Abstract
A vast array of challenging environments are inhabited by mammals, such as living in confined spaces where oxygen levels are likely to be low. Species can exhibit adaptations in basal metabolic rate (BMR) to exploit such unique niches. In this study we use 801 species to determine the relationship between BMR and burrow use in mammals. We included pre-existing data for mammalian BMR and 16 life history traits. Overall, mammalian BMR is dictated primarily by environmental ambient temperature. There were no significant differences in BMR of terrestrial, semi-fossorial and fossorial mammals, suggesting that species occupying a subterranean niche do not exhibit baseline metabolic costs on account of their burrowing lifestyle. Fossorial mammals likely show instantaneous metabolic responses to low oxygen in tunnels, rather than exhibit adaptive long-term responses in their BMR.
Subject terms: Ecophysiology, Evolutionary ecology
Introduction
Metabolic rate—the amount of energy expended by an animal over a specific period of time—is often referred to as the currency of life1,2. Understanding energy expenditure and energetic budgets is an important component, therefore, in explaining how species are adapted to their environment and any associated niche partitioning3. Moreover, metabolic rate can be used to predict the environmental conditions, such as temperature and elevation, that a species can tolerate4,5. Metabolic rate can also be used to determine aspects of an organism’s thermal neutral zone (TNZ), which is the range of temperatures at which the metabolic rate of an organism is at its minimum in response to ambient temperature6.
Basal metabolic rate (BMR) is a standardised measure, whereby metabolic activity is measured in non-lactating, non-reproductive, post-absorptive adults, whilst individuals are at rest during their natural rest phase of a 24-h cycle and within their thermoneutral zone7. BMR can, however, change throughout the annual cycle, exhibiting a degree of plasticity. Such plasticity enables organisms to alter their BMR in response to certain life-history events8–10. Natural environmental changes, such as seasonal variability in temperature, often manifests in behavioural and physiological responses like migration events, deposition of body fat and moulting11,12, hibernation13,14, torpor15 and more general alterations to activity patterns16. Mammals exhibit a variety of responses to a wide range of environmental conditions and thus provide an ideal model system on which to adopt macro-level approaches to the study of metabolic rate, both as an adaptation to different environments and life-histories.
Life histories frequently result in specific physiological adaptations and traits that are associated with a particular environment17,18. A number of factors have been found to influence BMR including diet, type of reproduction, and habitat, often interacting together in complex ways, leading to variation in physiological traits19,20. One other such example is living in confined spaces such as burrows and dens, where ambient oxygen levels are likely to be lower than outside21. This reduction in oxygen availability can present a challenge for species living in such environments. Despite the associated low oxygen levels, burrows, dens and crevices play an important role in the life history of many mammalian species22. Burrows can be used throughout the active period of the daily cycle, and/or as a safe retreat in which to sleep during the rest phase of the day. Moreover, they can be used seasonally to provide a place to safely raise young, or used more frequently as a place to live and possibly interact with conspecifics. Shelters create a locally contained environment that buffers the animal inside from external bioclimatic conditions, creating a microclimate around the individual or colony21,23,24.
Extreme adaptations in physiology, morphology and anatomy are evident in many species that exhibit a fossorial lifestyle. A lower BMR than similar sized closely related species has been observed in small mammalian species such as grey short-tailed opossums (Monodelphis domestica) and naked mole-rats (Heterocephalus glaber)25,26. These observations are suggested to be related to the respiratory stress hypothesis which proposes that low oxygen levels in burrows is thought to reduce gas exchange in oxygen-depleted environments and reduce heat storage. As a result, oxygen usage and metabolic rate are reduced27–30. This study will explore the potential impact on BMR of leading a fossorial lifestyle, and the effects on BMR of the degree of time spent underground. While the effects of hypoxia as a result of subterranean living have been studied in connection with energy expenditure and metabolism in individual species, particularly subterranean rodents, it has yet to be explored in a wider mammalian context. The aim of this macro-comparative phylogenetically informed study was to establish the effects of the use of burrows as a driver for low BMR in subterranean species in the broad context of the mammalian order. Due to the respiratory stress hypothesis, which suggests that hypoxic conditions found in burrows lead to poor gas exchange and thus lower metabolic rates29,30, we hypothesised that semi-fossorial and entirely fossorial species will exhibit lower BMRs than more surface-dwelling species, after accounting for body mass and phylogeny.
Results
The phylogenetic least squares model (PGLS) was strongly influenced by phylogeny; the maximum likelihood estimate of Pagel’s Lambda (λ) was 0.95 for the top models. Pagel’s λ for the top models is significantly different from 0 (p < 0.001), which indicates that variation in BMR is associated more strongly with phylogeny rather than specific life history traits (Fig. 1). Thus, close relatives are likelier to have similar BMR than distant relatives.
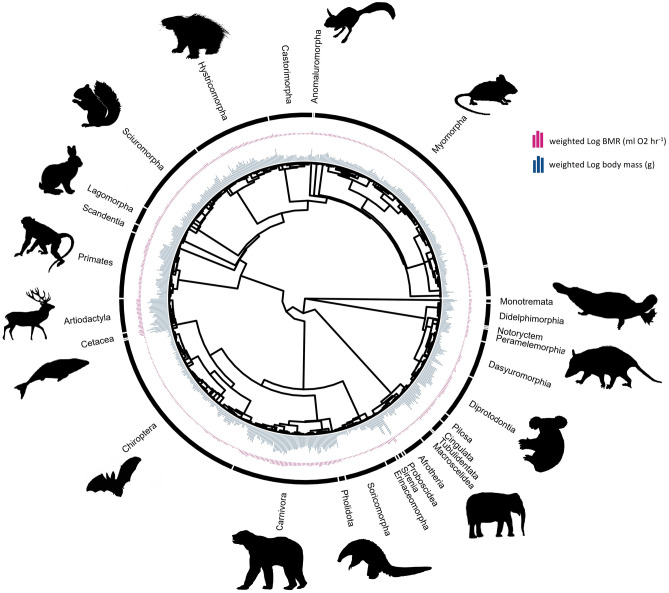
Figure 1.
Phylogenetic tree showing all 801 mammalian species used in this study. The tree was obtained from the Open Tree of Life (OTL) and used in the PGLS and MCMCglmm. The inner bar plot represents weighted log body mass (g), and the outer bar plot represents weighted log BMR (ml O2 h−1). Mammalian order is shown in black around the phylogeny. BMR is strongly linked to phylogeny (Pagel’s λ; p < 0.001), and closely related species and clades will have similar BMRs compared to other individuals.
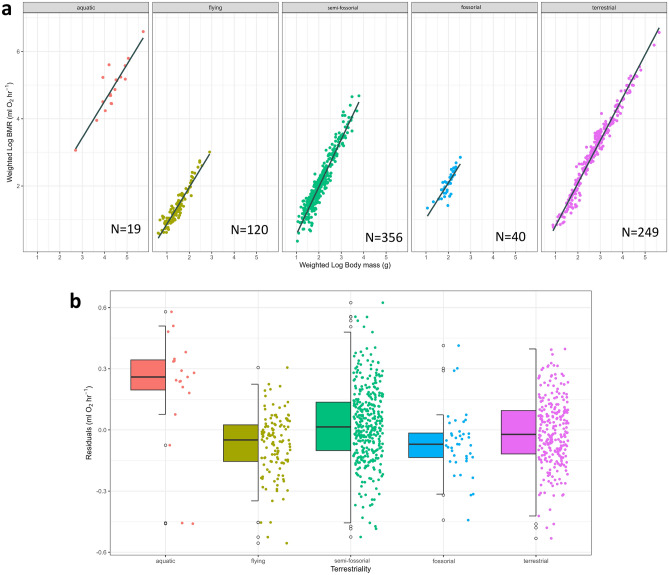
The PGLS models established that the best-supported traits to explain variation in BMR are weighted log body mass (se = 0.01, z = 65.56, p < 0.05), annual temperature (se = 0.001, z = 2.71, p < 0.05), diurnal temperature range (se = 0.003, z = 3.45, p < 0.05), paternal care (se = 0.03, z = 3.05, p < 0.05) and isothermality (se = 0.0005, z = 2.46, p < 0.05) (Supplementary Table 5). BMR increased with body mass and decreased with increasing annual temperature, diurnal temperature range, isothermality and the presence of paternal care. Aquatic mammal BMR was found to be significantly higher than terrestrial (se = 0.07, z = 3.35, p < 0.05), semi-fossorial (se = 0.07, z = 3.26, p < 0.05) or fossorial (se = 0.08, z = 2.43, p < 0.05) categories (Fig. 2). All other traits were not retained in the final models (Supplementary Table 5).
Figure 2.
(a) Weighted log basal metabolic rate (BMR) (ml O2 h−1) against weighted log body mass (g) for each 801 mammal species in the study, across terrestrial categories. Regression lines are in black and confidence intervals are present in grey. Aquatic mammals demonstrated significantly higher BMRs for a given body mass than all other terrestrial groups, apart from ‘flying’ in the PGLS. No other groups were significantly different. (b) Box and jitter plot showing the residuals of weighted log basal metabolic rate (BMR) (ml O2 h−1) against weighted log body mass (g) across five terrestriality categories for all 801 mammal species used in this study.
The MCMCglmm established that weighted log body mass, annual temperature and diurnal temperature range were still significant even when taking into consideration intra-species variation (taking multiple studies per species into account) (Supplementary Table 6). Of the habitat categories, the aquatic category remained significantly different from the terrestrial, semi-fossorial and fossorial categories. Paternal care and isothermality were no longer retained as significant factors. Phylogenetic heritability (H2) was 0.95—close to 1—suggesting that BMR is strongly determined by phylogeny, and thus supporting the outcome of the PGLS (Fig. 3).
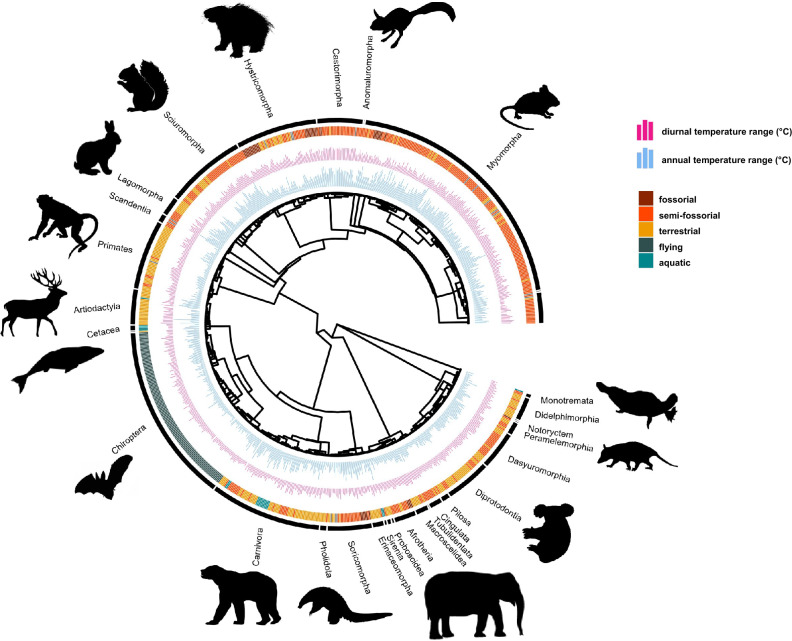
Figure 3.
Phylogenetic tree showing all 801 mammalian species used in this study. The tree was obtained from OTL and used in the PGLS and MCMCglmm. The bar plots represent the significant predictors of BMR in the PGLS in conditionally averaged models for the PGLS and MCMCglmm. The inner bar plot represents annual temperature range (°C), and the outer bar plot represents diurnal temperature (°C). Terrestriality is colour coded and shown around the phylogeny, and mammalian order is shown in black.
Discussion
Overall, this study concludes that mammalian basal metabolic rate (BMR) was primarily driven by body mass and environmental temperature parameters; diurnal temperature and annual temperature range. Living underground was not found to influence BMR, and only aquatic mammals had a significantly increased BMR compared to terrestrial, fossorial and semi-fossorial species. A significantly lower BMR was found when paternal care was present, and as paternal care was shown to be a significant predictor trait in the PGLS but not the MCMCglmm, this suggests that elements of inter-specific variation, but not intra-specific variation, can be explained by paternal care of young.
Neither the semi-fossorial nor fossorial mammalian categories demonstrated a different BMR to terrestrial species and, therefore, there does not appear to be an associated BMR trace with living underground, either continuously or periodically. It is possible that species occupying a subterranean niche do not pay any baseline metabolic costs as a result of potential hypoxia and respiratory stress within burrows. This may facilitate certain species being able to occupy an underground shelter at night or during other periods of the day, whilst also spending significant amounts of time above ground. Moreover, it is possible that burrows may not be as physiologically challenging as previously suggested. Based on available data, burrow O2 and CO2 levels do not support the pronounced hypoxic conditions hypothesised in subterranean burrow systems31. Whilst further studies have demonstrated the pronounced hypoxia may not be as well established31, variation in soil type and structure, water content and animal activity may still influence gas concentrations in burrows at times. Furthermore, as the BMR measurements used in this analysis were likely carried out under normoxic conditions, and therefore will reflect BMR analysed under normothermic conditions, the values may not fully reflect the evolutionary adaptations of metabolic rate of an animal living in closed burrow systems with lower than ambient O2 levels.
Fossorial and semi-fossorial mammals, as well as aquatic mammals, experience periodic hypoxia that likely necessitates adaptations in basal physiology. Species that live for long periods in low hypoxic conditions may rely on phenotypic plasticity, which may be the case in both semi-fossorial and fossorial mammals. This means that to live in low O2 levels, BMR does not show a consistent directional adaptation, but rather there may be requirements for plasticity in metabolic rate, such as up-regulation or down-regulation of metabolism16. Furthermore, as per the respiratory stress hypothesis, individuals show morphological adaptations to enhance oxygen transfer from blood to metabolic active tissues, rather than physiological adaptations29,30. Hypoxia may determine maximal metabolism and this influence may not be detectable in measurements of BMR32. This contrasts with aquatic mammals that will experience conditions with limited oxygen for short bursts of time during dive events spanning 30 min to a few hours at a time33. These species will hold their breath in order to forage, socialise and move in water and are, therefore, experiencing periodic extremely low oxygen levels as pre-dive O2 stores are used during the dive, and CO2 accumulates34. It is possible that the difference between intensity and duration of exposure may result in a difference in metabolic traces. Species that are exposed regularly, but not continuously, to intense hypoxia likely have physiological mechanisms that they rely on to live in low oxygen conditions, such as increased oxygen affinity of haemoglobin molecules. Rapid short-term responses in metabolic rate have been observed in birds and mammals. Laughing doves (Streptopelia senegalensis) demonstrate short-term thermal acclimation capacities35,36, and when comparing two species of flycatchers, pied flycatchers (Ficedula hypoleuca) with a more variable metabolic rate demonstrated the ability to be more adaptive to climate change and spread their ranges further than collared flycatchers (Ficedula albicollis), which exhibited a more limited scope for metabolic change37. One species of subterranean rodent, Spalacopus cyanus, was exposed to low external temperatures in a laboratory experiment out of their normal range and, following repeated exposure, showed an increase in metabolic rate and thermogenic capacity in response to low temperatures, thus demonstrating acclimation38. Thus, it is possible that even in the case of species that are living entirely underground, physiology is still largely dictated by ambient temperatures above ground. Luna et al. demonstrated that ambient temperature plays a similar role in the evolution of residual BMR in terrestrial and subterranean rodents, suggesting that responses to modal ambient temperature are similar on a large scale, regardless of the thermal stability of the burrow systems39. The mechanistic process and how the temperature changes are sensed is still not very well understood. Short-term responses, instead of a permanent metabolic trace, may be important for both long-term and short-term energy management. There has been little to no research into what proportion of energy management is attributed to metabolic response to external and internal triggers, so it is unknown when and how much subterranean species may use this ‘activation to hypoxia’ response. As the hypothesis that hypoxic and hypercapnic conditions in burrow systems leads to respiratory stress29, the presence of a lower BMR even due to short-term exposure precludes hypoxia as an influencer of mammalian BMR. As such, thermal factors such as ambient surface and burrow temperatures appear to have a greater influence on BMR, and thermal physiological factors such as body temperature39,40. As climatic variables seem to be a greater predictor for metabolic rate, this shows that there may be an adaptive hierarchy, with the need to be adapted to the ambient temperature of their habitat being more important than the hypoxic conditions of their environment.
Climatic variables were shown to explain the observed differences between categories in BMR; annual temperature and diurnal temperature range were significant predictors retained in both the PGLS and the MCMCglmm models. Environmental temperature, therefore, seems to be a consistently significant predictor of variation in metabolic rate in this study. Climate may have an important influence on BMR due to most land-based mammals being comparatively more static in their movements across the land and in their home ranges41. This relative restriction in long-range movements compared to volant and aquatic mammals means that any changes in environmental temperature experienced will likely require physiological coping mechanisms due to the inability to evade unfavourable conditions. However, due to dominance of Chiropterans in the flying category it is not possible to separate whether the results of this group are influenced solely by phylogeny, and therefore no conclusions can be drawn from this. Mammals may utilise tactics such as hibernation and torpor. Species that do not employ these will likely need seasonal acclimations in BMR in response to these seasonal changes in the environment range8–10. Similarly, plasticity allows an organism to respond to changes in both daily and annual environmental conditions16. This, in turn, may also lead to changes in thermoneutral zones (TNZs), shifting to lower ranges in winter compared to summer, for example, reddish-grey mouse lemurs (Microcebus griseorufus) and striped hamsters (Cricetulus barabensis) have demonstrated adjustments to their TNZs in response to environmental variability in temperature, thus demonstrating that at large scales, ambient temperature can be associated with underground ambient temperature41–44.
Environmental temperature is an important determinant of BMR. Some mammalian species have very specific climatic adaptations and are, therefore, living in highly conserved temperature ranges such as Arctic foxes (Vulpes lagopus) and dromedary camels (Camelus dromedarius)45,46. Other mammals rely on very specific changes in temperatures and day length as triggers for hibernation, migration, and breeding47–50. Changes to the environmental temperatures that these species are experiencing are likely to have knock-on effects and alter population range changes, as well as the persistence of populations in certain areas. This highlights the importance of climate change and the large-scale effects that small changes in unseasonal environmental temperature may have on the diversity and distribution of mammalian species on the planet. It is important to take into consideration that sub-surface temperatures could not be obtained for the distribution ranges of the burrowing species used in this study. A future step would be to determine the sub-surface soil temperature at the correct burrowing depth of semi-fossorial and fossorial species to accurately quantify the temperatures experienced in the burrows and to further understand the impact of fossorial lifestyles on BMR.
Body mass was found to be a significant predictor of BMR. That adult body mass accounts for much of the variation in BMR has been well-established, where body mass increases so does BMR51. For the remaining, or residual variation, paternal care and isothermality were found to be significant predictors of mammalian BMR in the PGLS analyses but did not remain within the top MCMCglmms. Paternal care and isothermality may not influence BMR when taking into account variation between studies. The MCMCglmm may be used as a proxy for intra-species variation and could indicate that in this study, BMR varied enough within species to negate the effects on BMR.
This macro-comparative mammal study demonstrates that environmental temperature (annual temperature and diurnal temperature range) is an important determining factor when exploring differences in mammalian BMR and therefore strongly infers whether a mammal is physiologically suited to living in a particular place or habitat. There is no detectable trace in the BMR of mammalian species affiliated with living a subterranean lifestyle. This lack of a physiological trace suggests that hypoxia does not explain variation in BMR, but that inter-specific variation might play a role, and this variability may in part, explain why burrowing lifestyles evolve a number of times across multiple mammalian orders, all over the world.
Methods
Metabolic data
Data was collated from the literature, primarily using the dataset from Genoud et al.52. Genoud et al.52 created a highly quality controlled dataset to determine the value of BMR data and focussed on a methodological review of how basal metabolic rate (BMR; ml O2 h−1) is experimentally collected. In total, Genoud et al. collated the body mass and BMR measurements of 800 extant mammalian species across 30 taxonomic orders, including relevant information on the conditions under which the data were collected52. BMR has been debated with regard to its efficacy in drawing evolutionary conclusions, and a number of studies recommend the use of a number of physiological metrics in tandem53,54. However, BMR is still widely used, having been found to correlate with a range of ecological, physiological and life-history variables, as well as phylogeny55–57. Additional studies published since Genoud et al.52 were then added to the dataset for the present study. We searched for papers from 2015 to March 2021 using the search terms “mammal”, “BMR”, “RMR”, “basal metabolic rate” and “resting metabolic rate” on Google Scholar and Web of Science.
Essential inclusion criteria for our additional studies matched that of Genoud et al.52; fasted (post-absorptive), non-reproductive, adult, at thermoneutrality, during natural circadian resting phase, and at rest. Furthermore, we later limited inclusion to studies that had determined BMR using respirometry and additionally, only included BMR of non-domesticated species.
A total of 40 additional papers—published since 2018—were found to fit the criteria for inclusion in this study. If an aspect of the essential criteria was missing, contact was attempted via email to lead authors to obtain any missing information (N = 14). As with the Genoud et al.52 dataset, for the additional studies that did not fit the inclusion criteria, or where information regarding the criteria could not be obtained through email, these studies were not included. A total of nine authors replied with sufficient details so that the BMR values could then be included in the full data set and following this, 29 new BMR entries were added to the database. This consisted of 26 studies, assessing 28 species (across nine mammalian orders), nine species of which were novel in the database. In total, 801 mammal species across 1586 studies were used in the final dataset for this study.
Life history trait data
Data on life history traits (LHTs) were selected based on known relevance and influence on BMR (Supplementary Tables 1, 2). The definitions for all life history traits collected were based on those outlined from the databases and books from which we obtained the relevant data (Supplementary Table 3)58–66. Life history traits included were: activity pattern, life span (months), brain mass (g), population density (/km2), annual number of litters, litter size, gestation (days), terrestriality, breeding seasonality, mating strategy, paternal care, upper elevation limit (m), diet and annual recruitment (/year). For the explanatory trait in the present study—terrestriality—the definitions and categories were outlined based on existing and accepted definitions, adjusting them where required to be more suitable in the context of mammals and the primary aim of the present study (Table 1). We included an intermediary group of species that are not obligately subterranean but do spend a significant amount of time in a burrow, den or crevice, which was termed semi-fossorial. This intermediate group provided the opportunity to detect if there was a potential gradient, and whether the species that fell into this group demonstrate a similar metabolic trace to the fossorial species. Definitions of terrestriality were initially tested through pilot data and allocation of definitions using 12 scientists at the start and end of the data collection to ensure that the definitions were robust, and to test for interobserver reliability in the use of these terms.
Table 1.
List of definitions created for this study, to outline different terrestrial strategies: fossorial, semi-fossorial, terrestrial, flying and aquatic.
| Terrestrial category | Definition |
|---|---|
| Fossorial—below ground only | Spends approx. whole life underground. Primarily digs/creates own closed burrow systems, which creates a controlled microclimate. Will show evidence of morphological and physiological adaptations to subterranean life, such as regression of eyesight or developed forelimbs adapted for digging |
| Semi-fossorial—below and on the ground | Spend significant time underground: using burrow at least once during a daily cycle (for sleeping, sheltering, eating, etc.). May create own burrow or may make use of existing burrows/crevices/cavities that are open or covered, but not completely closed. Some degree of a microclimate is created. May or may not hibernate |
| Terrestrial—on the ground only | Spends whole life above ground. Does not dig/create/make use of burrows or underground systems. Does not live in a controlled microclimate |
| Flying | Mammals capable of powered sustained flight. May or may not make use of cavities/crevices/caves but do not dig/create underground systems. Some degree of a microclimate may or may not be created |
| Aquatic | Mammals that are found predominantly in freshwater or marine environments. May or may not leave water for brief periods of time within a daily cycle. May or may not make use of cavities/crevices/caves but do not dig/create underground systems. Some degree of a microclimate may or may not be created |
Explanations for any exceptions to the rule are also given. See Supplementary for Exceptions.
Numerous sources were accessed to obtain LHTs for all species for which we had BMR data. Where relevant—for continuous traits—a mean value was used as this was most readily available in databases and in books. In other cases, a maximum value was used as this again was most commonly found in databases. A detailed full list of sources can be found in the Supplementary Material (Supplementary Table 1). Any LHT with less than 60% of the data available was removed from the database and thus only nine of the initial 15 LHTs were retained (Supplementary Table 3).
Bioclimatic variables were obtained from the WorldClim—Global Climate Data website67 and were extracted using the ‘raster’ package in R version 4.1.1. The seven bioclimatic variables extracted were: annual precipitation (mm), precipitation seasonality (mm), annual temperature (°C), annual temperature range (°C), diurnal temperature range (°C), temperature seasonality (°C) and isothermality (°C) (Supplementary Table 3). This data was extracted at 2.5-min (of a longitude/latitude degree) spatial resolution (4.5 km at the equator)67. The median of each distinct geographical population for each species was extracted, and following this, the mean of the median was calculated for species that had more than one distinct geographic distribution.
Statistical analysis
All calculations and statistical analyses were performed using the statistical software R version 4.1.168.
Obtaining BMR and body mass values
Log values for BMR (LogBMR) and body mass (LogBody mass) were used to account for the large range in the body mass of the species being used in this study (from 2.2 g (Suncus etruscus) to 4037.5 kg (Orcinus orca), and to reduce the skewness of the data51.
The database contained multiple studies per species in most cases, and therefore multiple associated BMR and body mass values at the species level. As multiple entries per species cannot be used in a phylogenetic least squares model (PGLS), a single mean value for both BMR and body mass was required. All 1586 studies in the final dataset were included, and for each of the 801 mammal species, where multiple studies per species were present a weighted average (for both BMR and body mass) was calculated. The larger the sample number used in a study, the more accurate the estimate69, so species mean values of BMR and body mass were weighted by the sample size for each study so that larger studies would contribute more to the overall mean value for a species. Thus, using LogBMR and LogBody mass, weighted basal metabolic rate (wLogBMR) and body mass (wLogBody mass) were obtained for inclusion in the PGLS.
Co-linearity
A generalised variance inflation factor (GVIF) analysis, undertaken in a stepwise fashion, was used to determine multicollinearity between continuous and categorical LHTs. This identified three collinear traits (annual temperature range, temperature seasonality and annual recruitment), which were subsequently omitted from further analyses (Supplementary Table 4).
PGLS
The database contained multiple studies per species; this duplication of species meant that a two-pronged approach was used. A PGLS was first used to determine the evolutionary association between BMR and the remaining LHTs within a phylogenetic context70. PGLS models were fitted using the phylolm function in the package ‘phylom’ and the ‘caper’ package71,72. All possible model combinations with up to five predictors were fitted using the pdredge function in package ‘MuMIn’73. AIC values were given, which measure the overall goodness of fit of each model, but not the significance of individual parameters74. All models with AIC values < 2 were retained and conditional model averaging then determined the importance of each trait present in these top ranking models75.
Pagel’s Lambda was estimated by maximum likelihood to evaluate the strength of the phylogenetic signal within the top models. This value ranges from 0 to 1 where 0 suggests no phylogenetic signal in the data and 1 indicates that phylogeny explains all the variation in mammalian BMR and is consistent with a Brownian motion model of trait evolution76.
MCMCglmm
To confirm the results from the PGLS were robust, a generalised linear mixed-effects model using a Markov chain Monte Carlo approach under a Bayesian statistical framework (MCMCglmm) was applied in the ‘MCMCglmm’ package77. This approach was used to incorporate the multiple studies per species that were present in the database and thus account for intra-specific variation. A MCMCglmm was performed on the LogBMR for all 1586 studies in the database. This approach fits individual-level data whilst controlling for relationships in species traits due to common ancestry78. Phylogeny was included as a random effect within the mode based on mammalian order and species levels, to control for variation due to factors related to phylogeny. A single consensus tree was used, and 130,000 iterations were applied with 100 thinning intervals and 30,000 burn-in. In the main model, study-level BMR and body mass values were logged (LogBMR and Log body mass), thus producing one LogBMR and Log body mass value per study. Predicted BMR was calculated by regressing LogBMR against Log body mass. This was first run using the scaling coefficient for the whole dataset (0.74), and later a separate scaling coefficient for each terrestrial group was obtained to create unique predicted BMR values for each category (fossorial = 1.07, terrestrial = 1.28, semi-fossorial = 1.42, flying = 1.09 and aquatic = 1.11). The results from both did not differ significantly and the results presented are those obtained using the overall scaling coefficient.
LogBMR was set as a dependent variable, and independent variables were the significant predictor variable that were identified in the PGLS, namely Log body mass, annual temperature, diurnal temperature range, paternal care and terrestriality. Significance of the predictor variables carried forward from the PGLS were determined, and a heritability value (H2) were also obtained. The statistical significance of the genetic influence on BMR was assessed using 95% confidence intervals (CI) for the heritability estimates which is the transmission of the phenotypic variability within a population from generation to generation.
A mammalian phylogenetic tree was constructed in R using the package ‘rotl’. A taxonomic name resolution service (TNRS) was used to match taxon names in the database to the Open Tree Taxonomy identifiers. The Open Tree of Life (OTL) was used to create a final tree along with the backbone tree of the Open Tree Taxonomy (OTT). For replicability and standardisation reasons we have not altered the taxonomy of the tree. We carried out this analysis on 21/12/23 and the OTL and OTT reflect the mammalian phylogenetic tree as it was at this point in time.
Ethics
All data was collected from pre-existing and published studies, therefore no permits or licenses were required for this study.
Supplementary Information
Acknowledgements
The authors are grateful for the researchers and data collectors involved in all the papers used to create the database in this study. They thank the Portugal lab group (RHUL) for pilot testing data and ensuring the robustness of life history definitions used for our data collection. They thank Craig White for useful discussions. They acknowledge funding from the Natural Environment Research Council under Grant NE\L002485\1.
Author contributions
Conceptualisation, methodology and planning, H.N.M., J.T. and S.J.P. Data collected by H.N.M and J.T. Methodological development, H.N.M., J.T. and S.J.P. Data analysis was performed by H.N.M. All authors contributed to the intellectual interpretation of the results. The initial draft of the paper was written by H.N.M., with contribution and edits by all authors.
Data availability
All data are available as an Electronical Supplementary File.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-61595-1.
References
- 1.Pettersen AK, White CR, Marshall DJ. Metabolic rate covaries with fitness and the pace of the life history in the field. Proc. R. Soc. B Biol. Sci. 2016;283(1831):3–23. doi: 10.1098/rspb.2016.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White CR, Kearney MR. Determinants of inter-specific variation in basal metabolic rate. J. Compar. Physiol. B. 2013;183(1):1–26. doi: 10.1007/s00360-012-0676-5. [DOI] [PubMed] [Google Scholar]
- 3.Mathot KJ, Dingemanse NJ, Nakagawa S. The covariance between metabolic rate and behaviour varies across behaviours and thermal types: Meta-analytic insights. Biol. Rev. 2019;94(3):1056–1074. doi: 10.1111/brv.12491. [DOI] [PubMed] [Google Scholar]
- 4.Biro PA, Stamps JA. Do consistent individual differences in metabolic rate promote consistent individual differences in behavior? Trends Ecol. Evol. 2010;25(11):653–659. doi: 10.1016/j.tree.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Mathot KJ, Dingemanse NJ. Energetics and behavior: Unrequited needs and new directions. Trends Ecol. Evol. 2015;30(4):199–206. doi: 10.1016/j.tree.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Khaliq I, Hof C, Prinzinger R, Böhning-Gaese K, Pfenninger M. Global variation in thermal tolerances and vulnerability of endotherms to climate change. Proc. R. Soc. B Biol. Sci. 2014;281(1789):1097. doi: 10.1098/rspb.2014.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Speakman JR, McDevitt RM, Cole KR. Measurement of basal metabolic rates: Don’t lose sight of reality in the quest for comparability. Physiol. Zool. 1993;66(6):1045–1049. doi: 10.1086/physzool.66.6.30163753. [DOI] [Google Scholar]
- 8.Renouf D, Gales R. Seasonal variation in the metabolic rate of harp seals: Unexpected energetic economy in the cold ocean. Can. J. Zool. 1994;72(9):1625–1632. doi: 10.1139/z94-216. [DOI] [Google Scholar]
- 9.Jingsong L, Ming L. Phenotypic flexibility of metabolic rate and organ masses among tree sparrows Passer montanus in seasonal acclimatization. Acta Zool. Sin. 2006;52(3):469–477. [Google Scholar]
- 10.Lawler JP, White RG. Seasonal changes in metabolic rates in muskoxen following twenty-four hours of starvation. Rangifer. 1997;17(3):135–138. doi: 10.7557/2.17.3.1365. [DOI] [Google Scholar]
- 11.Boone RB, Thirgood SJ, Hopcraft JGC. Serengeti wildebeest migratory patterns modelled from rainfall and new vegetation growth. Ecology. 2006;87(8):1987–1994. doi: 10.1890/0012-9658(2006)87[1987:SWMPMF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Naidoo R, Chase MJ, Beytell P, Du Preez P, Landen K, Stuart-Hill G, Taylor R. A newly discovered wildlife migration in Namibia and Botswana is the longest in Africa. Oryx. 2016;50(1):138–146. doi: 10.1017/S0030605314000222. [DOI] [Google Scholar]
- 13.Rutovskaya MV, Diatroptov ME, Kuznetzova EV, Anufriev AI, Feoktistova NY, Surov AV. The dynamics of body temperature of the Eastern European hedgehog (Erinaceus roumanicus) during winter hibernation. Biol. Bull. 2019;46(9):1136–1145. doi: 10.1134/S1062359019090127. [DOI] [Google Scholar]
- 14.Bearman-Brown LE, Baker PJ, Scott D, Uzal A, Evans L, Yarnell RW. Over-winter survival and nest site selection of the west-European hedgehog (Erinaceus europaeus) in arable dominated landscapes. Animals. 2020;10(9):1449. doi: 10.3390/ani10091449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Withers PC, Richardson KC, Wooller RD. Metabolic physiology of euthermic and torpid honey possums, Tarsipes rostratus. Austral. J. Zool. 1989;37(6):685–693. doi: 10.1071/ZO9890685. [DOI] [Google Scholar]
- 16.Norin T, Metcalfe NB. Ecological and evolutionary consequences of metabolic rate plasticity in response to environmental change. Philos. Trans. R. Soc. B. 2019;374(1768):1–80. doi: 10.1098/rstb.2018.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovegrove BG. The zoogeography of mammalian basal metabolic rate. Am. Nat. 2000;156(2):201–219. doi: 10.1086/303383. [DOI] [PubMed] [Google Scholar]
- 18.Lovegrove BG. The influence of climate on the basal metabolic rate of small mammals: A slow-fast metabolic continuum. J. Compar. Physiol. B. 2003;173:87–112. doi: 10.1007/s00360-002-0309-5. [DOI] [PubMed] [Google Scholar]
- 19.Arends A, McNab BK. The comparative energetics of ‘caviomorph’ rodents. Compar. Biochem. Physiol. A Mol. Integr. Physiol. 2001;130(1):105–122. doi: 10.1016/S1095-6433(01)00371-3. [DOI] [PubMed] [Google Scholar]
- 20.McNab BK. An analysis of the factors that influence the level and scaling of mammalian BMR. Compar. Biochem. Physiol. A Mol. Integr. Physiol. 2008;151(1):5–28. doi: 10.1016/j.cbpa.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 21.McNab BK. The influence of body size on the energetics and distribution of fossorial and burrowing mammals. Ecology. 1979;60(5):1010–1021. doi: 10.2307/1936869. [DOI] [Google Scholar]
- 22.Hofstede L, Dziminski MA. Greater bilby burrows: Important structures for a range of species in an arid environment. Austral. Mammal. 2017;39(2):227–237. doi: 10.1071/AM16032. [DOI] [Google Scholar]
- 23.Buck CL, Barnes BM. Temperatures of hibernacula and changes in body composition of arctic ground squirrels over winter. J. Mammal. 1999;80(4):1264–1276. doi: 10.2307/1383177. [DOI] [Google Scholar]
- 24.Jorgensen EE. Small mammal use of microhabitat reviewed. J. Mammal. 2004;85(3):531–539. doi: 10.1644/BER-019. [DOI] [Google Scholar]
- 25.Singer D. Metabolic adaptation to hypoxia: Cost and benefit of being small. Respir. Physiol. Neurobiol. 2004;141(3):215–228. doi: 10.1016/j.resp.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Li M, Pan D, Sun H, Zhang L, Cheng H, Shao T, Wang Z. The hypoxia adaptation of small mammals to plateau and underground burrow conditions. Anim. Models Exp. Med. 2021;4(4):319–328. doi: 10.1002/ame2.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darden TR. Respiratory adaptations of a fossorial mammal, the Plains pocket gopher (Thomomys bottae) J. Compar. Physiol. 1972;78:121–137. doi: 10.1007/BF00693609. [DOI] [Google Scholar]
- 28.Withers PC. Models of diffusion-mediated gas exchange in animal burrows. Am. Nat. 1978;112:1101–1112. doi: 10.1086/283349. [DOI] [Google Scholar]
- 29.Ar A, Arieli R, Shkolnik A. Blood-gas properties and function in the fossorial mole rat under normal and hypoxic–hypercapnic atmospheric conditions. Respir. Physiol. 1977;30:201–218. doi: 10.1016/0034-5687(77)90031-7. [DOI] [PubMed] [Google Scholar]
- 30.Arieli R, Nevo E. Hypoxic survival differs between two mole rat species (Spalax ehrenbergi) of humid and arid habitats. Compar. Biochem. Physiol. A Physiol. 1991;100:543–545. doi: 10.1016/0300-9629(91)90367-L. [DOI] [PubMed] [Google Scholar]
- 31.Burda H, Šumbera R, Begall S. Microclimate in burrows of subterranean rodents—Revisited. In: Burda H, Šumbera R, Begall S, editors. Subterranean Rodents: News from Underground. Springer; 2007. pp. 21–33. [Google Scholar]
- 32.Lutz PL, Hochachka PW. Hypoxia defense mechanisms: A comparison between diving reptiles and mammals. In: Lutz PL, Hochachka PW, editors. Surviving Hypoxia. CRC Press; 2022. pp. 459–469. [Google Scholar]
- 33.Berenbrink M. The role of myoglobin in the evolution of mammalian diving capacity—The August Krogh principle applied in molecular and evolutionary physiology. Compar. Biochem. Physiol. A Mol. Integr. Physiol. 2021;252:110843. doi: 10.1016/j.cbpa.2020.110843. [DOI] [PubMed] [Google Scholar]
- 34.Simon M, Johnson M, Tyack P, Madsen PT. Behaviour and kinematics of continuous ram filtration in bowhead whales (Balaena mysticetus) Proc. R. Soc. B Biol. Sci. 2009;276(1674):3819–3828. doi: 10.1098/rspb.2009.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKechnie AE, Wolf BO. Partitioning of evaporative water loss in white-winged doves: Plasticity in response to short-term thermal acclimation. J. Exp. Biol. 2004;207(2):203–210. doi: 10.1242/jeb.00757. [DOI] [PubMed] [Google Scholar]
- 36.McKechnie AE, Chetty K, Lovegrove BG. Phenotypic flexibility in the basal metabolic rate of laughing doves: Responses to short-term thermal acclimation. J. Exp. Biol. 2007;210(1):97–106. doi: 10.1242/jeb.02615. [DOI] [PubMed] [Google Scholar]
- 37.McFarlane SE, Ålund M, Sirkiä PM, Qvarnström A. Difference in plasticity of resting metabolic rate—The proximate explanation to different niche breadth in sympatric Ficedula flycatchers. Ecol. Evol. 2018;8(9):4575–4586. doi: 10.1002/ece3.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nespolo RF, Bacigalupe LD, Rezende EL, Bozinovic F. When nonshivering thermogenesis equals maximum metabolic rate: Thermal acclimation and phenotypic plasticity of fossorial Spalacopus cyanus (Rodentia) Physiol. Biochem. Zool. 2001;74(3):325–332. doi: 10.1086/320420. [DOI] [PubMed] [Google Scholar]
- 39.Luna F, Naya H, Naya DE. Understanding evolutionary variation in basal metabolic rate: An analysis in subterranean rodents. Compar. Biochem. Physiol. A Mol. Integr. Physiol. 2017;206:87–94. doi: 10.1016/j.cbpa.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Luna F, Antenucci CD, Bozinovic F. Comparative energetics of the subterranean Ctenomys rodents: Breaking patterns. Physiol. Biochem. Zool. 2009;82(3):226–235. doi: 10.1086/597526. [DOI] [PubMed] [Google Scholar]
- 41.Kobbe S, Nowack J, Dausmann KH. Torpor is not the only option: Seasonal variations of the thermoneutral zone in a small primate. J. Compar. Physiol. B. 2014;184(6):789–797. doi: 10.1007/s00360-014-0834-z. [DOI] [PubMed] [Google Scholar]
- 42.Zhao ZJ, Chi QS, Liu QS, Zheng WH, Liu JS, Wang DH. The shift of thermoneutral zone in striped hamster acclimated to different temperatures. PLoS ONE. 2014;9(1):84396. doi: 10.1371/journal.pone.0084396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson AL, Brown M, Downs CT. Seasonal variation in metabolic rate of a medium-sized frugivore, the Knysna turaco (Tauraco corythaix) J. Therm. Biol. 2011;36(3):167–172. doi: 10.1016/j.jtherbio.2011.01.004. [DOI] [Google Scholar]
- 44.Gorman ML, Speakman JR. Seasonal variation in the resting metabolic rate of male wood mice Apodemus sylvaticus from two contrasting habitats 15 km apart. J. Compar. Physiol. B. 1997;167(3):229–239. doi: 10.1007/s003600050069. [DOI] [PubMed] [Google Scholar]
- 45.Prestrud P. Adaptations by the arctic fox (Alopex lagopus) to the polar winter. Arctic. 1991;1:132–138. [Google Scholar]
- 46.Fesseha H, Desta W. Dromedary camel and its adaptation mechanisms to desert environment: A review. Int. J. Zool. Stud. 2020;5(2):23–28. [Google Scholar]
- 47.Carey HV, Andrews MT, Martin SL. Mammalian hibernation: Cellular and molecular responses to depressed metabolism and low temperature. Physiol. Rev. 2003;83(4):1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- 48.Deutsch CJ, Reid JP, Bonde RK, Easton DE, Kochman HI, O’Shea TJ. Seasonal movements, migratory behavior, and site fidelity of West Indian manatees along the Atlantic Coast of the United States. Wildl. Monogr. 2003;151:1–77. [Google Scholar]
- 49.McNutt JW, Groom R, Woodroffe R. Ambient temperature provides an adaptive explanation for seasonal reproduction in a tropical mammal. J. Zool. 2019;309(3):153–160. doi: 10.1111/jzo.12712. [DOI] [Google Scholar]
- 50.Hart DW, Alharbi YS, Bennett NC, Schoeman KS, Amor NM, Mohammed OB, Alagaili AN. Shedding light on the role of photoperiod, rainfall and ambient temperature on the breeding physiology of male lesser Egyptian jerboa (Jaculus jaculus) from central Saudi Arabia. J. Zool. 2020;311(3):217–226. doi: 10.1111/jzo.12776. [DOI] [Google Scholar]
- 51.Clarke A, Rothery P, Isaac NJ. Scaling of basal metabolic rate with body mass and temperature in mammals. J. Anim. Ecol. 2010;79(3):610–619. doi: 10.1111/j.1365-2656.2010.01672.x. [DOI] [PubMed] [Google Scholar]
- 52.Genoud M, Isler K, Martin RD. Comparative analyses of basal rate of metabolism in mammals: Data selection does matter. Biol. Rev. 2018;93(1):404–438. doi: 10.1111/brv.12350. [DOI] [PubMed] [Google Scholar]
- 53.Mitchell D, Snelling EP, Hetem RS, Maloney SK, Strauss WM, Fuller A. Revisiting concepts of thermal physiology: Predicting responses of mammals to climate change. J. Anim. Ecol. 2018;87(4):956–973. doi: 10.1111/1365-2656.12818. [DOI] [PubMed] [Google Scholar]
- 54.Portugal SJ, Green JA, Halsey LG, Arnold W, Careau V, Dann P, Frappell PB, Grémillet D, Handrich Y, Martin GR, Ruf T. Associations between resting, activity, and daily metabolic rate in free-living endotherms: No universal rule in birds and mammals. Physiol. Biochem. Zool. 2016;89(3):251–261. doi: 10.1086/686322. [DOI] [PubMed] [Google Scholar]
- 55.White CR, Seymour RS. Does basal metabolic rate contain a useful signal? Mammalian BMR allometry and correlations with a selection of physiological, ecological, and life-history variables. Physiol. Biochem. Zool. 2004;77(6):929–941. doi: 10.1086/425186. [DOI] [PubMed] [Google Scholar]
- 56.White CR, Blackburn TM, Martin GR, Butler PJ. Basal metabolic rate of birds is associated with habitat temperature and precipitation, not primary productivity. Proc. R. Soc. B Biol. Sci. 2007;274(1607):287–293. doi: 10.1098/rspb.2006.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naya DE, Spangenberg L, Naya H, Bozinovic F. How does evolutionary variation in basal metabolic rates arise? A statistical assessment and a mechanistic model. Evolution. 2013;67(5):1463–1476. doi: 10.1111/evo.12042. [DOI] [PubMed] [Google Scholar]
- 58.Bennie JJ, Duffy JP, Inger R, Gaston KJ. Biogeography of time partitioning in mammals. Proc. Natl. Acad. Sci. 2014;111(38):13727–13732. doi: 10.1073/pnas.1216063110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burger JR, George MA, Leadbetter C, Shaikh F. The allometry of brain size in mammals. J. Mammal. 2019;100(2):276–283. doi: 10.1093/jmammal/gyz043. [DOI] [Google Scholar]
- 60.DeCasien AR, Thompson NA, Williams SA, Shattuck MR. Encephalization and longevity evolved in a correlated fashion in Euarchontoglires but not in other mammals. Evolution. 2018;72(12):2617–2631. doi: 10.1111/evo.13633. [DOI] [PubMed] [Google Scholar]
- 61.Lukas D, Clutton-Brock TH. The evolution of social monogamy in mammals. Science. 2013;341(6145):526–530. doi: 10.1126/science.1238677. [DOI] [PubMed] [Google Scholar]
- 62.Tucker MA, Ord TJ, Rogers TL. Evolutionary predictors of mammalian home range size: Body mass, diet and the environment. Glob. Ecol. Biogeogr. 2014;23(10):1105–1114. doi: 10.1111/geb.12194. [DOI] [Google Scholar]
- 63.Stockley P, Hobson L. Paternal care and litter size coevolution in mammals. Proc. R. Soc. B Biol. Sci. 2016;283(1829):1471–2954. doi: 10.1098/rspb.2016.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kleiman DG, Geist V, McDade MC. Grzimek’s Animal Life Encyclopedia. Mammals I–IV. Gale; 2003. [Google Scholar]
- 65.IUCN. The IUCN Red List of Threatened Species. Version (2020-3). https://www.iucnredlist.org (Accessed 01 May 2020) (2020).
- 66.Parr CS, Wilson MN, Leary MP, Schulz KS, Lans MK, Walley ML, Hammock JA, Goddard MA, Rice MJ, Studer MM, Holmes JT. The encyclopedia of life v2: Providing global access to knowledge about life on earth. Biodivers. Data J. 2014;2:10–79. doi: 10.3897/BDJ.2.e1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fick SE, Hijmans RJ. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017;37(12):4302–4315. doi: 10.1002/joc.5086. [DOI] [Google Scholar]
- 68.R Core Team. R: A Language and Environment for Statistical Computing. https://www.R-project.org/ (R Foundation for Statistical Computing, 2021).
- 69.Clark-Carter D. Measures of central tendency. In: Peterson P, Baker E, McGraw B, editors. International Encyclopedia of Education. Elsevier; 2010. pp. 264–266. [Google Scholar]
- 70.Symonds MRE, Blomberg SP. A primer on phylogenetic generalised least squares. In: Garamszegi L, editor. Modern Phylogenetic Comparative Methods and Their Application in Evolutionary Biology. Springer; 2014. pp. 105–130. [Google Scholar]
- 71.Ho, L. S. T. et al. Phylolm: Phylogenetic Linear Regression. R Package Version 2.1 (2014).
- 72.Orme, D., Freckleton, R., Thomas, G., Petzoldt, T., Fritz, S. & Isaac, N. Caper: Comparative Analyses of Phylogenetics and Evolution in R. R Package Version 0.4 (2011).
- 73.Barton, K. & Barton, M. K. Package ‘MuMIn’. R Package. Version 1 (2019).
- 74.Angert AL, Crozier LG, Rissler LJ, Gilman SE, Tewksbury JJ, Chunco AJ. Do species’ traits predict recent shifts at expanding range edges? Ecol. Lett. 2011;14:677–689. doi: 10.1111/j.1461-0248.2011.01620.x. [DOI] [PubMed] [Google Scholar]
- 75.Grueber CE, Nakawa S, Laws RJ, Jamieson IG. Multimodel inference in ecology and evolution: Challenges and solutions. J. Evol. Biol. 2011;24:699–711. doi: 10.1111/j.1420-9101.2010.02210.x. [DOI] [PubMed] [Google Scholar]
- 76.Freckleton P, Harvey H, Pagel M. Phylogenetic analysis and comparative data: A test and review of evidence. Am. Nat. 2002;160:712–726. doi: 10.1086/343873. [DOI] [PubMed] [Google Scholar]
- 77.Hadfield JD. MCMC methods for multiresponse generalized linear mixed models: The MCMCglmm R package. J. Stat. Softw. 2010;33:1–22. doi: 10.18637/jss.v033.i02. [DOI] [Google Scholar]
- 78.Garamszegi LZ. Uncertainties due to within species variation in comparative studies: Measurement errors and statistical weights. In: Garamszegi LZ, editor. Modern Phylogenetic Comparative Methods and their Application in Evolutionary Biology. Springer; 2014. pp. 157–199. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available as an Electronical Supplementary File.