Abstract
Delayed cerebral ischemia (DCI) is a serious, life-threatening, complication affecting patients who have survived the initial bleeding from a ruptured intracranial aneurysm. Due to the challenging diagnosis, potential DCI prognostic markers should be of value in clinical practice. According to recent reports isoprostanes and red blood cell distribution (RDW) showed to be promising in this respect. We conducted a prospective study of 27 aSAH patients and control group (n = 8). All patients from the study group were treated within the first day of the initial bleeding. We collected data regarding clinical status and results of biochemical, and radiological examinations. We measured cerebrospinal fluid (CSF) concentration of 8-iso-prostaglandin F2α (F2-IsoP) and RDW on days 1, 3, and 5. Both CSF F2-IsoP level and RDW-SD measured on day 1 were significant predictors of DCI. The receiver operating characteristics curve for DCI prediction based on the multivariate model yielded an area under the curve of 0.924 (95% CI 0.824–1.000, p < 0.001). In our study, the model based on the combination of RDW and the level of isoprostanes in CSF on the first day after the initial bleeding showed a prognostic value for DCI prediction. Further studies are required to validate this observation.
Subject terms: Neuro-vascular interactions, Biomarkers, Neurology
Introduction
Aneurysmal subarachnoid haemorrhage (aSAH) is a serious, life-threatening medical condition. Roughly 10% of patients with aSAH die before receiving medical assistance and nearly 40% of them do not survive the first 30 days of hospitalisation1,2. Furthermore, one-third of the patients experience severe complications that can result in functional impairment3. One of the adverse events present in 20–30% of the SAH patients is delayed cerebral ischemia (DCI)4,5. The diagnosis of DCI is based on clinical evaluation, radiological imaging and laboratory tests. DCI is defined as the occurrence of new neurological deficit (e.g., apraxia, aphasia, hemi-inattention, hemiparesis) and/or deterioration in level of consciousness shown as a drop in Glasgow Coma Scale (GCS) of 2 or more points. These symptoms must last for at least 1 h and should not occur following the exclusion of aneurysm from circulation. The diagnosis of DCI is only possible after ruling out other potential causes of the deterioration, such as hypotension, electrolyte imbalance, infection or re-bleeding6–8. Although the specific pathomechanism of DCI remains unclear, many authors emphasise that its cause is complex and comprises microcirculatory disturbances, microthrombosis, inflammation, blood-barrier disruption, electrolyte imbalances and cerebral autoregulation disorders.
According to one of the most likely theories, metabolic changes associated with extravasated blood led to an inflammation in the arterial walls and oxidative stress (OS)9–11. OS is a condition marked by increased levels of toxic oxygen species (TOS), reactive oxygen species (ROS) and endogenous antioxidants. The elevated concentration of ROS, leads to predominance of oxidation reaction and finally, to disruption of redox signalling (RS)12. RS regulates “redox switches” which are diverse mechanisms which influence endothelium and vasoconstriction13–18. As a result, there are local perfusion disruptions which may be responsible for the clinical picture of DCI. Since ROS levels have been associated with F2-isoprostanes (F2-IsoPs), both have been postulated as oxidative stress markers19,20.
Another factor that has been associated with inflammation and thus with OS, confirmed in various diseases, is red blood cell (RBC) anisocytosis21–23, which may be expressed as a standard deviation (SD) or as a coefficient of variation (CV) of red cell distribution width (RDW)24. Uneven RBC size may be caused by oxidative stress, which is present after aSAH25. Disturbances in RBC size affect the microcirculation. Several studies have pointed to the role of the presence of microclots in cerebral infarction, and microthromboembolism has been postulated to be one of the mechanisms of DCI26,27. The RDW can be assessed as part of a routine complete blood count and is therefore easy to integrate into the daily routine of clinical practice. In an analysis conducted in 2022, we showed that SAH patients with poor outcomes had significantly greater RDW-CV on admission (13.9% vs 12.8%, p = 0.016)28.
Therefore, in this study, we investigated the potential value of cerebrospinal fluid (CSF) concentration of F2-isoprostanes (reflecting the oxidative stress) and erythrocyte anisocytosis (resulting in their reduced deformability, which affects the microcirculation) as predictors of DCI in aSAH patients.
Material and methods
Ethical statement
The local Ethical Committee of Medical University of Lodz gave a positive opinion on the research protocol (number RNN/280/13/KE). Written informed consent was obtained from patients prior to their inclusion in the study. In the case of unconscious patients, the consent was obtained in accordance with the regulations governing clinical studies as mandated by Polish law. The study was performed in accordance with Good Clinical Practice and the Declaration of Helsinki.
Patients
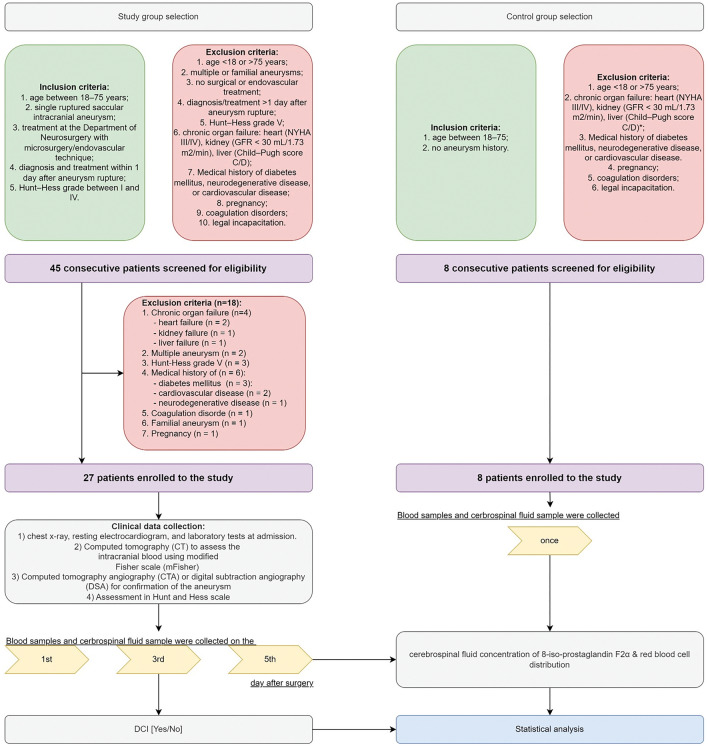
We performed a prospective analysis of 27 consecutive aSAH patients. The patients were operated on for a ruptured cerebral aneurysm within 24 h after the bleeding between May 2013 and January 2018. The patient information was collected as a part of an ongoing prospective database of clinical, biochemical, and radiological data from patients with confirmed aSAH. Moreover, we included a control group consisting of 8 volunteers (4 (50%) females). The inclusion and exclusion criteria for both groups are collected in Fig. 1.
Figure 1.
Algorithm of the study. CT computed tomography, CTA computed tomography angiography, DCI delayed cerebral ischemia, DSA digital subtraction angiography, GFR glomerular filtration rate, NYHA New York heart association classification.
Clinical assessment
After patient inclusion routine radiological (chest x-ray, resting electrocardiogram) and laboratory test were performed. In each case the amount of subarachnoid blood was confirmed on computed tomography (CT) according to Modified Fisher scale (mFisher) scale. Computed tomography angiography (CTA) or digital subtraction angiography (DSA) were performed to confirm the presence of the aneurysm. The patients’ clinical state was assessed according to the Hunt and Hess scale. A decision about the treatment strategy (microsurgery and endovascular intervention) was made by a multidisciplinary team. In each case we performed, routine neuroimaging examination at 12 h after aneurysm surgery.
Patients after aSAH have been subjected to the standard procedure for preventing DCI and vasospasm according to the applicable AHA recommendations29. Thus, in all patients after aSAH nimodypine was administered and euvolemia was maintained to prevent DCI. When clinical deterioration was observed the suspicion of DCI was taken into account. DCI was diagnosed in every case of otherwise unexplainable new symptoms of confusion or drop in the level of consciousness (by ≥ 2 point in GCS), with or without accompanying focal neurologic deficits. Therefore, the first step was to rule out other causes of patient's deterioration and to perform radiological diagnostics for the signs of vasospasm, via transcranial Doppler ultrasound and, if necessary, digital subtraction angiography. In most severe cases, in unconscious patients, intracranial pressure and cerebral perfusion pressure monitoring were used first.
The neurological examination was performed at discharge and after 1 and 12 months (in a routine out-patient clinic follow-up), by a neurosurgeon who did not have access to the study data. Glasgow Outcome Scale (GOS) and the modified Rankin Scale (mRS) were used30,31.
Specimen collection
CSF was obtained via lumbar puncture on days 1, 3, and 5 post-surgery. Blood samples were drawn each time from a new venipuncture, at intervals matching the CSF sampling. The samples were aliquoted then centrifuged for 10 min at 7000g to remove particulates and snap frozen in liquid nitrogen, and stored at − 80 °C until further analysis. Blood samples were collected on the 1st, 3rd and 5th day after surgery, and directly passed along to the laboratory for analysis. Regarding the control group, single CSF samples and blood samples were collected and processed in exactly the same way.
Detection of free form of F2-IsoPs (8-iso prostaglandin F2α)
We performed the analysis of 27 samples in the study group and in 8 samples in the control group. Free form of F2-IsoPs in CSF was quantified using STAT-8- Isoprostane ELISA Kit (Cayman Chemical, Ann Arbor, Michigan, USA). The analysis was conducted according to the manufacturer’s protocol. All samples were measured in duplicates. Synergy 2 Multi-Mode Reader (BioTek Instruments, Inc., Winooski, VT, USA) and the dedicated software were used for plate readings.
Detection of erythrocytes anisocytosis (RDW-CV; RDW-SD)
RDW-CV and RDW-SD were analysed in routine morphology tests performed after the operation. Plasma samples (5–10 mL of morning sample taken following ≥ 8 h of fasting) were collected from each patient into commercially available ethylenediaminetetraacetic acid (EDTA) treated tubes as a part of a routine examination.
Analytical recovery studies for 8-iso prostaglandin F2α, RDW-CV and RDW-SD
Analytical recovery studies were carried out using CSF samples from 3 different time points assessing the F2-IsoPs level from 3 different time points assessing the RDW-CV and RDW-SD, revealing that the recovery rate ranged from 95.9 to 97.8%.
Statistical analysis
The analysis was performed in R statistical software (version 4.3.1)32 and plots were generated using the ggplot2 package33. Shapiro–Wilk test was used to evaluate the normal distribution, while Levene’s test was used to verify the homogeneity of variance between groups. Continuous variables with normal distribution were expressed as means ± SD and were analysed using parametric tests (ANOVA with Tukey’s post-hoc test); otherwise, as the values were expressed as medians with interquartile range and were analysed with nonparametric tests (Kruskal–Wallis test with Dunn’s post-hoc test).
The predictive potential of single factors and more complex models were assessed using receiver operating characteristic (ROC) curves with calculation of the area under the curve (AUC) (using the pROC package34). The optimal thresholds were identified according to Youden’s index. Finally, we performed multivariable analysis using stepwise logistic regression; the final model was evaluated for stability using the bootstrap approach (using the Generalized Linear Models and boot package35–37). For all analyses, the p values < 0.05 were considered as statistically significant.
Sample size calculation
The minimal sample size required for this study was determined using initial data from a cohort of the first 5 aSAH patients, who developed DCI and first 5 who did not. This calculation was based on RDW-CV, RDW-SD, and CSF ISOP measured on the first day post-surgery. We have made additional assumptions: alpha of 0.05, power of 0.8, the ratio of DCI to nonDCI patients of 2:3. Furthermore, we decided to incorporate a 10% excess into our sample size calculation to account for potential data loss or variability. Consequently n = 25 was established as the minimum number required to ensure statistical robustness and validity of the study outcomes (Table S1).
Results
The patients’ clinical condition
As it was shown on Fig. 1, 45 patients were screened for eligibility and 27 were included in further investigations. Their clinical and laboratory data are summarized in Table S2.
The study group comprised 16 (59.26%) males, while the control group had 4 (50%) males. The aneurysms were located most often in the anterior part of the circle of Willis (n = 24 (88.9%). DCI was diagnosed in 13 (48.15%) patients and the median day of DCI onset was day 5. The DCI diagnosis was made by a multidisciplinary team, consisting of neurosurgeons and a radiologist.
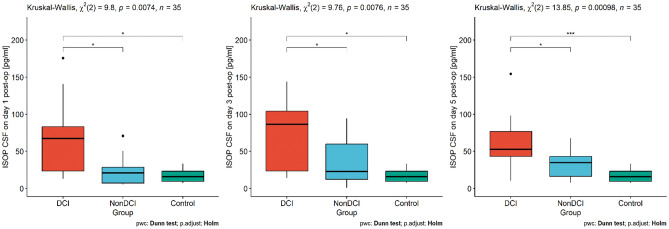
First, we assessed the dependencies between DCI, nonDCI and the control group in terms of RDW-SD, RDW-CV and F2-IsoP levels. Analysis indicated a statistically significant difference in RDW-CV between the tested groups (see Fig. 2). Moreover, we noted that CSF F2-IsoP levels on day 1 and day 5 were associated with DCI occurrence, comparing to non-DCI patients and the controls (see Fig. 3). Interestingly, on day 3 its concentration differs only between DCI and control group (see Fig. 3).
Figure 2.
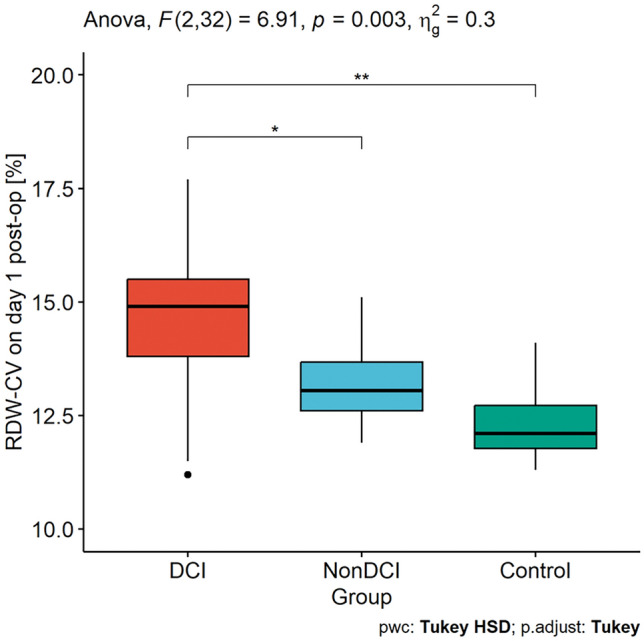
Differences in RDW-CV between the DCI, nonDCI and control groups. DCI delayed cerebral ischemia, RDW-CV red cell distribution width—coefficient of variation, *p < 0.05; **p < 0.01.
Figure 3.
The dependencies in CSF IsoP concentration between the DCI, nonDCI and control groups measured on 1st, 3rd, 5th day after surgery. CSF cerebrospinal fluid, DCI delayed cerebral ischemia, ISOP 8-iso-prostaglandin F2α, *p < 0.05; **p < 0.01; ***p < 0.001.
Next, we performed a univariate analysis and assessed the relation between clinical, radiological, laboratory features and F2-IsoP levels regarding DCI occurrence. The results are presented in Table 1. In the next step, we performed a multivariate analysis (backward stepwise logistic regression). To the analysis we included only those variables that had shown to be significant in the univariate analysis. The results are presented in Table 1.
Table 1.
The univariate and multivariate analyses assessing the relation between clinical, radiological, laboratory features and F2-IsoP levels regarding DCI occurrence.
| Total n = 27 | DCI n = 13 (48.1%) | Without DCI n = 14 (51.9%) | Univariate OR (95% CI) | P value | Multivariate OR (95% CI) | P value | |
|---|---|---|---|---|---|---|---|
| Age (years) | 61 (50–66) | 63 (61–70) | 54 (43–61) | 1.05 (0.99–1.12) | 0.099 | ||
| Sex (male) | 11 (41%) | 6 (46%) | 5 (36%) | 1.53 (0.32–7.22) | 0.582 | ||
| Location (anterior circulation) | 24 (89%) | 13 (100%) | 11 (79%) | N.A | 1.000 | ||
| Treatment (surgical treatment) | 24 (89%) | 13 (100%) | 11 (79%) | N.A | 1.000 | ||
| Hunt and Hess scale | |||||||
| I | 1 (4%) | 0 (0%) | 1 (7.1%) | 1.73 (0.47–6.4) | 0.405 | ||
| II | 8 (30%) | 3 (23%) | 5 (35.8%) | ||||
| III | 17 (63%) | 10 (77%) | 7 (50%) | ||||
| IV | 1 (4%) | 0 (0%) | 1 (7.1%) | ||||
| mFisher grade | |||||||
| I | 3 (11%) | 0 (0%) | 3 (21.5%) | 3.57 (1.14–11.13) | 0.028 | ||
| II | 4 (15%) | 0 (0%) | 4 (28.5%) | ||||
| III | 4 (15%) | 3 (23%) | 1 (7.2%) | ||||
| IV | 16 (59%) | 10 (77%) | 6 (42.8%) | ||||
| Intracerebral haemorrhage (ICH) | 5 (18.5%) | 2 (15.4%) | 3 (21.4%) | 0.66 (0.09–4.8) | 0.687 | ||
| Hydrocephalus (HCP) | 14 (51.8%) | 8 (61.5%) | 6 (42.8%) | 2.13 (0.45–9.94) | 0.334 | ||
| Anisocytosis parameters | |||||||
| RDW-CV (%CV)—day 1 | 13.8 ± 1.5 13 (12–15) | 14.5 ± 1.8 14 (13–15) | 13.2 ± 0.9 13(12–13) | 1.92 (1.02–3.61) | 0.042 | 1.289 (1.041–1.597) | 0.020 |
| RDW-SD (fl)—day 1 | 43.5 ± 8.9 42 (39–50) | 47.2 ± 10 49 (44–53) | 40.1 ± 6 40 (38–42) | 1.11 (1–1.23) | 0.049 | ||
| RDW-CV (%CV)—day 3 | 12.5 ± 0.8 12 (11–13) | 12.6 ± 0.9 12 (11–13) | 12.4 ± 0.8 12 (11–12) | 1.32 (0.55–3.16) | 0.525 | ||
| RDW-SD (fl)—day 3 | 42.1 ± 4.5 42 (39–44) | 41.6 ± 6 40 (38–44) | 42.5 ± 2.5 43(40–44) | 0.95 (0.8–1.13) | 0.581 | ||
| RDW-CV (%CV)—day 5 | 12.9 ± 0.9 12 (12–13) | 12.8 ± 1 12 (11–13) | 12.9 ± 0.8 13 (12–13) | 0.84 (0.36–1.94) | 0.690 | ||
| RDW-SD (fl)—day 5 | 41.7 ± 4 40 (39–44) | 41.5 ± 4 40 (38–43) | 41.9 ± 3.6 42 (39–44) | 0.97 (0.8–1.17) | 0.774 | ||
| ISOP CSF conc | |||||||
| Day 1 (pg/ml) | 43.9 ± 42 25 (16–67) | 65.7 ± 50 67 (23–83) | 23.7 ± 19 20 (7–28) | 1.04 (1–1.08) | 0.028 | 1.094 (1.005–1.191) | 0.039 |
| Day 3 (pg/ml) | 49.3 ± 37 43 (16–80) | 65.8 ± 39 76 (21–94) | 36.4 ± 30 22 (12–59) | 1 (0.98–1) | 0.878 | ||
| Day 5 (pg/ml) | 46 ± 32 43 (22–53) | 61.7 ± 52 (43–76) | 31.4 ± 17 34 (16–43) | 1.05 (1.004–1.097) | 0.031 | ||
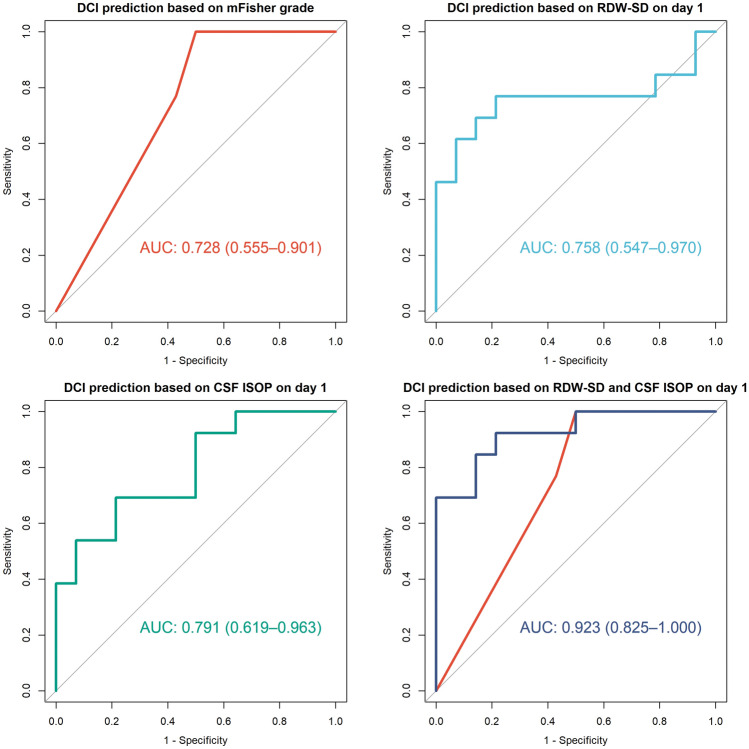
We noted that day 1 CSF F2-IsoP concentration and day 1 RDW-SD value were the most important predictors for DCI. The receiver operating characteristic curve for DCI prediction based on multivariate models had the area under the curve (AUC: 0.923, 95% CI 0.825–1.000, p < 0.001; Fig. 4). To verify the stability of the model, we employed a bootstrap approach with 1000 iterations, obtaining the mean AUC value of the model of 0.919 (95% CI 0.725–0.977). In the second step we investigated the backward stepwise logistic regression models, which with a combination with the clinical features provided the highest prognostic value regarding the occurrence of DCI. ROC curves based on backward stepwise logistic regression showed that the best model for DCI prediction on the basis of the clinical markers incorporated only mFisher grade (AUC: 0.728, 95% CI 0.532–0.924, p = 0.023; see Fig. 4).
Figure 4.
Receiver operating characteristic (ROC) curve for predicting DCI based on mFisher grade, RDW-SD on day 1 post-op and CSF F2-IsoP concentration on day 1 as well as the regression model based on both biomarkers. AUC area under the curve, ISOP F2-isoprostane.
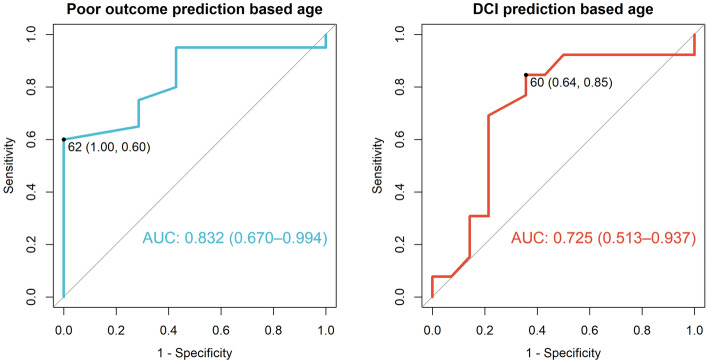
Furthermore, we investigated which variables had been associated with poor long-term outcomes. To reach this purpose, we dichotomized the quality-of-life scales according to the need for assistance in everyday life (i.e., GOS scores 4–5 vs. 1–3 and mRS scores 0–3 vs. 4–6). The backward stepwise logistic regression showed that age (OR 1.1 (95% CI 1.01–1.2), p = 0.02) with a cut-off point of 62 years (Fig. 5), DCI (OR 23.41 (95% CI 1.8–303.7), p = 0.01) and male gender (OR 15.68 (95% CI 1.15–213.68), p = 0.03) were the most important predictors for poor outcomes according to GOS and mRS.
Figure 5.
Receiver operating characteristic (ROC) curve with AUC values (with 95% confidence intervals) and optimal cut-off points (with specificity and sensitivity; based on Youden’s index) of age for prediction of: poor outcomes (in the terms of GOS at 12 month) and DCI.
Moreover, we decided to investigate the correlation between the patients’ age and DCI, as according to the literature older individuals are prone to DCI38. According to the ROC curve and the best cut-off point was 60 years old (see Fig. 5): (AUC: 0.725, 95% CI 0.518–0.932, p = 0.033).
Using the same dichotomization, we also checked the correlation between the patients’ age and IsoP levels on day 5, noting a statistical trend between them. The U Mann–Whitney test result was borderline, yet non-significant (p = 0.07).
Discussion
The main conclusion of this study is that elevated ISOP CSF concentration and erythrocyte anisocytosis (expressed as RDW-CV) measured on the first day after the aSAH surgery may be significant markers of an increased DCI risk. Based on this finding, we developed a predictive model for DCI occurrence incorporating both these factors. According to the literature, the model parameters meet the criteria for high predictive accuracy with AUC of 0.924 (95% CI 0.824–1.000; p < 0.001)39. Notably, we showed the potential role of oxidative stress in the DCI and predictive potential of IsoPs (as an oxidative stress biomarker) and erythrocytes anisocytosis (expressed by RDW-CV value) as an independent risk factor for DCI following aSAH.
In previous studies, it was shown that most important clinical predictors for DCI are: age (40–59 y. o.), thick SAH (high Fisher grade) and aneurysm located in the anterior circulation40. Our data are in line with these reports. We found that the most significant clinical parameter was the mFisher scale score (Grade 3 with 1.0 sensitivity and 0.5 specificity; Grade 4 with 0.769 sensitivity and 0.571 specificity; AUC 0.728 95% CI 0.532–0.924). However, no objective laboratory predictor is used in clinical practice. The amount of blood after SAH directly correlates with the severity of oxidative stress and DCI. It should be also mentioned that the blood in the subarachnoid space not only plays a role in the development of DCI, but also has an impact on early brain injury being the cause of neural damage in the first 72 h after the aneurysm rupture41,42. The extravasated blood increases intracranial pressure (ICP), leads to a decrease in the cerebral perfusion pressure (CPP) and impairs autoregulation causing cell death and brain oedema43. Further consequences might be microthrombosis, altered ionic homeostasis, excitotoxicity, oxidative stress and energy dysfunction secondary to cortical spreading depolarization44–46. All those events would add up to the DCI pathophysiology. There is no doubt that having an easily accessible biomarker of high prognostic value for DCI prediction would be of great assistance in the DCI prediction and diagnosis, on the other hand indicating a group of aSAH patients without the risk of DCI and thus reducing the cost of their hospitalization. As it seems, providing our observations are confirmed in the further studies, we could hope that our model will be useful as a biomarker for an early DCI detection.
RDW indicates that red blood cells of different sizes are present in the blood smear. RBC heterogeneity reflects the state of erythropoiesis and has been shown to correlate with inflammatory and thrombotic diseases such as ischaemic stroke47–49. When erythropoiesis is disturbed, RBC deformability is reduced, which impairs microcirculation50. Several studies have shown that RDW correlates well with in-hospital mortality, has a good ability to predict cerebral infarction and can also be used as a prognostic marker for aSAH patients51–53, which is in line with our findings. Subarachnoid haemorrhage causes high OS and generation of reactive oxygen species, which are directly reflected by isoprostanes formation18,54. The regulation of OS is particularly important for erythropoiesis55 with any impairment leading to elevated RDW values and microcirculatory disturbances. We therefore draw attention to OS as a cause of inflammation, microcirculatory disturbances and cerebral vasospasm after aSAH, which are all observed in DCI. We also point out that RDW and CSF isoprostanes concentration may improve the accuracy of DCI prediction with an AUC of 0.923.
At this stage of knowledge, it is difficult to point directly to the role of F2-IsoP and RDW after aSAH. We believe that both may be related to direct brain injury after aSAH leading to DCI. On the other hand, it may also show that there is a dynamic imbalance between ROS production and scavenging, leading to accumulation of OS, which may be a knowledge gap in the pathophysiology of DCI and requires future research.
The increase in oxidative stress is mostly a result of the lysis of red blood cells (RBCs) released into the subarachnoid space. Physiologically, the extravasated erythrocytes derived haemoglobin (Hb) and free heme are bound to haptoglobin (Hp) that neutralizes the strong oxidative capacity of heme–iron and prepares it for phagocytosis. In aSAH those mechanisms become rapidly exhausted and only some of the Hb and free heme originating from erythrocytes is neutralized by Hp. They collapse even faster in older patients due to insufficient antioxidant mechanisms in this age group56. Thus, free heme and iron in the extracellular space are the principal source of oxidative stress and reactive oxygen species (ROS).
The isoprostanes are prostaglandin-like compounds formed in vivo from the free radical-catalysed peroxidation of cell membrane arachidonic acid without the direct action of cyclooxygenase (COX) enzymes. Their concentration directly correlates with ROS production; thus, they were classified as oxidative stress biomarkers with potent biological activity57. In our research, we found that CSF IsoPs concentration on day 1 after surgery was elevated in the DCI group. From one side the level of IsoPs informs us about the severity of neuronal damage caused by oxidative stress as the concentration was higher in the DCI group versus non-DCI and control and from the other, their biological potential to affect the DNA synthesis in the smooth muscle cells, as well as mitogenesis and stimulation of platelets aggregation, fibroblast proliferation and vasoconstriction, all of which may also play a role in DCI pathophysiology58. In our work, we also showed that poor outcome and DCI occurrence were more frequently observed in older patients with the cut-off point at 60 years, as it already had been suggested in the literature59. The ROS formation increase is well-known to be age-related, whereas the age-dependent functional decline of antioxidant mechanisms in the population is also observed60,61. We would also draw attention to the trend between age and IsoPs level on day 5 as it suggests that on day 5, which was the median day of DCI onset in our cohort, there is an increase of ROS. In our opinion it is related to the insufficient antioxidant reaction that is observed in older patients. The dynamic balance between the production and elimination of ROS in patients with DCI might be shifted towards the ROS formation. This issue needs more investigation.
The pathophysiological mechanisms leading to DCI include microcirculatory disturbances and microthromboembolism. Some studies propose that anisocytosis may be associated with both of these mechanisms. According to the study by Pato et al.62, patients with RDW above 14% had significantly decreased RBC deformability. In an animal model study, lower blood flow was associated with reduced plasticity of RBC63. Although the exact mechanism remains unclear, one hypothesis proposes that erythrocytes with reduced deformability, may be recognized by macrophages, which secret the agents causing vasoconstriction and accumulation of platelets and leukocytes. Aarts et al.64 suggest that erythrocytes with reduced deformability were also responsible for increased platelet adhesion which could be a potential cause of aggravated microthromboembolism. However, association of RCB deformability and RDW is not fully understood as there is only one study confirming the existence of this relationship.
Many authors indicated that RDW-CV and RDW-SD show an upward trend with advanced age, however, the exact mechanism of how aging influences anisocytosis is not known64–67. This may potentially explain the association of RDW with poor outcome in various diseases. In our multivariate analysis, the combination of isoprostanes and RDW turned out to be a very good predictor of DCI (AUC: 0.924, 95% CI 0.824–0.1, p < 0.01). Additionally, RDW-SD values were also greater in patients over 59 years of age.
Anisocytosis has been identified as a risk factor of poor outcome in many diseases, also being associated with age48. In 2023r. Lukito et al.53 performed meta-analysis in which they found that high RDW value was significantly correlated with poor functional outcome, mortality and DCI. Importantly enough, our study findings are consistent with analyses involving other possible biomarkers and RDW. Researchers looking at factors such as neutrophil-to-lymphocyte ratio (NLR) or systemic inflammatory response index (SIRI) also found a correlation between RDW and DCI68,69. Interestingly, an analysis performed by Ingacio et al.70 demonstrated that RDW holds greater significance in predicting the DCI than NLR does.
A certain drawback of our work is that in the study protocol we employed the Hunt-Hess scale while WFNS scale is usually preferred in clinical analyses, albeit the Hunt-Hess scale is still widely used in clinical practice. We would like to draw attention to the fact that it is difficult to collect CFS samples at strict time points in aSAH patients. This may render incorporating suggested CSF biomarkers into the routine clinical practice somewhat difficult. Nonetheless, we believe that our efforts are sensible from a clinical point of view. Establishing a biochemical marker or screening test detecting DCI should facilitate minimally invasive DCI monitoring and prompt implementation of the treatment as well as reduce the costs of hospitalization. Finally, this could kindle a future search for a specific treatment directly preventing DCI.
Conclusions
According to the results of our analysis, the combination of RDW-CV value and the concentration of IsoPs in CSF on the first day after surgery for a ruptured intracranial aneurysm, can serve as prognostic factors in DCI.
Strengths and limitations of the study
In this study, we aimed to assess prognostic biomarkers for DCI following aSAH, specifically focusing on CSF 8-iso-Prostaglandin F2α and erythrocyte anisocytosis. A key strength of our study is its prospective design with prespecified inclusion and exclusion criteria, which allowed us to collect reliable data systematically until reaching the minimal sample size determined by prior calculations. Biomarker measurements were conducted at three distinct time points, and we have incorporated 12-month follow-up to enhance the robustness of our findings.
Despite these strengths, we acknowledge several limitations. This exploratory study was conducted at a single centre with the limited power of multivariate analysis, which affect the generalizability of the results. Thus, a confirmatory multicentre study with a larger sample size is necessary to confirm and expand upon our findings.
Supplementary Information
Author contributions
Conceptualization—W.K. Methodology—W.K.; B.M. Analysis—W.K.; Z.K.; P.M.; B.M.; S.B. Investigation—W.K.; Z.K.; P.M.; B.M.; P.B.; B.E.J. Writing original draft preparation—W.K.; Z.K.; S.B. Writing—review and editing—W.K.; Z.K.; S.B.; J.D.J. Project administration—W.K. Supervision—W.K.; J.D.J. All authors reviewed and accepted the manuscript.
Funding
National Science Center and Ministry of Science and Higher Education provided financial support in the form of PRELUDIUM VI; Project ID 507/1-121-03/507-10-075/UMO-2013/11/N/NZ4/00273.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-61956-w.
References
- 1.Rinkel GJ, Algra A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2011;10(4):349–356. doi: 10.1016/s1474-4422(11)70017-5. [DOI] [PubMed] [Google Scholar]
- 2.Hijdra A, Braakman R. Aneurysmal subarachnoid hemorrhage. Complications and outcome in a hospital population. Stroke. 1987;18:1061–1067. doi: 10.1161/01.STR.18.6.1061. [DOI] [PubMed] [Google Scholar]
- 3.Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41(8):e519–e536. doi: 10.1161/STROKEAHA.110.581975. [DOI] [PubMed] [Google Scholar]
- 4.Vergouwen MD, Vermeulen M, Coert BA, Stroes ES, Roos YB. Microthrombosis after aneurysmal subarachnoid hemorrhage: An additional explanation for delayed cerebral ischemia. J. Cereb. Blood Flow Metab. 2008;28(11):1761–1770. doi: 10.1038/jcbfm.2008.74. [DOI] [PubMed] [Google Scholar]
- 5.Frontera JA, Claassen J, Schmidt JM, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: The modified fisher scale. Neurosurgery. 2006;59(1):21–27. doi: 10.1227/01.neu.0000243277.86222.6c. [DOI] [PubMed] [Google Scholar]
- 6.Dankbaar JW, et al. Relationship between vasospasm, cerebral perfusion, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Neuroradiology. 2009;51(12):813–819. doi: 10.1007/s00234-009-0575-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vergouwen MDI, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke. 2010;41(10):2391–2395. doi: 10.1161/strokeaha.110.589275. [DOI] [PubMed] [Google Scholar]
- 8.Claassen J, Bernardini GL, Kreiter K, Bates J, Du Y, Copeland D, et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage. The Fisher scale revisited. Stroke. 2001;32:2012–2020. doi: 10.1161/hs0901.095677. [DOI] [PubMed] [Google Scholar]
- 9.Pradilla G, Garzon-Muvdi T, Ruzevick JJ, Bender M, Edwards L, Momin EN. Systemic L-citrulline prevents cerebral vasospasm in haptoglobin 2–2 transgenic mice after subarachnoid hemorrhage. Neurosurgery. 2012;70:747–757. doi: 10.1227/NEU.0b013e3182363c2f. [DOI] [PubMed] [Google Scholar]
- 10.Lin C-L, Kwan A-L, Dumont AS, Su YF, Kassell NF, Wang CJ, et al. Attenuation of experimental subarachnoid hemorrhage-induced increases in circulating intercellular adhesion molecule-1 and cerebral vasospasm by the endothelin-converting enzyme inhibitor CGS 26303. J. Neurosurg. 2007;106:442–448. doi: 10.3171/jns.2007.106.3.442. [DOI] [PubMed] [Google Scholar]
- 11.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010;49:603–616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sies H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 14.Brune B, Schmidt KU, Ullrich V. Activation of soluble guanylate cyclase by carbon monoxide and inhibition by superoxide anion. Eur. J. Biochem./FEBS. 1990;192:683–688. doi: 10.1111/j.1432-1033.1990.tb19276.x. [DOI] [PubMed] [Google Scholar]
- 15.Sayed N, Baskaran P, Ma X, van den Akker F, Beuve A. Desensitization of soluble guanylyl cyclase, the NO receptor, by S-nitrosylation. Proc. Natl. Acad. Sci. U S A. 2007;104:12312–12317. doi: 10.1073/pnas.0703944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Watts SW, Banes AK, Galligan JJ, Fink GD, Chen AF. NADPH oxidase-derived superoxide augments endothelin- 1-induced venoconstriction in mineralocorticoid hypertension. Hypertension. 2003;42:316–321. doi: 10.1161/01.HYP.0000084853.47326.F2. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Fink GD, Watts SW, Northcott CA, Galligan JJ, Pagano PJ, et al. Endothelin-1 increases vascular superoxide via endothelin (A)-NADPH oxidase pathway in low-renin hypertension. Circulation. 2003;107:1053–1058. doi: 10.1161/01.CIR.0000051459.74466.46. [DOI] [PubMed] [Google Scholar]
- 18.Hernanz R, Briones AM, Salaices M, Alonso MJ. New roles for old pathways? A circuitous relationship between reactive oxygen species and cyclooxygenase in hypertension. Clin. Sci. (Lond.) 2014;126:111–121. doi: 10.1042/CS20120651. [DOI] [PubMed] [Google Scholar]
- 19.Mallat Z, et al. Elevated levels of 8-iso-prostaglandin F2alpha in pericardial fluid of patients with heart failure: a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation. 1998;28:1536–1539. doi: 10.1161/01.CIR.97.16.1536. [DOI] [PubMed] [Google Scholar]
- 20.Yan Z, Mas E, Mori TA, Croft KD, Barden AE. A significant proportion of F2-isoprostanes in human urine are excreted as glucuronide conjugates. Anal. Biochem. 2010;403:126–128. doi: 10.1016/j.ab.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Oustamanolakis P, et al. Measurement of reticulocyte and red blood cell indices in the evaluation of anemia in inflammatory bowel disease. J. Crohn's Colitis. 2011;5(4):295–300. doi: 10.1016/j.crohns.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Horta-Baas G, Romero-Figueroa MS. Clinical utility of red blood cell distribution width in inflammatory and non-inflammatory joint diseases. Int. J. Rheum. Dis. 2018 doi: 10.1111/1756-185x.13332. [DOI] [PubMed] [Google Scholar]
- 23.Lucijanic, , et al. The degree of anisocytosis predicts survival in patients with primary myelofibrosis. Acta Haematol. 2016;136(2):98–100. doi: 10.1159/000445247. [DOI] [PubMed] [Google Scholar]
- 24.Lippi G, Pavesi F, Bardi M, Pipitone S. Lack of harmonization of red blood cell distribution width (RDW). Evaluation of four hematological analyzers. Clin. Biochem. 2014;47:1100–1103. doi: 10.1016/j.clinbiochem.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Stein SC, Browne KD, Chen XH, Smith DH, Graham DI. Thromboembolism and delayed cerebral ischemia after subarachnoid hemorrhage: An autopsy study. Neurosurgery. 2006;59(4):781–787. doi: 10.1227/01.NEU.0000227519.27569.45. [DOI] [PubMed] [Google Scholar]
- 26.Romano JG, Rabinstein AA, Arheart KL, et al. Microemboli in aneurysmal subarachnoid hemorrhage. J. Neuroimaging. 2008;18(4):396–401. doi: 10.1111/j.1552-6569.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- 27.Ycas JW, Horrow JC, Horne BD. Persistent increase in red cell size distribution width after acute diseases: A biomarker of hypoxemia? Clin. Chim. Acta. 2015;448:107–117. doi: 10.1016/j.cca.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Bobeff EJ, et al. Plasma amino acids may improve prediction accuracy of cerebral vasospasm after aneurysmal subarachnoid haemorrhage. J. Clin. Med. 2022;11(2):380. doi: 10.3390/jcm11020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, Patel AB. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43(6):1711–1737. doi: 10.1161/str.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- 30.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/S0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 31.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 32.R Core Team. R: A Language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (2023).
- 33.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer; 2016. [Google Scholar]
- 34.Robin X, et al. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedman J, Tibshirani R, Hastie T. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 2010;33(1):1–22. doi: 10.18637/jss.v033.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canty, A. & Ripley, B. boot: Bootstrap R (S-Plus) Functions. R package version 1.3-30 (2024).
- 37.Davison AC, Hinkley DV. Bootstrap Methods and Their Applications. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- 38.Lee H, Perry JJ, et al. Clinical prediction of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2018;1:1–8. doi: 10.3171/2018.1.JNS172715. [DOI] [PubMed] [Google Scholar]
- 39.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010;5(9):1315–1316. doi: 10.1097/JTO.0b013e3181ec173d. [DOI] [PubMed] [Google Scholar]
- 40.Sehba FA, Hou J, Pluta RM, Zhang JH. The importance of early brain injury after subarachnoid hemorrhage. Prog. Neurobiol. 2012;97:14–37. doi: 10.1016/j.pneurobio.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kusaka G, Ishikawa M, Nanda A, Granger DN, Zhang JH. Signaling pathways for early brain injury after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2004;24(8):916–925. doi: 10.1097/01.WCB.0000125886.48838.7E. [DOI] [PubMed] [Google Scholar]
- 42.Gaasch M, et al. Cerebral autoregulation in the prediction of delayed cerebral ischemia and clinical outcome in poor-grade aneurysmal subarachnoid hemorrhage patients. Crit. Care Med. 2018;46(5):774–780. doi: 10.1097/CCM.0000000000003016I. [DOI] [PubMed] [Google Scholar]
- 43.de Oliveira Manoel AL, et al. The critical care management of poor-grade subarachnoid haemorrhage. Crit. Care. 2016;20:21. doi: 10.1186/s13054-016-1193-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chou SH, et al. Elevated peripheral neutrophils and matrix metalloproteinase 9 as biomarkers of functional outcome following subarachnoid hemorrhage. Transl. Stroke Res. 2011;2(4):600–607. doi: 10.1007/s12975-011-0117-x.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakowitz OW, et al. Clusters of spreading depolarizations are associated with disturbed cerebral metabolism in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2013;44(1):220–223. doi: 10.1161/STROKEAHA.112.672352. [DOI] [PubMed] [Google Scholar]
- 46.Gorni D, Finco A. Oxidative stress in elderly population: A prevention screening study. Aging Med. (Milton) 2020;3(3):205–213. doi: 10.1002/agm2.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch. Pathol. Lab. Med. 2009;133(4):628–632. doi: 10.5858/133.4.628. [DOI] [PubMed] [Google Scholar]
- 48.Danese E, Lippi G, Montagnana M. Red blood cell distribution width and cardiovascular diseases. J. Thorac. Dis. 2015;7(10):E402–E411. doi: 10.3978/j.issn.2072-1439.2015.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turcato G, Cappellari M, Follador L, et al. Red blood cell distribution width is an independent predictor of outcome in patients undergoing thrombolysis for ischemic stroke. Semin. Thromb. Hemost. 2017;43(1):30–35. doi: 10.1055/s-0036-1592165. [DOI] [PubMed] [Google Scholar]
- 50.Patel KV, Mohanty JG, Kanapuru B, Hesdorffer C, Ershler WB, Rifkind JM. Association of the red cell distribution width with red blood cell deformability. Adv. Exp. Med. Biol. 2013;765:211–216. doi: 10.1007/978-1-4614-4989-8_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang YL, Han ZJ, De HuZ. Red blood cell distribution width and neutrophil to lymphocyte ratio are associated with outcomes of adult subarachnoid haemorrhage patients admitted to intensive care unit. Ann. Clin. Biochem. 2017;54(6):696–701. doi: 10.1177/0004563216686623. [DOI] [PubMed] [Google Scholar]
- 52.Ignacio KHD, Diestro JDB, Enriquez CAG, et al. Predictive value of hematologic inflammatory markers in delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2022;160:e296–e306. doi: 10.1016/j.wneu.2022.01.014. [DOI] [PubMed] [Google Scholar]
- 53.Lukito PP, Lie H, Angelica V, Wijovi F, Nathania R, July J. Red-cell distribution width as a prognostic marker for aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. World Neurosurg. X. 2023;23(19):100202. doi: 10.1016/j.wnsx.2023.100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schildknecht S, Karreman C, Daiber A, Zhao C, Hamacher J, et al. Autocatalytic nitration of prostaglandin endoperoxide synthase-2 by nitrite inhibits prostanoid formation in rat alveolar macrophages. Antioxid. Redox Signal. 2012;15(17):1393–1406. doi: 10.1089/ars.2011.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid. Redox Signal. 2008;10(11):1923–1940. doi: 10.1089/ars.2008.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montuschi P, Barnes PJ, Roberts LJ., 2nd Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18(15):1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 57.Wiśniewski K, et al. Isoprostanes as potential cerebral vasospasm biomarkers. Neurol. Neurochir. Pol. 2018;52(6):643–651. doi: 10.1016/j.pjnns.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 58.Meydani SN, Wu D, Santos MS, Hayek MG. Antioxidants and immune response in aged persons: Overview of present evidence. Am. J. Clin. Nutr. 1995;62(6 Suppl):1462S–1476S. doi: 10.1093/ajcn/62.6.1462S. [DOI] [PubMed] [Google Scholar]
- 59.De la Fuente M. Effects of antioxidants on immune system ageing. Eur. J. Clin. Nutr. 2002;56(Suppl 3):S5–8. doi: 10.1038/sj.ejcn.1601476. [DOI] [PubMed] [Google Scholar]
- 60.Rautalin I, Juvela S, Martini ML, Macdonald RL, Korja M. Risk factors for delayed cerebral ischemia in good-grade patients with aneurysmal subarachnoid hemorrhage. J. Am. Heart. Assoc. 2022;11(23):e027453. doi: 10.1161/JAHA.122.027453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patel K, et al. Association of the Red cell distribution width with red blood cell deformability. Oxyg. Transport Tissue. 2012;2:211–216. doi: 10.1007/978-1-4614-4989-8_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simchon S, Jan KM, Chien S. Influence of reduced red cell deformability on regional blood flow. Am. J. Physiol. 1987;253:H898–H903. doi: 10.1152/ajpheart.1987.253.4.H898. [DOI] [PubMed] [Google Scholar]
- 63.Aarts PA, Bolhuis PA, Sakariassen KS, Heethaar RM, Sixma JJ. Red blood cell size is important for adherence of blood platelets to artery subendothelium. Blood. 1983;62(1):214–217. doi: 10.1182/blood.V62.1.214.214. [DOI] [PubMed] [Google Scholar]
- 64.Lippi G, et al. Between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch. Pathol. Lab. Med. 2009;133(4):628–632. doi: 10.5858/133.4.628. [DOI] [PubMed] [Google Scholar]
- 65.Qiao R, Yang S, Yao B, Wang H, Zhang J, Shang H. Complete blood count reference intervals and age- and sex-related trends of North China Han population. Clin. Chem. Lab. Med. 2014;52:1025–1032. doi: 10.1515/cclm-2012-0486. [DOI] [PubMed] [Google Scholar]
- 66.Lippi G, Salvagno GL, Guidi GC. Red blood cell distribution width is significantly associated with aging and gender. Clin. Chem. Lab. Med. (CCLM) 2014 doi: 10.1515/cclm-2014-0353. [DOI] [PubMed] [Google Scholar]
- 67.Patel KV, et al. Red cell distribution width and mortality in older adults: A meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65(3):258–265. doi: 10.1093/gerona/glp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi M, Yang C, Tang QW, Xiao LF, Chen ZH, Zhao WY. The prognostic value of neutrophil-to-lymphocyte ratio in patients with aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis of observational studies. Front. Neurol. 2021 doi: 10.3389/fneur.2021.745560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yun S, Yi HJ, Lee DH, Sung JH. Systemic inflammation response index and systemic immune-inflammation index for predicting the prognosis of patients with aneurysmal subarachnoid hemorrhage. J. Stroke Cerebrovasc. Dis. 2021 doi: 10.1016/j.jstrokecerebrovasdis.2021.105861. [DOI] [PubMed] [Google Scholar]
- 70.Ignacio KHD, Diestro JDB, Enriquez CAG, et al. Predictive value of hematologic inflammatory markers in delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2022;160:e296–e306. doi: 10.1016/j.wneu.2022.01.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.