG-treated-CIE-exposed mice underscore the significant impact of P2X7R inhibition on the transcriptional landscape within brain microvessels during chronic EtOH exposure.
In chronic inflammation and neurodegenerative diseases, enhanced P2X7R shedding has been observed [71–73]. Additionally, in vitro dendritic cell P2X7R stimulation with ATP can trigger shedding of microvesicles carrying the P2X7R itself, suggesting a regulatory role of P2X7R signaling in its own shedding process [74]. Earlier, we showed that in vitro treatment of lung epithelial cells with EtOH increased P2X7R shedding [18]. In this study, we observed a substantial increase in the circulatory P2X7R levels in CIE-exposed animals, which was significantly reduced pharmacologic or genetic P2X7R inhibition (Fig. 3A and B). The observed results add to the growing body of evidence, implicating P2X7R shedding in the regulation of P2X7R signaling and activity.
P-glycoprotein (P-gp) is an efflux transporter with a crucial role in the transport of substances across the BBB. Chronic alcohol exposure significantly increases P-gp mRNA and protein expression in vitro [75]. TNF-α and endothelin-1 exposure also stimulates P-gp expression [76]. We found a significant increase in P-gp levels in the blood of CIE-exposed mice reflecting BBB injury. However, treatment with BBG did not alter P-gp levels in CIE-exposed animals, suggesting that P2X7R signaling may not regulate P-gp expression (Fig. 4). More recently, Arnaud-Sampaio and colleagues have shown that P2X7R isoform B has higher efflux activity than P2X7R A, which may be mediated by P-gp and other ABC transporters [77].
P2X7R are ATP-gated cation channel receptors and undisputedly serve as gatekeepers of inflammation [63, 78]. P2X7R-dependent ATP release contributes to increased extracellular ATP levels in osteoclast and osteoblast cultures, highlighting autocrine/paracrine role of P2X7R signaling [79]. Studies have highlighted the importance of P2X7R-mediated ATP release in initiating and amplifying inflammatory responses in the CNS and peripheral tissues [80]. Similarly, we found increased serum ATP level in CIE-exposed mice. The BBG-treated or P2X7R−/−-CIE-exposed mice exhibited significantly lower serum ATP levels compared to CIE-exposed mice (Fig. 5). This suggests that inhibition or genetic knockout of the P2X7R reduces eATP release, a neuroinflammatory messenger, mitigating neuroinflammatory responses associated with chronic alcohol exposure [81].
Several studies have reported that P2X7R stimulation by ATP triggers EV shedding with significant change in their size [20, 21]. Moreover, EV cargo composition in various cell types, including immune cells and neurons is influenced by P2X7R stimulation by ATP [22–24, 82]. In the context of chronic alcohol exposure, limited research has explored the role of P2X7R in EV regulation and its implications for alcohol-induced pathology. Pfeiffer and colleagues have noted that ATP-dependent P2X7R activation results in P38-MAPK-facilitated cytoskeletal restructuring, leading to EV release [83]. We found a drastically increased number of EVs in CIE-exposed mice, which was significantly lowered in both BBG-treated and P2X7R−/− CIE-exposed mice (Fig. 7). These data indicate a potential role of P2X7R in EV biogenesis and secretion due to alcohol exposure in vivo. The mechanism of increasing EV generation and their content changes are of considerable interest to investigate.
Interestingly, Ibáñez and collogues have reported EtOH-induced EV secretion with significant alterations in lipid metabolism and EV enrichment with inflammatory-related proteins and miRNAs in BV2 microglia and astrocytes [25, 84]. Studies have reported changes in EV composition after alcohol exposure in liver and lung cells [85, 86]. EV-mediated ATP signaling plays an important role in regulating inflammatory responses and immune cell activation [87, 88]. Along with other components, ATP itself is present in released EVs [23, 89]. Our study showed reduced serum EV-ATP levels following P2X7R inhibition or knockout in CIE-exposed mice (Fig. 6), potentially mitigating the pro-inflammatory and pathological effects associated with chronic alcohol exposure.
EVs can cross BBB, carry exchange between CNS and blood, and regulate neuroinflammation [90–92]. P2X7R overactivation leads to mitochondrial damage, causing the release of mitochondrial content, including Ca2+, ATP, and mtDNA into the extracellular environment. [17, 93]. Chronic alcohol exposure of hepatocyte causes release of EVs, containing mtDNA fragments, which act as DAMPs with proinflammatory properties, activating autocrine and paracrine signaling pathways [28]. Of note, EtOH-induced mtDNA damage is known to be involved in the release of exosomes enriched with damaged mtDNA in vitro [94]. Recent reports have demonstrated the presence of mtDNA fragments in hepatocyte-derived EVs after EtOH-exposure in vivo and in patients with fatty liver conditions [26, 27]. Our prior study showed that P2X7R inhibition reduces mtDNA copy numbers in EVs from EtOH-treated lung epithelial cells in vitro [18]. In this study, we report a significant increase in mtDNA copy numbers in isolated EVs from CIE-exposed animals (Fig. 8A, B), whereas P2X7R inhibition or knockout significantly reduced mtDNA content in CIE-exposed mice. It is known that cytosolic mtDNA-mediated NLRP3 inflammasome activation is associated with caspase-1 activation and IL-1β/IL-18 production [95, 96]. The increased expression of caspase-1 and IL-1β (Fig. 2A, Table 1) in isolated brain microvesicles further supports this observation. Additionally, mtDNA is known to activate TLR9-MyD88 downstream signaling, causing NF-κB activation, which triggers the production of pro-inflammatory cytokines and chemokines [97, 98]. Increased mtDNA concentration in isolated EVs and significantly increased gene expression of tnf-α, il-6, il-1β, cxcl1, cxcl2, and cxcr5 from EtOH-exposed groups corroborate these findings (Fig. 2A, Table 1). In earlier studies, the presence of mtDNA in EVs was detected using gene-specific probes for mt-ND2, mt-ATP8, mt-Cox2, and mt-RNR2 [46, 99]. These genes are involved in mitochondrial respiration [100]. In the present study, significantly increased mtDNA concentration in EVs and proinflammatory changes in the periphery and BBB of CIE-exposed group suggest that the mtDNA carried by EVs acts as DAMP and may cause an exacerbation of neuroinflammation [99, 101].
Our observations imply that P2X7R signaling may play a role in regulating EV release and their cargo contents. While the specific mechanisms underlying P2X7R-mediated regulation of mitochondrial stress and escape of mtDNA in EVs remain to be fully elucidated, our findings suggest a potential link between P2X7R signaling, mitochondrial dysfunction, and EV dynamics in the context of chronic alcohol exposure and neuroinflammation. Future studies of the mechanism of EV release, link to P2X7R activation, and cross organ communication by EVs may pave the way to future treatment interventions.
Conclusion
The present study delved into understanding the impact of P2X7R signaling on neuroinflammatory responses and BBB injury induced by CIE exposure. Blockade of P2X7R channels resulted in reduced eATP release, enhanced BBB function, and downregulation of genes associated with inflammation, apoptosis, vasodilation, and platelet activation, underscoring the critical role of P2X7R in CIE-induced neuroinflammation. Furthermore, inhibition or genetic knockout of P2X7R led to altered EV dynamics, such as reduced quantity and eATP and mtDNA levels, suggesting a potential regulatory role of P2X7R signaling in mitigating chronic alcohol-induced pro-inflammatory effects associated with EVs. These findings contribute to understanding the complex interplay between P2X7R signaling, peripheral and neuro-inflammation, BBB integrity, and circulating EV biology in the context of in vivo chronic alcohol exposure.
Acknowledgements
Parts of some figures were created with Biorender.com.
Funding
This work was supported by funding from The National Institute of Health (R01 DA040619, 1R01AA030841 (Y. P.)
Abbreviations
- BBB
Blood–brain barrier
- EV
Extracellular vesicle
- CIE
chronic intermittent ethanol (CIE)
- BBG
Brilliant Blue G
- BEC
Blood ethanol concentration
- eATP
Extracellular ATP
- P7X7R
Purinergic receptor P2X7
- DAMPs
Damage-associated molecular patterns
- NLRP3
Nod-like receptor pyrin domain containing 3
- EtOH
Ethanol
- BMVECs
Brain microvascular endothelial cells
- mtDNA
Mitochondrial DNA
- mt-ATP-8
Mitochondrially encoded ATP synthase membrane subunit 8
- mt-ND2
NADH dehydrogenase 2
- mt-COX2
Cytochrome c oxidase subunit II
- mt-RNR2
16S ribosomal RNA
- HBSS
Hank’s balanced salt solution
- P-gp
P-glycoprotein
- KC/GRO
Keratinocyte chemoattractant (KC)/human growth-regulated oncogene (GRO)
- TNF-α
Tumor necrosis factor alpha
- IFN-γ
Interferon gamma
- IL-1β
Interleukin 1 beta
- IL-2
Interleukin-2
- IL-4
Interleukin-4
- IL-5
Interleukin-5
- IL-6
Interleukin-6
- IL-10
Interleukin-10
- IL12p70
Interleukin-12 p70
Footnotes
Conflict of interest
None of the authors has any potential financial conflict of interest related to this manuscript.
Additional Declarations: No competing interests reported.
Ethics declarations
All procedures were performed in accordance with the National Institutes of Health Guideline for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the Temple University (IACUC: 5142)
Consent for publication
Not applicable.
Contributor Information
Namdev S. Togre, Temple University
Naveen Melaka, Temple University.
Priyanka S. Bhoj, Temple University
Nikhita Mogadala, Temple University.
Malika Winfield, Temple University.
Jayshil Trivedi, Temple University.
Deborah Grove, Temple University.
Sudhir Kotnala, Temple University.
Slava S Rom, Temple University.
Uma Sriram, Temple University.
Yuri Persidsky, Temple University.
Data availability
All data generated or analyzed during this study are included in this published article or its supplementary information files.
References
- 1.Global status report on alcohol and health. 2018. In. Edited by Poznyak V, D. R.
- 2.Zhang W, Liu R, Chen Y, Wang M, Du J. Crosstalk between Oxidative Stress and Exosomes. Oxid Med Cell Longev 2022, 2022:3553617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pervin Z, Stephen JM. Effect of alcohol on the central nervous system to develop neurological disorder: pathophysiological and lifestyle modulation can be potential therapeutic options for alcohol-induced neurotoxication. AIMS Neurosci. 2021;8(3):390–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrino D, Branca JJV, Becatti M, Paternostro F, Morucci G, Gulisano M, Di Cesare Mannelli L, Pacini A. Alcohol-Induced Blood-Brain Barrier Impairment: An In Vitro Study. Int J Environ Res Public Health. 2021;18(5):2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.León BE, Kang S, Franca-Solomon G, Shang P, Choi DS. Alcohol-Induced Neuroinflammatory Response and Mitochondrial Dysfunction on Aging and Alzheimer’s Disease. Front Behav Neurosci. 2021;15:778456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montagne A, Barnes Samuel R, Sweeney Melanie D, Halliday Matthew R, Sagare Abhay P, Zhao Z, Toga Arthur W, Jacobs Russell E, Liu Collin Y, Amezcua L, et al. Blood-Brain Barrier Breakdown in the Aging Human Hippocampus. Neuron. 2015;85(2):296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei J, Dai Y, Wen W, Li J, Ye LL, Xu S, Duan DD. Blood-brain barrier integrity is the primary target of alcohol abuse. Chemico-Biol Interact. 2021;337:109400. [DOI] [PubMed] [Google Scholar]
- 8.Huang X, Hussain B, Chang J. Peripheral inflammation and blood-brain barrier disruption: effects and mechanisms. CNS Neurosci Ther. 2021;27(1):36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takata F, Nakagawa S, Matsumoto J, Dohgu S. Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction. Front Cell Neurosci. 2021;15:661838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnstock G. Introduction to Purinergic Signalling in the Brain. In: Glioma Signaling. Edited by Barańska J. Cham: Springer International Publishing; 2020: 1–12. [Google Scholar]
- 11.Illes P, Xu G-Y, Tang Y. Purinergic Signaling in the Central Nervous System in Health and Disease. Neurosci Bull. 2020;36(11):1239–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sedgwick AE, D’Souza-Schorey C. The biology of extracellular microvesicles. Traffic. 2018;19(5):319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliveira-Giacomelli Á, Petiz LL, Andrejew R, Turrini N, Silva JB, Sack U, Ulrich H. Role of P2X7 Receptors in Immune Responses During Neurodegeneration. Front Cell Neurosci 2021, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gicquel T, Victoni T, Fautrel A, Robert S, Gleonnec F, Guezingar M, Couillin I, Catros V, Boichot E, Lagente V. Involvement of purinergic receptors and NOD-like receptor-family protein 3-inflammasome pathway in the adenosine triphosphate-induced cytokine release from macrophages. Clin Exp Pharmacol Physiol. 2014;41(4):279–86. [DOI] [PubMed] [Google Scholar]
- 15.Le Daré B, Ferron P-J, Gicquel T. The Purinergic P2X7 Receptor-NLRP3 Inflammasome Pathway: A New Target in Alcoholic Liver Disease? Int J Mol Sci. 2021;22(4):2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asatryan L, Ostrovskaya O, Lieu D, Davies DL. Ethanol differentially modulates P2X4 and P2X7 receptor activity and function in BV2 microglial cells. Neuropharmacology. 2018;128:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mekala N, Gheewala N, Rom S, Sriram U, Persidsky Y. Blocking of P2X7r Reduces Mitochondrial Stress Induced by Alcohol and Electronic Cigarette Exposure in Brain Microvascular Endothelial Cells. Antioxidants. 2022;11(7):1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mekala N, Trivedi J, Bhoj P, Togre N, Rom S, Sriram U, Persidsky Y. Alcohol and e-cigarette damage alveolar-epithelial barrier by activation of P2X7r and provoke brain endothelial injury via extracellular vesicles. Cell Communication Signal. 2024;22(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heldt NA, Seliga A, Winfield M, Gajghate S, Reichenbach N, Yu X, Rom S, Tenneti A, May D, Gregory BD, et al. Electronic cigarette exposure disrupts blood-brain barrier integrity and promotes neuroinflammation. Brain Behav Immun. 2020;88:363–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carotti V, Rigalli JP, van Asbeck-van der Wijst J, Hoenderop JGJ. Interplay between purinergic signalling and extracellular vesicles in health and disease. Biochem Pharmacol. 2022;203:115192. [DOI] [PubMed] [Google Scholar]
- 21.Drago F, Lombardi M, Prada I, Gabrielli M, Joshi P, Cojoc D, Franck J, Fournier I, Vizioli J, Verderio C. ATP Modifies the Proteome of Extracellular Vesicles Released by Microglia and Influences Their Action on Astrocytes. Front Pharmacol. 2017;8:910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golia MT, Gabrielli M, Verderio C. P2X(7) Receptor and Extracellular Vesicle Release. Int J Mol Sci 2023, 24(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lombardi M, Gabrielli M, Adinolfi E, Verderio C. Role of ATP in Extracellular Vesicle Biogenesis and Dynamics. Front Pharmacol 2021, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pegoraro A, De Marchi E, Ferracin M, Orioli E, Zanoni M, Bassi C, Tesei A, Capece M, Dika E, Negrini M, et al. P2X7 promotes metastatic spreading and triggers release of miRNA-containing exosomes and microvesicles from melanoma cells. Cell Death Dis. 2021;12(12):1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ibáñez F, Montesinos J, Area-Gomez E, Guerri C, Pascual M. Ethanol Induces Extracellular Vesicle Secretion by Altering Lipid Metabolism through the Mitochondria-Associated ER Membranes and Sphingomyelinases. Int J Mol Sci 2021, 22(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyapati RK, Tamborska A, Dorward DA, Ho GT. Advances in the understanding of mitochondrial DNA as a pathogenic factor in inflammatory diseases. F1000Res. 2017;6:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samuvel DJ, Li L, Krishnasamy Y, Gooz M, Takemoto K, Woster PM, Lemasters JJ, Zhong Z. Mitochondrial depolarization after acute ethanol treatment drives mitophagy in living mice. Autophagy. 2022;18(11):2671–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Mambro T, Pellielo G, Agyapong ED, Carinci M, Chianese D, Giorgi C, Morciano G, Patergnani S, Pinton P, Rimessi A. The Tricky Connection between Extracellular Vesicles and Mitochondria in Inflammatory-Related Diseases. Int J Mol Sci. 2023;24(9):8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao H, Zhang X, Dai Z, Feng Y, Li Q, Zhang JH, Liu X, Chen Y, Feng H. P2X7 Receptor Suppression Preserves Blood-Brain Barrier through Inhibiting RhoA Activation after Experimental Intracerebral Hemorrhage in Rats. Sci Rep. 2016;6:23286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Zhu Y, Wang J, Dong L, Liu S, Li S, Wu Q. Purinergic signaling: A gatekeeper of blood-brain barrier permeation. Front Pharmacol. 2023;14:1112758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao B, Pei J, Li H, Yang G, Shi X, Zhang Z, Wang H, Zheng Z, Liu Y, Zhang J. Inhibition of P2X7R alleviates neuroinflammation and brain edema after traumatic brain injury by suppressing the NF-κB/NLRP3 inflammasome pathway. J Neurorestoratology 2024:100106. [Google Scholar]
- 32.Yang F, Zhao K, Zhang X, Zhang J, Xu B. ATP Induces Disruption of Tight Junction Proteins via IL-1 Beta-Dependent MMP-9 Activation of Human Blood-Brain Barrier < i > In Vitro. Neural Plasticity 2016, 2016:8928530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahn YH, Tang Y, Illes P. The neuroinflammatory astrocytic P2X7 receptor: Alzheimer’s disease, ischemic brain injury, and epileptic state. Expert Opin Ther Targets. 2023;27(9):763–78. [DOI] [PubMed] [Google Scholar]
- 34.Becker HC. Positive relationship between the number of prior ethanol withdrawal episodes and the severity of subsequent withdrawal seizures. Psychopharmacology. 1994;116(1):26–32. [DOI] [PubMed] [Google Scholar]
- 35.Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28(12):1829–38. [DOI] [PubMed] [Google Scholar]
- 36.Karkhanis AN, Rose JH, Huggins KN, Konstantopoulos JK, Jones SR. Chronic intermittent ethanol exposure reduces presynaptic dopamine neurotransmission in the mouse nucleus accumbens. Drug Alcohol Depend. 2015;150:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carrara-Nascimento PF, Lopez MF, Becker HC, Olive MF, Camarini R. Similar Ethanol Drinking in Adolescent and Adult C57BL/6J Mice After Chronic Ethanol Exposure and Withdrawal. Alcoholism: Clin Experimental Res. 2013;37(6):961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffin WC 3rd, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin Exp Res. 2009;33(11):1893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yousif S, Marie-Claire C, Roux F, Scherrmann JM, Declèves X. Expression of drug transporters at the blood-brain barrier using an optimized isolated rat brain microvessel strategy. Brain Res. 2007;1134(1):1–11. [DOI] [PubMed] [Google Scholar]
- 40.Hartz AM, Notenboom S, Bauer B. Signaling to P-glycoprotein-A new therapeutic target to treat drug-resistant epilepsy? Drug News Perspect. 2009;22(7):393–7. [DOI] [PubMed] [Google Scholar]
- 41.Mohd Abd Razak MR, Norahmad NA, Md Jelas NH, Jusoh B, Muhammad A, Mohmad Misnan N, Zainol M, Thayan R, Syed Mohamed AF. Preliminary study on the expression of endothelial cell biology related genes in the liver of dengue virus infected mice treated with Carica papaya leaf juice. BMC Res Notes. 2019;12(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olson SA, Osborn BK, Cotton ME, Krocker JD, Koami H, White N, Cardenas JC. Fibrinogen Fragment X Mediates Endothelial Barrier Disruption via Suppression of VE-Cadherin. J Surg Res. 2024;293:639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Bruggen S, Kraisin S, Van Wauwe J, Bomhals K, Stroobants M, Carai P, Frederix L, Van De Bruaene A, Witsch T, Martinod K. Neutrophil peptidylarginine deiminase 4 is essential for detrimental age-related cardiac remodelling and dysfunction in mice. Philosophical Trans Royal Soc B: Biol Sci. 2023;378(1890):20220475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kao Y-C, Chang Y-W, Lai CP, Chang N-W, Huang C-H, Chen C-S, Huang H-C, Juan H-F. Ectopic ATP synthase stimulates the secretion of extracellular vesicles in cancer cells. Commun Biology. 2023;6(1):642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soo CY, Song Y, Zheng Y, Campbell EC, Riches AC, Gunn-Moore F, Powis SJ. Nanoparticle tracking analysis monitors microvesicle and exosome secretion from immune cells. Immunology. 2012;136(2):192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazo S, Noren Hooten N, Green J, Eitan E, Mode NA, Liu QR, Zonderman AB, Ezike N, Mattson MP, Ghosh P, et al. Mitochondrial DNA in extracellular vesicles declines with age. Aging Cell. 2021;20(1):e13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Byappanahalli AM, Noren Hooten N, Vannoy M, Mode NA, Ezike N, Zonderman AB, Evans MK. Mitochondrial DNA and inflammatory proteins are higher in extracellular vesicles from frail individuals. Immun Ageing. 2023;20(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pardridge WM. The Isolated Brain Microvessel: A Versatile Experimental Model of the Blood-Brain Barrier. Front Physiol 2020, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Linville RM, Sklar MB, Grifno GN, Nerenberg RF, Zhou J, Ye R, DeStefano JG, Guo Z, Jha R, Jamieson JJ, et al. Three-dimensional microenvironment regulates gene expression, function, and tight junction dynamics of iPSC-derived blood–brain barrier microvessels. Fluids Barriers CNS. 2022;19(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chai AB, Callaghan R, Gelissen IC. Regulation of P-Glycoprotein in the Brain. Int J Mol Sci 2022, 23(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andrejew R, Oliveira-Giacomelli Á, Ribeiro DE, Glaser T, Arnaud-Sampaio VF, Lameu C, Ulrich H. The P2X7 Receptor: Central Hub of Brain Diseases. Front Mol Neurosci 2020, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu YJ, Wang C. A review of the regulatory mechanisms of extracellular vesicles-mediated intercellular communication. Cell Commun Signal. 2023;21(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neupane SP. Neuroimmune Interface in the Comorbidity between Alcohol Use Disorder and Major Depression. Front Immunol. 2016;7:655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adams C, Conigrave JH, Lewohl J, Haber P, Morley KC. Alcohol use disorder and circulating cytokines: A systematic review and meta-analysis. Brain Behav Immun. 2020;89:501–12. [DOI] [PubMed] [Google Scholar]
- 55.Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40(2):140–55. [DOI] [PubMed] [Google Scholar]
- 56.Mangano EN, Litteljohn D, So R, Nelson E, Peters S, Bethune C, Bobyn J, Hayley S. Interferon-γ plays a role in paraquat-induced neurodegeneration involving oxidative and proinflammatory pathways. Neurobiol Aging. 2012;33(7):1411–26. [DOI] [PubMed] [Google Scholar]
- 57.Shigemoto-Mogami Y, Hoshikawa K, Sato K. Activated Microglia Disrupt the Blood-Brain Barrier and Induce Chemokines and Cytokines in a Rat in vitro Model. Front Cell Neurosci 2018, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asquith M, Pasala S, Engelmann F, Haberthur K, Meyer C, Park B, Grant KA, Messaoudi I. Chronic Ethanol Consumption Modulates Growth Factor Release, Mucosal Cytokine Production, and MicroRNA Expression in Nonhuman Primates. Alcoholism: Clin Experimental Res. 2014;38(4):980–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asatryan L, Khoja S, Rodgers KE, Alkana RL, Tsukamoto H, Davies DL. Chronic ethanol exposure combined with high fat diet up-regulates P2X7 receptors that parallels neuroinflammation and neuronal loss in C57BL/6J mice. J Neuroimmunol. 2015;285:169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hofman P, Hoyng P, vanderWerf F, Vrensen GFJM, Schlingemann RO. Lack of Blood–Brain Barrier Properties in Microvessels of the Prelaminar Optic Nerve Head. Investig Ophthalmol Vis Sci. 2001;42(5):895–901. [PubMed] [Google Scholar]
- 61.Allt G, Lawrenson JG. Is the pial microvessel a good model for blood-brain barrier studies? Brain Res Brain Res Rev. 1997;24(1):67–76. [DOI] [PubMed] [Google Scholar]
- 62.Huang C, Chi X-s, Li R, Hu X, Xu H-x, Li J-m, Zhou D. Inhibition of P2X7 Receptor Ameliorates Nuclear Factor-Kappa B Mediated Neuroinflammation Induced by Status Epilepticus in Rat Hippocampus. J Mol Neurosci. 2017;63(2):173–84. [DOI] [PubMed] [Google Scholar]
- 63.Wang M, Deng X, Xie Y, Chen Y. Astaxanthin Attenuates Neuroinflammation in Status Epilepticus Rats by Regulating the ATP-P2X7R Signal. Drug Des Devel Ther. 2020;14(null):1651–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen X, Hu J, Jiang L, Xu S, Zheng B, Wang C, Zhang J, Wei X, Chang L, Wang Q. Brilliant Blue G improves cognition in an animal model of Alzheimer’s disease and inhibits amyloid-β-induced loss of filopodia and dendrite spines in hippocampal neurons. Neuroscience. 2014;279:94–101. [DOI] [PubMed] [Google Scholar]
- 65.Calzaferri F, Ruiz-Ruiz C, de Diego AMG, de Pascual R, Méndez-López I, Cano-Abad MF, Maneu V, de los Ríos C, Gandía L, García AG. The purinergic P2X7 receptor as a potential drug target to combat neuroinflammation in neurodegenerative diseases. Med Res Rev. 2020;40(6):2427–65. [DOI] [PubMed] [Google Scholar]
- 66.Monif M, Reid CA, Powell KL, Drummond KJ, O’Brien TJ, Williams DA. Interleukin-1β has trophic effects in microglia and its release is mediated by P2X7R pore. J Neuroinflamm. 2016;13(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grygorowicz T, Dąbrowska-Bouta B, Strużyńska L. Administration of an antagonist of P2X7 receptor to EAE rats prevents a decrease of expression of claudin-5 in cerebral capillaries. Purinergic Signal. 2018;14(4):385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharp AJ, Polak PE, Simonini V, Lin SX, Richardson JC, Bongarzone ER, Feinstein DL. P2×7 deficiency suppresses development of experimental autoimmune encephalomyelitis. J Neuroinflammation. 2008;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Inose Y, Kato Y, Kitagawa K, Uchiyama S, Shibata N. Activated microglia in ischemic stroke penumbra upregulate MCP-1 and CCR2 expression in response to lysophosphatidylcholine derived from adjacent neurons and astrocytes. Neuropathology. 2015;35(3):209–23. [DOI] [PubMed] [Google Scholar]
- 70.Zhao H, Chen Y, Feng H. P2X7 Receptor-Associated Programmed Cell Death in the Pathophysiology of Hemorrhagic Stroke. Curr Neuropharmacol. 2018;16(9):1282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang ZF, Wu W, Hu HB, Li ZY, Zhong M, Zhang L. P2X7 receptor as the regulator of T-cell function in intestinal barrier disruption. World J Gastroenterol. 2022;28(36):5265–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Savio LEB, de Andrade Mello P, da Silva CG, Coutinho-Silva R. The P2X7 Receptor in Inflammatory Diseases: Angel or Demon? Front Pharmacol. 2018;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang XH, Xie X, Luo XG, Shang H, He ZY. Inhibiting purinergic P2X7 receptors with the antagonist brilliant blue G is neuroprotective in an intranigral lipopolysaccharide animal model of Parkinson’s disease. Mol Med Rep. 2017;15(2):768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pizzirani C, Ferrari D, Chiozzi P, Adinolfi E, Sandonà D, Savaglio E, Di Virgilio F. Stimulation of P2 receptors causes release of IL-1beta-loaded microvesicles from human dendritic cells. Blood. 2007;109(9):3856–64. [DOI] [PubMed] [Google Scholar]
- 75.Theile D, Schmidt TT, Haefeli WE, Weiss J. In-vitro evaluation of chronic alcohol effects on expression of drug-metabolizing and drug-transporting proteins. J Pharm Pharmacol. 2013;65(10):1518–25. [DOI] [PubMed] [Google Scholar]
- 76.Bauer B, Hartz AMS, Miller DS. Tumor Necrosis Factor α and Endothelin-1 Increase P-Glycoprotein Expression and Transport Activity at the Blood-Brain Barrier. Mol Pharmacol. 2007;71(3):667–75. [DOI] [PubMed] [Google Scholar]
- 77.Arnaud-Sampaio VF, Bento CA, Glaser T, Adinolfi E, Ulrich H, Lameu C. P2X7 receptor isoform B is a key drug resistance mediator for neuroblastoma. Front Oncol 2022, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhattacharya A, Biber K. The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia. 2016;64(10):1772–87. [DOI] [PubMed] [Google Scholar]
- 79.Brandao-Burch A, Key ML, Patel JJ, Arnett TR, Orriss IR. The P2X7 Receptor is an Important Regulator of Extracellular ATP Levels. Front Endocrinol 2012, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 Receptor in Infection and Inflammation. Immunity. 2017;47(1):15–31. [DOI] [PubMed] [Google Scholar]
- 81.Di Virgilio F, Vultaggio-Poma V, Falzoni S, Giuliani AL. Extracellular ATP: A powerful inflammatory mediator in the central nervous system. Neuropharmacology. 2023;224:109333. [DOI] [PubMed] [Google Scholar]
- 82.Bianco F, Pravettoni E, Colombo A, Schenk U, Möller T, Matteoli M, Verderio C. Astrocyte-Derived ATP Induces Vesicle Shedding and IL-1β Release from Microglia1. J Immunol. 2005;174(11):7268–77. [DOI] [PubMed] [Google Scholar]
- 83.Pfeiffer ZA, Aga M, Prabhu U, Watters JJ, Hall DJ, Bertics PJ. The nucleotide receptor P2X7 mediates actin reorganization and membrane blebbing in RAW 264.7 macrophages via p38 MAP kinase and Rho. J Leukoc Biol. 2004;75(6):1173–82. [DOI] [PubMed] [Google Scholar]
- 84.Ibáñez F, Montesinos J, Ureña-Peralta JR, Guerri C, Pascual M. TLR4 participates in the transmission of ethanol-induced neuroinflammation via astrocyte-derived extracellular vesicles. J Neuroinflammation. 2019;16(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Andres J, Smith LC, Murray A, Jin Y, Businaro R, Laskin JD, Laskin DL. Role of extracellular vesicles in cell-cell communication and inflammation following exposure to pulmonary toxicants. Cytokine Growth Factor Rev. 2020;51:12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rahman MA, Patters BJ, Kodidela S, Kumar S. Extracellular Vesicles: Intercellular Mediators in Alcohol-Induced Pathologies. J Neuroimmune Pharmacol. 2020;15(3):409–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dave KM, Zhao W, Hoover C, D’Souza A, Manickam S. Extracellular Vesicles Derived from a Human Brain Endothelial Cell Line Increase Cellular ATP Levels. AAPS PharmSciTech. 2021;22(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baxter AA. Stoking the Fire: How Dying Cells Propagate Inflammatory Signalling through Extracellular Vesicle Trafficking. Int J Mol Sci. 2020;21(19):7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Graner MW. Extracellular vesicles in cancer immune responses: roles of purinergic receptors. Semin Immunopathol. 2018;40(5):465–75. [DOI] [PubMed] [Google Scholar]
- 90.Banks WA, Sharma P, Bullock KM, Hansen KM, Ludwig N, Whiteside TL. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int J Mol Sci. 2020;21(12):4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Upadhya D, Shetty AK. Promise of extracellular vesicles for diagnosis and treatment of epilepsy. Epilepsy Behav. 2021;121(Pt B):106499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Upadhya D, Shetty AK. Extracellular Vesicles as Therapeutics for Brain Injury and Disease. Curr Pharm Des. 2019;25(33):3500–5. [DOI] [PubMed] [Google Scholar]
- 93.Martínez-García JJ, Martínez-Banaclocha H, Angosto-Bazarra D, de Torre-Minguela C, Baroja-Mazo A, Alarcón-Vila C, Martínez-Alarcón L, Amores-Iniesta J, Martín-Sánchez F, Ercole GA, et al. P2X7 receptor induces mitochondrial failure in monocytes and compromises NLRP3 inflammasome activation during sepsis. Nat Commun. 2019;10(1):2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sadikot RT, Bedi B, Li J, Yeligar SM. Alcohol-induced mitochondrial DNA damage promotes injurious crosstalk between alveolar epithelial cells and alveolar macrophages. Alcohol. 2019;80:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12(3):222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhong F, Liang S, Zhong Z. Emerging Role of Mitochondrial DNA as a Major Driver of Inflammation and Disease Progression. Trends Immunol. 2019;40(12):1120–33. [DOI] [PubMed] [Google Scholar]
- 97.Imajo M, Tsuchiya Y, Nishida E. Regulatory mechanisms and functions of MAP kinase signaling pathways. IUBMB Life. 2006;58(5–6):312–7. [DOI] [PubMed] [Google Scholar]
- 98.Marongiu L, Gornati L, Artuso I, Zanoni I, Granucci F. Below the surface: The inner lives of TLR4 and TLR9. J Leukoc Biol. 2019;106(1):147–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Byappanahalli AM, Omoniyi V, Noren Hooten N, Smith JT, Mode NA, Ezike N, Zonderman AB, Evans MK. Extracellular vesicle mitochondrial DNA levels are associated with race and mitochondrial DNA haplogroup. iScience. 2024;27(1):108724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stuart JA, Brown MF. Mitochondrial DNA maintenance and bioenergetics. Biochim et Biophys Acta (BBA) - Bioenergetics. 2006;1757(2):79–89. [DOI] [PubMed] [Google Scholar]
- 101.Todkar K, Chikhi L, Desjardins V, El-Mortada F, Pépin G, Germain M. Selective packaging of mitochondrial proteins into extracellular vesicles prevents the release of mitochondrial DAMPs. Nat Commun. 2021;12(1):1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or its supplementary information files.