Abstract
Background.
In early stages, individuals with Primary Progressive Aphasia (PPA) report language symptoms while scoring within norm in formal language tests. Early intervention is important due to the progressive nature of the disease.
Method.
We report a single case study of an individual with logopenic variant PPA (lvPPA). We tested whether letter fluency, used as a therapy task, can improve lexical retrieval when combined with tDCS to either the left inferior-frontal gyrus (IFG) or the left inferior parietal lobe (IPL), administered in two separate therapy phases separated by a wash-out period of three months.
Outcomes and results.
We observed increases in number of words retrieved during a letter fluency task in trained and untrained letters, when letter fluency therapy (LeFT) was administered with anodal tDCS. When LeFT was combined with left IFG stimulation, words produced in a letter fluency task were lower frequency and higher age of acquisition after treatment, compared to before treatment and there was also an increase in accuracy and response times in an untrained picture-naming task.
Conclusions.
The results indicate that letter fluency therapy combined anodal tDCS is effective in improving lexical retrieval, particularly when left IFG stimulation was used. Effects generalize beyond the trained task, albeit slowing down of responses in picture naming. This task may provide a useful clinical intervention strategy for patients with mild anomia, who are not challenged enough by traditional naming therapies.
Keywords: Primary Progressive Aphasia, Verbal fluency, Aphasia therapy, transcranial Direct Current Stimulation (tDCS), Lexical retrieval
1. INTRODUCTION
In early stages of Primary Progressive Aphasia (PPA), patients complain of communication difficulties, while scoring within norm in formal language assessments (Czarnecki et al., 2008). Given the progressive nature of the disease, it is important to intervene early (Croot, Nickels, Laurence, & Manning, 2009). However, there is a lack of evidence-based treatment tools suitable for individuals with mild language impairments. In the present single case study, we test the efficacy of letter fluency therapy (LeFT) combined anodal transcranial Direct Current Stimulation (tDCS) for a highly functioning individual with subjective complaints of word finding difficulty and a diagnosis of early-stage logopenic variant PPA. We compare treatment response with tDCS administered to the left IFG and the left IPL, which is known to show significant atrophy in lvPPA (Gorno-Tempini, Dronkers, et al., 2004).
1.1. Clinical profiles of individuals with PPA
PPA is a primary language disorder that occurs in individuals with frontotemporal lobar degeneration (FTLD) or Alzheimer’s disease (AD), as a consequence of progressive atrophy that predominantly affects the left hemisphere (Mesulam et al., 2008). PPA affects 1 to 6 people per 100,000 due to FTLD, and additional people with AD pathology (Grossman, 2014). PPA’s onset is more common in individuals aged between 40 and 60 years old (Grossman, 2014; Johnson et al., 2005; Nickels & Croot, 2014), an age at which individuals are still actively working. Furthermore, life expectancy after diagnosis ranges between 7 and 10 years (albeit there exist reports of 23 years) (Mesulam et al., 2008), and thus there is a long-lasting impact on quality of life (Medina & Weintraub, 2007).
PPA is diagnosed when a progressive language impairment occurs, and this is the most prominent symptom for at least the first two years of the disease, as well as the one with the greatest impact on participation in activities of daily living (Mesulam, 2001; Gorno-Tempini et al., 2011). In the current article, we present a case of an individual with logopenic variant PPA (lvPPA). The most prominent features of lvPPA are word finding difficulties and impaired repetition of phrases and sentences, which may be linked to poor verbal short-term memory. Speech rate is reduced and with pauses, which can be attributed to word finding difficulties. Naming errors are typically no response, circumlocutions, or phonological paraphasias (Gorno-Tempini et al., 2011). Typically, brain imaging abnormalities are detected in the temporoparietal junction, including in posterior temporal areas, the angular and supramarginal gyri (Gorno-Tempini, Dronkers, et al., 2004). Over time, brain atrophy extends to frontal and anterior temporal areas (Rogalski et al., 2011), in accordance with the progressive deterioration of fluency and auditory word comprehension (Grossman, 2014). Brain pathology studies indicate that degeneration is linked to the presence of Alzheimer’s disease in a large proportion of cases (Mesulam et al., 2008), but also with ubiquitin immunoreactivity (FTLD-U) and tau positive inclusions (Grossman, 2010).
1.2. Interventions for naming deficits in PPA
Despite the efforts to characterize the pathological origins of PPA, there are no disease-modifying treatments that may reverse the disease or hamper its progression. However, behavioural intervention studies have provided promising results, showing that speech and language therapy can result in improved language performance as well as a reduction in the progression of naming deterioration for trained words (Jokel, Graham, Rochon, & Leonard, 2014; Meyer et al., 2013). Picture naming is the treatment task most used in this body of research. During treatment, naming performance has been facilitated using semantic, phonemic, orthographic, and gestural cues (Beeson et al., 2011; Evans, Quimby, Dickey, & Dickerson, 2016; Jokel, Cupit, Rochon, & Leonard, 2009; Macoir et al., 2015; Meyer, Getz, Brennan, Hu, & Friedman, 2015). Henry et al. (2013) tested effects of lexical retrieval cascade therapy, which included picture naming with self-generated semantic, phonemic, and orthographic cues, as well as generation of semantic features, semantic subcategories, and generation of new items within subcategories. Finally, some studies focused on improving retrieval of orthographic word forms (e.g., Tsapkini & Hillis, 2013).
Most studies report improved naming performance for trained words (e.g., Beeson et al., 2011; Evans et al., 2016; Jokel et al., 2009), and maintenance of therapy effects has been reported for up to six months (Heredia, Sage, Ralph, & Berthier, 2009; Jokel, Rochon, & Leonard, 2006). Improved retrieval of untrained words occurs sparingly. Henry and colleagues (2013) report a case of a person with semantic variant PPA (svPPA) showing treatment related increases in retrieval of words of trained and untrained items from untrained semantic categories, in a semantic fluency task.
Henry and colleagues (2013) included one individual with lvPPA in their study, who showed improved word retrieval in conversation after treatment. Beeson et al. (2011) also included one individual with lvPPA in their therapy study. Treatment used a variety of tasks (picture naming, verbal fluency, retrieval of semantic features, and home activities) and yielded improvements of words within trained categories, increased speaking rate, and increased number of correct information units in connected speech. While these two studies show promising effects of the two therapy approaches implemented, each one employed a variety of tasks during intervention. Therapy effects may be a result of just one task or of the combination of the different tasks. In the current study, we isolate verbal fluency (specifically, letter fluency, in the oral modality), and examine effects of intervention focusing on this specific task.
1.3. Verbal fluency tasks
Verbal fluency tasks have been widely used in neuropsychological assessments, including the Multi-lingual Aphasia Examination (Benton, Hamsher, & Sivan, 1994). In letter fluency tasks, the examiner gives one minute to the subjects to say as many words as they can recall starting with a specific letter (typically, F, A, and S), excluding proper nouns, numbers, and the same root with different inflectional morphemes. The number of words produced is predicted by both vocabulary size and the updating ability, that is, the executive function responsible for constant monitoring and tracking of representations in working memory (Shao, Janse, Visser, & Meyer, 2014). Fluency tasks can tell apart people with PPA and other neurodegenerative diseases from healthy individuals, as well as predict which individuals with Mild Cognitive Impairment (MCI) may be more likely to develop Alzheimer’s disease (AD) (Forbes-McKay, Ellis, Shanks, & Venneri, 2005; Marczinski & Kertesz, 2006; Vita et al., 2014).
Studies of individuals with brain lesions have shown that lesions to the frontal lobe impair letter fluency disproportionately, when compared to category fluency (e.g., fruits, animals, and vegetables) (Henry & Crawford, 2004). Conversely, greater difficulties in category fluency when compared to letter fluency are found in individuals with temporal lobe lesions (Baldo, Schwartz, Wilkins, & Dronkers, 2006). Studies with healthy controls indicate that both letter and category fluency are associated with activation of frontal (Basho, Palmer, Rubio, Wulfeck, & Müller, 2007; Birn et al., 2010; Fu et al., 2002; Heim, Eickhoff, & Amunts, 2008) and parietal areas (Birn et al., 2010; Vitali et al., 2005). In category fluency tasks, switching behaviours between semantic sub-categories are associated with left IFG activation (Hirshorn & Thompson-Schill, 2006). Neuromodulation studies also show that transcranial Direct Current Stimulation (tDCS) to the left dorsolateral pre-frontal cortex enhances performance in letter fluency (Pereira et al., 2013). Inhibition of the same region using repeated TMS (Transcranial Magnetic Stimulation) hinders performance in letter fluency (Smirni et al., 2017). Altogether, this research indicates that letter fluency and category fluency rely on frontotemporoparietal regions, with some specific processes occurring in non-overlapping regions.
1.4. tDCS in PPA research
Transcranial Direct Current Stimulation (tDCS) is a non-invasive neuromodulation technique where a small, continuous current is administered over the scalp. This current can change the resting membrane potential of neurons in stimulated areas (Kuo, Polanía, & Nitsche, 2016), and may enhance language performance in healthy individuals, stroke, and PPA (see de Aguiar, Paolazzi, & Miceli, 2015; Fridriksson et al., 2018; Monti et al., 2012; Sebastian, Tsapkini, & Tippett, 2016; Tsapkini et al., 2018). In individuals with PPA, tDCS was used to enhance word retrieval across several studies. Cotelli et al. (2014) showed that 10 sessions of real anodal stimulation to the left dorsolateral prefrontal cortex paired with anomia therapy in a between-subjects design improved oral naming more than sham in nonfluent variant PPA (nvPPA). Tsapkini et al. (2018) reported similar augmentation in spelling outcomes for trained and untrained words in 36 PPA participants (nfv, lv, and svPPA) after anodal tDCS over the left IFG in a within-subjects design. Roncero et al. (2017) paired anomia therapy (cued with repetition) with anodal tDCS to the left inferior parieto-temporal region in individuals with all three PPA variants. The above studies report greater change in naming after real stimulation when compared to sham, indicating that tDCS can be successfully used to improve lexical retrieval in individuals with PPA. Others, however, did not include a sham condition in their design. Hung et al. (2017) provided modified semantic feature analysis (SFA) during anodal tDCS to the left temporo-parietal cortex to patients with PPA and Alzheimer’s disease. Patients improved in naming accuracy for trained items and maintained better performance for trained items when compared to untrained items after 6 months. Importantly, considering studies with only behavioural therapy and these non-controlled studies, a pattern still emerges: improvement in naming of untrained items seems to occur only when therapy is combined with tDCS (Cotelli et al., 2020).
Other sham-controlled studies attempted to enhance verbal fluency using a single session of tDCS. Studies with young, healthy individuals targeted largely overlapping regions, with anodal stimulation administered to the motor cortex (Meinzer et al., 2014), pre-motor cortex (Pisoni et al., 2018), DLPFC (Vannorsdall et al., 2012), or Brocás area (Cattaneo, Pisoni, & Papagno, 2011). These studies found enhancement of performance with tDCS compared to sham in the fluency tasks tested, including both letter and category fluency (Cattaneo et al., 2011), category fluency (Meinzer et al., 2014; Vannorsdall et al., 2012), and letter fluency (Pisoni et al., 2018). Other sham-controlled studies did not replicate the effects of anodal tDCS to the DLPFC on letter fluency (Radman et al., 2018), or to the left IFG on either category or letter fluency (Vannorsdall et al., 2016; Westwood et al., 2018).
In clinical populations, tDCS was administered without concurrent behavioural treatment to enhance both letter and category fluency in one individual with hypoxic-ischemic brain injury (Boggio and colleagues, 2009), and in a group of left-hemisphere stroke patients (Santos et al., 2013), with anodal stimulation to the left DLPFC and cathodal stimulation to the left motor cortex, respectively. While these studies did not compare tDCS to sham, both studies report improved performance after treatment. Penolazzi and colleagues (2015) administered 5 sessions of left DLPFC anodal tDCS combined with a cognitive training that included letter fluency to individuals with Alzheimer’s disease. No improvement was detected in either the tDCS of sham therapy phases. In another study, tDCS was reported to enhance performance of individuals with Parkinson’s disease during a letter fluency task, when administered to the left IFG, but not the left temporoparietal cortex (Pereira et al., 2013). Finally, in a previous sham-controlled study of anodal tDCS over the left IFG in PPA (Tsapkini et al., 2014), three participants with PPA (two nvPPA and one lvPPA) performed a written letter fluency task during therapy. All three patients produced more words after written letter fluency therapy with tDCS compared to sham.
In summary, effects of tDCS on verbal fluency have been variable, with some difficulty replicating findings in healthy controls, and the lack of evidence for efficacy from controlled studies in clinical populations. Furthermore, given the broad cognitive nature of verbal fluency tasks, it is important to study whether improvements in verbal fluency translate to general improvements in lexical retrieval, or are specific to performance in fluency tasks.
The main aim of the current study is to developed a treatment protocol using oral verbal fluency and inquire if oral verbal fluency can be used as a therapy task to enhance lexical retrieval of an individual with PPA. We study which aspects of language processing are associated with improvements in letter fluency by examining treatment-related changes to the psycholinguistic properties of the words produced during a letter fluency task. Furthermore, tDCS to the left IFG can enhance effects of written letter fluency therapy (Tsapkini et al., 2014). In addition, the left IPL is known to show significant atrophy in lvPPA (Gorno-Tempini, Dronkers, et al., 2004) and to be involved in both letter and category fluency (Birn et al., 2010; Vitali et al., 2005). This way, both IFG and IPL stimulation with tDCS may be effective adjuvants when combined with letter fluency therapy, either via to increase function of intact or relatively atrophic regions, which are both functionally relevant in relation to the treatment task. Thus, in the present study, we tested whether two stimulation protocols, with tDCS to the left IFG and left IPL, are differentially effective in enhancing the effects of oral verbal fluency therapy.
2. MATERIALS AND METHODS
2.1. Participant
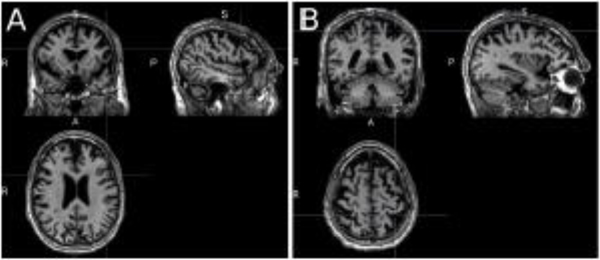
AGG was a 72-year-old individual at the time of testing, with 16 years of education (Bachelor’s degree). He complained of difficulties findings words, while at the same time rating himself high (4/5) in his overall speaking and comprehension abilities. He attended the assessments with his wife, well-groomed, with euthymic mood. His MRI scan was compared quantitatively with scans of age-matched controls using MRIcloud (Mori et al., 2016). Atrophy was reduced and indeed only significant in the left frontal lobe (see Figure 1, panel A). The mild pattern of atrophy is consistent with the fact that his language difficulties were mild and self-rating of language was rather high. His output was fluent, albeit with reduced speech rate. He presented word-finding difficulties and occasional phonemic paraphasias in spontaneous speech, while scoring within norm in the Boston Naming Test (25/30) but was impaired in action naming (Hopkins Action Naming Assessment: 25/35). His errors in the action naming task consisted mainly of semantic paraphasias (6/10, e.g. sweeping for vacuuming), with 2 phonemic errors (e.g. drubble, dribbing for dribble), and 2 no responses. Performance in a more extensive object naming assessment with 252 items (see section 2.3.3) confirmed impaired object naming, with lower naming accuracy compared to a group of controls (t(6)=−7.55, p<0.001, using Crawfordś modified t test, Crawford & Howell, 1998). His conceptual knowledge of objects and actions was preserved (Pyramids and Palm Trees test: 15/15; Kissing and Dancing test: 14/15). His syntactic knowledge was generally good, with sentence comprehension errors limited to comprehension of object relatives (SOAP test 37/40). Sentence repetition was impaired, with 1/5 sentences fully accurate, but 31/37 words accurate. Errors in sentence repetition consisted of omissions or substitutions leading to grammatical sentences (e.g. The teacher bought three gloves instead of A teacher bought three pairs of gloves) and one paragrammatic response (The rabbit was given to the child from the fireman instead of The rabbit was given to the child by the fireman).
Figure 1.
T1 MRI scan at the time of enrolment in the research. Panel A: MRI scan highlighting the frontal lobe; Panel B: Scan highlighting the parietal lobe. Significant atrophy in comparison to controls was restricted to the left frontal lobe.
FTD-CDR Clinical Dementia Rating scale severity scores at the start of therapy reflected mild impairments (score 0.5) in community affairs, home and hobbies, and language (Knopman et al., 2008). PPA Variant was diagnosed taking into account the language profile consistent with lvPPA (Gorno-Tempini et l., 2011; Gorno-Tempini, Murray, Rankin, Weiner, & Miller, 2004). It is clear that his imaging findings were, at the state of enrolment, not typical of lvPPA as parietal, rather than frontal atrophy, would be expected. However, after the completion this research AGG continued to progress with a language profile characteristic of lvPPA whereby he showed decay in naming and repetition, and an increase in phonemic errors, while maintaining comprehension abilities. Individuals with nfvPPA, instead, tend to show more generalized decay (Rogalski et al., 2011), and thus we argue that a diagnosis of lvPPA, based on performance in language tests, is the one that best describes AGG’s language profile.
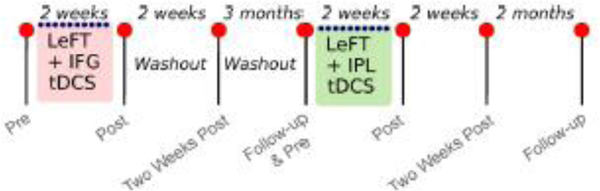
2.2. Study design
Therapy was administered in two separate treatment phases, each consisting of ten 1-hour sessions, administered 5 times per week (see Figure 2). During both treatment phases, AGG received Letter Fluency Therapy (LeFT) paired with tDCS. Treatment details are described in the next section. The two therapy phases were identical in relation to the behavioural treatment, but differed in terms of the location of stimulation (left IFG during phase 1 and left IPL during phase 2) and were separated by a washout period of three months. As the two treatment phases share the behavioural intervention, findings of improvement in performance in language tasks in both phases supports the idea that LeFT combined with either one of the stimulation protocols, can improve language processing. In addition, the comparison of the two phases is relevant to test if either tDCS protocol is more effective than the other one. While we also aimed to include a Sham phase, our participant did not wish to receive Sham. This way, differences in treatment response across phases only inform us about whether LeFT combined with stimulation to the IFG is differentially beneficial compared to LeFT combined with stimulation to the IPL, but not about the overall efficacy of tDCS compared to Sham or no stimulation.
Figure 2.
Study design. Assessment moments are indicated as red dots, and treatment sessions as blue dots.
Assessments were conducted before, immediately after, two weeks after, and at a follow-up time after each therapy phase. This follow-up was of three months for Phase 1, but only two months for Phase 2, to accommodate the participant’s availability. The 3-month follow-up assessment after Phase 1, also corresponded to the pre-treatment assessment for Phase 2.
2.2.1. Behavioural therapy: Letter Fluency Therapy (LeFT)
In the present study, intervention consists solely of oral letter fluency activities. The main ingredient of treatment was repeated attempts at retrieving as many words as possible starting with each of the trained letters. AGG was given periods of 1–3 minutes (increasing across therapy sessions) to perform this task. Previous research indicates that the responses in letter fluency at the beginning of the response window depend on rapid access of words from the lexicon, while later responses require strategies for effortful lexical search (Fernaeus & Almkvist, 1998). Hence, by increasing the time available to retrieve words for each letter during treatment we hoped to pose high demands on lexical search. Occasional errors were ignored, if AGG proceeded with accurate performance immediately afterwards, to avoid interrupting successful lexical search. However, when several errors occurred subsequently, AGG was interrupted briefly and the kind of error was pointed out.
In addition, to facilitate performance, we showed AGG an alphabet board. With it, we prompted AGG to think of combinations of the target with other letters, when we had exhausted what he could retrieve spontaneously. For homework activities, AGG generated lists of words for each of the trained letters, in both oral and written form, while timing himself in periods of 2–5 minutes per letter. While not initially instructed to do so, AGG consulted a dictionary during his homework to expand the vocabulary used in the letter fluency task. We then instructed him to do the same during the second therapy phase, to replicate as much of the behavioural treatment as possible. No other facilitation strategies were implemented. To ensure treatment fidelity, the procedures within each session steps were described and noted in a checklist, the time given to practice with each letter was timed, and each session was audio recorded. A trained research assistant reviewed all recordings, ensuring that all items (the letters) were practiced as planned. AGG was not undergoing other forms of therapy while participating in this study.
2.2.2. tDCS parameters
In the first 20 minutes of each session, AGG received anodal tDCS at 2mA, delivered using 5×5cm electrodes. The anode was placed over the left IFG during Phase 1 and over the left IPL during Phase 2. The left IFG was localized using the F7 co-ordinate of the international 10–20 system (Homan, 1988). The left IPL was localized using the TP3 electrode (Herwig, Satrapi, & Schönfeldt-Lecuona, 2003; Homan, 1988). The cathode was placed over the right cheek in both therapy phases. No blinding between the two stimulation protocols was implemented.
2.2.2. Outcome measures: Number of words retrieved during letter fluency
The primary outcome measure was the number of words retrieved in a letter fluency task, assessed in 1-minute periods, for each of 24 letters. AGG was asked to produce as many words as he could think of starting with the letter provided, while avoiding proper names, numbers, and using the same root with different inflections. Three sets of eight letters were created: one to use in treatment, and two untrained sets (one to serve as control, and the other one reserved for treatment in Phase 2). The three sets were matched for the total number of words present in the CELEX database starting with each target letter, the average frequency of those same words, the number of vowels and consonants (2 vowels in each set), and the average number of words retrieved before the start of the study protocol (see Table 2 for details) (Baayen, Piepenbrock, & Gulikers, 1995). Answers were scored as incorrect if they did not begin with the target letter, if they were a proper name or a number, if they were repetitions of a previously produced word or a different inflection of a same stem produced before, and if they contained phonemic paraphasias or consisted of neologisms. At the start of the study protocol, there were no significant differences between the three sets of letters in number of words retrieved, or in any of the variables used to match sets. The letters ‘x’ and ‘z’ were not included, as these have the fewest word counts in the CELEX database.
Table 2.
Matching of the three sets of letters
| N words in CELEX (mean ± sd) | Frequency mean (mean ± sd) | Pre-therapy N words (mean ± sd) | |
|---|---|---|---|
| Set 1 (trained in Phase 1) |
1368.000±520.042 | 36.419±36.009 | 10.750±3.991 |
| Set 2 (trained in Phase 2) |
1120.750±1033.190 | 33.393±41.178 | 9.5001±604 |
| Set 3 (Untrained) |
1330.875±1162.015 | 35.406±21.485 | 11.000±2.928 |
Table 2. Matching of three sets of 8 letters prior to the start of the treatment protocol. Three sets including 8 letters each were matched by the number of words existing in the CELEX database starting with that letter, the mean frequency of those words, and by how many words AGG produced before therapy.
While it was not possible to collect multiple baselines for all 24 letters prior to the beginning of this study, letter fluency had been assessed for three letters (FAS) on three occasions before the start of the study protocol, without any intervention between them. Letter fluency was found to be within norm (FAS sum = 47, 43, 40, measured 8 months, 3 months, and immediately before therapy, respectively), and so was category fluency for animals (17, 18, 14, same assessment times; according to norms from Tombaugh, Kozak, & Rees, 1999). Performance is relatively stable but with a trend to decrease. Given these data and the degenerative nature of PPV, no spontaneous improvement was expected to occur and positive changes may be attributed to the treatment protocol.
2.2.3. Outcome measures: Psycholinguistic properties of words retrieved during letter fluency
In addition to the number of words retrieved during the letter fluency task, we were interested in understanding at which level of psycholinguistic processing were any changes to word retrieval occurring. Therefore, we also studied the psycholinguistic properties of words produced by AGG during the letter fluency task, for trained and untrained letters. For this purpose, we studied two variables that have been associated in the literature with processing and impairments in the phonological output lexicon (Carroll & White, 1973): word (log) frequency (Baayen et al., 1995), and age of acquisition (AoA; Kuperman, Stadthagen-Gonzalez, & Brysbaert, 2012). In addition, we studied two variables associated with processing at the semantic level: imageability (Bird, Lambon Ralph, Patterson, & Hodges, 2000; Luzzatti et al., 2002; Rofes et al., 2017) and concreteness (e.g., Grossman & Ash, 2004). This approach has been previously used to differentiate language profiles of people with different PPA variants (Rofes et al., 2019) and to characterize language processing during fluency tasks in individuals with Alzheimeŕs Disease (Rofes et al., in press).
Imageability norms were obtained with a 6,377-word database, combining norms from Coltheart (1981), Juhasz, Lai, and Woodcock (2015), and Stadthagen-Gonzalez and Davis (2006) to increase the availability of norms for specific words. As the database of Coltheart (1981) is the largest of these three, it was taken as the default when the same word appeared in more than one database. Concreteness norms were obtained from the database by Brysbaert, Warriner, and Kuperman (2014). Singular word forms were used to query the databases for age of acquisition and imageability, as the databases did not include ratings of the same words in the plural form (e.g., apples). We included frequency scores for plural words when they were present in the database.
2.2.4. Outcome measures: Examining transfer to an untrained task: timed picture naming
To study improvement in lexical retrieval in an untrained task, we administered a timed object naming test, using 252 coloured Snodgrass and Vanderwart’s pictures (Rossion & Pourtois, 2004). Stimuli were presented using ePrime (v. 3.0). We presented a fixation cross on the screen for 500ms, followed by the picture. An internal microphone captured latency between picture presentation and the start of AGG’s oral naming, and any incorrectly named items or extraneous noises between picture presentation and naming (e.g. “um”) were documented. When a response was detected, the examiner moved on to the next item manually. AGG was instructed to name each of the pictures as quickly and accurately as possible. This task was administered before, after, and at the two/three-month follow-up time after therapy. Given AGG’s high level of performance, the first response was used for scoring of accuracy, and responses including phonemic errors were also considered incorrect. Hesitations (in the form of interjections) followed immediately by a correct response were counted as correct. Reaction times calculations included only correct responses.
2.3. Analyses
For all variables related to the sets of letters tested in the letter fluency task (number of words produced, log frequency, AoA, imageability, and concreteness) we used Friedman ANOVAs to test whether there were any significant changes across the 4 assessment times related to each therapy phase (pre-, post-, two weeks post-, two/three months post-therapy). For posthoc analyses, we conducted pairwise comparisons between assessment times using the Wilcoxon sign ranks test for the same letters. Each letter in a set provided a separate data point in the sample of trained or untrained letters. In addition, we inquire if changes from pre- to each of the post-therapy time points are significantly different between Phase 1 and Phase 2. For these comparisons, we used Mann-Whitney U test for the different letters in each set. P-values were corrected for multiple comparisons.
For the untrained picture naming task, we compared performance between the three time points, separately for Phase 1 and 2, using the Cochran’s Q test for accuracy and Friedman ANOVA for oral reaction times (after verification that the reaction time data did not follow a normal distribution). Follow-up pairwise comparisons between timepoints were done using McNemar X2 tests for accuracy and Wilcoxon sign rank tests for oral reaction times. We also contrasted the changes obtained in the two phases, both from pre- to immediately post-therapy (post- minus pre-therapy accuracy or reaction times), and from pre- to two/three months post-therapy (two/three months post- minus pre-therapy accuracy or reaction times) using McNemar X2 tests for accuracy data and the Wilcoxon sign ranks test for reaction times. For oral reaction times, only items with correct responses in all measurements were considered (N=89). P-values were corrected for multiple comparisons. All work was conducted with the formal approval of the local human ethics committee. AGG provided written informed consent prior to participating in this research.
3. RESULTS
3.1. Changes in number of words retrieved during letter fluency
Across all assessment times combined, AGG produced 2668 correct words in the fluency task and 419 errors (16% errors). Errors consisted mostly of repetitions of a previously uttered word (53% of all errors), failure to follow the instructions (e.g., producing proper names or numerals, 15%), a different inflectional form of a previously uttered word (near, nearest), neologisms (e.g. ignominist or unarrive, 11%), phonemic paraphasias (e.g. synthenic, 8%), and production of words in a different language (e.g. doppia, 1%).
3.1.1. Trained letters
In the first therapy phase (IFG+LeFT), the number of words retrieved for each letter in the trained set changed significantly (X2(3, N=8)=20.392, p<0.01) (see Figure 3, panel A). Words per letter retrieved increased significantly from pre to post-therapy (Z=0, p<0.05), and remained significantly above pre-therapy levels at 2-weeks post-therapy (Z=0, p<0.05) and at the 3-months follow-up assessment (Z=1.5, p<0.05). There was also a significant change across measurements for letters trained during Phase 2 (IPL+LeFT) (X2(3, N=8)= 19.735, p<0.01). The number of words retrieved increased from pre-to post therapy (Z=0, p<0.05), remaining significantly above pre-therapy measurements after two weeks (Z=0, p<0.05), but not at the two months follow-up (Z=5, p=0.280). For trained letters, change was greater with IFG+LeFT than in IPL+LeFT from pre- to immediately post-therapy (U=56, p<0.05) but not from pre- to two weeks after therapy (U=52, p=0.110), or from pre- to follow-up (U=46, p=0.460).
Figure 3.
Letter fluency counts for trained and untrained letters. The y axis represents, for the 8 letters of each set, the number of words retrieved averaged across letters. The x axis represents the different assessment times. Panel A: trained letters; Panel B: untrained letters.
3.1.2. Untrained letters
The two untrained sets of phase 1 were sets 2 and 3. There were significant changes across Phase 1 (IFG+LeFT) measurements for those untrained sets (X2(3, N=16)=19.735, p<0.001) (see Figure 3, panel B). Significant changes were observed in untrained sets from Pre to Post-therapy (Z=14.5, p<0.01), and to two weeks post therapy (Z=8.5, p<0.01), but not from pre to the three months follow-up (Z=54.5, p=0.925). The two untrained sets of Phase 2 (IPL+LeFT) were Set 1 (that had been trained during Phase 1) and Set 3 (never trained). There were significant changes in number of letters retrieved across Phase 2 measurement (X2(3, N=16)=11.266, p<0.05). There were significant increases in accuracy for untrained sets from pre- to post-therapy (Z=18.5, p<0.05) and from pre-therapy to two weeks after therapy (Z=12, p<0.05). The number of words retrieved at the two months follow-up after therapy Phase 2 (IPL+LeFT) was not significantly different from before therapy (Z=47.5, p=0.916). No differences in the change observed between the assessment points of the two phases occurred for the untrained sets in this measure (pre to post: U=160.5, p=0.224; to 2 weeks: U=172.5, p=0.094; to follow-up: U=120, p=0.776).
3.2. Changes in psycholinguistic properties of words retrieved during letter fluency
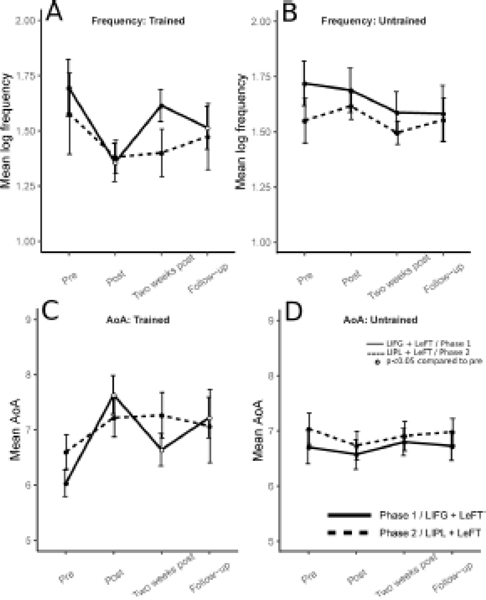
3.2.1. Lexical-phonological properties: frequency and Age of Acquisition (AoA)
In the first therapy phase (IFG+LeFT), for trained letters, there were significant changes in the frequency of words produced across measurements (X2(3, N=8)=13.050, p<0.01), with word frequency decreasing significantly from pre to post-therapy (Z=36, p<0.01), and three months after therapy (Z=33, p<0.05), but not in the two week post-therapy measurement (Z=24, p=0.461) (see Figure 4, panel A). No changes in word frequencies were observed for the untrained sets (X2(3, N=16)=1.875, p=0.599) (Figure 4, panel B). In AoA, there were significant differences for trained letters across measurements (X2(3, N=8)=11.85, p<0.05), with increase in AoA of words produced from pre- to post-therapy (Z=0, p<0.01), from pre- to two weeks post-therapy (Z=2, p<0.05) and to the three months post-therapy follow-up (Z=3, p<0.05) (Figure 4, panel C). No changes in AoA were observed for the untrained sets (X2(3, N=16)=1.425, p=1) (Figure 4, panel D).
Figure 4.
Lexical-phonological properties for words retrieved in letter fluency for trained and untrained letters. In each panel the y axis represents, for the 8 letters of each set, the average value of the psycholinguistic variable examined, averaged across letters. The x axis represents the different assessment times, with “Pre-phase 2” also corresponding to three months after the end of phase 1. Panels A and B: log frequency, for trained and untrained letters, respectively. Panels C and D: Age of Acquisition, for trained and untrained letters, respectively.
In the second therapy phase (IPL+LeFT), there were no significant changes in the frequency of words produced, either for trained letters (X2(3, N=8)=4.05,p=0.256), or untrained letters (X2(3, N=16)=5.1, p=0.165). The same was true for AoA (trained: X2(3, N=8)=6.75, p=0.161; untrained: X2(3, N=16)=5.475, p=0.280) (Figure 4, panels A to D).
There were no significant differences between phase 1 and phase 2 in the changes observed in frequency of words retrieved during letter fluency, for trained (from pre to post: U=27, p=0.645; from pre to two weeks post: U=33, p=0.959; from pre to follow-up: U=24, p=0.442) or for untrained sets (from pre to post: U=87, p=0.128; from pre to two weeks post: U=107, p=0.445; from pre to follow-up: U=105, p=0.402).
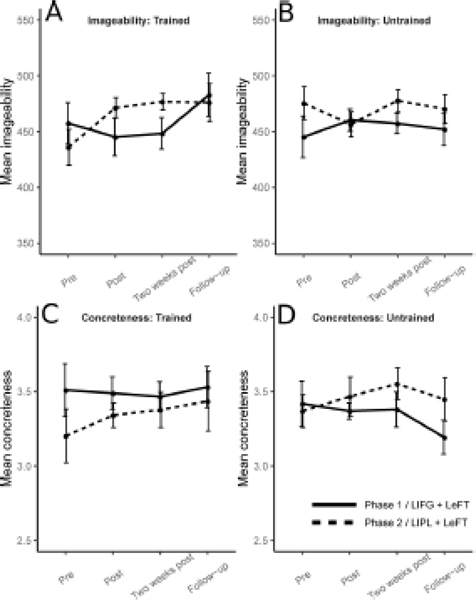
3.2.2. Lexical-semantic properties: imageability and concreteness
There were no significant changes in either phase in the imageability of words retrieved during letter fluency tasks for trained (Phase 1: X2(3, N=8)=3.9, p=0.545; Phase 2: X2(3, N=8)=4.1, p=0.481) or untrained letters (Phase 1: X2(3, N=16)=3.15, p=0.738; Phase 2: X2(3, N=16)=1.453, p=1.386) (Figure 5, panels A and B, respectively). The same absence of change was observed for concreteness of words produced, both for trained (Phase 1: X2(3, N=8)=1.8, p=1.230; Phase 2: X2(3, N=8)=1.8, p=1.230) and untrained letters (Phase 1: X2(3, N=16)=0.877, p=1.662; Phase 2: X2(3, N=16)=5.48, p=0.230) (Figure 5, panels C and D, respectively).
Figure 5.
Lexical-semantic properties for words retrieved in letter fluency for trained and untrained letters. In each panel the y axis represents, for the 8 letters of each set, the average value of the psycholinguistic variable examined, averaged across letters. The x axis represents the different assessment times, with “Pre-phase 2” also corresponding to three months after the end of phase 1. Panels A and B: Imageability, for trained and untrained letters, respectively. Panels C and D: Concreteness, for trained and untrained letters, respectively.
There were also no significant differences between Phase 1 and Phase 2 in changes to the imageability of words produced from pre- to either post-therapy measurement (Trained: pre to post U=16, p=0.481, pre to two weeks post U=11, p=0.084, pre to follow-up U=29, p=0.240; Untrained: pre to post U=157, p=0.861, pre to two weeks post U=145, p=1.617, pre to follow-up U=130, p=2.867).
3.3. Transfer to an untrained task: changes in timed picture naming task
In the picture naming task, AGG produced, across all assessments, 1512 response trials, with 233 errors (15% errors). Almost half of these errors were words semantically related to the target (42%), fragments of the target error (15%) or other phonemic errors (13%), fragments that could not be linked to the target word (12%), mixed errors (6%), null responses (6%), or unrelated words (4%). The proportion of errors of each type did not change significantly across the assessment moments.
During Phase 1 (IFG+LeFT), AGG’s performance changed significantly both in accuracy (X2(2, N=252)=11.534, p<0.01) and response times (X2(2, N=89)=39.258, p<0.001) in the untrained timed object naming task. There was a significant increase in accuracy from pre to post-therapy (X2(1, N=252)=7.843, p<0.01), which was maintained at the three-month follow-up (X2(1, N=252)=6.685, p<0.01) (see Figure 6, panel A). Reaction times were significantly longer immediately (Z=1050.5, p<0.001) and three months after treatment (Z=720, p<0.001) when compared to before treatment (Figure 6) (Figure 6, panel B).
Figure 6.
Change in naming accuracy and reaction times. Each y axis represents the change from pre-therapy in accuracy (panel A) and reaction times (panel B) in naming pictures of untrained stimuli. The x axis represents the different post-therapy assessment times.
During Phase 2 there were no significant changes in either accuracy (X2(2, N=252)=1.037, p=0.595) or reaction times (X2(2, N=89)=3.994, p=0.271) in the untrained timed object naming task.
Changes in accuracy in the untrained object naming task were significantly larger in Phase 1 than during Phase 2, both immediately after therapy (X2(3, N=252)=9.817, p<0.05), and at the follow-up assessment (X2(3, N=252)=28.214, p<0.001). Changes in reaction times were significantly larger in phase 1 when compared to Phase 2 immediately treatment (Z=2855.5, p<0.001) and at the two/three month follow up (Z=2858.5, p<0.001).
4. DISCUSSION
In the present study we asked whether Letter Fluency Therapy (LeFT) could result in improvements in lexical retrieval, for trained and untrained letters, and in an untrained naming task. We also inquired which levels of language processing (lexical-semantic vs. lexical-phonological) were changed by examining the psycholinguistic properties of words produced during letter fluency. Furthermore, we tested whether anodal tDCS administered to the left IFG and left IPL were differentially effective in enhancing treatment effects.
For the trained letter sets, with left IFG stimulation combined with LeFT, we found significant improvements immediately after treatment, which were sustained at two weeks, and three months after treatment. With left IPL stimulation, effects for trained letters were significant immediately and 2 weeks, but not 2 months after therapy. Change from pre- to immediately after therapy was greater with left IFG compared to left IPL stimulation. For the untrained letter sets, improvement was similar between stimulation conditions: there were significant improvements after both therapy phases immediately after treatment, and gains were sustained at two weeks, but not three months after treatment. Furthermore, after stimulation to the left IFG combined with LeFT we observed decreases in the frequency of words produced for trained letters (immediately and three months after therapy) and increases in Age of Acquisition (AoA) (in all post-therapy assessments). There were no changes in imageability and concreteness in either phase. Finally, there were significant increases in accuracy and reaction times in the untrained object naming task, both immediately and three months after IFG but not after IPL stimulation combined with LeFT. Changes in naming accuracy and reaction times were larger after IFG+LeFT than IPL+LeFT treatment.
4.1. Does LeFT improve lexical retrieval?
First and foremost, treatment using LeFT combined with anodal tDCS had a positive outcome, as it resulted in improvements in letter fluency and in naming. We will argue that treatment resulted in improvement of AGG’s lexical retrieval abilities, which may have been facilitated by left IFG, but not IPL, stimulation. There are increases in the number of words produced in letter fluency tasks for trained and untrained letters, after both therapy phases. We get support for our hypothesis of generalized improvement in lexical retrieval in the finding that AGG’s accuracy in an untrained picture naming task also increased, albeit only after left IFG stimulation.
Improvements in naming could also be explained by an improvement in other language processing levels, namely in semantic processing (Boyle & Coelho, 1995). We found that while AGG’s number of words retrieved in letter fluency increases, the frequency and age of acquisition of words produced decreased in Phase 1, with LIFG stimulation. Effects of word frequency and of age of acquisition on performance in language tasks have been interpreted as a reflecting the functioning of the phonological output lexicon (Carroll & White, 1973; Ellis & Young, 1988; Rofes et al., 2015). On the contrary, there were no changes in imageability or concreteness, variables that have long been associated with the integrity of semantic processing (e.g., Ellis & Young, 1988; Luzzatti et al., 2002; Grossman & Ash, 2004).
The increase in accuracy in picture naming after Phase 1 only, was accompanied by a lengthening of response times in the picture naming test. This means that there was a speed-accuracy trade-off, whereby, AGG prioritized accuracy over speed in the post-therapy assessments. This signals a change in AGG’s behaviour from before to after therapy. Hence, the trade-off is the reflection of the strategic processing and indeed successful implementation of lexical retrieval strategies. In addition, response times may have increased due to deterioration of motor speech, which occurs over time in individuals with lvPPA (Rogalski et al., 2011; Grossman, 2014) and may be related to the distribution of pauses in narrative speech (Mack et al., 2015). However, given that the increase in reaction times was only found in Phase 1, that it occurred within a period of only two weeks, and there were no other signs of motor deterioration, it is unlikely that deterioration of motor speech accounts for the change in reaction times.
Considering that the treatment task and the outcome assessment task overlap (with the exception of the feedback and cueing alphabet board provided during treatment), it could be seen as a concern that effects observed are a mere result of task practice (Iyer et al., 2005). Task practice effects are not, however, a negative outcome. For instance, practicing a functionally relevant task improves communication in individuals with PPA (Volkmer et al., 2020). In the context of tasks designed to engage lexical retrieval, letter fluency tasks provide a rather naturalistic setting, as retrieval is unaided by visual or auditory stimuli. Often, during a conversation, a patient may be unable to retrieve a word, yet be aware of partial orthographic or phonological information about the same word (Goodglass et al., 1997). Translating knowledge of the first letter of a word into phonemic knowledge is considered a crucial skill for self-cueing of word retrieval (Bruce & Howard, 1988). A fluency task can mimic such tip-of-the tong phenomena, and is then closer to functional language usage compared to other (picture supported) tasks which are often used in aphasia therapy. Practicing this task may then, in principle, facilitate functional communication. Given that AGG was a high-functioning individual already at the start of intervention (with self-ratings close to ceiling), we could not test accurately if there was indeed improvement in functional communication. Nonetheless, such improvements were informally reported by his wife and friends, after the first but not the second treatment phase. In addition, our observations of improvement in naming accuracy in the untrained picture naming task, support the claim that benefits obtained from intervention improved language performance beyond the treated task.
Importantly, AGG maintained improvements for up to three months after therapy, for trained letters, and two weeks, for untrained letters. Maintenance of these therapy-related gains is particularly noteworthy in the context of a progressive language impairment. Altogether, these findings indicate that letter fluency therapy was successful in enhancing AGG’s lexical retrieval, for both trained and untrained letters, and provide the basis for future group studies.
4.2. Is anodal tDCS over the left IFG differentially effective to enhance therapy effects than anodal tDCS over the left IPL?
Change in letter fluency for trained letters from pre- to immediately after therapy was greater with left IFG compared to left IPL stimulation. In addition, while LeFT combined with IFG stimulation led to effects sustained until the follow-up assessment, this was not the case for LeFT combined with IPL stimulation. It is of course important to consider the possibility that the order of treatment may have played a role in the differences observed between stimulation conditions: left IPL stimulation was given in phase 2, and AGG could have been more habituated to the therapy or assessment tasks at this stage and have less potential to improve. However, his performance in the letter fluency task, as showed in Figure 3, returned to levels alike those before phase 1 at the start of phase 2. Hence, it is unlikely that his potential to show improvement was substantially smaller in phase 2, when receiving IPL stimulation. This way, the results suggest a greater benefit in pairing LeFT with IFG compared to IPL stimulation. This finding is in line with Pereira et al. (2013), who reported enhanced performance in letter fluency in individuals with Parkinsońs disease after left IFG stimulation but not left IPL stimulation. It is possible and theoretically sound that some enhancement of treatment effects with stimulation to the left IPL is also present, given its role in both letter and category fluency (Birn et al., 2010; Vitali et al., 2005). However, the current study did not include a sham phase; hence, we did not measure the potential advantage of the left IPL stimulation condition in relation to no stimulation or sham.
Changes in the frequency of the words retrieved in letter fluency were only significant after LeFT was combined with left IFG stimulation, and not in the IPL stimulation phase. This is in line with research indicating that the left IFG is particularly involved in lexical retrieval (Sharp et al., 2005), and supports our claim that stimulation facilitated changes at this level of processing. The IFG triangularis, in particular, has been shown to be responsible for active retrieval of information stored in the temporal areas (Petrides, 1995; Thompson-Schill et al., 1997). In addition, a recent study from our group showed that tDCS over the left IFG triangularis improved the retrieval of lexical representations by modulating the functional connectivity between the left IFG and the left inferior temporal gyrus, an area involved in long-term storage of lexical representations (Ficek et al., 2018). This is probably achieved via structural connections between the IFG and the inferior temporal gyrus through the extreme capsule (Zhao et al. in press). However, the degree of change in frequency and AoA was not significantly different between the two treatment phases, and there were no changes in semantic variables, in either phase. Altogether, these results suggest that the mechanisms of change may have been similar across phases, with change occurring at the lexical level. In addition, results also indicate that such treatment related changes in processing were enhanced to a greater extent and therefore more robust with IFG stimulation.
Changes in letter fluency for untrained letters were not significantly different between stimulation conditions. Altogether, we can state that the IFG stimulation combined with LeFT resulted in stronger and longer sustained effects for trained letters, but not in greater generalization to untrained letters. It should be considered if a benefit that is specific to trained items is clinically meaningful, as it may not translate to improvements in everyday communication. We argue that the absence of differences between stimulation conditions for untrained letters does not diminish, in itself, the potential relevance of administering left IFG tDCS along with LeFT. Differently from conventional naming therapies, letter fluency therapy is not given in specific words; rather, letters are used as stimuli and the patient can produce a rich variety of lexical items. Also, in conventional naming therapies, including all possible words as trained items is not clinically feasible, and thus generalization is particularly important. In contrast, with LeFT, including all letters of the language in the treatment sessions is not difficult. Because of these differences, within task-generalization is not as important in LeFT as it would be in conventional naming therapy.
A potentially more meaningful kind of generalization is transfer of effects to an untrained task. This kind of generalization may have a greater impact in functional communication, as it signals transfer of treatment benefits beyond the context in which treatment is provided. Changes in accuracy in the untrained object naming task were significantly larger in Phase 1 (with left IFG stimulation) than during Phase 2 (left IPL stimulation), both immediately after therapy, and at the follow-up assessment. Our results thus suggest that this transfer may be more substantial when LeFT is paired with left IFG tDCS, compared to left IPL tDCS.
The recent meta-analysis of studies testing improvement in naming abilities in PPA after language training and tDCS (Cotelli et al., 2020) reports that oral naming of untrained items only improves when treatment is combined with tDCS. The current study adds that stimulation protocols may not all be equally effective in inducing such generalized improvement: we find improvement in the untrained picture naming task only when LeFT is combined with left IFG tDCS. Nonetheless, in addition to differences in the stimulation protocol, the difference in gain across phases in this untrained picture naming task may also be a product of different pre-therapy performance levels across phases in this particular task, due to sustained improvement coming from phase 1. This way, we also agree with Cotelli and colleagues (2020), and suggest that future studies using randomized allocation to different treatment orders, or inversion of the treatment protocol within a single subject should be conducted, to lead to a definite conclusion as to whether transfer to untrained contexts is indeed larger with left IFG tDCS compared to left IPL tDCS, and if either approach is more beneficial than Sham.
5. CONCLUSION
There is an increasing awareness of the role of speech and language therapy in PPA (Taylor et al., 2009) and the potential augmentation of language therapy with tDCS (Tsapkini et al., 2018). Letter fluency is a challenging task even for individuals that perform within norm. Here we show for the first time that practicing letter fluency in the oral modality, combined with anodal tDCS, results in improved word retrieval. Importantly, this approach led to improvements not only for treated letters, but performance improved also when retrieving words with untreated letters, and in an untreated picture naming task (where a trade-off between speed and accuracy may be occurring). Treatment effects for trained letters and the transfer to the untrained naming task were more prominent when LeFT was combined with tDCS administered to the left IFG compared to the left IPL. These findings highlight a potential of this task for promoting generalized improvement in lexical retrieval, particularly if combined with left IFG stimulation.
Furthermore, improvements obtained with this approach show maintenance for up to three months, even in the presence of a degenerative disease. Given the progressive nature of PPA, and the difficulty in providing therapies that are challenging enough for individuals who are at early stages of the disease, this study provides preliminary yet important evidence of a potentially useful treatment approach for individuals with mild language impairment. Future research including a Sham phase, a cross-over design, and a larger cohort of patients is recommended to evaluate effects of IFG and IPL stimulation over Sham and to replicate the findings reported in this article.
Table 1.
Diagnostic assessment
| Task | Before Phase 1 | After Phase 1 | Before Phase 2 | After Phase 2 | |
|---|---|---|---|---|---|
| Conceptual | Object concepts (PPT) | 15/15 | 15/15 | 15/15 | 15/15 |
| Action concepts (Kissing and dancing test) | 14/15 | 15/15 | 13/15 | 14/15 | |
| Lexico-semantic | Naming nouns (BNT) | 27/30, 27/30 | 27/30, 29/30 | 27/30, 28/30 | 26/30, 26/30 |
| Naming verbs (HANA) | 25/35 | 27/35 | 28/35 | 27/35 | |
| Word reading - high imageability, high frequency (PALPA 31) | 20/20 | NA | 20/20 | 19/20 | |
| Word reading - high imageability, low frequency (PALPA 31) | 20/20 | NA | 18/20 | 18/20 | |
| Word reading - low imageability, high frequency (PALPA 31) | 19/20 | NA | 18/20 | 19/20 | |
| Word reading - low imageability, low frequency (PALPA 31) | 18/20 | NA | 18/20 | 16/20 | |
| Word reading - regular words (PALPA 35) | 29/30 | NA | 29/30 | 30/30 | |
| Word reading - exception words (PALPA 35) | 27/30 | NA | 26/30 | 27/30 | |
| Spelling nouns (JHU dysgraphia battery) | NA | 12/12 | 12/12 | 12/12 | |
| Spelling verbs (JHU dysgraphia battery) | NA | 12/12 | 12/12 | 12/12 | |
| Grammatical | Sentence anagrams (actives) | NA | 5/5 | 5/5 | 5/5 |
| Sentence anagrams (passives) | NA | 5/5 | 3/5 | 4/5 | |
| Sentence comprehension (actives, SOAP) | 10/10 | 10/10 | 10/10 | 10/10 | |
| Sentence comprehension (passives, SOAP) | 10/10 | 10/10 | 10/10 | 9/10 | |
| Sentence comprehension (subject relatives, SOAP) | 10/10 | 10/10 | 10/10 | 10/10 | |
| Sentence comprehension (object relatives, SOAP) | 7/10 | 8/10 | 6/10 | 8/10 | |
| Sentence repetition (whole sentence score) | 1/5 | 1/5 | 1/5 | 1/5 | |
| Sentence repetition (N correct words) | 31/37 | 30/37 | 28/37 | 33/37 | |
| Cognitive screening | Digit span forward | 4 | 4.5 | 3.5 | 3.5 |
| Digit span backward | 4 | 3 | 3.5 | 3 | |
| Spatial span forward | 6 | 6.5 | 5.5 | 4 | |
| Spatial span backward | 4.5 | 5 | 6 | 5 | |
| Verbal Learning (RAVLT - sum of 5 first trials) | 39 | 48 | 37 | 46 | |
| Category fluency (fruits/animals/vegetables/actions) | 36 | 31 | 35 | 36 |
Table 1. AGG’s scores in a diagnostic assessment. PPT: Pyramids and Palm Trees test (Howard & Patterson, 1992); BNT: Boston Naming Test (Kaplan, Goodglass, Weintraub, & Goodglass, 1983); HANA: Hopkins Assessment for Naming Actions (Breining et al., 2015); PALPA: Psycholinguistic Assessment of Language Processing in Aphasia (Kay, Lesser, & Coltheart, 1996); SOAP: Subject Object Actives Passives (Love & Oster, 2002); RAVLT: Rey Auditory Verbal Learning Test (Schmidt, 1996).
ACKNOWLEDGEMENTS
We would like to thank AGG for participating in this research, and for sharing his delightful stories. AGG is a wonderful story-teller with both his words and his drawings. At his request, we are thrilled to share the poem he wrote during his participation in this study, in between the two treatment phases (see Appendix 1).
This work was supported by grants from the Science of Learning Institute at Johns Hopkins University and by the National Institutes of Health (National Institute of Deafness and Communication Disorders) through award R01 DC014475 to KT.
Appendix 1: Poem written by AGG
I am embracing my aphasia
“Man was made for joy and woe;And when this we rightly know,Thro’ the world we safely go.
Joy and woe are woven fine,A clothing for the soul divine.Under every grief and pineRuns a joy with silken twine.”
This is a part of the poem “Auguries of Innocence,” 1803 by William Blake.
I was reading a biography of Kahlil Gibran. The author’s name is Paul-Gordon Chandler. (An Aside: Paul-Gordon invited me to participate as an artist in his “Caravan Project” called “Amen.” It was 2015. I made a statue and a lady bought the statue and now, the statue is in the St. Albans High School in Washington, DC, on the grounds of the National Cathedral.)
When Paul-Gordon was working in London, he met Malcolm Muggeride, the English writer, mystic, and the former BBC commentator. Muggeridge told Paul-Gordon over his fascinating and at times troubled life that William Blake had experienced profound states of celebration and desperation.
“Under every grief and pineRuns a joy with silken twine.”
Kahlil Gibran met the great French sculptor, August Rodin, and Rodin introduced Kahlil to William Blake’s poetry. Blake and Gibran understood that Joy and Suffering are the two poles between which the current of life passes.
Kahlil wrote, “For he who has not looked on Sorrow will never see Joy.”
Now, I have been diagnosed with “Primary Progressive Aphasia,” a part of dementia that undermines my speaking to others.
When I was diagnosed, I realized that my speaking will be comprimised over my life.
And reading the biography of Gibran, I also realized my “compromised speech” that’s part of my Life’s Sorrow and will see Joy again. Every Day.
Today, I am celebrating with my wife, family, and friends. And, we will celebrate and continue with Joy in our Life!
Footnotes
DISCLOSURE OF INTEREST
The authors report no conflict of interest.
REFERENCES
- Baayen RH, Piepenbrock R, & Gulikers L. (1995). The CELEX lexical database (release 2). Distributed by the Linguistic Data Consortium, University of Pennsylvania. [Google Scholar]
- Baldo JV, Schwartz S, Wilkins D, & Dronkers NF (2006). Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. Journal of the International Neuropsychological Society, 12(06), 896–900. [DOI] [PubMed] [Google Scholar]
- Basho S, Palmer ED, Rubio MA, Wulfeck B, & Müller R-A (2007). Effects of generation mode in fMRI adaptations of semantic fluency: Paced production and overt speech. Neuropsychologia, 45(8), 1697–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson PM, King RM, Bonakdarpour B, Henry ML, Cho H, & Rapcsak SZ (2011). Positive effects of language treatment for the logopenic variant of primary progressive aphasia. Journal of Molecular Neuroscience: MN, 45(3), 724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL, Hamsher KS, & Sivan AB (1994). Multilingual aphasia examination (3rd ed.). Lutz, FL: Psychological Assessment Resources (PAR). [Google Scholar]
- Bird H, Lambon Ralph MA, Patterson K, & Hodges JR (2000). The rise and fall of frequency and imageability: noun and verb production in semantic dementia. Brain and Language, 73(1), 17–49. [DOI] [PubMed] [Google Scholar]
- Birn RM, Kenworthy L, Case L, Caravella R, Jones TB, Bandettini PA, & Martin A. (2010). Neural systems supporting lexical search guided by letter and semantic category cues: a self-paced overt response fMRI study of verbal fluency. NeuroImage, 49(1), 1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Macedo E. C. de, Pascual-Leone A, Muñoz JMT, SchWartzman JS, & Fregni F. (2009). Neuromodulation in hypoxic-ischemic injury. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation, 2(3), 179–181. [DOI] [PubMed] [Google Scholar]
- Boyle M, & Coelho CA (1995). Application of Semantic Feature Analysis as a Treatment for Aphasic Dysnomia. American Journal of Speech Language Pathology, 4(4), 94–98. [Google Scholar]
- Breining BL, Tippett DC, Davis C, Posner J, Sebastian R, Oishie K, & ... (2015). Assessing dissociations of object and action naming in acute stroke. Paper Presented at the Clinical Aphasiology Conference, Monterey, CA. [Google Scholar]
- Bruce C, & Howard D. (1988). Why don’t Broca’s aphasics cue themselves? An investigation of phonemic cueing and tip of the tongue information. Neuropsychologia, 26(2), 253–264. [DOI] [PubMed] [Google Scholar]
- Brysbaert M, Warriner AB, & Kuperman V. (2014). Concreteness ratings for 40 thousand generally known English word lemmas. Behavior Research Methods, 46(3), 904–911. [DOI] [PubMed] [Google Scholar]
- Carroll JB, & White MN (1973). Word frequency and age of acquisition as determiners of picture-naming latency. Quarterly Journal of Experimental Psychology, 25(1), 85–95. [Google Scholar]
- Cattaneo Z, Pisoni A, & Papagno C. (2011). Transcranial direct current stimulation over Broca’s region improves phonemic and semantic fluency in healthy individuals. Neuroscience, 183, 64–70. [DOI] [PubMed] [Google Scholar]
- Coltheart M. (1981). The MRC psycholinguistic database. The Quarterly Journal of Experimental Psychology, 33(4), 497–505. [Google Scholar]
- Cotelli M, Manenti R, Ferrari C, Gobbi E, Macis A, & Cappa SF (2020). Effectiveness of language training and non-invasive brain stimulation on oral and written naming performance in Primary Progressive Aphasia: A meta-analysis and systematic review. Neuroscience & Biobehavioral Reviews, 108, 498–525. [DOI] [PubMed] [Google Scholar]
- Cotelli M, Manenti R, Petesi M, Brambilla M, Cosseddu M, Zanetti O, … Borroni B. (2014). Treatment of primary progressive aphasias by transcranial direct current stimulation combined with language training. Journal of Alzheimer’s Disease: JAD, 39(4), 799–808. [DOI] [PubMed] [Google Scholar]
- Crawford JR, & Howell DC (1998). Comparing an individual’s test score against norms derived from small samples. The Clinical Neuropsychologist, 12(4), 482–486. [Google Scholar]
- Croot K, Nickels L, Laurence F, & Manning M. (2009). Impairment-and activity/participation-directed interventions in progressive language impairment: Clinical and theoretical issues. Aphasiology, 23(2), 125–160. [Google Scholar]
- Czarnecki K, Duffy JR, Nehl CR, Cross SA, Molano JR, Jack CR, … Boeve BF (2008). Very Early Semantic Dementia With Progressive Temporal Lobe Atrophy: An 8-Year Longitudinal Study. Archives of Neurology, 65(12), 1659–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Aguiar V, Paolazzi CL, & Miceli G. (2015). tDCS in post-stroke aphasia: the role of stimulation parameters, behavioral treatment and patient characteristics. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 63, 296–316. [DOI] [PubMed] [Google Scholar]
- Ellis AW, & Young AW (1988). Human Cognitive Neuropsychology. Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Evans WS, Quimby M, Dickey MW, & Dickerson BC (2016). Relearning and Retaining Personally-Relevant Words using Computer-Based Flashcard Software in Primary Progressive Aphasia. Frontiers in Human Neuroscience, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernaeus S-E, & Almkvist O. (1998). Word Production: Dissociation of Two Retrieval Modes of Semantic Memory Across Time. Journal of Clinical and Experimental Neuropsychology, 20(2), 137–143. 10.1076/jcen.20.2.137.1170 [DOI] [PubMed] [Google Scholar]
- Ficek BN, Wang Z, Zhao Y, Webster KT, Desmond JE, Hillis AE, … Tsapkini K. (2018). The effect of tDCS on functional connectivity in primary progressive aphasia. NeuroImage: Clinical. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes-McKay KE, Ellis AW, Shanks MF, & Venneri A. (2005). The age of acquisition of words produced in a semantic fluency task can reliably differentiate normal from pathological age-related cognitive decline. Neuropsychologia, 43(11), 1625–1632. 10.1016/j.neuropsychologia.2005.01.008 [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Rorden C, Elm J, Sen S, George MS, & Bonilha L. (2018). Transcranial Direct Current Stimulation vs Sham Stimulation to Treat Aphasia After Stroke: A Randomized Clinical Trial. JAMA Neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CHY, Morgan K, Suckling J, Williams SCR, Andrew C, Vythelingum GN, & McGuire PK (2002). A functional magnetic resonance imaging study of overt letter verbal fluency using a clustered acquisition sequence: greater anterior cingulate activation with increased task demand. NeuroImage, 17(2), 871–879. [PubMed] [Google Scholar]
- Goodglass H, Wingfield A, Hyde MR, Gleason JB, Bowles NL, & Gallagher RE (1997). The importance of word-initial phonology: Error patterns in prolonged naming efforts by aphasic patients. Journal of the International Neuropsychological Society, 3(2), 128–138. [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, … Miller BL (2004). Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology, 55(3), 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, … Grossman M. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Murray RC, Rankin KP, Weiner MW, & Miller BL (2004). Clinical, cognitive and anatomical evolution from nonfluent progressive aphasia to corticobasal syndrome: a case report. Neurocase, 10(6), 426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M. (2010). Primary progressive aphasia: clinicopathological correlations. Nature Reviews.Neurology, 6(2), 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Murray. (2014). Biomarkers in the primary progressive aphasias. Aphasiology, 28(8–9), 922–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Murray, & Ash S. (2004). Primary Progressive Aphasia. Neurocase (Psychology Press), 10(1), 3–18. [DOI] [PubMed] [Google Scholar]
- Heim S, Eickhoff SB, & Amunts K. (2008). Specialisation in Broca’s region for semantic, phonological, and syntactic fluency? NeuroImage, 40(3), 1362–1368. [DOI] [PubMed] [Google Scholar]
- Henry JD, & Crawford JR (2004). A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology, 18(2), 284–295. [DOI] [PubMed] [Google Scholar]
- Henry ML, Rising K, DeMarco AT, Miller BL, Gorno-Tempini ML, & Beeson PM (2013). Examining the value of lexical retrieval treatment in primary progressive aphasia: two positive cases. Brain and Language, 127(2), 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia CG, Sage K, Ralph MAL, & Berthier ML (2009). Relearning and retention of verbal labels in a case of semantic dementia. Aphasiology, 23(2), 192–209. [Google Scholar]
- Herwig U, Satrapi P, & Schönfeldt-Lecuona C. (2003). Using the International 10–20 EEG System for Positioning of Transcranial Magnetic Stimulation. Brain Topography, 16(2), 95–99. [DOI] [PubMed] [Google Scholar]
- Hirshorn EA, & Thompson-Schill SL (2006). Role of the left inferior frontal gyrus in covert word retrieval: neural correlates of switching during verbal fluency. Neuropsychologia, 44(12), 2547–2557. [DOI] [PubMed] [Google Scholar]
- Homan RW (1988). The 10–20 electrode system and cerebral location. American Journal of EEG Technology, 28(4), 269–279. [Google Scholar]
- Howard D, & Patterson K. (1992). Pyramids and palm trees: a test of semantic access from pictures and words (Thames Valley Test Company, Bury St. Edmunds, UK: ). [Google Scholar]
- Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, & Wassermann EM (2005). Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology, 64(5), 872–875. [DOI] [PubMed] [Google Scholar]
- Johnson JK, Diehl J, Mendez MF, Neuhaus J, Shapira JS, Forman M, … Miller BL (2005). Frontotemporal Lobar Degeneration: Demographic Characteristics of 353 Patients. Archives of Neurology, 62(6), 925–930. [DOI] [PubMed] [Google Scholar]
- Jokel R, Cupit J, Rochon E, & Leonard C. (2009). Relearning lost vocabulary in nonfluent progressive aphasia with MossTalk Words®. Aphasiology, 23(2), 175–191. [Google Scholar]
- Jokel R, Graham NL, Rochon E, & Leonard C. (2014). Word retrieval therapies in primary progressive aphasia. Aphasiology, 28(8–9), 1038–1068. [Google Scholar]
- Jokel R, Rochon E, & Leonard C. (2006). Treating anomia in semantic dementia: improvement, maintenance, or both? Neuropsychological Rehabilitation, 16(3), 241–256. [DOI] [PubMed] [Google Scholar]
- Juhasz BJ, Lai Y-H, & Woodcock ML (2015). A database of 629 English compound words: ratings of familiarity, lexeme meaning dominance, semantic transparency, age of acquisition, imageability, and sensory experience. Behavior Research Methods, 47(4), 1004–1019. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S, & Goodglass H. (1983). Boston naming test. Philadelphia: Lea & Febiger. [Google Scholar]
- Kay J, Lesser R, & Coltheart M. (1996). Psycholinguistic assessments of language processing in aphasia (PALPA): An introduction. Aphasiology, 10(2), 159–180. [Google Scholar]
- Knopman DS, Kramer JH, Boeve BF, Caselli RJ, Graff-Radford NR, Mendez MF, … Mercaldo N. (2008). Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain, 131(11), 2957–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman V, Stadthagen-Gonzalez H, & Brysbaert M. (2012). Age-of-acquisition ratings for 30,000 English words. Behavior Research Methods, 44(4), 978–990. 10.3758/s13428-012-0210-4 [DOI] [PubMed] [Google Scholar]
- Love T, & Oster E. (2002). On the Categorization of Aphasic Typologies: The SOAP (A Test of Syntactic Complexity). Journal of Psycholinguistic Research, 31(5), 503–529. 10.1023/A:1021208903394 [DOI] [PubMed] [Google Scholar]
- Luzzatti C, Raggi R, Zonca G, Pistarini C, Contardi A, & Pinna G-D (2002). Verb–noun double dissociation in aphasic lexical impairments: The role of word frequency and imageability. Brain and Language, 81(1–3), 432–444. [DOI] [PubMed] [Google Scholar]
- Mack JE, Chandler SD, Meltzer-Asscher A, Rogalski E, Weintraub S, Mesulam M-M, & Thompson CK (2015). What do pauses in narrative production reveal about the nature of word retrieval deficits in PPA? Neuropsychologia, 77, 211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macoir J, Leroy M, Routhier S, Auclair-Ouellet N, Houde M, & Jr RL (2015). Improving verb anomia in the semantic variant of primary progressive aphasia: the effectiveness of a semantic-phonological cueing treatment. Neurocase, 21(4), 448–456. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, & Kertesz A. (2006). Category and letter fluency in semantic dementia, primary progressive aphasia, and Alzheimer’s disease. Brain and Language, 97(3), 258–265. [DOI] [PubMed] [Google Scholar]
- Medina J, & Weintraub S. (2007). Depression in Primary Progressive Aphasia. Journal of Geriatric Psychiatry and Neurology, 20(3), 153–160. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Lindenberg R, Sieg MM, Nachtigall L, Ulm L, & Flöel A. (2014). Transcranial direct current stimulation of the primary motor cortex improves word-retrieval in older adults. Frontiers in Aging Neuroscience, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM (2001). Primary progressive aphasia. Annals of Neurology, 49(4), 425–432. [PubMed] [Google Scholar]
- Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, … Bigio EH (2008). Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Annals of Neurology, 63(6), 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Getz H, Snider S, Sullivan K, Long S, Turner R, & Friedman R. (2013). Remediation and Prophylaxis of Anomia in Primary Progressive Aphasia. Procedia, Social and Behavioral Sciences, 94, 275–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Getz HR, Brennan DM, Hu TM, & Friedman RB (2015). Telerehabilitation of anomia in primary progressive aphasia. Aphasiology. Retrieved from http://www.tandfonline.com/doi/abs/10.1080/02687038.2015.1081142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti A, Ferrucci R, Fumagalli M, Mameli F, Cogiamanian F, Ardolino G, & Priori A. (2012). Transcranial direct current stimulation (tDCS) and language. Journal of Neurology, Neurosurgery, and Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wu D, Ceritoglu C, Li Y, Kolasny A, Vaillant MA, ... & Miller MI. (2016). MRICloud: delivering high-throughput MRI neuroinformatics as cloud-based software as a service. Computing in Science & Engineering, 18(5), 21–35. [Google Scholar]
- Nickels L, & Croot K. (2014). Understanding and living with primary progressive aphasia: Current progress and challenges for the future. Aphasiology, 28(8–9), 885–899. [Google Scholar]
- Penolazzi B, Bergamaschi S, Pastore M, Villani D, Sartori G, & Mondini S. (2015). Transcranial direct current stimulation and cognitive training in the rehabilitation of Alzheimer disease: A case study. Neuropsychological Rehabilitation, 25(6), 799–817. [DOI] [PubMed] [Google Scholar]
- Pereira JB, Junqué C, Bartrés-Faz D, Martí MJ, Sala-Llonch R, Compta Y, … Tolosa E. (2013). Modulation of verbal fluency networks by transcranial direct current stimulation (tDCS) in Parkinson’s disease. Brain Stimulation, 6(1), 16–24. [DOI] [PubMed] [Google Scholar]
- Petrides M. (1995). Functional organization of the human frontal cortex for mnemonic processing. Evidence from neuroimaging studies. Annals of the New York Academy of Sciences, 769, 85–96. [DOI] [PubMed] [Google Scholar]
- Pisoni A, Mattavelli G, Papagno C, Rosanova M, Casali AG, Lauro R, & J L. (2018). Cognitive Enhancement Induced by Anodal tDCS Drives Circuit-Specific Cortical Plasticity. Cerebral Cortex, 28(4), 1132–1140. [DOI] [PubMed] [Google Scholar]
- Radman N, Britz J, Buetler K, Weekes BS, Spierer L, & Annoni J-M (2018). Dorsolateral Prefrontal Transcranial Direct Current Stimulation Modulates Language Processing but Does Not Facilitate Overt Second Language Word Production. Frontiers in Neuroscience, 12, 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rofes A, de Aguiar V, & Miceli G. (2015). A minimal standardization setting for language mapping tests: an Italian example. Neurological Sciences, 36(7), 1113–1119. [DOI] [PubMed] [Google Scholar]
- Rofes A, De Aguiar V, Ficek B, Wendt H, Webster K, & Tsapkini K. (2019). The role of word properties in performance on fluency tasks in people with primary progressive aphasia. Journal of Alzheimer’s Disease, 68(4), 1521–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rofes A, de Aguiar V, Jonkers R, Oh SJ, Dede G, Sung JE (in press; ). What drives task performance during animal fluency in people with Alzheimer’s disease? Frontiers in Psychology, Language Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rofes A, Zakariás L, Ceder K, Lind M, Johansson MB, Aguiar V. de, … Howard D. (2017). Imageability ratings across languages. Behavior Research Methods, 1–11. [DOI] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Weintraub S, & Mesulam MM (2011). Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology, 76(21), 1804–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossion B, & Pourtois G. (2004). Revisiting Snodgrass and Vanderwart’s object pictorial set: the role of surface detail in basic-level object recognition. Perception, 33(2), 217–236. [DOI] [PubMed] [Google Scholar]
- Santos MD, Gagliardi RJ, Mac-Kay APMG, Boggio PS, Lianza R, Fregni F, … Fregni F. (2013). Transcranial direct-current stimulation induced in stroke patients with aphasia: a prospective experimental cohort study. Sao Paulo Medical Journal, 131(6), 422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. (1996). Rey auditory verbal learning test: A handbook. Western Psychological Services Los; Angeles, CA. [Google Scholar]
- Sebastian R, Tsapkini K, & Tippett DC (2016). Transcranial direct current stimulation in post stroke aphasia and primary progressive aphasia: Current knowledge and future clinical applications. NeuroRehabilitation, 39(1), 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Janse E, Visser K, & Meyer AS (2014). What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Frontiers in Psychology, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Scott SK, Cutler A, & Wise RJS (2005). Lexical retrieval constrained by sound structure: The role of the left inferior frontal gyrus. Brain and Language, 92(3), 309–319. [DOI] [PubMed] [Google Scholar]
- Smirni D, Turriziani P, Mangano GR, Bracco M, Oliveri M, & Cipolotti L. (2017). Modulating phonemic fluency performance in healthy subjects with transcranial magnetic stimulation over the left or right lateral frontal cortex. Neuropsychologia, 102, 109–115. [DOI] [PubMed] [Google Scholar]
- Stadthagen-Gonzalez H, & Davis CJ (2006). The Bristol norms for age of acquisition, imageability, and familiarity. Behavior Research Methods, 38(4), 598–605. [DOI] [PubMed] [Google Scholar]
- Taylor C, Kingma RM, Croot K, & Nickels L. (2009). Speech pathology services for primary progressive aphasia: Exploring an emerging area of practice. Aphasiology, 23(2), 161–174. [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, & Farah MJ (1997). Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proceedings of the National Academy of Sciences, 94(26), 14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN, Kozak J, & Rees L. (1999). Normative Data Stratified by Age and Education for Two Measures of Verbal Fluency: FAS and Animal Naming. Archives of Clinical Neuropsychology, 14(2), 167–177. [PubMed] [Google Scholar]
- Tsapkini K, Frangakis C, Gomez Y, Davis C, & Hillis AE (2014). Augmentation of spelling therapy with transcranial direct current stimulation in primary progressive aphasia: Preliminary results and challenges. Aphasiology, 28((8–9), 1112–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K, & Hillis AE (2013). Spelling intervention in post-stroke aphasia and primary progressive aphasia. Behavioural Neurology, 26(1–2), 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini Kyrana, Webster KT, Ficek BN, Desmond JE, Onyike CU, Rapp B, … Hillis AE (2018). Electrical brain stimulation in different variants of primary progressive aphasia: A randomized clinical trial. Alzheimer’s & Dementia (New York, N. Y.), 4, 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannorsdall TD, Schretlen DJ, Andrejczuk M, Ledoux K, Bosley LV, Weaver JR, … Gordon B. (2012). Altering Automatic Verbal Processes with Transcranial Direct Current Stimulation. Frontiers in Psychiatry, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannorsdall TD, van Steenburgh JJ, Schretlen DJ, Jayatillake R, Skolasky RL, & Gordon B. (2016). Reproducibility of tDCS Results in a Randomized Trial: Failure to Replicate Findings of tDCS-Induced Enhancement of Verbal Fluency. Cognitive and Behavioral Neurology: Official Journal of the Society for Behavioral and Cognitive Neurology, 29(1), 11–17. [DOI] [PubMed] [Google Scholar]
- Vita MG, Marra C, Spinelli P, Caprara A, Scaricamazza E, Castelli D, … Quaranta D. (2014). Typicality of words produced on a semantic fluency task in amnesic mild cognitive impairment: linguistic analysis and risk of conversion to dementia. Journal of Alzheimer’s Disease: JAD, 42(4), 1171–1178. [DOI] [PubMed] [Google Scholar]
- Vitali P, Abutalebi J, Tettamanti M, Rowe J, Scifo P, Fazio F, … Perani D. (2005). Generating animal and tool names: An fMRI study of effective connectivity. Brain and Language, 93(1), 32–45. [DOI] [PubMed] [Google Scholar]
- Volkmer A, Spector A, Meitanis V, Warren JD, & Beeke S. (2020). Effects of functional communication interventions for people with primary progressive aphasia and their caregivers: a systematic review. Aging & mental health, 24(9), 1381–1393. [DOI] [PubMed] [Google Scholar]
- Westwood SJ, & Romani C. (2018). Null Effects on Working Memory and Verbal Fluency Tasks When Applying Anodal tDCS to the Inferior Frontal Gyrus of Healthy Participants. Frontiers in Neuroscience, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Ficek B, Webster K, Wang Z, Desmond JE, Frangakis CE, Caffo B, Hillis AE, Faria A, & Tsapkini K. White-matter integrity predicts electrical stimulation and language therapy effects in primary progressive aphasia. Neurorehabilitation and Neural Repair, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]