Abstract
Orthognathic surgery may induce hemorrhage resulting from nasal mucosal injury or from maxillary osteotomy sites, and if severe, the hemorrhage may cause airway obstruction. The patient in this case report was a 27-year-old woman who underwent Le Fort I and bilateral sagittal split osteotomies under general anesthesia. There were no abnormal intraoperative vital signs. However, immediately after the patient was returned to the ward, significant bleeding that required frequent suctioning was observed in the oral cavity. As the bleeding persisted, the surgeon attempted to insert epinephrine-soaked gauze and polyvinyl acetal sponges into both nasal cavities, but hemostasis was difficult to obtain. To achieve hemostasis by compression/closure at the choana and maintain nasal patency, we inserted a modified cuffed endotracheal tube to serve as a transnasal airway and a choanal hemostatic balloon. This method resulted in hemostasis. The volume of blood loss after returning to the ward was approximately 420 mL. The transnasal airway and choanal balloon was useful for airway management and the prevention of intranasal bleeding into the lower pharyngeal regions. Furthermore, the method was simple and minimally invasive, suggesting its clinical usefulness in similar situations.
Keywords: Modified endotracheal tube, Choanal hemostasis, Le Fort I osteotomy, Epistaxis, Hemorrhage, Nasal.
Orthognathic surgery or surgical/anesthetic procedures than induce substantial epistaxis postoperatively require swift, appropriate management to avoid potentially catastrophic complications. We report a patient in whom the use of a modified endotracheal tube (ETT) with a cuff as a transnasal airway and a choanal hemostasis balloon was effectively applied for emergent epistaxis control following orthognathic surgery. The patient provided written consent to publish the details of this case.
CASE PRESENTATION
The patient was a 27-year-old woman (height 165 cm, weight 51.3 kg, body mass index 18.8 kg/m2) scheduled to undergo nasally intubated general anesthesia for orthognathic surgery. Given her maxillary prognathia and mandibular retrognathia, Le Fort I and bilateral sagittal split osteotomies were planned. The patient had a history of gastroesophageal reflux disease (GERD) that was treated 4 years previously with lansoprazole, but because of lansoprazole-induced exanthema, she was no longer taking that medication and denied any GERD symptoms. No other allergies were reported. There were no abnormalities noted on her preoperative electrocardiogram (ECG), posteroanterior chest radiograph, or pulmonary function test. Neither hematology nor blood biochemistry tests showed any abnormalities, particularly with her platelet count or coagulation pathways. Upon her physical evaluation, her mouth opening range was 35 mm, and she had a Mallampati class II airway. She was assigned an American Society of Anesthesiologists physical status of class I.
Food intake was prohibited starting at 2100 the night before surgery. The patient was only allowed clear liquids and drank 500 mL of water 2 hours before surgery. Upon entering the operating room, standard anesthesia monitors were applied that consisted of a noninvasive blood pressure cuff, pulse oximeter, ECG, capnometer, electroencephalographic monitor, and neuromuscular monitor. Oxygen 6 L/min was administered via face mask prior to induction of general anesthesia. Peripheral intravenous (IV) access was obtained in the dorsum of the left hand and the right forearm. An arterial line was obtained at the right radial artery for continuous blood pressure measurements.
After sufficient preoxygenation, continuous infusions of remifentanil 0.5 lg/kg/min and propofol using the target-controlled infusion (TCI) function were started. The patient was induced once the effective-site concentration of propofol was 1.9 lg/mL, and rocuronium 40 mg was then administered. After confirming paralysis via neuromuscular monitoring (a train-of-four 0 count), intranasal disinfection and nasal passage patency confirmation were conducted using cotton swabs immersed in 0.01% benzalkonium chloride disinfectant solution. A preformed, cuffed, 6.5-mm nasal ETT was inserted through the left nasal cavity and advanced smoothly with no signs of epistaxis. Tracheal intubation was performed under direct vision, and according to the Cormack-Lehane classification, the obtained view was grade 2. The ETT was advanced, and intubation completed successfully without difficulty. Thoracic auscultation did not show any abnormal respiratory sounds. Subsequently, an 8-French scale (Fr) enteral feeding tube was inserted through the right nasal cavity and fixed at a position 55 cm from the nostril with no signs of pharyngeal or nasal hemorrhage. Subsequently, betamethasone 4 mg and ampicillin 1 g were administered IV. After placement of the gauze throat pack, surgery was started.
General anesthesia was maintained with a continuous infusion of remifentanil (0.15–0.35 lg/kg/min) and a propofol TCI (2.5–3.0 lg/mL; 6–7 mg/kg/h) along with oxygen (1 L/min) and air (2 L/min). For local anesthesia, 10 mL of 1% lidocaine with 1:100,000 epinephrine was administered for each arch (totals: 20 mL, lidocaine 200 mg, epinephrine 0.2 mg). After incision and mucoperiosteal flap elevation for the maxilla, hypotensive anesthesia was started using sevoflurane (1.5%–3.0%) and a nitroglycerin infusion (1–5 lg/kg/min). Hypotensive anesthesia was continued until mandibular segmentation was completed. The patient’s vital signs remained stable and within acceptable limits throughout the surgery which was completed without complications. The total volume of IV fluids administered was 3,400 mL, consisting of 2,050 mL of acetated Ringer s solution containing 1% glucose and 1,350 mL of a hydroxyethyl starch preparation (molecular weight: 130,000). The total volume of blood loss was 556 mL, and the operative time was 6 hours and 27 minutes.
After the completion of surgery, the throat pack gauze was removed, and any fluids retained in the oral cavity and posterior oropharynx were evacuated to verify the absence of hemorrhage. After confirmation of spontaneous ventilations and an active response to stimulation, the ETT was partially withdrawn for use as a transnasal airway. The ETT in the left nasal cavity was withdrawn under direct vision using a laryngoscope until the end of the ETT was behind the uvula. A direct view into the oral cavity ensured that the end of the ETT was placed so as not to induce the vomiting reflex. The ETT was then cut 1 cm from the nostril; the length from the end of the ETT to the cut surface was about 11 cm. A 10-Fr endotracheal suction catheter was inserted into the transnasal airway to evacuate any blood or secretions retained in the pharynx. Notably, no pharyngeal or nasal hemorrhage was observed at this time.
The patient was returned to the ward given that she was awake with stable cardiovascular and respiratory vital signs. Immediately upon her return to the ward, she began to cough intermittently. Active bleeding was observed in her oral cavity, requiring frequent suctioning. The surgeon attempted to insert epinephrine-soaked gauze and polyvinyl acetal sponges (Ivalon, Medsorb Dominicana, SA) into both nasal cavities, but the bleeding persisted.
To help achieve hemostasis by providing compression/closure at the choana and to establish a more secure airway, we quickly modified a cuffed nasal ETT to serve as another transnasal airway and a choanal hemostatic balloon upon cuff inflation. Initially, the ETT was cut at a position approximately 11 cm from the distal end (Figure 1A). The pilot balloon tube was cut obliquely at a point before its connection with the previously removed ETT segment (Figure 1B) and inserted into the side hole leading to the ETT cuff (Figure 1C). After inflating the pilot balloon with air and confirming cuff inflation, the reconnected pilot balloon tube was fixed with cyanoacrylate (Figure 1D). The epinephrine-soaked gauze and sponges were removed immediately before the modified ETT with its deflated cuff was inserted through the right naris. The modified ETT was advanced so that the length from its distal tip to the nostril approximated 10 cm, and 4 mL of air was then injected back into the cuff for fixation of the airway (Figure 2). The inflated cuff was likely located around the posterior choana (Figure 2B). As a result, hemostasis was achieved, leading to a decrease in coughing frequency to approximately once every 5 minutes. The volume of blood loss after the patient’s return to the ward was approximately 420 mL. Her coughing had disappeared almost completely 3 hours later, and there was no evidence of bleeding noted intraorally. The oral sputum was suctioned at appropriate times.
Figure 1.
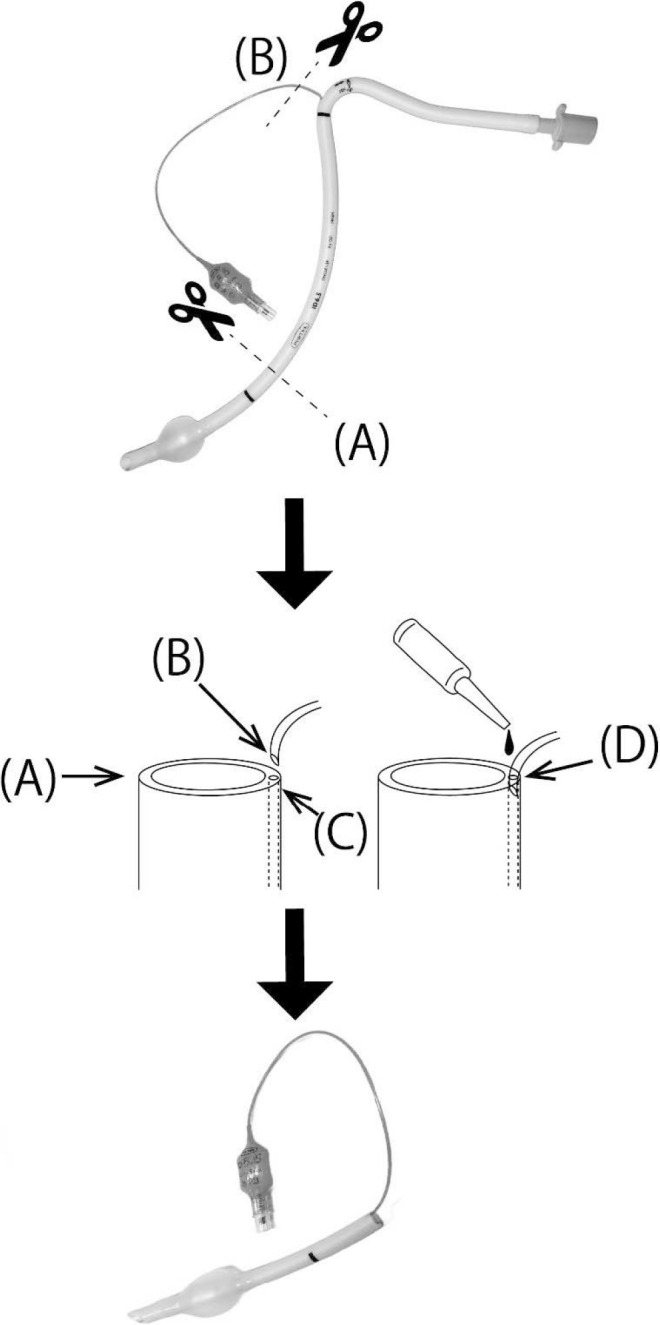
Modified Nasal Endotracheal Tube (ETT) With a Reattached Pilot Balloon
A, Nasal ETT cut approximately 11 cm from the distal (cuffed) end. B, Pilot balloon tube cut obliquely from the proximal end of the ETT. C, Side hole of the ETT leading to the cuff. D, Cut pilot balloon tube inserted into the ETT side hole and fixed with cyanoacrylate adhesive.
Figure 2.
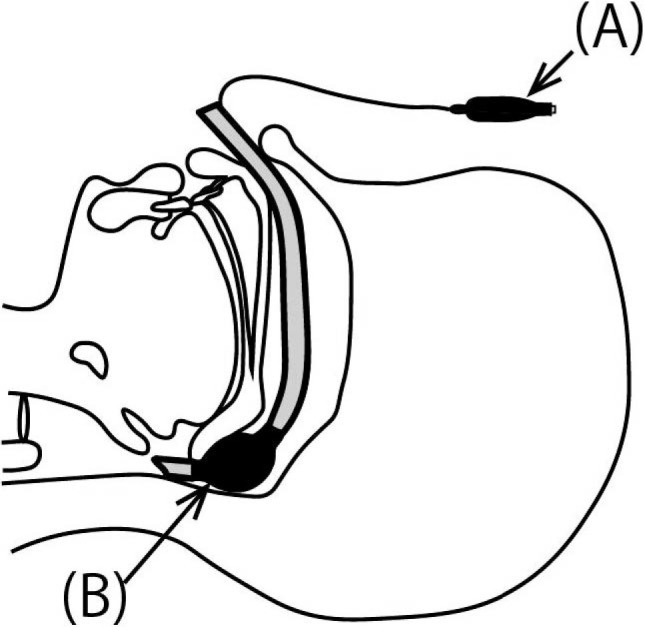
Placement of the Modified Nasal Endotracheal Tube (ETT)
A, After insertion of the modified nasal ETT, the cuff was inflated via a pilot balloon. B, The inflated cuff was located around the posterior choana.
At 0930 on the first postoperative day, the patient reported marked pressure/pain associated with the transnasal airways and balloon as well as pharyngeal pain. The transnasal airways and the remaining epinephrine-soaked gauze and sponges were removed after confirming the absence of epistaxis and presence of a patent airway. On the seventh postoperative day, the patient complained of nasal obstruction and consulted otorhinolaryngology in our university hospital. Retained blood clots and mucosal crusting were removed bilaterally from the patient’s nasal cavities as promptly as possible, and 0.118% tramazoline nasal spray was administered, reducing nasal obstruction.
DISCUSSION
As demonstrated by this patient who experienced substantial epistaxis of more than 1000 mL, Le Fort type I maxillary osteotomies1 may induce hemorrhage from injury to the nasal mucosa or because of the surgical procedure itself. Such hemorrhage related to nasopharyngeal injury may cause significant loss of the airway if it is marked.2 In 90% of episodes of epistaxis, the bleeding is derived from the Kiesselbach area.3 Because of venous epistaxis, it is easy to achieve hemostasis. However, hemorrhage from the posterior nasal cavity occurs in less than 10% of epistaxis episodes, and hemostasis is often difficult because of arterial epistaxis.3 Massive hemorrhage often occurs via the trauma to the posterior area of the middle nasal concha or sphenopalatine artery branch.3,4
Bleeding from the nasal cavity noted in this case was likely due to a vascular injury that occurred during the surgical procedure. The nasopalatine, nasoseptal, and sphenopalatine arteries commonly bleed excessively after maxillary osteotomies.5 According to a review of case reports of severe postoperative bleeding in Le Fort I osteotomies, the greater palatine artery, lesser palatine artery, and pterygoid venous plexus are the most frequently involved vessels.6 Although the blood vessel that was being compressed was not identified in this case, the inflated cuff was able to stop the bleeding by compressing a wide area of the posterior nasal cavity. In patients with epistaxis, compression is performed using gauze containing a vasoconstrictor, such as epinephrine, and an anesthetic.3 When hemostasis is difficult, hemostatic packing with a silver nitrate stick, absorbable gelatin, or oxycellulose can be used.7 Concerning choanal packing, a tampon can be used, or a urinary (ie, Foley) catheter with an inflatable balloon can also be placed in some cases.4 However, the hemostasis rate for posterior epistaxis by packing is 30%8 to 50%.9 In the present case, there was no tampon available, so epinephrine-soaked gauze and polyvinyl acetal sponges were used as substitutes. However, hemostasis was still impossible to achieve. Given that a Foley catheter cannot be used as an airway, it was not used in this case.
The modified ETT serving as a transnasal airway with a choanal hemostatic balloon in the present case facilitated the insertion of a suction catheter and maintained airway patency. If the material of the ETT is soft, the lumen may become narrow because of pressure when it is inserted into the nasal cavity. However, because the aspiration catheter could pass through the lumen smoothly, it was found that there was no luminal stenosis. In addition, this method prevents blood from the nasal cavity from flowing through the choana and to the posterior nasopharynx once the cuff is in place and inflated. Furthermore, pharyngeal blood or secretions may be easily evacuated by a suction catheter after inserting a transnasal airway, making it useful for preventing airway stenosis or occlusion. After a Le Fort type I osteotomy there is a risk of nasal obstruction due to swelling of the nasal mucosa and bleeding from the surgical field. Patency of the nasal airways is essential for nasal breathing and the suction of pharyngeal secretions. If bleeding is poorly controlled, preparation for emergent reintubation and/or placement of a supraglottic airway would be needed to promote airway security so that the bleeding can then be addressed.
The modified ETT was cut short for ease of postoperative management like insertion of a suction catheter. In this case, the tube was cut 11 cm from the tip in similar fashion to the ETT on the left. This allowed its use as a nasopharyngeal airway for suction, inhalation of supplemental oxygen via a face mask, and choanal hemostasis by inflating the cuff if needed. The pilot balloon tube was glued to the proximal end of the side hole leading to the ETT cuff. The tube length was cut 1 cm longer than the nostril to allow us to fix the tube with tape and prevent it from migrating back into the nasal cavity.
When adopting the use of this modified ETT as a transnasal device, the following points must be considered: the pharyngeal reflex may be triggered if the cuff is located within the oropharynx, and hemostatic effectiveness may be reduced if the cuff is incorrectly positioned in this manner. Furthermore, it is necessary to confirm whether the pilot tube is accurately inserted and fixed to the side hole of the indwelling tube to allow the cuff to inflate without leakage prior to inserting the modified ETT.
Reported choanal balloon-related complications include excessive compression-related Eustachian tube dysfunction, occlusion of the nasolacrimal duct, tissue necrosis, sinusitis related to bacterial infection, endotoxin shock, endocarditis, myocardial infarction, and cerebrovascular disorder.10 Therefore, insertion times should be kept at as short as effectively possible. This device was used for approximately 15 hours in this case without any complications. For subsequent hemostasis, arterial ligation and arterial embolization by angiography should be considered.10
CONCLUSION
A cuffed nasal ETT was quickly modified and used for airway management and to prevent significant postoperative nasal hemorrhage from occluding the airway. This method was simple and minimally invasive, suggesting its potential clinical usefulness in similar situations.
REFERENCES
- 1.Arai Y, Hasegawa A, Kameda A, et al. A case of nasal mucosa cautery with reintubation under pharyngeal suction for massive epistaxis after extubation. Anesth Prog. 2021; 68: 235–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teichgraeber JF, Rappaport NH, Harris JH Jr. The radiology of upper airway obstruction in maxillofacial trauma. Ann Plast Surg. 1991; 27:103–109. [DOI] [PubMed] [Google Scholar]
- 3.Kucik CJ, Clenney T Management of epistaxis. Am Fam Physician. 2005; 71:305–311. [PubMed] [Google Scholar]
- 4.Koh E, Frazzini VI, Kagetsu NJ Epistaxis: vascular anatomy, origins, and endovascular treatment. AJR Am J Roentgenol. 2000; 174:845–851. [DOI] [PubMed] [Google Scholar]
- 5.Bell WH Modern Practice in Orthognathic and Reconstructive Surgery. Vol 1. WB Saunders Co; 1992:100–127. [Google Scholar]
- 6.Lanigan DT, West RA Management of postoperative hemorrhage following the Le Fort I maxillary osteotomy. J Oral Maxillofac Surg. 1984; 42:367–75. [DOI] [PubMed] [Google Scholar]
- 1.Tan LK, Calhoun KH Epistaxis. Med Clin North Am. 1999; 83:43–56. [DOI] [PubMed] [Google Scholar]
- 2.Klotz DA, Winkle MR, Richmon J, Hengerer AS Surgical management of posterior epistaxis: a changing paradigm. Laryngoscope. 2002; 112:1577–1582. [DOI] [PubMed] [Google Scholar]
- 9.Shargorodsky J, Bleier BS, Holbrook EH, et al. Outcomes analysis in epistaxis management: development of a therapeutic algorithm. Otolaryngol Head Neck Surg. 2013; 149:390–398. [DOI] [PubMed] [Google Scholar]
- 10.Wong AS, Anat DS Epistaxis: a guide to assessment and management. J Fam Pract. 2018; 67:E13–E20. [PubMed] [Google Scholar]


