Abstract
Purpose
P2Y12 receptor inhibitors are drugs that decrease the risk of stent thrombosis and lower the long-term risk of non-stent-related myocardial infarction and stroke. They inhibit the binding of adenosine diphosphate (ADP) to the P2Y12 receptor and effectively reduce platelet reactivity. However, considerable variability in the pharmacodynamics response contributes to a failure of antiplatelet therapy; this phenomenon is especially notorious for older drugs, such as clopidogrel. Some genetic polymorphisms associated with these drugs’ metabolic pathway, especially in the CYP2C19 gene, can significantly decrease antiplatelet efficacy. There are few reports on the variability stemming from the target of this drug class that is the P2Y12 receptor itself.
Results and conclusion
This review summarizes the results of research that focus on the influence of P2Y12 genetic polymorphisms on the pharmacodynamics and the efficacy of P2Y12 inhibitors. We found that the conclusions of the studies are unequivocal, and despite several strong candidates, such as G52T (rs6809699) or T744C (rs2046934), they may not be independent predictors of the inadequate response to the drug. Most probably, P2Y12 genetic polymorphisms contribute to the effect exerted by other gene variants (such as CYP2C19*2/*3/*17), drug interactions, or patient habits, such as smoking. Also, epigenetic modifications, such as methylation or miRNA levels, may play a role in the efficacy of antiplatelet treatment.
Keywords: Platelet aggregation inhibitors; Purinergic P2Y receptor antagonists; Polymorphism, Genetic; Clopidogrel
Introduction
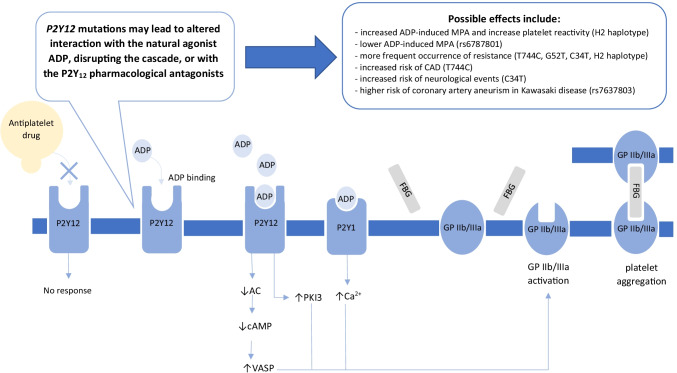
Combined with aspirin, P2Y12 receptor inhibitors are used in dual antiplatelet therapy (DAPT). This treatment aims to prevent thrombotic events after percutaneous coronary intervention (PCI) or myocardial infarction (MI) and stroke [1]. The basis of the therapeutic action of this drug class is the inhibition of adenosine diphosphate (ADP) binding to the P2Y12 receptor. Two platelet receptors, P2Y1 and P2Y12, react to ADP in physiological conditions and induce platelet aggregation [2]. The introduction of P2Y12 inhibitors attenuates ADP interaction with its platelet receptor and effectively reduces platelet reactivity (Fig. 1).
Fig. 1.
Activation mechanism of P2Y12 receptor through agonist. ADP, adenosine diphosphate; AC, adenyl cyclase; CAD, coronary artery disease; cAMP, cyclic adenosine monophosphate; FBG, fibrinogen; MPA, maximal platelet aggregation; PKI 3, phosphoinositide 3-kinase; VASP, vasodilator-stimulated phosphoprotein
Besides the most widely used clopidogrel, newer P2Y12 inhibitors gain interest, and their use increases (Table 1). For example, Chest Pain-Myocardial Infarction Registry in the United States noted that ticagrelor use in patients with ST-segment-elevation myocardial infarction (STEMI) increased from 18.0 to 44.0% [3]. Last year, selatogrel (ACT-246475), a novel subcutaneous P2Y12 inhibitor, completed phase 2 studies [4, 5]. The declining use of clopidogrel stems from a sizeable observed variability in the therapeutic response. The “clopidogrel resistance” phenomenon can affect as much as 16–50% of the population [6], leading to the failure of antiplatelet therapy. Significant contributors to this resistance are genetic polymorphisms of CYP450 enzymes, especially CYP2C19 [7]. The presence of loss-of-function CYP2C19 alleles increases the risk of major adverse cardiovascular events (MACE) during DAPT with clopidogrel [8–11]. However, some authors claim that alterations in the P2Y12 gene could also play a role in thrombotic events during antiplatelet therapy (Tables 2 and 3) [12, 13]. As these polymorphisms may affect the receptor’s functionality, its potential influence may translate to other P2Y12 inhibitors. Therefore, this narrative review focuses on presenting available data on the influence of P2Y12 genetic polymorphism on the efficacy and safety of currently used antiplatelet drugs.
Table 1.
Antiplatelet drugs’ characterization
| Drug | Administration route | Metabolic activation | Activating enzymes and reaction type | Inhibition | Reference |
|---|---|---|---|---|---|
| Ticlopidine | Oral | + | CYP 2B6, 2C19, 1A2, 3A4 (oxidation) | Irreversible | [14, 15] |
| Clopidogrel | Oral | + | CYP 2C19, 1A2, 2B6, 3A4 (oxidation) | Irreversible | [15–18] |
| Prasugrel | Oral | + | Esterases (hydrolysis), CYP 3A5, 2B6, 2C9 and 2C19 (oxidation) | Irreversible | [15, 19–22] |
| Vicagrel | Oral | + | Carbohydrate esterase family 2 and paraoxonase 1 (hydrolysis), CYP 2C19, 3A4, 2B6 (oxidation) | Irreversible | [23, 24] |
| Selatogrel | Subcutaneous | - | - | Reversible | [25, 26] |
| Elinogrel | Oral/intravenous | - | - | Reversible | [27, 28] |
| Ticagrelor* | Oral | ± | CYP 3A4 (oxidation) | Reversible | [29, 30] |
| Cangrelor | Bolus or intravenous infusion | - | - | Reversible | [31] |
*Parent compound is pharmacologically active
Table 2.
Genetic polymorphisms in the P2Y12 gene sequence with possible influence on the efficacy of antithrombotic drugs. Data taken from the dbSNP database (ncbi.nlm.nih.gov/snp/). Global allele frequencies (AF) taken from the Ensembl database, 1000 Genomes Project Phase 3 (ensembl.org). The alleles leading to a possible function alteration are in bold
| Accession number | Position | Common name | Region | Change | AF | Modification | Function alteration |
|---|---|---|---|---|---|---|---|
| rs6798347 | g.1387C > T | - | Promoter | G > A |
G: 0.711 A: 0.289 |
Promoter modification | Possible lower incidence of HTPR (as part of H1 haplotype) [32] |
| rs1907637 | g.2707 T > C | - | Promoter | G > A |
G: 0.903 A: 0.097 |
Promoter modification | May influence baseline platelet aggregation [33] |
| rs6787801 | g.7804 T > G | - | Intron | T > C |
T: 0.527 G: 0.473 |
Intron variant | Possible lower incidence of HTPR (as part of H1 haplotype) [32] |
| rs9859552 | g.16549C > T | - | Intron | G > T |
G: 0.938 T: 0.062 |
Intron variant | Noticeable but not significantly greater platelet reactivity [34] |
| rs3732759 | g.32620 T > C | - | Intron | A > G |
A: 0.678 G: 0.322 |
Intron variant | Occurs more frequently in patients with CVD |
| rs7428575 | g.32922A > C | - | Intron | T > G |
T: 0.678 G: 0.322 |
Intron variant | Increased risk of CHD [35] |
| rs6801273 | g.44715A > G | - | Intron | T > C |
T: 0.583 C: 0.417 |
Intron variant | Possible lower incidence of HTPR (as part of H1 haplotype) [32] |
| rs10935838 | g.49298 T > C | i-C139T | Intron | C > T |
C: 0.868 A: 0.132 |
Intron variant | Presence increases maximum platelet aggregation and reduction in cAMP (in H2 haplotype) [13] |
| rs2046934 | g.49903C > T | i-T744C / T744C | Intron | T > C |
T: 0.868 C: 0.132 |
Intron variant | Possible lower inhibition of platelet aggregation, higher peak platelet aggregation, elevated platelet reactivity [36, 37] |
| rs5853517 | g.49960del | i-ins801A | Intron | Ins A |
A: 0.868 AA: 0.132 |
Intron variant, insertion/ deletion |
Presence increases maximum platelet aggregation and reduction in cAMP (in H2 haplotype) [13] |
| rs6785930 | g.50929C > T | C34T |
Exon 2 (short transcript) |
C > T |
C: 0.758 T: 0.242 |
Missense (Asn > Lys) |
Lower incidence of HTPR (as part of H1 haplotype) [32] |
| rs6809699 | g.50947 T > G | G52T |
Exon 2 (short transcript) |
G > T |
G: 0.912 T: 0.088 |
Synonymous (Gly > Gly) |
Presence increases maximum platelet aggregation and reduction in cAMP (in H2 haplotype) [13] |
CVD cardiovascular disease, HTPR high on-treatment platelet reactivity
Table 3.
The influence of chosen genetic polymorphism on the platelet reactivity and the efficacy of the antiplatelet treatment
| Reference | Studied P2Y12 SNPs | Study group | Drug tested | Observed effects | Pharmacodynamic assessment | Results |
|---|---|---|---|---|---|---|
| In vitro assessment only | ||||||
| [13] | T744C, C139T, 801A, G52T, C34T | 98 healthy volunteers (Caucasian origin) | None (ADP reactivity test only) | Assessment of maximal platelet aggregation between P2Y12 genotypes and haplotypes | Photometric method | The H2 haplotype was related to increased maximal platelet aggregation after ADP exposition |
| [38] | T744C, C139T, 801A, G52T, C34T | 158 healthy volunteers (of Korean origin) | None (ADP reactivity test only) | Assessment of the relationship between ADP-induced MPA and studied SNPs | LTA/platelet-rich plasma turbidimetry | ADP-induced maximal platelet aggregation was not affected by three intronic P2Y12 and C34T polymorphisms. However, the authors noticed a correlation between the TT genotype of G52T polymorphism and higher ADP-induced maximal platelet aggregation |
| [33] | T744C, G52T, C34T, rs1907637, rs79320243, rs10935842, rs6787801, rs6801273, rs16863323 | 196 healthy volunteers (Chinese origin) | Ticagrelor, in vitro test only | Assessment of MPA and relative inhibition of platelet aggregation | LTA/platelet-rich plasma turbidimetry | Any genetic variations both in the P2Y12 and G-protein beta 3 subunit were not related to differences in platelet inhibition after partial ex vivo blockade using ticagrelor. Several P2Y12 polymorphisms and one haplotype (haplotype D) were related to differences in baseline platelet aggregation, but no studied polymorphism affected response to ticagrelor in the ex vivo study |
| [36] | T744C, rs6798347, rs6787801, rs9859552, rs6801273 | 242 healthy volunteers (mostly of Caucasian origin) | Cangrelor, in vitro test only | Assessment of MPA and relative inhibition of platelet aggregation | LTA/platelet-rich plasma turbidimetry | The minor C allele of rs6787801 was related to lower ADP-induced maximal platelet aggregation. rs9859552 AA genotype was related to decreased response to cangrelor therapy when compared with CC genotype. Haplotypes analysis presented results similar to the results of SNPs |
| [37] | T744C, C34T | 29 healthy volunteers (mostly of Caucasian origin) | Cangrelor, in vitro test only | Assessment of TRAP-mediated platelet activation by PAC1 binding and CD62P expression, and relative inhibition of platelet aggregation | Flow cytometry, VerifyNow P2Y12 assay | Patients with H2/H2 haplotypes showed greater platelet inhibition during cangrelor exposition. No consistent effects of the C34T and T744C polymorphisms separately were found |
| Influence of studied P2Y12 SNPs on platelet reactivity or clinical outcome | ||||||
| [39] | T744C | 77 ACS patients & 101 healthy volunteers (of Moroccan origin) | Clopidogrel, 300 mg LD & 75 mg MD | Association between genotypes and ACS risk and CR, defined as 208 PRU and above or < 20% inhibition of platelet aggregation after 7 days of treatment | VerifyNow P2Y12 assay | The C allele of studied polymorphism was more frequent among patients resistant to therapy. Relation of C allele to observed ACS was statistically significant |
| [35] | T744C, rs7428575, rs3732759 | 178 CHD patients and 182 healthy controls (Chinese origin) | Clopidogrel, 300 mg LD & 75 mg MD | Assessment of the decrease in MPA. CR defined as a < 10% decrease from baseline after 10 days of treatment | LTA/platelet-rich plasma turbidimetry | Significant differences in genotype and allele frequencies of T744C and rs3732759 between the case and control groups were observed. Haplotype TCA was related to increased coronary artery disease risk. Responsive to clopidogrel therapy group consisted of coronary artery disease patients with higher frequencies of TT genotype of T744C and lower frequencies of GG genotype of rs3732759 compared to patients with CR |
| [40] | T744C | 40 ACS patients and 40 age-matched healthy controls (origin unspecified) | Clopidogrel; exact dosing regimen unspecified |
CR defined as persistence of HPR (ADP-Ag > 70%) Comparison of allele frequencies between patients and controls; prevalence of CR according to the genotype C |
LTA/platelet-rich plasma turbidimetry | Carriers of T744C C allele showed increased platelet reactivity after ADP activation |
| [41] | T744C | 191 patients with ischemic stroke (Chinese origin) | Clopidogrel 300 mg LD & 75 mg MD | CR defined as a < 10% decrease in MPA after 5 days of treatment | LTA/platelet-rich plasma turbidimetry | The C allele in T744C P2RY12 polymorphism was considered to decrease the risk of CR. Additionally, CR was found to be related to increased risk of hypertension |
| [42] | T744C | 268 patients with ischemic stroke (Chinese origin) | Clopidogrel, 75 mg | Primary endpoints: transient ischemic attack, ischemic stroke, myocardial infarction or vascular-related mortality | - | There was significant association between A allele of the T744C P2Y12 polymorphism and present adverse effects |
| [32] | T744C, C34T, rs6798347, rs6787801, rs6801273 | 180 patients with ACS (Chinese origin) | Clopidogrel, 75 mg MD, with or without 300/600 mg LD | HTPR defined by platelet inhibition > 30% | Thromboelastography | Authors determined six haplotypes on basis of the selected P2RY12 and CYP2C19 polymorphisms (named as H0—H5). They discovered, that combination of few P2RY12 variations rather than T744C alone, associated with different response to treatment with clopidogrel |
| [43] | T744C, G52T, C34T | 146 patients with CAD (Han Chinese origin) | Clopidogrel, 75 mg MD | HTPR defined by MPA > = 50% | LTA/platelet-rich plasma turbidimetry | There was no association between studied polymorphism and HTPR or recurrence of major adverse cardiac events |
| [44] | T744C, C34T | 124 patients with acute myocardial infarction (Caucasian origin) | Clopidogrel, 75 mg MD | CR defined as values > 45% in LTA or 298 AUC/min in Multiplate | LTA/platelet-rich plasma turbidimetry and Multiplate analyzer | There was not any statistically significant association between P2RY12 receptor polymorphisms (T744C, C34T) and response to antiplatelet therapy with clopidogrel |
| [45] | T744C | 60 patients with ACS (Mexican origin) | Clopidogrel 300 mg LD & 75 mg MD | CR defined as persistence of HPR (ADP-Ag > 70%) | LTA/platelet-rich plasma turbidimetry | T744C polymorphism showed no association with clopidogrel resistance |
| [46] | T744C | 100 patients with CAD and ACS or chronic stable angina (North Indian origin) | Clopidogrel 75 mg MD, 300 mg LD in one subject | Assessment of the decrease in MPA. CR defined as a < 10% decrease from baseline, semi-responders as 10–29% reduction, responders as > 30% reduction | LTA/platelet-rich plasma turbidimetry | H1/H1 haplotype (P2Y12 polymorphism) was related to CR. CR was not related to single ADP receptor P2Y1 and P2Y12 gene polymorphisms |
| [47] | T744C | 1419 ACS patients (origin unspecified) | Clopidogrel 600 mg LD & 75 mg MD | CR defined as persistence of HPR (ADP-Ag > 70% or Arachidonic acid-AG > 20%) after 6 days | LTA/platelet-rich plasma turbidimetry | T744C polymorphism was not associated with higher platelet reactivity |
| [48] | T744C | 597 patients with ACS (origin unspecified) | Clopidogrel 600 mg LD | CR defined as persistence of HPR (ADP-Ag > 70%) after LD; also VASP value and P-selectin surface expression | LTA/platelet-rich plasma turbidimetry, VASP phosphorylation, flow cytometry | T774C polymorphism was not associated with CR |
| [49] | T744C | 120 patients scheduled for elective PCI (mixed origin) | Clopidogrel 300 mg LD & 75 mg MD | CR defined as a < 10% decrease from baseline after 3 months | LTA/platelet-rich plasma turbidimetry, flow cytometry | No divergence in polymorphism frequencies between resistant and nonresistant patients was observed. No significant differences were observed also in response to aspirin or to clopidogrel in patients grouped accordingly to their genotype |
| [50] | T744C | 119 patients with CAD (origin unspecified) | Clopidogrel 300 mg LD & 75 mg MD or 75 mg MD only | MPA assessment and platelet activation with fibrinogen, and P-selectin expression | LTA/platelet-rich plasma turbidimetry, flow cytometry | T744C polymorphism was not related to differences in response to clopidogrel at early or late stage of therapy |
| [51] | T744C, C34T | 222 patients with ACS (Chinese Han origin) | Clopidogrel 300 mg LD & 75 mg MD | HPR cut-off point has been described as ≥ 208 PRU | VerifyNow P2Y12 assay | Poor response to clopidogrel was based on coexistence of CYP2B6*9 and T744C and coexistence of CYP2B6*1B and T744C |
| [52] | G52T, C34T | 503 STEMI patients (Chinese Han origin) | Clopidogrel 300 mg LD & 75 mg MD | Primary endpoint was a composite of cardiovascular death, nonfatal MI, vessel revascularization, and S. Safety endpoint was incidence of major bleeding events | - | G52T minor allele was factor responsible for major bleedings |
| [53] | G52T, C34T | 473 patients with PAD | Clopidogrel, 75 mg (in 137 patients) | Primary endpoint of neurological events (ischemic stroke, carotid endartectomy, carotid stenting) | - | Clopidogrel treated patients with at least one C34T T allele showed 4.02-fold higher risk for neurological events comparing to carriers of only C34T C alleles. None of the studied polymorphisms was related to all-cause mortality |
| [54] | G52T, C34T | 498 patients with ACS | Clopidogrel 300 mg LD & 75 mg MD | CR defined as < 10% decrease from baseline after 7 days of treatment. Secondary outcome comprised adverse cardiovascular events | AggRAM system | Patients with T allele at G52T or C34T polymorphisms, presented significantly higher risk of CR* and presence of cardiovascular events in comparison to wild-type patients. Patients with the T variations in C34T presented also significantly increased risk of post percutaneous coronary intervention and higher recurrence risk of cardiovascular diseases |
| [55] | G52T | 557 patients with CAD and 43 healthy volunteers (origin unspecified) | Clopidogrel 300, 450 or 600 mg LD & 75 mg MD (in patients only) | Nonresponsiveness defined as impedance above 5 Ω | Impedance aggregometry | Patients with H2/H2 haplotype were more often nonresponders and showed stronger platelet aggregation than subjects with at least one H1. Haplotype H2/H2 was also related with increased CR |
| [56] | T744C | 26 patients with acute MI and 102 healthy volunteers (Western Indian origin) | Clopidogrel 300 mg LD & 75 mg MD | Assessment of MPA and inhibition of platelet aggregation before, 24 h and 6 days of treatment | LTA/platelet-rich plasma turbidimetry | Beside T744C P2Y12 polymorphism MDR1 and CYP2C19 SNPs were taken into consideration. T744C and CYP2C19*2 mutant alleles were related to clopidogrel resistance, compared to wild type |
| [57] | T744C | 112 patients scheduled for PCI (Iranian origin) | Clopidogrel 600 mg LD & 150 and 75 mg MD | Responsiveness to clopidogrel defined a decrease from baseline value; < 10% for nonresponders, 10 – 30% for semiresponders, > 30% for responders | LTA/platelet-rich plasma turbidimetry | No significant association between response to clopidogrel treatment and P2Y12 polymorphism was observed |
| [56] | T744C | 26 patients with acute MI and 102 healthy volunteers (Western Indian origin) | Clopidogrel 300 mg LD & 75 mg MD | Assessment of MPA and inhibition of platelet aggregation before, 24 h and 6 days of treatment | LTA/platelet-rich plasma turbidimetry | Beside T744C P2Y12 polymorphism MDR1 and CYP2C19 SNPs were taken into consideration. T744C and CYP2C19*2 mutant alleles were related to clopidogrel resistance, compared to wild type |
| Comparison of P2Y12 SNP prevalence in study populations | ||||||
| [58] | T744C, C34T | 696 patients with CAD (Caucasian origin) | Unspecified | Assessment of bleeding complications defined as major bleeding, intercranial bleeding, or clinically over bleeding, minor bleeding relative to studied polymorphisms | - | Studied polymorphisms were related to bleeding incidents |
| [59] | T744C, C34T | 105 patients with premature MI, and 132 patients without CAD (Caucasian origin) | Unspecified | Assessment of SNP frequencies in study groups and evaluation of cardiovascular risk factors | - | No effect of P2Y12 polymorphism was observed |
| [60] | T744C, G52T, rs10935838 rs5853517 | A total of 1982 patients and matched controls: with/without MI, with/without transient ischemic attacks, with/without deep venous thrombosis (mostly Caucasian origin) | Unspecified | Comparison of SNPs prevalence between cases and controls | - | Haplotype H2 was significantly related to lower risk of incident deep vein thrombosis and pulmonary embolism, compared to haplotype H1. There was no association between the P2RY12 polymorphisms or the H2 haplotype with possible myocardial infarction or ischemic stroke |
| [61] | T744C, G52T, rs9859538, rs1491974, rs7637803 | 776 patients with Kawasaki disease and 1335 healthy controls (Southern Chinese origin) | - | Assessment of P2Y12 SNP association with coronary aneurism in Kawasaki disease | - | No significant association between any of studied polymorphism and Kawasaki disease was found. There was however significant association between TT genotype of rs7637803 and higher risk of coronary artery aneurysm risk in Kawasaki disease patients. Authors claimed that it could be used as biomarker for prediction of giant coronary artery aneurysm |
| [62] | T744C, G52T, rs9859538, rs1491974, rs7637803 | 759 patients with Kawasaki disease (Chinese Han origin) | - | Association of studied SNPs with intravenous immunoglobulin resistance | - | After including such factors as gender and age, carriers of the rs6809699 (G52T) C allele were at lower risk of intravenous immunoglobulin resistance. There was no significant association between four other polymorphism and sensitivity to intravenous immunoglobulin |
| [63] | T744C, G52T, rs6801273, rs6798347 | 122 patients with MI (subdivided according to the age when MI occurred) and 235 healthy controls | Unspecified | Assessment of studied SNPs prevalence between study groups | - | Studied P2RY12 polymorphisms did not show any significant correlations to CR |
ACS acute coronary syndrome, CHD coronary heart disease, CR clopidogrel resistance, HTPR high on-treatment platelet reactivity, LD loading dose, LTA light transmittance aggregometry, MD maintenance dose, MPA maximum platelet aggregation rate, PAD peripheral artery disease, PCI percutaneous coronary intervention, STEMI ST-elevation myocardial infarction, ST stent thrombosis
H1/H2 Haplotype and Related Alleles
In 2003, Fontana et al. [13] identified that some healthy volunteers had significantly greater ADP-induced maximal platelet aggregation (MPA). The authors performed genotyping and found that four of the studied polymorphisms (G52T (rs6809699), i-C139T (rs10935838), i-T744C (rs2046934), and i-ins801A (rs5853517); Table 2) were in linkage disequilibrium. When a subject carried G variant in G52T, C in i-C139T, T in i-T744C, and lacked insertion in 801A, he carried H1 haplotype. Otherwise, an H2 haplotype was present. P2Y12 genotyping revealed that greater MPA and a more significant reduction in cAMP concentration correlated with the presence of H2 haplotype.
Further investigations showed that the H2 haplotype could be related to thrombotic-related diseases. For example, a case–control study showed that patients with peripheral arterial disease are more likely to be H2 carriers (30% in cases vs. 21% in healthy controls) [12]. Likewise, a recent China-based study revealed that 26.3% of subjects with cerebral infarction carried H2 haplotype, while this variant occurred less frequently (16.7%) in healthy controls [64]. The most widely used allele indicative of the H1/H2 haplotype is the intronic variant T744C. Several case–control studies conducted on patients with acute coronary syndrome (ACS) or coronary artery disease observed that the mutant C allele was more frequent in the ACS group: 22.73% vs. 19.13% [39], 25.4% vs. 31.5% [35], and 27.5% vs. 2.5% [40] in cases and controls, respectively. These findings confirm that H2 polymorphisms can be connected with cardiovascular diseases and influence platelet reactivity. Consequently, they may alter the efficacy of the antiplatelet treatment with P2Y12 inhibitors.
H1/H2 Haplotype Alleles and Clopidogrel
Two meta-analyses investigated the association of P2Y12 single nucleotide polymorphisms (SNPs) linked with H1/H2 haplotypes and the efficacy of clopidogrel. The first one focused on the risk of clopidogrel resistance, defined either as lower than 10% platelet inhibition or ADP-induced platelet aggregation greater than 70% [65]. The authors found that under the dominant model, only G52T increased the odds ratio of clopidogrel resistance (OR = 1.45, 95% CI: 1.14–1.85, p = 0.003 for G52T). The other investigated SNP, T744C, did not influence the odds of inadequate response to clopidogrel. The second meta-analysis, by Zhao et al. [66], showed a significant association of T744C and ischemic events in the Han Chinese population but not in the Caucasian population. Moreover, the significance was observed only under a recessive model (CC vs. CT + TT, OR = 3.32, 95% CI: 1.62–6.82, p = 0.001). In contrast, Liu et al. [41] found that in the case of patients with ischemic stroke, the presence of the C allele was associated with a lower incidence of clopidogrel resistance (OR = 0.407, 95% CI: 0.191–0.867, p = 0.018). Another study on the population of Asian patients with symptomatic extracranial or intracranial stenosis showed a significant adverse influence of the T allele. When a patient carried the T allele, there were significantly greater odds of transient ischemic attack, ischemic stroke, MI, or vascular-related mortality during a 1-year follow-up (OR: 2.01, 95% CI: 1.10–3.67, p = 0.041) [42].
Despite these reports, a number of studies failed to notice any influence of T744C on the prevalence of HTPR or adverse events [32, 34, 39, 43–50, 58, 67, 68]. It is also possible that the effect of T744C on platelet function and the efficacy of clopidogrel treatment may depend on the coexistence of other factors, such as compliance, drug interactions, and comorbidities [51, 69, 70]. For example, a study on 222 ACS patients showed that the concomitance of a mutant allele of T744C and polymorphic alleles of CYP2B6 (*9 and *1B) were associated with high platelet reactivity and poor response to clopidogrel [71].
Another SNP associated with H1/H2 haplotype, G52T, can negatively impact PCI outcomes in elderly patients. In a recent study that included 811 patients aged ≥ 75 years of age, TT homozygotes had a higher risk of bleeding during a 1-year follow-up compared with GG and GT genotypes (adjusted HR: 3.87, 95% CI: 1.41–10.68, p = 0.009) [72]. Although T744C was not one of the investigated variants, the T allele in G52T is associated with the H2 haplotype. Therefore, the observation of Cha et al. could translate to the combined effect of this haplotype on the efficacy of DAPT with clopidogrel (75 mg) and aspirin (100 mg).
H1/H2 Haplotype Alleles and Other P2Y12 Inhibitors
Available resources regarding the influence of H1/H2 on the efficacy of other P2Y12 inhibitors are less abundant than for clopidogrel. One study investigated the role of two alleles comprising H1/H2 haplotypes (T744C and G52T) on ticagrelor’s ex vivo antiplatelet effect [33]. The carriers of the T744C and G52T mutant alleles had significantly lower baseline platelet aggregation. However, there were no statistically significant differences in peak and late aggregation or inhibition of platelet aggregation at low (15 μM) and high (50 μM) ticagrelor concentrations.
In a cangrelor-focused study, Bouman et al. [36] investigated ex vivo peak and late platelet aggregation at low and high drug concentrations. The carriers of the T744C CC genotype exhibited higher peak platelet aggregation and lower peak inhibition of platelet aggregation at low (0.05 µM) cangrelor concentrations but the differences were not statistically significant. In contrast, Oestreich et al. [37] reported a significant recessive effect of H2 haplotype, represented by the T744C variant, on platelet reactivity stimulated by thrombin receptor–activating peptide (TRAP). The response to TRAP was markedly reduced (25–42%) in H2/H2 carriers whose isolated platelets were subjected to cangrelor.
C34T and P2Y12 Inhibitors
Two of the meta-analyses cited above investigated the influence of C34T (rs6785930) on the efficacy of clopidogrel therapy. Both found a significant relationship between the presence of the T allele and the risk of insufficient response to the drug. In the first analysis, the presence of the T allele increased over two-fold the risk of clopidogrel resistance (OR = 2.30, 95% CI: 1.50–3.51, p = 0.0001) [65]. In the other study, the odds ratio of ischemic events was 1.7 (95% CI: 1.22–2.36, p = 0.002) in carriers of the T allele, but the effect was significant in Han Chinese population only [66]. Also, this polymorphism was not associated with an increased risk of bleeding events.
Lack of the influence of C34T on clopidogrel therapy was reported for European populations [34, 44]. Ulehlova et al. [44] did not observe significant differences in the frequencies of C34T alleles between clopidogrel-resistant patients with AMI and the entire investigated group. This polymorphism also seems not to influence the endothelial function or arterial wall properties [73]. However, an interesting observation was reported for smokers [74]. Carriers of mutant C34T allele with coronary artery disease (CAD) undergoing PCI who also smoked had a higher risk of reaching the primary endpoint (death from cardiovascular causes, nonfatal MI, revascularization, stroke, recurrent cardiac ischemia, or transient ischemic attack) than the CC homozygotes (HR 2.23, 95% CI: 1.05–6.01, p = 0.04).
The influence of the C34T allele on the efficacy of ticagrelor may also be insignificant. Although there are no clinical reports, the ex vivo study showed that there might be a difference in platelet aggregation and the inhibition caused by ticagrelor [33]. The CT heterozygotes had significantly lower platelet aggregation and greater inhibition of platelet aggregation at the highest studied ticagrelor concentrations than TT homozygotes.
Other P2Y12 Haplotypes and Alleles
Besides H1/H2, some reports distinguish other haplotypes that may influence platelet reactivity. Nie et al. [32] investigated SNPs in the promoter and regulatory region of the P2Y12 gene. The authors inferred six commonly occurring haplotypes of four SNPs—rs6798347 (C > t), rs6787801 (T > c), rs6801273 (A > g), and rs6785930 (C > t). Among the haplotypes labeled H0–H5, haplotype H1 (tcgt) had a lower incidence of high-on-treatment platelet reactivity (HTPR) in all of the 180 patients with ACS compared with the reference H0 (CTAC) haplotype (adjusted OR 0.13, 95% CI: 0.03–0.68, p = 0.016). The influence was significant after adjusting for other covariates—patients’ demographics and CYP2C19 LoF alleles.
In a study with patients with ischemic stroke who underwent a stenting procedure, two P2Y12 SNPs, rs6787801 and rs6798347, comprised three haplotypes [42]. The patients were on DAPT with clopidogrel and aspirin and were monitored for the occurrence of ischemic events for 12 months after the procedure. None of the distinguished haplotypes was associated with a greater risk of subsequent vascular events.
Li et al. [33] distinguished six common haplotypes (A–F) from nine investigated P2Y12 SNPs. One of the haplotypes (‘D’, TAATAGCCT) included, among others, the minor C allele of T744C, the major C allele of C34T, and the minor T allele of G52T. The carriers of this haplotype exhibited significantly lower baseline platelet aggregation than the most common haplotype. However, it did not translate to significantly lower aggregation or lower inhibition of platelet reactivity caused by ticagrelor. Hence, the authors concluded that the investigated P2Y12 haplotypes do not influence the antiplatelet effect of the drug.
Bouman et al. [36] distinguished six common haplotypes (A–F) from five SNPs in the P2Y12 gene. The studies SNPs were rs6798347, rs6787801, rs9859552, rs6801273, and T744C (rs2046934). The authors found that the C haplotype, comprising the minor T744C C allele and the major variants of the other SNPs, had the most significant influence on cangrelor-induced platelet aggregation. Both peak and late ex vivo platelet aggregation were greater than the reference haplotype under low and high cangrelor concentrations. At the same time, the C haplotype had lower inhibition of platelet aggregation, but the effect was most pronounced 360 s after stimulation with ADP.
Another study showed that the minor G of rs3732759 (A > G) allele occurred more frequently in patients with cardiovascular diseases treated with clopidogrel than in healthy controls (42.7% vs. 26.7%, OR = 1.43, 95% CI: 1.05–1.93, p = 0.021) [35]. This observation was also confirmed for the frequency of clopidogrel resistance, as 54.2% of the resistant and only 28.6% of sensitive patients carried GG genotype (p = 0.017). Decreased sensitivity to clopidogrel translated to the occurrence of MACE. The patients with MACE were more likely to carry the rs3732759 GG genotype. The authors paired three analyzed SNPs into four common haplotypes in the same paper. It allowed capturing the interplay between the SNPs. Although the rs3732759 GG negatively influenced clopidogrel therapy, the only haplotype associated with an increased risk of CHD was TCA (rs7428575 T, rs2046934 C, and rs3732759 A; OR: 1.57, 95% CI: 1.14–2.17, p = 0.005).
Recently, Rath et al. [34] investigated patients with 103 ischemic stroke or transient ischemic attack and investigated the influence of selected SNPs on the platelet reactivity and the degree of aggregation inhibition during clopidogrel therapy. The minor T allele (rs9859552) carriers appeared to have greater platelet reactivity values; the effect was not statistically significant.
Epigenetic Studies on P2Y12
Epigenetic studies are a new direction to identify the mechanism of variable response to antiplatelet therapy. Several reports suggest that methylations of promoters or other epigenetic changes contribute to the altered expression of P2Y12 and could be responsible for the epigenetic mechanisms of clopidogrel resistance. Su et al. [75] reported that DNA methylation levels of two cytosine-phosphate-guanine (CpG) dinucleotides on P2Y12 promoter (CpG1 and CpG2) were related to platelet activity measured by the VerifyNow P2Y12 assay in patients with CAD. Lower methylation of two CpGs indicated the poorer clopidogrel response in alcohol-abusing status. Moreover, lower methylation levels of CpG1 correlated with higher platelet activity in the active smokers and patients with albumin concentrations 35 g/L. In the study by Li et al. [76], the impact of the P2Y12 promoter DNA methylation on the recurrence of ischemic events was evaluated in patients with cerebrovascular disease. Among sixteen tested CpG dinucleotides, three CpG sites (CpG11 and CpG12 + 13) showed lower methylation levels, which correlated with ADP inhibition rate and ADP-induced platelet–fibrin cloth strength measured by thromboelastography. The lower methylation status of CpG11 and CpG12 + 13 was also associated with an increased risk of clinical events, including vascular-related mortality, ischemic stroke, transient ischemic attack, or MI.
Evidence suggests that platelet-related miRNAs could play a pivotal role as biomarkers of antiplatelet therapy efficacy because they act as modulators of P2Y12 expression. miRNAs are small non-coding sequences of nucleotides that bind to mRNA sites, block transcription, and, in consequence, cause a decrease in protein production [77]. Landry et al. [78] reported that the P2Y12 receptor is a target of miRNA-223, and complexes of Argonaute 2 protein with miRNA-223 may be involved in regulating P2Y12 receptor expression in platelets. These results seem to align with the study by Chyrchel et al. [79], who reported that miRNA-223 expression in plasma was elevated in patients with ACS exhibiting increased platelet inhibition in response to DAPT with aspirin plus clopidogrel, prasugrel, or ticagrelor. The effect was stronger for newer P2Y12 antagonists. Similarly, an association between decreased platelet miRNA-223 levels and high on-treatment platelet reactivity in patients treated with clopidogrel was confirmed in other studies [80, 81]. Alteration in platelet aggregation due to reduced P2Y12 expression caused by miRNA-126 inhibition was confirmed in patients with ACS [82]. In another study on that group of patients, the statistically significant connection between miRNA-29 and miRNA-34 expression levels and P2Y12R gene polymorphism (A > G, rs3732759) was reported [83]. Syam et al. [84] noticed a significant association of high miRNA-26a platelet expression but not DNA methylation of the P2Y12 gene on platelet reactivity in patients with acute STEMI undergoing clopidogrel therapy. The role of an elevated miRNA-92a as a biomarker of an increased risk of ACS was confirmed in coronary heart disease patients with type 2 diabetes mellitus [85]. However, as Kramer et al. [86] claimed, further studies are needed to elaborate a standardized protocol for miRNA sample handling to minimize preanalytical variability and the kinetics of platelet miRNA release in response to platelet activation.
Summary
With an inadequate response to P2Y12 inhibitors, thrombotic events may occur, leading to treatment failure. As shown in Table 3, some authors claim that alterations in the P2Y12 gene could also play a role in thrombotic events during antiplatelet therapy. This review outlines that the H2 haplotype could be most strongly related to thrombotic-related diseases and possibly influences platelet reactivity. However, several studies showed that only G52T, one of the polymorphisms comprising H1/H2 haplotypes, increased the odds of clopidogrel resistance, simultaneously exposing the lack of influence of T744C polymorphism. The coexistence of other factors should be considered regarding platelet function and the efficacy of clopidogrel treatment. The non-genetic factors include the origin of study population or smoking status. While for the newer antiplatelet drugs, the reports are scarce and inconclusive, and some clopidogrel co-factors are well described. For example, the two-step activation process involves several CYP450 enzymes, among which CYP2C19 is regarded as the most influential [7, 87]. The carriers of CYP2C19 alleles are at the greater risk of MACE, MI, or stent thrombosis. The association is so pronounced that some clinicians postulate guided antiplatelet therapy that includes an option of switching from clopidogrel to newer, CYP2C19-independent alternatives [88]. As CYP2C19 is crucial in the second step of the activation, its inhibitors may also decrease clopidogrel’s efficacy. For example, in a recent meta-analysis, it was shown that concomitant intake of proton pump inhibitors increases the risk of MACE by 63% in CYP2C19 loss-of-function allele carriers (95% CI: 1.31–2.03, p < 0.0001) [89]. These findings indicate that studies on P2Y12 polymorphisms in patients treated with clopidogrel should account for the CYP2C19 genotype.
Recent evidence indicates that epigenetic factors such as P2Y12 DNA methylation and miRNAs play a role in the observed variability of platelet reactivity and may serve as biomarkers of response to P2Y12 inhibitors. Lower DNA methylation of the P2Y12 gene promoter increases the risk of resistance to antiplatelet therapy, while decreased miRNA levels are connected to high on-treatment platelet reactivity and ischemic events in patients treated with P2Y12 inhibitors. Due to a broad range of platelet miRNAs, further studies are needed on a reliable miRNA-based biomarker to assess the in vivo platelet activation and response to P2Y12 inhibitors.
Conclusions
The current review underlines the multifactorial causes of the observed response variability to P2Y12 receptor inhibitors. Most of the reported P2Y12 genetic polymorphisms (e.g., T allele in G52T) may be responsible for changes in the structure of the P2Y12 receptor, therefore decreasing the efficacy of antiplatelet therapy. However, the impact of some factors (e.g., T744C, C34T, smoking) on antiplatelet therapy is not yet fully determined, and their coexistence may lead to resistance to antiplatelet therapy.
Author Contribution
All authors contributed to the study conception. The first draft of the manuscript was written by D. Danielak, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.ESC guidelines on dual antiplatelet therapy (DAPT). European Society of Cardiology. 2017. https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/2017-focused-update-on-dual-antiplatelet-therapy-dapt. Accessed 9 Jun 2021.
- 2.Ferri N, Corsini A, Bellosta S. Pharmacology of the new P2Y12 receptor inhibitors: insights on pharmacokinetic and pharmacodynamic properties. Drugs. 2013;73:1681–1709. doi: 10.1007/s40265-013-0126-z. [DOI] [PubMed] [Google Scholar]
- 3.Faridi KF, Garratt KN, Kennedy KF, et al. Physician and hospital utilization of P2Y12 inhibitors in ST-segment-elevation myocardial infarction in the United States: a study from the National Cardiovascular Data Registry’s Research to practice initiative. Circ Cardiovasc Qual Outcomes. 2020;13:e006275. doi: 10.1161/CIRCOUTCOMES.119.006275. [DOI] [PubMed] [Google Scholar]
- 4.Sinnaeve P, Fahrni G, Schelfaut D, et al. Subcutaneous selatogrel inhibits platelet aggregation in patients with acute myocardial infarction. J Am Coll Cardiol. 2020;75:2588–2597. doi: 10.1016/j.jacc.2020.03.059. [DOI] [PubMed] [Google Scholar]
- 5.Milluzzo RP, Franchina GA, Capodanno D, Angiolillo DJ. Selatogrel, a novel P2Y12 inhibitor: a review of the pharmacology and clinical development. Expert Opin Investig Drugs. 2020;29:537–546. doi: 10.1080/13543784.2020.1764533. [DOI] [PubMed] [Google Scholar]
- 6.Mallouk N, Labruyère C, Reny J-L, et al. Prevalence of poor biological response to clopidogrel: a systematic review. Thromb Haemost. 2012;107:494–506. doi: 10.1160/TH11-03-0202. [DOI] [PubMed] [Google Scholar]
- 7.Danielak D, Karaźniewicz-Łada M, Komosa A, et al. Influence of genetic co-factors on the population pharmacokinetic model for clopidogrel and its active thiol metabolite. Eur J Clin Pharmacol. 2017;73:1623–1632. doi: 10.1007/s00228-017-2334-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswas M, Kali SK. Association of CYP2C19 Loss-of-function alleles with major adverse cardiovascular events of clopidogrel in stable coronary artery disease patients undergoing percutaneous coronary intervention: meta-analysis. Cardiovasc Drugs Ther. 2021. [DOI] [PubMed]
- 9.Pereira NL, Rihal C, Lennon R, et al. Effect of CYP2C19 genotype on ischemic outcomes during oral P2Y12 inhibitor therapy: a meta-analysis. JACC Cardiovasc Interv. 2021;14:739–750. doi: 10.1016/j.jcin.2021.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou X, Shi J, Sun H. Gene polymorphism of cytochrome P450 2C19*2 and clopidogrel resistance reflected by platelet function assays: a meta-analysis. Eur J Clin Pharmacol. 2014;70:1041–1047. doi: 10.1007/s00228-014-1714-x. [DOI] [PubMed] [Google Scholar]
- 11.Alakbarzade V, Huang X, Ster IC, McEntagart M, Pereira AC. High on-clopidogrel platelet reactivity in ischaemic stroke or transient ischaemic attack: systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2020;29:104877. doi: 10.1016/j.jstrokecerebrovasdis.2020.104877. [DOI] [PubMed] [Google Scholar]
- 12.Fontana P, Gaussem P, Aiach M, Fiessinger J-N, Emmerich J, Reny J-L. P2Y12 H2 haplotype is associated with peripheral arterial disease: a case-control study. Circulation. 2003;108:2971–2973. doi: 10.1161/01.CIR.0000106904.80795.35. [DOI] [PubMed] [Google Scholar]
- 13.Fontana P, Dupont A, Gandrille S, et al. Adenosine diphosphate-induced platelet aggregation is associated with P2Y12 gene sequence variations in healthy subjects. Circulation. 2003;108:989–995. doi: 10.1161/01.CIR.0000085073.69189.88. [DOI] [PubMed] [Google Scholar]
- 14.Kim M-J, Jeong ES, Park J-S, et al. Multiple cytochrome P450 isoforms are involved in the generation of a pharmacologically active thiol metabolite, whereas paraoxonase 1 and carboxylesterase 1 catalyze the formation of a thiol metabolite isomer from ticlopidine. Drug Metab Dispos. 2014;42:141–152. doi: 10.1124/dmd.113.053017. [DOI] [PubMed] [Google Scholar]
- 15.Farid NA, Kurihara A, Wrighton SA. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J Clin Pharmacol. 2010;50:126–142. doi: 10.1177/0091270009343005. [DOI] [PubMed] [Google Scholar]
- 16.Sangkuhl K, Klein TE, Altman RB. Clopidogrel pathway. Pharmacogenet Genomics. 2010;20:463–465. doi: 10.1097/FPC.0b013e3283385420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farid NA, Payne CD, Small DS, et al. Cytochrome P450 3A inhibition by ketoconazole affects prasugrel and clopidogrel pharmacokinetics and pharmacodynamics differently. Clin Pharmacol Ther. 2007;81:735–741. doi: 10.1038/sj.clpt.6100139. [DOI] [PubMed] [Google Scholar]
- 18.Kazui M, Nishiya Y, Ishizuka T, et al. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010;38:92–99. doi: 10.1124/dmd.109.029132. [DOI] [PubMed] [Google Scholar]
- 19.Rehmel JLF, Eckstein JA, Farid NA, et al. Interactions of two major metabolites of prasugrel, a thienopyridine antiplatelet agent, with the cytochromes P450. Drug Metab Dispos. 2006;34:600–607. doi: 10.1124/dmd.105.007989. [DOI] [PubMed] [Google Scholar]
- 20.Farid NA, Smith RL, Gillespie TA, et al. The disposition of prasugrel, a novel thienopyridine, in humans. Drug Metab Dispos. 2007;35:1096–1104. doi: 10.1124/dmd.106.014522. [DOI] [PubMed] [Google Scholar]
- 21.Williams ET, Bacon JA, Bender DM, et al. Characterization of the expression and activity of carboxylesterases 1 and 2 from the beagle dog, cynomolgus monkey, and human. Drug Metab Dispos. 2011;39:2305–2313. doi: 10.1124/dmd.111.041335. [DOI] [PubMed] [Google Scholar]
- 22.Dean L. Prasugrel therapy and CYP genotype. In: Pratt VM, Scott SA, Pirmohamed M, Esquivel B, Kane MS, Kattman BL, et al., editors. Med Genet Summ. Bethesda (MD): National Center for Biotechnology Information (US); 2012. http://www.ncbi.nlm.nih.gov/books/NBK425796/. Accessed 16 Dec 2021. [PubMed]
- 23.Li X, Liu C, Zhu X, et al. Evaluation of tolerability, pharmacokinetics and pharmacodynamics of vicagrel, a novel P2Y12 antagonist, in healthy Chinese volunteers. Front Pharmacol. 2018;9:643. doi: 10.3389/fphar.2018.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y-D, Zhang H, Zhan Y, et al. Pharmacokinetics, mass balance, and metabolism of [14C]vicagrel, a novel irreversible P2Y12 inhibitor in humans. Acta Pharmacol Sin. 2021;42:1535–1546. doi: 10.1038/s41401-020-00547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ufer M, Huynh C, van Lier JJ, Caroff E, Fischer H, Dingemanse J. Absorption, distribution, metabolism and excretion of the P2Y12 receptor antagonist selatogrel after subcutaneous administration in healthy subjects. Xenobiotica. 2020;50:427–434. doi: 10.1080/00498254.2019.1646440. [DOI] [PubMed] [Google Scholar]
- 26.Beavers CJ, Effoe SA, Dobesh PP. Selatogrel: a novel subcutaneous P2Y12 inhibitor. J Cardiovasc Pharmacol. 2022;79:161–7. doi: 10.1097/FJC.0000000000001079. [DOI] [PubMed] [Google Scholar]
- 27.Haberstock-Debic H, Andre P, Mills S, Phillips DR, Conley PB. A clopidogrel-insensitive inducible pool of P2Y12 receptors contributes to thrombus formation: inhibition by elinogrel, a direct-acting, reversible P2Y12 antagonist. J Pharmacol Exp Ther. 2011;339:54–61. doi: 10.1124/jpet.111.184143. [DOI] [PubMed] [Google Scholar]
- 28.Angiolillo DJ, Welsh RC, Trenk D, et al. Pharmacokinetic and pharmacodynamic effects of elinogrel: results of the platelet function substudy from the intravenous and oral administration of elinogrel to evaluate tolerability and efficacy in nonurgent percutaneous coronary intervention patients (INNOVATE-PCI) trial. Circ Cardiovasc Interv. 2012;5:347–356. doi: 10.1161/CIRCINTERVENTIONS.111.965608. [DOI] [PubMed] [Google Scholar]
- 29.Husted S, Emanuelsson H, Heptinstall S, Sandset PM, Wickens M, Peters G. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur Heart J. 2006;27:1038–1047. doi: 10.1093/eurheartj/ehi754. [DOI] [PubMed] [Google Scholar]
- 30.BRILINTA (ticagrelor) - Product Monograph. AstraZeneca Canada Inc. 2022. https://www.astrazeneca.ca/content/dam/az-ca/downloads/productinformation/brilinta-product-monograph-en.pdf. Accessed 19 Jul 2022.
- 31.European Medicines Agency. Kengrexal. 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/kengrexal. Accessed 16 Dec 2021.
- 32.Nie X-Y, Li J-L, Zhang Y, et al. Haplotype of platelet receptor P2RY12 gene is associated with residual clopidogrel on-treatment platelet reactivity. J Zhejiang Univ Sci B. 2017;18:37–47. doi: 10.1631/jzus.B1600333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li M-P, Tang J, Wen Z-P, et al. Influence of P2Y12 polymorphisms on platelet activity but not ex-vivo antiplatelet effect of ticagrelor in healthy Chinese male subjects. Blood Coagul Fibrinolysis. 2015;26:874–881. doi: 10.1097/MBC.0000000000000308. [DOI] [PubMed] [Google Scholar]
- 34.Rath CL, Jørgensen NR, Wienecke T. Clopidogrel responder status is uninfluenced by CYP2C19*2 in Danish patients with stroke. PLoS One. 2020;15:e0236260. doi: 10.1371/journal.pone.0236260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang H-H, Chen Y, Gao C-Y. Associations of P2Y12R gene polymorphisms with susceptibility to coronary heart disease and clinical efficacy of antiplatelet treatment with clopidogrel. Cardiovasc Ther. 2016;34:460–467. doi: 10.1111/1755-5922.12223. [DOI] [PubMed] [Google Scholar]
- 36.Bouman HJ, van Werkum JW, Rudez G, et al. The influence of variation in the P2Y12 receptor gene on in vitro platelet inhibition with the direct P2Y12 antagonist cangrelor. Thromb Haemost. 2010;103:379–386. doi: 10.1160/TH09-06-0367. [DOI] [PubMed] [Google Scholar]
- 37.Oestreich JH, Steinhubl SR, Ferraris SP, Loftin CD, Akers WS. Effect of genetic variation in P2Y12 on TRAP-stimulated platelet response in healthy subjects. J Thromb Thrombolysis. 2014;38:372–379. doi: 10.1007/s11239-014-1058-5. [DOI] [PubMed] [Google Scholar]
- 38.Kim K-A, Song W-G, Lee H-M, Joo H-J, Park J-Y. Effect of P2Y1 and P2Y12 genetic polymorphisms on the ADP-induced platelet aggregation in a Korean population. Thromb Res. 2013;132:221–226. doi: 10.1016/j.thromres.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Hassani Idrissi H, Hmimech W, El Khorb N, Akoudad H, Habbal R, Nadifi S. Does i–T744C P2Y12 polymorphism modulate clopidogrel response among Moroccan acute coronary syndromes patients? Genet Res Int. 2017;2017:9532471. doi: 10.1155/2017/9532471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zoheir N, Abd Elhamid S, Abulata N, El Sobky M, Khafagy D, Mostafa A. P2Y12 receptor gene polymorphism and antiplatelet effect of clopidogrel in patients with coronary artery disease after coronary stenting. Blood Coagul Fibrinolysis. 2013;24:525–531. doi: 10.1097/MBC.0b013e32835e98bf. [DOI] [PubMed] [Google Scholar]
- 41.Liu R, Zhou Z-Y, Chen Y-B, et al. Associations of CYP3A4, NR1I2, CYP2C19 and P2RY12 polymorphisms with clopidogrel resistance in Chinese patients with ischemic stroke. Acta Pharmacol Sin. 2016;37:882–888. doi: 10.1038/aps.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X-Q, Ma N, Li X-G, et al. Association of PON1, P2Y12 and COX1 with recurrent ischemic events in patients with extracranial or intracranial stenting. PLoS One. 2016;11:e0148891. doi: 10.1371/journal.pone.0148891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ou W, He Y, Li A, Liu B, Jin L. Genotype frequencies of CYP2C19, P2Y12 and GPIIIa polymorphisms in coronary heart disease patients of Han ethnicity, and their impact on clopidogrel responsiveness. Int Heart J. 2016;57:586–592. doi: 10.1536/ihj.16-006. [DOI] [PubMed] [Google Scholar]
- 44.Ulehlova J, Slavik L, Kucerova J, Krcova V, Vaclavik J, Indrak K. Genetic polymorphisms of platelet receptors in patients with acute myocardial infarction and resistance to antiplatelet therapy. Genet Test Mol Biomark. 2014;18:599–604. doi: 10.1089/gtmb.2014.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isordia-Salas I, Olalde-Román MJ, Santiago-Germán D, de la Peña NC, Valencia-Sánchez JS. The impact of CYP3A5*1/*3, PIA1/A2 and T744C polymorphisms on clopidogrel and acetylsalicylic acid response variability in Mexican population. Thromb Res. 2012;130:e67–72. doi: 10.1016/j.thromres.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 46.Kar R, Meena A, Yadav BK, Yadav R, Kar SS, Saxena R. Clopidogrel resistance in North Indian patients of coronary artery disease and lack of its association with platelet ADP receptors P2Y1 and P2Y12 gene polymorphisms. Platelets. 2013;24:297–302. doi: 10.3109/09537104.2012.693992. [DOI] [PubMed] [Google Scholar]
- 47.Giusti B, Gori AM, Marcucci R, et al. Cytochrome P450 2C19 loss-of-function polymorphism, but not CYP3A4 IVS10+12G/A and P2Y12 T744C polymorphisms, is associated with response variability to dual antiplatelet treatment in high-risk vascular patients. Pharmacogenet Genomics. 2007;17:1057–1064. doi: 10.1097/FPC.0b013e3282f1b2be. [DOI] [PubMed] [Google Scholar]
- 48.Cuisset T, Frere C, Quilici J, et al. Role of the T744C polymorphism of the P2Y12 gene on platelet response to a 600-mg loading dose of clopidogrel in 597 patients with non-ST-segment elevation acute coronary syndrome. Thromb Res. 2007;120:893–899. doi: 10.1016/j.thromres.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 49.Lev EI, Patel RT, Guthikonda S, Lopez D, Bray PF, Kleiman NS. Genetic polymorphisms of the platelet receptors P2Y(12), P2Y(1) and GP IIIa and response to aspirin and clopidogrel. Thromb Res. 2007;119:355–360. doi: 10.1016/j.thromres.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Lack of association between the P2Y12 receptor gene polymorphism and platelet response to clopidogrel in patients with coronary artery disease. Thromb Res. 2005;116:491–497. doi: 10.1016/j.thromres.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Liu X, Luo Y, Lai Y, et al. Effect of genetic and coexisting polymorphisms on platelet response to clopidogrel in Chinese Han patients with acute coronary syndrome. J Genet. 2016;95:231–237. doi: 10.1007/s12041-016-0618-1. [DOI] [PubMed] [Google Scholar]
- 52.Zhang J-H, Wang J, Tang X-F, et al. Effect of platelet receptor gene polymorphisms on outcomes in ST-elevation myocardial infarction patients after percutaneous coronary intervention. Platelets. 2016;27:75–79. doi: 10.3109/09537104.2015.1034096. [DOI] [PubMed] [Google Scholar]
- 53.Ziegler S, Schillinger M, Funk M, et al. Association of a functional polymorphism in the clopidogrel target receptor gene, P2Y12, and the risk for ischemic cerebrovascular events in patients with peripheral artery disease. Stroke. 2005;36:1394–1399. doi: 10.1161/01.STR.0000169922.79281.a5. [DOI] [PubMed] [Google Scholar]
- 54.Li M, Wang H, Xuan L, et al. Associations between P2RY12 gene polymorphisms and risks of clopidogrel resistance and adverse cardiovascular events after PCI in patients with acute coronary syndrome. Medicine (Baltimore) 2017;96:e6553. doi: 10.1097/MD.0000000000006553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Staritz P, Kurz K, Stoll M, Giannitsis E, Katus HA, Ivandic BT. Platelet reactivity and clopidogrel resistance are associated with the H2 haplotype of the P2Y12-ADP receptor gene. Int J Cardiol. 2009;133:341–345. doi: 10.1016/j.ijcard.2007.12.118. [DOI] [PubMed] [Google Scholar]
- 56.Shalia KK, Shah VK, Pawar P, Divekar SS, Payannavar S. Polymorphisms of MDR1, CYP2C19 and P2Y12 genes in Indian population: effects on clopidogrel response. Indian Heart J. 2013;65:158–167. doi: 10.1016/j.ihj.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Namazi S, Kojuri J, Khalili A, Azarpira N. The impact of genetic polymorphisms of P2Y12, CYP3A5 and CYP2C19 on clopidogrel response variability in Iranian patients. Biochem Pharmacol. 2012;83:903–908. doi: 10.1016/j.bcp.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Motovska Z, Kvasnicka J, Hajkova J, et al. Platelet gene polymorphisms and risk of bleeding in patients undergoing elective coronary angiography: a genetic substudy of the PRAGUE-8 trial. Atherosclerosis. 2010;212:548–552. doi: 10.1016/j.atherosclerosis.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 59.Motovska Z, Kvasnicka J, Widimsky P, et al. Platelet glycoprotein GP VI 13254C allele is an independent risk factor of premature myocardial infarction. Thromb Res. 2010;125:e61–64. doi: 10.1016/j.thromres.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 60.Zee RYL, Michaud SE, Diehl KA, et al. Purinergic receptor P2Y, G-protein coupled, 12 gene variants and risk of incident ischemic stroke, myocardial infarction, and venous thromboembolism. Atherosclerosis. 2008;197:694–699. doi: 10.1016/j.atherosclerosis.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 61.Lu Z, Xu Y, Fu L, et al. P2RY12:rs7637803 TT variant genotype increases coronary artery aneurysm risk in Kawasaki disease in a southern Chinese population. J Gene Med. 2019;21:e3066. doi: 10.1002/jgm.3066. [DOI] [PubMed] [Google Scholar]
- 62.Wang Z, Xu Y, Zhou H, et al. Association between P2RY12 gene polymorphisms and IVIG resistance in Kawasaki patients. Cardiovasc Ther. 2020;2020:3568608. doi: 10.1155/2020/3568608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pina-Cabral LB, Carvalhais V, Mesquita B, et al. Myocardial infarction before and after the age of 45: possible role of platelet receptor polymorphisms. Rev Port Cardiol. 2018;37:727–735. doi: 10.1016/j.repc.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 64.Lu S-J, Zhou X-S, Zheng Q, Chen H-L, Geng Y-L. Platelet membrane receptor P2Y12 H1/H2 polymorphism is highly associated with cerebral infarction: a case–control study. Neuropsychiatr Dis Treat. 2018;14:2225–2231. doi: 10.2147/NDT.S171213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cui G, Zhang S, Zou J, Chen Y, Chen H. P2Y12 receptor gene polymorphism and the risk of resistance to clopidogrel: a meta-analysis and review of the literature. Adv Clin Exp Med. 2017;26:343–349. doi: 10.17219/acem/63745. [DOI] [PubMed] [Google Scholar]
- 66.Zhao K, Yang M, Lu Y, et al. P2Y12 polymorphisms and the risk of adverse clinical events in patients treated with clopidogrel: a meta-analysis. Drug Res. 2019;69:23–31. doi: 10.1055/a-0622-8110. [DOI] [PubMed] [Google Scholar]
- 67.Liang Z-Y, Han Y-L, Zhang X-L, Li Y, Yan C-H, Kang J. The impact of gene polymorphism and high on-treatment platelet reactivity on clinical follow-up: outcomes in patients with acute coronary syndrome after drug-eluting stent implantation. EuroIntervention. 2013;9:316–327. doi: 10.4244/EIJV9I3A53. [DOI] [PubMed] [Google Scholar]
- 68.Saiz-Rodríguez M, Belmonte C, Caniego JL, et al. Influence of CYP450 enzymes, CES1, PON1, ABCB1, and P2RY12 polymorphisms on clopidogrel response in patients subjected to a percutaneous neurointervention. Clin Ther. 2019;41:1199–1212.e2. doi: 10.1016/j.clinthera.2019.04.037. [DOI] [PubMed] [Google Scholar]
- 69.Würtz M, Grove EL. Interindividual variability in the efficacy of oral antiplatelet drugs: definitions, mechanisms and clinical importance. Curr Pharm Des. 2012;18:5344–5361. doi: 10.2174/138161212803251925. [DOI] [PubMed] [Google Scholar]
- 70.Gebel JM. Heterogeneity of efficacy and safety of antiplatelet therapy in cardiovascular and cerebrovascular disease. Am J Cardiovasc Drugs. 2010;10:115–124. doi: 10.2165/11319580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 71.Coccheri S. Antiplatelet therapy: controversial aspects. Thromb Res. 2012;129:225–229. doi: 10.1016/j.thromres.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 72.Cha J-J, Park JH, Joo HJ, et al. Impact of genetic variants on clinical outcome after percutaneous coronary intervention in elderly patients. Aging. 2021;13:6506–24. doi: 10.18632/aging.202799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siasos G, Kioufis S, Oikonomou E, et al. Impact of C34T P2Y12 genotype on endothelial function and arterial stiffness in patients after percutaneous coronary intervention receiving clopidogrel. Int J Cardiol. 2014;177:1073–1075. doi: 10.1016/j.ijcard.2014.10.060. [DOI] [PubMed] [Google Scholar]
- 74.Siasos G, Kioufis S, Oikonomou E, et al. Impact of C34T P2Y12 ADP receptor polymorphism and smoking status on cardiovascular outcome in coronary artery disease patients receiving clopidogrel. Int J Cardiol. 2016;210:161–163. doi: 10.1016/j.ijcard.2016.02.129. [DOI] [PubMed] [Google Scholar]
- 75.Su J, Li X, Yu Q, et al. Association of P2Y12 gene promoter DNA methylation with the risk of clopidogrel resistance in coronary artery disease patients. Biomed Res Int. 2014;2014:450814. doi: 10.1155/2014/450814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li X-G, Ma N, Wang B, et al. The impact of P2Y12 promoter DNA methylation on the recurrence of ischemic events in Chinese patients with ischemic cerebrovascular disease. Sci Rep. 2016;6:34570. doi: 10.1038/srep34570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Agarwal V, Bell GW, Nam J-W, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015;4:e05005. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Landry P, Plante I, Ouellet DL, Perron MP, Rousseau G, Provost P. Existence of a microRNA pathway in anucleate platelets. Nat Struct Mol Biol. 2009;16:961–966. doi: 10.1038/nsmb.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chyrchel B, Totoń-Żurańska J, Kruszelnicka O, et al. Association of plasma miR-223 and platelet reactivity in patients with coronary artery disease on dual antiplatelet therapy: a preliminary report. Platelets. 2015;26:593–597. doi: 10.3109/09537104.2014.974527. [DOI] [PubMed] [Google Scholar]
- 80.Shi R, Ge L, Zhou X, et al. Decreased platelet miR-223 expression is associated with high on-clopidogrel platelet reactivity. Thromb Res. 2013;131:508–513. doi: 10.1016/j.thromres.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Y-Y, Zhou X, Ji W-J, et al. Decreased circulating microRNA-223 level predicts high on-treatment platelet reactivity in patients with troponin-negative non-ST elevation acute coronary syndrome. J Thromb Thrombolysis. 2014;38:65–72. doi: 10.1007/s11239-013-1022-9. [DOI] [PubMed] [Google Scholar]
- 82.Kaudewitz D, Skroblin P, Bender LH, et al. Association of microRNAs and YRNAs with platelet function. Circ Res. 2016;118:420–432. doi: 10.1161/CIRCRESAHA.114.305663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rytkin E, Mirzaev K, Bure I, et al. MicroRNAs as novel biomarkers for P2Y12 – inhibitors resistance prediction. Pharmacogenomics Pers Med. 2021;14:1575–1582. doi: 10.2147/PGPM.S324612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Syam H, Sukmawan R, Dharma S, et al. Epigenetic interaction of miRNA-26a and P2Y12 gene DNA methylation on platelet reactivity under clopidogrel and their impact to the coronary flow after primary PCI in STEMI. Eur Heart J. 2020;41:ehaa946.1547. doi: 10.1093/ehjci/ehaa946.1547. [DOI] [Google Scholar]
- 85.Wang W, Li Z, Zheng Y, Yan M, Cui Y, Jiang J. Circulating microRNA-92a level predicts acute coronary syndrome in diabetic patients with coronary heart disease. Lipids Health Dis. 2019;18:22. doi: 10.1186/s12944-019-0964-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krammer TL, Mayr M, Hackl M. MicroRNAs as promising biomarkers of platelet activity in antiplatelet therapy monitoring. Int J Mol Sci. 2020;21:E3477. doi: 10.3390/ijms21103477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Biswas M, Sukasem C, Khatun Kali MS, Ibrahim B. Effects of the CYP2C19 LoF allele on major adverse cardiovascular events associated with clopidogrel in acute coronary syndrome patients undergoing percutaneous coronary intervention: a meta-analysis. Pharmacogenomics. 2022;23:207–220. doi: 10.2217/pgs-2021-0098. [DOI] [PubMed] [Google Scholar]
- 88.Galli M, Franchi F, Rollini F, et al. Genetic testing in patients undergoing percutaneous coronary intervention: rationale, evidence and practical recommendations. Expert Rev Clin Pharmacol. 2021;14:963–978. doi: 10.1080/17512433.2021.1927709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Biswas M, Rahaman S, Biswas TK, Ibrahim B. Risk of major adverse cardiovascular events for concomitant use of clopidogrel and proton pump inhibitors in patients inheriting CYP2C19 loss-of-function alleles: meta-analysis. Int J Clin Pharm. 2021;43:1360–1369. doi: 10.1007/s11096-021-01261-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.