Abstract
Purpose
Breast cancer treatment is associated with weight gain, and obesity and its related cardiometabolic and hormonal risk factors have been associated with poorer outcomes. Dietary intervention may address these risk factors, but limited research has been done in the setting of metastatic breast cancer requiring systemic therapy.
Methods
Women with metastatic breast cancer on stable treatment were randomized 2:1 to an 8-week intervention (n = 21) or control (n = 11). The intervention included weekly assessment visits and an ad libitum whole-food, plant-based (WFPB) diet with provided meals. Cardiometabolic, hormonal, and cancer markers were assessed at baseline, 4 weeks, and 8 weeks.
Results
Within the intervention group, mean weight decreased by 6.6% (p < 0.01) after 8 weeks. Fasting insulin decreased from 16.8 uIU/L to 11.2 uIU/L (p < 0.01), concurrent with significantly reduced insulin resistance. Total cholesterol decreased from 193.6 mg/dL to 159 mg/dL (p < 0.01), and low-density lipoprotein (LDL) cholesterol decreased from 104.6 mg/dL to 82.2 mg/dL (p < 0.01). Total testosterone was unchanged, but free testosterone trended lower within the intervention group (p = 0.08) as sex hormone binding globulin increased from 74.3 nmol/L to 98.2 nmol/L (p < 0.01). There were no significant differences in cancer progression markers at week 8, although mean CA 15-3, CA 27.29, and CEA were lower in the intervention group (p = 0.53, p = 0.23, and p = 0.54, respectively) compared to control, when adjusted for baseline.
Conclusion
WFPB dietary changes during treatment for metastatic breast cancer are well tolerated and significantly improve weight, cardiometabolic and hormonal parameters. Longer studies are warranted to assess the durability of changes.
Trial registration First registered at Clinicaltrials.gov (NCT03045289) on February 7, 2017.
Keywords: Diet, Nutrition, Breast cancer, Plant-based diet, Vegan diet, Obesity
Background
While many anti-neoplastic treatments are associated with weight loss, treatment for breast cancer (BC) is consistently associated with weight gain [1]. A 1997 review reported that significant weight gain occurs in 50% to 96% of women receiving chemotherapy for early stage BC, with a common weight gain of five to 13.6 pounds [2]. Little has changed since then [3]. Excess weight and weight gain remain common even through advanced BC. Among women receiving chemotherapy for metastatic disease, rates of obesity are comparable to or even higher than rates of obesity in the general population [4].
Obesity at diagnosis as well as excess weight gain after diagnosis has been associated with both BC-specific mortality and overall mortality [5–9]. In addition, obesity and its related cardiometabolic comorbidities contribute to higher symptom burden and reduced quality of life [10, 11]. Given this, it is not surprising that one survey found > 90% of patients with breast cancer who also have overweight or obesity reported being “somewhat” or “very” concerned about their weight [3].
Excess weight is often comorbid with elevated insulin and insulin resistance, blood glucose, cholesterol, sex hormones, and IGF-1. These may independently worsen risk of BC progression and mortality as well as reduce quality of life [12, 13]. Beyond cancer, it is well established that several of these comorbidities are risk factors for cardiovascular events [14], and cardiovascular disease is one of the leading causes of mortality (> 40%) among BC survivors [15].
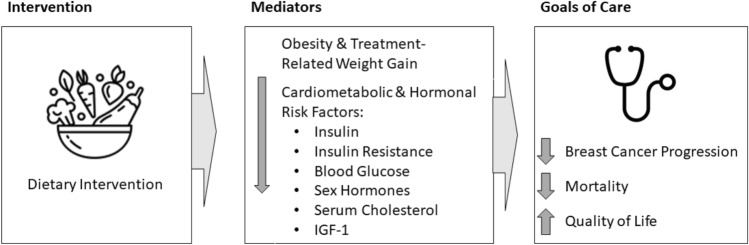
Dietary therapy can affect both obesity and its related cardiometabolic and hormonal risk factors (Fig. 1). A plant-predominant dietary pattern, lower in processed foods, is commonly recommended by many organizations, including the American Institute for Cancer Research [16] and the American Cancer Society [17]. At the end of the spectrum of plant-predominant dietary patterns is a whole-food, plant-based (WFPB) diet that minimizes or entirely avoids animal-based foods, highly processed foods, added fats and sugars. Interventions integrating this type of dietary approach have resulted in substantial weight loss [18, 19], regression of coronary atherosclerosis [20, 21], lowered cholesterol [22] and blood pressure [23] as well as reduced insulin resistance [24].
Fig. 1.
Potential mediators connecting dietary intake with goals of care. Dietary intervention may affect breast cancer-related goals of care through several potential mediators, some of which are shown here. This article details how a whole-food, plant-based intervention affects these mediators. Feasibility of the intervention and its effect on quality of life outcomes are published separately [42]
Patients with breast cancer, frequently concerned about their weight, are highly interested in nutrition information [25, 26]. Unfortunately, only limited research has investigated how dietary intervention affects BC-related outcomes [27, 28]. The findings of two large interventions [29, 30] suggest that weight loss, or diet and lifestyle change large enough to produce weight loss, may be necessary to impact cancer outcomes. Numerous other diet and lifestyle interventions have targeted weight loss among subjects with early stage breast cancer [31–40], but these studies have not been large enough or long enough to determine the effect of weight loss on recurrence or mortality and usually enrolled cancer survivors who already completed treatment.
Women with metastatic breast cancer on systemic therapy have largely been excluded from dietary intervention research, but with improved survival rates and an aging population, there is predicted to be 169,000 women living with metastatic breast cancer by 2025, up from 140,000 in 2018 [41]. Cancer burden is more easily tracked in metastatic breast cancer and there is a far higher risk of cancer progression and mortality compared to earlier stages. This presents an opportunity to understand how diet and lifestyle interventions may affect cancer-related outcomes within a shorter timeframe.
Given this background, we designed a pilot study to explore the feasibility and preliminary effects of a whole-food, plant-based dietary intervention in women with metastatic breast cancer. Findings relating to feasibility and effects on quality of life are published separately, while this report focuses on weight, cardiometabolic, and hormonal biomarkers.
Methods
Women with metastatic breast cancer were recruited between February 2018 to March 2022 from oncology clinics at the University of Rochester Medical Center (URMC) and by flyers and announcements at local support groups in Rochester, NY. Women with stage 4 breast cancer with any ER/PR/HER2 status who were expected to live at least 6 months and who were on a stable treatment regimen for the past 6 weeks, with no planned treatment changes in the near future, were eligible for the study. Exclusions included inability to tolerate a normal diet, an active malabsorption syndrome or eating disorder, uncontrolled diarrhea, recent consumption of a vegan diet, major surgery within 2 months, current insulin, sulfonylurea, or warfarin use, glomerular filtration rate (GFR) < 30 mL/min/1.73 m2 or serum potassium > 5.3 mmol/L on two lab tests within 90 days, current smoking, illicit drug use, more than 7 alcoholic drinks per week, food intolerances to plant-based foods, or psychiatric disorder impairing ability to give consent.
Subjects were randomized 2:1 to two arms: whole food, plant-based (WFBP) intervention (n = 21) or usual diet control (n = 11). Subjects in the WFPB arm received 3 prepared meals and one side dish per day for 8 weeks, weekly assessment visits with the study physicians (TC and/or EKC), and a weekly phone call from a study physician (EC). Weekly assessment visits included education, coaching, and evaluation of adverse events or other medical changes. The ad libitum WFPB diet consisted of fruits, vegetables, whole grains, legumes, nuts and seeds. The diet excluded animal products and added oils/solid fats. Subjects were encouraged to eat as much and as often as they wanted to be comfortably full. They were encouraged to add their own food in addition to, or in place of, the provided food, as long as it was ‘on-plan.’ A daily multivitamin (Centrum Women) was provided to all subjects in both arms.
Subjects in the control arm continued their usual diets for 8 weeks and received phone calls from a study physician at weeks 2 and 6 to assess for adverse events and treatment changes. As an incentive to maintain participation, control subjects received condensed educational resources related to the WFPB diet and 2 weeks of prepared study meals after completing their final 8-week assessments.
Testing procedures
All subjects had study visits and blood draws at baseline, week 4, and week 8. Weight and height were measured with subjects in light clothing, without shoes, on a Detecto Apex clinical digital scale with mechanical stadiometer. Blood pressure was measured with an automated blood pressure cuff with subjects seated quietly by themselves for 5 min before the monitor measured blood pressure three times, with 2 min between each measure. The average of the three blood pressures was recorded. Blood samples were drawn with subjects in a fasted state, in the morning, and tested using standard procedures at the CLIA certified URMC Clinical Laboratory. Blood tests included a complete metabolic panel, complete blood count, total and free testosterone, estradiol, sex hormone binding globulin (SHBG), dehydroepiandrosterone (DHEA), insulin and insulin-like growth factor-1 (IGF-1), insulin-like growth factor-1 binding protein (IGFBP-3), cholesterol panel, carcinoembryonic antigen (CEA), cancer antigen 27.29 (CA 27.29), and cancer antigen 15-3 (CA 15-3).
Statistical analysis
Descriptive statistics (e.g., mean standard deviation [SD], n, percent) were used to evaluate distributions of patients’ clinical and sociodemographic variables to assess balance between treatment arm and control. For outcome measures (weight, BMI, cardiometabolic measures, biomarkers), the distributions were first evaluated graphically for normality and outliers. Mean, SD, and the range were calculated at baseline, 4 weeks, and 8 weeks by study arm to assess balance at baseline, within group changes at 4 and 8 weeks. Changes in outcome values from baseline to 4 and 8 weeks within each study arm were assessed by paired t test. Analysis of covariance model with arm as the main factor and corresponding baseline levels as the covariate was used to evaluate the effects of the WFPB intervention on the weight, BMI and cardiometabolic and biomarker outcomes at 8 weeks. The results were further evaluated in linear mixed effect model incorporating all three time points. Between-group difference in change from the baseline to 8 weeks was estimated by difference in marginal means at 8 weeks. The effect size (ES) was calculated as ratio of mean between group difference in change from baseline to the baseline SD. Additionally, since distribution of some of the markers did not fully follow Gaussian normal distribution, the within group and between group changes were also assessed by nonparametric tests. Results based on both parametric and nonparametric analyses were in agreement and supported the same conclusions. p values from the parametric analysis are shown. Statistical significance was set at two-sided alpha = 0.05 level. Data were analyzed using SAS/STAT software version 9.4 (SAS Inc, Cary, NC, USA).
Results
Thirty of 32 (94%) randomized subjects completed their study participation. One subject was lost to follow-up immediately after being randomized to the control arm. One intervention subject was withdrawn by study investigators in March 2020 shortly after the baseline assessment due to the onset of the COVID-19 pandemic shutdown (Fig. 2). By our prespecified definition of compliance, 95% of subjects were compliant with the dietary prescription, and 100% of the subjects attended at least 6 of the 8 weekly assessment visits. Feasibility and dietary changes are detailed separately [42].
Fig. 2.
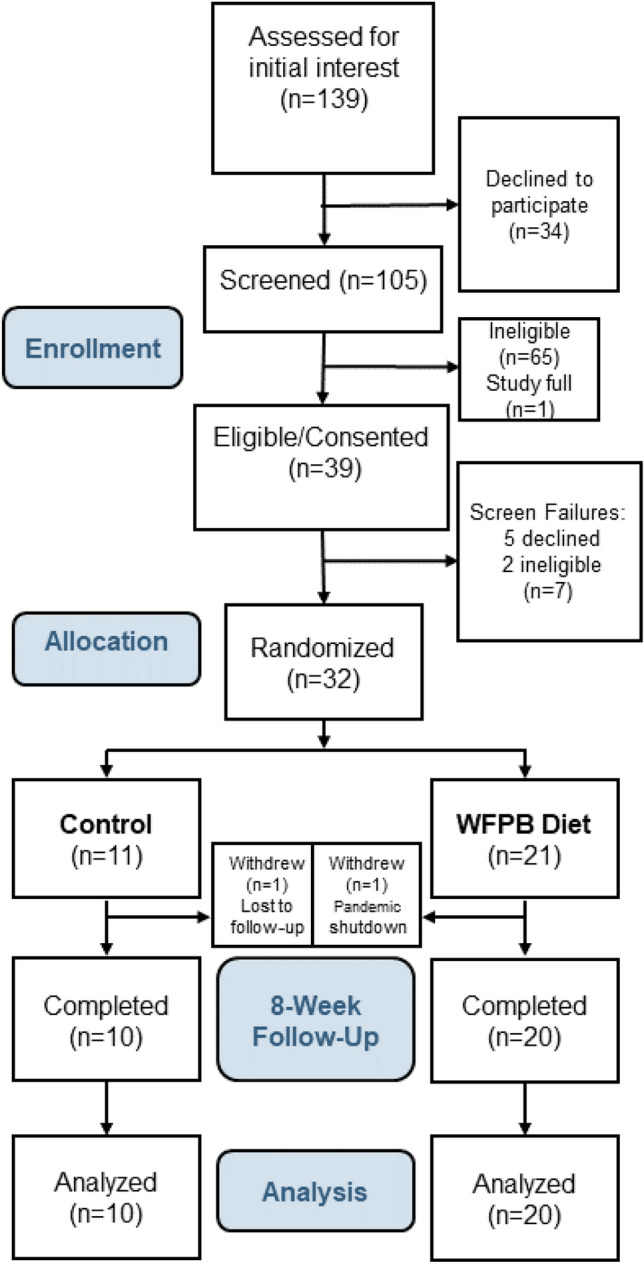
CONSORT diagram
The characteristics of the 31 subjects who completed baseline assessments are shown in Table 1. Of these subjects, 29% had BMIs categorized as normal (BMI 18.5–24.9 kg/m2), 32.3% as overweight (BMI 25–29.9 kg/m2), and 38.7% as obese (BMI ≥ 30 kg/m2). Almost all had hormone receptor positive breast cancer, and the most common treatment regimen was a cyclin-dependent kinase 4/6 inhibitor and an aromatase inhibitor. The most common site of metastasis was bone.
Table 1.
Baseline characteristics
| Control (10) | Intervention (21) | ||
|---|---|---|---|
| Age | Mean (SD) | 64.2 (8.9) | 59.1 (11) |
| Race | White, % (n) | 100.0 (10) | 90.5 (19) |
| Black, % (n) | 0 | 4.8 (1) | |
| No answer, % (n) | 0 | 4.8 (1) | |
| Ethnicity | Not Hispanic/Latino, % (n) | 100.0 (10) | 95.2 (20) |
| No answer, % (n) | 0 | 4.8 (1) | |
| Marital Status | Married, % (n) | 70.0 (7) | 66.7 (14) |
| Divorced, % (n) | 20.0 (2) | 14.3 (3) | |
| Single, % (n) | 10.0 (1) | 14.3 (3) | |
| Widowed, % (n) | 0 | 4.8 (1) | |
| Employment Status | Currently employed outside home, % (n) | 30.0 (3) | 28.6 (6) |
| Self-employed, % (n) | 0 | 9.5 (2) | |
| Retired, % (n) | 40.0 (4) | 19.0 (4) | |
| Disability, % (n) | 10.0 (1) | 14.3 (3) | |
| Homemaker, % (n) | 20.0 (2) | 19.0 (4) | |
| Not Working—Other, % (n) | 0 | 9.5 (2) | |
| BMI at Study Baseline | Mean, Kilograms/m2 (SD) | 28.4 (4.4) | 30.2 (7.2) |
| Age at First Breast Cancer Diagnosis | Mean (SD) | 52.9 (11.7) | 49.4 (10.9) |
| Years Elapsed Since First Diagnosis | Mean (SD) | 11.2 (7.9) | 9.7 (6.4) |
| Years Elapsed Since Diagnosis of Metastatic Breast Cancer | Mean (SD) | 5.3 (6.0) | 2.2 (1.8) |
| Hormone Receptor Status | ER + , % (n) | 100.0 (10) | 95.2 (20) |
| PR + , % (n) | 90.0 (9) | 81.0 (17) | |
| HER2 + , % (n) | 30.0 (3) | 28.6 (6) | |
| Location of Metastases | Bone, % (n) | 70.0 (7) | 90.5 (19) |
| Lung, % (n) | 40.0 (4) | 38.1 (8) | |
| Brain, % (n) | 10.0 (1) | 14.3 (3) | |
| Liver, % (n) | 20.0 (2) | 4.8 (1) | |
| Other, % (n) | 60.0 (6) | 33.3 (7) | |
| Cancer Therapy | Palbociclib, % (n) | 30.0 (3) | 47.6 (10) |
| Abemaciclib, % (n) | 10.0 (1) | 9.5 (2) | |
| Ribociclib, % (n) | 0 | 4.8 (1) | |
| Trastuzumab, % (n) | 20.0 (2) | 23.8 (5) | |
| Pertuzumab, % (n) | 10.0 (1) | 19.0 (4) | |
| Capecitabine, % (n) | 10.0 (1) | 4.8 (1) | |
| Letrozole, % (n) | 30.0 (3) | 61.9 (13) | |
| Anastrozole, % (n) | 30.0 (3) | 4.8 (1) | |
| Exemestane, % (n) | 10.0 (1) | 9.5 (2) | |
| Fulvestrant, % (n) | 20.0 (2) | 14.3 (3) | |
| Denosumab, % (n) | 10.0 (1) | 47.6 (10) | |
| Zoledronic acid, % (n) | 0 | 4.8 (1) | |
| Leuprolide, % (n) | 0 | 9.5 (2) |
Results for 20 intervention and 10 control subjects with complete data are shown in Table 2. Mean weight among intervention subjects decreased from 177.5 lbs to 165.7 lbs at 8 weeks, or a 6.6% decrease, which represents an average loss of approximately 1.5 lbs a week. BMI decreased from 29.7 to 27.8 kg/m2. When adjusted for baseline, intervention subjects lost 9 lbs more than control subjects (p ≤ 0.01, effect size − 0.21) and lost 1.7 kg/m2 more from their BMI (p ≤ 0.01, effect size − 0.26). Concurrently, mean total cholesterol level decreased 17.7% and mean LDL cholesterol levels decreased 21.4% to 82.2 mg/dL within the intervention group. Compared to control, mean total cholesterol levels decreased by 35.3 mg/dL (p ≤ 0.01, effect size − 0.93) and mean LDL levels decreased by 23.5 mg/dL (p ≤ 0.01, effect size − 0.75) in the intervention group.
Table 2.
Outcomes between baseline and 8 weeks in intervention and control groups
| Outcome | Intervention diet | Usual diet control | Between group differences in change at week 8 (adjusted for baseline value)d | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 8 | Baseline | Week 8 | Diff | Effect size | p value | |
| Weight (lbs) | 177.5 | 165.7* | 159.9 | 158.8 | − 9.0 | − 0.21 | < 0.01 |
| BMI | 29.7 | 27.8* | 28.4 | 28.2 | − 1.7 | − 0.25 | < 0.01 |
| Cardiometabolic outcomes | |||||||
| Total Cholesterol (mg/dL)a | 193.6 | 159.4* | 174.6 | 181.4 | − 35.3 | − 0.93 | < 0.01 |
| LDL Cholesterol (mg/dL)a | 104.6 | 82.2* | 92.3 | 97.9 | − 23.5 | − 0.75 | < 0.01 |
| Triglycerides (mg/dL)a | 111.1 | 113.0 | 106.7 | 118.4 | − 8.8 | − 0.21 | 0.55 |
| HDL (mg/dL)a | 66.7 | 54.7* | 60.8 | 59.8 | − 9.3 | − 0.60 | < 0.01 |
| Systolic BP (mmHg)b | 113.2 | 110.3 | 111.0 | 113.3 | − 3.9 | − 0.33 | 0.28 |
| Diastolic BP (mmHg)b | 71.3 | 68.8 | 65.3 | 66.7 | − 1.9 | − 0.16 | 0.50 |
| Glucose (mg/dL) | 101.5 | 93.8 | 114.4 | 117.3 | − 11.9 | − 0.45 | 0.16 |
| Insulin (uIU/L) | 16.8 | 11.2* | 11.4 | 12.1 | − 3.8 | − 0.39 | 0.12 |
| HOMA-IR | 4.4 | 2.7* | 3.2 | 3.5 | − 1.3 | − 0.43 | 0.10 |
| Hormonal markers | |||||||
| Sex Hormone Binding Globulin (nmol/L) | 74.3 | 98.2* | 89.0 | 78.9* | 33.4 | 0.84 | < 0.01 |
| Total Testosterone (ng/dL) | 23.7 | 23.7 | 16.8 | 15.6 | 2.7 | 0.19 | 0.21 |
| Free Testosterone (ng/dL) | 0.49 | 0.32 | 0.25 | 0.28 | − 0.01 | − 0.03 | 0.90 |
| DHEA (ug/dL) | 110.9 | 106.8 | 56.5 | 53.7 | 2.8 | 0.06 | 0.72 |
| IGF-1 (ng/mL) | 173.8 | 156.4* | 150.5 | 144.7 | − 7.9 | − 0.16 | 0.38 |
| IGFBP-3 (ng/mL) | 4966 | 4874 | 4415 | 4418 | − 23.7 | − 0.02 | 0.92 |
| Blood counts | |||||||
| White Blood Cells (1000/uL) | 3.7 | 3.3 | 4.8 | 4.7 | − 0.7 | − 0.37 | 0.06 |
| Neutrophils (1000/uL) | 2.0 | 1.9 | 3.0 | 2.8 | − 0.4 | − 0.25 | 0.16 |
| Lymphocytes (1000/uL) | 1.1 | 1.0 | 1.3 | 1.3 | − 0.1 | − 0.17 | 0.38 |
| Hemoglobin (g/dL) | 12.3 | 12.2 | 12.1 | 12.4 | − 0.3 | − 0.27 | 0.37 |
| Platelet (1000/uL) | 215 | 206 | 222 | 223 | − 12.0 | − 0.18 | 0.34 |
| Cancer progression markers | |||||||
| CA 27.29 (U/mL)c | 25.7 | 24.6 | 84.9 | 97.5 | − 5.3 | − 0.08 | 0.23 |
| CA 15-3 (U/mL)c | 22.3 | 22.7 | 90.8 | 111.2 | − 5.2 | − 0.07 | 0.53 |
| CEA (ng/mL) | 3.1 | 3.2 | 7.9 | 10.2 | − 0.5 | − 0.07 | 0.54 |
Values are means
*p < 0.05 for within-group change
aOne intervention subject was excluded from the cholesterol analysis due to having stopped her cholesterol medication midway through the study
bOne intervention subject was excluded from the blood pressure analysis due to missing a baseline measurement
cOne intervention subject was excluded from the cancer marker analysis due to being an extreme outlier
dThe mean between-group difference in change from the baseline to 8 weeks was estimated by marginal means calculated via linear mixed effect model
Blood pressure was at optimal levels in both groups at baseline with no statistically significant changes during the intervention, although blood pressure trended lower in the intervention group. Compared to baseline, mean fasting blood glucose levels were lower within the intervention group at 8 weeks, but this did not meet significance (p = 0.11). Although baseline insulin was within the normal range (3–25 uIU/mL), decreases were noted within the intervention group, from 16.8 uIU/mL to 11.2 uIU/mL (p < 0.01). Insulin resistance, as calculated by HOMA-IR, decreased in the intervention group, from 4.4 to 2.7 (p = 0.01).
Sex hormone binding globulin increased within the intervention group (p ≤ 0.01), likely related to weight loss in this group [43]. SHBG happened to decrease within the control group without a known cause (p = 0.05). When adjusted for baseline, the intervention group saw a 33.4 nmol/L increase in sex hormone binding globulin compared to control (p ≤ 0.01, effect size 0.84). DHEA was not statistically different in either group at 8 weeks. Accordingly, while changes in total testosterone did not reach statistical significance in either group, free testosterone was lower within the intervention group at 8 weeks, though this was not statistically significant (p = 0.08). Estradiol was undetectable at baseline in the majority of subjects given that natural or chemically induced menopause was common, as reflected by the fact that 74% of subjects were on an aromatase inhibitor. Insulin-like growth factor 1 (IGF-1) significantly decreased by 10% within the intervention group (p = 0.01), but the between-group difference was not statistically significant.
White blood cells (WBCs) were slightly lower at 8 weeks within the intervention group, a difference that approached statistical significance (p = 0.06) when compared to the control group. Most participants were on therapy that strongly affects white blood cell counts, and blood draws were not done at the same point in each treatment cycle. The change in WBCs did not appear to be clinically significant, and a mechanistic relationship to the dietary intervention was not established. Hemoglobin and platelets were not significantly different within either group or between the groups from baseline to 8 weeks. There was no statistically significant difference in changes between the groups in serum sodium, potassium chloride, bicarbonate, and calcium, or alanine transaminase (ALT), aspartate aminotransferase (AST), or serum total protein. Kidney function as measured by creatinine and the estimated glomerular filtration rate were not significantly different, but urea nitrogen was significantly lower at 8 weeks within the intervention group (p ≤ 0.01).
In the intervention group, the cancer markers CA 27.29, CA 15-3, and CEA were in the normal range at baseline and were not statistically significantly different at 8 weeks within the intervention group or between the two groups.
Adverse events related to the intervention were infrequent and mild. Three intervention subjects had grade 2 hypotension during the study with mild symptoms (Two on antihypertensives and one on an extensive pain control regimen) and were referred to their routine care providers for medication adjustments. One control subject experienced lightheadedness following a blood draw. Other adverse events (mild, transient neutropenia, aphthous ulcer, transient, mild hyponatremia) were deemed related to medications. One subject in each group had the dose of their primary cancer therapy reduced due to adverse events typical of their medication.
Discussion
This is one of the first studies to demonstrate both feasibility and clinically important improvements from a dietary change in metastatic breast cancer patients receiving systemic therapy. Results from this study showed that our whole-food, plant-based dietary intervention promotes significant weight loss and improves several cardiometabolic and hormonal risk factors among women with metastatic breast cancer. Specifically, subjects in the intervention group, who had a baseline BMI of 29.7 kg/m2, lost 1–2 pounds a week and saw significant improvements, both clinically and statistically, in cholesterol, insulin, insulin resistance, sex hormone binding globulin, and IGF-1.
Several other nutrition and lifestyle trials for cancer survivors, conducted after subjects had completed their primary cancer treatment, have demonstrated feasibility and weight loss, but most show lower or significantly slower weight loss than this trial [31–40]. Weight loss in this study reflects the large nutritional changes achieved, described in a separate report [42]. While this amount of weight loss may be larger and/or faster compared to previous cancer interventions, the rate of weight loss is consistent with other interventions using whole-food, plant-based diets, even when no prepared food is provided [18, 44]. It is also consistent with recommendations for individuals with excess weight in the general population to target 1 to 2 pounds of weight loss per week during their weight loss efforts [45].
The weight loss was due to large, intentional dietary changes, without signs or symptoms of concurrently progressing disease or cachexia, and it was achieved without portion or calorie restriction or mandated exercise. Coaching included frequent recommendations to eat greater volumes of food and eat more frequently, while choosing foods that were ‘on plan.’ When comparing the final 3-day food diary with the baseline 3-day food diary, intervention subjects had 16% greater dietary intake (solid plus liquid intake) in terms of weight but consumed 26% fewer calories, suggesting significantly reduced calorie density of the study diet compared to their baseline diets.
Consistent with weight loss, the cardiometabolic and hormonal milieu improved within the intervention group. These changes likely relate to a convergence of mechanisms. Weight loss has been shown to increase SHBG [43, 46], lower free androgens [43], and improve cardiometabolic risk factors including insulin, insulin resistance, and cholesterol levels [47, 48]. However, the specific plant-based dietary composition also likely played a role. Dietary patterns with substantial increases in dietary fiber and substantial reductions in saturated fat and/or animal protein have been tested in various human trials and found to modulate serum cholesterol [49, 50], insulin resistance [51, 52], sex hormones [53–55], and IGF-1 [56].
Whether this weight loss or risk factor modification improves cancer-specific progression or mortality is unknown. Barnard et al. [57] found that, in post-menopausal women with overweight or obesity, a 2-week intervention consisting of an ad libitum whole-food, plant-predominant diet and exercise resulted in significantly reduced estradiol, insulin, and IGF-1. When comparing subjects’ pre- and post-intervention serum in vitro, using 3 estrogen receptor-positive breast cancer cell lines, there was a significant decrease in cell growth and increase in apoptosis concurrent with improvements in biomarkers following the diet and lifestyle intervention.
In our study, there were no significant changes in cancer markers at 8 weeks, although the intervention group showed a more stable trend compared to the control group. Among participants who did not have elevated cancer markers during the study, 50% of control participants and 46% of intervention participants previously had elevated markers, suggesting a relatively similar percentage of participants in both groups who had cancer markers that reflected cancer activity. The normal baseline levels among the intervention group, along with our small sample size, limited our ability to detect larger changes. The differences in baseline levels between the two groups were made more likely by the possibility of wide variation in cancer marker levels combined with our smaller sample size. In addition to these limitations, the short duration of the trial makes changes in cancer markers more difficult to interpret given the possibility of spurious results in the first 4–6 weeks following therapy changes [58].
This randomized controlled trial has numerous limitations and strengths. The study duration limits our ability to know whether these findings are sustainable, and whether these findings affect risks of cancer progression or mortality. This brief intervention was not intended to support long-term behavior change, and participants’ outcomes will not be followed over time. The size of the study, particularly the smaller control group, limits our ability to detect smaller differences in outcomes. In addition, the lack of racial diversity as well as the overrepresentation of hormone-receptor positive breast cancer both limit generalizability. The control group was not matched in terms of time with, and attention from, study staff, making it harder to isolate the effects of the dietary changes from the effects of the overall intervention. Strengths of the study include the very large dietary changes achieved and high retention rate, presumably related to the intensity of the intervention and the provided food.
Conclusion
Our whole-food, plant-based intervention among women with metastatic breast cancer is feasible and results in clinically significant improvements in weight and related cardiometabolic and hormonal risk factors. This is one of the first RCTs to demonstrate that dietary changes during systemic treatment are well tolerated and result in these clinically important improvements. This is particularly relevant for this population, which is highly interested in nutrition and concerned with treatment-related weight gain, its comorbid conditions, and its implications for cancer-related outcomes. Trials of longer duration are required to understand the sustainability of these findings as well as their effects on cancer progression and mortality.
Acknowledgements
The authors would like to acknowledge Kelly Koch for her work coordinating a portion of this study and Laurie Taillie for her role in food provision.
Abbreviations
- BC
Breast cancer
- CA 27.29
Cancer antigen 27.29
- CA 15-3
Cancer antigen 15-3
- CEA
Carcinoembryonic antigen
- DHEA
Dehydroepiandrosterone
- HOMA-IR
Homeostatic Model Assessment of Insulin Resistance
- IGF-1
Insulin-like growth factor-1
- IGFBP-3
Insulin-like growth factor-1 binding protein-3
- LDL
Low-density lipoprotein
- SHBG
Sex hormone binding globulin
- WFPB
Whole-food, plant-based
Author contributions
TMC contributed to conceptualization; funding acquisition; investigation; methodology; project administration; resources; supervision; and writing—original draft; EKC contributed to conceptualization; data curation; investigation; methodology; project administration; resources; supervision; and writing—review & editing; EC contributed to data curation; formal analysis; methodology; resources; and writing—review & editing; LMB contributed to data curation; formal analysis; investigation; project administration; and writing—review & editing; NW contributed to data curation; investigation; methodology; project administration; resources; and writing—review & editing; JJG contributed to data curation; formal analysis; and writing—review & editing; JF contributed to conceptualization; methodology; and writing—review & editing; AH, MS, MCJ and RGM contributed to resources and writing—review & editing; KMM contributed to funding acquisition; resources; and writing—review & editing; LJP contributed to conceptualization; methodology; project administration; resources; supervision; visualization; and writing—review & editing. All authors read and approved the final manuscript.
Funding
This work was supported by the Highland Hospital Foundation, with donations from The Ladybug Foundation, T. Colin Campbell Center for Nutrition Studies and multiple individuals. Support was also provided by US National Institutes of Health (UG1-CA189961). Funders had no role in study design, collection, analysis, and interpretation of data, or writing of the report and there were no restrictions regarding the submission of the report for publication. Angle, PLC provided parsortix testing kits at no charge as well as services related to result analysis.
Data availability
The data underlying this article are available by request at https://gitlab-public.circ.rochester.edu/WFPB-breast-cancer/biomarkers.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the U.S. Common Rule. The study protocol was approved by the University of Rochester Research Subject Review Board (ClinicalTrials.gov identifier: NCT03045289; registration February 7, 2017). Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
TMC: Royalties from general interest books about plant-based nutrition (Benbella Books, Penguin Random House) and income from a lifestyle medicine practice, Thomas M. Campbell, MD PLLC; EKC: Conflicts of spouse (TMC); AH: MJH Healthcare Holdings (OncLive), Mediflix (Skipta/Informa); RGM: Consultant for Fujirebio Diagnostics. Research funding from Angle plc. The rest of the authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Makari-Judson G, Braun B, Jerry DJ, Mertens WC. Weight gain following breast cancer diagnosis: implication and proposed mechanisms. World J Clin Oncol. 2014;5(3):272–282. doi: 10.5306/wjco.v5.i3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demark-Wahnefried W, Rimer BK, Winer EP. Weight gain in women diagnosed with breast cancer. J Am Diet Assoc. 1997;97(5):519–526. doi: 10.1016/s0002-8223(97)00133-8. [DOI] [PubMed] [Google Scholar]
- 3.Ee C, Cave AE, Naidoo D, Bilinski K, Boyages J. Weight before and after a diagnosis of breast cancer or ductal carcinoma in situ: a national Australian survey. BMC Cancer. 2020;20(1):113. doi: 10.1186/s12885-020-6566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ligibel JA, Huebner L, Rugo HS, Burstein HJ, Toppmeyer DL, Anders CK, Ma C, Barry WT, Suman V, Carey LA, et al. Physical activity, weight, and outcomes in patients receiving chemotherapy for metastatic breast cancer (C40502/alliance) JNCI Cancer Spectr. 2021 doi: 10.1093/jncics/pkab025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Lu W, Zheng W, Gu K, Chen Z, Zheng Y, Shu XO. Obesity and weight change in relation to breast cancer survival. Breast Cancer Res Treat. 2010;122(3):823–833. doi: 10.1007/s10549-009-0708-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwan ML, Chen WY, Kroenke CH, Weltzien EK, Beasley JM, Nechuta SJ, Poole EM, Lu W, Holmes MD, Quesenberry CP, Jr, et al. Pre-diagnosis body mass index and survival after breast cancer in the After Breast Cancer Pooling Project. Breast Cancer Res Treat. 2012;132(2):729–739. doi: 10.1007/s10549-011-1914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ewertz M, Jensen MB, Gunnarsdottir KA, Hojris I, Jakobsen EH, Nielsen D, Stenbygaard LE, Tange UB, Cold S. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29(1):25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 8.Conroy SM, Maskarinec G, Wilkens LR, White KK, Henderson BE, Kolonel LN. Obesity and breast cancer survival in ethnically diverse postmenopausal women: the Multiethnic Cohort Study. Breast Cancer Res Treat. 2011;129(2):565–574. doi: 10.1007/s10549-011-1468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Playdon MC, Bracken MB, Sanft TB, Ligibel JA, Harrigan M, Irwin ML. Weight gain after breast cancer diagnosis and all-cause mortality: systematic review and meta-analysis. J Natl Cancer Inst. 2015;107(12):2djv275. doi: 10.1093/jnci/djv275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imayama I, Alfano CM, Neuhouser ML, George SM, Wilder Smith A, Baumgartner RN, Baumgartner KB, Bernstein L, Wang CY, Duggan C, et al. Weight, inflammation, cancer-related symptoms and health related quality of life among breast cancer survivors. Breast Cancer Res Treat. 2013;140(1):159–176. doi: 10.1007/s10549-013-2594-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheean P, Gomez-Perez S, Joyce C, Vasilopoulos V, Bartolotta MB, Robinson P, Lo S, Lomasney L. Body composition, serum biomarkers of inflammation and quality of life in clinically stable women with estrogen receptor positive metastatic breast cancer. Nutr Cancer. 2019;71(6):981–991. doi: 10.1080/01635581.2019.1595053. [DOI] [PubMed] [Google Scholar]
- 12.Kang C, LeRoith D, Gallagher EJ. Diabetes, obesity, and breast cancer. Endocrinology. 2018;159(11):3801–3812. doi: 10.1210/en.2018-00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallagher EJ, LeRoith D. Hyperinsulinaemia in cancer. Nat Rev Cancer. 2020;20(11):629–644. doi: 10.1038/s41568-020-0295-5. [DOI] [PubMed] [Google Scholar]
- 14.Lee K, Kruper L, Dieli-Conwright CM, Mortimer JE. The impact of obesity on breast cancer diagnosis and treatment. Curr Oncol Rep. 2019;21(5):41–41. doi: 10.1007/s11912-019-0787-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradshaw PT, Stevens J, Khankari N, Teitelbaum SL, Neugut AI, Gammon MD. Cardiovascular disease mortality among breast cancer survivors. Epidemiology. 2016;27(1):6–13. doi: 10.1097/ede.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Cancer Research Fund/American Institute for Cancer Research: Continuous Update Project Expert Report 2018. Recommendations and public health and policy implications. Available at dietandcancerreport.org. In
- 17.Rock CL, Thomson C, Gansler T, Gapstur SM, McCullough ML, Patel AV, Andrews KS, Bandera EV, Spees CK, Robien K, et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin. 2020;70(4):245–271. doi: 10.3322/caac.21591. [DOI] [PubMed] [Google Scholar]
- 18.Wright N, Wilson L, Smith M, Duncan B, McHugh P. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7(3):e256. doi: 10.1038/nutd.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnard ND, Levin SM, Yokoyama Y. A systematic review and meta-analysis of changes in body weight in clinical trials of vegetarian diets. J Acad Nutr Diet. 2015 doi: 10.1016/j.jand.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, Sparler S, Armstrong WT, Ports TA, Kirkeeide RL, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280(23):2001–2007. doi: 10.1001/jama.280.23.2001. [DOI] [PubMed] [Google Scholar]
- 21.Esselstyn CB, Jr, Ellis SG, Medendorp SV, Crowe TD. A strategy to arrest and reverse coronary artery disease: a 5-year longitudinal study of a single physician's practice. J Fam Pract. 1995;41(6):560–568. [PubMed] [Google Scholar]
- 22.Yokoyama Y, Levin SM, Barnard ND. Association between plant-based diets and plasma lipids: a systematic review and meta-analysis. Nutr Rev. 2017;75(9):683–698. doi: 10.1093/nutrit/nux030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KW, Loh HC, Ching SM, Devaraj NK, Hoo FK. Effects of vegetarian diets on blood pressure lowering: a systematic review with meta-analysis and trial sequential analysis. Nutrients. 2020 doi: 10.3390/nu12061604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banaszak M, Gorna I, Przyslawski J. Non-pharmacological treatments for insulin resistance: effective intervention of plant-based diets-a critical review. Nutrients. 2022 doi: 10.3390/nu14071400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88(3):674–684. doi: 10.1002/(SICI)1097-0142(20000201)88:3<674::AID-CNCR26>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 26.Oostra DL, Burse NR, Wolf LJ, Schleicher E, Mama SK, Bluethmann S, Schmitz K, Winkels RM. Understanding nutritional problems of metastatic breast cancer patients: opportunities for supportive care through eHealth. Cancer Nurs. 2021;44(2):154–162. doi: 10.1097/NCC.0000000000000788. [DOI] [PubMed] [Google Scholar]
- 27.Demark-Wahnefried W, Schmitz KH, Alfano CM, Bail JR, Goodwin PJ, Thomson CA, Bradley DW, Courneya KS, Befort CA, Denlinger CS, et al. Weight management and physical activity throughout the cancer care continuum. CA Cancer J Clin. 2018;68(1):64–89. doi: 10.3322/caac.21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ligibel JA, Alfano CM, Hershman D, Ballard RM, Bruinooge SS, Courneya KS, Daniels EC, Demark-Wahnefried W, Frank ES, Goodwin PJ, et al. Recommendations for obesity clinical trials in cancer survivors: American Society of Clinical Oncology Statement. J Clin Oncol. 2015;33(33):3961–3967. doi: 10.1200/JCO.2015.63.1440. [DOI] [PubMed] [Google Scholar]
- 29.Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK, Goodman MT, Giuliano AE, Karanja N, McAndrew P, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study. J Natl Cancer Inst. 2006;98(24):1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 30.Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, Rock CL, Kealey S, Al-Delaimy WK, Bardwell WA, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women's Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298(3):289–298. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rock CL, Flatt SW, Byers TE, Colditz GA, Demark-Wahnefried W, Ganz PA, Wolin KY, Elias A, Krontiras H, Liu J, et al. Results of the exercise and nutrition to enhance recovery and good health for you (ENERGY) trial: a behavioral weight loss intervention in overweight or obese breast cancer survivors. J Clin Oncol. 2015;33(28):3169–3176. doi: 10.1200/JCO.2015.61.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stolley M, Sheean P, Gerber B, Arroyo C, Schiffer L, Banerjee A, Visotcky A, Fantuzzi G, Strahan D, Matthews L, et al. Efficacy of a weight loss intervention for african american breast cancer survivors. J Clin Oncol. 2017;35(24):2820–2828. doi: 10.1200/JCO.2016.71.9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reeves MM, Terranova CO, Eakin EG, Demark-Wahnefried W. Weight loss intervention trials in women with breast cancer: a systematic review. Obes Rev. 2014;15(9):749–768. doi: 10.1111/obr.12190. [DOI] [PubMed] [Google Scholar]
- 34.Santa-Maria CA, Coughlin JW, Sharma D, Armanios M, Blackford AL, Schreyer C, Dalcin A, Carpenter A, Jerome GJ, Armstrong DK, et al. The effects of a remote-based weight loss program on adipocytokines, metabolic markers, and telomere length in breast cancer survivors: the POWER-Remote Trial. Clin Cancer Res. 2020;26(12):3024–3034. doi: 10.1158/1078-0432.CCR-19-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodwin PJ, Segal RJ, Vallis M, Ligibel JA, Pond GR, Robidoux A, Findlay B, Gralow JR, Mukherjee SD, Levine M, et al. The LISA randomized trial of a weight loss intervention in postmenopausal breast cancer. NPJ Breast Cancer. 2020;6:6. doi: 10.1038/s41523-020-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demark-Wahnefried W, Rogers LQ, Gibson JT, Harada S, Fruge AD, Oster RA, Grizzle WE, Norian LA, Yang ES, Della Manna D, et al. Randomized trial of weight loss in primary breast cancer: Impact on body composition, circulating biomarkers and tumor characteristics. Int J Cancer. 2020;146(10):2784–2796. doi: 10.1002/ijc.32637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cortesi L, Sebastiani F, Iannone A, Marcheselli L, Venturelli M, Piombino C, Toss A, Federico M. Lifestyle Intervention on body weight and physical activity in patients with breast cancer can reduce the risk of death in obese women: The EMILI Study. Cancers (Basel) 2020 doi: 10.3390/cancers12071709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell KL, Van Patten CL, Neil SE, Kirkham AA, Gotay CC, Gelmon KA, McKenzie DC. Feasibility of a lifestyle intervention on body weight and serum biomarkers in breast cancer survivors with overweight and obesity. J Acad Nutr Diet. 2012;112(4):559–567. doi: 10.1016/j.jada.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 39.Befort CA, Kimler BF, Bantis LE, Phillips TA, Fabian CJ. Effects of weight loss and weight regain on circulating biomarkers in overweight/obese breast cancer survivors enrolled in a weight loss trial in the rural midwest. Cancer Epidemiol Biomark Prev. 2020;29(7):1321–1328. doi: 10.1158/1055-9965.EPI-19-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenlee H, Lew DL, Hershman DL, Newman VA, Hansen L, Hartman SJ, Korner J, Shi Z, Sardo Molmenti CL, Sayegh A, et al. Phase II feasibility study of a weight loss intervention in female breast and colorectal cancer survivors (SWOG S1008) Obesity (Silver Spring) 2018;26(10):1539–1549. doi: 10.1002/oby.22269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallicchio L, Devasia TP, Tonorezos E, Mollica MA, Mariotto A. Estimation of the numbers of individuals living with metastatic cancer in the United States. J Natl Cancer Inst. 2022 doi: 10.1093/jnci/djac158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell EK, Campbell TM, Culakova E, Blanchard L, Wixom N, Guido J, Fetten J, Huston A, Shayne M, Janelsins MC, et al. A whole food, plant-based randomized controlled trial in metastatic breast cancer: feasibility, nutrient, and patient-reported outcomes. Res Sq (preprint) 2023 doi: 10.21203/rs.3.rs-3606685/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aubuchon M, Liu Y, Petroski GF, Thomas TR, Polotsky AJ. The impact of supervised weight loss and intentional weight regain on sex hormone binding globulin and testosterone in premenopausal women. Syst Biol Reprod Med. 2016;62(4):283–289. doi: 10.1080/19396368.2016.1177619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell EK, Fidahusain M, Campbell TM. Evaluation of an eight-week whole-food plant-based lifestyle modification program. Nutrients. 2019 doi: 10.3390/nu11092068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.NHLBI Obesity Education Initiative Expert Panel on the Identification Evaluation and Treatment of Overweight and Obesity in Adults.: Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. In., vol. NIH Publication No. 98–4083: National Institutes of Health
- 46.Rock CL, Pande C, Flatt SW, Ying C, Pakiz B, Parker BA, Williams K, Bardwell WA, Heath DD, Nichols JF. Favorable changes in serum estrogens and other biologic factors after weight loss in breast cancer survivors who are overweight or obese. Clin Breast Cancer. 2013;13(3):188–195. doi: 10.1016/j.clbc.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andersen RE, Wadden TA, Bartlett SJ, Vogt RA, Weinstock RS. Relation of weight loss to changes in serum lipids and lipoproteins in obese women. Am J Clin Nutr. 1995;62(2):350–357. doi: 10.1093/ajcn/62.2.350. [DOI] [PubMed] [Google Scholar]
- 48.Schenk S, Harber MP, Shrivastava CR, Burant CF, Horowitz JF. Improved insulin sensitivity after weight loss and exercise training is mediated by a reduction in plasma fatty acid mobilization, not enhanced oxidative capacity. J Physiol. 2009;587(Pt 20):4949–4961. doi: 10.1113/jphysiol.2009.175489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferdowsian HR, Barnard ND. Effects of plant-based diets on plasma lipids. Am J Cardiol. 2009;104(7):947–956. doi: 10.1016/j.amjcard.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 50.Sirtori CR, Agradi E, Conti F, Mantero O, Gatti E. Soybean-protein diet in the treatment of type-II hyperlipoproteinaemia. Lancet. 1977;1(8006):275–277. doi: 10.1016/S0140-6736(77)91823-2. [DOI] [PubMed] [Google Scholar]
- 51.Smith GI, Yoshino J, Kelly SC, Reeds DN, Okunade A, Patterson BW, Klein S, Mittendorfer B. High-Protein intake during weight loss therapy eliminates the weight-loss-induced improvement in insulin action in obese postmenopausal women. Cell Rep. 2016;17(3):849–861. doi: 10.1016/j.celrep.2016.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riccardi G, Giacco R, Rivellese AA. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr. 2004;23(4):447–456. doi: 10.1016/j.clnu.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 53.Hamalainen EK, Adlercreutz H, Puska P, Pietinen P. Decrease of serum total and free testosterone during a low-fat high-fibre diet. J Steroid Biochem. 1983;18(3):369–370. doi: 10.1016/0022-4731(83)90117-6. [DOI] [PubMed] [Google Scholar]
- 54.Goldin BR, Woods MN, Spiegelman DL, Longcope C, Morrill-LaBrode A, Dwyer JT, Gualtieri LJ, Hertzmark E, Gorbach SL. The effect of dietary fat and fiber on serum estrogen concentrations in premenopausal women under controlled dietary conditions. Cancer. 1994;74(3 Suppl):1125–1131. doi: 10.1002/1097-0142(19940801)74:3+<1125::aid-cncr2820741521>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 55.Bagga D, Ashley JM, Geffrey SP, Wang HJ, Barnard RJ, Korenman S, Heber D. Effects of a very low fat, high fiber diet on serum hormones and menstrual function. Implications for breast cancer prevention. Cancer. 1995;76(12):2491–2496. doi: 10.1002/1097-0142(19951215)76:12<2491::aid-cncr2820761213>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 56.Key TJ. Diet, insulin-like growth factor-1 and cancer risk. Pro Nutr Soc. 2011 doi: 10.1017/S0029665111000127. [DOI] [PubMed] [Google Scholar]
- 57.Barnard RJ, Gonzalez JH, Liva ME, Ngo TH. Effects of a low-fat, high-fiber diet and exercise program on breast cancer risk factors in vivo and tumor cell growth and apoptosis in vitro. Nutr Cancer. 2006;55(1):28–34. doi: 10.1207/s15327914nc5501_4. [DOI] [PubMed] [Google Scholar]
- 58.Chu WG, Ryu DW. Clinical significance of serum CA15-3 as a prognostic parameter during follow-up periods in patients with breast cancer. Ann Surg Treat Res. 2016;90(2):57–63. doi: 10.4174/astr.2016.90.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available by request at https://gitlab-public.circ.rochester.edu/WFPB-breast-cancer/biomarkers.