Abstract
Introduction
Immunoglobulin A nephropathy (IgAN) has been reported to coexist with hepatitis B virus (HBV) infection. Despite the clinical significance of this association, there is a lack of comprehensive research investigating the impact of various common conditions following HBV infection and the potential influence of anti-HBV therapy on the progression of IgAN.
Methods
We investigated 3 distinct states of HBV infection, including chronic HBV infection, resolved HBV infection, and the deposition of hepatitis B antigens in renal tissue, in a follow-up database of 1961 patients with IgAN. IgAN progression was defined as a loss of estimated glomerular filtration rate (eGFR) >40%. Multivariable cause-specific hazards models to analyze the relationship between HBV states and IgAN progression.
Results
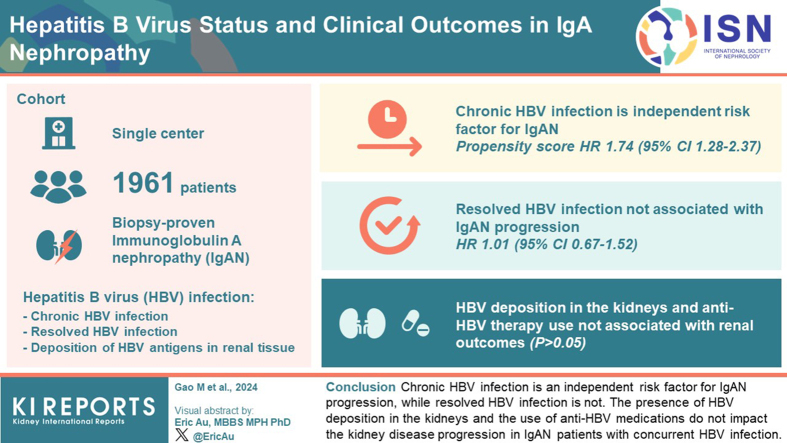
Chronic HBV infection was identified as an independent risk factor for IgAN progression, supported by both prematching analysis (hazard ratio [HR], 1.61; 95% confidence interval [CI], 1.06–2.44; P = 0.024) and propensity-score matching analysis (HR, 1.74; 95% CI 1.28–2.37; P < 0.001). Conversely, resolved HBV infection showed no significant association with IgAN progression (HR, 1.01; 95% CI 0.67–1.52; P = 0.969). Moreover, the presence of HBV deposition in the kidneys and the utilization of anti-HBV therapy did not appear to be significant risk factors for renal outcomes (P > 0.05).
Conclusion
Chronic HBV infection is an independent risk factor for IgAN progression, whereas resolved HBV infection is not. In patients with IgAN, management of concurrent chronic HBV infection should be enhanced. The presence of HBV deposition in the kidneys and the use of anti-HBV medications do not impact the kidney disease progression in patients with IgAN with concurrent HBV infection.
Keywords: antihepatitis B virus therapy, chronic hepatitis B virus infection, IgA nephropathy, kidney disease progression
Graphical abstract
IgAN is characterized by the accumulation of IgA immune complexes in the mesangial area of the renal glomerulus, and is the most prevalent form of primary glomerulonephritis worldwide,.1 The incidence rate varies among different regions.2 Within 20 years after its onset, approximately 20% to 40% of individuals with IgAN may develop end-stage kidney disease.3 IgAN has been documented in patients with various comorbidities, including chronic HBV infection.4 As a significant global public health issue, about 296 million people live with chronic HBV infection.5 Notably, the long-term cooccurrence of IgAN and HBV infection is expected to rise in incidence, particularly in regions endemic to southeastern Asia. Although the etiological role and association analysis of HBV in IgAN have been continuously studied, some conclusions are controversial.6,7 Some early clinical studies suggested that hepatitis B did not play a role in the pathogenesis of IgAN,8,9 or even did not affect the prognosis of IgAN.10 However, some recent retrospective studies suggested clinicopathological differences between hepatitis B surface antigen (HBsAg)-positive IgAN and primary IgAN without HBV infection. Hepatitis B infection is an independent risk factor for IgAN progression.11
However, it is noteworthy that HBV can persist despite achieving serological resolution. Patients ever infected with HBV may experience viral reactivation under specific circumstances.12 Therefore, a comprehensive investigation into the different states of HBV infection and their respective implications for the clinical characteristics and prognosis of IgAN is of utmost importance. In the treatment of high-risk patients with IgAN who did not resolve from maximal supportive therapy, current guidelines recommend adding steroids. Nevertheless, caution must be exercised due to the potential risk of HBV reactivation when there is concomitant nonreplicative chronic HBV infection during immunosuppressant use. The simultaneous administration of antiviral agents in the context of high-risk progressive IgAN raises clinical concerns about potential exacerbation of kidney progression.
Considering these concerns, we conducted a cohort study aimed at evaluating the impact of HBV infection with differing states on IgAN, as well as the prognosis of anti-HBV drugs in patients with IgAN with HBV infection.
Methods
Study Population
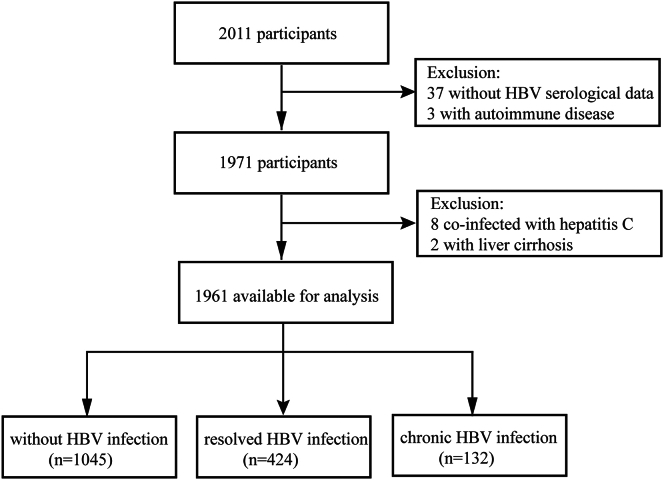
A total of 2011 individuals who received a diagnosis of biopsy-proven IgAN between the 1994 and 2020 were identified from the prospective IgAN database meticulously maintained at Peking University First Hospital. These patients underwent regular follow-up examinations at intervals ranging from 3 to 6 months. Patients who lacked HBV serological data were excluded from the analysis. In addition, patients with liver cirrhosis, hepatitis C infection, and other forms of secondary IgAN were deliberately excluded from the study. The recruitment process is visually summarized in Figure 1, presenting a detailed flowchart.
Figure 1.
Flow diagram of the screening and enrollment of study participants.
All participants provided written informed consent for the use of their clinical and pathological data in future studies when they entered the IgAN cohort. The protocol was reviewed and approved by the institute's Ethics Committee (2021Y148), and no additional informed consent was required.
Clinical and Pathologic Information
At the time of renal biopsy, baseline demographics and clinical features were examined, including age, gender, body mass index, blood pressure, urinary sediment microscopy, 24-hour urine protein excretion, albumin-to-creatinine ratio, serum levels of IgA and C3, creatinine, uric acid, lipid profiles, and albumin. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.13 Furthermore, a comprehensive panel of serological markers pertaining to HBV was obtained for analysis. These markers encompassed HBsAg, hepatitis B surface antibody, hepatitis B e antigen, hepatitis B e antibody, antihepatitis B core antibody, and quantification of HBV-DNA copies.
Pathologic lesions in our IgAN database were reviewed and reevaluated according to the updated 2016 Oxford classification.14 HBV antigen deposition in renal tissue was detected using the frozen section immunofluorescence method.
Comprehensive data on posttreatment observations, encompassing clinical progression, and renal function were recorded. The administration of these medications was documented, including antiviral agents for hepatitis B, renin-angiotensin-aldosterone system inhibitors, corticosteroids, and other immunosuppressive agents. Immunosuppressives were used as follows within the first 6 months postkidney biopsy: persistent proteinuria (>1 g/day) despite optimal blood pressure control and maximum dosage of renin-angiotensin-aldosterone system inhibitors, corticosteroid therapy was added. In cases of >25% crescents on biopsy and progressive GFR loss, steroids were combined with either cyclophosphamide or mycophenolate mofetil.
Definition of HBV States and Disease Outcome
The focus of our study centered on 3 distinct states of HBV in patients with IgAN. According to the American Association for the Study of Liver Diseases 2018 hepatitis B guideline, chronic HBV infection was defined as the persistence of HBsAg for a duration exceeding 6 months following the acute phase of infection.15 Resolved HBV infection refers to individuals who have been previously exposed to HBV (exhibiting seropositivity for antihepatitis B core antibody), while demonstrating undetectable levels of both HBsAg and HBV-DNA in their serum, with or without seroconversion to hepatitis B surface antibody.16,17 In our study, the deposition of hepatitis B antigens in renal tissue refers to the detection of 2 antigens, HBsAg and HbcAg, utilizing immunofluorescence staining. Follow-up was defined as the interval between renal biopsy and the last outpatient visit. The primary end point of this study is defined as a loss of eGFR >40%.
Statistical Analyses
Categorical variables were expressed in terms of proportions (%), whereas continuous variables were reported as either the mean (SD) or median (interquartile range) depending on their distribution. To assess differences between groups, 1-way analysis of variance was employed for normally distributed variables, Mann-Whitney U test or Kruskal-Wallis test was used for skewed distributions, and chi-square or Fisher exact tests were performed for categorical variables. Cox regression models were used to estimate HRs and their 95% CIs to analyze the relationship between different HBV states and renal end point. Sensitivity analyses were conducted to further validate the robustness of the results, which involved performing Cox regression after 1:4 propensity-score matching and removing data with missing covariates prior to multiple imputation for Cox regression. In addition, subgroup analyses were performed to further verify the reliability of the results. Cumulative renal survival rates were calculated according to the Kaplan-Meier method (log-rank test).
Before statistical analyses were performed, multiple imputation method was applied to handle missing data using fully conditional specification implemented by MICE algorithm as described by Van Buuren and Groothuis-Oudshoorn.18
All statistical analyses were performed using Free Statistics software version 1.9 and the R software packages (http://www.R-project.org. The R Foundation). A significance threshold of P < 0.05 (2-sided) was applied to determine statistical significance.
Results
Baseline Clinical Characteristics of Study Population
A total of 1961 patients were enrolled in this study. The prevalence of hepatitis B infection was determined to be 28.35%, encompassing both current (6.73%) and previous infections (21.62%). The baseline characteristics of all study participants, stratified by their HBV states, are detailed in Table 1. The mean age and body mass index of the population were 35.6 ± 12.1 years and 24.5 ± 3.9 kg/m2, respectively. Patients with chronic HBV infection had a mean age of 37.1 ± 10.2 years, whereas those with resolved HBV infection had a mean age of 39.5 ± 12.1 years. Both groups exhibited a significantly higher age compared to the IgAN without HBV infection group (P < 0.001). There were no discernible differences in gender distribution among the 3 groups.
Table 1.
Demographics and clinical features of patients
| Characteristics | No infection (group 1 = 1405) | Resolved infection (group 2 = 424) | Chronic infection (group 3 = 132) | P value |
|---|---|---|---|---|
| Age, yr | 34.3 ± 12.0 | 39.5 ± 12.1 | 37.1 ± 10.2 | <0.001 |
| BMI, kg/m2 | 24.4 ± 4.0 | 24.5 ± 3.5 | 25.1 ± 4.0 | 0.159 |
| SBP, mm Hg | 124.4 ± 15.5 | 125.8 ± 16.8 | 124.4 ± 13.5 | 0.257 |
| DBP, mm Hg | 78.7 ± 11.3 | 79.6 ± 11.6 | 79.5 ± 10.6 | 0.342 |
| Prodromic infection, (%) | 467 (33.2) | 128 (30.2) | 29 (22.0) | 0.021 |
| Gross hematuria, n (%) | 360 (25.6) | 91 (21.5) | 21 (15.9) | 0.016 |
| Nephrotic syndrome (%) | 15 (8.5) | 31 (7.3) | 106 (7.6) | 0.640 |
| Proteinuria, g/24h | 1.3 (0.7, 2.6) | 1.2 (0.6, 2.2) | 1.3 (0.6, 2.4) | 0.723 |
| ACR, mg/g | 707 (331, 1455) | 633 (321, 1393) | 759 (312, 1396) | 0.994 |
| eGFR, ml/min per 1.73 m2 | 84.2 (55.4, 107.0) | 74.1 (50.3, 102.0) | 80.0 (58.9, 100.5) | 0.003 |
| Serum creatinine, μmol/l | 90.0 (72.0, 126.0) | 97.1 (70.9, 134.5) | 97.0 (74.5, 120.2) | 0.291 |
| Uric acid, μmol/l | 377.1 ± 104.2 | 374.9 ± 100.2 | 384.5 ± 101.8 | 0.433 |
| Serum albumin, g/l | 37.7 ± 6.1 | 37.9 ± 5.9 | 36.6 ± 5.7 | 0.096 |
| LDL-C, mmol/l | 2.9 ± 1.2 | 2.9 ± 1.0 | 2.9 ± 1.1 | 0.722 |
| Total cholesterol, mmol/l | 5.1 ± 1.7 | 5.0 ± 1.4 | 5.0 ± 1.3 | 0.451 |
| Triglycerides, mmol/l | 1.7 (1.1, 2.5) | 1.7 (1.1, 2.6) | 1.7 (1.1, 2.5) | 0.624 |
| Plasma IgA, g/l | 3.1 (2.4, 3.9) | 3.1 (2.5, 3.9) | 3.1 (2.5, 4.0) | 0.710 |
| C3, n (%) | 0.011 | |||
| < 0.6 (g/l) | 10 (0.7) | 4 (0.9) | 3 (2.3) | |
| < 0.8 (g/l) | 110 (7.8) | 31 (7.3) | 20 (15.2) | |
| Immunosuppressor, n (%) | 609 (43.3) | 164 (38.9) | 39 (29.5) | 0.004 |
| Oxford classification | ||||
| M1, n (%) | 577 (41.1) | 157 (37.0) | 76 (57.6) | < 0.001 |
| E1, n (%) | 436 (31.0) | 136 (32.1) | 52 (39.4) | 0.142 |
| S1, n (%) | 912 (64.9) | 277 (65.3) | 82 (62.1) | 0.789 |
| T1-T2, n (%) | 428 (30.5) | 135 (31.8) | 54 (40.9) | 0.007 |
| C1-C2, n (%) | 822 (58.5) | 254 (59.9) | 78 (59.1) | 0.071 |
ACR, albumin-to-creatinine ratio; BMI, body mass index; C, presence of crescent; DBP, diastolic blood pressure; E, endocapillary hypercellularity; eGFR, estimated glomerular filtration rate; LDL-C, low-density lipoprotein cholesterol; M, mesangial hypercellularity; S, segmental glomerulosclerosis/adhesion; SBP, Systolic blood pressure; T, severity of tubular atrophy/interstitial fibrosis.
Missing data (as a percentage of observations): BMI 0.05%, IgA/C3 level 29.8%, serum lipid 3.8%, uric acid 1%.
In comparison to the IgAN without HBV group, patients with chronic HBV infection exhibited a significantly lower incidence of gross hematuria (15.9% vs. 25.6%, P = 0.014) and prodromic infection (22.0% vs. 33.2%, P = 0.016) at disease onset. Conversely, the resolved HBV infection group did not exhibit statistically significant differences in these aspects compared to the other groups. Moreover, the chronic HBV infection group showed a significantly lower level of complement C3 (P < 0.001). Patients with resolved HBV infection, when compared to the IgAN without HBV group, demonstrated lower eGFR level (74.1 vs. 84.2 ml/min per 1.73 m2, P = 0.001) at the time of kidney biopsy. Notably, there were no significant differences observed among the 3 groups in terms of blood pressure, urine protein, serum lipid, uric acid, and IgA levels.
Compared to the IgAN without HBV group, the chronic HBV infection group displayed a significantly higher proportion of tubular atrophy/interstitial fibrosis(T) lesions (40.9% vs. 30.5%, P = 0.028) and mesangial hypercellularity (M) lesions (57.6% vs. 41.1%, P < 0.001) in terms of pathological features. However, this significant difference was not observed in the resolved infection group.
Outcome and Analysis of Factors Associated With Kidney End Point
During a median follow-up period of 51 months (interquartile range: 28–91 months), a total of 341 patients (17.4%) within the study cohort reached renal endpoint. The results from multivariable cox regression analyses are presented in Table 2. After adjustment for, age, blood pressure, baseline eGFR, albumin-to-creatinine ratio, uric acid, lipid profiles, level of IgA, use of immunosuppressants, and pathology Oxford classification, chronic HBV infection demonstrated significant associations with the progression of IgAN (HR, 1.61; 95% CI, 1.06–2.44; P = 0.024), whereas resolved HBV infection demonstrated no significant associations with renal endpoint (HR, 1.01; 95% CI, 0.67–1.52; P = 0.969). Other factors, including systolic blood pressure (HR, 1.03; 95% CI, 1.01–1.05; P = 0.01), eGFR (HR, 0.97; 95% CI, 0.96–0.98; P = 0.010), and albumin-to-creatinine ratio (HR, 1.03; 95% CI. 1.02–1.05; P < 0.001), as well as the severity of M, S, and T lesions in the Oxford classification of renal pathology exert a significant influence on the outcomes of renal progression.
Table 2.
Risk factors for renal endpoint (eGFR decrease >40%)
| Variable | HR (95% CI) | P |
|---|---|---|
| Resolved HBV infection | 1.01 (0.67∼1.52) | 0.969 |
| Chronic HBV infection | 1.61 (1.06∼2.44) | 0.024 |
| Systolic blood pressure | 1.03 (1.01∼1.05) | 0.010 |
| Diastolic blood pressure | 0.99 (0.98∼1.02) | 0.334 |
| eGFR | 0.97 (0.96∼0.98) | <0.001 |
| Albumin-to-creatinine ratio | 1.03 (1.02∼1.05) | <0.001 |
| Uric acid | 1.00 (0.95∼1.06) | 0.106 |
| Triglycerides | 1.05 (0.98∼1.38) | 0.303 |
| IgA | 0.97 (0.88∼1.06) | 0.527 |
| Immunosuppressor | 1.11 (0.88∼1.41) | 0.024 |
| Oxford classification | ||
| M | 133 (1.05-1.68) | 0.016 |
| E | 0.98(0.78-1.25) | 0.893 |
| S | 1.90(1.43-2.54) | <0.001 |
| T | 1.76(1.35-2.28) | <0.001 |
| C | 1.05(0.83-1.34) | 0.684 |
C, presence of crescent; CI, confidence interval; E, endocapillary hypercellularity; eGFR, estimated glomerular filtration rate; HBV, hepatitis B virus; HR, hazard ratio; M, mesangial hypercellularity; S, segmental glomerulosclerosis/adhesion; T, severity of tubular atrophy/interstitial fibrosis.
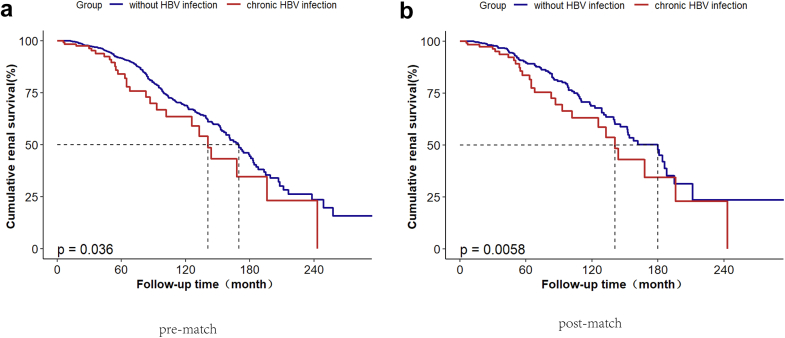
Sensitivity Analyses and Survival Analyses
A further 1:4 propensity-score matching approach was employed to ensure comparability between the chronic HBV infection group and the IgAN without HBV infection group (Supplementary Table S1). The analysis revealed consistent findings, reinforcing the significant association between chronic HBV infection and disease progression in IgAN (HR, 1.74; 95% CI, 1.28–2.37; P < 0.001). This association was further substantiated by a Kaplan-Meier survival analysis, which demonstrated a notable difference in renal survival between the chronic HBV infection group and the non-HBV infection group. Notably, patients with chronic HBV infection showed a worse prognosis compared to those without HBV infection in both prematching analysis (P = 0.036) and propensity-score-matching analysis (P = 0.006) (Figure 2). Furthermore, to address missing covariate data, an additional Cox regression analysis was conducted on the original dataset prior to imputation, which included a total of 1294 patients. Again, this analysis confirmed the persistent association between chronic HBV infection and disease progression (HR, 1.62; 95% CI, 1.07–2.44; P = 0.022). Similarly, the analysis indicated that resolved HBV infection did not exhibit any significant associations with the renal end point before imputation (HR, 1.01; 95% CI, 0.77–1.34; P = 0.926), as illustrated in Table 3.
Figure 2.
(a) Comparisons of Kaplan-Meier renal survival curves between IgAN with chronic HBV infection and those without HBV infection pre-matching analysis; (b) post propensity-score-matching analysis.
Table 3.
Sensitivity analysis of the association between HBV infection states and outcome
| Variable | Post imputation |
P-value | Before imputation |
P-value |
|---|---|---|---|---|
| HR(95% CI) | HR (95% CI) | |||
| Without HBV infection | 1 (Ref) | 1 (Ref) | ||
| Resolved HBV infection | 1.09 (0.83–1.42) | 0.556 | 1.01 (0.77–1.34) | 0.926 |
| Chronic HBV infection | 1.62 (1.07–2.44) | 0.022 | ||
| Before PSM | 1.61 (1.06–2.44) | 0.024 | ||
| After PSM | 1.74 (1.28–2.37) | <0.001 |
CI, confidence interval; HBV, hepatitis B virus; HR, hazard ratio; PSM, propensity score matching.
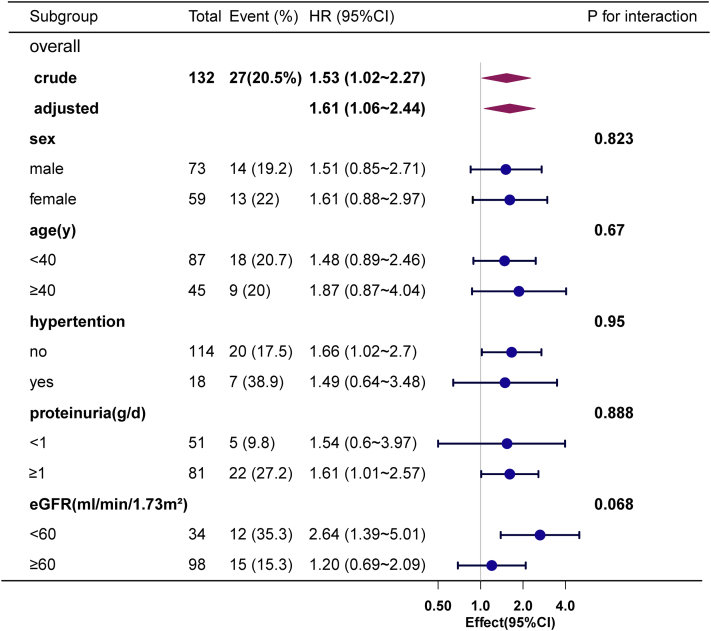
The results of the subgroup analysis and the summarization of the interaction suggest that no significant interactions were observed among the subgroups stratified by gender, age, hypertension, proteinuria, and eGFR (Figure 3). These sensitivity and subgroup analyses provide robust evidence supporting the significant role of chronic HBV infection in IgAN disease progression.
Figure 3.
Subgroup analysis on the correlation between chronic HBV infection and the progression of IgAN.
The Impact of HBV Deposition in Renal Tissue and Anti-HBV Therapy on Renal Outcome
Subsequently, we examined the impact of HBV deposition in renal tissue on clinical manifestations and prognosis in 556 individuals with IgAN and a history of HBV infection. Renal tissue deposition of hepatitis B antigen was observed in 33 patients, of which 15 had chronic hepatitis B infection and 18 had resolved hepatitis B infection. Among 1405 cases of IgAN without a history of HBV infection, 30 cases were randomly tested for hepatitis B antigen staining, resulting in no detectable deposition of hepatitis B antigen in renal tissue. As depicted in Table 4, patients with IgAN and concurrent renal HBV deposits exhibited a tendency for younger age (P = 0.003), heightened susceptibility to prodromic infections (P = 0.012), and lower C3 levels (P = 0.001). Notably, the prevalence of M, E, and T lesions in the renal pathology of the HBV renal deposition group was the lowest. The level of IgG, IgM, and C1q had no significant differences between the 2 groups.
Table 4.
The clinical and pathological characteristics of HBVwith or without deposition in renal
| Variables | Renal tissue |
Without HBV deposition |
P-value | |
|---|---|---|---|---|
| HBV deposition (n = 33) | Chronic infection (n = 116) | Resolved infection (n = 407) | ||
| Age | 32.6 ± 11.7 | 38.2 ± 10.1 | 39.7 ± 12.0 | 0.003 |
| Female | 13 (39.4) | 51 (44) | 195 (47.9) | 0.546 |
| Hypertension, n (%) | 3 (9.1) | 27 (23.3) | 103 (25.3) | 0.099 |
| BMI, kg/m2 | 23.8 ± 3.5 | 25.2 ± 3.9 | 24.5 ± 3.5 | 0.086 |
| Prodromic infection, n (%) | 16 (48.5) | 25 (21.6) | 116 (28.5) | 0.012 |
| Gross hematuria, n (%) | 8 (24.2) | 19 (16.4) | 85 (20.9) | 0.448 |
| Proteinuria, g/24h | 2.0 ± 2.5 | 1.8 ± 2.0 | 1.9 ± 2.4 | 0.857 |
| Uric acid, μmol/l | 363.6 ± 92.4 | 386.9 ± 102.5 | 375.2 ± 99.8 | 0.394 |
| eGFR, ml/min/1.73 m2 | 86.8 (66.0, 105.0) | 78.0 (57.6, 98.6) | 73.7 (50.0, 102.1) | 0.422 |
| IgA, g/l | 3.5 (2.3, 4.2) | 3.1 (2.6, 4.2) | 3.2 (2.4, 3.9) | 0.714 |
| C3, g/l | 0.8 (0.8, 1.0) | 0.9 (0.8, 1.0) | 1.0 (0.9, 1.2) | 0.001 |
| Antiviral medications, n (%) | 5 (15.2) | 36 (31) | 3 (0.7) | <0.001 |
| immunosuppressor, n (%) | 16 (48.5) | 30 (25.9) | 157 (38.8) | 0.001 |
| renal pathology | ||||
| IgM, n (%) | 20 (60.6) | 56(49.6) | -- | 0.318 |
| IgG, n (%) | 5 (15.2) | 22 (19.5) | -- | 0.819 |
| C1Q, n (%) | 4 (12.1) | 15 (13.3) | -- | 0.999 |
| Oxford classification | ||||
| M, n (%) | 10 (31.2) | 68 (59.6) | 147 (36.7) | 0.001 |
| E, n (%) | 9 (28.1) | 47 (41.2) | 127 (31.7) | 0.141 |
| S, n (%) | 18 (56.2) | 75 (65.8) | 262 (65.3) | 0.537 |
| T, n (%) | 8(25.0) | 48(42.1) | 130(32.4) | 0.041 |
| C, n (%) | 12(37.5) | 69(60.0) | 251(61.7) | 0.011 |
BMI, body mass index; eGFR, estimated glomerular filtration rate; HBV, hepatitis B virus.
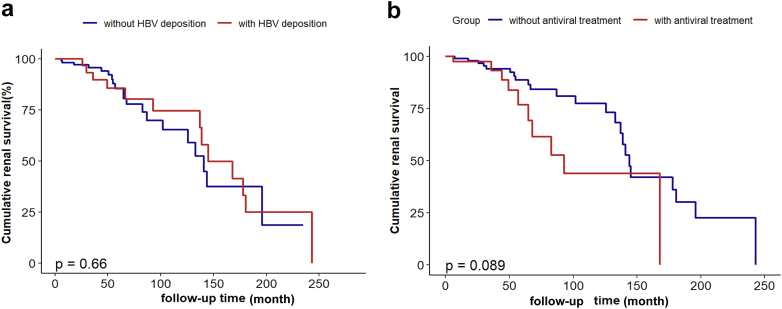
Nevertheless, functional survival analysis using the Kaplan-Meier method yielded no significant differences between the HBV deposition and non-HBV deposition groups in terms of renal survival (P = 0.660). Conversely, the presence of replicative HBV infection has been consistently associated with a notable reduction in the cumulative renal survival rates (P = 0.029) (Supplementary Figure S1). In addition, we assessed the potential impact of targeted antiviral therapy against HBV on renal outcomes. Among a subset of 132 individuals diagnosed with chronic HBV infection, 44 patients received antiviral therapy. Significantly, the utilization of these antiviral agents did not result in noteworthy disparities in cumulative renal survival when compared to the untreated group (P = 0.089) (Figure 4).
Figure 4.
(a) A comparison of the Kaplan–Meier renal survival curves for IgAN with HBV deposition and without deposition; (b) A comparison of the Kaplan–Meier renal survival curves for the patients who received antiviral treatment and those who did not receive antiviral treatment.
Furthermore, among the 556 individuals with IgAN and a history of HBV infection, 173 cases (11 with chronic HBV infection and 162 with resolved HBV infection) tested negative for HBV-DNA. These individuals received immunosuppressive agents without concurrent antiviral prophylaxis. Notably, 2 cases (1.2%) of hepatitis B reactivation were observed during the follow-up period. In one case, a patient with chronic hepatitis B infection exhibited elevated HBV-DNA levels, reaching 9.8 x 105 copies/ml, accompanied by abnormal liver function. The second case involved a serologic conversion of hepatitis B markers, transitioning from initial antihepatitis B core antibody positivity to subsequent positivity for HBsAg, hepatitis B e antibody, and antihepatitis B core antibody.
Discussion
To our knowledge, this study is the first comprehensive investigation examining the impact of different states of HBV infection on the clinical characteristics and prognosis of IgAN. The large-scale examination utilized a meticulously maintained follow-up database for patients with IgAN, providing valuable insights into the topic. The primary objective was to elucidate the distinct states of HBV infection and their consequential effects on the clinical features and disease progression in individuals with IgAN. Furthermore, we endeavored to explore the clinical significance of renal HBV deposition in IgAN and assess the potential impact of antiviral medications on the progression of renal function.
Consistent with a previous study conducted in 2021,19 our research findings provide evidence that chronic HBV infection represents an independent risk factor for the progression of IgAN. In addition, our study reveals that resolved HBV infection does not contribute to the disease progression of IgAN. An investigation conducted in 2020, which suggested that every HBV infection status posed a risk factor for chronic kidney disease.20 The observed discrepancies in conclusions between our study and the cross-sectional study conducted in 2020 may be attributed to methodological and population differences. Furthermore, our study revealed notable clinicopathological differences between patients with chronic HBV infection and those without HBV infection. Specifically, we observed a higher proportion of mesangial hypercellularity and renal tubulointerstitial lesions in the chronic HBV infection group, suggesting that HBV infection may exacerbate renal damage in individuals with IgAN. In addition, we observed a significant difference in C3 levels between the chronic HBV infection group and the IgAN group without signs of HBV infection, with lower C3 levels in HBV-infected individuals. Moreover, we found that 33 patients with deposition of HBV in the kidneys appeared to be more associated with precursor infections and reduced C3 levels. The complement activation plays a crucial role in the pathogenesis of IgAN.21,22 Although the precise interplay between chronic HBV infection and complement remains largely unknown, several studies have documented reduced levels of complement components (including C3, C4, C7, and C9) in individuals suffering from chronic HBV infection,23,24 thus indicative of a possible activation of the complement system. Notably, the extent to which the involvement of the complement pathway contributes to the pathogenesis of IgAN in the setting of HBV infection remains to be elucidated and requires comprehensive investigation and rigorous evaluation.
The presence of HBV deposition in mesangial proliferative glomerulonephritis with IgA deposits raises the question whether it should be classified as HBV-associated renal disease or as an overlapping coincidental occurrence of IgAN and HBV-related renal tissue deposition. Early studies have shown that HBsAg deposition in IgAN is colocalized with IgA, and HBV can be detected in mesangial cell nuclei and cytoplasm. These findings suggest a potential pathogenic role of HBV in the development of IgAN.6 Some studies proposed that HBV could directly damage renal tissue through in situ replication, thereby promoting the pathogenesis of IgAN.25 In contrast to the established mechanistic association observed between HBV infection and membranous nephropathy,26 the underlying mechanism by which HBV induces IgAN remains elusive and lacks recognition. In recent years, several investigations pertaining to IgAN and chronic HBV potentially offer valuable insights into the intricate mechanisms involved in this pathogenic process. Genome-wide DNA methylation analysis of CD4+ T cells in patients with IgAN identified a hypermethylation region containing VTRNA2-1, a noncoding RNA.27 VTRNA2-1 activates PKR, leading to CREB phosphorylation and IL-6 expression. This pathway is upregulated in patients with IgAN, resulting in elevated IL-6 levels.28 Involvement of IL-6 plays a critical role in the IgA deposition process of IgAN.29 PKR is typically activated by both viral and bacterial RNA species. Interestingly, an independent study demonstrated that HBsAg augments the glucose/cAMP/PKR/CREB signaling pathway.30 Consequently, it would be prudent to explore potential contributions of HBsAg, particularly when considering genetic factors, in the pathogenesis of IgAN through this signaling cascade in future investigations. In addition, in recent years, significant progress has been made in studying the gut microbiota in IgAN, as well as in chronic HBV-related liver diseases.31,32 The gut microbiota may play a certain interactive role between these 2 diseases, providing another plausible pathway worth investigating further.
Contrary to expectations, our study found no evidence of worse renal outcomes in patients with IgAN with concurrent HBV infection despite the presence of HBV deposits in renal tissue. Therefore, the deposition of hepatitis B antigen in kidneys is not a reliable factor for assessing the impact of HBV on renal prognosis in IgAN. Instead, HBV-DNA might serve as a more suitable indicator. In addition, our study demonstrated that the use of antihepatitis B medications does not worsen the progression of IgAN with chronic HBV infection. It is important to note that current guidelines recommend antiviral treatment for replicative viral infections (hepatitis B e antigen-positive and/or viral DNA levels >2000 IU/ml) in kidney disease. Antiviral prophylaxis is indicated for patients with chronic HBV infection who are receiving a drug regimen with an intermediate or high risk of HBV reactivation.33, 34, 35 A cohort study conducted in Taiwan in 2018 revealed that untreated HBV infection was associated with an elevated risk of end-stage renal disease in patients with chronic kidney disease. Conversely, treatment with nucleos(t)ide analogues was linked to a reduced risk of end-stage renal disease.36 These findings should alleviate concerns about the deterioration of renal function when administering antiviral therapy for HBV in progressive patients with IgAN receiving immunosuppressive therapy. They reinforce the importance of promptly recognizing and managing HBV infection in patients with IgAN in order to prevent HBV reactivation and subsequent disease progression.
Despite the relatively large-scale nature of our study on IgAN, it is essential to acknowledge certain limitations. First, our study predominantly encompassed participants from the Han ethnic group residing in northern China. It is vital to consider that the prevalence of both HBV infection and IgAN may vary among distinct ethnicities. To enhance the generalizability and scientific rigor of our findings, it is essential to conduct further research encompassing diverse populations from different regions and ethnic backgrounds. Second, the absence of information pertaining to changes in antiviral medication types and discontinuation hindered a comprehensive evaluation of their efficacy. Consequently, a holistic assessment of the effectiveness of these medications could not be fully realized. In addition, the third hepatitis B infection status studied, also known as renal tissue HBV deposition, cannot be ruled out in some cases due to contamination of renal tissue with HBV from serum after renal puncture injury to blood vessels, resulting in false-positive renal tissue HBV staining. This may introduce some bias. Third, owing to the unavailability of patients with IgAN with concomitant acute HBV infection, a meticulous evaluation of the status of such infection could not be incorporated.
Conclusion
In conclusion, our study provides evidence that chronic HBV infection leads to distinct clinical and pathological features, increasing the risk of IgAN. However, resolved HBV infections do not pose similar risks. Renal deposition of HBV does not correlate with adverse renal outcomes, and antiviral therapy tailored for HBV does not suggest a risk factor in kidney function worsening. Our findings highlight the importance of considering HBV infection status when managing patients with IgAN and call for further research to elucidate underlying mechanisms and investigate potential benefits of targeted antiviral therapy in individuals with concomitant HBV infection and IgAN.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported by the National Science Foundation of China (82022010, 82131430172, 82370709, 81970613, 82070733, 82000680); Beijing Natural Science Foundation (Z190023); Academy of Medical Sciences--Newton Advanced Fellowship (NAFR13∖1033); Fok Ying Tung Education Foundation (171030); Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (2019-I2M-5-046, 2020-JKCS-009); National High Level Hospital Clinical Research Funding (Interdisciplinary Clinical Research Project of Peking University First Hospital, 2022CR41); and China International Medical Foundation (Z-2017-26-2202-2).
Author Contributions
M-zG conceived the study, contributed to study design, oversaw analysis, and wrote the manuscript. X-jZ and HZ contributed to funding and data acquisition, provided intellectual input, and reviewed/edited the manuscript. YL and XW contributed to data analysis/interpretation. PC and L-lX contributed to data acquisition and study conceptualization. J-cL, F-YH, S-fS, and L-jL contributed to study design, provided intellectual input, and reviewed the manuscript. X-jZ is the guarantor of this work and takes responsibility for final responsibility for the decision to submit for publication.
Footnotes
Table S1. Clinical features of patients after 1:4 propensity score matching.
Figure S1. Comparison of Kaplan-Meier renal survival curves for IgAN with and without HBV replication.
STROBE Statement.
Supplementary Materials
Table S1. Clinical features of patients after 1:4 propensity score matching
Figure S1. Comparison of Kaplan-Meier renal survival curves for IgAN with and without HBV replication
STROBE Statement.
References
- 1.Pattrapornpisut P., Avila-Casado C., Reich H.N. IgA nephropathy: core curriculum 2021. Am J Kidney Dis. 2021;78:429–441. doi: 10.1053/j.ajkd.2021.01.024. [DOI] [PubMed] [Google Scholar]
- 2.Schena F.P., Nistor I. Epidemiology of IgA nephropathy: a global perspective. Semin Nephrol. 2018;38:435–442. doi: 10.1016/j.semnephrol.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 3.He J.W., Zhou X.J., Hou P., et al. Potential roles of oral microbiota in the pathogenesis of immunoglobin A nephropathy. Front Cell Infect Microbiol. 2021;11 doi: 10.3389/fcimb.2021.652837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saha M.K., Julian B.A., Novak J., Rizk D.V. Secondary IgA nephropathy. Kidney Int. 2018;94:674–681. doi: 10.1016/j.kint.2018.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu Y.C., Huang D.Q., Nguyen M.H. Global burden of hepatitis B virus: current status, missed opportunities and a call for action. Nat Rev Gastroenterol Hepatol. 2023;20:524–537. doi: 10.1038/s41575-023-00760-9. [DOI] [PubMed] [Google Scholar]
- 6.Lai K.N., Lai F.M., Tam J.S. IgA nephropathy associated with chronic hepatitis B virus infection in adults: the pathogenetic role of HBsAg. J Pathol. 1989;157:321–327. doi: 10.1002/path.1711570409. [DOI] [PubMed] [Google Scholar]
- 7.Wang N.S., Wu Z.L., Zhang Y.E., Liao L.T. Existence and significance of hepatitis B virus DNA in kidneys of IgA nephropathy. World J Gastroenterol. 2005;11:712–716. doi: 10.3748/wjg.v11.i5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakopoulou L., Stefanaki K., Zeis P.M., et al. The frequency of hepatitis B virus infection in Greek patients with various types of glomerulonephritis. Eur J Epidemiol. 1994;10:737–742. doi: 10.1007/bf01719291. [DOI] [PubMed] [Google Scholar]
- 9.Iida H., Izumino K., Asaka M., Fujita M., Takata M., Sasayama S. IgA nephropathy and hepatitis B virus. IgA nephropathy unrelated to hepatitis B surface antigenemia. Nephron. 1990;54:18–20. doi: 10.1159/000185803. [DOI] [PubMed] [Google Scholar]
- 10.Sun I.O., Hong Y.A., Park H.S., et al. Clinical characteristics and treatment of patients with IgA nephropathy and hepatitis B surface antigen. Ren Fail. 2013;35:446–451. doi: 10.3109/0886022x.2013.775659. [DOI] [PubMed] [Google Scholar]
- 11.Wang R., Wu Y., Zheng B., et al. Clinicopathological characteristics and prognosis of hepatitis B associated membranous nephropathy and idiopathic membranous nephropathy complicated with hepatitis B virus infection. Sci Rep. 2021;11 doi: 10.1038/s41598-021-98010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y., Zheng M. Hepatitis B virus persistence and reactivation. BMJ. 2020;370:m2200. doi: 10.1136/bmj.m2200. [DOI] [PubMed] [Google Scholar]
- 13.Kong X., Ma Y., Chen J., et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating glomerular filtration rate in the Chinese population. Nephrol Dial Transplant. 2013;28:641–651. doi: 10.1093/ndt/gfs491. [DOI] [PubMed] [Google Scholar]
- 14.Trimarchi H., Barratt J., Cattran D.C., et al. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91:1014–1021. doi: 10.1016/j.kint.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Terrault N.A., Lok A.S.F., McMahon B.J., et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myint A., Tong M.J., Beaven S.W. Reactivation of hepatitis B virus: a review of clinical guidelines. Clin Liver Dis (Hoboken) 2020;15:162–167. doi: 10.1002/cld.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Etienne S., Vosbeck J., Bernsmeier C., Osthoff M. Prevention of hepatitis B reactivation in patients receiving immunosuppressive therapy: a case series and appraisal of society guidelines. J Gen Intern Med. 2023;38:490–501. doi: 10.1007/s11606-022-07806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Buuren S., Groothuis-Oudshoorn K. mice: multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45:1–67. doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 19.Wang K., Yu Z., Huang Y., et al. Clinicopathological features, risk factors, and outcomes of immunoglobulin A nephropathy associated with hepatitis B virus infection. J Nephrol. 2021;34:1887–1896. doi: 10.1007/s40620-021-01004-2. [DOI] [PubMed] [Google Scholar]
- 20.Du Y., Zhang S., Hu M., et al. Association between hepatitis B virus infection and chronic kidney disease: a cross-sectional study from 3 million population aged 20 to 49 years in rural China. Med (Baltim) 2019;98 doi: 10.1097/md.0000000000014262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maillard N., Wyatt R.J., Julian B.A., et al. Current understanding of the role of complement in IgA nephropathy. J Am Soc Nephrol. 2015;26:1503–1512. doi: 10.1681/asn.2014101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roos A., Rastaldi M.P., Calvaresi N., et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006;17:1724–1734. doi: 10.1681/asn.2005090923. [DOI] [PubMed] [Google Scholar]
- 23.Baidya A., Khatun M., Mondal R.K., et al. Hepatitis B virus suppresses complement C9 synthesis by limiting the availability of transcription factor USF-1 and inhibits formation of membrane attack complex: implications in disease pathogenesis. J Biomed Sci. 2022;29:97. doi: 10.1186/s12929-022-00876-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu C., Song H., Xu F., Yi W., Liu F., Liu X. Hepatitis B virus inhibits the expression of complement C3 and C4, in vitro and in vivo. Oncol Lett. 2018;15:7459–7463. doi: 10.3892/ol.2018.8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang N.S., Wu Z.L., Zhang Y.E., Guo M.Y., Liao L.T. Role of hepatitis B virus infection in pathogenesis of IgA nephropathy. World J Gastroenterol. 2003;9:2004–2008. doi: 10.3748/wjg.v9.i9.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long J.D., Rutledge S.M., Sise M.E. Autoimmune kidney diseases associated with chronic viral infections. Rheum Dis Clin North Am. 2018;44:675–698. doi: 10.1016/j.rdc.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Sallustio F., Serino G., Cox S.N., et al. Aberrantly methylated DNA regions lead to low activation of CD4+ T-cells in IgA nephropathy. Clin Sci (Lond) 2016;130:733–746. doi: 10.1042/cs20150711. [DOI] [PubMed] [Google Scholar]
- 28.Sallustio F., Picerno A., Cimmarusti M.T., et al. Elevated levels of IL-6 in IgA nephropathy patients are induced by an epigenetically driven mechanism modulated by viral and bacterial RNA. Eur J Intern Med. 2023;118:108–117. doi: 10.1016/j.ejim.2023.07.045. [DOI] [PubMed] [Google Scholar]
- 29.Sallustio F., Picerno A., Montenegro F., Cimmarusti M.T., Di Leo V., Gesualdo L. The human virome and its crosslink with glomerulonephritis and IgA nephropathy. Int J Mol Sci. 2023:24. doi: 10.3390/ijms24043897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y., Wang B., Ou X., et al. Small hepatitis B virus surface antigen promotes hepatic gluconeogenesis via enhancing glucagon/cAMP/protein kinase A/CREB signaling. J Virol. 2022;96 doi: 10.1128/jvi.01020-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen Y., Wu S.D., Chen Y., et al. Alterations in gut microbiome and metabolomics in chronic hepatitis B infection-associated liver disease and their impact on peripheral immune response. Gut Microbes. 2023;15 doi: 10.1080/19490976.2022.2155018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He J.W., Zhou X.J., Lv J.C., Zhang H. Perspectives on how mucosal immune responses, infections and gut microbiome shape IgA nephropathy and future therapies. Theranostics. 2020;10:11462–11478. doi: 10.7150/thno.49778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau G., Yu M.L., Wong G., et al. APASL clinical practice guideline on hepatitis B reactivation related to the use of immunosuppressive therapy. Hepatol Int. 2021;15:1031–1048. doi: 10.1007/s12072-021-10239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kidney Disease: Improving Global Outcomes KDIGO 2021 Clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;2021:S1–S276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 35.European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y.C., Li C.Y., Tsai S.J., Chen Y.C. Nationwide cohort study suggests that nucleos(t)ide analogue therapy decreases dialysis risk in Taiwanese chronic kidney disease patients acquiring hepatitis B virus infection. World J Gastroenterol. 2018;24:917–928. doi: 10.3748/wjg.v24.i8.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.