Abstract
Interstitial lung disease (ILD) is a significant complication of many systemic autoimmune rheumatic diseases (SARDs), although the clinical presentation, severity and outlook may vary widely between individuals. Despite the prevalence, there are no specific guidelines addressing the issue of screening, diagnosis and management of ILD across this diverse group. Guidelines from the ACR and EULAR are expected, but there is a need for UK-specific guidelines that consider the framework of the UK National Health Service, local licensing and funding strategies. This article outlines the intended scope for the British Society for Rheumatology guideline on the diagnosis and management of SARD-ILD developed by the guideline working group. It specifically identifies the SARDs for consideration, alongside the overarching principles for which systematic review will be conducted. Expert consensus will be produced based on the most up-to-date available evidence for inclusion within the final guideline. Key issues to be addressed include recommendations for screening of ILD, identifying the methodology and frequency of monitoring and pharmacological and non-pharmacological management. The guideline will be developed according to methods and processes outlined in Creating Clinical Guidelines: British Society for Rheumatology Protocol version 5.1.
Keywords: guideline, interstitial lung disease, connective tissue disease, UK, scope
Why the guideline is needed
Systemic autoimmune rheumatic diseases (SARDs) is a term that covers a broad spectrum of clinical conditions of autoimmune aetiology [1]. Interstitial lung disease (ILD) is a common manifestation of several SARDs and is associated with excess morbidity and mortality.
SARDs associated with ILD include RA, SSc, SS, idiopathic inflammatory myopathy (IIM), MCTD, SLE, ANCA-associated vasculitis (AAV) and axial SpA (axSpA). The terms CTD and collagen vascular disease have been historically used to describe this group of conditions and particularly SSc, SS, IIM, MCTD and SLE. However, CTD and collagen vascular disease are not reflective of our current understanding of the pathophysiology or organ involvement of these conditions and the term SARD is therefore preferred for the purposes of this guideline.
There are currently no UK guidelines related to the management of SARD-ILD and there is a concern that the condition may be underdiagnosed and consequently undertreated. It is important to understand that within the broad spectrum of SARD-ILD, distinct clinical phenotypes such as rapidly progressive ILD and progressive pulmonary fibrosis (PPF) (also termed progressive fibrosing ILD) have specific diagnostic and management considerations.
Strategies for screening people with existing SARDs for ILD are not clearly defined. In RA, many people have subclinical ILD and it is unclear if abnormalities on imaging in the absence of clinical symptoms should influence treatment and the frequency of monitoring. Screening for ILD also has the potential to overdiagnose minor interstitial lung abnormalities as ILD, the prognostic significance of which is unclear.
The data supporting the use of commonly used immunomodulatory strategies and newer therapies (e.g. antifibrotic drugs) in the treatment of SARD-ILD is steadily growing [2–4]. Antifibrotic drugs are now recommended in the National Institute for Health and Care Excellence (NICE) guideline for pulmonary fibrosis–ILD [5] and it is vital that all healthcare professionals caring for people with SARD-ILD are aware of the appropriate use of such treatment.
A EULAR task force is expected to publish its guideline for SARD-ILD in 2024 and the ACR published their summary guidance in August 2023 [6]. However, there is a need for UK-specific guidance that takes into account the resources of the UK health service, including local licencing and funding policies. The guideline will therefore be a useful document to support local and national policy-making in the UK.
This guideline will be developed using the methods and processes outlined in Creating Clinical Guidelines: British Society for Rheumatology Protocol [7].
Facts and figures
SARDs are estimated to account for ≈30% of ILDs [8]. Among those with SARD, the incidence of ILD varies. Interstitial lung abnormalities in RA are common, but only 5–11% are thought to result in clinically significant symptoms [9, 10]. ILD is estimated to affect 39–72% with MCTD, 44–50% with SSc, 33–50% with IIM, 12–21% with primary SS [11], 3–40% with AAV [12–14], 3–10% with SLE [11] and 7–30% with axSpA [15, 16]. The outlook is highly variable following SARD-ILD diagnosis. Life expectancy can vary widely, e.g. from 8 years post-diagnosis in RA-ILD [17] to 6-month survival rates of 33–66% in those with rapidly progressive melanoma differentiation-associated protein 5–positive IIM-ILD [18].
Current practice
Current practice is variable across the UK [19]. Most people with SARD-ILD are managed in secondary or tertiary care settings, with dual-specialty rheumatology–respiratory clinics and multidisciplinary teams (MDTs) operating in some, but not all, centres. Previous disease-specific British Society for Rheumatology (BSR) guidelines have advocated screening for ILD in all people with SSc [20] and those with high-risk IIM subtypes [21], but no such recommendations have been made in RA or other SARDs.
The NICE idiopathic pulmonary fibrosis (IPF) guideline states that people with suspected ILD should be referred to a specialist centre for confirmation of the diagnosis and development of a treatment plan by an MDT with relevant clinical expertise and training [22]. The approach to SARD-ILD diagnosis can vary depending on the availability of resources and expertise. Some medical therapies are used off-licence and reimbursement is often dependent on validation from the treating tertiary centre, but varies by region.
Immunosuppressive medication is frequently used in the treatment of SARD-ILD, including corticosteroids, azathioprine, mycophenolate mofetil, abatacept, tocilizumab, rituximab and cyclophosphamide [2, 3, 23–25]. Antifibrotic medications are now approved by NICE for use in people with progressive non-IPF ILD and may be used alongside immunomodulatory therapies [5].
In RA, methotrexate (MTX) is a recommended first-line treatment for active joint disease. However, it is still sometimes avoided in those with RA-ILD due to concerns regarding MTX pneumonitis, which carries a higher mortality in people with reduced pulmonary reserve [26]. This remains an area of controversy and will be examined in detail in the guideline.
Who the guideline is for?
This guideline is for any health professional in the UK who cares directly for people with SARD-ILD, including rheumatologists, pulmonologists, rheumatology and respiratory specialist nurses, radiologists, primary care clinicians, allied health professionals, specialty trainees, pharmacists and other stakeholders such as patient organizations and charities.
Equality considerations
People of all ethnicities are affected by SARD-ILD, meaning language and cultural barriers will have to be addressed to ensure all receive equitable access to care and high-quality education and treatment.
What will the guideline cover?
This guideline is being developed to provide recommendations on the screening, diagnosis, monitoring, pharmacological and non-pharmacological management of adults with SARD-ILD in the UK.
Groups that will be covered
Overarching principles will cover people with ILD associated with RA, SSc, SS, IIM, MCTD, SLE, AAV and axSpA. Another group included at the scoping stage are those people with ILD in whom clinical and serological profiles raise suspicion of SARD but do not meet classification criteria of a specific diagnosis. This will include patients in which ILD is the first, the predominant or the sole manifestation of SARD. This group remains poorly defined and previous labels include interstitial pneumonia with autoimmune features (IPAF), undifferentiated CTD-associated ILD, lung-dominant CTD and autoimmune-featured ILD. Each term has different but overlapping criteria. For the purposes of this guideline, this group will be referred to as ‘ILD with suspected SARD’ in order to ensure that our literature search includes relevant evidence for all the aforementioned terms.
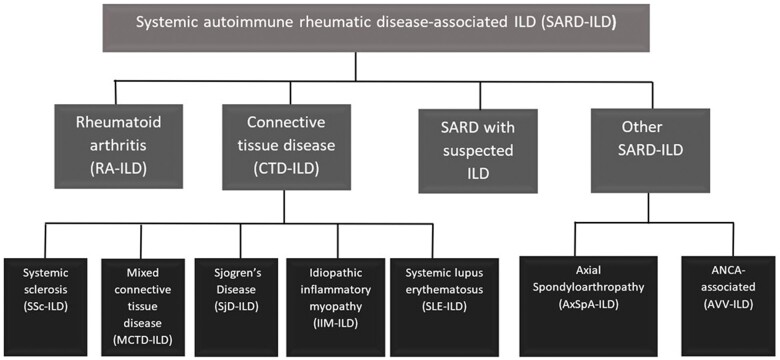
For the purposes of the literature review, we elected to divide SARDs into the following four groups (Fig. 1): RA-ILD, CTD-ILD (SSc, IIM, MCTD, SS and SLE-related ILD), other (AAV and axSpA-related ILD) and ILD with suspected SARD. We acknowledge that these categories have limitation, and do not reflect clinical manifestations or the underlying pathophysiology of these conditions.
Figure 1.
Chart demonstrating the types of SARD-ILD that will be included in the guideline. These have been grouped into four sections to aid the organization of the systematic review
Groups that will not be covered
This guideline is not intended to cover people with idiopathic pulmonary fibrosis or other causes of ILD such as asbestosis, silicosis, hypersensitivity pneumonitis, radiation or non-anti-rheumatic drug-induced ILD.
A statement on the management of pulmonary sarcoidosis has recently been published by the British Thoracic Society (BTS) [27], and while sarcoidosis is an important cause of ILD, management of its multisystem manifestations is beyond the scope of this guideline.
This guideline will not cover the diagnosis and management of SARD-ILD in paediatric populations (<16 years of age). ILD is a rare complication of SARD-ILD in children and there is insufficient literature available upon which to make any evidence-based recommendations. Also, the management of pulmonary hypertension and other complications will not be covered.
Settings
The guideline is being developed primarily for use in rheumatology and respiratory secondary and tertiary care settings but will also be of interest to other specialities involved in shared care, including primary care.
Related guidance
2023—ACR guideline for the screening and monitoring of interstitial lung disease in people with systemic autoimmune rheumatic disease (publication pending).
2008—Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society [28].
2022—American Thoracic Society (ATS), European Respiratory Society (ERS), Japanese Respiratory Society (JRS), and Asociación Latinoamericana de Tórax (ALAT) guideline: idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline [29].
2021—Interstitial lung disease associated with autoimmune rheumatic diseases: checklists for clinical practice (Italian guideline—Delphi process) [30].
2020—Japanese 2020 guide for the diagnosis and treatment of interstitial lung disease associated with connective tissue disease [31].
RA-ILD:
2022—Spanish Societies of Rheumatology and Pneumology and Thoracic Surgery recommendations for the management of rheumatoid arthritis–related interstitial lung disease. Part 2: treatment [32].
SSc-ILD:
2017—Update of EULAR recommendations for the treatment of systemic sclerosis [33].
2023—Treatment of systemic sclerosis-associated interstitial lung disease: evidence-based recommendations. an official American Thoracic Society clinical practice guideline [34].
2016—BSR and British Health Professionals in Rheumatology guideline for the treatment of systemic sclerosis [20].
IIM-ILD:
2022—BSR guideline on management of paediatric, adolescent and adult patients with idiopathic inflammatory myopathy [21].
Sjögren’s-ILD:
2021—ACR consensus guidelines for evaluation and management of pulmonary disease in Sjögren’s [35].
Sarcoidosis:
2021—European Respiratory Society clinical practice guidelines on treatment of sarcoidosis [36].
2021—BTS clinical statement on pulmonary sarcoidosis [27].
2020—Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline [37].
Key issues and draft questions
The following key issues and draft questions have been identified by the working group and will be used to develop more detailed questions to be addressed following a systematic search of the literature.
-
Which people with known SARD should be screened for ILD?
How often should screening take place?
What modalities should be used for screening?
How should subclinical ILD be managed when identified in people with SARD?
What pharmacological management should be considered in SARD-ILD?
What non-pharmacological therapies are of clinical benefit?
Should any anti-rheumatic drugs be avoided or used with caution in those at risk of SARD-ILD?
How should people newly diagnosed with ILD, with positive SARD-associated autoantibodies but no other clinical manifestations of a SARD be managed?
Proposed guideline structure
Overarching principles
Screening:
Who should be screened? Does this differ according to underlying SARD or by antibody profile?
-
What tests should be performed to screen for ILD?
Clinical examination
Chest X-ray
High-resolution computed tomography (HRCT) of the chest
Lung function tests
Six-minute walk test
Lung ultrasound
-
How do we determine prognosis?
Autoantibody profile
Lung function test parameters
Imaging features on HRCT chest
Quantitative HRCT chest analyses
Functional exercise capacity tests
Soluble biomarkers
Patient reported outcome measures
Monitoring:
-
What complications should we screen for?
Pulmonary hypertension (due to ILD)
Gastro-oesophageal reflux disease
Glucocorticoid-related complications
Progressive pulmonary fibrosis
-
At what frequency and by which modes should people with SARD-ILD be monitored?
Frequency of lung function tests
Frequency of HRCT chest imaging
-
How should we define deterioration and improvement?
When to escalate or de-escalate therapy
Management:
How should management of the underlying SARD be balanced with the management of ILD?
Are any anti-rheumatic drugs associated with the development or worsening existing SARD-ILD?
How should those with ILD found to have positive autoantibodies without underlying SARD be managed?
-
Specific interventions to be examined are:
Glucocorticoids—dose and route
-
Conventional synthetic DMARDs:
Mycophenolate mofetil
MTX
Leflunomide
Azathioprine
Calcineurin inhibitors
Cyclophosphamide
-
Biologic DMARDs:
Janus kinase inhibitors
Interleukin-6 inhibitors (e.g. tocilizumab)
TNF inhibitors
Abatacept
Anti-CD20 monoclonal antibodies (e.g. rituximab)
IVIG
Anti-fibrotic therapies
Proton pump inhibitors
Plasma exchange
Extra-corporeal membrane oxygenation
Lung transplant
Haematopoietic stem cell transplant
Physiotherapy and pulmonary rehabilitation
Antimicrobial prophylaxis
In order to produce evidence-based recommendations for specific treatments, a systematic literature search will be undertaken using the Population, Intervention, Comparison and Outcomes (PICO) framework.
Population
The population includes patients with RA-ILD, CTD-ILD (SSc, IIM, MTD, SS and SLE), other SARD-ILD (AAV, axSpA) and ILD with suspected SARD.
Comparison
Comparison will be against either placebo or standard-of-care therapy.
Outcomes
The primary outcome to be examined will be progression as measured by the decline in pulmonary function tests. Secondary outcomes will include mortality, acute exacerbation rate, radiological progression, quality of life and potential serious adverse effects of the intervention.
Narrative/consensus recommendations will also be produced to cover the following aspects of management:
Palliative care/symptomatic management
Oxygen therapy
Vaccinations
Alternative therapies—hyperbaric oxygen therapy, essential oils, acupuncture, hypnotherapy, ayurveda, homeopathy, naturopathy, Chinese or Oriental medicine, dietary supplements
Smoking
Nutrition
Approaches to audit of the guideline
Audit tools will be created to enable guideline users to easily assess local service delivery.
Guideline working group
Muhammad Nisar (chair), consultant rheumatologist; Ahmed Fahim, consultant respiratory physician; Anjali Crawshaw, consultant respiratory physician; Carol Fielding, patient representative; Caroline Cotton, consultant rheumatologist; Claire Riddell, consultant rheumatologist; Clive Kelly, consultant rheumatologist; Cristiana Sieiro Santos, consultant rheumatologist; Elizabeth Perry, consultant rheumatologist; Fasihul Khan, consultant respiratory physician; Gouri Koduri, consultant rheumatologist; Harsha Gunawardena, consultant rheumatologist; Helen Morris, ILD specialist nurse; Jennifer Hannah, rheumatology specialist trainee; Julie Dawson, consultant rheumatologist; Kerri-Marie Heenan, respiratory specialist trainee; Lisa G. Spencer, consultant respiratory physician; Liz Ball, consultant rheumatologist; Marium Naqvi, highly specialist pharmacist; Mark Garton, consultant rheumatologist; Mia Rodziewicz, rheumatology specialist trainee; Nazia Chaudhuri, consultant respiratory physician; Peter George, consultant respiratory physician; Puja Mehta, rheumatology specialist trainee; Richard Conway, consultant rheumatologist; Sara Carty, consultant rheumatologist; Sarah Cox, patient representative; Shaney Barrett, consultant respiratory physician; Sujal Desai, consultant radiologist
Contributor Information
Jennifer Hannah, Academic Rheumatology, Faculty of Life Sciences and Medicine, King’s College London, London, UK.
Mia Rodziewicz, Centre for Musculoskeletal Research, University of Manchester, Manchester, UK.
Puja Mehta, Centre for Inflammation and Tissue Repair, University College London, London, UK.
Kerri-Marie Heenan, Department of Respiratory Medicine, Northern Health and Social Care Trust, Antrim, UK.
Elizabeth Ball, Department of Rheumatology, Belfast Health and Social Care Trust, Belfast, UK.
Shaney Barratt, Department of Respiratory Medicine, Bristol Medical School, Bristol, UK.
Sara Carty, Department of Rheumatology, Great Western Hospitals NHS Foundation Trust, Swindon, UK.
Richard Conway, Department of Rheumatology, Trinity College Dublin, Dublin, Ireland.
Caroline V Cotton, Department of Rheumatology, Liverpool University Hospitals NHS Foundation Trust, Liverpool, UK.
Sarah Cox, Patient Representative, London, UK.
Anjali Crawshaw, Department of Respiratory Medicine, University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK.
Julie Dawson, Department of Rheumatology, St Helens Hospital, Saint Helens, UK.
Sujal Desai, Radiology Department, Royal Brompton Hospital, London, UK.
Ahmed Fahim, Department of Respiratory Medicine, New Cross Hospital, Wolverhampton, UK.
Carol Fielding, Patient Representative, London, UK.
Mark Garton, Department of Rheumatology, Royal Shrewsbury Hospital, Shrewsbury, UK.
Peter M George, Department of Respiratory Medicine, Royal Brompton Hospital, London, UK.
Harsha Gunawardena, North Bristol NHS Trust, Academic Rheumatology, Bristol, UK.
Clive Kelly, Department of Rheumatology, James Cook University Hospital, Middlesbrough, UK.
Fasihul Khan, Department of Respiratory Medicine, University Hospitals of Leicester NHS Trust, Leicester, UK.
Gouri Koduri, Department of Rheumatology, Southend University Hospital NHS Foundation Trust, Southend-on-Sea, Essex, UK.
Helen Morris, Department of Respiratory Medicine, Wythenshawe Hospital, Manchester, UK.
Marium Naqvi, Department of Respiratory Medicine, Guy’s and St Thomas’ Hospitals NHS Trust, London, UK.
Elizabeth Perry, Department of Rheumatology, University Hospitals Bristol and Weston NHS Foundation Trust, Bristol, UK.
Claire Riddell, Department of Rheumatology, Belfast Health and Social Care Trust, Belfast, UK.
Cristiana Sieiro Santos, Department of Rheumatology, Complejo Asistencial Universitario de Leon, Leon, Spain.
Lisa G Spencer, Department of Respiratory Medicine, Aintree University Hospital, Liverpool, UK.
Nazia Chaudhuri, School of Medicine, University of Ulster, Coleraine, UK.
Muhammad K Nisar, Rheumatology Department, Luton, Dunstable University Hospital, Luton, UK.
Data availability
No new data were generated or analysed in support of this article.
Authors’ contributions
All authors have contributed equally to the manuscript preparation.
Funding
This work was supported by the British Society for Rheumatology.
Disclosure statement: M.R. has received research funding from North West England Medical Research Council, Roche Pharma, Eli Lilly, UCB Pharma, Novartis, the University of Liverpool and the University of Manchester (paid to employer) and conference fees from UCB. S.B. has received speaker fees and funding for refreshments from Boehringer Ingelheim. N.C. has received a research grant from Boehringer Ingelheim (paid to employer); consultancy fees from Vicore Pharma AB, Bridge Biotherapeutics, Boehringer Ingelheim, tranScrip, the limbic, NICE and PPD and speaker fees from Boehringer Ingelheim. R.C. has received research funding from AbbVie, Novartis, Nordic Pharma and Celltrion (paid to employer); conference fees from AbbVie and Janssen and honoraria from Janssen, AbbVie, Galapagos, Fresenius Kabi, Viatris and UCB. A.C. has received speaker honoraria at educational meetings from Boehringer Ingelheim and AstraZeneca. S.D. is co-founder and co-clinical director of DMC Radiology Reporting, course tutor for Boehringer Ingelheim Interstitial Disease Courses and an external expert steering committee member for AstraZeneca (Trastuzumab deruxtecan phase IIIb/IV trial). A.F. has received speaker fees from Boehringer Ingelheim, Roche and Chiesi. M.G. has applied for research funding from Pfizer for an interventional multicentre UK study of tofacitinib in RA-related ILD. P.G. has received a research grant from Boehringer Ingelheim (paid to employer); conference fees from Boehringer Ingelheim and honoraria from Boehringer Ingelheim, Roche, AstraZeneca, GSK, Cipla, Teva and Avalyn; is part of the Data and Safety Monitoring Board for a GSK phase 3 study of SSc-ILD and is Senior Medical Director at Brainomix and receives a salary and stock options. H.G. has received honoraria for consultancy work from Boehringer Ingelheim. C.K. has received honoraria for consultancy work from Boehringer Ingelheim. G.K. has received speaker fees from Boehringer Ingelheim. P.M. has received honoraria from UCB, Pfizer, Boehringer Ingelheim and EUSA Pharma and a research grant from GSK (paid to employer). M.N. has received financial support for materials and sponsorship of regional ILD meetings from Boehringer Ingelheim (paid to employer) and speaker honoraria from Boehringer Ingelheim and Action for Pulmonary Fibrosis. E.P. has received financial reimbursement from Boehringer Ingelheim for consultancy work and funding from Eli Lilly for conference attendances. C.R. has received funding to attend regional ILD meetings from Boehringer Ingelheim. L.G.S. has received fees for advisory work from Daiichi-Sankyo (unrelated to this work). M.K.N. has received conference fees, speaker fees and honoraria from AbbVie, Amgen, BMS, Celgene, Fresenius Kabi, Galapagos Biotech, Eli Lilly, MSD, Nordic Pharma, Novartis, Pfizer, Roche, Chugai and UCB. All other authors have no conflicts of interest to declare.
References
- 1. Guthridge JM, Wagner CA, James JA.. The promise of precision medicine in rheumatology. Nat Med 2022;28:1363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maher TM, Tudor VA, Saunders P. et al. Rituximab versus intravenous cyclophosphamide in patients with connective tissue disease-associated interstitial lung disease in the UK (RECITAL): a double-blind, double-dummy, randomised, controlled, phase 2b trial. Lancet Respir Med 2023;11:45–54. [DOI] [PubMed] [Google Scholar]
- 3. Khanna D, Lin CJF, Furst DE. et al. Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2020;8:963–74. [DOI] [PubMed] [Google Scholar]
- 4. Distler O, Highland KB, Gahlemann M. et al. Nintedanib for systemic sclerosis–associated interstitial lung disease. N Engl J Med 2019;380:2518–28. [DOI] [PubMed] [Google Scholar]
- 5. National Institute for Health and Care Excellence. Nintedanib for treating progressive fibrosing interstitial lung diseases. London: National Institute for Health and Care Excellence, 2021. https://www.nice.org.uk/guidance/ta747 (1 November 2023, date last accessed). [Google Scholar]
- 6. American College of Rheumatology. Interstitial lung disease clinical practice guidelines. https://rheumatology.org/interstitial-lung-disease-guideline (16 February 2024, date last accessed)
- 7. British Society for Rheumatology. Creating clinical guidelines: our protocol. https://www.rheumatology.org.uk/Portals/0/Documents/Guidelines/Guidelines%20Protocol%20edited%20-%20Dec23%20FINAL.pdf?ver=-Kk1kW7Pat7ncErckPrzTw%3d%3d (19 January 2024, date last accessed).
- 8. Mittoo S, Gelber AC, Christopher-Stine L. et al. Ascertainment of collagen vascular disease in patients presenting with interstitial lung disease. Respir Med 2009;103:1152–8. Aug [DOI] [PubMed] [Google Scholar]
- 9. Olson AL, Swigris JJ, Sprunger DB. et al. Rheumatoid arthritis–interstitial lung disease–associated mortality. Am J Respir Crit Care Med 2011;183:372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dawson J, Fewins H, Desmond J, Lynch M, Graham D.. Fibrosing alveolitis in patients with rheumatoid arthritis as assessed by high resolution computed tomography, chest radiography, and pulmonary function tests. Thorax 2001;56:622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joy GM, Arbiv OA, Wong CK. et al. Prevalence, imaging patterns and risk factors of interstitial lung disease in connective tissue disease: a systematic review and meta-analysis. Eur Respir Rev 2023;32:220210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Villeneuve T, Faguer S, Collot S, Pugnet G, Prévot G.. HRCT imaging of pulmonary involvement in granulomatosis with polyangiitis and microscopic polyangiitis at disease onset and during follow-up. Semin Arthritis Rheum 2023;63:152307. [DOI] [PubMed] [Google Scholar]
- 13. Arulkumaran N, Periselneris N, Gaskin G. et al. Interstitial lung disease and ANCA-associated vasculitis: a retrospective observational cohort study. Rheumatology (Oxford) 2011;50:2035–43. [DOI] [PubMed] [Google Scholar]
- 14. Sada K-e, Yamamura M, Harigai M. et al. Classification and characteristics of Japanese patients with antineutrophil cytoplasmic antibody-associated vasculitis in a nationwide, prospective, inception cohort study. Arthritis Res Ther 2014;16:R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Özdemir O, Gülsün Akpınar M, İnanıcı F, Hasçelik HZ.. Pulmonary abnormalities on high-resolution computed tomography in ankylosing spondylitis: relationship to disease duration and pulmonary function testing. Rheumatol Int 2012;32:2031–6. [DOI] [PubMed] [Google Scholar]
- 16. El Maghraoui A, Dehhaoui M.. Prevalence and characteristics of lung involvement on high resolution computed tomography in patients with ankylosing spondylitis: a systematic review. Pulm Med 2012;2012:965956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Raimundo K, Solomon JJ, Olson AL. et al. Rheumatoid arthritis–interstitial lung disease in the United States: prevalence, incidence, and healthcare costs and mortality. J Rheumatol 2019;46:360–9. [DOI] [PubMed] [Google Scholar]
- 18. Niu Q, Zhao L-Q, Ma W-L. et al. A new predictive model for the prognosis of MDA5+ DM-ILD. Front Med 2022;9:908365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Interstitial Lung Disease Interdisciplinary Network. Interstitial lung disease service evaluation. 2023. https://ild-in.org.uk/learning-resources/ild-in-service-evaluation/ (22 January 2024, date last accessed).
- 20. Denton CP, Hughes M, Gak N. et al. BSR and BHPR guideline for the treatment of systemic sclerosis. Rheumatology (Oxford) 2016;55:1906–10. [DOI] [PubMed] [Google Scholar]
- 21. Oldroyd AGS, Lilleker JB, Amin T. et al. British Society for Rheumatology guideline on management of paediatric, adolescent and adult patients with idiopathic inflammatory myopathy. Rheumatology (Oxford) 2022;61:1760–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Institute for Health and Care Excellence. Idiopathic pulmonary fibrosis in adults: diagnosis and management. London: National Institute for Health and Care Excellence, 2013. https://www.nice.org.uk/guidance/cg163 (11 December 2023, date last accessed). [PubMed]
- 23. Vadillo C, Nieto MA, Romero-Bueno F. et al. Efficacy of rituximab in slowing down progression of rheumatoid arthritis-related interstitial lung disease: data from the NEREA Registry. Rheumatology (Oxford) 2020;59:2099–108. [DOI] [PubMed] [Google Scholar]
- 24. Fernández-Díaz C, Castañeda S, Melero-González RB. et al. Abatacept in interstitial lung disease associated with rheumatoid arthritis: national multicenter study of 263 patients. Rheumatology (Oxford) 2020;59:3906–16. [DOI] [PubMed] [Google Scholar]
- 25. Md Yusof MY, Kabia A, Darby M. et al. Effect of rituximab on the progression of rheumatoid arthritis–related interstitial lung disease: 10 years’ experience at a single centre. Rheumatology (Oxford) 2017;56:1348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Conway R, Low C, Coughlan RJ, O’Donnell MJ, Carey JJ.. Methotrexate and lung disease in rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheumatol 2014;66:803–12. [DOI] [PubMed] [Google Scholar]
- 27. Thillai M, Atkins CP, Crawshaw A. et al. BTS clinical statement on pulmonary sarcoidosis. Thorax 2021;76:4–20. Jan 1 [DOI] [PubMed] [Google Scholar]
- 28. Bradley B, Branley HM, Egan JJ. et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax 2008;63(Suppl 5):v1–58. [DOI] [PubMed] [Google Scholar]
- 29. Raghu G, Remy-Jardin M, Richeldi L. et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2022;205:e18–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bosello SL, Beretta L, Del Papa N. et al. Interstitial lung disease associated with autoimmune rheumatic diseases: checklists for clinical practice. Front Med 2021;8:732761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kondoh Y, Makino S, Ogura T. et al. 2020 guide for the diagnosis and treatment of interstitial lung disease associated with connective tissue disease. Respir Investig 2021;59:709–40. [DOI] [PubMed] [Google Scholar]
- 32. Narváez J, Díaz Del Campo Fontecha P, Brito García N. et al. SER-SEPAR recommendations for the management of rheumatoid arthritis-related interstitial lung disease. Part 2: Treatment. Reumatol Clin 2022;18:501–12. [DOI] [PubMed] [Google Scholar]
- 33. Kowal-Bielecka O, Fransen J, Avouac J. et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis 2017;76:1327–39. Aug [DOI] [PubMed] [Google Scholar]
- 34. Raghu G, Montesi SB, Silver RM. et al. Treatment of systemic sclerosis-associated interstitial lung disease: evidence-based recommendations. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2024;209:137–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee AS, Scofield RH, Hammitt KM. et al. Consensus guidelines for evaluation and management of pulmonary disease in Sjögren’s. Chest 2021;159:683–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baughman RP, Valeyre D, Korsten P. et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J 2021;58:2004079. [DOI] [PubMed] [Google Scholar]
- 37. Crouser ED, Maier LA, Wilson KC. et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2020;201:e26–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this article.