Abstract
Introduction
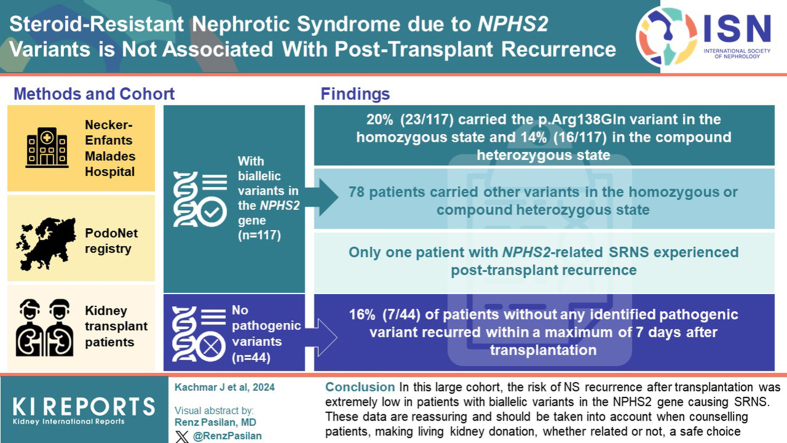
Unlike idiopathic nephrotic syndrome (NS), hereditary podocytopathies are not expected to recur after kidney transplantation. However, some reports of posttransplant recurrence of NS in patients carrying variants in the NPHS2 gene have been described, notably with the p.Arg138Gln variant, which is more prevalent in Europe. The objective of this study was to assess the risk of recurrence after kidney transplantation in a large cohort of patients with biallelic NPHS2 pathogenic variants.
Methods
Since January 2010, 61 patients identified at Necker-Enfants Malades Hospital and 56 enrolled in the PodoNet Registry with biallelic variants in the NPHS2 gene were transplanted and were compared with 44 transplanted children with steroid-resistant NS (SRNS) without any identified pathogenic variant.
Results
Of the 117 patients, 23 carried the p.Arg138Gln variant in the homozygous state and 16 in the compound heterozygous state. The other 78 patients carried different variants in the homozygous (n = 44) or compound heterozygous state. Only 1 patient with NPHS2-related SRNS experienced posttransplant recurrence (median follow-up of cohort 8.5 years [2.5–15]). Conversely, 7 of 44 patients (16%) without any identified pathogenic variant recurred within a maximum of 7 days after transplantation (median follow-up 8.9 years [0.6–13.9]).
Conclusion
In this large cohort, the risk of patients with causative variants in the NPHS2 gene to develop NS recurrence after kidney transplantation was extremely low. This is coherent with the pathophysiology of intrinsic slit-diaphragm disease. These data are reassuring and should be considered when counselling patients, making living kidney donation, whether related or not, a safe choice.
Keywords: glomerular disease, hereditary nephrotic syndrome, kidney transplantation, NPHS2 gene, posttransplant recurrence, steroid-resistant nephrotic syndrome
Graphical abstract
NS is a major cause of glomerulopathy in children and adults. It is responsible for 15% of kidney failure (KF) in patients aged less than 25 years.1 Recurrence of NS after transplantation is a challenging condition that may occur in 30% to 50% of cases.2, 3, 4, 5, 6, 7, 8 In the past 2 decades, genetic analyses performed in patients with SRNS allowed researchers and clinicians to classify them into 2 groups: patients with a pathogenic variant in a podocyte gene and those without any identified pathogenic variant. Patients with confirmed hereditary NS are not expected to experience disease recurrence after transplantation, whereas patients without any identified pathogenic variant are more likely to experience recurrence because NS is associated, in some of these cases, with an immune-related circulating factor.5, 6, 7,9, 10, 11 One strong predictor of recurrence after transplantation is initial steroid-sensitivity, which indicates probable immune etiology.7,12,13
The NPHS2 gene (NM_014625.4) encodes podocin, a protein that is expressed at the slit diaphragm in podocytes.14,15 Variants in NPHS2 were first identified in 200015 and account for 12% to 26% of SRNS cases,10,16, 17, 18 more precisely 40% to 57% of familial cases, and 6% to 26% of sporadic cases.6,10,19 Pathogenic variants in the NPHS2 gene are the most commonly detected pathogenic variants in children older than 4 months with SRNS.20 Patients carrying biallelic variants in the NPHS2 gene typically reach KF between the neonatal period and the second decade, with a mean age of 6.6 years.16,19,21,22 Most transplanted patients will never experience recurrence.5, 6, 7,9, 10, 11 However, some cases of recurrence of NS after kidney transplantation in patients carrying variants in the NPHS2 gene have been described, notably with the p.Arg138Gln variant, the most prevalent variant in Europe.6,10,16,23, 24, 25 This has clinical implications, particularly when it comes to genetic counselling and living kidney donation.
In order to determine whether the p.Arg138Gln variant or other NPHS2 variants were associated with recurrence of NS after transplantation, we investigate herein a large cohort of patients with homozygous or compound heterozygous variants in the NPHS2 gene. We compared the outcome between the following: (i) patients carrying the NPHS2 p.Arg138Gln variant, (ii) patients carrying other NPHS2 pathogenic variants, and (iii) patients with SRNS and no identified pathogenic variant.
Methods
Patients
Data were collected from patients with SRNS referred to Necker-Enfants Malades Hospital for genetic testing from January 2010 to March 2018. The independent validation group comprised patients from the PodoNet Registry (www.podonet.org) enrolled from 2011 to 2020. Genetic testing identified biallelic variants in the NPHS2 gene in 175 patients referred to Necker-Enfants Malades and 181 from the PodoNet registry; 61 and 56, respectively, of whom received a kidney transplant and were included in the study. They were compared to 44 patients with primary SRNS who had received a kidney transplant and who had no pathogenic variant identified by gene panel testing. One patient with the p.Arg229Gln and p.Arg138Gln variants at the compound heterozygous state was excluded from the NPHS2 group because this association is not pathogenic.26 This patient was included in the group with no identified pathogenic variant.
We divided the patients into 3 groups as follows: (i) patients bearing the p.Arg138Gln NPHS2 variant in trans to another pathogenic variant, (ii) patients with other biallelic pathogenic NPHS2 variants, and (iii) patients with no identified pathogenic variant.
The following baseline characteristics were collected and compared: sex (classification of male or female based on biological distinction), familial or sporadic cases, age at diagnosis, kidney histology, age at KF, and age at transplantation. Recurrence was defined as reappearance of nephrotic-range proteinuria (≥200 mg/mmol or ≥2.0 g/g)7 after transplantation, with no other apparent cause (such as rejection, infection, drug toxicity, etc.), whether in the short term or long term. Follow-up period was until March 2019 for Necker-Enfants Malades patients and until February 2021 for PodoNet patients.
Genetic Testing
Molecular diagnosis in patients with pathogenic variants in the NPHS2 (NM_014625.4) gene was performed either by direct Sanger sequencing of NPHS2 exons and flanking regions, by next generation sequencing of a panel of genes involved in renal disease, or by whole exome sequencing. On the other hand, in the group with primary SRNS without an identified pathogenic variant, all patients underwent genetic analysis of an updated version of a panel of known genes involved in kidney disease at the time of the study (Supplementary Table S1).
Statistics
All analyses were performed using the Prism GraphPad 8.0.0 software (GraphPad Software, San Diego, CA). Values are expressed as mean±SD for continuous, normally distributed variables, median (25th–75th percentile) for skewed variables and number (percentages) for qualitative variables. Quantitative variables were compared across groups using t-test for normally distributed variable or Mann-Whitney test for skewed variables. Proportions were compared by the Fisher exact 2-tailed test. Kaplan-Meier curve for recurrence-free survival was obtained with Prism GraphPad 8.0.0 software as well. P-values <0.05 were considered statistically significant.
Results
Epidemiology
Epidemiological data are shown in Table 1. Since 2010, we identified 356 patients with biallelic variants in the NPHS2 gene and SRNS, 117 of whom received a kidney transplant. Of the transplanted patients, 39 (33%) carried the p.Arg138Gln variant, in the homozygous (n = 23) or compound heterozygous state (n = 16). The other 78 patients carried homozygous or compound heterozygous NPHS2 variants other than the p.Arg138Gln variant. During the same period, we enrolled 44 children with primary SRNS who had received a kidney transplant and who had no identified pathogenic variant.
Table 1.
Baseline characteristics of patients
| Characteristics of patients | p.R138Q (n = 39) | Other variants (n = 78) | No pathogenic variant (n = 44) |
|---|---|---|---|
| Homozygous variants | 23 | 43 | - |
| Male, n (%) | 19 (49%) | 44 (56%) | 29 (66%) |
| Familial cases (2 cases/3 cases/NC) | 8 (6/0/2) | 27 (9/4/15) | 5 (5/0) |
| Age at first manifestation (Median/1Q/3Q/NC) | 0.7/0.1/3.4/0 | 1.8/0.5/6.3/1 | 9.0/3.4/25.2/0 |
| Kidney histology | |||
| MCD | 7 (18%) | 13 (17%) | 28 (64%) |
| FSGS | 17 (44%) | 42 (54%) | 9 (20%) |
| DMS | 1 (2%) | 1 (1%) | 2 (5%) |
| Other | 5 (13%)a | 9 (11%)b | 0 |
| NC | 9 (23%) | 13 (17%) | 5 (11%) |
| Age at KF (Median/1Q/3Q/NC) | 7.3/4.2/12.6/0 | 9.2/5.6/14.0/6 | 19.0/6.9/33.3/6 |
| Age at kidney transplantation (Median/1Q/3Q/NC) | 9.0/6.2/13.1/2 | 10.7/7.6/14.8/4 | 20.5/9.1/36.5/2 |
DMS, diffuse mesangial sclerosis; FSGS, focal segmental glomerulosclerosis; KF, kidney failure; MCD, minimal change disease; NC, not communicated.
Mesangial proliferative glomerulonephritis (1), mesangiocapillary glomerulonephritis (3), Alport syndrome (1).
Mesangial proliferative glomerulonephritis (4), mesangiocapillary glomerulonephritis (3), C3 glomerulopathy / membranoproliferative glomerulonephritis (1), no lesions (1).
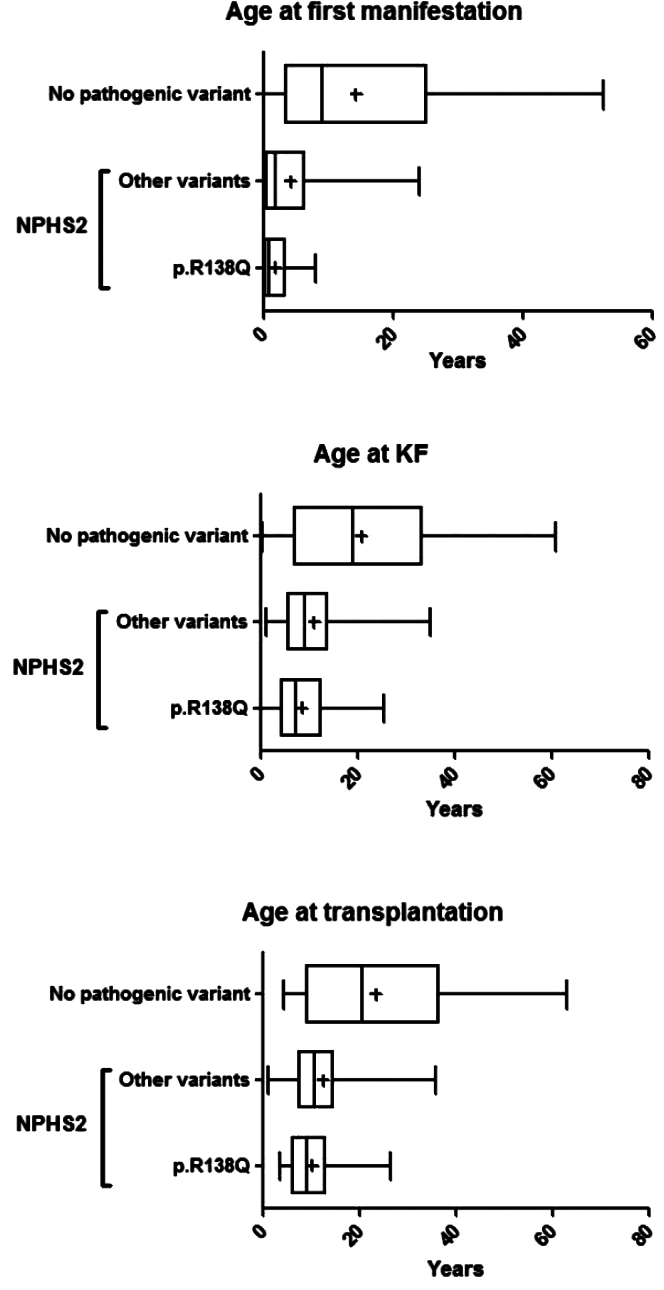
Family history was lacking for 15 patients with identified NPHS2 variants. Assuming their family history was negative, unsurprisingly, familial forms were still more represented in the groups with identified NPHS2 variants compared to the group with no pathogenic variant (30% vs. 11%, respectively, P = 0.005). In addition, age at diagnosis was higher in the group without any identified pathogenic variant compared to the group with homozygous or compound heterozygous p.Arg138Gln variant or 2 other NPHS2 variants (9.0 years vs. 0.7 years and 1.8 years, respectively; P < 0.0001) (Table 1, Figure 1). Kidney biopsy examination at diagnosis found mainly focal segmental glomerulosclerosis (FSGS) lesions in patients with NPHS2 variants whereas minimal change disease lesions were predominant in patients without any identified pathogenic variant. The age of progression to KF was lower in the group with NPHS2 biallelic variants (7.3 and 9.2 years in the p.Arg138Gln variant group and other variants in NPHS2 group, respectively, vs. 19.0 years in the group without any identified pathogenic variant; P = 0.0002) (Table 1, Figure 1). Similarly, median age at kidney transplantation was higher in the group with no pathogenic variant compared to the groups with p.Arg138Gln variant and other NPHS2 variants (20.5, 9.0, and 10.7 years, respectively; P < 0.0001) (Table 1, Figure 1).
Figure 1.
Age at first manifestation, KF and kidney transplantation in patients with identified NPHS2 variants and those with no identified pathogenic variant. Patients without any pathogenic variant identified tend to develop KF and undergo kidney transplantation later than patients with biallelic pathogenic variants. KF, kidney failure.
Genetic Analysis
Thirty-nine patients bore the p.Arg138Gln variant, including 23 at the homozygous state. The 16 other patients had compound heterozygous variants with either a missense variant (n = 5), a nonsense variant (n = 8) or splice variant (n = 3) (Table 2).
Table 2.
NPHS2 variants with p.R138Q
| Variants | n = 39 |
|---|---|
| p.R138Q (c.413G>A) / p.R138X (c.412C>T) | 2 |
| p.R138Q (c.413G>A) / p.R138Q (c.413G>A) | 23 |
| p.R138Q (c.413G>A) / c.451+2T>A | 1 |
| p.R138Q (c.413G>A) / p.V165X (c.460_467insT) | 1 |
| p.R138Q (c.413G>A) / p.R168H (c.503G>A) | 2 |
| p.R138Q (c.413G>A) / c.535-1G>T | 1 |
| p.R138Q (c.413G>A) / p.V180M (c.538G>A) | 2 |
| p.R138Q (c.413G>A) / p.D183Y (c.547G>T) | 1 |
| p.R138Q (c.413G>A) / p.R286Tfs∗17 (c.855_856del) | 5 |
| p.R138Q (c.413G>A) / c.873+2T>A | 1 |
Of the remaining patients with NPHS2 variants other than p.Arg138Gln, 44 had a homozygous variant, mainly p.Val260Glu (n = 9) (Table 3). The additional 34 patients had compound heterozygous variants of which 21 involved the p.Arg229Gln variant (associated with the p.Ala284Val variant located on exon 7 in 10 patients). Among the 156 variants present in 78 patients, there were 31 nonsense variants, 36 frameshift variants, 83 missense variants, 5 splice variants, and 1 small in-phase deletion (Table 3).
Table 3.
NPHS2 variants other than p.R138Q
| Other variants | n = 78 |
|---|---|
| p.E87X (c.259G>T) / p.E87X (c.259G>T) | 5 |
| p.E87X (c.259G>T) / c.535-1G>C | 1 |
| p.R138X (c.412C>T) / p.R138X (c.412C>T) | 5 |
| p.G140Dfs∗41 (c.419del) / p.R36Pfs∗34 (c.104dup) | 2 |
| p.G140Dfs∗41 (c.419del) / p.G140Dfs∗41 (c.419del) | 4 |
| c.451+3 A>T / c.451+3 A>T | 1 |
| p.L156Cfs∗25 (c.460delT) / p.L156Cfs∗25 (c.460delT) | 2 |
| p.V165X (c.460-467insT) / p.V165X (c.460-467insT) | 1 |
| p.L156Ffs∗11 (c.467dup) / p.E87X (c.259G>T) | 1 |
| p.L156Ffs∗11 (c.467dup) / p.L156Ffs∗11 (c.467dup) | 2 |
| p.L156Ffs∗11 (c.467dup) / p.R168S (c.502C>A) | 1 |
| p.L156Ffs∗11 (c.467dup) / p.V180M (c.538G>A) | 1 |
| p.R168C (c.502C>T) / p.R168C (c.502C>T) | 1 |
| p.R168H (c.503G>A) / p.G92C (c.274G>T) | 2 |
| p.R168H (c.503G>A) / p.R168H(c.503G>A) | 1 |
| p.L169P (c.506T>C) / p.L169P (c.506T>C) | 2 |
| p.P118L (c.535C>T) / p.P118L(c.535C>T) | 4 |
| p.P118L (c.535C>T) / p.R238S (c.714G>T) | 1 |
| p.V180M (c.538G>A) / p.V180M (c.538G>A) | 2 |
| p.Q215X (c.643C>T) / p.I114del (c.342_344delTCA) | 1 |
| p.Q219X (c.655C>T) / p.Q219X (c.655C>T) | 1 |
| p.R229Q (c.686G>A) / p.A284V (c.851C>T) | 10 |
| p.R229Q (c.686G>A) / p.R286Tfs∗17 (c.855_856del) | 2 |
| p.R229Q (c.686G>A) / p.R291W (c.871C>T) | 2 |
| p.R229Q (c.686G>A) / p.A297V (c.890C>T) | 1 |
| p.R229Q (c.686G>A) / p.E310K (c.928G>A) | 1 |
| p.R229Q (c.686G>A) / p.F344Lfs∗4 (c.1032delT) | 5 |
| p.V260E (c.779T>A) / p.V260E (c.779T>A) | 9 |
| p.R286Tfs∗17 (c.855_856del) / p.R286Tfs∗17 (c.855_856del) | 2 |
| p.R286Tfs∗17 (c.855_856del) / p.R322X (c.964C>T) | 2 |
| c.873+1 G>T / c.873+1 G>T | 1 |
| p.L347X (c.948delT) / p.L347X (c.948delT) | 1 |
| p.P343S (c.1027C>T) / p.S120P (c.358T>C) | 1 |
Recurrence
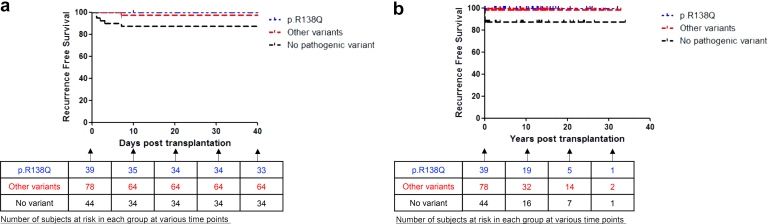
In our cohort of 117 transplanted patients with NPHS2 gene pathogenic variants, 1 patient experienced recurrence after transplantation. The other 116 patients had no recurrence of disease, whether in the short term or long term (median follow-up of cohort 8.5 [2.5–15] years) (Figure 2). Conversely, 7 of 44 patients without any identified variant experienced recurrence within a maximum of 7 days after transplantation (median follow-up 8.9 [0.6–13.9] years). Graft loss due to recurrence occurred in 3 of these cases.
Figure 2.
(a) Recurrence free survival in patients with identified NPHS2 variants compared to those with no identified pathogenic variant, in the short term. (b) Recurrence free survival in patients with identified NPHS2 variants compared to those with no identified pathogenic variant, in the long term. Other than in 1 patient, no recurrence was observed with short and long term follow-up in patients with biallelic pathogenic variants in NPHS2. All recurrences occurred within 7 days post transplantation, whether in the sole patient with biallelic variants in NPHS2 or in the cohort of patients without pathogenic variants.
The patient who experienced recurrence in the cohort of patients with NPHS2 pathogenic variants was a case from the PodoNet registry carrying the NPHS2 homozygous p.Leu347∗ variant who was reported in 2003 by Billing et al.24 This patient was a 4-year-old girl who presented with SRNS at the age of 6 months characterized by heavy proteinuria (4.4 g/l) as well as hematuria and hypogammaglobulinemia. Her kidney biopsy had revealed FSGS lesions. She developed KF and received a kidney from her mother who was heterozygous for the p.Leu347∗ variant. On day 7, she developed progressive proteinuria up to 2.4 g/g which responded well to methylprednisolone pulse therapy, increased cyclosporin dosage and ramipril therapy. Graft biopsy did not show any anomaly.
In the cohort of patients with NPHS2 variants, 13 other patients had posttransplant reappearance of proteinuria. However, in all these cases, the cause of proteinuria was established and was not related to initial podocytopathy. In fact, 2 patients experienced venous graft thrombosis, 8 patients had graft rejection (acute in 4 cases, chronic in 3 cases, and combined acute and chronic lesions in 1 case) and 3 patients had posttransplant lymphoproliferative disease. These conditions led to graft loss. Of note, of these 13 patients, 5 patients had a second transplantation. Three experienced no recurrence of proteinuria or NS after the second transplantation. One patient had a primary nonfunction of graft upon second transplantation and received a third transplantation, which eventually led to chronic dysfunction due to calcineurin inhibitors nephrotoxicity, rejection, and thrombotic microangiopathy. Finally, the fifth patient had a second transplantation and experienced graft loss 8 years later, but information regarding the cause of graft loss was lacking.
Discussion
We report herein the results of posttransplant recurrence of NS from a large cohort of patients with SRNS related to pathogenic variants in the NPHS2 gene. In our experience, posttransplant recurrence in patients with biallelic variants in the NPHS2 gene causing SRNS seems extremely rare, whether in the short term or long term.
In patients with SRNS, which is a leading cause of KF in children,1 recurrence after transplantation is a common complication, which poses clinical challenges.2, 3, 4, 5 Although the pathophysiology of recurrence in patients with idiopathic NS seems scientifically consistent (presence of a circulating factor), it appears more difficult to understand in patients with SRNS related to hereditary podocytopathy confirmed by the identification of causal variants. In fact, hereditary glomerular diseases referred to as podocytopathies are caused by defects in genes encoding key podocyte components such as slit diaphragm proteins, nuclear and transcription factor, actin cytoskeleton and signaling factors, mitochondrial proteins, and metabolic and lysosomal proteins.27 With NPHS2 pathogenic variants, abnormal or absent podocin in native kidneys causes the NS.15 Therefore, after transplantation with a healthy kidney containing normal podocin, recurrence is not expected in accordance with the disease’s pathophysiology.
Recurrence of proteinuria after transplantation has been well-described in patients homozygous for Fin-major pathogenic variant in the NPHS1 gene, which leads to complete absence of nephrin in native kidneys and the formation of antinephrin antibodies.28 Correspondingly, those patients respond well to immunosuppression and plasmapheresis.29 The term recurrence is a misnomer in these cases because it is not a relapse of hereditary NS but rather a “posttransplant de novo glomerulopathy.” However, NS after transplantation caused by antibodies has never been described in patients with NPHS2 gene pathogenic variants and posttransplant proteinuria.6,23,25 Podocin is a hairpin-like protein, composed of cytoplasmic extensions, which are intracellular.19 This could therefore potentially explain why, contrary to nephrin, a large extracellular immunoglobulin-like protein, podocin is not immunogenic.
In the past, any NPHS2 pathogenic variant combined with the single nucleotide polymorphism p.Arg229Gln was thought to trigger genetic SRNS. However, Tory et al. have shown that the p.Arg229Gln polymorphism only causes SRNS when combined in trans with some pathogenic variants on exon 7 or 8.26 In fact, 1 patient in our cohort had a p.Arg229Gln polymorphism combined with a p.Arg138Gln variant on exon 5 and was initially misdiagnosed as having genetic NS. She developed proteinuria 7 days after kidney transplantation. We now know that her genotype was not causative of the SRNS, which was likely of immune origin. This explains the early recurrence of NS after transplantation. Indeed, patients who have a p.Arg229Gln polymorphism combined with a NPHS2 variant other than a pathogenic variant on exon 7 or 8 should not be considered to have NS due to an identified genetic cause.
There have been only 9 reports of patients with biallelic variants in the NPHS2 gene presenting with recurrence after kidney transplantation. Bertelli et al. were the first to report 2 cases of recurrence in patients with homozygous p.Arg138Gln pathogenic variant.25 One of the patients had recurrence 10 days after transplantation but the second one occurred 300 days after transplantation, with kidney biopsy demonstrating FSGS. The later onset of recurrence of NS in that patient alludes to an alternative diagnosis, possibly secondary FSGS. It is to be noted that no antipodocin antibodies were found in these 2 patients although both responded to plasmapheresis and cyclophosphamide pulses, suggesting an immune process.25 Caridi et al. also related 2 cases of recurrence after transplantation in patients with biallelic pathogenic NPHS2 variants (homozygous p.Gly140Aspfs∗41 variant and compound heterozygous variants p.[Val180Met;Leu156Phefs∗11]), with few details regarding recurrence however.30 Soon after, 2 other cases were described with homozygous p.Leu347∗ variant and compound heterozygous variants p.[Leu347∗X;p.Lys126Asn].16,24 One of these cases actually was the patient from the PodoNet registry we report here, who had an initial steroid-resistant disease whereas recurrence of proteinuria after kidney transplantation responded to increased immunosuppression. Two other cases of recurrence after transplantation in patients with homozygous p.Arg138∗ variant were reported as well, one occurring 2 years after transplantation and the other 4 years later, with FSGS demonstrated on kidney biopsy in both cases.6,23 Again, the later onset of recurrence in these 2 cases alludes to a different diagnosis such as secondary FSGS. Finally, Hocker et al. also published a case report of a pediatric patient with compound heterozygous NPHS2 variants, including the p.Arg138Gln variant on 1 allele, who developed biopsy-proven recurrence of FSGS 10 years after transplantation when cyclosporin was converted to sirolimus,31 which seems unlikely to be related to the initial podocytopathy.
Accurately assessing the risk of recurrence has clinical repercussions and can impact genetic counselling, especially in the case of living donor transplantation. If recurrence is possible, living donors should be informed regarding possible disease reappearance in recipient and less favorable graft outcomes. Also, one can hypothesize that kidney transplantation with a living related donor who has a heterozygous variant in the NPHS2 gene might increase the risk of recurrence in the kidney graft. However, of the cases describing recurrence in kidney transplant in patients with biallelic NPHS2 variants, 4 occurred with a deceased donor,16,23,25 whereas 3 had a living related donor (mother).6,16,24,31 In any case, these data should be interpreted in light of the proportion of kidney transplants from living or deceased donors, which is not precisely known.
Subsequently, there have been studies which challenged these findings and showed that recurrence is less frequently observed in genetic SRNS when compared to idiopathic SRNS.5,7,9, 10, 11 However, our study is the first to precisely describe the short-term and long-term posttransplant evolution of a large cohort of patients with SRNS related to pathogenic variants in the NPHS2 gene, in particular the p.Arg138Gln variant, which has a high prevalence in Europe.6,10
In the present study, only 1 patient out of 117 experienced disease recurrence after transplantation. The other 116 patients did not have recurrence, whether in the short term or long term (up to 32 years after transplantation for 1 patient), with a median follow-up of cohort of 8.5 (2.5–15) years. These results are reassuring and concordant with our knowledge of this genetic disease’s pathophysiology which is localized to podocytes.
In the control group, all recurrences were observed within a maximum of 7 days after transplantation. This matches previous studies which also show early recurrence of SRNS after transplantation in patients with no genetic cause,5,9,11,32 and is in line with the hypothesis of a circulating factor. This circulating factor has not yet been identified although possible candidates have been proposed, such as hemopexin, antiactin, soluble urokinase receptor, anti-CD40 autoantibody, cardiotrophin-like cytokine 1, anti-UCHL1, anti-annexin A2, and more recently, antinephrin antibodies.33, 34, 35, 36, 37, 38, 39, 40 It is to be noted as well that in our study, recurrence rate was lower than what is described in the literature. Recurrence in our cohort was observed in 16% of patients with no identified pathogenic variant, whereas recurrence rates up to 50% are usually described in SRNS.2, 3, 4, 5, 6, 7, 8 This can be explained by a selection bias; patients in our control group were referred for genetic testing and preferentially presented with multidrug resistant NS, less typical of idiopathic NS, which is usually steroid-sensitive. Some of these patients might have a genetic cause of NS that has not yet been identified, which could explain the lower recurrence rate.
In regard to the case reports described above of recurrence of NS after transplantation in patients with NPHS2 pathogenic variants, we may hypothesize that some of these patients have a cooccurrence of an immune process and a genetic podocytopathy, considering early recurrence and response to immunosuppression. In fact, this could potentially explain why in the patient from the PodoNet registry, NS was steroid-resistant in native kidneys whereas proteinuria after transplantation responded to corticosteroids and increased immunosuppression. Recently, it has been shown that circulating nephrin autoantibodies may be found during active disease in a subset of patients with minimal change disease, supporting an immune etiology.38 It would thus be interesting to measure nephrin autoantibodies in patients with hereditary podocytopathies who have recurrence after transplantation. If these autoantibodies are positive, this could help confirm our hypothesis of an immune process causing recurrence of disease rather than the hereditary podocytopathy.
There are a few limitations to our study. We lacked information regarding some patients, especially given that several of them had been transplanted many years before being referred for genetic testing. For example, details concerning the type of donor (living or deceased) were not available for all patients. However, because only 1 recurrence was observed in the group with NS due to NPHS2 variants, this has no impact on our findings.
Conclusion
In the cohort presented here, the risk of recurrence after transplantation appeared extremely low in patients with biallelic variants in the NPHS2 gene causing SRNS. No recurrences were observed in patients with p.Arg138Gln variant. These data are reassuring and should be considered when counselling patients. According to our findings in this large cohort, kidney transplantation in NPHS2 patients is safe, both with deceased kidney donation or living kidney donation, whether related or not.
Appendix
List of Members of the PodoNet Network
Algeria: Mounia Boutaba, Algiers; Austria: Dagmar Csaiscich, Vienna; Belarus: Sergay Baiko; Chile: Marta Azocar, Santiago; Lily Quiroz, Santiago; Colombia: Lina Maria Serna Higuita, Medellín; Czech Republic: Jiří Dušek, Prague; France: Bruno Ranchin, Lyon; Adriane Zaloszyc, Strasbourg; Georgia: Tinatin Davitaia, Tbilisi; Germany: Jutta Gellermann, Berlin; Jun Oh, Hamburg; Anette Melk, Hannover; Franz Schaefer, Heidelberg; Hagen Staude, Rostock; Greece: Nikoleta Printza, Thessaloniki; Hungary: Kalman Tory, Budapest; Iran: Alaleh Gheissari, Isfahan; Italy: Giuseppe Remuzzi, Bergamo; Andrea Pasini, Bologna; Gian Marco Ghiggeri, Genova; Gianluigi Ardissino, Milano; Elisa Benetti, Padova; Francesco Emma, Rome; Roberta Camilla, Torino; Kazakhstan: Nazym Nigmatullina, Astana; Lebanon: Bilal Aoun, Beirut; Chebl Mourani, Beirut; Pauline Abou-Jaoudé, Byblos; Lithuania: Augustina Jankauskiene, Vilnius; Poland: Anna Wasilewska, Bialystok; Lidia Hyla Klekot, Chorzow; Aleksandra Zurowska, Gdansk; Dorota Drozdz, Krakow; Marcin Tkaczyk, Lodz; Przemysław Sikora, Lublin; Danuta Ostalska, Poznan; Andrzej Brodkiewicz, Szczecin; Mieczyslaw Litwin, Warsaw; Małgorzata Panczyk-Tomaszewska, Warsaw; Anna Medyńska, Wroclaw; Maria Szczepanska, Zabrze; Portugal: Alberto Caldas Afonso, Porto; Helena Jardim, Porto; Romania: Adrian Lungu, Bucharest; Russia: Alexej Tsygin, Moscow; Larisa Prikhodina, Moscow; Serbia: Dusan Paripovic, Belgrade; Radovan Bogdanovic, Belgrade; Sweden: Rafael T. Krmar, Stockholm; Syria: Bassam Saeed, Damascus; Turkey: Ali Anarat, Adana; Ayse Balat, Gaziantep; Z. Esra Baskin, Ankara; Nilgun Cakar, Ankara; Ozlem Erdogan, Ankara; Birsin Özcakar, Ankara; Fatih Ozaltin, Ankara; Onur Sakallioglu, Ankara; Oguz Soylemezoglu, Ankara; Sema Akman, Antalya; Faysal Gok, Gulhane; Salim Caliskan, Istanbul; Cengiz Candan, Istanbul; Alev Yilmaz, Istanbul; Betul Sozeri, Izmir; Ipek Akil, Manisa; Pelin Ertan, Manisa; Ozan Özkaya, Samsun; Mukaddes Kalyoncu, Trabzon; United Arab Emirates: Martin Bitzan, Dubai; Ukraine: Svitlana Formina, Kiev; Roman Sobko, Lviv.
Disclosure
BL-Z reports personal fees from Takeda and personal fees from Astra Zeneca, outside the submitted work. All the other authors declared no competing interests.
Acknowledgments
This study has been supported by ERKNet, the European Rare Kidney Disease Reference Network. ERKNet is co-funded by the European Union within the framework of the Third Health Program “ERN-2016—Framework Partnership Agreement 2017–2021.”
PodoNet has received funding from the German Ministry of Education and Research, the EU 7th Framework Program (EURenOmics; grant 2012-305608), the German Research Foundation (Scha 477/11-1), the Polish Ministry of Science and Education (grant N402631840), and the Scientific and Technological Research Council of Turkey (TUBITAK) (grant 108S417).
Footnotes
Table S1. List of 228 genes from our panel of known genes involved in kidney diseases. Genes involved in FSGS are underlined in bold.
Contributor Information
Guillaume Dorval, Email: guillaume.dorval@inserm.fr.
PodoNet Network:
Mounia Boutaba, Dagmar Csaiscich, Sergay Baiko, Marta Azocar, Lily Quiroz, Lina Maria Serna Higuita, Jiří Dušek, Bruno Ranchin, Adriane Zaloszyc, Tinatin Davitaia, Jutta Gellermann, Jun Oh, Anette Melk, Franz Schaefer, Hagen Staude, Nikoleta Printza, Kalman Tory, Alaleh Gheissari, Giuseppe Remuzzi, Andrea Pasini, Gian Marco Ghiggeri, Gianluigi Ardissino, Elisa Benetti, Francesco Emma, Roberta Camilla, Nazym Nigmatullina, Bilal Aoun, Chebl Mourani, Pauline Abou-Jaoudé, Augustina Jankauskiene, Anna Wasilewska, Lidia Hyla Klekot, Aleksandra Zurowska, Dorota Drozdz, Marcin Tkaczyk, Przemysław Sikora, Danuta Ostalska, Andrzej Brodkiewicz, Mieczyslaw Litwin, Małgorzata Panczyk-Tomaszewska, Anna Medyńska, Maria Szczepanska, Alberto Caldas Afonso, Helena Jardim, Adrian Lungu, Alexej Tsygin, Larisa Prikhodina, Dusan Paripovic, Radovan Bogdanovic, Rafael T. Krmar, Bassam Saeed, Ali Anarat, Ayse Balat, Z. Esra Baskin, Nilgun Cakar, Ozlem Erdogan, Birsin Özcakar, Fatih Ozaltin, Onur Sakallioglu, Oguz Soylemezoglu, Sema Akman, Faysal Gok, Salim Caliskan, Cengiz Candan, Alev Yilmaz, Betul Sozeri, Ipek Akil, Pelin Ertan, Ozan Özkaya, Mukaddes Kalyoncu, Martin Bitzan, Svitlana Formina, and Roman Sobko
Supplementary Material
Table S1. List of 228 genes from our panel of known genes involved in kidney diseases. Genes involved in FSGS are underlined in bold.
References
- 1.Smith J.M., Stablein D.M., Munoz R., Hebert D., McDonald R.A. Contributions of the transplant registry: the 2006 annual report of the North American pediatric renal trials and collaborative studies (NAPRTCS) Pediatr Transplant. 2007;11:366–373. doi: 10.1111/j.1399-3046.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- 2.Senggutuvan P., Cameron J.S., Hartley R.B., et al. Recurrence of focal segmental glomerulosclerosis in transplanted kidneys: analysis of incidence and risk factors in 59 allografts. Pediatr Nephrol. 1990;4:21–28. doi: 10.1007/BF00858431. [DOI] [PubMed] [Google Scholar]
- 3.Mahesh S., Del Rio M., Feuerstein D., et al. Demographics and response to therapeutic plasma exchange in pediatric renal transplantation for focal glomerulosclerosis: a single center experience. Pediatr Transplant. 2008;12:682–688. doi: 10.1111/j.1399-3046.2007.00880.x. [DOI] [PubMed] [Google Scholar]
- 4.Cochat P., Fargue S., Mestrallet G., et al. Disease recurrence in paediatric renal transplantation. Pediatr Nephrol. 2009;24:2097–2108. doi: 10.1007/s00467-009-1137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morello W., Puvinathan S., Puccio G., et al. Post-transplant recurrence of steroid resistant nephrotic syndrome in children: the Italian experience. J Nephrol. 2020;33:849–857. doi: 10.1007/s40620-019-00660-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber S., Gribouval O., Esquivel E.L., et al. NPHS2 mutation analysis shows genetic heterogeneity of steroid-resistant nephrotic syndrome and low post-transplant recurrence. Kidney Int. 2004;66:571–579. doi: 10.1111/j.1523-1755.2004.00776.x. [DOI] [PubMed] [Google Scholar]
- 7.Ding W.Y., Koziell A., McCarthy H.J., et al. Initial steroid sensitivity in children with steroid-resistant nephrotic syndrome predicts post-transplant recurrence. J Am Soc Nephrol. 2014;25:1342–1348. doi: 10.1681/ASN.2013080852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber S., Tonshoff B. Recurrence of focal-segmental glomerulosclerosis in children after renal transplantation: clinical and genetic aspects. Transplantation. 2005;80(1 Suppl):S128–134. doi: 10.1097/01.tp.0000187110.25512.82. [DOI] [PubMed] [Google Scholar]
- 9.Maas R.J., Deegens J.K., van den Brand J.A., Cornelissen E.A., Wetzels J.F. A retrospective study of focal segmental glomerulosclerosis: clinical criteria can identify patients at high risk for recurrent disease after first renal transplantation. BMC Nephrol. 2013;14:47. doi: 10.1186/1471-2369-14-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jungraithmayr T.C., Hofer K., Cochat P., et al. Screening for NPHS2 mutations may help predict FSGS recurrence after transplantation. J Am Soc Nephrol. 2011;22:579–585. doi: 10.1681/ASN.2010010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basiratnia M., Yavarian M., Torabinezhad S., Erjaee A. NPHS2 gene in steroid-resistant nephrotic syndrome: prevalence, clinical course, and mutational spectrum in South-West Iranian children. Iran J Kidney Dis. 2013;7:357–362. [PubMed] [Google Scholar]
- 12.Miura K., Ando T., Kanda S., et al. Response to steroid and immunosuppressive therapies may predict post-transplant recurrence of steroid-resistant nephrotic syndrome. Pediatr Transplant. 2022;26 doi: 10.1111/petr.14103. [DOI] [PubMed] [Google Scholar]
- 13.Francis A., Prestidge C., Kausman J., et al. Impact of initial steroid response on transplant outcomes in children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2022;37:1149–1156. doi: 10.1007/s00467-021-05270-x. [DOI] [PubMed] [Google Scholar]
- 14.Roselli S., Heidet L., Sich M., et al. Early glomerular filtration defect and severe renal disease in podocin-deficient mice. Mol Cell Biol. 2004;24:550–560. doi: 10.1128/MCB.24.2.550-560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boute N., Gribouval O., Roselli S., et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–354. Published correction appears in Nat Genet. 2000;25:125. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 16.Ruf R.G., Lichtenberger A., Karle S.M., et al. Patients with mutations in NPHS2 (podocin) do not respond to standard steroid treatment of nephrotic syndrome. J Am Soc Nephrol. 2004;15:722–732. doi: 10.1097/01.asn.0000113552.59155.72. [DOI] [PubMed] [Google Scholar]
- 17.Santin S., Bullich G., Tazón-Vega B., et al. Clinical utility of genetic testing in children and adults with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2011;6:1139–1148. doi: 10.2215/CJN.05260610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guaragna M.S., Lutaif A.C., Piveta C.S., et al. NPHS2 mutations account for only 15% of nephrotic syndrome cases. BMC Med Genet. 2015;16:88. doi: 10.1186/s12881-015-0231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benoit G., Machuca E., Antignac C. Hereditary nephrotic syndrome: a systematic approach for genetic testing and a review of associated podocyte gene mutations. Pediatr Nephrol. 2010;25:1621–1632. doi: 10.1007/s00467-010-1495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadowski C.E., Lovric S., Ashraf S., et al. A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2015;26:1279–1289. doi: 10.1681/ASN.2014050489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinkes B., Vlangos C., Heeringa S., et al. Specific podocin mutations correlate with age of onset in steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2008;19:365–371. doi: 10.1681/ASN.2007040452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinkes B.G., Mucha B., Vlangos C.N., et al. Nephrotic syndrome in the first year of life: two thirds of cases are caused by mutations in 4 genes (NPHS1, NPHS2, WT1, and LAMB2) Pediatrics. 2007;119:e907–e919. doi: 10.1542/peds.2006-2164. [DOI] [PubMed] [Google Scholar]
- 23.Becker-Cohen R., Bruschi M., Rinat C., et al. Recurrent nephrotic syndrome in homozygous truncating NPHS2 mutation is not due to anti-podocin antibodies. Am J Transplant. 2007;7:256–260. doi: 10.1111/j.1600-6143.2006.01605.x. [DOI] [PubMed] [Google Scholar]
- 24.Billing H., Müller D., Ruf R., et al. NPHS2 mutation associated with recurrence of proteinuria after transplantation. Pediatr Nephrol. 2004;19:561–564. doi: 10.1007/s00467-003-1408-6. [DOI] [PubMed] [Google Scholar]
- 25.Bertelli R., Ginevri F., Caridi G., et al. Recurrence of focal segmental glomerulosclerosis after renal transplantation in patients with mutations of podocin. Am J Kidney Dis. 2003;41:1314–1321. doi: 10.1016/s0272-6386(03)00364-0. [DOI] [PubMed] [Google Scholar]
- 26.Tory K., Menyhárd D.K., Woerner S., et al. Mutation-dependent recessive inheritance of NPHS2-associated steroid-resistant nephrotic syndrome. Nat Genet. 2014;46:299–304. doi: 10.1038/ng.2898. [DOI] [PubMed] [Google Scholar]
- 27.Bierzynska A., Soderquest K., Koziell A. Genes and podocytes - new insights into mechanisms of podocytopathy. Front Endocrinol (Lausanne) 2014;5:226. doi: 10.3389/fendo.2014.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patrakka J., Ruotsalainen V., Reponen P., et al. Recurrence of nephrotic syndrome in kidney grafts of patients with congenital nephrotic syndrome of the Finnish type: role of nephrin. Transplantation. 2002;73:394–403. doi: 10.1097/00007890-200202150-00013. [DOI] [PubMed] [Google Scholar]
- 29.Kuusniemi A.M., Qvist E., Sun Y., et al. Plasma exchange and retransplantation in recurrent nephrosis of patients with congenital nephrotic syndrome of the Finnish type (NPHS1) Transplantation. 2007;83:1316–1323. doi: 10.1097/01.tp.0000262569.27890.64. [DOI] [PubMed] [Google Scholar]
- 30.Caridi G., Bertelli R., Di Duca M., et al. Broadening the spectrum of diseases related to podocin mutations. J Am Soc Nephrol. 2003;14:1278–1286. doi: 10.1097/01.asn.0000060578.79050.e0. [DOI] [PubMed] [Google Scholar]
- 31.Hocker B., Knüppel T., Waldherr R., Schaefer F., Weber S., Tönshoff B. Recurrence of proteinuria 10 years post-transplant in NPHS2-associated focal segmental glomerulosclerosis after conversion from cyclosporin A to sirolimus. Pediatr Nephrol. 2006;21:1476–1479. doi: 10.1007/s00467-006-0148-9. [DOI] [PubMed] [Google Scholar]
- 32.Cheong H.I., Han H.W., Park H.W., et al. Early recurrent nephrotic syndrome after renal transplantation in children with focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2000;15:78–81. doi: 10.1093/ndt/15.1.78. [DOI] [PubMed] [Google Scholar]
- 33.Wei C., El Hindi S., Li J., et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952–960. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shoji J., Mii A., Terasaki M., Shimizu A. Update on recurrent focal segmental glomerulosclerosis in kidney transplantation. Nephron. 2020;144(suppl 1):65–70. doi: 10.1159/000510748. [DOI] [PubMed] [Google Scholar]
- 35.Pukajlo-Marczyk A., Zwolinska D. Involvement of hemopexin in the pathogenesis of proteinuria in children with idiopathic nephrotic syndrome. J Clin Med. 2021;10:3160. doi: 10.3390/jcm10143160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jamin A., Berthelot L., Couderc A., et al. Autoantibodies against podocytic UCHL1 are associated with idiopathic nephrotic syndrome relapses and induce proteinuria in mice. J Autoimmun. 2018;89:149–161. doi: 10.1016/j.jaut.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Chebotareva N., Cao V., Vinogradov A., et al. A cross-sectional study of antibodies to ubiquitin proteasome system in different glomerulopathies. Clin Nephrol. 2023;99:78–84. doi: 10.5414/CN110897. [DOI] [PubMed] [Google Scholar]
- 38.Watts A.J.B., Keller K.H., Lerner G., et al. Discovery of autoantibodies targeting nephrin in minimal change disease supports a novel autoimmune etiology. J Am Soc Nephrol. 2022;33:238–252. doi: 10.1681/ASN.2021060794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musante L., Candiano G., Bruschi M., et al. Circulating anti-actin and anti-ATP synthase antibodies identify a sub-set of patients with idiopathic nephrotic syndrome. Clin Exp Immunol. 2005;141:491–499. doi: 10.1111/j.1365-2249.2005.02862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye Q., Zhang Y., Zhuang J., et al. The important roles and molecular mechanisms of annexin A(2) autoantibody in children with nephrotic syndrome. Ann Transl Med. 2021;9:1452. doi: 10.21037/atm-21-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.