Abstract
Frailty is a multidimensional clinical syndrome characterized by low physical activity, reduced strength, accumulation of multiorgan deficits, decreased physiological reserve, and vulnerability to stressors. Frailty has key social, psychological, and cognitive implications. Frailty is accelerated by uremia, leading to a high prevalence of frailty in patients with advanced chronic kidney disease (CKD) and end-stage kidney disease (ESKD) as well as contributing to adverse outcomes in this patient population. Frailty assessment is not routine in patients with CKD; however, a number of validated clinical assessment tools can assist in prognostication. Frailty assessment in nephrology populations supports shared decision-making and advanced communication and should inform key medical transitions. Frailty screening and interventions in CKD or ESKD are a developing research priority with a rapidly expanding literature base.
Keywords: assessment, communication, frailty, kidney disease, rehabilitation
Frailty is a clinical syndrome characterized by immobility and inactivity, reduced strength and muscle loss, accumulation of multiple end-organ impairments, and decreased physiological reserve.1,2 Frailty is a state of accelerated and pathological aging which is prevalent and clinically relevant in nephrology populations. Pathophysiology reflects an accumulation of vascular, inflammatory, nutritional and age-related insults, all of which are compounded by the presence of comorbidity and uremia.3 People living with frailty experience increased functional dependency and key social and cognitive sequelae that interact with physical manifestations.4,5 Frailty threatens independence and quality of life, and predicts nonroutine recovery and increased healthcare utilization patterns.6 In nephrology settings, frailty is associated with accelerated disease progression, dialysis and transplant complications, as well as increased mortality risk.7,8 National and international data registries indicate that among patients with ESKD, those aged >65 years represent the largest and most rapidly growing patient population, accounting for up to 45% of people accepted for renal replacement therapy.9, 10, 11 Although poorly captured by registry data, frailty likely contributes substantially to the increased case complexity noted among patient populations referred for nephrology care.12 This emphasizes the importance of recognizing frailty in kidney disease populations.
Other reviews of frailty in kidney disease have been presented. This current paper offers new insights including an emphasis on the nonphysical aspects of frailty, including social frailty, cognitive frailty, and emotional/psychological frailty which emerge as primary concerns to people living with frailty. We compare the CKD experience of frailty with other chronic end-organ disease populations and explore the complex interaction of sarcopenia and estimated glomerular filtration rate calculations which obscure assessment of both kidney disease and frailty in CKD settings. We also explore the lived experience of frailty and significance of self-identified frailty and implications for medical decision-making. This current review incorporates the updated literature, particularly new clinical practice guidelines and interventions for frailty management. We describe how frailty has implications for caregiver burden and healthcare utilization patterns, including dialysis withdrawal. The review explores validated tools for frailty assessment to complement nephrology practice and highlights key risk factors and clinical markers that should alert clinicians to the presence of frailty. Lastly, we outline how recognition of frailty in nephrology settings may improve communication and access to palliative and supportive care.
Prevalence of Frailty in Nephrology Populations: an Epidemic of Public Health Significance
Frailty is highly prevalent among nephrology populations, occurring at more than twice the rate compared to community-dwelling older adults and increasing with CKD progression.13, 14, 15, 16 Compared to chronic disease populations with heart and lung pathology, patients with kidney disease demonstrate greater functional disability.17 Systematic reviews examining frailty in kidney disease report that frailty prevalence varies with population demographics, research settings, and diagnostic definitions. We refer to our recently published scoping review which provides an overview of frailty prevalence by nephrology population and choice of frailty assessment tool.18 A number of studies report variation in frailty prevalence using different screening tools within the same study population, allowing a comparison of frailty metrics.19, 20, 21, 22 Among patients with advanced CKD, frailty ranges from 42.6% to 74.1% in patients with stage 4 CKD and 53.8 to 4.7% in predialysis populations.18,23,24 Frailty appears to develop alongside kidney disease progression with incidence rate of 44.5% described in one heterogenous CKD cohort.25 Rates as high as 82% have been described in hemodialysis (HD) populations26 and up to 75% in patients incident to peritoneal dialysis.27 Duration of HD appears to predict severity of frailty.28 Functional assessments are frequently utilized as a proxy measure of frailty in the nephrology literature.29 Studies that have examined functional status longitudinally report increased dependency in activities of daily living within 3 months30 and 12 months31 of dialysis initiation. Longitudinal assessments of Fried frailty within HD cohorts demonstrate that progression of frailty occurs as often as frailty remission, although the individual frailty domains of physical inactivity and grip strength decline significantly with time.32, 33, 34 Conservatively managed populations have notably been underexamined for frailty; a single study reports prevalence of 62%.21 A growing body of evidence examining frailty in kidney transplantation informs waitlisting and transplantation practices.35,36 Frailty within kidney transplant centers has been described in a number of studies with many indicating prevalence of about 20% among waitlisted candidates and following successful transplantation.36, 37, 38, 39, 40, 41, 42
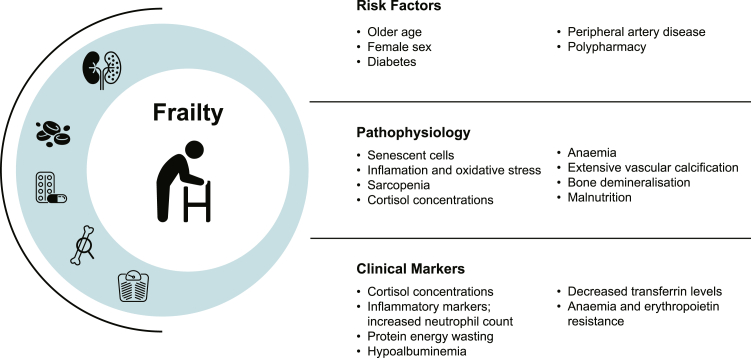
In addition to kidney disease, risk factors for frailty include older age, female sex, diabetes, and peripheral artery disease.32,43,44 Polypharmacy is also associated with frailty, demonstrating a dose-response relationship, and in particular predicting greater risk of exhaustion and physical inactivity.45 Presence of these risk factors should alert clinicians to the increased probability of frailty and also offering insights into pathophysiologic processes. Few studies have explored how frailty behaves in kidney disease models with high inflammatory and immunosuppressive burden such as glomerulonephritis; these conditions provide opportunity for further study and enhanced understanding of frailty in CKD.46 Recognition of frailty and its risk factors may improve clinical prognostication and strengthen shared decision-making.
Pathophysiology of Frailty
Frailty pathogenesis may be characterized by the accumulation of senescent cells in multiple tissues and proinflammatory signaling, leading to a concept of “inflammageing”47. The pathophysiological mechanisms and relationship between organ systems remains poorly defined and largely speculative. Cell senescence is believed to be triggered by multiple stimuli, including telomere shortening, accumulation of DNA damage, oncogene activation, epigenetic alterations, mitochondrial dysfunction, and environmental factors. Senescent cells may acquire a secretory phenotype and produce inflammatory signals that act in a paracrine fashion to mediate cellular death in neighboring cells. The aging immune system exhibits a reduction in T-lymphocyte production, blunted B-cell-mediated antibody response, and reduced phagocytic activity among neutrophils and macrophages that fails to adequately respond to acute inflammatory or infective threat.48 Inflammation appears to play a central role in frailty pathogenesis through an aberrant, low-grade inflammatory response that persists beyond the removal of the inciting inflammatory stimulus. Several inflammatory mediators, including interleukin-6, C-reactive protein, tumor-necrosis factor α, and advanced glycation end-products have been implicated in aging, chronic disease, and mortality.47,49 Changes in gut microbiota and permeability are thought to contribute to a chronic proinflammatory state.50 Inflammation mediates T-cell senescence, bone loss, and physical and cognitive decline.47 Oxidative stress and inflammation promote imbalances in protein metabolism, infiltration of intermuscular fat (myosteatosis), and decreased type II myofibers contributing to sarcopenia and insulin resistance.51,52 Hyperactivity of the hypothalamic-pituitary-axis contributes to neuronal deterioration associated with aging, manifest as chronic raised diurnal cortisol concentrations, increased stress responsiveness, and vulnerability to stressors.53 Persistently high levels of cortisol contribute to catabolism, loss of muscle mass, anorexia, weight loss, and reduced energy expenditure.54, 55, 56 Changes in synaptic function, protein transport, and mitochondrial function particularly in the prefrontal cortex and the hippocampus coincide with functional changes to microglial cells—the central nervous system equivalent of macrophages—producing hyper-responsive inflammatory changes to neuronal death that are implicated in cognitive decline, impaired learning and are postulated to have an important role in the pathophysiology of delirium.53,57, 58, 59
CKD is an intensely inflammatory state characterized by metabolic dysregulation and protein catabolism due to uremia and metabolic acidosis, insulin resistance, accumulation of advanced glycation end-products, oxidative stress, anemia and impaired oxygen delivery, extensive vascular calcification, bone demineralization, and frequent comorbid infection.60 Dysregulation of renin-angiotensin activity is implicated in the induction of reactive oxygen species, cellular hypertrophy and apoptosis, fibroblast proliferation and collagen synthesis with end-organ effects, which manifest in cardiac and vascular tissues. The central role of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers in the prevention or regression of age-associated and CKD-related disease such as ischemic heart disease, left ventricular hypertrophy, atrial fibrillation, stroke, and diabetes offers indirect evidence for the role of renin-angiotensin in systemic disease and frailty.61 Abnormalities within renin-angiotensin are implicated in sarcopenia through impaired muscle regeneration and accelerated proteolysis.62 Uremia, insulin resistance, hypogonadism, and vitamin D deficiency are also contributary to sarcopenia.63,64 Myostatin is elevated in uremic states and impairs muscle regeneration and promotes muscle degradation.65 Sarcopenia increases progressively with loss of renal function in patients with CKD, reflecting type II fiber atrophy, mitochondrial depletion, and altered protein turnover.64 Metabolic acidosis, inflammation, anorexia, and malnutrition further aggravate this cycle of deconditioning.60,66,67 Derangements of the hypothalamic-pituitary axis are prominent in kidney disease states and may be implicated in the development and progression of frailty.68 Serum cyanate, which exists in equilibrium with urea, promotes protein carbamylation, contributing to erythropoietin resistance, renal fibrosis, disordered immune response, insulin resistance, and vascular damage.69
Protein-energy wasting is frequently identified in patients with CKD and ESKD, where metabolic derangements coincide with nutritional deficiencies, characterized by loss of systemic body protein and energy stores. Clinical criteria for identifying catabolism include low serum albumin, weight loss and low body mass index, reductions in fat and muscle mass, and identification of deficient dietary intake.70 Dietary restrictions, dysgeusia, and uremic nausea or vomiting may contribute to anorexic states, socioeconomic factors, and depression (Figure 1).
Figure 1.
Risk factors, pathophysiological mechanisms, and clinical markers of frailty in the kidney disease context.
Recognizing the role of inflammation in CKD or frailty has important clinical implications. Clinicians should consider the possibility of frailty where inflammatory markers are elevated alongside sarcopenia and malnutrition in patients with CKD. In particular, hypoalbuminemia, decreased transferrin levels, increased neutrophil count, elevated c-reactive protein, and the presence of erythropoietin resistance frequently accompany frailty.71 Visceral adiposity predicts not only increased risk of frailty but also progressive renal function decline, offering opportunity for early metabolic intervention. Moreover, in the setting of CKD and ESKD, reductions in serum creatinine may reflect sarcopenia and hypercatabolism rather than improvements in renal function or dialysis adequacy. In frail and sarcopenic populations, alternative measures of renal function which are independent of muscle mass, emerge as the optimal research tool. Observational studies using cystatin-C report an independent association with frailty and glomerular filtration rate.14,72,73
Clinical Implications of Frailty in Kidney Disease
The presence of frailty in patients with nondialysis CKD predicts a number of adverse patient outcomes (see Table 1). Frailty predicts an approximately 2- to 6-fold increase in risk of death, hospitalization, and progression to dialysis.14,74 Both objective measures of frailty and subjective assessments are associated with not-for-dialysis conservatively managed choice.75,76, 77, 78, 79 Whether assessed objectively or subjectively, frailty appears to increase the probability of initiation of in-center rather than home-based dialysis modality.75,80 Frailty in home dialysis populations predicts technique failure and death.81 A single study has examined survival outcomes specifically in frail patients undertaking dialysis compared to conservative care and reported no differences in survival outcomes when adjusted for sex, comorbidity, or age.75
Table 1.
Adverse outcomes associated with frailty among kidney disease populations
| Study population | Outcomes of interest | HR/OR, risk difference (95% CI) | References |
|---|---|---|---|
| CKD | Choice of RRT over nondialysis conservative care | OR 0.62 (0.51–0.75) | Pyart et al.75 |
| Dialysis initiation | HR 5.42 (1.06– 27.64) | Vezza et al.82 | |
| In-center dialysis over home therapy | OR 3.41 (1.56– 7.44) | Brar et al.80 | |
| Hospitalization | HR 18.80 (2.36– 150.00) | Vezza et al.82 | |
| All-cause mortality | HR 4.28 (1.22– 14.9) | Ali et al.83 | |
| HR 1.96 (1.47– 2.61) | Brar et al.80 | ||
| HR 1.51 (1.10– 2.10) | Delgado et al.84 | ||
| HR 2.32 (0.23– 23.12) | Vezza et al.82 | ||
| HR 3.0 (2.2– 4.1) | Wilhelm-Leen et al.16 | ||
| Death or dialysis | HR 2.5 (1.4– 4.4) | Roshanravan et al.14 | |
| HD | Earlier dialysis commencement | OR 1.44 (1.23– 1.68) | Bao et al.85 |
| OR 1.16 per frailty symptom (1.06– 1.28) | Tamura et al.86 | ||
| Falls | HR 3.09 (1.38– 6.90) | McAdams-DeMarco et al.87 | |
| HR 2.1 (1.21– 3.92) | Yadla et al.26 | ||
| Emergency department visits | HR 2.20 (1.58– 3.08) | Garcia-Canton et al.88 | |
| IRR 2.78 (1.70– 4.60) | Li- et al.89 | ||
| HR 2.28 (1.30– 3.98) | Vinson et al.90 | ||
| Time to first hospitalization | HR 1.26 (1.09– 1.45) | Bao et al.85 | |
| Hospitalization | HR 2.09 (1.38– 3.18) | Garcia-Canton et al.88 | |
| HR 1.80 (1.38– 2.36) | Lee et al.91 | ||
| HR 1.43 (1.00– 2.03) | McAdams-DeMarco et al.8 | ||
| HR 1.35 (1.20– 1.53) | Nixon et al.92 | ||
| HR 2.31 (1.24– 4.32) | Van Loon et al.93 | ||
| HR 2.06 (1.18– 3.58) | Yadla et al.26 | ||
| Hospitalization (LOS >2 weeks post kidney transplantation) | HR 2.02 (1.20– 3.40) | Chu et al.34 | |
| 12-month mortality | HR 7.22 (2.47– 21.13) | Van Loon et al.93 | |
| All-cause mortality | HR 1.57 (1.25– 1.97) | Bao et al.85 | |
| HR 3.77 (1.10– 12.92) | Fitzpatrick et al.94 | ||
| HR 2.34 (1.39– 3.95) | Garcia-Canton et al.88 | ||
| HR 2.16 (1.41– 3.29) | Johansen et al.95 | ||
| HR 5.22 (2.28– 11.97) | Johansen et al.33 | ||
| HR 2.37 (1.11– 5.02) | Lee et al.91 | ||
| HR 4.10 (1.09– 15.43) | Li et al.89 | ||
| HR 2.60 (1.04– 6.49) | McAdams-DeMarco et al.8 | ||
| HR 2.15 (1.63– 2.85) | Nixon et al.92 | ||
| Postkidney transplant mortality | HR 2.27 (1.11– 4.65) | Chu et al.34 | |
| HR 2.30 (1.12– 4.74) | Nastasi et al.96 | ||
| No survival advantage to dialysis where frailty is present | HR 1.2 (0.69–2.06) | Pyart et al.75 | |
| Home therapy (Home HD or PD) | Composite technique failure and death | HR 2.10 (1.09– 3.99) | Brar et al.81 |
| Peritoneal dialysis | All-cause mortality | HR 1.79 (1.09– 2.94) | Chan et al.97 |
| HR 12.2 (2.27– 65.5) | Kamijo et al.98 | ||
| Kidney transplant recipient | Postoperative complications | HR 14.54 (7.90– 21.18) | Schopmeyer et al.99 |
| RR 2.14 (1.01– 4.54) | Dos Santos Mantovani et al.41 | ||
| Delayed graft function | RR 1.94 (1.13– 3.36) | Garonzik-Wang et al.100 | |
| Early hospital readmission | RR 1.61 (1.18– 2.19) | McAdams-DeMarco et al.101 | |
| Immunosuppression intolerance | HR 1.29 (1.01– 1.66) | McAdams-Demarco et al.102 | |
| All-cause mortality | HR 2.17 (1.01– 4.65) | McAdams-DeMarco et al.103 | |
| HR 2.61 (1.14– 5.97) | McAdams-DeMarco et al.104 |
CKD, chronic kidney disease; HD, hemodialysis; HR, hazard ratio; LOS, length of stay; OR, odds ratio; PD, peritoneal dialysis.
Predialysis frailty predicts earlier dialysis commencement, possibly reflecting misidentification of the manifestations of frailty as uremic symptoms.85,86 Presence of frailty while undergoing maintenance dialysis treatment is associated with excess infections and cardiovascular events as well as dialysis complications including prolonged postdialysis recovery time, increased interdialytic weight gain, hypoalbuminemia, and reduced medication adherence.105, 106, 107, 108 Studies report that frailty is associated with higher probability of initial and recurrent vascular access failure.109,110 A limited number of cross-sectional studies have compared the risk of frailty progression based on maintenance dialysis modality; most of these studies conclude no difference between HD and PD, but patient selection bias confounds the interpretation of this data.7,85,91,93,111, 112, 113, 114, 115, 116 Frailty in PD populations remains a neglected area of research activity.18,29 Studies of dialysis populations consistently report 1.4- to 3-fold increased risk of hospitalization and emergency department presentations and 2- to 5-fold hazard ratio for mortality, when adjusted for age and comorbidity.7,8,33,44,74 Hospitalization contributes to frailty progression and deterioration in frailty scores.32 Decreased physical performance and frailty are associated with reduced likelihood of transplant referral and waitlisting, and increased removal from waitlist and death on waitlist.38,40,117, 118, 119, 120 Studies enrolling patients with frailty incident to dialysis report transplantation rates of just 4%.93 International data registries confirm that frailty and its surrogate measures increase the odds of dialysis withdrawal as primary cause of death.121, 122, 123 Falls and particularly hip fracture-related mortality are also common frailty sequelae, with one study describing a 25% and 58% 30-day and 1-year mortality rate, respectively, for patients undergoing HD.87
Risk stratification using frailty assessment demonstrates increasing uptake and much research interest.18,124,125 Transplant centers that assess frailty as part of clinical practice demonstrate better patient-level outcomes, including waitlist mortality and death-censored graft loss.126 Such improvements in patient outcomes appear to come at the cost of reduced access to transplantation among frail candidates, although a number of centers did describe provision of additional social and home support, along with prehabilitation.124 Importantly, such benefits were only realized where validated frailty assessment tools were utilized, illustrating the risk of discrimination associated with subjective measures of frailty.126 Presence of frailty at transplantation is predicted by dialysis duration before transplantation.28 Frailty at the time of transplantation is associated with postoperative complications, including delayed graft function, extended hospital length-of-stay, readmission, immunosuppression intolerance, graft loss, and death with graft function.41,99,100,102,127,128 Frailty may be improved by kidney transplantation, following a period of early postoperative exacerbation.129
Frailty is associated with increased symptom burden and impaired quality of life. Qualitative studies of community-dwelling elderly populations report themes of progressive deterioration and vulnerability, loss of meaning, agency, and social identity.130,131 Social isolation and geographic contraction are common with community mobility and participation in life-spaces threatened by the experience of CKD and further disrupted by the introduction of dialysis.132,133 Frailty independently predicts worse health-related quality of life, symptom burden, and depression.24,31,134, 135 The lived experience of frailty in CKD for both patients and their caregivers deserve further examination. Pilot work suggests patients experience prominent fatigue and lack of social support and prioritize the ability to live independently.136
Frailty in CKD is independently associated with poorer cognitive function, increasing in prevalence across CKD stages and highly prevalent in dialysis populations.137,138 Impairment is more frequently vascular rather than amyloid-related in nature, with implications for executive function, attention, behavior control, and working memory.139, 140, 141 Coincident depression worsens cognitive performance further.142 These frailty manifestations have implications for therapeutic engagement, ability to self-care, adherence to therapy, and management of complex medication regimens. Nephrologists increasingly face questions of decision-making capacity. Skilled practitioners will recognize frailty as an opportunity for advanced communication, offer supported decision-making, and timely advance care planning.
Frailty is an important clinical phenomenon whose presence outperforms biochemical assessment in predicting patient-level outcomes. Recognizing frailty and individualizing management decisions to prevent its progression is a crucial skill for improving patient wellbeing and optimizing healthcare utilization. Improvement in dialysis outcomes has stalled in recent years; recognition of frailty before dialysis initiation offers opportunity to substantially improve morbidity and mortality events. Rapid progression of frailty precipitated by dialysis commencement reinforces prompt access to transplant work-up as a valid key performance indicator for ensuring equitable care. Accelerated aging in CKD populations has implications for healthcare policy, justifying earlier access to Aged Care Assessment, support, and funding. Healthcare systems that fail to proactively address frailty within our aging chronic disease population risk overwhelming healthcare expenditure and compromised quality of care.
Diagnostic Approaches to Frailty
A large number of frailty metrics which have been validated in nephrology populations exist for use but vary by frailty conceptualization and resource demands (Table 2). Frailty should be objectively assessed, noting that subjective frailty judgements risk misclassification and discrimination and do not afford the same improved patient level outcomes following their use.126,143 The emerging body of literature in nephrology research settings appears to favor use of Fried frailty phenotype, which defines frailty based on presence of 3 or more features of weight loss or sarcopenia, exhaustion, weakness, slowness, and low physical activity.1 It allows definition of a prefrail state to facilitate earlier intervention. Fried frailty can be feasibly incorporated into routine nephrology outpatient and dialysis assessments, noting that weight is regularly assessed and that fatigue and low activity or poor mobility can be triangulated from many symptom assessment tools.24 Slowness may be assessed over 10 meters or by 6-minute walk distance, while grip strength can be rapidly assessed with inexpensive equipment. Several nephrology care models have embedded these assessments with success, acknowledging that in these well-resourced settings, Fried frailty assessments have been largely performed by auxiliary allied health professionals attached to the nephrology outpatient clinic.24,144
Table 2.
Comparison of frailty assessment tools validated for use in kidney disease populations
| Frailty metric | Strengths as a diagnostic tool | Limitations as a diagnostic tool |
|---|---|---|
| Fried frailty phenotype1 | Objective Robust data across different CKD states and settings |
Fatigue and weight/fluid status components may vary with dialysis timing Potentially cumbersome |
| Clinical frailty scale145 | Easy to use Quantitative Potential for retrospective assessment Smart-phone app |
Relies on subjective clinical impression. Heavily focused on disability. Over-categorizes frailty in dialysis (Minimum frailty scale 3) Insensitive tool in kidney disease populations |
| Frailty Index146 | Quantitative Electronic form available |
Focused on disability and comorbidity |
| Comprehensive Geriatric Review2 | Gold standard for frailty diagnosis and management Only tool to incorporate cognitive, social and caregiver components. |
Resource intensive and arduous for patients |
| Short Physical Performance Battery147 | Objective Lower limb function highly predictive of outcomes in kidney disease populations |
Potentially cumbersome Unable to be utilized in some patients with lower limb amputation or bedbound status |
| Hand-grip strength148 | Can be performed in patients with lower limb amputation or bedbound status | Lower limb disability more predictive of outcome in kidney disease populations |
| Voorend’s CKD geriatric assessment tool149 | Holistic assessment of objective physical function, functional dependence, polypharmacy, cognition, nutrition, social and caregiver appraisal | Resource intensive and potentially arduous for patients/caregivers Frailty assessments are relevant in nongeriatric (younger) patient age groups |
CKD, chronic kidney disease.
The 9-point Clinical Frailty Scale comprises a standard set of clinical descriptors to capture frailty based on reported mobility and activities of daily living.145 It is available as a smartphone App and endorsed by clinicians working with frail CKD populations for its usability.125 It has excellent construct validity and interrater reliability and performs with prognostic accuracy in dialysis populations.116 It may, however, be insufficiently sensitive to identify relevant changes in frailty dynamics in CKD populations.19,46
The Frailty Index allows disability and comorbidity to be quantitatively captured with good construct validity and feasibility in outpatient settings, as well as opportunity for electronic data capture.146 In nephrology settings, it has been most frequently operationalized in nondialysis CKD populations where competing comorbidities were of equal or equivalent relevance.18,29
Comprehensive geriatric review is the gold standard frailty assessment and cornerstone of geriatric medicine, capturing functional dependence, polypharmacy, sensory deficits, and falls history alongside social, cognitive, and psychological domains.2 An objective physical measure of frailty is assessed in a short physical performance battery of tests. This structured appraisal of geriatric syndromes affords development of an integrated multidisciplinary treatment plan and evaluation of progression and subsequent adjustment of care needs. Comprehensive geriatric assessment (CGA) improves awareness of holistic and palliative care needs while facilitating appropriate multidisciplinary referral and advance care planning.92,150 In geriatric settings, CGA adoption and subsequent management in acute hospital settings is associated with superior patient outcomes, including performance status, avoidance of residential aged care admission, cognition, and mortality.151 This process of structured multidisciplinary appraisal of health needs has been used successfully outside of geriatric medicine settings;152,153 however, its use in routine nephrology practice is limited by its resource-heavy demands and assessment burden to consumers. Studies in CKD and HD populations to date demonstrate its feasibility for implementation in nephrology settings with its ability to identify overburdened caregivers.27,92,154 Pilot studies indicate that use of CGA may substantially improve functional independence and avoidance of nursing home admission within median 48.5 days of assessment.155
The short physical performance battery has also been used as a stand-alone assessment of frailty in several studies, particularly favored in studies examining participants with advanced CKD and transplant candidates.18 Briefly, short physical performance battery examines lower extremity function through standing balance assessment, repeated chair stand maneuver, and gait speed (usually over 4 m or 15 ft). In ambulatory geriatric settings, it demonstrates association with mortality, quality of life, and functional decline as well as responsiveness to intervention.147 Performing differently from Fried frailty assessment, short physical performance battery impairment predicts dialysis modality choice, home dialysis technique failure, all-cause mortality, and posttransplant mortality; and is a potentially modifiable objective measure for renal replacement therapy risk prediction.22,80,81,96 Handgrip strength might also offer prognostic utility, demonstrating correlation with gait speed and kidney transplant assessment outcomes, as well as sensitivity to change over acute admission.125,156,157 However, impairment of lower limb function, rather than upper limb function, is most predictive of outcomes in CKD studies.22,158
To date, there remains no consensus on the most appropriate tool for frailty assessment in nephrology populations. Voorend and colleagues propose a definitive CKD geriatric assessment tool comprising functional assessment of activities of daily living, objective physical assessment based on handgrip strength, symptom assessment by patient-reported outcome measures, cognitive appraisal using Montreal Cognitive Assessment, frailty assessment by Clinical Frailty Scale, nutritional assessment, polypharmacy review, and measurement of caregiver burden.149 This tool can be completed within an hour utilizing both patient questionnaire and nurse-administered components.
It is likely that different instruments offer distinctive advantages that lend themselves to specific clinical settings. Frailty is critically dynamic, with some studies reporting clinically meaningful changes (improvement or decline) in performance at 3-month or longer intervals.32,159,160 Fluctuations in frailty status over the course of hospitalization events, dialysis treatments, and transplantation justify frequent and repeated assessments at critical transitions in care.30,156 The challenge for nephrology workforces is to familiarize themselves with different aspects of frailty assessment and embed this is in clinical practice.
Intervention for CKD/Frailty: Renal Rehabilitation
Guidelines recommend screening for frailty in elderly patients with advanced CKD to direct in-depth geriatric assessment and rehabilitation.161 Currently, there is limited evidence to guide maintenance or improvement in frailty in nephrology contexts. This contrasts with cardiology settings where an “essential frailty toolkit” and international consensus statements endorse frailty and cognitive assessment followed by multidisciplinary evidence-based primary, secondary, and tertiary prevention strategies.162, 163, 164 The Kidney Disease Improving Global Outcomes initiative recommends physical activity and exercise in a graduated, supervised, and individualized program.165 Exercise guidelines specific to PD populations have recently been published by the International Society of Peritoneal Dialysis in collaboration with the Global Renal Exercise Network.166 Exercise-based interventions for patients undergoing HD may be either intradialytic or home-based interdialytic programs, with equal benefits observed in performance parameters in both modalities.167 Long-term outcomes such as hospitalization and mortality have not been examined to date. A number of trials and systematic reviews in nondialysis CKD settings have demonstrated that exercise intervention improves inflammatory markers, physical fitness, muscular functioning, and cardiovascular dimensions; also slows or resolves progression of kidney disease and proteinuria as well as improving renal hemodynamics.168, 169, 170, 171, 172, 173, 174, 175 Psychological parameters, sleep quality, and quality of life also improved.168 Few studies have examined the impact of exercise intervention on frail nephrology populations; recent scoping review identified just 3 randomized controlled trials, in their infancy.18 The current Ex-FRAIL trial and CYCLE-HD will examine the impact of home-based exercise and interdialytic cycling on frailty in CKD, noting some difficulties with retention and recruitment.176, 177, 178 Yamaguchi and colleagues report considerable durability of the impact on performance status and self-efficacy when frail patients were offered flexible (both home-based and interdialytic) exercise program.179 Acute inpatient geriatric rehabilitation for CKD and HD populations demonstrates equivalent outcomes to non-CKD elderly adults but remains underutilized.180, 181, 182
Successful frailty interventions are likely to be broader in scope than exercise alone, addressing the psycho-emotional-cognitive aspects of frailty alongside physical manifestations. The Japanese Society of Renal Rehabilitation proposes “a long-term comprehensive program consisting of exercise therapy, diet therapy and water management, drug therapy, education, psychological and mental support to alleviate the physical and mental effects of kidney disease and dialysis therapy, prolong life, and improve psychological and occupational circumstances”.183 Its guidelines emphasize the psycho-emotional-social needs of this vulnerable patient population, endorsing multidisciplinary and multimodal interventions that “exhaust all support options to help kidney disease patients smoothly achieve social rehabilitation instead of simply implementing exercise therapy”. Within the geriatric literature, there is evidence to support the use of multicomponent interventions incorporating social, nutritional, and cognitive elements in addition to exercise program for improving frailty, cognitive, social, and emotional outcomes in frail and prefrail community-dwelling elderly.184, 185, 186 These programs demonstrate high recruitment and retention rates, suggesting acceptability among consumers. An ambitious randomized controlled trial of exercise intervention alongside psychological and nutritional care in predialysis patients with CKD to examine the impact on frailty, hospitalization, and mortality has been proposed.187 The published protocol does not specify details of the dietary intervention. There is evidence to support the use of megestrol acetate and dietary supplements in this patient population.188,189 Dietary recommendations suggest that 1.2g/kg/day protein intake is appropriate in end-stage renal disease, where efficient dialysis or quality of life can be prioritized over CKD progression.70
Outcomes pertaining to frailty assessment are likely to be poorly captured, reflecting poor attention to patient priorities within research agendas. Consumer-focused research such as the Standardised Outcomes in Nephrology initiative and James Lind Alliance prioritize patient perspectives in research and clinical outcomes, noting that neither have specifically explored frailty perspectives in nephrology populations.190,191 The lived experience of frailty and CKD remains unexamined. The implementation of CGA and multidisciplinary follow-up appears to enhance education, training, and awareness of palliative care needs among clinicians serving this patient population while facilitating delivery of holistic care plans, which incorporate discussions of treatment options through appropriate referral and advance care planning.92,150,192 Although not formally measured, studies evaluating CGA in nephrology populations frequently report improvement and individualization in treatment options and reinforced social supports.150,192,193 Interventions addressing the socioeconomic determinants of health and wellbeing offer opportunity to address key inequities characterizing the experience of frailty.136 Patient care priorities include provision of emotional and practical support, maintenance of mobility, and supported decision-making.29 Caregiver needs remain undiscovered with recent systematic reviews highlighting the high level of burden involved in caring for people with advanced CKD.194,195 Health economics studies should be used to capture efficiency or intervention, with cost of frailty intervention compared against healthcare utilization patterns. Early data suggest that exercise intervention in HD populations is cost-effective, affording avoidance of hospitalization and additional care costs, noting that participants with frailty were not specifically identified or evaluated in this analysis.196
Conclusions
Frailty in the kidney disease context is an important clinical syndrome characterized by uremic symptom burden and a subjective psycho-emotional-social experience, with specific implications for healthcare utilization and caregiver burden. Although underdiagnosed, frailty is highly prevalent and directs important clinical outcomes. Recognition of frailty emerges as a core nephrology skill that is crucial to the assessment of patients with CKD, for those approaching dialysis, and planning for renal transplantation. Successful frailty intervention is likely longitudinal and multidisciplinary, addressing the multisystemic components of frailty while supporting shared decision-making and advance care planning. Future nephrology research should incorporate evaluations of frailty status to add meaning to key patient-level outcomes. This includes database registries which require frailty data capture to furnish understanding of big data. Frailty in the CKD context and its implications for healthcare delivery should be a clinical and research priority for nephrology workforces, public health systems, and policy writers.
Disclosure
All the authors declared no competing interests.
References
- 1.Fried L.P., Tangen C.M., Walston J., et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Clegg A., Young J., Iliffe S., Rikkert M.O., Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kooman J.P., Broers N.J., Usvyat L., et al. Out of control: accelerated aging in uremia. Nephrol Dial Transplant. 2013;28:48–54. doi: 10.1093/ndt/gfs451. [DOI] [PubMed] [Google Scholar]
- 4.Ruan Q., Yu Z., Chen M., Bao Z., Li J., He W. Cognitive frailty, a novel target for the prevention of elderly dependency. Ageing Res Rev. 2015;20:1–10. doi: 10.1016/j.arr.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Bunt S., Steverink N., Olthof J., van der Schans C.P., Hobbelen J.S.M. Social frailty in older adults: a scoping review. Eur J Ageing. 2017;14:323–334. doi: 10.1007/s10433-017-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue Q.L. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansen K.L., Chertow G.M., Jin C., Kutner N.G. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 8.McAdams-DeMarco M.A., Law A., Salter M.L., et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61:896–901. doi: 10.1111/jgs.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.USRDS, NIDDK, NIH. Chapter 1: Incidence, prevalence, patient characteristics, and treatment modalities. https://usrds-adr.niddk.nih.gov/2022/end-stage-renal-disease/1-incidence-prevalence-patient-characteristics-and-treatment-modalities
- 10.ANZDATA Registry . 43rd Report, Chapter 5: Peritoneal Dialysis. Australia and New Zealand Dialysis and Transplant Registry; Adelaide, Australia: 2020. http://www.anzdata.org.au [Google Scholar]
- 11.Hole B., Gilg J., Casula A., Methven S., Castledine C. Chapter 1 UK renal replacement therapy adult incidence in 2016: national and centre-specific analyses. Nephron. 2018;139(suppl 1):13–46. doi: 10.1159/000490959. [DOI] [PubMed] [Google Scholar]
- 12.Tonelli M., Wiebe N., Manns B.J., et al. Comparison of the complexity of patients seen by different medical subspecialists in a universal health care system. JAMA Netw Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterky E., Stegmayr B.G. Elderly patients on haemodialysis have 50% less functional capacity than gender- and age-matched healthy subjects. Scand J Urol Nephrol. 2005;39:423–430. doi: 10.1080/00365590500199319. [DOI] [PubMed] [Google Scholar]
- 14.Roshanravan B., Khatri M., Robinson-Cohen C., et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60:912–921. doi: 10.1053/j.ajkd.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shlipak M.G., Stehman-Breen C., Fried L.F., et al. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43:861–867. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 16.Wilhelm-Leen E.R., Hall Y.N., KT M., Chertow G.M. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med. 2009;122 doi: 10.1016/j.amjmed.2009.01.026. 664-71.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartmann E.L., Kitzman D., Rocco M., et al. Physical function in older candidates for renal transplantation: an impaired population. Clin J Am Soc Nephrol. 2009;4:588–594. doi: 10.2215/CJN.03860808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennard A.L., Rainsford S., Glasgow N.J., Talaulikar G.S. Use of frailty assessment instruments in nephrology populations: a scoping review. BMC Geriatr. 2023;23:449. doi: 10.1186/s12877-023-04101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Worthen G., Vinson A., Cardinal H., et al. Prevalence of frailty in patients referred to the kidney transplant waitlist. Kidney360. 2021;2:1287–1295. doi: 10.34067/KID.0001892021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baddour N.A., Robinson-Cohen C., Lipworth L., et al. The surprise question and self-rated health are useful screens for frailty and disability in older adults with chronic kidney disease. J Palliat Med. 2019;22:1522–1529. doi: 10.1089/jpm.2019.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goto N.A., van Loon I.N., Morpey M.I., et al. Geriatric assessment in elderly patients with end-stage kidney disease. Nephron. 2019;141:41–48. doi: 10.1159/000494222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nixon A.C., Bampouras T.M., Pendleton N., Mitra S., Dhaygude A.P. Diagnostic accuracy of frailty screening methods in advanced chronic kidney disease. Nephron. 2019;141:147–155. doi: 10.1159/000494223. [DOI] [PubMed] [Google Scholar]
- 23.Chowdhury R., Peel N.M., Krosch M., Hubbard R.E. Frailty and chronic kidney disease: a systematic review. Arch Gerontol Geriatr. 2017;68:135–142. doi: 10.1016/j.archger.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Kennard A., Rainsford S., Hamilton K., Glasgow N., et al. Matching nephrology care to the needs of vulnerable patients: assessing frailty in patients with chronic kidney disease. Nephrology. Forthcoming. 2023 [Google Scholar]
- 25.Ghazi L., Yaffe K., Tamura M.K., et al. Association of 24-hour ambulatory blood pressure patterns with cognitive function and physical functioning in CKD. Clin J Am Soc Nephrol. 2020;15:455–464. doi: 10.2215/CJN.10570919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yadla M., John J.P., Mummadi M. A study of clinical assessment of frailty in patients on maintenance hemodialysis supported by cashless government scheme. Saudi J Kidney Dis Transplant. 2017;28:15–22. doi: 10.4103/1319-2442.198102. [DOI] [PubMed] [Google Scholar]
- 27.van Loon I.N., Goto N.A., Boereboom F.T.J., Bots M.L., Verhaar M.C., Hamaker M.E. Frailty screening tools for elderly patients incident to dialysis. Clin J Am Soc Nephrol. 2017;12:1480–1488. doi: 10.2215/CJN.11801116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosoku A., Uchida J., Iwai T., et al. Frailty is associated with dialysis duration before transplantation in kidney transplant recipients: a Japanese single-center cross-sectional study. Int J Urol. 2020;27:408–414. doi: 10.1111/iju.14208. [DOI] [PubMed] [Google Scholar]
- 29.Hurst H., Young H.M.L., Nixon A.C., Ormandy P., Brettle A. scoping review author collaborative for outcomes and care priorities for older people with ACKD. Outcomes and care priorities for older people living with frailty and advanced chronic kidney disease: a multi-professional scoping review. Age Ageing. 2022;51:afac296. doi: 10.1093/ageing/afac296. [DOI] [PubMed] [Google Scholar]
- 30.Kurella Tamura M., Covinsky K.E., Chertow G.M., Yaffe K., Landefeld C.S., McCulloch C.E. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jafari M., Kour K., Giebel S., Omisore I., Prasad B. The burden of frailty on mood, cognition, quality of life, and level of independence in patients on hemodialysis: Regina hemodialysis frailty study. Can J Kidney Health Dis. 2020;7 doi: 10.1177/2054358120917780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansen K.L., Dalrymple L.S., Delgado C., et al. Factors associated with frailty and its trajectory among patients on hemodialysis. Clin J Am Soc Nephrol. 2017;12:1100–1108. doi: 10.2215/CJN.12131116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansen K.L., Delgado C., Kaysen G.A., et al. Frailty among patients receiving hemodialysis: evolution of components and associations with mortality. J Gerontol A Biol Sci Med Sci. 2019;74:380–386. doi: 10.1093/gerona/gly206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu N.M., Deng A., Ying H., et al. Dynamic frailty before kidney transplantation: time of measurement matters. Transplantation. 2019;103:1700–1704. doi: 10.1097/TP.0000000000002563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harhay M.N., Rao M.K., Woodside K.J., et al. An overview of frailty in kidney transplantation: measurement, management and future considerations. Nephrol Dial Transplant. 2020;35:1099–1112. doi: 10.1093/ndt/gfaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAdams-DeMarco M.A., Chu N.M., Segev D.L. Frailty and long-term post-kidney transplant outcomes. Curr Transplant Rep. 2019;6:45–51. doi: 10.1007/s40472-019-0231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McAdams-DeMarco M.A., Ying H., Thomas A.G., et al. Frailty, inflammatory markers, and waitlist mortality among patients with end-stage renal disease in a prospective cohort study. Transplantation. 2018;102:1740–1746. doi: 10.1097/TP.0000000000002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haugen C.E., Chu N.M., Ying H., et al. Frailty and access to kidney transplantation. Clin J Am Soc Nephrol. 2019;14:576–582. doi: 10.2215/CJN.12921118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shrestha P., Haugen C.E., Chu N.M., et al. Racial differences in inflammation and outcomes of aging among kidney transplant candidates. BMC Nephrol. 2019;20:176. doi: 10.1186/s12882-019-1360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novais T., Pongan E., Gervais F., et al. Pretransplant comprehensive geriatric assessment in older patients with advanced chronic kidney disease. Nephron. 2021;145:692–701. doi: 10.1159/000517342. [DOI] [PubMed] [Google Scholar]
- 41.Dos Santos Mantovani M., Coelho de Carvalho N., Archangelo T.E., et al. Frailty predicts surgical complications after kidney transplantation. A propensity score matched study. PLoS One. 2020;15 doi: 10.1371/journal.pone.0229531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McAdams-DeMarco M.A., Olorundare I.O., Ying H., et al. Frailty and postkidney transplant health-related quality of life. Transplantation. 2018;102:291–299. doi: 10.1097/TP.0000000000001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker S.R., Brar R., Eng F., et al. Frailty and physical function in chronic kidney disease: the CanFIT study. Can J Kidney Health Dis. 2015;2:32. doi: 10.1186/s40697-015-0067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee H.J., Son Y.J. Prevalence and associated factors of frailty and mortality in patients with end-stage renal disease undergoing hemodialysis: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18073471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kimura H., Kalantar-Zadeh K., Rhee C.M., Streja E., Sy J. Polypharmacy and frailty among hemodialysis patients. Nephron. 2021;145:624–632. doi: 10.1159/000516532. [DOI] [PubMed] [Google Scholar]
- 46.Floyd L., Byrne L., Morris A.D., Nixon A.C., Dhaygude A. The limitations of frailty assessment tools in ANCA-associated vasculitis. J Frailty Aging. 2023;12:139–142. doi: 10.14283/jfa.2023.14. [DOI] [PubMed] [Google Scholar]
- 47.Ferrucci L., Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller R.A. The aging immune system: primer and prospectus. Sci (N Y NY) 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 49.Rocha V.Z., Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 50.Picca A., Fanelli F., Calvani R., et al. Gut dysbiosis and muscle aging: searching for novel targets against sarcopenia. Mediators Inflamm. 2018;2018 doi: 10.1155/2018/7026198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cruz-Jentoft A.J., Sayer A.A. Sarcopenia. Lancet. 2019;393:2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 52.Bonanni A., Mannucci I., Verzola D., et al. Protein-energy wasting and mortality in chronic kidney disease. Int J Environ Res Public Health. 2011;8:1631–1654. doi: 10.3390/ijerph8051631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brivio P., Paladini M.S., Racagni G., Riva M.A., Calabrese F., Molteni R. From healthy aging to frailty: in search of the underlying mechanisms. Curr Med Chem. 2019;26:3685–3701. doi: 10.2174/0929867326666190717152739. [DOI] [PubMed] [Google Scholar]
- 54.Khan K.T., Hemati K., Donovan A.L. Geriatric physiology and the frailty syndrome. Anesthesiol Clin. 2019;37:453–474. doi: 10.1016/j.anclin.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Attaix D., Mosoni L., Dardevet D., Combaret L., Mirand P.P., Grizard J. Altered responses in skeletal muscle protein turnover during aging in anabolic and catabolic periods. Int J Biochem Cell Biol. 2005;37:1962–1973. doi: 10.1016/j.biocel.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Varadhan R., Walston J., Cappola A.R., Carlson M.C., Wand G.S., Fried L.P. Higher levels and blunted diurnal variation of cortisol in frail older women. J Gerontol A Biol Sci Med Sci. 2008;63:190–195. doi: 10.1093/gerona/63.2.190. [DOI] [PubMed] [Google Scholar]
- 57.Bishop N.A., Lu T., Yankner B.A. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464:529–535. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cunningham C., Wilcockson D.C., Campion S., Lunnon K., Perry V.H. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Gool W.A., van de Beek D., Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375:773–775. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- 60.Wang X.H., Mitch W.E. Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol. 2014;10:504–516. doi: 10.1038/nrneph.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abadir P.M. The frail renin-angiotensin system. Clin Geriatr Med. 2011;27:53–65. doi: 10.1016/j.cger.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moorthi R.N., Avin K.G. Clinical relevance of sarcopenia in chronic kidney disease. Curr Opin Nephrol Hypertens. 2017;26:219–228. doi: 10.1097/MNH.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Avin K.G., Moorthi R.N. Bone is not alone: the effects of skeletal muscle dysfunction in chronic kidney disease. Curr Osteoporos Rep. 2015;13:173–179. doi: 10.1007/s11914-015-0261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fahal I.H. Uraemic sarcopenia: aetiology and implications. Nephrol Dial Transplant. 2014;29:1655–1665. doi: 10.1093/ndt/gft070. [DOI] [PubMed] [Google Scholar]
- 65.Avin K.G., Chen N.X., Organ J.M., et al. Skeletal muscle regeneration and oxidative stress are altered in chronic kidney disease. PLoS One. 2016;11 doi: 10.1371/journal.pone.0159411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Workeneh B.T., Mitch W.E. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr. 2010;91:1128s–1132s. doi: 10.3945/ajcn.2010.28608B. [DOI] [PubMed] [Google Scholar]
- 67.Zelle D.M., Klaassen G., van Adrichem E., Bakker S.J., Corpeleijn E., Navis G. Physical inactivity: a risk factor and target for intervention in renal care. Nat Rev Nephrol. 2017;13:152–168. doi: 10.1038/nrneph.2016.187. [DOI] [PubMed] [Google Scholar]
- 68.Meuwese C.L., Carrero J.J. Chronic kidney disease and hypothalamic-pituitary axis dysfunction: the chicken or the egg? Arch Med Res. 2013;44:591–600. doi: 10.1016/j.arcmed.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 69.Gorisse L., Jaisson S., Piétrement C., Gillery P. Carbamylated proteins in renal disease: aggravating factors or just biomarkers? Int J Mol Sci. 2022;23 doi: 10.3390/ijms23010574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hanna R.M., Ghobry L., Wassef O., Rhee C.M., Kalantar-Zadeh K. A practical approach to nutrition, protein-energy wasting, sarcopenia, and cachexia in patients with chronic kidney disease. Blood Purif. 2020;49:202–211. doi: 10.1159/000504240. [DOI] [PubMed] [Google Scholar]
- 71.Kaysen G.A. Biochemistry and biomarkers of inflamed patients: why look, what to assess. Clin J Am Soc Nephrol. 2009;4(suppl 1):S56–S63. doi: 10.2215/CJN.03090509. [DOI] [PubMed] [Google Scholar]
- 72.Dalrymple L.S., Katz R., Rifkin D.E., et al. Kidney function and prevalent and incident frailty. Clin J Am Soc Nephrol. 2013;8:2091–2099. doi: 10.2215/CJN.02870313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jacobs A., Benraad C., Wetzels J., Rikkert M.O., Kramers C. Clinical Relevance of Differences in glomerular filtration rate Estimations in Frail Older People by creatinine- vs. cystatin C-Based Formulae. Drugs Aging. 2017;34:445–452. doi: 10.1007/s40266-017-0460-z. [DOI] [PubMed] [Google Scholar]
- 74.Karnabi P., Massicotte-Azarniouch D., Ritchie L.J., Marshall S., Knoll G.A. Physical frailty and functional status in patients with advanced chronic kidney disease: a systematic review. Can J Kidney Health Dis. 2023;10 doi: 10.1177/20543581231181026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pyart R., Aggett J., Goodland A., et al. Exploring the choices and outcomes of older patients with advanced kidney disease. PLOS ONE. 2020;15 doi: 10.1371/journal.pone.0234309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown M.A., Collett G.K., Josland E.A., Foote C., Li Q., Brennan F.P. CKD in elderly patients managed without dialysis: survival, symptoms, and quality of life. Clin J Am Soc Nephrol. 2015;10:260–268. doi: 10.2215/CJN.03330414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kumarasinghe A.P., Chakera A., Chan K., et al. Incorporating the clinical frailty scale into routine outpatient nephrology practice: an observational study of feasibility and associations. Intern Med J. 2020;51:1269–1277. doi: 10.1111/imj.14892. [DOI] [PubMed] [Google Scholar]
- 78.Foote C., Morton R.L., Jardine M., et al. COnsiderations of Nephrologists when suggesting dialysis in elderly patients with renal failure (CONSIDER): a discrete choice experiment. Nephrol Dial Transplant. 2014;29:2302–2309. doi: 10.1093/ndt/gfu257. [DOI] [PubMed] [Google Scholar]
- 79.Voorend C.G.N., van Oevelen M., Verberne W.R., et al. Survival of patients who opt for dialysis versus conservative care: a systematic review and meta-analysis. Nephrol Dial Transplant. 2022;37:1529–1544. doi: 10.1093/ndt/gfac010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brar R.S., Whitlock R.H., Komenda P.V.J., et al. Provider perception of frailty is associated with dialysis decision making in patients with advanced CKD. Clin J Am Soc Nephrol. 2021;16:552–559. doi: 10.2215/CJN.12480720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brar R., Whitlock R., Komenda P., et al. The impact of frailty on technique failure and mortality in patients on home dialysis. Perit Dial Int. 2019;39:532–538. doi: 10.3747/pdi.2018.00195. [DOI] [PubMed] [Google Scholar]
- 82.Vezza C., Vettoretti S., Caldiroli L., Bergamaschini L., Messa P., Cesari M. Use of the frailty index in older persons with chronic kidney disease. J Am Med Dir Assoc. 2019;20:1179–1180. doi: 10.1016/j.jamda.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 83.Ali H., Abdelaziz T., Abdelaal F., Baharani J. Assessment of prevalence and clinical outcome of frailty in an elderly predialysis cohort using simple tools. Saudi J Kidney Dis. 2018;29:63–70. doi: 10.4103/1319-2442.225175. [DOI] [PubMed] [Google Scholar]
- 84.Delgado C., Grimes B.A., Glidden D.V., Shlipak M., Sarnak M.J., Johansen K.L. Association of Frailty based on self-reported physical function with directly measured kidney function and mortality. BMC Nephrol. 2015;16:203. doi: 10.1186/s12882-015-0202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bao Y., Dalrymple L., Chertow G.M., Kaysen G.A., Johansen K.L. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172:1071–1077. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kurella Tamura M., O’Hare A.M., McCulloch C.E., Johansen K.L. Signs and symptoms associated with earlier dialysis initiation in nursing home residents. Am J Kidney Dis. 2010;56:1117–1126. doi: 10.1053/j.ajkd.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McAdams-DeMarco M.A., Suresh S., Law A., et al. Frailty and falls among adult patients undergoing chronic hemodialysis: a prospective cohort study. BMC Nephrol. 2013;14:224. doi: 10.1186/1471-2369-14-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garcia-Canton C., Rodenas A., Lopez-Aperador C., et al. Frailty in hemodialysis and prediction of poor short-term outcome: mortality, hospitalization and visits to hospital emergency services. Ren Fail. 2019;41:567–575. doi: 10.1080/0886022X.2019.1628061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Y., Zhang D., Ma Q., Diao Z., Liu S., Shi X. The impact of frailty on prognosis in elderly hemodialysis patients: a prospective cohort study. Clin Interv Aging. 2021;16:1659–1667. doi: 10.2147/CIA.S329665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vinson A.J., Bartolacci J., Goldstein J., Swain J., Clark D., Tennankore K.K. Predictors of need for first and recurrent emergency medical service transport to emergency department after dialysis initiation. Prehosp Emerg Care. 2020;24:822–830. doi: 10.1080/10903127.2019.1701157. [DOI] [PubMed] [Google Scholar]
- 91.Lee S.Y., Yang D.H., Hwang E., et al. The prevalence, association, and clinical outcomes of frailty in maintenance dialysis patients. J Ren Nutr. 2017;27:106–112. doi: 10.1053/j.jrn.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 92.Nixon A.C., Brown J., Brotherton A., et al. Implementation of a frailty screening programme and Geriatric Assessment Service in a nephrology centre: a quality improvement project. J Nephrol. 2021;34:1215–1224. doi: 10.1007/s40620-020-00878-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Loon I.N., Goto N.A., Boereboom F.T.J., et al. Geriatric assessment and the relation with mortality and hospitalizations in older patients starting dialysis. Nephron. 2019;143:108–119. doi: 10.1159/000501277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fitzpatrick J., Sozio S.M., Jaar B.G., et al. Frailty, body composition and the risk of mortality in incident hemodialysis patients: the Predictors of Arrhythmic and cardiovascular Risk in End Stage Renal Disease study. Nephrol Dial Transplant. 2019;34:346–354. doi: 10.1093/ndt/gfy124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Johansen K.L., Dalrymple L.S., Glidden D., et al. Association of performance-based and self-reported function-based definitions of frailty with mortality among patients receiving hemodialysis. Clin J Am Soc Nephrol. 2016;11:626–632. doi: 10.2215/CJN.03710415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nastasi A.J., McAdams-DeMarco M.A., Schrack J., et al. Pre-kidney transplant lower extremity impairment and post-kidney transplant mortality. Am J Transplant. 2018;18:189–196. doi: 10.1111/ajt.14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chan G.C., N G J.K., Chow K.M., et al. Interaction between central obesity and frailty on the clinical outcome of peritoneal dialysis patients. PloS One. 2020;15(10) doi: 10.1371/journal.pone.0241242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kamijo Y., Kanda E., Ishibashi Y., Yoshida M. Sarcopenia and frailty in PD: impact on mortality, malnutrition, and inflammation. Perit Dial Int. 2018;38:447–454. doi: 10.3747/pdi.2017.00271. [DOI] [PubMed] [Google Scholar]
- 99.Schopmeyer L., El Moumni M., Nieuwenhuijs-Moeke G.J., Berger S.P., Bakker S.J.L., Pol R.A. Frailty has a significant influence on postoperative complications after kidney transplantation-a prospective study on short-term outcomes. Transpl Int. 2019;32:66–74. doi: 10.1111/tri.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garonzik-Wang J.M., Govindan P., Grinnan J.W., et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. 2012;147:190–193. doi: 10.1001/archsurg.2011.1229. [DOI] [PubMed] [Google Scholar]
- 101.McAdams-DeMarco M.A., Law A., Salter M.L., et al. Frailty and early hospital readmission after kidney transplantation. Am J Transplant. 2013;13:2091–2095. doi: 10.1111/ajt.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McAdams-DeMarco M.A., Law A., Tan J., et al. Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation. 2015;99:805–810. doi: 10.1097/TP.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McAdams-DeMarco M.A., Law A., King E., et al. Frailty and mortality in kidney transplant recipients. Am J Transplant. 2015;15:149–154. doi: 10.1111/ajt.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McAdams-DeMarco M.A., Ying H., Olorundare I., et al. Individual frailty components and mortality in kidney transplant recipients. Transplantation. 2017;101:2126–2132. doi: 10.1097/TP.0000000000001546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chao C.T., Huang J.W. COGENT (COhort of GEriatric Nephrology in NTUH) study group. Geriatric syndromes are potential determinants of the medication adherence status in prevalent dialysis patients. PeerJ. 2016;4 doi: 10.7717/peerj.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Poveda V., Filgueiras M., Miranda V., Santos-Silva A., Paúl C., Costa E. Frailty in end-stage renal disease patients under dialysis and its association with clinical and biochemical markers. J Frailty Aging. 2017;6:103–106. doi: 10.14283/jfa.2017.14. [DOI] [PubMed] [Google Scholar]
- 107.Fitzpatrick J., Sozio S.M., Jaar B.G., et al. Frailty, age, and postdialysis recovery time in a population new to hemodialysis. Kidney360. 2021;2:1455–1462. doi: 10.34067/KID.0001052021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chao C.T., Hsu Y.H., Chang P.Y., et al. Simple self-report FRAIL scale might be more closely associated with dialysis complications than other frailty screening instruments in rural chronic dialysis patients. Nephrology (Carlton) 2015;20:321–328. doi: 10.1111/nep.12401. [DOI] [PubMed] [Google Scholar]
- 109.Chao C.T., Chiang C.K., Huang J.W., Hung K.Y., COGENT study group Self-reported frailty among end-stage renal disease patients: A potential predictor of dialysis access outcomes. Nephrology (Carlton) 2017;22:333–334. doi: 10.1111/nep.12961. [DOI] [PubMed] [Google Scholar]
- 110.Lin H.C., Cai Z.S., Huang J.T., Chen M.J. The correlation between frailty and recurrent vascular access failure in the elderly maintenance hemodialysis patients. Int J Gerontol. 2020;14:159–162. [Google Scholar]
- 111.van Munster B.C., Drost D., Kalf A., Vogtlander N.P. Discriminative value of frailty screening instruments in end-stage renal disease. Clin Kidney J. 2016;9:606–610. doi: 10.1093/ckj/sfw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Iyasere O., Brown E., Gordon F., et al. Longitudinal trends in quality of life and physical function in frail older dialysis patients: a comparison of assisted peritoneal dialysis and in-center hemodialysis. Perit Dial Int. 2019;39:112–118. doi: 10.3747/pdi.2018.00086. [DOI] [PubMed] [Google Scholar]
- 113.Kang S.H., Do J.Y., Jeong H.Y., Lee S.Y., Kim J.C. The clinical significance of physical activity in maintenance dialysis patients. Kidney Blood Press Res. 2017;42:575–586. doi: 10.1159/000480674. [DOI] [PubMed] [Google Scholar]
- 114.Moreno-Useche L., Urrego-Rubio J., Cadena-Sanabria M., Rodríguez Amaya R., Maldonado-Navas S., Ruiz Gonzalez C. Frailty syndrome in patients with chronic kidney disease at a dialysis Centre from Santander, Colombia. J Gerontol Geriatr. 2021;69:1–7. doi: 10.36150/2499-6564-N249. [DOI] [Google Scholar]
- 115.Jegatheswaran J., Chan R., Hiremath S., et al. Use of the FRAIL questionnaire in patients with end-stage kidney disease. Can J Kidney Health Dis. 2020;7 doi: 10.1177/2054358120952904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alfaadhel T.A., Soroka S.D., Kiberd B.A., Landry D., Moorhouse P., Tennankore K.K. Frailty and mortality in dialysis: evaluation of a clinical frailty scale. Clin J Am Soc Nephrol. 2015;10:832–840. doi: 10.2215/CJN.07760814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chan G.C., Ng J.K., Chow K.M., et al. Impact of frailty and its inter-relationship with lean tissue wasting and malnutrition on kidney transplant waitlist candidacy and delisting. Clin Nutr (Edinburgh, Scotland) 2021;40:5620–5629. doi: 10.1016/j.clnu.2021.09.023. [DOI] [PubMed] [Google Scholar]
- 118.Cheng X.S., Myers J., Han J., et al. Physical performance testing in kidney transplant candidates at the top of the waitlist. Am J Kidney Dis. 2020;76:815–825. doi: 10.1053/j.ajkd.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lorenz E.C., Cosio F.G., Bernard S.L., et al. The relationship between frailty and decreased physical performance with death on the kidney transplant waiting list. Prog Transplant (Aliso Viejo Calif) 2019;29:108–114. doi: 10.1177/1526924819835803. [DOI] [PubMed] [Google Scholar]
- 120.Manay P., Ten Eyck P., Kalil R., et al. Frailty measures can be used to predict the outcome of kidney transplant evaluation. Surgery. 2021;169:686–693. doi: 10.1016/j.surg.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Findlay M.D., Donaldson K., Doyle A., et al. Factors influencing withdrawal from dialysis: a national registry study. Nephrol Dial Transplant. 2016;31:2041–2048. doi: 10.1093/ndt/gfw074. [DOI] [PubMed] [Google Scholar]
- 122.Chen J.H.C., Brown M.A., Jose M., et al. Temporal changes and risk factors for death from early withdrawal within 12 months of dialysis initiation-a cohort study. Nephrol Dial Transplant. 2022;37:760–769. doi: 10.1093/ndt/gfab207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen J.C., Thorsteinsdottir B., Vaughan L.E., et al. End of life, withdrawal, and palliative care utilization among patients receiving maintenance hemodialysis therapy. Clin J Am Soc Nephrol. 2018;13:1172–1179. doi: 10.2215/CJN.00590118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McAdams-DeMarco M.A., Van Pilsum Rasmussen S.E., Chu N.M., et al. Perceptions and practices regarding frailty in kidney transplantation: results of a national survey. Transplantation. 2020;104:349–356. doi: 10.1097/TP.0000000000002779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kennard A., Glasgow N., Rainsford S., Talaulikar G. Frailty in advanced chronic kidney disease: perceptions and practices among treating clinicians. Ren Soc Australas J. 2022;18:44–50. doi: 10.33235/rsaj.18.2.44-50. [DOI] [Google Scholar]
- 126.Chen X., Liu Y., Thompson V., et al. Transplant centers that assess frailty as part of clinical practice have better outcomes. BMC Geriatr. 2022;22:82. doi: 10.1186/s12877-022-02777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McAdams-DeMarco M., Law A., King B., Walston J., Segev D. Frailty, immunosuppression tolerance, and outcomes in kidney transplant recipients. Transplantation. 2014;98:619;D2442. doi: 10.1097/00007890-201407151-02094. [DOI] [Google Scholar]
- 128.Kutner N.G., Zhang R., Bowles T., Painter P. Pretransplant physical functioning and kidney patients’ risk for posttransplantation hospitalization/death: evidence from a national cohort. Clin J Am Soc Nephrol. 2006;1:837–843. doi: 10.2215/CJN.01341005. [DOI] [PubMed] [Google Scholar]
- 129.McAdams-DeMarco M.A., Isaacs K., Darko L., et al. Changes in frailty after kidney transplantation. J Am Geriatr Soc. 2015;63:2152–2157. doi: 10.1111/jgs.13657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Skilbeck J.K., Arthur A., Seymour J. Making sense of frailty: an ethnographic study of the experience of older people living with complex health problems. Int J Older People Nurs. 2018;13 doi: 10.1111/opn.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Van Damme J., Neiterman E., Oremus M., Lemmon K., Stolee P. Perspectives of older adults, caregivers, and healthcare providers on frailty screening: a qualitative study. BMC Geriatr. 2020;20:65. doi: 10.1186/s12877-020-1459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Han M., Ye X., Preciado P., et al. Relationships between neighborhood walkability and objectively measured physical activity levels in hemodialysis patients. Blood Purif. 2018;45:236–244. doi: 10.1159/000485161. [DOI] [PubMed] [Google Scholar]
- 133.Bowling C.B., Muntner P., Sawyer P., et al. Community mobility among older adults with reduced kidney function: a study of life-space. Am J Kidney Dis. 2014;63:429–436. doi: 10.1053/j.ajkd.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nixon A.C., Bampouras T.M., Pendleton N., Mitra S., Brady M.E., Dhaygude A.P. Frailty is independently associated with worse health-related quality of life in chronic kidney disease: a secondary analysis of the Frailty Assessment in Chronic Kidney Disease study. Clin Kidney J. 2020;13:85–94. doi: 10.1093/ckj/sfz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Iyasere O.U., Brown E.A., Johansson L., et al. Quality of life and physical function in older patients on dialysis: a comparison of assisted peritoneal dialysis with hemodialysis. Clin J Am Soc Nephrol. 2016;11:423–430. doi: 10.2215/CJN.01050115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Crews D.C., Delaney A.M., Walker Taylor J.L., et al. Pilot intervention addressing social support and functioning of low socioeconomic status older adults with ESRD: the seniors optimizing community integration to advance better living with ESRD (SOCIABLE) study. Kidney Med. 2019;1:13–20. doi: 10.1016/j.xkme.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Coppolino G., Bolignano D., Gareri P., et al. Kidney function and cognitive decline in frail elderly: two faces of the same coin? Int Urol Nephrol. 2018;50:1505–1510. doi: 10.1007/s11255-018-1900-3. [DOI] [PubMed] [Google Scholar]
- 138.Kallenberg M.H., Kleinveld H.A., Dekker F.W., et al. Functional and cognitive impairment, frailty, and adverse health outcomes in older patients reaching ESRD-a systematic review. Clin J Am Soc Nephrol. 2016;11:1624–1639. doi: 10.2215/CJN.13611215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kurella Tamura M., Vittinghoff E., Hsu C.Y., et al. Loss of executive function after dialysis initiation in adults with chronic kidney disease. Kidney Int. 2017;91:948–953. doi: 10.1016/j.kint.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Drew D.A., Weiner D.E., Tighiouart H., et al. Cognitive function and all-cause mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2015;65:303–311. doi: 10.1053/j.ajkd.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sanchez-Fernandez M.D.M., Reyes Del Paso G.A., Gil-Cunquero J.M., Fernandez-Serrano M.J. Executive function in end-stage renal disease: acute effects of hemodialysis and associations with clinical factors. PLoS One. 2018;13 doi: 10.1371/journal.pone.0203424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Szeto C.C., Chan G.C., Ng J.K., et al. Depression and physical frailty have additive effect on the nutritional status and clinical outcome of Chinese peritoneal dialysis. Kidney Blood Press Res. 2018;43:914–923. doi: 10.1159/000490470. [DOI] [PubMed] [Google Scholar]
- 143.Salter M.L., Gupta N., Massie A.B., et al. Perceived frailty and measured frailty among adults undergoing hemodialysis: a cross-sectional analysis. BMC Geriatr. 2015;15:52. doi: 10.1186/s12877-015-0051-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dawson J.R., Ryan M., Brennan M., et al. The prevalence of frailty and malnutrition and association with dietary intake in people commencing a conservative kidney management pathway. Nephrology. https://anzsnasm.com/15844 Published 2022.
- 145.Rockwood K., Song X., MacKnight C., et al. A global clinical measure of fitness and frailty in elderly people. CMAJ Can Med Assoc J. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rockwood K., Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 147.Guralnik J.M., Simonsick E.M., Ferrucci L., et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 148.Innes E. Handgrip strength testing: a review of the literature. Asutralian Occup Ther J. 1999;46:120–140. doi: 10.1046/j.1440-1630.1999.00182.x. [DOI] [Google Scholar]
- 149.Voorend C.G.N., Joosten H., Berkhout-Byrne N.C., et al. Design of a consensus-based geriatric assessment tailored for older chronic kidney disease patients: results of a pragmatic approach. Eur Geriatr Med. 2021;12:931–942. doi: 10.1007/s41999-021-00498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Voorend C.G.N., Berkhout-Byrne N.C., Meuleman Y., Mooijaart S.P., Bos W.J.W., van Buren M. Perspectives and experiences of patients and healthcare professionals with geriatric assessment in chronic kidney disease: a qualitative study. BMC Nephrol. 2021;22:9. doi: 10.1186/s12882-020-02206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ellis G., Whitehead M.A., Robinson D., O’Neill D., Langhorne P. Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. BMJ (Clin Res Ed) 2011;343:d6553. doi: 10.1136/bmj.d6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Harari D., Hopper A., Dhesi J., Babic-Illman G., Lockwood L., Martin F. Proactive care of older people undergoing surgery (‘POPS’): designing, embedding, evaluating and funding a comprehensive geriatric assessment service for older elective surgical patients. Age Ageing. 2007;36:190–196. doi: 10.1093/ageing/afl163. [DOI] [PubMed] [Google Scholar]
- 153.Extermann M., Aapro M., Bernabei R., et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG) Crit Rev Oncol Hematol. 2005;55:241–252. doi: 10.1016/j.critrevonc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 154.Parlevliet J.L., Buurman B.M., Pannekeet M.M., et al. Systematic comprehensive geriatric assessment in elderly patients on chronic dialysis: a cross-sectional comparative and feasibility study. BMC Nephrol. 2012;13:30. doi: 10.1186/1471-2369-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li M., Porter E., Lam R., Jassal S.V. Quality improvement through the introduction of interdisciplinary geriatric hemodialysis rehabilitation care. Am J Kidney Dis. 2007;50:90–97. doi: 10.1053/j.ajkd.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lo D., Chiu E., Jassal S.V. A prospective pilot study to measure changes in functional status associated with hospitalization in elderly dialysis-dependent patients. Am J Kidney Dis. 2008;52:956–961. doi: 10.1053/j.ajkd.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 157.Moorthi R.N., Fadel W.F., Cranor A., et al. Mobility impairment in patients new to dialysis. Am J Nephrol. 2020;51:705–714. doi: 10.1159/000509225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Roshanravan B., Robinson-Cohen C., Patel K.V., et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol. 2013;24:822–830. doi: 10.1681/ASN.2012070702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hall R.K., Rutledge J., Luciano A., Hall K., Pieper C.F., Colón-Emeric C. Physical function assessment in older hemodialysis patients. Kidney Med. 2020;2:425–431. doi: 10.1016/j.xkme.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hladek M.D., Zhu J., Crews D.C., et al. Physical resilience phenotype trajectories in incident hemodialysis: characterization and mortality risk assessment. Kidney Int Rep. 2022;7:2006–2015. doi: 10.1016/j.ekir.2022.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Farrington K., Covic A., Nistor I., et al. Clinical Practice Guideline on management of older patients with chronic kidney disease stage 3b or higher (eGFR<45 mL/min/1.73 m2): a summary document from the European Renal Best Practice Group. Nephrol Dial Transplant. 2017;32:9–16. doi: 10.1093/ndt/gfw411. [DOI] [PubMed] [Google Scholar]
- 162.Afilalo J., Lauck S., Kim D.H., et al. Frailty in older adults undergoing aortic valve replacement: the FRAILTY-AVR study. J Am Coll Cardiol. 2017;70:689–700. doi: 10.1016/j.jacc.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 163.Ijaz N., Buta B., Xue Q.L., et al. Interventions for frailty among older adults with cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79:482–503. doi: 10.1016/j.jacc.2021.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Richter D., Guasti L., Walker D., et al. Frailty in Cardiology: Definition, Assessment and Clinical Implications for General Cardiology. A Consensus Document of the Council for Cardiology Practice (CCP), Association for Acute Cardio Vascular Care (ACVC): Association of Cardiovascular Nursing and Allied Professions, European Association of Preventive Cardiology, European Heart Rhythm Association, Council on Valvular Heart Diseases (VHD), Council on Hypertension (CHT), Council of Cardio-Oncology (CCO), Working Group (WG) Aorta and Peripheral Vascular Diseases, WG e-Cardiology, WG Thrombosis, of the European Society of Cardiology, European Primary Care Cardiology Society (EPCCS) Eur J Prev Cardiol. 2022;29:216–227. doi: 10.1093/eurjpc/zwaa167. [DOI] [PubMed] [Google Scholar]
- 165.Eknoyan G., Lameire N., Eckardt K., et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3:5–14. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 166.Bennett P.N., Bohm C., Harasemiw O., et al. Physical activity and exercise in peritoneal dialysis: International Society for Peritoneal Dialysis and the Global Renal Exercise Network practice recommendations. Perit Dial Int J Int Soc Perit Dial. 2022;42:8–24. doi: 10.1177/08968608211055290. [DOI] [PubMed] [Google Scholar]
- 167.Ferrari F., Andrade F.P., Teixeira M.S., et al. Efficacy of six exercise-based interventions for individuals undergoing hemodialysis: a network meta-analysis of randomized clinical trials. Nephrol Dial Transplant. 2023;38:2389–2406. doi: 10.1093/ndt/gfad083. [DOI] [PubMed] [Google Scholar]
- 168.Afsar B., Siriopol D., Aslan G., et al. The impact of exercise on physical function, cardiovascular outcomes and quality of life in chronic kidney disease patients: a systematic review. Int Urol Nephrol. 2018;50:885–904. doi: 10.1007/s11255-018-1790-4. [DOI] [PubMed] [Google Scholar]
- 169.Heiwe S., Jacobson S.H. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2014;64:383–393. doi: 10.1053/j.ajkd.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 170.Heiwe S., Jacobson S.H. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev. 2011;10 doi: 10.1002/14651858.CD003236.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pechter U., Ots M., Mesikepp S., et al. Beneficial effects of water-based exercise in patients with chronic kidney disease. Int J Rehabil Res. 2003;26:153–156. doi: 10.1097/00004356-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 172.Barcellos F.C., Santos I.S., Umpierre D., Bohlke M., Hallal P.C. Effects of exercise in the whole spectrum of chronic kidney disease: a systematic review. Clin Kidney J. 2015;8:753–765. doi: 10.1093/ckj/sfv099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Castaneda C., Gordon P.L., Uhlin K.L., et al. Resistance training to counteract the catabolism of a low-protein diet in patients with chronic renal insufficiency. A randomized, controlled trial. Ann Intern Med. 2001;135:965–976. doi: 10.7326/0003-4819-135-11-200112040-00008. [DOI] [PubMed] [Google Scholar]
- 174.Zhang L., Wang Y., Xiong L., Luo Y., Huang Z., Yi B. Exercise therapy improves eGFR, and reduces blood pressure and BMI in non-dialysis CKD patients: evidence from a meta-analysis. BMC Nephrol. 2019;20:398. doi: 10.1186/s12882-019-1586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Thompson S., Wiebe N., Padwal R.S., et al. The effect of exercise on blood pressure in chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials. PLOS ONE. 2019;14 doi: 10.1371/journal.pone.0211032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nixon A.C., Bampouras T.M., Gooch H.J., et al. Home-based exercise for people living with frailty and chronic kidney disease: A mixed-methods pilot randomised controlled trial. PLoS One. 2021;16 doi: 10.1371/journal.pone.0251652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nixon A.C., Bampouras T.M., Gooch H.J., et al. The EX-FRAIL CKD trial: a study protocol for a pilot randomised controlled trial of a home-based EXercise programme for pre-frail and FRAIL, older adults with Chronic Kidney Disease. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-035344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Young H.M.L., March D.S., Highton P.J., et al. Exercise for people living with frailty and receiving haemodialysis: a mixed-methods randomised controlled feasibility study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-041227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yamaguchi T., Yabe H., Mitake Y., Chishiki A., Katogi T., Fujii T. Effects of exercise therapy on the persistence of physical function, exercise habits, and self-efficacy after cessation of exercise in patients undergoing hemodialysis: a nonrandomized control trial. Ther Apher Dial. 2021;25:458–466. doi: 10.1111/1744-9987.13587. [DOI] [PubMed] [Google Scholar]
- 180.Farragher J., Einbinder Y., Oliver M.J., Nachman J., Chow E., Jassal S.V. Importance of early inpatient geriatric rehabilitation on outcomes in individuals on dialysis. Arch Phys Med Rehabil. 2020;101:227–233. doi: 10.1016/j.apmr.2019.08.472. [DOI] [PubMed] [Google Scholar]
- 181.Doyle E.M., Sloan J.M., Goodbrand J.A., et al. Association between kidney function, rehabilitation outcome, and survival in older patients discharged from inpatient rehabilitation. Am J Kidney Dis. 2015;66:768–774. doi: 10.1053/j.ajkd.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 182.Shinjo G., Otaka Y., Muraoka K., Ebata H., Liu M. Functional outcomes of rehabilitation in patients with subacute stroke on haemodialysis. J Rehabil Med. 2020;52:jrm00033. doi: 10.2340/16501977-2646. [DOI] [PubMed] [Google Scholar]
- 183.Yamagata K., Hoshino J., Sugiyama H., et al. Clinical practice guideline for renal rehabilitation: systematic reviews and recommendations of exercise therapies in patients with kidney diseases. Ren Replace Ther. 2019;5:28. doi: 10.1186/s41100-019-0209-8. [DOI] [Google Scholar]
- 184.Tarazona-Santabalbina F.J., Gómez-Cabrera M.C., Pérez-Ros P., et al. A multicomponent exercise intervention that reverses frailty and improves cognition, emotion, and social networking in the community-dwelling frail elderly: a randomized clinical trial. J Am Med Dir Assoc. 2016;17:426–433. doi: 10.1016/j.jamda.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 185.Yu R., Tong C., Ho F., Woo J. Effects of a multicomponent frailty prevention program in prefrail community-dwelling older persons: a randomized controlled trial. J Am Med Dir Assoc. 2020;21:294.e1–294.e10. doi: 10.1016/j.jamda.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 186.Cameron I.D., Fairhall N., Langron C., et al. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Med. 2013;11:65. doi: 10.1186/1741-7015-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chang J., Gao Y., Fang X.Y., Zhao S.M., Hou Y.P., Sun Q.M. Individualized intervention for frail non-dialysis elderly patients with chronic kidney disease: protocol for a randomized controlled trial. BMC Geriatr. 2020;20:159. doi: 10.1186/s12877-020-1491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yeh S.S., Marandi M., Thode H.C., Jr., et al. Report of a pilot, double-blind, placebo-controlled study of megestrol acetate in elderly dialysis patients with cachexia. J Ren Nutr. 2010;20:52–62. doi: 10.1053/j.jrn.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 189.Scott M.K., Shah N.A., Vilay A.M., Thomas J., 3rd, Kraus M.A., Mueller B.A. Effects of peridialytic oral supplements on nutritional status and quality of life in chronic hemodialysis patients. J Ren Nutr. 2009;19:145–152. doi: 10.1053/j.jrn.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 190.Tong A., Manns B., Hemmelgarn B., et al. Establishing core outcome domains in hemodialysis: report of the standardized outcomes in nephrology-hemodialysis (SONG-HD) consensus workshop. Am J Kidney Dis. 2017;69:97–107. doi: 10.1053/j.ajkd.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Parker S.G., Corner L., Laing K., et al. Priorities for research in multiple conditions in later life (multi-morbidity): findings from a James Lind Alliance Priority Setting Partnership. Age Ageing. 2019;48:401–406. doi: 10.1093/ageing/afz014. [DOI] [PubMed] [Google Scholar]
- 192.Moffatt H., Moorhouse P., Mallery L., Landry D., Tennankore K. Using the Frailty Assessment for Care Planning Tool (FACT) to screen elderly chronic kidney disease patients for frailty: the nurse experience. Clin Interv Aging. 2018;13:843–852. doi: 10.2147/CIA.S150673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hall R.K., Haines C., Gorbatkin S.M., et al. Incorporating geriatric assessment into a nephrology clinic: preliminary data from two models of care. J Am Geriatr Soc. 2016;64:2154–2158. doi: 10.1111/jgs.14262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Alshammari B., Noble H., McAneney H., Alshammari F., O’Halloran P. Factors associated with burden in caregivers of patients with end-stage kidney disease (A systematic review) Healthcare. 2021;9 doi: 10.3390/healthcare9091212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gilbertson E.L.K.R., Foote C., Kennard A.L., Jardine M. Gray NA Burden of caring and quality of life in caregivers of adult dialysis patients: a systematic review. Am J Kidney Dis. 2018 doi: 10.1053/j.ajkd.2018.09.006. Accepted for pulication. [DOI] [PubMed] [Google Scholar]
- 196.March D.S., Hurt A.W., Grantham C.E., et al. A cost-effective analysis of the CYCLE-HD randomized controlled trial. Kidney Int Rep. 2021;6:1548–1557. doi: 10.1016/j.ekir.2021.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]