Abstract
Candidates for prostate-specific membrane antigen (PSMA)-targeted radioligand therapy (RLT) of metastatic castration-resistant prostate cancer (mCRPC) frequently have “mismatch” lesions with pronounced 18-fluorodeoxyglucose ([18F]FDG) but attenuated PSMA ligand uptake on positron emission tomography (PET). However, no quantitative criteria yet exist to identify mismatch lesions and predict their response to RLT. To define such criteria, we retrospectively analyzed 267 randomly-selected glucometabolic mCRPC metastases from 22 patients. On baseline PET, we determined [18F]FDG and [68Ga]Ga-PSMA-11 maximum standardized uptake value (SUVmax), and calculated the [18F]FDG SUVmax/[68Ga]Ga-PSMA-11 SUVmax quotient (FPQ). From follow-up [18F]FDG PET after two lutetium-177-PSMA-617 RLT cycles, we evaluated the treatment response and categorized the lesions into three subgroups (partial remission, stable disease, progression) based on change in [18F]FDG SUVmax. Lastly, we compared the baseline PET variables in progressing versus non-progressing lesions. Variables differing significantly, and a score incorporating them, were assessed via receiver operator characteristic (ROC) curve analysis, regarding ability to predict lesional progression, with area under the curve (AUC) as metric. Cut-offs with optimal sensitivity and specificity were determined using the maximum value of Youden's index. Fifty-one of 267 lesions (19.1%) progressed, 102/267 (38.2%) manifested stable disease, and 114/267 (42.7%) partially responded after two RLT cycles. At baseline, median [68Ga]Ga-PSMA-11 SUVmax was significantly lower (p < 0.001), median FPQ significantly higher (p < 0.001), and median [18F]FDG SUVmax similar in progressing versus non-progressing lesions. [68Ga]Ga-PSMA-11 SUVmax and FPQ showed predictive power regarding progression (AUCs: 0.89, 0.90). An introduced clinical score combining both further improved predictive performance (AUC: 0.94). Optimal cut-offs to foretell progression were: [68Ga]Ga-PSMA-11 SUVmax < 11.09 (88.2% sensitivity, 81.9% specificity), FPQ ≥ 0.92 (90.2% sensitivity, 78.7% specificity), clinical score ≥ 6/9 points (88.2% sensitivity, 87.5% specificity). At baseline, a low [68 Ga]Ga-PSMA-11 SUVmax and a high FPQ predict early lesional progression under RLT; [18F]FDG SUVmax does not. A score combining [68 Ga]Ga-PSMA-11 SUVmax and FPQ predicts early lesional progression even more effectively and might therefore be useful to quantitatively identify mismatch lesions.
Keywords: mCRPC, PSMA, FDG, Mismatch, Radioligand therapy
Subject terms: Prostate cancer, Molecular medicine, Predictive markers
Introduction
Radioligand therapy (RLT) targeted at prostate-specific membrane antigen (PSMA) has emerged, and gained regulatory approval, as a promising new option for palliation and/or increased survival for many patients with metastatic castration-resistant prostate cancer (mCRPC)1–3. Also a target for positron emission tomography (PET) imaging of prostate cancer4–12, PSMA is a transmembrane glycoprotein that is typically, but not invariably, overexpressed on mCRPC cells13–15. Ample tumoral expression of this target, established on PET, is, unsurprisingly, the major eligibility requirement for PSMA-directed RLT of mCRPC1–3,16.
Prostate cancer cells with an aggressive phenotype, especially mCRPC cells, also frequently abundantly express glucose transporter 1 (GLUT1) due to heightened dependence on glucose intake and aerobic glycolysis for energy as mutations and tumor burden increase17,18. As on many other malignant cell types, GLUT1 overexpression on prostate cancer cells is reflected by uptake of the fluorine-18-conjugated glucose analogue fluorodeoxyglucose ([18F]FDG), shown on PET19. Higher [18F]FDG uptake now is well-recognized to correlate with prognostically-unfavorable pathological factors in prostate cancer in general20,21, and with poor outcomes in men receiving systemic treatment of mCRPC, including PSMA-targeted RLT18,22–25. As a result, [18F]FDG PET/computed tomography (CT) increasingly is being applied in mCRPC, to provide prognostic and/or predictive data3,16,26–29.
mCRPC lesions with high GLUT1 expression but attenuated or absent PSMA expression, as evidenced by uptake of the respectively-targeted radiotracers on PET, are termed “mismatch” lesions30. Due to the perceived reduced efficacy of RLT in mismatch lesions, the presence of such findings, indeed, sometimes even of a single mismatch lesion31, has been used as an exclusion criterion for PSMA-targeted RLT32–34. However, no quantitative uptake-related criteria based on both [18F]FDG PET and PSMA-targeted PET exist yet to identify such lesions and to predict their response to RLT. We therefore performed a retrospective analysis of mCRPC metastases seeking to use quantitative PET variables to identify PSMA ligand/[18F]FDG uptake profiles that predict RLT-refractory/resistant mCRPC lesions. Such characterization of mismatch lesions potentially will aid treatment-related decision-making in candidates for PSMA-targeted RLT.
Materials and methods
Endpoints
The first endpoint was each lesion’s uptake of [68Ga]Ga-PSMA-11 and of [18F]FDG, reflected by the maximum standardized uptake value (SUVmax) of each radiotracer, on PET scans acquired before [177Lu]Lu-PSMA-617 RLT, i.e. at baseline. As part of this endpoint, we also calculated the pre-RLT [18F]FDG SUVmax/[68Ga]Ga-PSMA-11 SUVmax quotient (FPQ) for each individual lesion.
The second endpoint was the evaluation of each lesion's response to two RLT cycles, based on changes in the [18F]FDG SUVmax between the baseline [18F]FDG PET/CT scan and a follow-up [18F]FDG scan acquired after the second cycle.
The third endpoint was the comparison of baseline [68Ga]Ga-PSMA-11 SUVmax, baseline [18F]FDG SUVmax, and baseline FPQ in the subgroup of progressing lesions versus the subgroup of lesions with disease stability or partial response after the two RLT administrations, and determination of adequate cut-off values of these variables to predict lesional outcome.
The last endpoint, based on these comparisons, was the development and preliminary assessment of a score to use baseline quantitative PET variables to predict whether a lesion would progress early in the course of RLT.
Lesions, patients, and ethics
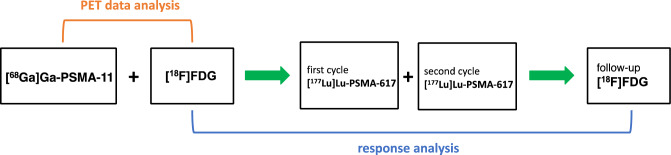
The analysis included a sample of [18F]FDG-positive lesions in consecutive eligible patients with mCRPC who received two cycles of [177Lu]Lu-PSMA-617 RLT. Eligible patients had to have available PET imaging data from three scans: (1) baseline (pre-RLT) [68Ga]Ga-PSMA-11 PET/CT, (2) baseline [18F]FDG PET/CT, and (3) follow-up [18F]FDG PET/CT acquired shortly after completion of the two RLT cycles (Fig. 1). All patients received [177Lu]Lu-PSMA-617 RLT within a prospective patient registry (REALITY Study, NCT04833517). The analyzed imaging and treatment took place at the Saarland University Medical Center between 21 January 2019 and 21 December 2022. The selection of [18F]FDG-positive target lesions was random and did not consider uptake in PSMA ligand PET/CT.
Figure 1.
Schematic summary of study design.
A total of 267 lesions from 22 men (up to 14 per patient) were included in this analysis. Table 1 shows characteristics of the lesions and of the study sample. The analyzed lesions were mostly (almost 80%) in the skeleton. The patients tended to have late-stage or end-stage disease and were heavily pretreated. They received the PSMA-targeted RLT on a compassionate use basis according to §13 (2b) of the German Pharmaceutical Act. Patients gave written informed consent, which also covered participation in the registry and permission for de-identified patient data to be published in scientific communications. The study was approved by the local institutional review board (ethics committee approval number 140/17, 13 July 2017).
Table 1.
Characteristics of the patients cohort and target lesions.
| Characteristic | Value |
|---|---|
| Age [yr] | 71 (55–84) |
| PSA at baseline [ng/mL], median (minimum–maximum) | 469 (0.08–2907) |
| ECOG performance status category at baseline, % (n) | |
| 0 | 14% (3) |
| 1 | 45% (10) |
| ≥ 2 | 41% (9) |
| Prior treatment, % [no.] | |
| Prostatectomy | 55% (12) |
| Radiation therapy | 64% (14) |
| ADT | 100% (22) |
| NAAD | 100% (22) |
| Chemotherapy | |
| Any | 77% (17) |
| Docetaxel | 77% (17) |
| Cabazitaxel | 45% (10) |
| Other | 14% (3) |
| Radium-223 | 14% (3) |
| [177Lu]Lu-PSMA-617 RLT activities [GBq], median (minimum–maximum) | |
| First activity | 8.6 (1.1–10.6) |
| Second activity | 8.2 (1.5–9.3) |
| Cumulative activity | 16.7 (2.6–19.4) |
| Target lesions category, n (%) | |
| Any | 267 (100%) |
| Lymph node | 49 (18.4%) |
| Bone | 212 (79.4%) |
| Liver | 6 (2.2%) |
| Number per patient | |
| Mean ± SD | 12 ± 4 |
| Median (minimum–maximum) | 14 (1–14) |
ADT androgen deprivation therapy, ECOG eastern cooperative oncology group, NAAD novel androgen access drugs, PSA, prostate-specific antigen.
PET/CT
Initial [68Ga]Ga-PSMA-11 PET/CT and [18F]FDG PET/CT were performed a mean ± standard deviation (SD) 25 ± 23 d and 18 ± 22 d, respectively, before PSMA-RLT started. The mean time between baseline [68Ga]Ga-PSMA-11 and baseline [18F]FDG PET/CT was 6 ± 8 d. The follow-up [18F]FDG PET/CT, acquired after two cycles PSMA-RLT, took place 122 ± 65 d after the baseline [18F]FDG PET/CT scan. Both [68Ga]Ga-PSMA-11 PET/CT and [18F]FDG PET/CT were performed ~ 60 min after intravenous injection of the radiotracer and a subsequent 500-mL infusion of NaCl 0.9%. A mean ± SD 142 ± 23 MBq of [68Ga]Ga-PSMA-11, 246 ± 42 MBq of [18F]FDG (baseline scan), and 259 ± 35 MBq of [18F]FDG (follow-up scan) were given. Patients fasted at least 4 h before each [18F]FDG infusion, and were instructed to void shortly before all PET/CT scans. Imaging was carried out applying standard protocols35,36. Whole-body PET images were acquired from vertex to mid-femur, using 3 min ([68Ga]Ga-PSMA-11) or 2 min ([18F]FDG) per bed position, with a 21.4-cm extended field-of-view. A Biograph 40 mCT scanner (Siemens Medical Solutions, Knoxville, TN, USA) was employed. For attenuation correction and anatomical localization, low-dose CT was performed together with the PET, using a 120-keV X-ray tube voltage. The tube current was modulated with CARE Dose4D software (Siemens Healthineers, Erlangen, Germany), with 30 mAs as the reference. PET datasets were reconstructed with an iterative 3-dimensional ordered-subset expectation maximization algorithm (3 iterations, 24 subsets) with gaussian filtering (5 mm full width at half maximum) and a 3 mm slice thickness. Besides attenuation correction, random, decay, and scatter correction were done.
PET/CT data analysis
Firstly, [18F]FDG-positive target lesions of mCRPC were randomly selected and analyzed at baseline [18F]FDG PET/CT. Subsequently, the same target lesions were analyzed on the baseline [68Ga]Ga-PSMA-11 PET/CT scan. Care was taken to exclude foci representing characteristic inflammatory changes on [18F]FDG PET/CT, e.g. pulmonary inflammatory changes or reactive-inflammatory lymph nodes. For each target lesion and both radiotracer SUVmax was quantified, applying Syngo.via (Siemens Healthineers, Erlangen, Germany). In addition, FPQ was calculated for each individual lesion by dividing SUVmax of [18F]FDG by SUVmax of [68Ga]Ga-PSMA-11.
RLT response classification
For each target lesion selected on baseline [18F]FDG PET/CT, SUVmax was analyzed on the follow-up scan after two cycles of [177Lu]Lu-PSMA-617 RLT. Response to the two cycles of [177Lu]Lu-PSMA-617 RLT was classified based on the change in lesional SUVmax (i.e., radiotracer uptake) from the baseline [18F]FDG scan to the follow-up [18F]FDG scan. Positron Emission Tomography Response Criteria in Solid Tumors (PERCIST)37 were used. According to these criteria, a ≥ 30% decrease from baseline SUVmax was defined as a partial response, a < 30% decrease to a < 30% increase, as stable disease, and a ≥ 30% increase, as progression.
Statistics
Descriptive statistics are reported as mean ± SD, median (minimum–maximum), and/or number (percentage) or vice versa, as applicable. The Mann–Whitney-U test was used to compare baseline SUVmax of each radiotracer and baseline FPQ between the subgroup of lesions progressing after RLT versus the subgroup of non-progressing lesions, i.e. those exhibiting stable disease or partial response. Statistical significance was set at p < 0.05. All statistical analyses were carried out using Prism version 8 (GraphPad Software, San Diego, CA, USA). Variables showing a significant difference between lesion response subgroups were used to develop a lesion response prediction score for clinical application. For this score, points were assigned for different values of the respective variables with the goal of achieving a stepwise increment in lesional progression risk as the score increased.
Receiver operator characteristic (ROC) curve analysis was carried out to assess predictive performance of the significant variables and of the score. The perfomance metric was the area under the ROC curve (AUC). For each variable and for the predictive score, the maximum value of the Youden's index (J) was used to determine the cut-off that attained optimal sensitivity and specificity.
Ethics approval and consent to participate
All procedures performed in the patients described herein were in accordance with the ethical standards of the Institutional and/or National Research Ethics Committees and with the 1964 Helsinki Declaration and its later amendments, or with comparable ethical standards. This analysis was approved by the Institutional Review Board of the Ärztekammer des Saarlandes/Saarbrücken (approval number: 140/17, approval date: 13 July 2017. This report does not include any animal studies. Written informed consent was obtained from all participants.
Results
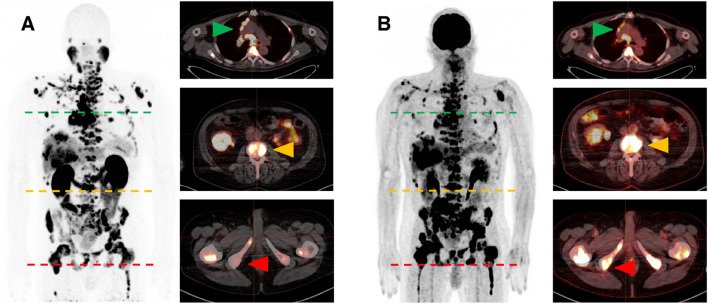
Lesional values were highly variable for [68Ga]Ga-PSMA-11 SUVmax, [18F]FDG SUVmax, and FPQ, as reflected by large SDs relative to mean values and by wide minimum–maximum ranges (Table 2, Figs. 2 and 3). At baseline, 72/267 lesions (27.0%) had greater [18F]FDG uptake than [68Ga]Ga-PSMA-11 uptake i.e. an FPQ > 1, and 195/267 lesions (73.0%), equal or lesser [18F]FDG uptake than [68Ga]Ga-PSMA-11 uptake, i.e. an FPQ ≤ 1. Representative images of lesions showing different patterns of [18F]FDG and [68Ga]Ga-PSMA-11 uptake are seen in Fig. 4.
Table 2.
PET variables of target lesions at baseline.
| Characteristic/finding | Value | ||
|---|---|---|---|
| Lesional SUVmax | |||
| Baseline [68Ga]Ga-PSMA-11 PET/CT | |||
| Median (minimum–maximum) | 17.03 (0.35–185.83) | ||
| Mean ± SD | 26.12 ± 29.98 | ||
| Baseline [18F]FDG PET/CT | |||
| Median (minimum–maximum) | 7.94 (2.28–44.00) | ||
| Mean ± SD | 9.95 ± 6.84 | ||
| Baseline FPQ | |||
| Median (minimum–maximum) | 0.51 (0.03–15.54) | ||
| Mean ± SD | 0.99 ± 1.69 | ||
| Category, % of study sample (n) | |||
| ≤ 1 | 73.0% (195/267) | ||
| > 1 | 27.0% (72/267) | ||
| Lesional responsea to RLT, % of study sample (n) | |||
| Partial response | 42.7% (114/267) | ||
| Stable disease | 38.2% (102/267) | ||
| Progression | 19.1% (51/267) | ||
| Non-progressive lesions (n = 216)b | Progressive lesions (n = 51) | p | |
|---|---|---|---|
| SUVmax, baseline [68Ga]Ga-PSMA-11 PET/CT | |||
| Median (minimum–maximum) | 20.77 (1.03–185.80) | 4.48 (0.35–48.25) | < 0.001 |
| Mean ± SD | 30.59 ± 31.45 | 7.19 ± 8.68 | |
| SUVmax, baseline [18F]FDG PET/CT | |||
| Median (minimum–maximum) | 8.15 (2.28–44.00) | 6.90 (3.69–27.37) | 0.289 |
| Mean ± SD | 10.22 ± 7.11 | 8.79 ± 5.47 | |
| FPQ, baseline PET/CT scans | |||
| Median (minimum–maximum) | 0.40 (0.03–3.81) | 1.36 (0.51–15.54) | < 0.001 |
| Mean ± SD | 0.60 ± 0.63 | 2.65 ± 3.16 | |
18F fluorine-18, 68Ga gallium-68, CT computed tomography, FDG fluorodeoxyglucose, FPQ [18F]FDG/[68Ga]Ga-PSMA-11 SUVmax quotient, PET positron emission tomography, PSMA prostate-specific membrane antigen, RLT radioligand therapy, SD standard deviation, SUVmax maximum standardized uptake value.
p values in bold were statistically significant at p < 0.05.
aLesional response classifications are based on change from baseline to post-2nd cycle of RLT in [18F]FDG SUVmax, utilizing PERCIST 1.0 criteria37.
b“Non-progressing lesions” include those showing partial response (n = 114, 52.8% of non-progressing lesions) or stable disease (n = 102, 47.2% of non-progressing lesions).
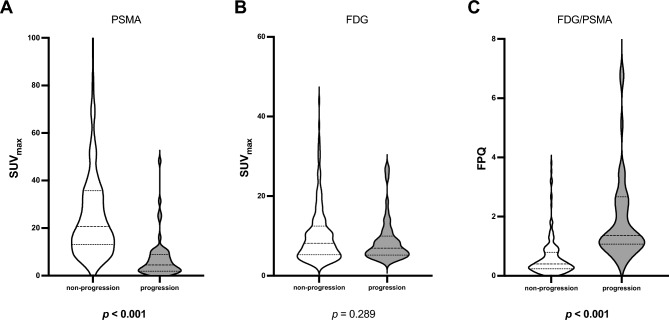
Figure 2.
Baseline PET variables by lesional response to 2 cycles of [177Lu]Lu-PSMA-617 RLT: (A) SUVmax on [68Ga]Ga-PSMA-11 PET/CT, (B) SUVmax on [18F]FDG PET/CT, and (C) FPQ, [18F]FDG SUVmax/[68Ga]Ga-PSMA-11 SUVmax quotient.
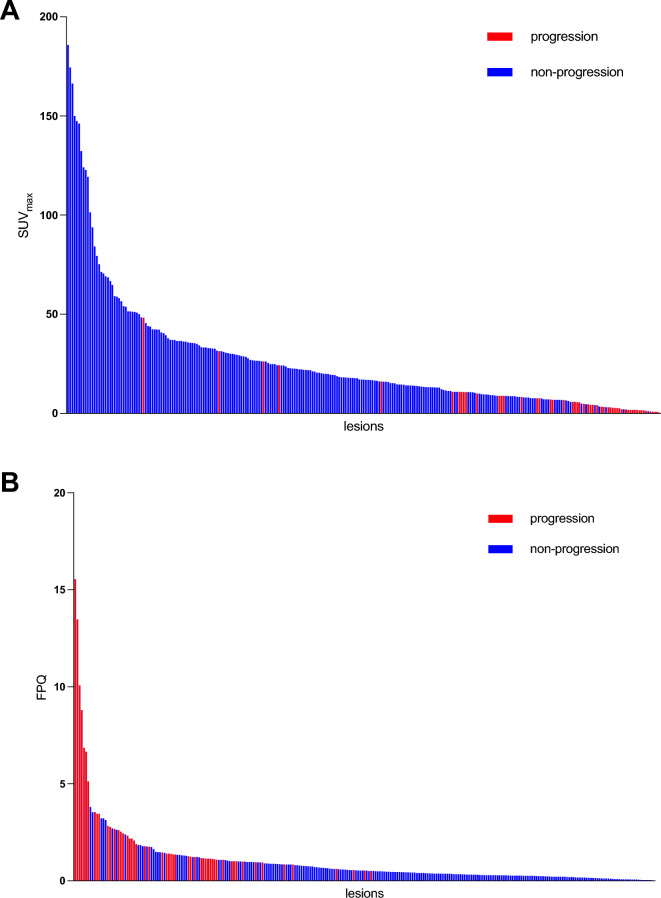
Figure 3.
Waterfall plots showing lesional response to 2 cycles of [177Lu]Lu-PSMA-617 RLT by value of the lesional (A) SUVmax on the baseline [68Ga]Ga-PSMA-11 PET/CT and (B) FPQ, [18F]FDG SUVmax/[68Ga]Ga-PSMA-11 SUVmax quotient.
Figure 4.
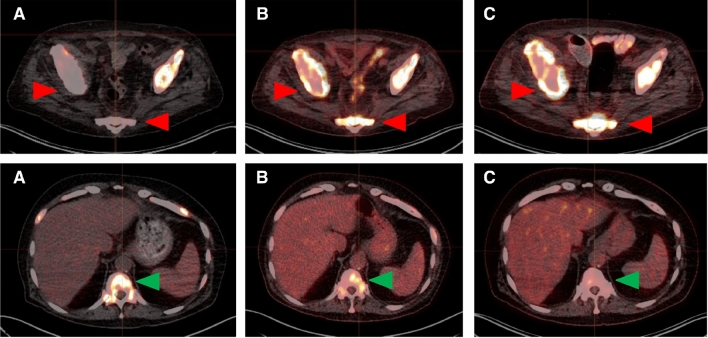
Representative whole-body and transversal slice images from (A) baseline [68Ga]Ga-PSMA-11 PET/CT scan and (B) baseline [18F]FDG-PET/CT scan of a mCRPC patient showing different patterns of [68Ga]Ga-PSMA-11 uptake and [18F]FDG uptake in different lesions. Green arrows denote lymph node metastases with intense [68Ga]Ga-PSMA-11 and moderate [18F]FDG uptake. Yellow arrows indicate bone metastases with intense uptake of both radiotracers. Red arrows indicate bone metastases with faint [68Ga]Ga-PSMA-11 uptake but intense [18F]FDG uptake.
After two cycles of [177Lu]Lu-PSMA-617 RLT, relatively few lesions showed PERCIST progression pertaining to glucose metabolism: [18F]FDG SUVmax increased ≥ 30% from baseline in 51/267 (19.1%) (Table 2). Of non-progressing lesions, a slight majority showed partial response (114/267, 42.7% of all analyzed lesions) versus stable disease (102/267, 38.2% of all analyzed lesions). Representative images of lesional responses are seen in Fig. 5.
Figure 5.
Representative transverse slice images showing lesion-based progression (first row) and partial response (second row) to 2 cycles of [177Lu]Lu-PSMA-617 RLT: (A) baseline [68Ga]Ga-PSMA-11 PET/CT, (B) baseline [18F]FDG PET/CT, and (C) follow-up [18F]FDG PET/CT. First row, red arrows: progressing bone metastases. These lesions had faint [68Ga]Ga-PSMA-11 uptake (SUVmax: 3.47 and 4.00, respectively) and intense [18F]FDG uptake (SUVmax: 11.17 and 8.67, respectively) in the baseline scans (FPQ: 3.22 and 2.17, respectively), and increased [18F]FDG uptake (SUVmax 16.87 and 12.37, respectively; 51% higher and 43% higher, respectively, than at baseline) in the follow-up scan. Second row, green arrows: bone metastasis showing partial response. The lesion, which had intense [68Ga]Ga-PSMA-11 uptake (SUVmax: 22.74) and moderate [18F]FDG uptake (SUVmax: 6.60) in the respective baseline scans (FPQ: 0.29), had decreased [18F]FDG uptake (SUVmax 4.52, 32% lower than at baseline) in the follow-up scan.
At baseline, [68Ga]Ga-PSMA-11 SUVmax was significantly lower in lesions that went on to progress after two RLT cycles than in lesions that went on to show stable disease or partial response (7.19 ± 8.68 vs. 30.59 ± 31.45, p < 0.001). Conversely, baseline [18F]FDG SUVmax did not differ between these subgroups (8.79 ± 5.47 vs. 10.22 ± 7.11, p = 0.29) (Fig. 2A and B). However, the FPQ, i.e. the ratio of [18F]FDG uptake to [68Ga]Ga-PSMA-11 uptake, in progressing lesions significantly exceeded that in non-progressing lesions (2.65 ± 3.16 vs. 0.60 ± 0.63, p < 0.001) (Fig. 2C). Figure 3 depicts as waterfall plots the lesions’ individual values for baseline [68Ga]Ga-PSMA-11 SUVmax and baseline FPQ. No lesion with a baseline [68Ga]Ga-PSMA-11 SUVmax ≥ 50 or a baseline FPQ < 0.5 showed [18F]FDG metabolic imaging progression.
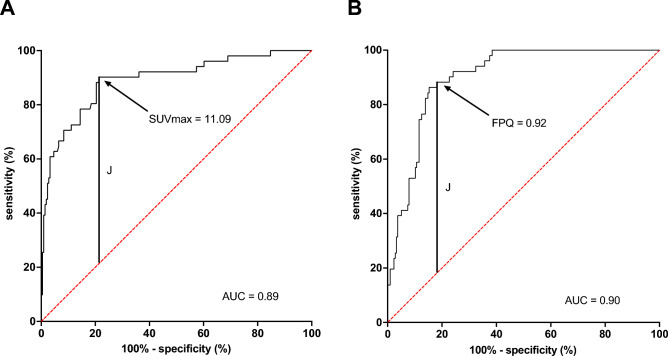
On ROC curve analysis, baseline [68Ga]Ga-PSMA-11 SUVmax and baseline FPQ showed similar performance to discriminate between lesions that would progress after two RLT cycles versus lesions that would not: respective AUCs were 0.89 versus 0.90. The respective maximum values of the Youden's index (J) identified a baseline [68Ga]Ga-PSMA-11 SUVmax of < 11.09 (Fig. 6A) or a baseline FPQ of ≥ 0.92 (Fig. 6B) as the threshold values of these variables that had optimal sensitivity and specificity in distinguishing lesional progression versus non-progression. At these thresholds, the two variables again showed comparable performance: respectively, 88.2% sensitivity and 81.9% specificity (OR 34.0), versus 90.2% sensitivity and 78.7% specificity (OR 34.0).
Figure 6.
ROC curves showing the performance to predict lesional progression of (A) lesional SUVmax on the baseline [68Ga]Ga-PSMA-11 PET/CT scan or (B) FPQ, [18F]FDG SUVmax /[68Ga]Ga-PSMA-11 SUVmax quotient. The respective maximum values (J) of the Youden's index identified a [68Ga]Ga-PSMA-11 SUVmax of < 11.09 (sensitivity 88.2%, specificity 81.9%) or an FPQ of ≥ 0.92 (sensitivity 90.2%, specificity 78.7%) as optimal threshholds.
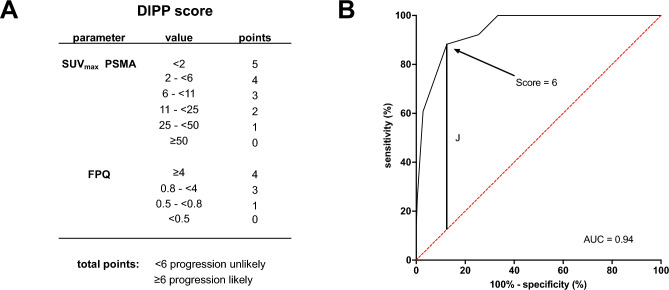
A clinical score incorporating the two variables, the dual imaging progression prediction (DIPP) score, was developed (Fig. 7A). Using ROC curve analysis, the DIPP score achieved an AUC of 0.94 to predict lesional response (Fig. 7B). The maximum value of the Youden's index (J) determined a DIPP score ≥ 6 on the 0–9-point scale to be the optimal threshold denoting high risk of lesional progression. With 88.2% sensitivity and 87.5% specificity (OR 52.5), this DIPP score threshold appeared to have comparable sensitivity, but appreciably improved specificity, relative to each of its component variables at their optimal thresholds.
Figure 7.
(A) Dual imaging progression prediction (DIPP) score and (B) ROC curve showing the score’s performance to predict lesional progression. The maximum value of the Youden’s index (J) identified a DIPP score of ≥ 6 on a 0–9-point scale (sensitivity 88.2%, specificity 87.5%) as showing optimal predictive performance among DIPP score values.
Discussion
This study is, to our knowledge, the first yet published that addresses the unmet need for lesion-based quantification of mismatch, by analyzing the relationship of baseline [68Ga]Ga-PSMA-11 and baseline [18F]FDG PET variables to RLT response, and introducing a scoring system that appears to effectively predict early lesional progression.
Our key finding was that efficient prediction of lesional response to RLT may be provided by considering not just the "traditional" eligibility criterion of PSMA ligand uptake, i.e. SUVmax, but an additional PET variable, the FPQ, i.e. the ratio of [18F]FDG SUVmax to [68Ga]Ga-PSMA-11 SUVmax. Even better prediction may be achieved by using the DIPP score introduced here, which combines these two variables, and which can be easily and quickly calculated.
Our observation that the relationship of [18F]FDG SUVmax to [68Ga]Ga-PSMA-11 SUVmax i.e. the FPQ, predicted lesional response to PSMA-targeted RLT, aligns with the findings of a number of studies suggesting predictive and prognostic power in prostate cancer of the [18F]FDG SUVmax of a lesion or of the total tumor burden. These studies showed these variables to be associated with negative disease characteristics and poor patient outcomes19–21,38–40, including in men with mCRPC18,22,41,42, and those undergoing PSMA-targeted RLT25,29. Thus, our analysis furnishes additional evidence buttressing use of [18F]FDG PET/CT in screening candidates for PSMA-targeted RLT.
However, our study also supplies evidence supporting the (unsurprising) primacy of PSMA ligand uptake in predicting lesional response to PSMA-targeted RLT. We noted that baseline [18F]FDG SUVmax alone did not differ between progressing and non-progressing lesions. By contrast, baseline [68Ga]Ga-PSMA-11 SUVmax was a strong predictor of lesional progression to PSMA-targeted RLT both by itself (at a cut-off of < 11.09, 88.2% sensitivity and 81.9% specificity) and as part of the FPQ (at a cut-off of ≥ 0.92, 90.2% sensitivity and 78.7% specificity). Moreover, considering PSMA ligand uptake as an absolute value as well as a relative value (i.e. as the denominator of the FPQ), combined into the DIPP score, further strengthened predictive performance (at a cut-off of ≥ 6/9 points, 88.2% sensitivity and 87.5% specificity).
Also of interest, there was an 80% rate of lesional stable disease or partial response in our study, notwithstanding the high degree of glucometabolism in many of the analyzed lesions. These observations support the notion that RLT may be beneficial even in lesions with high [18F]FDG uptake, so long as a sufficient proportion of cells has adequate PSMA expression—a factor that may be readily discerned using the FPQ. The benefit of RLT in such cases also has been suggested by others29,43.
A routine implementation of [18F]FDG PET/CT in clinical practice to screen candidates for PSMA-targeted RLT certainly brings additional information compared with a (contrast-enhanced) diagnostic CT but may have to be weighed against the potential financial burden regarding additional costs.
Based on our data, a mismatch lesion with insufficient PSMA expression and potential resistance to PSMA-targeted RLT can be identified by using the variables PSMA PET-derived SUVmax and FPQ. Compared to [68Ga]Ga-PSMA-11 SUVmax or FPQ alone, a score combining both further strengthens predictive performance. We propose that a mismatch finding can be identified with a DIPP score of ≥ 6. Quantitatively indentifying mismatch lesions may facilitate individualized decision-making regarding PSMA-targeted RLT and the design of individualized mCRPC management strategies. However, the feasibility of implementing the DIPP score into clinical routine needs to be evaluated. We recommend studies, ideally prospective, in larger patient cohorts verifying our observations and addressing the clinical impact of this approach.
This analysis has several limitations. Firstly, the retrospective, single-center study design may impose bias and decrease generalizability and transferability. Other cutoff values would be expected for the use of different PSMA ligands or other reconstruction settings. Secondly, a possible selection bias cannot be excluded, as only a (pre-)selected number of patients who participated in the REALITY study received baseline and follow-up [18F]FDG PET/CT. The results of the present study should be treated with caution when compared with outcome data from other studies as our criterion of lesional progression was based on [18F]FDG PET/CT which is not established for prostate cancer such as response based on PSMA PET/CT or structural changes as reflected by Response Criteria in Solid Tumors44. Furthermore, our definition of progression was formulated on a lesional basis, not a patient basis. Further studies should be undertaken to consider relationships of PSMA ligand uptake and [18F]FDG uptake across the total tumor burden, e.g. in form of whole-body PET variables such as total lesion PSMA and total lesion glycolysis. These variables should be analyzed for their association with patient-based RLT response or overall survival. Lastly, we did not validate the DIPP score in a sample of lesions separate from those used to develop the score. The score's predictive performance should be confirmed in this way.
Conclusions
A low baseline SUVmax on [68Ga]Ga-PSMA-11 PET/CT and high baseline FPQ, which incorporates the former as well as baseline [18F]FDG SUVmax, predict lesional progression early in the course of RLT, whereas baseline [18F]FDG SUVmax by itself does not. Compared to [68Ga]Ga-PSMA-11 SUVmax or FPQ alone, a score combining both predicts early lesional progression even more effectively and might therefore be useful to quantitatively identify mismatch lesions.
Abbreviations
- 18F
Fluorine-18
- 68 Ga
Gallium-68
- 177Lu
Lutetium-177
- ADT
Androgen deprivation therapy
- AUC
Area under the receiver operator characteristic curve
- CT
Computed tomography
- DIPP
Dual imaging progression prediction
- ECOG
Eastern cooperative oncology group
- FDG
Fluorodeoxyglucose
- NAAD
Novel androgen axis drugs
- PERCIST
Positron emission tomography response criteria in solid tumors
- PET
Positron emission tomography
- p.i.
Post-injection
- PSA
Prostate-specific antigen
- PSMA
Prostate-specific membrane antigen
- RLT
Radioligand therapy
- ROC
Receiver operator characteristic
- SD
Standard deviation
- SUVmax
Maximum standardized uptake value
Author contributions
Conceptualization, F.R., C.B.,and S.E.; methodology, F.R. and C.B..; formal analysis, F.R., F.K. C.B. and S.D.; investigation, F.R., C.B., M.B., S.D., A.S.-S., S.M. , S.P. and S.E.; data curation, F.R., C.B. and F.K.; writing—original draft preparation, F.R., C.B. and R.J.M.; writing—review and editing, all authors; visualization, F.R. and C.B.; supervision, S.E. All authors have read and agreed to the published version of the manuscript. All patients have given written consent to publication.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2021;385:1091–1103. doi: 10.1056/NEJMoa2107322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804. doi: 10.1016/S0140-6736(21)00237-3. [DOI] [PubMed] [Google Scholar]
- 3.Khreish F, Ghazal Z, Marlowe RJ, Rosar F, Sabet A, Maus S, et al. 177 Lu-PSMA-617 radioligand therapy of metastatic castration-resistant prostate cancer: Initial 254-patient results from a prospective registry (REALITY Study) Eur. J. Nucl. Med. Mol. Imaging. 2022;49:1075–1085. doi: 10.1007/s00259-021-05525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet. 2020;395:1208–1216. doi: 10.1016/S0140-6736(20)30314-7. [DOI] [PubMed] [Google Scholar]
- 5.Pienta KJ, Gorin MA, Rowe SP, Carroll PR, Pouliot F, Probst S, et al. A Phase 2/3 prospective multicenter study of the diagnostic accuracy of prostate specific membrane antigen PET/CT with (18)F-DCFPyL in prostate cancer patients (OSPREY) J. Urol. 2021 doi: 10.1097/JU.0000000000001698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris MJ, Rowe SP, Gorin MA, Saperstein L, Pouliot F, Josephson D, et al. Diagnostic performance of (18)F-DCFPyL-PET/CT in men with biochemically recurrent prostate cancer: Results from the CONDOR phase III, multicenter study. Clin. Cancer Res. 2021 doi: 10.1158/1078-0432.CCR-20-4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afshar-Oromieh A, da Cunha ML, Wagner J, Haberkorn U, Debus N, Weber W, et al. Performance of [(68)Ga]Ga-PSMA-11 PET/CT in patients with recurrent prostate cancer after prostatectomy-a multi-centre evaluation of 2533 patients. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:2925–2934. doi: 10.1007/s00259-021-05189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giesel FL, Will L, Kesch C, Freitag M, Kremer C, Merkle J, et al. Biochemical recurrence of prostate cancer: Initial results with [(18)F]PSMA-1007 PET/CT. J. Nucl. Med. 2018;59:632–635. doi: 10.2967/jnumed.117.196329. [DOI] [PubMed] [Google Scholar]
- 9.Hohberg M, Kobe C, Krapf P, Tager P, Hammes J, Dietlein F, et al. Biodistribution and radiation dosimetry of [(18)F]-JK-PSMA-7 as a novel prostate-specific membrane antigen-specific ligand for PET/CT imaging of prostate cancer. EJNMMI Res. 2019;9:66. doi: 10.1186/s13550-019-0540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietlein F, Kobe C, Vazquez SM, Fischer T, Endepols H, Hohberg M, et al. An (89)Zr-labeled PSMA tracer for PET/CT imaging of prostate cancer patients. J. Nucl. Med. 2022;63:573–583. doi: 10.2967/jnumed.121.262290. [DOI] [PubMed] [Google Scholar]
- 11.Rosar F, Khreish F, Marlowe RJ, Schaefer-Schuler A, Burgard C, Maus S, et al. Detection efficacy of [(89)Zr]Zr-PSMA-617 PET/CT in [(68)Ga]Ga-PSMA-11 PET/CT-negative biochemical recurrence of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 2023 doi: 10.1007/s00259-023-06241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burgard C, Hoffmann MA, Frei M, Buchholz HG, Khreish F, Marlowe RJ, et al. Detection efficacy of (68)Ga-PSMA-11 PET/CT in biochemical recurrence of prostate cancer with very low PSA Levels: A 7-year, two-center "real-world" experience. Cancers (Basel) 2023 doi: 10.3390/cancers15051376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright GL, Jr, Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol. Oncol. 1995;1:18–28. doi: 10.1016/1078-1439(95)00002-y. [DOI] [PubMed] [Google Scholar]
- 14.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 15.Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52:637–640. doi: 10.1016/s0090-4295(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 16.Kratochwil C, Fendler WP, Eiber M, Hofman MS, Emmett L, Calais J, et al. Joint EANM/SNMMI procedure guideline for the use of (177)Lu-labeled PSMA-targeted radioligand-therapy ((177)Lu-PSMA-RLT) Eur. J. Nucl. Med. Mol. Imaging. 2023 doi: 10.1007/s00259-023-06255-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eidelman E, Twum-Ampofo J, Ansari J, Siddiqui MM. The metabolic phenotype of prostate cancer. Front. Oncol. 2017;7:131. doi: 10.3389/fonc.2017.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauckneht M, Bertagna F, Donegani MI, Durmo R, Miceli A, De Biasi V, et al. The prognostic power of 18F-FDG PET/CT extends to estimating systemic treatment response duration in metastatic castration-resistant prostate cancer (mCRPC) patients. Prostate Cancer Prostatic Dis. 2021;24:1198–1207. doi: 10.1038/s41391-021-00391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meziou S, Ringuette Goulet C, Hovington H, Lefebvre V, Lavallee E, Bergeron M, et al. GLUT1 expression in high-risk prostate cancer: Correlation with (18)F-FDG-PET/CT and clinical outcome. Prostate Cancer Prostatic Dis. 2020;23:441–448. doi: 10.1038/s41391-020-0202-x. [DOI] [PubMed] [Google Scholar]
- 20.Lavallee E, Bergeron M, Buteau FA, Blouin AC, Duchesnay N, Dujardin T, et al. Increased prostate cancer glucose metabolism detected by (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in localised Gleason 8–10 prostate cancers identifies very high-risk patients for early recurrence and resistance to castration. Eur. Urol. Focus. 2019;5:998–1006. doi: 10.1016/j.euf.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Beauregard JM, Blouin AC, Fradet V, Caron A, Fradet Y, Lemay C, et al. FDG-PET/CT for pre-operative staging and prognostic stratification of patients with high-grade prostate cancer at biopsy. Cancer Imaging. 2015;15:2. doi: 10.1186/s40644-015-0038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suman S, Parghane RV, Joshi A, Prabhash K, Bakshi G, Talole S, et al. Therapeutic efficacy, prognostic variables and clinical outcome of (177)Lu-PSMA-617 PRLT in progressive mCRPC following multiple lines of treatment: Prognostic implications of high FDG uptake on dual tracer PET-CT vis-a-vis Gleason score in such cohort. Br. J. Radiol. 2019;92:20190380. doi: 10.1259/bjr.20190380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khreish F, Ribbat K, Bartholoma M, Maus S, Stemler T, Hierlmeier I, et al. Value of combined PET imaging with [(18)F]FDG and [(68)Ga]Ga-PSMA-11 in mCRPC patients with worsening disease during [(177)Lu]Lu-PSMA-617 RLT. Cancers (Basel) 2021 doi: 10.3390/cancers13164134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michalski K, Mix M, Meyer PT, Ruf J. Determination of whole-body tumour burden on [68Ga]PSMA-11 PET/CT for response assessment of [177Lu]PSMA-617 radioligand therapy: A retrospective analysis of serum PSA level and imaging derived parameters before and after two cycles of therapy. Nuklearmedizin. 2019;58:443–450. doi: 10.1055/a-1035-9052. [DOI] [PubMed] [Google Scholar]
- 25.Pathmanandavel S, Crumbaker M, Nguyen A, Yam AO, Wilson P, Niman R, et al. The prognostic value of posttreatment (68)Ga-PSMA-11 PET/CT and (18)F-FDG PET/CT in metastatic castration-resistant prostate cancer treated with (177)Lu-PSMA-617 and NOX66 in a Phase I/II Trial (LuPIN) J. Nucl. Med. 2023;64:69–74. doi: 10.2967/jnumed.122.264104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adnan A, Basu S. Concept proposal for a six-tier integrated dual tracer PET-CT (68Ga-PSMA and FDG) image scoring system ('Pro-PET' score) and examining its potential implications in metastatic castration-resistant prostate carcinoma theranostics and prognosis. Nucl. Med. Commun. 2021;42:566–574. doi: 10.1097/MNM.0000000000001371. [DOI] [PubMed] [Google Scholar]
- 27.Adnan A, Basu S. Comparison of dual-tracer PET and CT features to conventional risk categories in assessing response to (177)Lu-PSMA-617 therapy for metastatic prostate adenocarcinoma with urinary bladder involvement. J. Nucl. Med. Technol. 2020;48:148–153. doi: 10.2967/jnmt.119.235960. [DOI] [PubMed] [Google Scholar]
- 28.Alberts I, Schepers R, Zeimpekis K, Sari H, Rominger A, Afshar-Oromieh A. Combined [68 Ga]Ga-PSMA-11 and low-dose 2-[18F]FDG PET/CT using a long-axial field of view scanner for patients referred for [177Lu]-PSMA-radioligand therapy. Eur. J. Nucl. Med. Mol. Imaging. 2023;50:951–956. doi: 10.1007/s00259-022-05961-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michalski K, Ruf J, Goetz C, Seitz AK, Buck AK, Lapa C, et al. Prognostic implications of dual tracer PET/CT: PSMA ligand and [(18)F]FDG PET/CT in patients undergoing [(177)Lu]PSMA radioligand therapy. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:2024–2030. doi: 10.1007/s00259-020-05160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosar F, Ribbat K, Ries M, Linxweiler J, Bartholoma M, Maus S, et al. Neuron-specific enolase has potential value as a biomarker for [(18)F]FDG/[(68)Ga]Ga-PSMA-11 PET mismatch findings in advanced mCRPC patients. EJNMMI Res. 2020;10:52. doi: 10.1186/s13550-020-00640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–833. doi: 10.1016/S1470-2045(18)30198-0. [DOI] [PubMed] [Google Scholar]
- 32.Hotta M, Gafita A, Czernin J, Calais J. Outcome of patients with PSMA PET/CT screen failure by VISION criteria and treated with (177)Lu-PSMA therapy: A multicenter retrospective analysis. J. Nucl. Med. 2022;63:1484–1488. doi: 10.2967/jnumed.121.263441. [DOI] [PubMed] [Google Scholar]
- 33.Groener D, Schneider S, Baumgarten J, Happel C, Klimek K, Mader N, et al. Baseline [(68)Ga]Ga-PSMA-11 PET/CT before [(177)Lu]Lu-PSMA-617 radioligand therapy: Value of PSMA-uptake thresholds in predicting targetable lesions. Cancers (Basel) 2023 doi: 10.3390/cancers15020473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jadvar H. The VISION forward: Recognition and implication of PSMA-/(18)F-FDG+ mCRPC. J. Nucl. Med. 2022;63:812–815. doi: 10.2967/jnumed.121.263274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S, et al. (68)Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging. 2017;44:1014–1024. doi: 10.1007/s00259-017-3670-z. [DOI] [PubMed] [Google Scholar]
- 36.Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging. 2015;42:328–354. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009;50(Suppl 1):122S–S150. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meirelles GS, Schoder H, Ravizzini GC, Gonen M, Fox JJ, Humm J, et al. Prognostic value of baseline [18F] fluorodeoxyglucose positron emission tomography and 99mTc-MDP bone scan in progressing metastatic prostate cancer. Clin. Cancer Res. 2010;16:6093–6099. doi: 10.1158/1078-0432.CCR-10-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen K, Liu B, Zhou X, Ji Y, Chen L, Wang Q, et al. The evolving role of (18)F-FDG PET/CT in diagnosis and prognosis prediction in progressive prostate cancer. Front. Oncol. 2021;11:683793. doi: 10.3389/fonc.2021.683793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oyama N, Akino H, Suzuki Y, Kanamaru H, Miwa Y, Tsuka H, et al. Prognostic value of 2-deoxy-2-[F-18]fluoro-D-glucose positron emission tomography imaging for patients with prostate cancer. Mol. Imaging Biol. 2002;4:99–104. doi: 10.1016/s1095-0397(01)00065-6. [DOI] [PubMed] [Google Scholar]
- 41.Fox JJ, Gavane SC, Blanc-Autran E, Nehmeh S, Gonen M, Beattie B, et al. Positron emission tomography/computed tomography-based assessments of androgen receptor expression and glycolytic activity as a prognostic biomarker for metastatic castration-resistant prostate cancer. JAMA Oncol. 2018;4:217–224. doi: 10.1001/jamaoncol.2017.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jadvar H, Desai B, Conti PS. Sodium 18F-fluoride PET/CT of bone, joint, and other disorders. Semin. Nucl. Med. 2015;45:58–65. doi: 10.1053/j.semnuclmed.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Current K, Meyer C, Magyar CE, Mona CE, Almajano J, Slavik R, et al. Investigating PSMA-targeted radioligand therapy efficacy as a function of cellular PSMA levels and intratumoral PSMA heterogeneity. Clin. Cancer Res. 2020;26:2946–2955. doi: 10.1158/1078-0432.CCR-19-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.