Abstract
Lysosomal and synaptic dysfunctions are hallmarks in neurodegeneration and potentially relevant as biomarkers, but data on early Parkinson’s disease (PD) is lacking. We performed targeted mass spectrometry with an established protein panel, assessing autophagy and synaptic function in cerebrospinal fluid (CSF) of drug-naïve de novo PD, and sex-/age-matched healthy controls (HC) cross-sectionally (88 PD, 46 HC) and longitudinally (104 PD, 58 HC) over 10 years. Multiple markers of autophagy, synaptic plasticity, and secretory pathways were reduced in PD. We added samples from prodromal subjects (9 cross-sectional, 12 longitudinal) with isolated REM sleep behavior disorder, revealing secretogranin-2 already decreased compared to controls. Machine learning identified neuronal pentraxin receptor and neurosecretory protein VGF as most relevant for discriminating between groups. CSF levels of LAMP2, neuronal pentraxins, and syntaxins in PD correlated with clinical progression, showing predictive potential for motor- and non-motor symptoms as a valid basis for future drug trials.
Subject terms: Parkinson's disease, Parkinson's disease
Introduction
Parkinson’s disease (PD) is an increasingly prevalent, progressive, and complex neurodegenerative disease that lacks a conclusive panel of biomarkers that assess the rate, trait, fate, and state1–4 of the disease. PD diagnosis is still clinically based and usually made when the majority of affected neurons have already degenerated. In this context, non-motor symptoms like hyposmia and isolated REM (rapid eye movement) sleep behavior disorder (iRBD) are very valuable in identifying those at risk of developing the disease. iRBD is very specific, and therefore, the most powerful prodromal entity of PD. It is characterized by dream-enacting behaviors during REM sleep and a high conversion rate to an α-synucleinopathy, including PD and dementia with Lewy bodies and multiple system atrophy (MSA)5.
PD, mostly but not exclusively, affects dopaminergic neurons, especially in the substantia nigra pars compacta. α-synuclein (αSyn) plays a key role in its pathology6 and is physiologically localized mainly in presynaptic terminals of neurons. In certain synucleinopathies, including PD and iRBD7, it accumulates within the neuronal soma, described as Lewy body inclusions8. Nevertheless, the utility of quantifying total levels of αSyn as a biomarker is limited. It is lower in the cerebrospinal fluid (CSF) and plasma of PD patients compared to healthy controls, but no longitudinal changes or predictive potential have been detected9. α-synuclein seeding aggregation assays (αSyn-SAAs) were recently established that diagnose α-synucleinopathy in PD with high sensitivity and specificity, therefore enabling the identification of molecular heterogeneity and increased PD risk among patients. SAAs have not yet been qualified as progression markers and they have not been sufficiently linked to the specific pathophysiology10. Further, they are binary assays, helpful for diagnostics but not for tracking changes over time. Therefore, many questions remain to be answered. Research for effective diagnostic and prognostic biomarkers in early and prodromal disease stages needs to be widened11.
Synaptic dysfunction is a hallmark of many neurodegenerative diseases, including PD. Multiple established risk factors and causative PD genes are known to influence synaptic functioning12. Lysosomal dysfunction is also implicated in PD: multiple variants in lysosomal storage disorder genes are associated with an increased risk of PD13 and previous studies suggest that there are alterations of the autophagic and endolysosomal system in PD14. Furthermore, accumulation of autophagic vacuoles is evident in PD patients’ brain cells, accompanied by a decrease in endolysosomal markers like LAMP215,16.
Assessing lysosomal and synaptic functioning biomarkers in PD could potentially improve diagnostic and prognostic accuracy by focusing on the early disease phase and enabling treatment response monitoring in future drug trials. Previous attempts to assess synaptic functioning are based on research in synaptic proteins in CSF of patients with Alzheimer’s disease17–19. Some earlier studies, mainly focused on PD, either found no differences between PD and controls or had inconsistent results20–25.
Data on de novo PD and longitudinal analysis are generally lacking and no studies on prodromal PD stages are available to date.
We sought to fill this gap by investigating a combined panel of lysosomal and synaptic markers validated in different cohorts26–28. Over a 10-year follow-up, we used this approach to longitudinally assess differently expressed proteins in CSF between subjects with de novo PD and sex- and age-matched healthy controls (cross-sectional 88 PD, 46 HC; longitudinal 104 PD, 58 HC) from our ongoing, prospective, single-center de novo Parkinson’s disease (DeNoPa) cohort29. Further, we added samples from prodromal subjects with video-polysomnography diagnosed iRBD (cross-sectional 9, longitudinal 12) as an exploratory approach. We integrated our data into one30,31 of the two31,32 newly proposed neuronal αSyn-disease integrated staging systems and correlated the results with the available longitudinal clinical data. Linear mixed modelling was performed to analyze progression over time, and we evaluated the predictive and discriminative potential of these markers with machine learning algorithms.
Results
The DeNoPa cohort
We analyzed CSF samples from subjects of our longitudinal de novo PD (DeNoPa) cohort and included, from baseline, 88 PD patients (62 men, 70.5%) and 46 healthy controls (HC) (34 men, 73.9%) for whom CSF samples were available at all time points. The mean age was 65 years ( ± 9.8) for PD and 66 years ( ± 7.2) for HC. The mean Hoehn and Yahr Stage in the PD group was 2 ( ± 0.4), and the mean MDS-UPDRS part III score was 23 ( ± 11.4). MMSE (mini-mental state examination) did not significantly differ between PD and HC (28 in both groups), but the MoCA (Montreal cognitive assessment) score was lower in PD (PD: 24, healthy controls: 26). Further, we included an exploratory test set of nine subjects with iRBD (2 men, 2.2%) mean age 65 ( ± 9). The demographics and clinical characteristics of the cohort are shown in Table 1.
Table 1.
Baseline demographics and some CSF biomarkers of the included subjects, SAA data displays positive and negative results of the available samples
| Baseline | HC (N = 46) | PD (N = 88) | RBD (N = 9) | Adj. p-values |
|---|---|---|---|---|
| Sex | 0.008* | |||
| - female | 12 (26.1%) | 26 (29.5%) | 7 (77.8%) | |
| - male | 34 (73.9%) | 62 (70.5%) | 2 (22.2%) | |
| Age | 0.989 | |||
| - Mean (SD) | 65.6 (7.23) | 65.136 (9.81) | 64.78 (8.97) | |
| BMI | 0.012* | |||
| - Mean (SD) | 26.97 (4.63) | 28.447 (4.71) | 24.99 (3.68) | |
| MDS-UPDRS-III | < 0.001* | |||
| - Mean (SD) | 0.63 (1.48) | 22.966 (11.44) | 3.11 (2.71) | |
| MDS-UPDRS total score | < 0.001* | |||
| - Mean (SD) | 3.39 (3.63) | 37.278 (16.76) | 18.11 (10.17) | |
| Hoehn & Yahr | < 0.001* | |||
| - Mean (SD) | 0.00 (0.00) | 2.045 (0.74) | 0.11 (0.33) | |
| MMSE total score | 0.278 | |||
| - Mean (SD) | 28.64 (1.18) | 28.207 (1.48) | 28.56 (0.88) | |
| MoCA total score | 0.013* | |||
| - Mean (SD) | 26.05 (2.33) | 24.257 (3.265) | 25.44 (2.60) | |
| CSF albumin quotient | 0.019* | |||
| - Mean (SD) | 7.65 (3.74) | 8.916 (4.507) | 6.03 (1.88) | |
| CSF β-amyloid (Aβ) | 0.154 | |||
| - Mean (SD) | 870.65 (211.07) | 870.401 (210.363) | 673.67 (65.68) | |
| CSF phospho-tau protein (pTau) | 0.066 | |||
| - Mean (SD) | 44.97 (16.72) | 42.192 (16.326) | 27.77 (3.36) | |
| CSF Total tau protein (tTau) | 0.099 | |||
| - Mean (SD) | 256.84 (122.39) | 235.632 (115.464) | 159.00 (16.82) | |
| CSF neurofilament light chains (NfL) | < 0.001* | |||
| - Mean (SD) | 536.28 (203.38) | 702.10 (405.19) | 361.83 (85.92) | |
| α-synuclein seeding aggregation assay (SAA) | 41 (−) out of 41 | 68/12 (+/−) out of 80 | 8/1 (+/−) out of 9 | < 0.001* |
PD subjects displayed a higher CSF albumin quotient, indicative of a blood-brain barrier dysfunction. Typical CSF Alzheimer’s disease markers (Aβ, t- and p-tau) showed no differences between the groups. As reported before, neurofilament light (NfL) was increased in PD samples. The P-value is adjusted for age and sex. PD Parkinson’s Disease, HC Healthy control, iRBD isolated REM sleep behavior disorder, n Number, sd Standard deviation, CSF Cerebrospinal fluid, MDS-UPDRS Movement Disorder Society – United Parkinson’s Disease Rating Scale, MMSE Mini-Mental State Examination, MoCa Montreal Cognitive Assessment Score.
*indicates statistically significant results.
For additional information on the differences between the subgroups see Supplementary Table 6.
We added our available α-synuclein–seed amplification assay (αSyn-SAA) data, based on high-throughput CSF αSyn-SAA, showing that 68 of 80 tested PD subjects were positive, as were 8 of the 9 iRBD subjects.
To increase the power of our longitudinal model, we also included additional samples from subjects with no available CSF samples at baseline. This resulted in a total of 12 iRBD (11 of 12 αSyn-SAA positive), 104 PD (87 of 100 αSyn-SAA positive), and 58 HC (all negative) subjects in our longitudinal model. Applying the neuronal αSyn-disease integrated staging system (NSD-ISS) led to four subjects categorized as stage NSD-2A (iRBD, S + D−), six in stage NSD-2B (iRBD, S + D + ) and 78 who met the criteria for stage 4 (PD, S + D + , moderate clinical impairment). See the methods section and Supplementary Table 4 and Supplementary Table 5 for details.
Synaptic and lysosomal biomarker levels
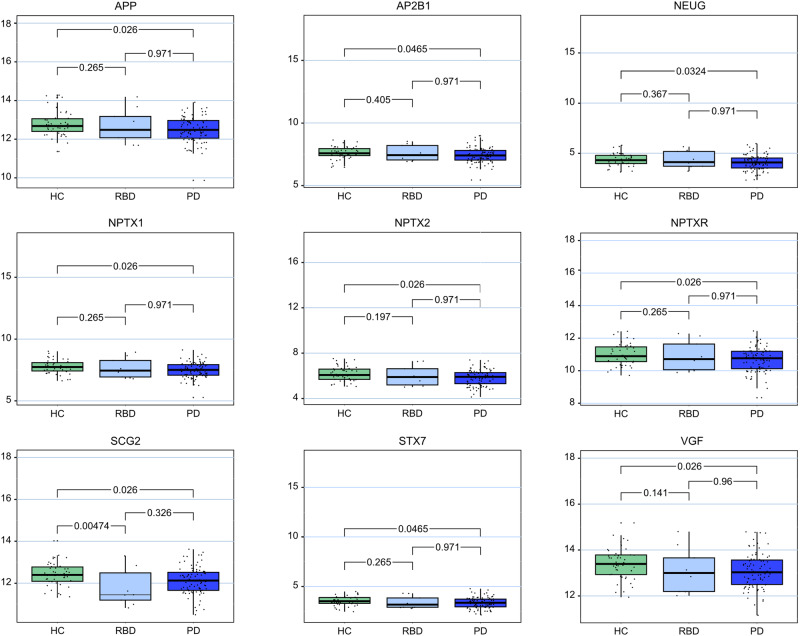
The application of a targeted mass spectrometry biomarker panel (Table 2) showed nine markers that were differentially expressed between PD and healthy controls: neurosecretory protein VGF, amyloid-beta precursor protein (APP), the neuronal pentraxins and its corresponding receptor (NPTX1, NPTX2, NPTXR), secretogranin-2, neurogranin, syntaxin-7 and AP-2 complex subunit beta (AP2B1). The markers displayed significantly lower CSF levels in PD compared to healthy controls. All these markers also showed lower CSF levels in iRBD, with significant results for secretogranin-2 (p = 0.01). The analysis showed no changes in protein levels between PD and iRBD.
Table 2.
Overview of the applied biomarker panel, the targeted peptide sequences, and the biological functions
| Protein | Abbreviation | Accession | Sequence | Function |
|---|---|---|---|---|
| Neurosecretory protein VGF | VGF | O15240 | AYQGVAAPFPK | Neurotransmitter secretion |
| NSEPQDEGELFQGVDPR | ||||
| Neuronal pentraxin receptor | NPTXR | O95502 | NNYMYAR | Glutamate receptor recruitment; synaptic plasticity |
| LVEAFGGATK | ||||
| Neuronal pentraxin-1 | NPTX1 | Q15818 | ETVLQQK | |
| CESQSTLDPGAGEAR | ||||
| Neuronal pentraxin-2 | NPTX2 | P47972 | VAELEDEK | |
| ETVVQQK | ||||
| Amyloid-beta precursor protein | APP | P05067 | VESLEQEAANER | Neuronal surface receptor |
| Secretogranin-2 | SCG2 | P13521 | ALEYIENLR | Neuroendocrine protein, biogenesis of secretory granules |
| VLEYLNQEK | ||||
| Neurogranin | NEUG | Q92686 | KGPGPGGPGGAGVAR | Binds calmodulin, enhances synaptic transmission |
| Syntaxin-7 | STX7 | O15400 | EFGSLPTTPSEQR | Vesicle trafficking |
| Syntaxin-1B | STX1B | P61266 | QHSAILAAPNPDEK | |
| AP-2 complex subunit beta | AP2B1 | P63010 | AVWLPAVK | Mediating endocytosis |
| IQPGNPNYTLSLK | ||||
| QVFLATWK | ||||
| Chromogranin-A | CMGA | P10645 | GLSAEPGWQAK | Aggregation and processing of secretory granules |
| EDSLEAGLPLQVR | ||||
| GangliosideGM2 activator | SAP3 | P17900 | EVAGLWIK | Binding gangliosides, stimulating GM2 degradation |
| IESVLSSSGK | ||||
| β-synuclein | SYUB | Q16143 | EGVVQGVASVAEK | Presynaptic functioning |
| γ-synuclein | SYUG | O76070 | ENVVQSVTSVAEK | |
| Complexin-2 | CPLX2 | Q6PUV4 | AALEQPCEGSLTRPK | Vesicle trafficking |
| Rab GDP dissociation inhibitor alpha | GDIA | P31150 | QLICDPSYIPDR | Vesicle trafficking |
| Phosphatidylethanolamine-binding protein 1 | PEBP1 | P30086 | NRPTSISWDGLDSGK | Regulatory protein, presynaptic functioning |
| LYEQLSGK | ||||
| Lysosome-associated membrane glycoprotein 2 | LAMP2 | P13473 | IPLNDLFR | Chaperone-mediated autophagy |
| Cathepsin-F | CATF | Q9UBX1 | TLLCSFQVLDELGR | Protein turnover, intracellular degradation |
| 1433E | 1433E | P62258 | IISSIEQK | Adapter proteins, modulating general and specific pathways by activity regulation of the binding partners |
| 1433 F | 1433 F | Q04917 | AVTELNEPLSNEDR | |
| 1433 T | 1433 T | P27348 | AVTEQGAELSNEER | |
| 1433Z | 1433Z | P63104 | VVSSIEQK |
Results are shown in Table 3 and box plots appear in Fig. 1.
Table 3.
Results of the assessed proteins in CSF samples, significantly differently expressed values are marked with *, P-value is adjusted for age, and sex and Benjamini-Hochberg FDR adjusted for multiple testing
| de novo PD | exploratory iRBD | |||||
|---|---|---|---|---|---|---|
| Protein | HC | PD | HC-PD adj. p-val. | iRBD | HC-iRBD adj. p-val. | PD-iRBD adj. p-val. |
| Secretogranin-2 | 12.42 | 12.12 | 0.03* | 11.79 | 0.01* | 0.43 |
| VGF | 13.42 | 13.09 | 0.03* | 13.12 | 0.12 | 0.92 |
| Neuronal Pentraxin receptor | 10.99 | 10.67 | 0.03* | 10.91 | 0.26 | 0.96 |
| Amyloid-beta precursor protein | 12.79 | 12.49 | 0.03* | 12.71 | 0.24 | 0.96 |
| Neuronal Pentraxin-2 | 6.16 | 5.83 | 0.03* | 5.99 | 0.17 | 0.96 |
| Neuronal Pentraxin-1 | 7.77 | 7.49 | 0.03* | 7.65 | 0.24 | 0.96 |
| Neurogranin | 4.36 | 4.08 | 0.03* | 4.36 | 0.39 | 0.96 |
| Syntaxin-7 | 3.53 | 3.34 | 0.05* | 3.37 | 0.26 | 0.96 |
| AP-2 complex subunit beta | 7.62 | 7.41 | 0.05* | 7.57 | 0.44 | 0.96 |
| β-synuclein | 3.29 | 3.10 | 0.12 | 2.99 | 0.11 | 0.47 |
| Syntaxin-1B | 3.69 | 3.48 | 0.07 | 3.51 | 0.26 | 0.96 |
| Chromogranin-A | 12.12 | 11.86 | 0.07 | 12.14 | 0.48 | 0.96 |
| Ganglioside GM2 activator | 12.70 | 12.59 | 0.27 | 12.43 | 0.22 | 0.82 |
| γ-synuclein | 4.48 | 4.40 | 0.43 | 4.13 | 0.12 | 0.43 |
| Complexin-2 | 5.86 | 5.71 | 0.16 | 5.75 | 0.37 | 0.96 |
| Rab GDP dissociation inhibitor alpha | 6.96 | 6.85 | 0.16 | 6.86 | 0.42 | 0.96 |
| Lysosome-associated membrane glycoprotein 2 | 14.04 | 13.93 | 0.34 | 14.09 | 0.56 | 0.96 |
| Phosphatidylethanolamine-binding protein 1 | 6.78 | 6.72 | 0.50 | 6.73 | 0.56 | 0.96 |
| 1433 F | 2.98 | 2.92 | 0.81 | 2.93 | 0.49 | 0.96 |
| 1433E | 3.75 | 3.77 | 0.92 | 3.94 | 0.70 | 0.96 |
| 1433 T | -0.34 | -0.33 | 0.95 | -0.74 | 0.21 | 0.43 |
| 1433Z | 5.59 | 5.65 | 0.41 | 5.42 | 0.45 | 0.54 |
| Cathepsin-F | 9.60 | 9.59 | 0.90 | 9.35 | 0.39 | 0.84 |
PD Parkinson’s Disease, HC Healthy control, iRBD Isolated REM sleep behavior disorder; based on the protein measurements at baseline [sample size: HC (N = 46); PD (N = 88)].
Statistically significant results are displayed in bold.
Fig. 1. Box plots of the significantly differently expressed proteins.
The center line displays the median, the box the interquartile range and the whiskers the minimum and maximum value of the data. HC Healthy controls, RBD isolated REM sleep behavior disorder, PD: Parkinson´s disease, APP Amyloid-beta precursor protein, AP2B1 AP-2 complex subunit beta, NEUG Neurogranin, NPTX1 Neuronal Pentraxin-1, NPTX2 Neuronal Pentraxin-2, NPTXR Neuronal Pentraxin receptor; SCG2: Secretogranin-2, STX7 Syntaxin-7; based on the protein measurements at baseline [sample size: HC (N = 46); PD (N = 88)].
Linear mixed models and longitudinal changes
The study includes longitudinal CSF samples collected during the follow-up visits every two years of a period of 10 years. The established linear mixed models, adjusted for age and sex, revealed no significant changes in the assessed peptides over time in the healthy controls, PD, or iRBD groups. As the PD subjects were de novo at baseline and started drug treatment during the follow-up, we wanted to monitor possible treatment effects on the marker levels and, therefore, analyzed the influence of the LEDD (levodopa equivalent daily dosage) on the marker panel by including it as a fixed effect term in the models. This showed no significant relationship to the medication. Only age and sex contributed significantly to the model. For all the assessed proteins the term TIME and the interaction term COHORT: TIME (COHORT = HC; PD; iRBD) are non-significant, indicating no significant changes over time between the groups and a stable expression level in CSF. Results can be found in Supplementary Table 3 and Supplementary Figure 7.
Correlation analysis
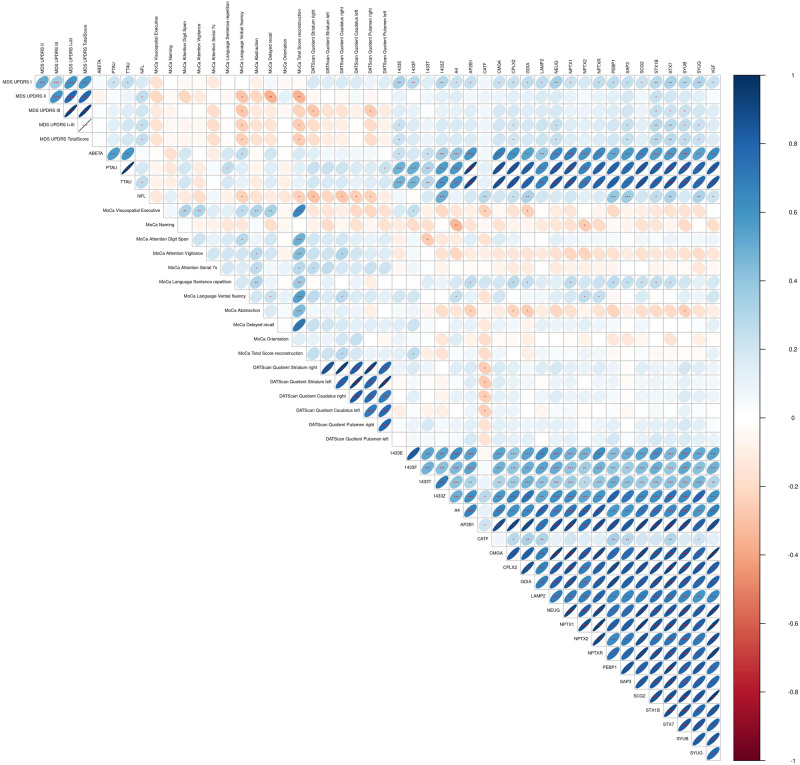
Multiple markers correlated with the available clinical and diagnostic data at baseline. This was especially the case for MDS-UPDRS part I: the rating of non-motor symptoms was strongly positively correlated with 20 markers, including VGF, NPTX1, NPTXR, neurogranin, γ-synuclein, β-synuclein, and AP2B1. Nine markers correlated positively with the MoCA domain “language sentence repetition”: SAP3, secretogranin-2, GDIA, PEBP1, AP2B1, syntaxin-7, syntaxin-1B, complexin-2, and NPTX1. Syntaxin-1B and 7 correlated positively with MDS-UPDRS parts I, II, III, and the total score, as well as with the MoCA domain “language sentence repetition”. This suggests a strong connection between the syntaxins and motor and non-motor impairment. The results from the dopamine-transporter–single-photon emission computed tomography (DAT-SPECT), namely the specific binding ratios of caudate nucleus and striatum on both sides, correlated negatively with Cathepsin-F. All correlations can be found in the correlation matrix (Figs. 2, 3).
Fig. 2. Correlation matrix of the measured panel and the available clinical data at baseline.
The colours of the circles represent positive (blue) and negative (red) correlations. The circle sizes represent the size of the absolute correlation coefficients, larger signifying stronger correlations. *p < 0.05 **p < 0.01 ***p < 0.001, The p-values are Benjamini-Hochberg adjusted for multiple testing. MDS-UPDRS Movement Disorder Society – United Parkinson’s Disease Rating Scale, MoCa: Montreal Cognitive Assessment Score, SBR: specific binding ratio, based on the protein measurements at baseline [sample size: HC (N = 46); PD (N = 88)].
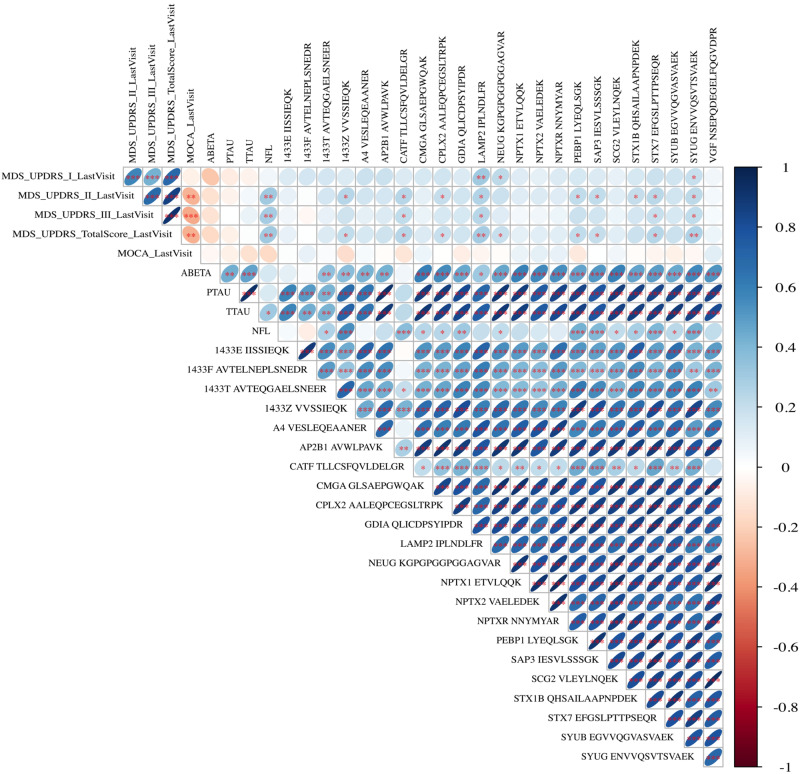
Fig. 3. Predictive potential of the analyzed markers evaluated by the correlation of the baseline CSF levels with the available clinical data after 10 years of follow-up.
The colours of the circles represent positive (blue) and negative (red) correlations. The circle sizes represent the size of the absolute correlation coefficients, larger signifying stronger correlations.*p < 0.05 **p < 0.01 ***p < 0.001, The p-values are Benjamini-Hochberg adjusted for multiple testing. Abbreviations: MDS-UPDRS = Movement Disorder Society – United Parkinson’s Disease Rating Scale, MoCa: Montreal Cognitive Assessment Score, based on the protein measurements at baseline [sample size: HC (N = 46); PD (N = 88)].
Predictive potential
The correlation of the baseline biomarker levels and the last available MDS-UPDRS scores after 10 years of follow-up revealed a predictive potential for several markers. Higher CSF levels at baseline predicted worse outcomes in MDS-UPDRS I for LAMP2, γ-synuclein, and Neurogranin. The markers Cathepsin-F, SAP3, LAMP2, PEBP1, Syntaxin-7, Syntaxin-1B, CPLX2, 1433Z, and γ-synuclein were predictive for MDS-UPDRS part II, Cathepsin-F, LAMP2, Syntaxin-7, and γ-synuclein for MDS-UPDRS part III, and Cathepsin-F, SAP3, LAMP2, PEBP1, Neurogranin, Syntaxin-7, CPLX2, 1433Z and γ-synuclein for the MDS-UPDRS total score. LAMP2 and γ-synuclein CSF baseline levels were predictive of the outcome in all MDS-UPDRS subscores. Cathepsin-F was predictive for all subscores of the MDS-UPDRS except for part I.
Machine learning approach and discrimination analysis
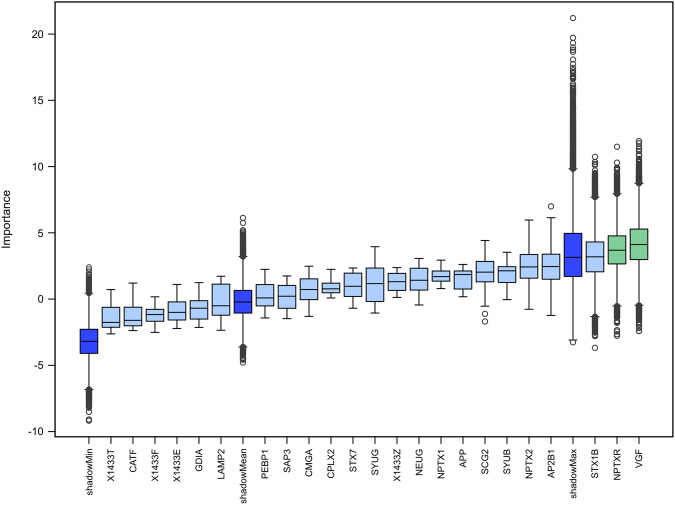
The Boruta analysis performed 100,000 iterations and the Boruta algorithm detected two proteins of high importance for the discrimination of the PD subjects from healthy controls, namely VGF and Neuronal pentraxin receptor (Fig. 4; based on the protein measurements at baseline (sample size: HC (N = 46); PD (N = 88)). Information on the classification accuracy is displayed in Supplementary Fig. 4a/b). Results of the discrimination analysis based on receiver operating characteristics (ROC) can be found in Supplementary Figure 7. The single markers showed Area Under the Curve (AUC) values around 0,6 between PD and HC.
Fig. 4. Results of the boruta algorithm.
The analysis revealed Neurosecretory protein VGF and Neuronal pentraxin receptor with significant relevance in discriminating between Parkinson´s Disease and Healthy controls out of 100.000 iterations, based on the protein measurements at baseline [sample size: HC (N = 46); PD (N = 88); The center line displays the median, the box the interquartile range and the whiskers the minimum and maximum value of the data.
Discussion
In prodromal and early PD, we need effective biomarkers that reflect specific pathophysiological processes and are directly connected to disease progression and clinical phenotypes. This led us to apply a well-established biomarker panel in the CSF of prodromal and manifest PD patients as well as healthy subjects. Synaptic and lysosomal dysfunction has been shown to play an important role in neurodegenerative diseases and the proteins included in the analyzed panel have been implicated in Alzheimer’s disease, PD, and other Parkinson syndromes26–28 Nevertheless, longitudinal data and studies in early and prodromal PD disease stages are lacking. To fill this gap, we (i) analyzed the potential of lysosomal and synaptic function markers to detect or monitor endolysosomal dysfunction and synaptic degeneration in an established cohort of de novo, unmedicated PD and matched healthy controls at baseline and longitudinally. We extended our study by including subjects with polysomnography (PSG)-verified iRBD in an exploratory approach. We further analyzed (ii) correlations with clinical data to evaluate the potential of these evaluated markers in routine clinical diagnostics and for monitoring therapeutic effects. The data were further tested for their potential to (iii) predict progression of motor and non-motor symptoms. Linear mixed modeling was performed to assess longitudinal changes and progression. Finally, we applied machine-learning algorithms to evaluate how the different markers discriminate between groups.
PD is a progressive neurodegenerative disease with synaptic loss28. Markers of synaptic dysfunction are increased in the CSF28 of patients with Alzheimer’s disease, while the levels of many markers (e.g. AP2B1, LAMP2, Secretogranin-2) were decreased in a cross-sectional PD cohort33.
In our approach, longitudinal samples of prodromal and de novo PD (NSD-ISS Stage 4 at baseline) subjects were assessed, identifying nine markers that were significantly decreased in CSF samples of PD subjects compared to healthy controls (VGF, APP, NPTX1/2 and NPTXR, neurogranin, secretogranin-2, syntaxin-7 and AP2B1). Interestingly, all these nine markers were lower in the iRBD, but only secretogranin-2 was already significantly decreased in prodromal iRBD (NSD-ISS stage 2 A/B). As we discuss below, the role of secretogranin-2 in vesicle-mediated transport could be important in early pathophysiological steps when αSyn aggregation takes place.
The correlation analysis of all measured markers showed a strong relationship between 20 markers and the baseline MDS-UPDRS part I score (that is, a mix of non-motor symptoms, like dementia, but also other features like sleep and depression, etc.). These included NPTX1 and the corresponding receptor, neurogranin, secretogranin-2, β- and γ-synuclein, and syntaxin-1B and 7.
From the MoCA score (cognition only), the domain “language sentence repetition” correlated the most with nine markers, including neuronal pentraxins, secretogranin-2, and the syntaxins. Syntaxin-1B and 7 also correlated positively with MDS-UPDRS parts I-III and total score. Cathepsin-F was the only marker correlating with several DAT-SPECT parameters.
As the longitudinal DeNoPa cohort collects extensive data every two years we analyzed longitudinal CSF samples over 10 years. The CSF levels of proteins, that were significantly differentially expressed at BL, remained at a stable expression level over time and showed no further significant change. The linear mixed model estimated the influence of dopaminergic drug intake, but found no significant correlation between the LEDD and the marker levels. As previously shown in many different studies, age and sex significantly influenced the model34. LAMP2 and γ-synuclein were the markers with the highest predictive potential regarding the progression of the MDS-UPDRS total score as well as its subscores (I, II, and III). The additional machine-learning approaches via the Boruta algorithm showed that the proteins VGF and NPTXR were most powerful in discriminating between PD and healthy controls.
Below we discuss the markers according to their biological function:
The neuronal pentraxins (NPTX1, NPTX2, NPTXR) are widely expressed at excitatory synapses and are very important in synaptic plasticity and in the clustering of glutamate receptors35.
Lower levels of the secreted glycoproteins NPTX1 and NPTX2 are related to more severe non-motor PD symptoms and cognitive deficits, measured by MDS-UPDRS part I, supporting previous findings in PD33,36,37.
NPTX1/2 is upregulated in the substantia nigra of PD subjects while NPTXR is downregulated. NPTX2, similar to αSyn, is present in Lewy bodies in the substantia nigra38. Lower NPTX2 CSF levels in PD could be connected to the accumulation in Lewy bodies, similarly to αSyn. NPTXR contributed significantly to our machine-learning model.
Neurogranin is a post-synaptic protein, a neuronal injury marker connected to calmodulin regulation. Higher CSF levels of neurogranin have been reported in Alzheimer’s disease19,21. However, in contrast to Alzheimer’s disease, CSF levels in PD were lower in our study compared to healthy controls and positively correlated with MDS-UPDRS part I.
In this study, CSF levels of markers associated with synaptic plasticity and presynaptic functioning showed lower values in PD compared to healthy controls, and correlated mostly with non-motor symptoms. Synaptic dysfunction seems to be already present in prodromal and early disease stages. It reflects motor as well as non-motor symptoms, including cognitive decline, which is in line with previous studies in neurodegenerative disorders28,39.
Neurosecretory protein VGF is a member of the chromogranin/secretogranin family. It regulates secretory pathways.
VGF levels were lower in PD in our study and the protein was marked as important by the machine-learning model, revealing a discriminatory potential between early PD and healthy controls. Available data for VGF reports decreased levels in dementia with Lewy bodies, amyotrophic lateral sclerosis, Alzheimer’s disease, and brain tissue of PD subjects33,40. VGF is also decreased in patient plasma samples. In a 6-hydroxydopamine-(6OHD)-induced lesions rat model (a common experimental PD animal model with lesions in the medial forebrain bundle), brain tissue and plasma samples were reduced compared with controls. Interestingly, these were restored by levodopa treatment41.
Secretogranin-2 regulates the packing and sorting of peptide hormones and neuropeptides into secretory vesicles and is generally considered a marker for secretory granules, dense-core vesicles in neurons and neuroendocrine cells42. Secretogranin-2 CSF levels were not only significantly lower in PD but also in iRBD compared with healthy controls, with the PD levels lying in between. They were also positively correlated with MDS-UPDRS part I and MoCA domain “language sentence repetition”.
Recently, the SCG2 gene was reported as a signal integrator of glutamate and dopamine inputs43. Lower CSF levels have been shown in PD as well as PD with GBA-(lysosomal enzyme glucocerebrosidase) mutation, the most common genetic risk factor for PD33. In a 6OHD rat model, increased SCG2 mRNA levels were found in brain tissue samples and validated via immunohistochemical staining. In addition, these levels even increased during chronic levodopa treatment43. Nevertheless, in our linear mixed-model analysis LEDD did not significantly influence Secretogranin-2 or VGF CSF levels.
The validation of decreased Secretogranin-2 levels in iRBD points towards its role early in the disease course during the loss of synapses and preceding neuronal degeneration. Its function is strongly connected to vesicle-mediated transport and lysosomal dysfunction, leading to a potential relation to αSyn and its aggregation.
The proteins syntaxin-7 and syntaxin-1B are relevant for vesicle trafficking and higher CSF levels of the syntaxins have been reported in Alzheimer’s disease44. Here, PD subjects had lower CSF levels and the markers also correlated positively with MDS-UPDRS part I, II, III and total score and the MoCA domain “language sentence repetition” and were predictive of MDS-UPDRS II and STX-7, as well as MDS-UPDRS part III and total score. Therefore, secretion and vesicular trafficking seem to reflect parts of PD pathophysiology, especially regarding the correlation with the clinical picture33.
Endolysosomal function and chaperon-mediated autophagy are key components of PD pathology33,45. Several markers are involved in these pathways according to our panel. Previous data reported increased LAMP2 CSF levels in Alzheimer’s disease and lower in PD compared to controls46. Here, LAMP2 levels were not significantly different between the groups but seemed to be a strong predictor for the clinical outcome in PD. LAMP2 CSF levels at baseline were predictive of higher scores in MDS-UPDRS I-III and the total score over the ten years of follow-up.
SAP3 is a lysosomal protein that catalyzes the degradation of gangliosides and is involved in α-synuclein proteostasis46. SAP3 levels were associated with worse clinical performance in PD, indicated by the positive correlations with MDS-UPDRS part I and total score and the MoCA domain “language sentence repetition”. Previous data reported higher CSF levels in Alzheimer’s disease and dementia with Lewy bodies and lower levels in PD33.
Cathepsin-F was predictive for MDS-UPDRS parts II, III, and total score and correlated with the DAT-SPECT quotient of N. caudatus and striatum on both sides. It is a member of the papain-like cysteine protease family. It is involved in protein degradation after endocytosis and presentation of protein fragments via major histocompatibility complex (MHC) class II to T lymphocytes47. Therefore, proteins of αSyn proteostatis are already altered in the early disease stages and are linked to immunological presentation, which is congruent with the known activity of αSyn-specific T-cell populations both prior to and at the onset of motor symptoms48.
Besides the illustration of specific disease pathways, biomarkers have clinically relevant implications. We found several markers, whose baseline CSF levels were correlated with motor and non-motor clinical symptoms, that were associated with clinical outcomes. MDS-UPDRS part I correlated with 20 proteins. It rates non-motor symptoms in PD including cognitive impairment, hallucinations, affective symptoms like anxiety and depression, sleeping problems, and vegetative symptoms like urinary problems, which are known to significantly impair disease-related quality of life49. Synaptic dysfunction seems to affect pronounced non-motor aspects of the disease, which is in line with findings showing a connection between synaptic dysfunction and cognitive decline in Alzheimer’s disease26.
Regarding prediction, LAMP2 levels were strongly connected with the performance of all MDS-UPDRS subscores and the total score. Cathepsin-F was associated with the outcome of all but part I of the MDS-UPDRS and correlated with DAT-SPECT results. LAMP2 seems to be an ideal marker to predict disease progression over time, for motor and non-motor symptoms, independent of drug therapy. Previous data showed that αSyn preformed fibrils impair autophagy flux resulting in the degradation of LAMP2 in activated microglia, forming a direct connection between synaptic dysfunction, αSyn pathology and neuroinflammation50.
As the described synaptic markers are correlated with cognitive functioning and are prominently altered in Alzheimer’s disease, it is interesting, that many correlated with the MoCA score, especially the part “language sentence repetition”. Studies show that sentence repetition and generation are altered in PD before51. Multiple markers from our panel correlated with pTau. This can be due to overall neurodegeneration, but it could also be speculated, that pTau itself is involved in synaptic dysfunction. Our panel showed more correlations with clinical parameters like MDS-UPDRS as pTau did. As it is indicated by previous publications in AD52, a possible future development could be combined panels of new markers with established biomarkers like pTau and β-amyloid could be an effective tool in patients’ stratification.
A strength of our study is the validated and established protein panel as well as the established study cohort. We present, longitudinal, high quality and deeply phenotyped clinical data from prodromal and PD subjects staged with the new NSD-ISS. This has been previously recommended by researchers in the field33.
Limitations include the small number of iRBD subjects in the exploratory group, a point we will address with our ongoing iRBD cohort in the near future. Other possible limitations are the lack of validation in corresponding brain or blood samples, and the panel itself. This may not be fully representative of all relevant autolysosome and synaptic processes in PD.
We included 104 PD subjects in our analysis, 100 with an available α-synuclein SAA testing. 13 (87%) of these PD subjects showed a positive αSyn-SAA result.
Possible reasons for the negative SAA results include inaccuracy in the Parkinson’s diagnosis (around 50% of the initial PD diagnosis in untreated subjects are wrong53), the sensitivity (82.6%) and specificity (88.2%) of CSF α-synuclein SAA at the time point of the initial diagnosis in other cohorts54,55 and the possible presence of genetic variants such as LRRK2. LRRK2 patients show less α-synuclein pathology and significantly fewer positive SAA results56. Our longitudinal cohort will be extensively genetically characterized in the future (thru GP2) and as part of our established brain donation program, we will receive some autopsy-conformed diagnoses in the future.
Autophagy, and lysosomal and synaptic dysfunction play a relevant role in PD pathology, but not all known synaptic markers showed the same importance in PD and prodromal subjects. LAMP2, Cathepsin-F and the Syntaxins were most predictive for the clinical outcome and progression over time. Neurosecretory protein VGF and the Neuronal pentraxin receptor were most able to discriminate the groups in our machine learning models and correlated with clinical measures. VGF showed already reduced levels in prodromal iRBD.
The validation of these markers in a larger cohort of prodromal PD subjects would be a promising way to assess new biomarker candidates for clinical trials and possible translation into clinical practice in the future.
Methods
The DeNoPa cohort
Recently diagnosed patients with PD and matched healthy controls were enrolled at the Paracelsus-Elena-Klinik, Kassel, Germany between 2009 and 2012. Participants had to be aged between 40 and 85 years old with newly diagnosed PD with at least two of the following criteria: resting tremor, bradykinesia, and rigidity according to UK Brain Bank Criteria; UKBBC)57. To be eligible for inclusion, participants had to meet the criteria for de novo PD: any exposure to L-dopa had to have been less than 2 weeks and not within the 4 weeks prior to study entry29. Reasons for exclusion were severe vascular encephalopathy, normal-pressure hydrocephalus (NPH) shown on magnetic resonance imaging (MRI) (when available at screening or when detected during imaging studies), evidence for multiple system atrophy (MSA) or progressive supranuclear palsy (PSP) as well as medication-induced PD.
Healthy individuals between 40 and 85 years, matched to the PD group by age, sex, and education level showing no pathological condition of the central nervous system and a negative family history of PD were included as controls. Biannual longitudinal clinical data were collected at baseline and at 2, 4, 6, 8, and 10 years of follow-up in 104 PD and 94 healthy controls (flow-chart see Supplementary Fig. 1a/b)29. Motor function in DeNoPa was assessed with the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) parts I, II, III, and total score. Cognitive decline was assessed using the Mini-Mental-State Examination (MMSE) and MoCA (Montreal cognitive assessment) in all patients at baseline, and 2, 4, 6, 8, and 10 years of follow-up.
Clinical diagnosis was reassessed at 24 month follow-up in the On-state for all patients at each follow-up by consensus of two teams of independent neurologists (CT/FS-D and BM/JE) and based on clinical examination and impression, reassessment of UPDRS, the effectiveness of dopaminergic medication to motor symptoms and the evaluation of cognitive decline, hallucinations, autonomic dysfunction and symptoms indicative for atypical PD58.
iRBD was diagnosed through video polysomnography by experienced raters (CT, FS-D, MLM) on two consecutive nights according to established criteria59,60. The LEDD (levodopa equivalent daily dosage) was calculated based on Tomlison et al.61.
The iRBD cohort is part of the DeNoPa cohort, designed as a single center, longitudinal, observational study, still ongoing and recruiting subjects in iRBD, but not in PD and HC. Follow-up visits take place every two years for each single subject, explaining the differing numbers between the follow-up visits in the flow chart. Supplementary Fig. 1b displays the current numbers at the time point of submission. Dropouts are subjects that decided not to continue in the study or did not have an RBD confirmed by video polysomnography (vPSG). Additional information on the iRBD subjects is shown in Supplementary Table 7, including the assessment of hyposmia/anosmia indicated by Sniffin sticks´ T(reshold)D(iscrimination)I(identification) score ( < 30 indicative for hyposmia), Becks Depression Inventory indecx (BDI; > 8 can indicate a depressive episode), Parkinson’s disease non-motor symptoms scale (PD-NMS; <10 describes mild non-motor symptoms), Mini-mental-state examination (MMSE; < 27 indicative for possible cognitive impairment).
Final diagnosis refers to the consensus diagnosis that was made up to 10 years of follow-up, including initial dopamine-transporter–single-photon emission computed tomography (DAT-SPECT), biannual clinical evaluations, levodopa challenge, lasting response to levodopa, and the emergence of advanced PD features such as motor fluctuations or levodopa–induced dyskinesias. Abnormal/pathological DAT-SPECT was determined by a specialist in nuclear medicine by visual inspection or quantification.
Sample collection
This study includes CSF samples taken at baseline and at the follow-up visits every two years. CSF was collected in polypropylene tubes (Sarstedt, Nümbrecht, Germany) directly after the plasma collection by lumbar puncture in the sitting position. Tubes were centrifuged at 2500 g at room temperature (20 °C) for 10 min and aliquoted and frozen within 30 min after collection at −80 °C until analysis. Before centrifugation, white and red blood cell counts in CSF were determined manually29,58. CSF β-amyloid 1–42, total tau protein (t-tau), phosphorylated tau protein (p-tau181) and neurofilament light chains (NFL) concentrations were measured by board-certified laboratory technicians, who were blinded to clinical data, using commercially available INNOTEST ELISA kits for the tau and Aβ markers (Fujirebio Europe, Ghent, Belgium) and the UmanDiagnostics NF-light® assay (UmanDiagnostics, Umeå, Sweden) for NFL. Total protein and albumin levels were measured by nephelometry (Dade Behring/Siemens Healthcare Diagnostics)58.
For the α-synuclein seeding aggregation assay (αSyn-SAA) the CSF samples were blindly analyzed in triplicate (40 μL/well) in a reaction mixture (0.3 mg/mL recombinant α-Syn (Amprion [California, USA]; catalog number S2020), 100 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) pH 6.50, 500 mM sodium chloride, 10 μM thioflavin T, and one bovine serum albumin (BSA)–blocked 2.4 mm silicon nitride G3 bead (Tsubaki-Nakashima [Georgia, USA]). Beads were blocked in 1% BSA 100 mM PIPES pH 6.50 and washed with 100 mM PIPES pH 6.50. The assay was performed in 96-well plates (Costar [New York, USA], catalog number 3916) using a FLUOstar Omega fluorometer (BMG [Ortenberg, Germany]). Plates were orbitally shaken (800 rpm for 1 min every 29 min at 37 °C). Results from the triplicates were considered input for a three-output probabilistic algorithm with sample labeling as “positive,” “negative,” or “inconclusive”, based on the parameters: Maximum fluorescence (Fmax), time to reach 50% Fmax (T50), slope, and the coefficient of determination for the fitting were calculated for each replicate using a sigmoidal equation available in Mars data analysis software (BMG). The time to reach the 5000 relative fluorescence units (RFU) threshold (TTT) was calculated with a user-defined equation in Mars62.
Consent to collect CSF samples was not successful for all the subjects, see Table 1 and Supplementary Table 4 for details.
Sample processing and storing followed a very strict protocol29. CSF samples were frozen in a period of less than 30 min and stored at −80 C° in aliquots for single use only. An electrical monitoring system ensures and guarantees a constant temperature, continuously documented and checked. For analysis, single use aliquots were shipped on dry ice.
Neuronal α-synuclein-disease integrated staging system (NSD-ISS)
Neuronal αSyn-disease is defined by the biological anchors S: presence of in vivo detected pathologic αSyn species, measured usually by αSyn seeding aggregation assays, (independent from clinical syndrome) and D: dopaminergic neuronal dysfunction, assessed by DAT-SPECT, leading to a proposed NSD Integrated Staging System (NSD-ISS) that includes clinical signs and symptoms. Stages 0−1 are defined by the presence of pathogenic variants in SNCA gene (Stage 0), S alone (Stage 1 A), or S and D (Stage 1B) without clinical signs/symptoms. The occurrence of clinical manifestations defines the transition to Stage 2 and higher. Stage 2 includes subtle signs/symptoms without functional impairment. Stages 2B-6 require S and D to be positive and for there to be stage-specific increases in functional impairment31. NSD-ISS was applied in all 88 participants where αSyn-SAA and DAT-SPECT were available (Supplementary Table 5).
Statistical analysis
All analyses were performed with the statistical software R (version 4.0.5). Baseline continuous variables were expressed as mean (standard deviation), median, and the range as given by the minimum and maximum values. Group comparisons were performed using the nonparametric Mann–Whitney Kruskall-Wallis test because some of the parameters had nonnormal distribution. For the binary variable “sex”, the count in each category is provided, and the Fisher exact Chi-square tests were used for comparison. Differential expression was assessed using the empirical Bayes approach as implemented in the Bioconductor limma package. Multiple
hypothesis testing corrections were performed by using Benjamini and Hochberg’s (BH) false discovery rate at a = 5%. Linear mixed models were used for longitudinal data analysis that allowed fitting only for random intercept models. They were implemented using the function lmer from the cran package lmerTest. The correlation between the assessed proteins and the clinical parameters was assessed via a nonparametric Spearman coefficient using the base R function cor.test from the cran psych package. Here again, the BH procedure was used to correct for multiplicity. To ensure a capture of the performance of every single marker in the longitudinal cohort in this first assessment of this panel in longitudinal de novo PD, we decided to not perform a dimension reduction.
For machine learning, the Boruta algorithm from the CRAN package Boruta was used, and algorithms were built around the random forest classification algorithm. It aims to capture all the relevant features in the dataset concerning the outcome variables of PD versus healthy controls. The algorithm adds randomness to the dataset by creating shuffled copies of all features (Shadow Features) and trains a random forest classifier on the extended dataset (original attributes plus shadow attributes), applying a feature importance measure (The Mean Decrease Accuracy), evaluating the importance of each feature. At every iteration, the Boruta algorithm checks whether a real feature is more important than others and removes features that are marked as highly unimportant. As a stopping rule, we used 100,000 iterations with a maximum of 500 random forests as indicated by the parameter maxRuns in the Boruta function. With actual CRAN implementation of Boruta, warm-up rounds are removed, and the multiple testing corrections are introduced, marking all features that are either strongly or weakly relevant to PD diagnosis.
Handling of missing data
The amount of missing data was small with n = 133 of overall n = 14421 measurements (0.92% of the missing cases) with less than 10% of the peptide show missing values. Most contributor was the protein LAMP2, especially on the last plate, thus it was excluded, concerning n = 35*2 peptides. The QCs can be found in Supplementary Table 1.
Supplementary Fig. 2 provide heat maps of the missing values across all time points and diagnosis.
Limma handles missing values like other linear model functions in R (missing at random (MAR)). For each peptide in the data matrix, the cases with missing values are removed from the data and the design matrix and the linear model is fitted to the non-missing values. If a particular regression coefficient cannot be estimated from the observed data for a particular peptide, then an NA value will be returned for that.
The Random forests (RF) use median imputation for missing non categorial variables. This has been proven as an effective imputation technique for MAR with a moderate amount of missing values63.
Standard protocol approvals, registrations, and patient consent
Approval was received from the local ethical standards committee on human experimentation for all human participants in all cohorts (FF 89/2008, FF 130/2012, MC 310/2010). Written informed consent for research was obtained from all study participants. DeNoPa is registered in the German Register for Clinical trials (DRKS00000540),
LC-MS/MS analysis
In summary, a mix of heavy standard peptides serving as an internal standard (JPT Peptide Technologies (Berlin, Germany; SpikeTides L). 25 µL (for concentration see Supplementary Table 1A) was added to 100 µL of CSF samples, which were then reduced, alkylated, digested, and desalted. The quantification was performed using liquid chromatography-tandem mass spectrometry (LC-MS/MS) with a micro-high-performance LC-MS/MS system (6495 Triple Quadrupole LC/MS system, Agilent Technologies) equipped with a Hypersil Gold reversed-phase column (100 × 2.1 mm, 1.9 µm particle size, Thermo Fisher Scientific). The spectra can be found in Supplementary Figure 5. For further details, see Supplementary Table 2, which describes the settings used. For detailed sample preparation, we refer to the following publications26,28.The method involved the measurement of a panel of 38 synaptic and lysosomal proteins as indicated in Table 2. The target selection and compilation of the panel is based on unbiased explorative quantitative proteomic approaches in AD and PD, that indicated the proteins as potential biomarkers for lysosomal and synaptic dysfunction in neurodegeneration. The panel was further refined after targeted application in different cohorts including PD33,40,64,65.
To monitor the assay’s performance, two different quality control (QC) samples comprising CSF pools were periodically injected, where one of them was used to adjust for potential plate differences (batches) and the second to evaluate the final analytical performance. Longitudinal samples from the same patients were run randomized on the same plates in order to minimize batch and run time variability. The analytical performance of the different proteins had a high precision within and between runs with a few exceptions (Supplementary Table 1A). Skyline 20.1 (MacCoss Lab Software) was utilized to analyze the mass spectrometric data. One peptide, the one with the best analytic performance, per target protein was selected for statistical analyses.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
H.Z. is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2022-01018 and #2019-02397), the European Union’s Horizon Europe research and innovation programme under grant agreement No 101053962, Swedish State Support for Clinical Research (#ALFGBG-71320), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the Alzheimer’s disease Strategic Fund and the Alzheimer’s Association (#ADSF-21-831376-C, #ADSF-21-831381-C, and #ADSF-21-831377-C), the Bluefield Project, the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022-0270), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), the European Union Joint Programme – Neurodegenerative Disease Research (JPND2021-00694), the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre, and the UK Dementia Research Institute at UCL (UKDRI-1003). We acknowledge support by the Open Access Publication Funds/transformative agreements of the Göttingen University.
Author contributions
M.B., B.M.: Conceptualization, Data acquisition, Data curation, Methodology, Writing –original draft. J.N., B.G. A.B.: Conduction of mass spectrometry, Conceptualization, Supervision, Writing – review & editing. M.D.: Data curation, Formal analysis, Methodology,– review & editing. M.E., C.T., H.Z., F.S.D.: Supervision, – review & editing. S.W.: Figure design. S.S., S.W., M.X., M.L.M.: Data acquisition – review & editing. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files. Additional information can be provided on reasonable request. Patient samples can be provided to other researchers for certain projects after contact with and upon availability approval of the team in Kassel.
Competing interests
M.B. has received funding from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – 413,501,650. C.T. has received honoraria for consultancy from Roche, and honoraria for educational lectures from UCB, and has received research funding for the PPMI study from Michael J. Fox Foundation and funding from the EU (Horizon 2020) and stipends from the (International Parkinson’s and Movement DisorderSociety) IPMDS. B.M. has received honoraria for consultancy from Roche, Biogen, AbbVie, UCB, and Sun Pharma Advanced Research Company. BM is a member of the executive steering committee of the Parkinson Progression Marker Initiative and PI of the Systemic Synuclein Sampling Study of the Michael J. Fox Foundation for Parkinson’s Research and has received research funding from the Deutsche Forschungsgemeinschaft (DFG), EU (Horizon 2020), Parkinson Fonds Deutschland, Deutsche Parkinson Vereinigung, Parkinson’s Foundation and the Michael J. Fox Foundation for Parkinson’s Research. M.L.M. has received honoraria for speaking engagements from Deutsche Parkinson Gesellschaft e.V. and royalties from Gesellschaft fur Medien + Kommunikation mbH + Co. FSD has received honoraria for speaking engagements from AbbVie, Bial, Ever Pharma, Medtronic and royalties from Elsevier and Springer. She served on an advisory board for Zambon and Stada Pharma. F.S.D. participated in Ad Boards for consultation: Abbvie, UCB, Bial, Ono, Roche and got honorary for lecturing: Stada Pharm, Abbvie, Alexion, Bial. S.S. received institutional salaries supported by the EU Horizon 2020 research and innovation program under grant agreement No. 863664 and by the Michael J. Fox Foundation for Parkinson’s Research under grant agreement No. MJFF-021923. He is supported by a PPMI Early Stage Investigators Funding Program fellowship of the Michael J. Fox Foundation for Parkinson’s Research under grant agreement No. MJFF-022656. H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). M.D., M.X., S.W., M.E., A.B., J.N., B.G. have no competing interests to report.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/25/2024
A Correction to this paper has been published: 10.1038/s41531-024-00726-x
Supplementary information
The online version contains supplementary material available at 10.1038/s41531-024-00714-1.
References
- 1.Mollenhauer B, et al. Longitudinal analyses of cerebrospinal fluid α-Synuclein in prodromal and early Parkinson’s disease. Mov. Disord. 2019;34:1354–1364. doi: 10.1002/mds.27806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall S, et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch. Neurol. 2012;69:1445–1452. doi: 10.1001/archneurol.2012.1654. [DOI] [PubMed] [Google Scholar]
- 3.Kang UJ, et al. Comparative study of cerebrospinal fluid α-synuclein seeding aggregation assays for diagnosis of Parkinson’s disease. Mov. Disord. 2019;34:536–544. doi: 10.1002/mds.27646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mollenhauer B, et al. Validation of Serum Neurofilament Light Chain as a Biomarker of Parkinson’s Disease Progression. Mov. Disord. 2020;35:1999–2008. doi: 10.1002/mds.28206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinzel S, et al. Update of the MDS research criteria for prodromal Parkinson’s disease. Mov. Disord. 2019;34:1464–1470. doi: 10.1002/mds.27802. [DOI] [PubMed] [Google Scholar]
- 6.Simuni T, et al. Baseline prevalence and longitudinal evolution of non-motor symptoms in early Parkinson’s disease: the PPMI cohort. J. Neurol. Neurosurg. Psychiatry. 2018;89:78–88. doi: 10.1136/jnnp-2017-316213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Högl B, Stefani A, Videnovic A. Idiopathic REM sleep behaviour disorder and neurodegeneration - an update. Nat. Rev. Neurol. 2018;14:40–55. doi: 10.1038/nrneurol.2017.157. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira LMA, et al. Alpha-synuclein research: defining strategic moves in the battle against Parkinson’s disease. NPJ Parkinsons Dis. 2021;7:65. doi: 10.1038/s41531-021-00203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulz I, et al. Systematic Assessment of 10 Biomarker Candidates Focusing on α-Synuclein-Related Disorders. Mov. Disord. 2021;36:2874–2887. doi: 10.1002/mds.28738. [DOI] [PubMed] [Google Scholar]
- 10.Siderowf A, et al. Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study. Lancet Neurol. 2023;22:407–417. doi: 10.1016/S1474-4422(23)00109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McFarland NR. Diagnostic Approach to Atypical Parkinsonian Syndromes. Contin. (Minneap. Minn.) 2016;22:1117–1142. doi: 10.1212/CON.0000000000000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soukup SF, Vanhauwaert R, Verstreken P. Parkinson’s disease: convergence on synaptic homeostasis. Embo J. 2018;37:e98960. doi: 10.15252/embj.201898960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robak LA, et al. Excessive burden of lysosomal storage disorder gene variants in Parkinson’s disease. Brain: a J. Neurol. 2017;140:3191–3203. doi: 10.1093/brain/awx285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boman A, et al. Distinct Lysosomal Network Protein Profiles in Parkinsonian Syndrome Cerebrospinal Fluid. J. Parkinsons Dis. 2016;6:307–315. doi: 10.3233/JPD-150759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez-Erviti L, et al. Chaperone-mediated autophagy markers in Parkinson disease brains. Arch. Neurol. 2010;67:1464–1472. doi: 10.1001/archneurol.2010.198. [DOI] [PubMed] [Google Scholar]
- 16.Murphy KE, et al. Lysosomal-associated membrane protein 2 isoforms are differentially affected in early Parkinson’s disease. Mov. Disord. 2015;30:1639–1647. doi: 10.1002/mds.26141. [DOI] [PubMed] [Google Scholar]
- 17.Brinkmalm A, et al. SNAP-25 is a promising novel cerebrospinal fluid biomarker for synapse degeneration in Alzheimer’s disease. Mol. Neurodegener. 2014;9:53. doi: 10.1186/1750-1326-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Öhrfelt A, et al. The pre-synaptic vesicle protein synaptotagmin is a novel biomarker for Alzheimer’s disease. Alzheimers Res Ther. 2016;8:41. doi: 10.1186/s13195-016-0208-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kvartsberg H, et al. Cerebrospinal fluid levels of the synaptic protein neurogranin correlates with cognitive decline in prodromal Alzheimer’s disease. Alzheimers Dement. 2015;11:1180–1190. doi: 10.1016/j.jalz.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Hall S, et al. Cerebrospinal fluid levels of neurogranin in Parkinsonian disorders. Mov. Disord. 2020;35:513–518. doi: 10.1002/mds.27950. [DOI] [PubMed] [Google Scholar]
- 21.Portelius E, et al. Cerebrospinal fluid neurogranin concentration in neurodegeneration: relation to clinical phenotypes and neuropathology. Acta Neuropathol. 2018;136:363–376. doi: 10.1007/s00401-018-1851-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wellington H, et al. Increased CSF neurogranin concentration is specific to Alzheimer disease. Neurology. 2016;86:829–835. doi: 10.1212/WNL.0000000000002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sjögren M, et al. The cerebrospinal fluid levels of tau, growth-associated protein-43 and soluble amyloid precursor protein correlate in Alzheimer’s disease, reflecting a common pathophysiological process. Dement Geriatr. Cogn. Disord. 2001;12:257–264. doi: 10.1159/000051268. [DOI] [PubMed] [Google Scholar]
- 24.Bereczki E, et al. Synaptic proteins in CSF relate to Parkinson’s disease stage markers. NPJ Parkinsons Dis. 2017;3:7. doi: 10.1038/s41531-017-0008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brinkmalm A, et al. Cerebrospinal fluid levels of SNAP-25 and SYT1 in Alzheimer’s and Parkinson’s disease. Alzheimer’s Dement. 2020;16:e044515. doi: 10.1002/alz.044515. [DOI] [Google Scholar]
- 26.Nilsson J, et al. Cerebrospinal fluid biomarker panel for synaptic dysfunction in Alzheimer’s disease. Alzheimers Dement (Amst.) 2021;13:e12179. doi: 10.1002/dad2.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsson J, et al. Cerebrospinal Fluid Biomarkers of Synaptic Dysfunction Are Altered in Parkinson’s Disease and Related Disorders. Mov. Disord. 2022;38:267–277. doi: 10.1002/mds.29287. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson J, et al. Cerebrospinal fluid biomarker panel of synaptic dysfunction in Alzheimer’s disease and other neurodegenerative disorders. Alzheimer’s Dement. 2022;19:1775–1784. doi: 10.1002/alz.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mollenhauer B, et al. Nonmotor and diagnostic findings in subjects with de novo Parkinson disease of the DeNoPa cohort. Neurology. 2013;81:1226–1234. doi: 10.1212/WNL.0b013e3182a6cbd5. [DOI] [PubMed] [Google Scholar]
- 30.Chahine LM, et al. Proposal for a Biologic Staging System of Parkinson’s Disease. J. Parkinsons Dis. 2023;13:297–309. doi: 10.3233/JPD-225111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simuni T, et al. Biological Definition of Neuronal alpha-Synuclein Disease: Towards an Integrated Staging System for Research. Lancet Neurol. 2023;23:178–190. doi: 10.1016/S1474-4422(23)00405-2. [DOI] [PubMed] [Google Scholar]
- 32.Höglinger GU, et al. A biological classification of Parkinson’s disease: the SynNeurGe research diagnostic criteria. The Lancet. Neurology. 2024;23:191–204. doi: 10.1016/S1474-4422(23)00404-0. [DOI] [PubMed] [Google Scholar]
- 33.Lerche S, et al. CSF Protein Level of Neurotransmitter Secretion, Synaptic Plasticity, and Autophagy in PD and DLB. Mov. Disord. 2021;36:2595–2604. doi: 10.1002/mds.28704. [DOI] [PubMed] [Google Scholar]
- 34.Bartl M, et al. Longitudinal Change and Progression Indicators Using the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale in Two Independent Cohorts with Early Parkinson’s Disease. J. Parkinsons Dis. 2021;12:437–452. doi: 10.3233/JPD-212860. [DOI] [PubMed] [Google Scholar]
- 35.Gómez de San José N, et al. Neuronal pentraxins as biomarkers of synaptic activity: from physiological functions to pathological changes in neurodegeneration. J. Neural Transm. (Vienna) 2022;129:207–230. doi: 10.1007/s00702-021-02411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu S, Bäckström D, Forsgren L, Trupp M. Alterations in Self-Aggregating Neuropeptides in Cerebrospinal Fluid of Patients with Parkinsonian Disorders. J. Parkinsons Dis. 2022;12:1169–1189. doi: 10.3233/JPD-213031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boiten WA, et al. Pathologically Decreased CSF Levels of Synaptic Marker NPTX2 in DLB Are Correlated with Levels of Alpha-Synuclein and VGF. Cells. 2020;10:38. doi: 10.3390/cells10010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moran LB, et al. Neuronal pentraxin II is highly upregulated in Parkinson’s disease and a novel component of Lewy bodies. Acta Neuropathol. 2008;115:471–478. doi: 10.1007/s00401-007-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camporesi E, et al. Fluid Biomarkers for Synaptic Dysfunction and Loss. Biomark. Insights. 2020;15:1177271920950319. doi: 10.1177/1177271920950319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rotunno MS, et al. Cerebrospinal fluid proteomics implicates the granin family in Parkinson’s disease. Sci. Rep. 2020;10:2479. doi: 10.1038/s41598-020-59414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cocco C, et al. VGF peptides as novel biomarkers in Parkinson’s disease. Cell Tissue Res. 2020;379:93–107. doi: 10.1007/s00441-019-03128-1. [DOI] [PubMed] [Google Scholar]
- 42.Wen G, et al. Proteomic characterization of secretory granules in dopaminergic neurons indicates chromogranin/secretogranin-mediated protein processing impairment in Parkinson’s disease. Aging (Albany N.Y.) 2021;13:20335–20358. doi: 10.18632/aging.203415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medhurst AD, et al. Up-regulation of secretoneurin immunoreactivity and secretogranin II mRNA in rat striatum following 6-hydroxydopamine lesioning and chronic L-DOPA treatment. Neuroscience. 2001;105:353–364. doi: 10.1016/S0306-4522(01)00190-7. [DOI] [PubMed] [Google Scholar]
- 44.Lleó A, et al. Changes in Synaptic Proteins Precede Neurodegeneration Markers in Preclinical Alzheimer’s Disease Cerebrospinal Fluid. Mol. Cell Proteom. 2019;18:546–560. doi: 10.1074/mcp.RA118.001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bandres-Ciga S, et al. The endocytic membrane trafficking pathway plays a major role in the risk of Parkinson’s disease. Mov. Disord. 2019;34:460–468. doi: 10.1002/mds.27614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sjödin S, et al. Endo-lysosomal proteins and ubiquitin CSF concentrations in Alzheimer’s and Parkinson’s disease. Alzheimers Res Ther. 2019;11:82. doi: 10.1186/s13195-019-0533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi GP, et al. Role for cathepsin F in invariant chain processing and major histocompatibility complex class II peptide loading by macrophages. J. Exp. Med. 2000;191:1177–1186. doi: 10.1084/jem.191.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindestam Arlehamn CS, et al. α-Synuclein-specific T cell reactivity is associated with preclinical and early Parkinson’s disease. Nat. Commun. 2020;11:1875. doi: 10.1038/s41467-020-15626-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goetz CG, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 50.Guo M, et al. Microglial exosomes facilitate α-synuclein transmission in Parkinson’s disease. Brain: a J. Neurol. 2020;143:1476–1497. doi: 10.1093/brain/awaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dick J, Fredrick J, Man G, Huber JE, Lee J. Sentence production in Parkinson’s disease. Clin. Linguist Phon. 2018;32:804–822. doi: 10.1080/02699206.2018.1444791. [DOI] [PubMed] [Google Scholar]
- 52.Nilsson, J. et al. Cerebrospinal fluid biomarker panel for synaptic dysfunction in a broad spectrum of neurodegenerative diseases. Brain: J. Neurol. (2024). Online ahead of print, 10.1093/brain/awae032. [DOI] [PMC free article] [PubMed]
- 53.Adler CH, et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology. 2014;83:406–412. doi: 10.1212/WNL.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oftedal L, Maple-Grødem J, Tysnes OB, Alves G, Lange J. Seed Amplification Assay as a Diagnostic Tool in Newly-Diagnosed Parkinson’s Disease. J. Parkinsons Dis. 2023;13:841–844. doi: 10.3233/JPD-230065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grossauer A, et al. α-Synuclein Seed Amplification Assays in the Diagnosis of Synucleinopathies Using Cerebrospinal Fluid-A Systematic Review and Meta-Analysis. Mov. Disord. Clin. Pr. 2023;10:737–747. doi: 10.1002/mdc3.13710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brockmann K, et al. CSF α-synuclein seed amplification kinetic profiles are associated with cognitive decline in Parkinson’s disease. NPJ Parkinsons Dis. 2024;10:24. doi: 10.1038/s41531-023-00627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson’s disease. Arch. Neurol. 1993;50:140–148. doi: 10.1001/archneur.1993.00540020018011. [DOI] [PubMed] [Google Scholar]
- 58.Mollenhauer B, et al. Monitoring of 30 marker candidates in early Parkinson disease as progression markers. Neurology. 2016;87:168–177. doi: 10.1212/WNL.0000000000002651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sateia MJ. International classification of sleep disorders. Chest. 2014;146:1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 60.Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- 61.Tomlinson CL, et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 62.Concha-Marambio L, et al. Accurate Detection of α-Synuclein Seeds in Cerebrospinal Fluid from Isolated Rapid Eye Movement Sleep Behavior Disorder and Patients with Parkinson’s Disease in the DeNovo Parkinson (DeNoPa) Cohort. Mov. Disord. 2023;38:567–578. doi: 10.1002/mds.29329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Breiman L. Random Forests. Mach. Learn. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 64.Nilsson J, et al. Cerebrospinal fluid biomarker panel of synaptic dysfunction in Alzheimer’s disease and other neurodegenerative disorders. Alzheimers Dement. 2023;19:1775–1784. doi: 10.1002/alz.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tijms BM, et al. Pathophysiological subtypes of Alzheimer’s disease based on cerebrospinal fluid proteomics. Brain: a J. Neurol. 2020;143:3776–3792. doi: 10.1093/brain/awaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files. Additional information can be provided on reasonable request. Patient samples can be provided to other researchers for certain projects after contact with and upon availability approval of the team in Kassel.