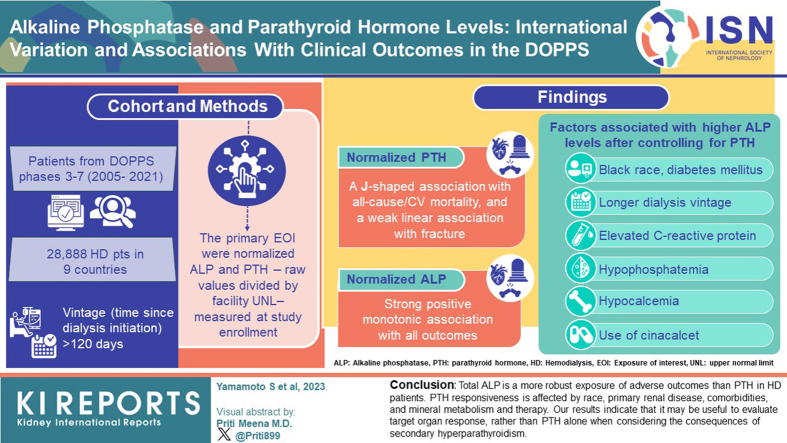
Abstract
Introduction
Secondary hyperparathyroidism (SHPT) increases the risk of fractures and cardiovascular (CV) disease in patients on hemodialysis (HD). The relationship between parathyroid hormone (PTH) and outcomes has been inconsistent, possibly due to variable bone responsiveness to PTH. The KDIGO guideline suggests monitoring total alkaline phosphatase (ALP), but the role of ALP versus PTH in the management of mineral and bone disorder (MBD) is not clear.
Methods
The analysis included 28,888 patients on HD in 9 countries in Dialysis Outcomes and Practice Patterns Study (DOPPS) phase 3 to 7 (2005–2021). The primary exposures of interest were normalized ALP and PTH, which are raw values divided by facility upper normal limit, measured at study enrollment. Cox models were used to estimate hazard ratios of all-cause or CV mortality and any or hip fracture adjusted for potential confounders. Linear mixed models, adjusted for potential confounders, were employed to investigate the relationship between normalized ALP levels and patient characteristics.
Results
Normalized PTH showed a J-shaped association with all-cause or CV mortality, and a weak linear association with fracture. In contrast, normalized ALP showed a strong association with all outcomes. Factors associated with higher ALP levels after controlling for PTH included Black race, longer dialysis vintage, diabetes mellitus, hypocalcemia, hypophosphatemia, elevated C-reactive protein (CRP), and the use of cinacalcet.
Conclusion
Total ALP is a more robust exposure of adverse outcomes than PTH in patients on HD. PTH responsiveness is affected by race, primary renal disease, comorbidities, and mineral metabolism and therapy. Our results indicate that it may be useful to evaluate target organ response, rather than PTH alone when considering the consequences of (SHPT).
Keywords: alkaline phosphatase, fractures, hemodialysis, mortality, parathyroid hormone
Graphical abstract
The MBD of chronic kidney disease (CKD) is an important risk factor for patient morbidity and mortality.1 The risk of fractures and CV disease increases as CKD progresses and is particularly high in patients receiving HD or peritoneal dialysis. SHPT may compromise both vascular and bone health. Elevated PTH levels associate with poor clinical outcomes, including fractures, CV disease, and overall mortality2, 3, 4, 5, 6, 7; however, the association between PTH and outcomes, especially fractures, is inconsistent across epidemiological studies.8, 9, 10, 11, 12
The target organ response to PTH is reduced in CKD, a condition sometimes referred to as “PTH resistance” or “PTH hyporesponsiveness.”13 Responsiveness to PTH could show important variability based on region, ethnicity, and patient-related variables such as comorbidities and medical therapies. Bone turnover markers are substances released from bone during the process of skeletal remodeling. Total ALP is the bone biomarker most assessed in routine clinical practice worldwide. ALP in the systemic circulation is a combination of different isoforms of this enzyme, approximately 50% of which originates from bone.14 Previous observational studies have demonstrated the correlation between serum ALP levels and mortality, hospitalization, CV events, and fractures in dialysis patients.15, 16, 17, 18 However, there have been no reports on the differences between ALP and PTH concerning the clinical outcomes in dialysis patients. By expressing the consequences of SHPT, it could be hypothesized that bone biomarkers such as ALP constitute better exposures of outcomes related to this condition, because it is not confounded by PTH resistance.
This study aimed to compare the associations of ALP and PTH with clinically relevant end points in patients receiving HD, by using data from the DOPPS. Further, we investigated the association between patient characteristics and normalized ALP levels.
Methods
Data Source
The DOPPS is a prospective cohort study of adult patients on chronic in-center HD in 21 countries, ongoing since 1996. Patients on maintenance HD were randomly selected from HD facilities in each country19,20; detailed information is included at http://www.dopps.org. The DOPPS includes data on patient demographics, socioeconomic measures, comorbidities, laboratory measures, medication prescriptions, and prospective follow-up for hospitalization and mortality.
Variables
The primary exposures of interest were normalized ALP and normalized PTH—raw values divided by facility upper normal limit—measured at study enrollment. All laboratory values, including ALP and PTH, comorbid conditions, and medication prescriptions were abstracted from medical records at study enrollment. Facility upper normal limits for laboratory values were captured by the study coordinator at each HD facility completing the DOPPS laboratory reference sheet. Hospitalizations and mortality (including cause) were captured prospectively throughout follow-up. CV mortality was defined as death due to one of the following primary causes: stroke, myocardial infarction, hyperkalemia, pericarditis, atherosclerotic heart disease, cardiomyopathy, cardiac arrhythmia, cardiac arrect, valvular heart disease, pulmonary edema due to exogenous fluid, congestive heart failure, cerebrovascular accident, ischemic brain damage/encephalophagy, and hypokalemia. Hip fracture was defined as hospitalizations with diagnosis code as “Hip Fracture” in any of the 6 diagnosis codes. Any fracture was defined as having hospitalizations with “Hip fracture” or “Other fracture” as diagnosis code, or with “Fracture repair” as procedure code.
Study Sample
In this analysis, patients from DOPPS phases 3 to 7 (2005–2022) with vintage (time since dialysis initiation) >120 days were included. Patients were excluded if they had missing data on ALP or PTH, severe inflammation (CRP >40 mg/l), cirrhosis of the liver, any 1 of 3 laboratory values (bilirubin, ALT, and AST) >1.5 facility upper normal limit, or any 3 of 4 laboratory values (bilirubin, ALT, AST, and ALP) above the facility upper normal limit. Facilities without upper limit of ALP or PTH were excluded. For analyses of cause-specific hospitalization and mortality, we further excluded patients dialyzing in HD facilities that did not sufficiently report causes of hospitalization or mortality. A detailed summary of the number of patients excluded for each reason is presented in Supplementary Figure S1.
Statistical Analysis
We summarized the distribution of ALP and PTH levels both before and after normalization to HD facility upper normal limit, by region (Europe, Japan, US) and by Black/nonblack race within the US. We described the distribution of HD facility upper normal limit of ALP and PTH levels, by region. To isolate the absolute normalized ALP independent of PTH, we compared normalized ALP levels across region and race within strata of normalized PTH levels.
We provided a descriptive summary of patient characteristics across categories of normalized ALP and normalized PTH, overall and by region or race. Because the skeletal responsiveness to PTH has been shown to depend on the severity of SHPT,21,22 descriptive analyses were conducted to illustrate the distribution of normalized ALP levels within categories of normalized PTH and across the following subgroups determined a priori: region or race, cause of end-stage kidney disease, autosomal dominant polycystic kidney disease (ADPKD), diabetes, active vitamin D (i.e., calcitriol or one of its synthetic analogs) use, calcimimetics use, CRP, serum phosphorus, and serum calcium.
To investigate the association more formally between patient characteristics and normalized ALP levels, we used linear mixed models with a random facility intercept to account for clustering and adjusted for potential confounders as fixed effects. To avoid overadjustment for mediators, models investigating case-mix exposures were adjusted for DOPPS phase, country, patient demographics, and comorbidity history, whereas models investigating laboratory exposures were additionally adjusted for other laboratory values, with the exception of CRP, which was rarely measured in some countries. The outcome variable (normalized ALP) was log-transformed due to the skewed distribution and to lessen the impact of extreme outliers on results. For presentation, coefficients were exponentiated and can thus interpreted as a ratio of means (e.g., 1.07 = 7% higher normalized ALP).23 Results are presented with and without adjustment for normalized PTH to evaluate exposures of higher normalized ALP with and without controlling for PTH.
To investigate the association between the 2 exposures (normalized ALP, normalized PTH) and time-to-first-event clinical outcomes, including all-cause or CV mortality and any or hip fracture, we used Cox regression, stratified by DOPPS phase and country, using a robust sandwich covariance estimator to account for facility clustering. A cause-specific hazards approach was used to handle competing risks. Time-at-risk begins at DOPPS enrollment and ends at the time of the event of interest, death, 7 days after leaving the facility due to transfer or change in modality, loss to follow-up, or administrative end of study phase (whichever occurred first). For all outcomes, only the first event per patient was considered. Hazard ratios (95% confidence interval) were estimated in models with increasing levels of stepwise adjustment to evaluate the impact of potential confounders.
In the primary analysis, models were adjusted for age, sex, Black race, dialysis vintage, 13 comorbid conditions (diabetes, hypertension, coronary artery disease, heart failure, cerebrovascular disease, peripheral vascular disease, other CV disease, gastrointestinal bleeding, lung disease, neurologic disease, psychiatric disorder, cancer, and recurrent cellulitis or gangrene), single pool Kt/V, body mass index, serum albumin, serum creatinine, and hemoglobin. In sensitivity analyses, we adjusted for additional variables that may be potential mediators in the causal pathway, including MBD markers (phosphorus and calcium) and MBD treatments (use of Ca-based phosphate binder, non-Ca-based phosphate binder, active vitamin D, and calcimimetics).
Akaike Information Criteria was used to compare the relative fit of Cox models (lower Akaike Information Criteria is better), including normalized PTH versus normalized ALP to help inform which marker better predicted clinical outcomes. In addition to studying each exposure individually, we similarly explored a single 25-category variable combining the 5 categories from each exposure, to help inform which exposure(s) is more strongly associated with time-to-event clinical outcomes.
We used multiple imputation, assuming data were missing at random, to impute missing covariate values using the Sequential Regression Multiple Imputation Method by IVEware.24 Results from 20 such imputed data sets were combined for the final analysis using Rubin’s formula.25 The proportion of missing data was below 5% for all covariates, with the exception of single pool Kt/V (24%), body mass index (8%), and ADPKD (6%). All analyses were conducted using SAS software, version 9.4 (SAS institute, Cary, NC).
Results
Determinants of ALP and PTH
Prior to normalizing values to facility upper normal limits, median (interquartile range) ALP values (in Units/l) were 232 (181–306) in Japan, 87 (66–118) in Europe, 92 (71–129) in US Black patients, and 91 (70–125) in US nonblack patients (Supplementary Figure S2A); and median (interquartile range) PTH values (in pg/ml) were 128 (69–216) in Japan, 227 (119–403) in Europe, 298 (191–537) in US Black patients, and 236 (147–388) in US nonblack patients (Supplementary Figure S2B). Most HD facilities in Europe and the US reported the ALP upper normal limit of 100 to 120 Units/l, compared to 350 Units/l in Japan (Supplementary Figure S3A). In contrast, the HD facility upper normal limit for PTH was similar across regions, at 65 to 75 pg/ml (Supplementary Figure S3B). The distribution of normalized ALP and normalized PTH —raw values divided by facility upper normal limit—indicated a positive association between elevated normalized ALP levels and increased PTH levels (Supplementary Figure S4).
In Tables 1 and 2, we summarize patient characteristics by normalized ALP and normalized PTH. Patients with higher ALP levels were younger, more likely to be female and of Black race, with longer dialysis vintage, and a greater burden of comorbidity. They also had lower levels of phosphorus and higher levels of CRP. Patients with higher levels of PTH had similar patient characteristics as those with higher ALP, except that phosphorus levels were higher in patients with higher PTH. Most of these trends were also observed after stratifying by region and race (Supplementary Table S1).
Table 1.
Patient characteristics, by categories of normalized ALP
| Characteristics | All |
Normalized ALP, x upper normal limit |
||||
|---|---|---|---|---|---|---|
| <0.5 |
0.5–0.74 |
0.75–0.99 |
1.0–1.49 |
1.5+ |
||
| (N = 31,701) | (n = 6258) | (n = 11,222) | (n = 6758) | (n = 5008) | (n = 2455) | |
| Demographics | ||||||
| Age, y | 64 ± 15 | 64 ± 15 | 65 ± 14 | 65 ± 14 | 64 ± 15 | 61 ± 16 |
| Sex (% male) | 61% | 68% | 64% | 58% | 53% | 49% |
| Race (% Black) | 4% | 3% | 4% | 4% | 4% | 6% |
| Vintage, y | 2.7 [0.9, 6.4] | 2.1 [0.8, 5.2] | 2.4 [0.8, 6.1] | 2.7 [1.0, 6.6] | 3.3 [1.2, 7.2] | 4.1 [1.5, 8.5] |
| Comorbidities | ||||||
| Diabetes | 42% | 37% | 41% | 44% | 46% | 46% |
| Hypertension | 86% | 87% | 87% | 86% | 86% | 85% |
| Coronary artery disease | 38% | 34% | 38% | 39% | 41% | 42% |
| Heart failure | 25% | 23% | 24% | 26% | 27% | 28% |
| Cerebrovascular disease | 16% | 15% | 16% | 16% | 16% | 16% |
| Peripheral vascular disease | 25% | 21% | 24% | 26% | 28% | 31% |
| Other CV disease | 30% | 28% | 29% | 31% | 33% | 33% |
| Gastrointestinal bleeding | 4% | 3% | 4% | 4% | 5% | 6% |
| Lung disease | 12% | 10% | 11% | 12% | 13% | 12% |
| Neurologic disease | 10% | 9% | 10% | 11% | 11% | 13% |
| Psychiatric disorder | 13% | 11% | 12% | 14% | 15% | 18% |
| Cancer | 14% | 14% | 14% | 14% | 13% | 13% |
| Recurrent cellulitis / gangrene | 8% | 5% | 7% | 9% | 10% | 13% |
| ADPKD | 5% | 6% | 5% | 4% | 4% | 3% |
| Body mass index, kg/m2 | 25.4 ± 5.9 | 25.4 ± 5.6 | 25.4 ± 5.9 | 25.3 ± 5.9 | 25.5 ± 6.3 | 25.4 ± 6.4 |
| <18 | 6% | 5% | 6% | 7% | 7% | 8% |
| 18–24 | 49% | 49% | 49% | 49% | 48% | 48% |
| 25–29 | 26% | 27% | 26% | 25% | 24% | 24% |
| 30+ | 19% | 18% | 18% | 19% | 21% | 20% |
| Biochemistry | ||||||
| Albumin, g/dl | 3.7 ± 0.5 | 3.8 ± 0.5 | 3.7 ± 0.5 | 3.7 ± 0.5 | 3.7 ± 0.5 | 3.6 ± 0.5 |
| Hemoglobin, g/dl | 11.2 ± 1.4 | 11.2 ± 1.4 | 11.2 ± 1.4 | 11.3 ± 1.4 | 11.3 ± 1.4 | 11.2 ± 1.5 |
| Phosphorus, mg/dl | 5.2 ± 1.6 | 5.4 ± 1.6 | 5.2 ± 1.6 | 5.1 ± 1.6 | 5.1 ± 1.6 | 5.0 ± 1.6 |
| <3.5 | 12% | 9% | 11% | 13% | 14% | 17% |
| 3.5–4.4 | 23% | 20% | 22% | 24% | 24% | 24% |
| 4.5–5.4 | 27% | 27% | 27% | 27% | 27% | 26% |
| 5.5–6.4 | 19% | 21% | 20% | 18% | 18% | 16% |
| 6.5+ | 19% | 22% | 19% | 18% | 17% | 16% |
| Total calcium, mg/dl | 8.9 ± 0.8 | 9.0 ± 0.8 | 8.9 ± 0.8 | 8.9 ± 0.8 | 8.9 ± 0.8 | 8.9 ± 0.9 |
| <8.4 | 21% | 18% | 20% | 20% | 22% | 26% |
| 8.4–9.4 | 57% | 58% | 57% | 58% | 57% | 51% |
| 9.5+ | 22% | 24% | 22% | 22% | 21% | 22% |
| CRP,a mg/l | 4.0 [1.3, 9.0] | 3.0 [1.0, 7.0] | 3.5 [1.1, 8.1] | 4.0 [1.4, 9.2] | 5.0 [2.0, 11.0] | 6.0 [3.0, 14.0] |
| <1 | 15% | 17% | 17% | 14% | 12% | 8% |
| 1–2.9 | 25% | 30% | 27% | 24% | 20% | 16% |
| 3–9.9 | 37% | 35% | 35% | 38% | 40% | 41% |
| 10+ | 23% | 18% | 21% | 24% | 28% | 36% |
| PTH, pg/ml | 213 [108, 390] | 162 [81, 288] | 197 [100, 338] | 226 [120, 411] | 278 [146, 517] | 379 [173, 841] |
| Alkaline phosphatase, U/l | 104 [73, 175] | 59 [50, 91] | 82 [71, 169] | 110 [97, 139] | 147 [129, 180] | 246 [200, 393] |
| Medication | ||||||
| Calcium based phosphate binder | 52% | 56% | 53% | 50% | 48% | 45% |
| Non-calcium based phosphate binder | 50% | 50% | 49% | 49% | 50% | 51% |
| Cinacalcet | 15% | 10% | 13% | 15% | 19% | 27% |
| Vitamin D | 61% | 59% | 61% | 61% | 61% | 62% |
ADPKD, autosomal dominant polycystic kidney disease; ALP, alkaline phosphatase; CRP, C-reactive protein; PTH, parathyroid hormone.
Mean ± SD, median [interquartile range], or prevalence (%) shown.
CRP was restricted to facilities routinely measuring CRP; US and Russia were excluded because CRP not commonly measured.
Table 2.
Patient characteristics, by categories of normalized PTH
| Characteristics | All |
Normalized PTH, x upper normal limit |
||||
|---|---|---|---|---|---|---|
| <1 |
1–3.9 |
4–5.9 |
6–8.9 |
9+ |
||
| (N = 31701) | (n = 4174) | (n = 14,620) | (n = 5113) | (n = 3723) | (n = 4071) | |
| Demographics | ||||||
| Age, y | 64 ± 15 | 65 ± 14 | 66 ± 14 | 64 ± 15 | 63 ± 15 | 59 ± 16 |
| Sex (% male) | 61% | 58% | 62% | 63% | 60% | 56% |
| Race (% Black) | 4% | 2% | 3% | 5% | 4% | 7% |
| Vintage, y | 2.7 [0.9, 6.4] | 2.8 [0.9, 7.3] | 2.4 [0.8, 5.9] | 2.5 [0.9, 5.9] | 2.9 [1.0, 6.6] | 3.8 [1.3, 7.8] |
| Comorbidity history | ||||||
| Diabetes | 42% | 41% | 44% | 45% | 40% | 35% |
| Hypertension | 86% | 83% | 87% | 88% | 87% | 86% |
| Coronary artery disease | 38% | 37% | 38% | 39% | 38% | 36% |
| Heart failure | 25% | 25% | 26% | 26% | 23% | 24% |
| Cerebrovascular disease | 16% | 16% | 17% | 15% | 15% | 12% |
| Peripheral vascular disease | 25% | 23% | 26% | 26% | 24% | 23% |
| Other cardiovascular disease | 30% | 31% | 32% | 30% | 28% | 28% |
| Gastrointestinal bleeding | 4% | 4% | 5% | 4% | 4% | 3% |
| Lung disease | 12% | 9% | 12% | 12% | 13% | 11% |
| Neurologic disease | 10% | 12% | 11% | 9% | 10% | 9% |
| Psychiatric disorder | 13% | 12% | 13% | 13% | 13% | 16% |
| Cancer | 14% | 15% | 14% | 14% | 13% | 11% |
| Recurrent cellulitis / gangrene | 8% | 8% | 8% | 8% | 8% | 8% |
| ADPKD | 5% | 4% | 5% | 6% | 5% | 5% |
| Lab/biometric markers | ||||||
| Body mass index, kg/m2 | 25.4 ± 5.9 | 24.0 ± 5.6 | 24.9 ± 5.7 | 26.1 ± 6.0 | 26.4 ± 6.2 | 26.6 ± 6.4 |
| <18 | 6% | 10% | 7% | 4% | 4% | 5% |
| 18–24 | 49% | 56% | 52% | 45% | 43% | 42% |
| 25–29 | 26% | 20% | 25% | 28% | 28% | 28% |
| 30+ | 19% | 14% | 16% | 22% | 24% | 25% |
| Albumin, g/dl | 3.7 ± 0.5 | 3.7 ± 0.5 | 3.7 ± 0.5 | 3.8 ± 0.5 | 3.8 ± 0.5 | 3.8 ± 0.5 |
| Hemoglobin, g/dl | 11.2 ± 1.4 | 11.1 ± 1.5 | 11.2 ± 1.4 | 11.3 ± 1.4 | 11.3 ± 1.4 | 11.3 ± 1.5 |
| Phosphorus, mg/dl | 5.2 ± 1.6 | 4.9 ± 1.6 | 5.0 ± 1.5 | 5.3 ± 1.6 | 5.5 ± 1.7 | 5.8 ± 1.8 |
| <3.5 | 12% | 16% | 14% | 11% | 9% | 8% |
| 3.5–4.4 | 23% | 24% | 25% | 22% | 19% | 16% |
| 4.5–5.4 | 27% | 26% | 28% | 27% | 27% | 24% |
| 5.5–6.4 | 19% | 19% | 18% | 20% | 21% | 20% |
| 6.5+ | 19% | 15% | 15% | 20% | 23% | 33% |
| Total calcium, mg/dL | 8.9 ± 0.8 | 9.1 ± 0.9 | 8.9 ± 0.7 | 8.9 ± 0.8 | 8.9 ± 0.8 | 9.0 ± 0.9 |
| <8.4 | 21% | 18% | 19% | 22% | 23% | 23% |
| 8.4–9.4 | 57% | 52% | 60% | 58% | 56% | 51% |
| 9.5+ | 22% | 30% | 20% | 20% | 21% | 26% |
| CRP,a mg/l | 4.0 [1.3, 9.0] | 3.3 [1.0, 8.6] | 3.5 [1.0, 8.4] | 4.2 [1.8, 9.4] | 4.5 [2.0, 9.9] | 5.0 [2.5, 10.5] |
| <1 | 15% | 19% | 18% | 12% | 10% | 7% |
| 1–2.9 | 25% | 27% | 27% | 24% | 24% | 20% |
| 3–9.9 | 37% | 33% | 34% | 40% | 41% | 45% |
| 10+ | 23% | 22% | 22% | 24% | 25% | 27% |
| PTH, pg/ml | 213 [108, 390] | 39 [24, 53] | 153 [107, 205] | 321 [285, 361] | 475 [425, 536] | 845 [683, 1164] |
| Alkaline phosphatase, U/l | 104 [73, 175] | 104 [69, 189] | 102 [71, 181] | 97 [73, 150] | 100 [76, 148] | 123 [88, 186] |
| Medication | ||||||
| Calcium based phosphate binder | 52% | 61% | 52% | 49% | 49% | 48% |
| Non-calcium based phosphate binder | 50% | 42% | 48% | 51% | 55% | 58% |
| Cinacalcet | 15% | 7% | 12% | 15% | 20% | 30% |
| Vitamin D | 61% | 53% | 57% | 65% | 67% | 68% |
ADPKD, autosomal dominant polycystic kidney disease; CRP, C-reactive protein; PTH, parathyroid hormone.
Mean ± SD, median [interquartile range], or prevalence (%) shown.
CRP was restricted to facilities routinely measuring CRP; US and Russia were excluded because CRP not commonly measured.
ALP, PTH, and Clinical Outcomes
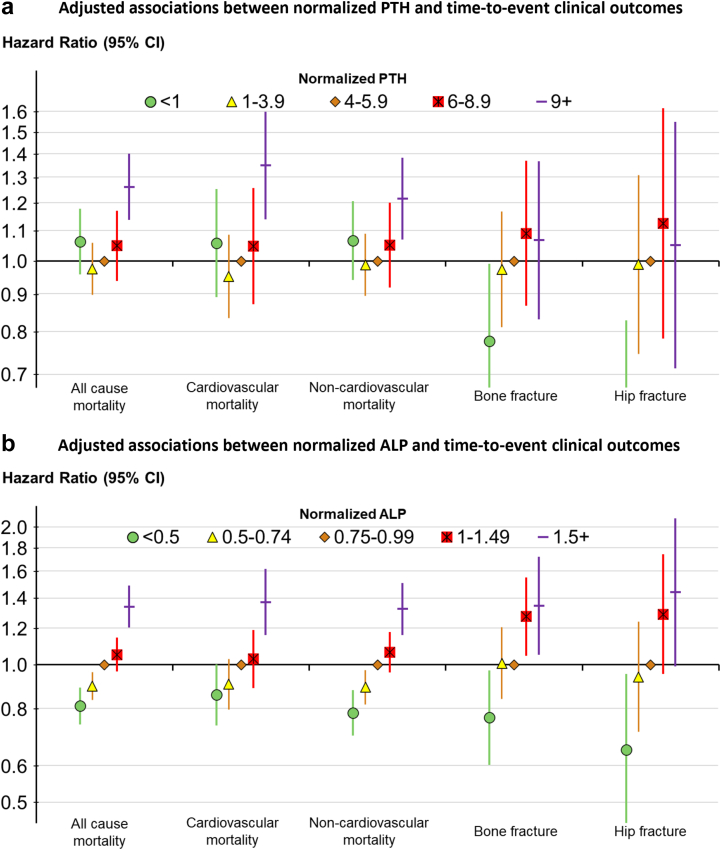
The associations between normalized PTH and normalized ALP and time-to-event clinical outcomes are illustrated in Figure 1. Normalized PTH had a J-shaped association with all-cause, CV and non-CV mortality, and a weak positive monotonic association with fracture (Figure 1a). Normalized ALP had a strong positive monotonic association with all outcomes, that is, patients with higher normalized ALP had higher rates of mortality (all cause, CV, and non-CV) and fracture (any bone and hip) (Figure 1b).
Figure 1.
Adjusted associations between (a) normalized ALP and (b) normalized PTH and time-to-event clinical outcomes. Cox models stratified by DOPPS phase and country, adjusted for demographics: age, sex, Black race, dialysis vintage, 13 comorbid conditions, single pool Kt/V, body mass index, serum albumin, serum creatinine, hemoglobin. Adjusted hazard ratio of PTH <1 for hip fracture was 0.52 (95% confidence interval: 0.33–0.83). ALP, alkaline phosphatase; DOPPS, Dialysis Outcomes and Practice Patterns Study; PTH, parathyroid hormone.
The impact of covariate adjustment on the hazard ratios is shown in Supplementary Table S2. Adjustment did not materially alter the J-shaped relationship between normalized PTH and outcomes, except for fractures, where the risk-relationship after adjustment for key confounders revealed a more linear association (Figure 1). Normalized ALP remained strongly and linearly associated with outcomes throughout adjustments. Akaike Information Criteria was lower for adjusted Cox models using normalized ALP (vs. normalized PTH) as the exposure; this finding was consistent for all 5 outcomes (Supplementary Table S3).
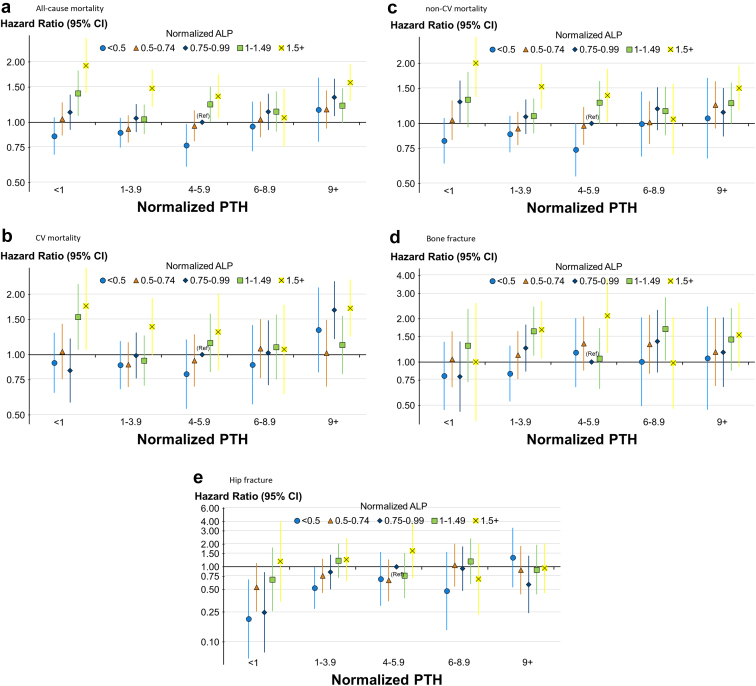
The combined association of normalized ALP and PTH with outcomes is illustrated in Figure 2. Using the center group (normalized ALP: 0.75–0.99 and normalized PTH 4.0–5.9) as the reference in the 5 × 5 = 25 group analysis, it is clear that ALP levels drive the associations with outcomes more than PTH levels. Sample sizes across the 25 groups ranged from 247 to 2576 (Supplementary Table S4).
Figure 2.
Adjusted associations between categories of normalized ALP and normalized PTH and time-to-event clinical outcomes. Cox models stratified by DOPPS phase and country, adjusted for demographics: age, sex, Black race, dialysis vintage, 13 comorbid conditions, single pool Kt/V, body mass index, serum albumin, serum creatinine, hemoglobin. (a) All cause mortality, (b) cardiovascular mortality, (c) non-cardiovascular mortality, (d) bone fracture, and (e) hip fracture. Reference group = normalized ALP: 0.75–0.99 and normalized PTH: 3–5.9. Sample sizes for each cell are provided in Supplementary Table S4. ALP, alkaline phosphatase; DOPPS, Dialysis Outcomes and Practice Patterns Study; PTH, parathyroid hormone.
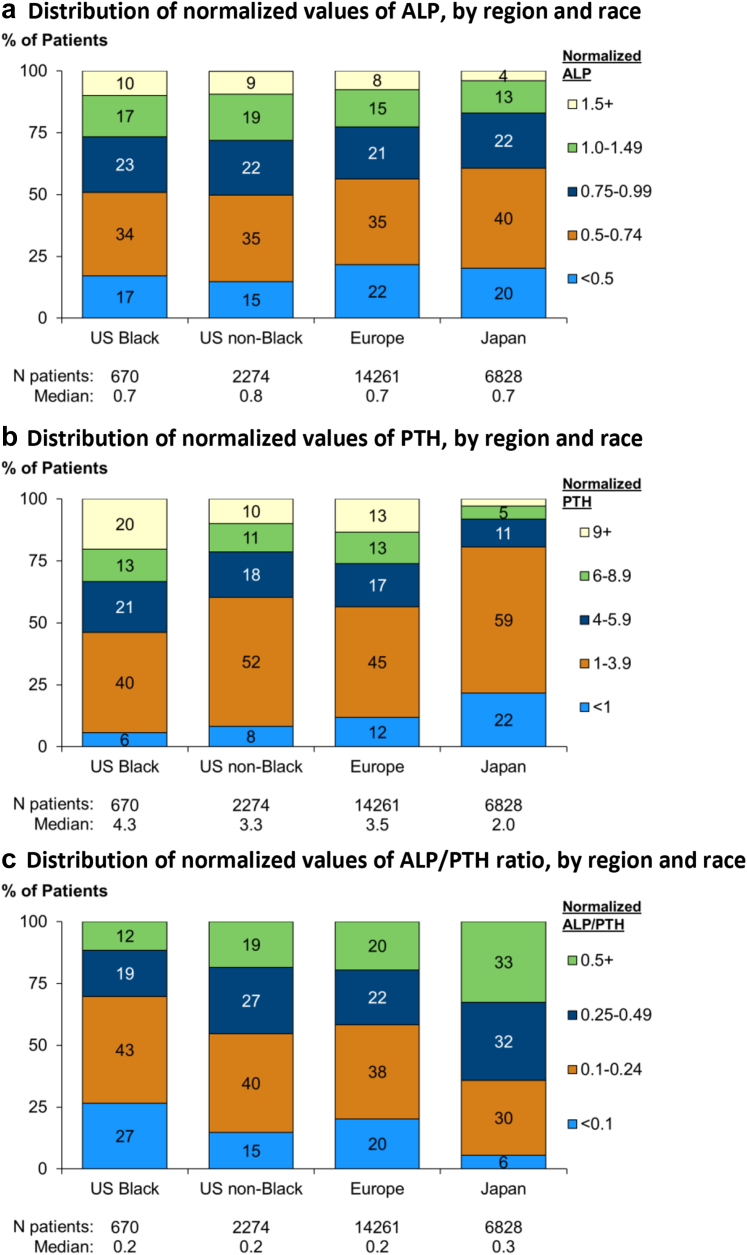
ALP, PTH, and ALP-to-PTH Ratio by Region and Ethnicity
Next, we investigated determinants of skeletal responsiveness to PTH in patients on HD. We used the normalized ALP-to normalized PTH ratio as a measure of skeletal responsiveness.
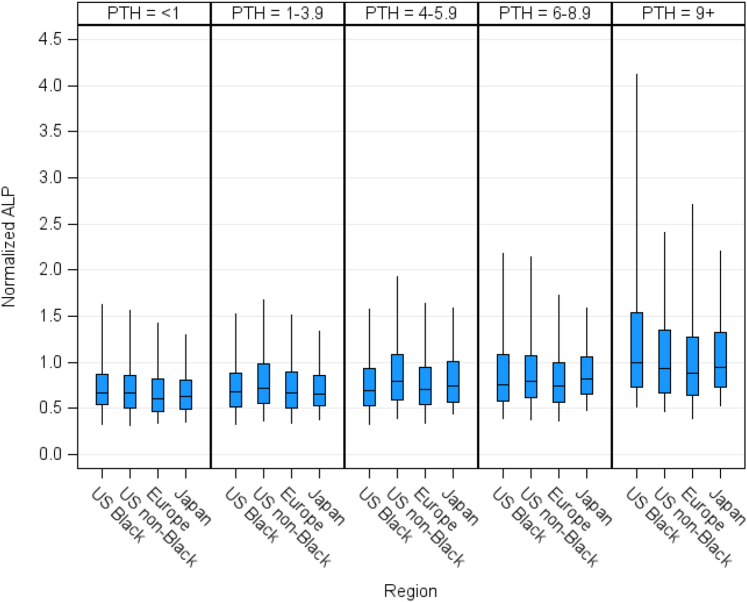
We present the distribution of normalized ALP, normalized PTH, and normalized ALP-to-PTH ratio in Figure 3, by region and race. Median normalized ALP was 0.7 overall and in Europe, Japan, and US Black and higher in US nonblack (0.8) patients. Median normalized PTH was 3.2 overall and lower in Japan (2.0) compared to Europe (3.5), US Black (4.3), and US nonblack (3.3) patients. The ALP-to-PTH ratio was higher in Japanese compared to European and US patients, with lower ALP-to-PTH ratio seen in US Black patients. To adjust for differences in PTH control between regions, we investigated normalized ALP levels within categories of normalized PTH. ALP levels were comparable between regions and by race within categories of PTH (Figure 4). In subgroup analysis, we observed lower ALP levels for any given level of PTH with hypercalcemia (calcium 9.5+ mg/dl), hyperphosphatemia (phosphorus 6.5+ mg/dl) and a diagnosis of ADPKD. Higher ALP for any given level of PTH was seen with diabetes mellitus, cinacalcet use, and elevated CRP. There were no differences in ALP by PTH categories with use of active vitamin D (Supplementary Figure S5).
Figure 3.
Distribution of normalized values of (a) ALP, (b) PTH, (c) ALP-to-PTH ratio, by region and race. All values normalized to facility upper normal limit. ALP, alkaline phosphatase; PTH, parathyroid hormone.
Figure 4.
Distribution of normalized ALP, stratified by region and race within categories of normalized PTH. All values normalized to facility upper normal limit. ALP, alkaline phosphatase; PTH, parathyroid hormone.
Lastly, we investigated patient factors associated with normalized ALP after controlling for PTH and other potential confounders (Table 3). Factors associated with lower ALP after adjustment were male sex, ADPKD, obesity, hypercalcemia, hyperphosphatemia, and phosphate binder use. Higher ALP was associated with Black race, longer dialysis vintage, diabetes mellitus, hypocalcemia, hypophosphatemia, elevated CRP, and cinacalcet use.
Table 3.
Patient characteristics associated with ALP, with and without adjustment for PTH
| Characteristics | Without PTH adjustment |
With PTH adjustment |
||
|---|---|---|---|---|
| Ratio of means | CI95 | Ratio of means | CI95 | |
| Demographics | ||||
| Patient age, per 10 y | 0.99 | (0.99, 0.99) | 1.00 | (1.00, 1.01) |
| Male | 0.90 | (0.89, 0.91) | 0.90 | (0.89, 0.91) |
| Black race | 1.11 | (1.07, 1.15) | 1.05 | (1.02, 1.09) |
| Vintage, per 1 y | 1.01 | (1.01, 1.01) | 1.01 | (1.01, 1.01) |
| Diabetes comorbidity | 1.04 | (1.03, 1.06) | 1.06 | (1.05, 1.07) |
| ADPKD | 0.93 | (0.90, 0.95) | 0.92 | (0.90, 0.94) |
| Body mass index, kg/m2 | ||||
| <18 | 1.05 | (1.02, 1.07) | 1.06 | (1.03, 1.08) |
| 18–24 | 1 | (ref) | 1 | (ref) |
| 25–29 | 0.96 | (0.95, 0.98) | 0.96 | (0.95, 0.97) |
| 30+ | 0.95 | (0.94, 0.97) | 0.94 | (0.93, 0.96) |
| Laboratory values | ||||
| Albumin, per 0.2 g/dl | 0.99 | (0.98, 0.99) | 0.99 | (0.98, 0.99) |
| Phosphorus, mg/dl | ||||
| <3.5 | 1.06 | (1.04, 1.08) | 1.08 | (1.06, 1.09) |
| 3.5–4.4 | 1 | (ref) | 1 | (ref) |
| 4.5–5.4 | 0.97 | (0.96, 0.99) | 0.96 | (0.95, 0.97) |
| 5.5–6.4 | 0.95 | (0.93, 0.96) | 0.92 | (0.91, 0.93) |
| 6.5+ | 0.93 | (0.92, 0.95) | 0.88 | (0.87, 0.89) |
| Calcium, mg/dl | ||||
| <8.4 | 1.05 | (1.03, 1.06) | 1.03 | (1.02, 1.05) |
| 8.4–9.4 | 1 | (ref) | 1 | (ref) |
| 9.5+ | 0.98 | (0.97, 1.00) | 0.98 | (0.97, 0.99) |
| CRP, mg/la | ||||
| <1 | 1 | (ref) | 1 | (ref) |
| 1–2.9 | 1.03 | (1.01, 1.06) | 1.03 | (1.00, 1.05) |
| 3–9.9 | 1.10 | (1.07, 1.13) | 1.09 | (1.06, 1.12) |
| 10+ | 1.17 | (1.14, 1.20) | 1.16 | (1.13, 1.19) |
| Medication | ||||
| Calcium based phosphate binder | 0.92 | (0.91, 0.93) | 0.92 | (0.91, 0.93) |
| Non-calcium phosphate binder | 0.98 | (0.97, 0.99) | 0.98 | (0.97, 0.98) |
| Cinacalcet | 1.17 | (1.15, 1.19) | 1.17 | (1.15, 1.19) |
| Vitamin D | 1.02 | (1.01, 1.03) | 1.02 | (1.01, 1.03) |
ADPKD, autosomal dominant polycystic kidney disease; ALP, alkaline phosphatase; CI95, 95% confidence interval; CRP, C-reactive protein; PTH, parathyroid hormone.
We used multivariable linear mixed models with a random facility intercept to account for clustering; and adjusted for potential confounders. For case-mix exposures, models were adjusted for DOPPS phase, country, patient demographics, and comorbidity history. For laboratory exposures, we additionally adjusted for other laboratory values except for CRP. The outcome variable (normalized ALP) was log-transformed due to the skewed distribution and to lessen the impact of extreme outliers on results. For presentation, coefficients were exponentiated and can thus be interpreted as a ratio of means (e.g., 1.07 = 7% higher normalized ALP).
CRP model was restricted to facilities that measure CRP often in countries other than US and Russia, and additionally adjusted for other laboratories.
Discussion
In this large, international cohort study on patients receiving HD, we demonstrate that total ALP is a more robust marker of adverse outcomes related to the syndrome of MBD of CKD than PTH. ALP after controlling for PTH is affected by race, primary renal disease, comorbidities, and mineral metabolism and therapy.
Associations of ALP and PTH With Adverse Outcomes
Investigating the associations of total ALP and PTH with relevant clinical endpoints, we found a J-shaped relationship between PTH and mortality, indicating the highest risk with increasing severity of SHPT. This aligns well with previous reports.9, 10, 11 In contrast, the relationship between total ALP and mortality was clearly linear. This is consistent with previous studies—high serum ALP levels associate with increased risk of all-cause mortality26, 27, 28 and of CV events.27,29 The combined association of ALP and PTH with all-cause and CV mortality showed that ALP had a stronger association with outcomes than PTH.
Interestingly, a more linear relationship between PTH and fracture risk was seen in this large, international cohort than what was previously reported.4,7,30 Published data on ALP and incident fractures, albeit limited, demonstrate a clear, linear relationship with risk,28,30 Our data also showed a monotonic association of serum ALP with fractures, and the effect was greater than PTH. These data are consistent with a high bone turnover state resulting in bone resorption and bone demineralization, with loss of skeletal integrity contributing to an increased risk of fractures.31
A high skeletal remodeling rate may contribute indirectly to CV disease and mortality, by providing a source of calcium and phosphate loading to the vasculature.32 Observational studies demonstrate a link between bone disease and vascular calcification in CKD. Low bone mass is associated with increase in coronary calcifications in patients on HD.33 Severity of arterial calcification showed positive associations with PTH in patients with CKD stage 4 to 5D.34 In addition, higher serum bone-specific ALP level associated with the presence of vascular calcification of hand arteries in male patients on HD.35 Patients with low bone volume also show greater progression of vascular calcification over time.36
Taken together, these findings demonstrate that ALP is more strongly associated with all-cause or CV mortality and any or hip fracture, than PTH. Our findings indicate that it may be more useful to consider target organ response to SHPT rather than PTH levels alone. Therefore, ALP may prove superior to PTH as a therapeutic target of MBD of CKD, but this hypothesis requires a prospective clinical trial.
Skeletal PTH Responsiveness in CKD
Skeletal responsiveness to PTH is impaired in CKD,22 as shown by reduced calcemic response to PTH infusion37 and impaired ability to recover from a hypocalcemia challenge.38 This has been used as an argument to target higher than normal PTH levels in patients with CKD.39,40 Variations in skeletal responsiveness to PTH could potentially explain the differences in optimal PTH targets demonstrated in Japanese41 compared to European or American patients.42 In the present study, we investigated variations in PTH responsiveness by region and race, using the ALP-to-PTH ratio as a measure of skeletal response. In our unadjusted analyses, Japanese patients had a higher ALP-to-PTH ratio, whereas US Black patients had the lowest values. However, when we adjusted for PTH control, by examining ALP levels within categories of PTH, the effects of region and race were greatly attenuated. Thus, we could not confirm differences in skeletal responsiveness to PTH based on region or race in this study.
Determinants of ALP
To identify determinants of skeletal PTH responsiveness, we investigated ALP levels within categories of PTH for prespecified patient subsets. An underlying diagnosis of ADPKD was associated with lower levels of ALP for any given level of PTH. This is consistent with previous findings of lower bone turnover markers and skeletal remodeling parameters in patients with ADPKD.43 In the present study, cinacalcet use was associated with higher levels of ALP for any given level of PTH, and cinacalcet remained a determinant of ALP after multivariate adjustment including PTH. Calcimimetics therapy may affect skeletal remodeling by modulation of the activity of the calcium-sensing receptor in bone.44,45 Hyperphosphatemia and hypercalcemia were both associated with lower ALP levels within categories of PTH. These findings could represent a lowering of skeletal responsiveness to PTH as an adaptive response aimed at reducing skeletal efflux of calcium and phosphate in situations of excess.
Strengths and Limitations
The comparison of absolute ALP level among regions and races as well as the determination of target levels are difficult due to multiple assays available and in use. In this study, we normalized serum levels of ALP and PTH to the upper normal limit in each HD facility and examined the association of PTH and ALP with outcomes using international data. The DOPPS study design allowed us to find distribution of bone responsiveness to PTH and the association with outcomes in an international cohort of real-world patients, like what physicians may encounter when rounding in the dialysis unit. However, we must acknowledge some important limitations. As with any observational study, the reported associations do not prove causality and may be affected by unmeasured confounders. ALP is derived not only from bone but liver dysfunction and inflammation. In patients on HD, it is known that elevated ALP includes small amount of liver fragment,46 but we excluded patients with hepatic cirrhosis, elevated hepatic markers, and high CRP level to discuss total ALP and bone metabolism in patients on HD. To discern differences in distribution and the impact on outcomes across countries over a prolonged period, we normalized serum levels of ALP and PTH to the upper normal limit. However, it is important to acknowledge that interassay variability may have played a role in misclassifying specific patients. Further, ALP and PTH (and covariates) were assessed at baseline only and thus, no time-updated analysis could be performed. Further studies will be needed to find the change of ALP and PTH with the intervention and benefits for the outcomes.
Conclusions
Total ALP was a more robust exposure of all-cause or CV mortality and any or hip fracture than PTH levels in this large, international cohort of patients on HD. Variations in the ALP-to-PTH ratio were mainly explained by the severity of SHPT, and we could not confirm any differences in skeletal responsiveness to PTH based on region or race. PTH responsiveness is affected by race, primary renal disease, comorbidities, and mineral metabolism and therapy. It may prove useful to consider target organ response, rather than PTH levels alone, when considering the consequences of SHPT.
Disclosure
SY has received honoraria from Kyowa Kirin, and research findings from Toray Medical Co. Ltd and Kaneka Medix Co. Ltd. JZ, AK, BB, and BR are employees for the nonprofit research organization Arbor Research Collaborative for Health, which has designed and carries out the DOPPS Program. Grants are made to Arbor Research Collaborative for Health and not to individual investigators. HK has received honoraria, consulting fees, and/or grant support from Bayer Yakuhin, Chugai Pharmaceutical, Kissei Pharmaceutical, Kyowa Kirin, Ono Pharmaceutical, and Sanwa Kagaku Kenkyusho. MV has received research funding from Dutch Kidney Foundation, European Union, Health Holland, Fresenius Medical Care, CSL Vifor, and Calciscon; and honoraria from Astra-Zeneca, Boehringer-Ingolheim, Kyowa Kirin, CSL Vifor, Otsuka, and Inozyme. SM has received honoraria for congress speech from Amgen and Vifor. EC has received consulting fees from IDS, DiaSorin, Nittobo, Fujirebio, bioMérieux, Werfen, Nephrolyx, and Snibe. PE has received institutional grants from Vifor FMC, Sanofi, and Amgen; honoraria from Vifor FMC and Amgen; and participation on Medice advisory board. MF has received honoraria, consulting fees, and/or grant support from Bayer Yakuhin, Chugai Pharmaceutical, Kissei Pharmaceutical, Kyowa Kirin, Ono Pharmaceutical, and Sanwa Kagaku Kenkyusho. All the other authors declared no conflicting interests.
Acknowledgments
This manuscript was directly supported by Kyowa Kirin. Global support for ongoing DOPPS Programs is provided without restriction on publications by a variety of funders (details in https://www.dopps.org/AboutUs/Support.aspx). This manuscript was directly supported by Kyowa Kirin Co. Global support for the ongoing DOPPS Programs is provided without restriction on publications by a variety of funders. For details see https://www.dopps.org/AboutUs/Support.aspx. All grants were made to Arbor Research Collaborative for Health and not to co-authors directly. None of the funders had any role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit this report for publication.
Data Availability Statement
Restrictions apply to the availability of the data analyzed in this study to preserve patient confidentiality. Data will be shared on request to the corresponding author with permission from the DOPPS investigators.
Author Contributions
All authors contributed study conception, design, analysis, and interpretation of data. Research idea and study design was by SY, HSJ, JZ, AK HK, MV, SM, EC, BB, BR, PE, and MF; data acquisition by BB and BR; data analysis/interpretation by SY, HSJ, JZ, AK HK, PE, and MF; statistical analysis by JZ and AK; drafting of the article: SY, HSJ, JZ, AK, and PE; and supervision or mentorship by BR and MF. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Footnotes
Figure S1. Flow chart of inclusion and exclusion criteria. ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transferase; CRP, C-reactive protein; DOPPS, Dialysis Outcomes and Practice Patterns Study; phase 3–7, 2005–2022; PTH, parathyroid hormone; vintage, time since dialysis initiation.
Figure S2. Distribution of raw (not normalized) values of (A) ALP, and (B) PTH, by region and race. ALP, alkaline phosphatase; PTH, parathyroid hormone.
Figure S3. Distribution of facility upper normal limit of (A) ALP, and (B) PTH by region. ALP, alkaline phosphatase; PTH, parathyroid hormone.
Figure S4. Distribution of normalized ALP, by normalized PTH. ALP, alkaline phosphatase; PTH, parathyroid hormone.
Figure S5. Normalized ALP by categories of normalized PTH by (A) primary cause of ESRD, (B) ADPKD, (C) diabetes, (D) CRP, (E) serum calcium, (F) serum phosphorus, (G) cinacalcet, and (H) active vitamin D. ADPKD, adult polycystic kidney disease; ALP, alkaline phosphatase; CRP, C-reactive protein; ESRD, end-stage renal disease; PTH, parathyroid hormone.
Table S1. Patient characteristics, by normalized ALP and normalized PTH, within each region and race. ALP, alkaline phosphatase; PTH, parathyroid hormone.
Table S2. Adjusted associations (HR, 95% confidence interval) between normalized ALP, normalized PTH, and time-to-event clinical outcomes, by level of adjustment. ALP, alkaline phosphatase; PTH, parathyroid hormone.
Table S3. Akaike Information Criteria for adjusted Cox models including normalized PTH vs. normalized ALP. ALP, alkaline phosphatase; PTH, parathyroid hormone.
Table S4. Sample size in each cell of the analysis (Figure 2) of the combined associations between normalized ALP, normalized PTH, and time-to-even clinical outcomes. ALP, alkaline phosphatase; PTH, parathyroid hormone.
STROBE Statement.
Supplementary Material
Figure S1. Flow chart of inclusion and exclusion criteria. ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transferase; CRP, C-reactive protein; DOPPS, Dialysis Outcomes and Practice Patterns Study; phase 3–7, 2005–2022; PTH, parathyroid hormone; vintage, time since dialysis initiation.
Figure S2. Distribution of raw (not normalized) values of (A) ALP, and (B) PTH, by region and race. ALP, alkaline phosphatase; PTH, parathyroid hormone.
Figure S3. Distribution of facility upper normal limit of (A) ALP, and (B) PTH by region. ALP, alkaline phosphatase; PTH, parathyroid hormone.
Figure S4. Distribution of normalized ALP, by normalized PTH. ALP, alkaline phosphatase; PTH, parathyroid hormone.
Figure S5. Normalized ALP by categories of normalized PTH by (A) primary cause of ESRD, (B) ADPKD, (C) diabetes, (D) CRP, (E) serum calcium, (F) serum phosphorus, (G) cinacalcet, and (H) active vitamin D. ADPKD, adult polycystic kidney disease; ALP, alkaline phosphatase; CRP, C-reactive protein; ESRD, end-stage renal disease; PTH, parathyroid hormone.
Table S1. Patient characteristics, by normalized ALP and normalized PTH, within each region and race. ALP, alkaline phosphatase; PTH, parathyroid hormone.
Table S2. Adjusted associations (HR, 95% confidence interval) between normalized ALP, normalized PTH, and time-to-event clinical outcomes, by level of adjustment. ALP, alkaline phosphatase; PTH, parathyroid hormone.
Table S3. Akaike Information Criteria for adjusted Cox models including normalized PTH vs. normalized ALP. ALP, alkaline phosphatase; PTH, parathyroid hormone.
Table S4. Sample size in each cell of the analysis (Figure 2) of the combined associations between normalized ALP, normalized PTH, and time-to-even clinical outcomes. ALP, alkaline phosphatase; PTH, parathyroid hormone.
STROBE Statement.
References
- 1.Moe S., Drueke T., Cunningham J., et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 2.Tentori F., Wang M., Bieber B.A., et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol. 2015;10:98–109. doi: 10.2215/CJN.12941213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamoto S., Karaboyas A., Komaba H., et al. Mineral and bone disorder management in hemodialysis patients: comparing PTH control practices in Japan with Europe and North America: the Dialysis Outcomes and Practice Patterns Study (DOPPS) BMC Nephrol. 2018;19:253. doi: 10.1186/s12882-018-1056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jadoul M., Albert J.M., Akiba T., et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2006;70:1358–1366. doi: 10.1038/sj.ki.5001754. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Martin J.L., Martinez-Camblor P., Dionisi M.P., et al. Improvement of mineral and bone metabolism markers is associated with better survival in haemodialysis patients: the COSMOS study. Nephrol Dial Transplant. 2015;30:1542–1551. doi: 10.1093/ndt/gfv099. [DOI] [PubMed] [Google Scholar]
- 6.Block G.A., Klassen P.S., Lazarus J.M., Ofsthun N., Lowrie E.G., Chertow G.M. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 7.Jansz T.T., Goto N.A., van Ballegooijen A.J., Willems H.C., Verhaar M.C., van Jaarsveld B.C. The prevalence and incidence of vertebral fractures in end-stage renal disease and the role of parathyroid hormone. Osteoporos Int. 2020;31:515–524. doi: 10.1007/s00198-019-05187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kidney disease: improving global outcomes CKDMBDWG KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009;113:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 9.Tentori F., Blayney M.J., Albert J.M., et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52:519–530. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Kalantar-Zadeh K., Kuwae N., Regidor D.L., et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 11.Lamina C., Kronenberg F., Stenvinkel P., et al. Association of changes in bone mineral parameters with mortality in haemodialysis patients: insights from the ARO cohort. Nephrol Dial Transplant. 2020;35:478–487. doi: 10.1093/ndt/gfz060. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi M., Fukagawa M., Fujii N., et al. Serum phosphate and calcium should be primarily and consistently controlled in prevalent hemodialysis patients. Ther Apher Dial. 2013;17:221–228. doi: 10.1111/1744-9987.12030. [DOI] [PubMed] [Google Scholar]
- 13.Evenepoel P., Bover J., Urena Torres P. Parathyroid hormone metabolism and signaling in health and chronic kidney disease. Kidney Int. 2016;90:1184–1190. doi: 10.1016/j.kint.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 14.Haarhaus M., Cianciolo G., Barbuto S., et al. Alkaline phosphatase: an old friend as treatment target for cardiovascular and mineral bone disorders in chronic kidney disease. Nutrients. 2022;14:2124. doi: 10.3390/nu14102124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z., Zhang X., Han F., et al. High alkaline phosphatase and low intact parathyroid hormone associate with worse clinical outcome in peritoneal dialysis patients. Perit Dial Int. 2021;41:236–243. doi: 10.1177/0896860820918131. [DOI] [PubMed] [Google Scholar]
- 16.Beddhu S., Baird B., Ma X., Cheung A.K., Greene T. Serum alkaline phosphatase and mortality in hemodialysis patients. Clin Nephrol. 2010;74:91–96. doi: 10.5414/cnp74091. [DOI] [PubMed] [Google Scholar]
- 17.Fan Y., Jin X., Jiang M., Fang N. Elevated serum alkaline phosphatase and cardiovascular or all-cause mortality risk in dialysis patients: a meta-analysis. Sci Rep. 2017;7 doi: 10.1038/s41598-017-13387-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abramowitz M., Muntner P., Coco M., et al. Serum alkaline phosphatase and phosphate and risk of mortality and hospitalization. Clin J Am Soc Nephrol. 2010;5:1064–1071. doi: 10.2215/CJN.08621209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pisoni R.L., Gillespie B.W., Dickinson D.M., Chen K., Kutner M.H., Wolfe R.A. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis. 2004;44(5 suppl 2):7–15. doi: 10.1053/j.ajkd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 20.DAG EWY, MAPES D.L., PORT F.K., et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS):an international hemodialysis study. Kidney Int. 2000;57:S74–S81. doi: 10.1046/j.1525-139x.2001.00043.x. [DOI] [Google Scholar]
- 21.Evenepoel P., Jorgensen H.S., Komaba H., et al. Lower bone turnover and skeletal PTH responsiveness in Japanese compared to European patients on hemodialysis. J Clin Endocrinol Metab. 2022;107:e4350–e4359. doi: 10.1210/clinem/dgac522. [DOI] [PubMed] [Google Scholar]
- 22.Wesseling-Perry K., Harkins G.C., Wang H.J., et al. The calcemic response to continuous parathyroid hormone (PTH)(1-34) infusion in end-stage kidney disease varies according to bone turnover: a potential role for PTH(7-84) J Clin Endocrinol Metab. 2010;95:2772–2780. doi: 10.1210/jc.2009-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee D.K. Data transformation: a focus on the interpretation. Korean J Anesthesiol. 2020;73:503–508. doi: 10.4097/kja.20137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor J.M., Cooper K.L., Wei J.T., Sarma A.V., Raghunathan T.E., Heeringa S.G. Use of multiple imputation to correct for nonresponse bias in a survey of urologic symptoms among African-American men. Am J Epidemiol. 2002;156:774–782. doi: 10.1093/aje/kwf110. [DOI] [PubMed] [Google Scholar]
- 25.Little R.J.A., DBR . John Wiley & Sons; 1987. Statistical Analysis With Missing Data. [Google Scholar]
- 26.Regidor D.L., Kovesdy C.P., Mehrotra R., et al. Serum alkaline phosphatase predicts mortality among maintenance hemodialysis patients. J Am Soc Nephrol. 2008;19:2193–2203. doi: 10.1681/ASN.2008010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blayney M.J., Pisoni R.L., Bragg-Gresham J.L., et al. High alkaline phosphatase levels in hemodialysis patients are associated with higher risk of hospitalization and death. Kidney Int. 2008;74:655–663. doi: 10.1038/ki.2008.248. [DOI] [PubMed] [Google Scholar]
- 28.Maruyama Y., Taniguchi M., Kazama J.J., et al. A higher serum alkaline phosphatase is associated with the incidence of hip fracture and mortality among patients receiving hemodialysis in Japan. Nephrol Dial Transplant. 2014;29:1532–1538. doi: 10.1093/ndt/gfu055. [DOI] [PubMed] [Google Scholar]
- 29.Shantouf R., Kovesdy C.P., Kim Y., et al. Association of serum alkaline phosphatase with coronary artery calcification in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:1106–1114. doi: 10.2215/CJN.06091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C., Chen X.M., Li Y., Zhou Y.L., Yan J.N., Du X.G. Factors and outcome of renal osteodystrophy-associated initial fragility fracture in end-stage renal disease patients. Kidney Dis (Basel) 2019;5:118–125. doi: 10.1159/000494924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malluche H., Faugere M.C. Renal bone disease 1990: an unmet challenge for the nephrologist. Kidney Int. 1990;38:193–211. doi: 10.1038/ki.1990.187. [DOI] [PubMed] [Google Scholar]
- 32.Evenepoel P., Opdebeeck B., David K., D’Haese P.C. Bone-vascular axis in chronic kidney disease. Adv Chronic Kidney Dis. 2019;26:472–483. doi: 10.1053/j.ackd.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Coen G., Ballanti P., Mantella D., et al. Bone turnover, osteopenia and vascular calcifications in hemodialysis patients. A histomorphometric and multislice CT study. Am J Nephrol. 2009;29:145–152. doi: 10.1159/000151769. [DOI] [PubMed] [Google Scholar]
- 34.Salam S., Gallagher O., Gossiel F., Paggiosi M., Eastell R., Khwaja A. Vascular calcification relationship to vascular biomarkers and bone metabolism in advanced chronic kidney disease. Bone. 2021;143 doi: 10.1016/j.bone.2020.115699. [DOI] [PubMed] [Google Scholar]
- 35.Ishimura E., Okuno S., Okazaki H., et al. Significant association between bone-specific alkaline phosphatase and vascular calcification of the hand arteries in male hemodialysis patients. Kidney Blood Press Res. 2014;39:299–307. doi: 10.1159/000355807. [DOI] [PubMed] [Google Scholar]
- 36.Malluche H.H., Blomquist G., Monier-Faugere M.C., Cantor T.L., Davenport D.L. High parathyroid hormone level and osteoporosis predict progression of coronary artery calcification in patients on dialysis. J Am Soc Nephrol. 2015;26:2534–2544. doi: 10.1681/ASN.2014070686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massry S.G., Coburn J.W., Lee D.B., Jowsey J., Kleeman C.R. Skeletal resistance to parathyroid hormone in renal failure. Studies in 105 human subjects. Ann Intern Med. 1973;78:357–364. doi: 10.7326/0003-4819-78-3-357. [DOI] [PubMed] [Google Scholar]
- 38.Llach F., Massry S.G., Singer F.R., Kurokawa K., Kaye J.H., Coburn J.W. Skeletal resistance to endogenous parathyroid hormone in pateints with early renal failure. A possible cause for secondary hyperparathyroidism. J Clin Endocrinol Metab. 1975;41:339–345. doi: 10.1210/jcem-41-2-339. [DOI] [PubMed] [Google Scholar]
- 39.Jorgensen H.S., Behets G., Viaene L., et al. Diagnostic accuracy of noninvasive bone turnover markers in renal osteodystrophy. Am J Kidney Dis. 2022;79:667–676.e1. doi: 10.1053/j.ajkd.2021.07.027. [DOI] [PubMed] [Google Scholar]
- 40.Sprague S.M., Bellorin-Font E., Jorgetti V., et al. Diagnostic accuracy of bone turnover markers and bone histology in patients with CKD treated by dialysis. Am J Kidney Dis. 2016;67:559–566. doi: 10.1053/j.ajkd.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 41.Fukagawa M., Yokoyama K., Koiwa F., et al. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial. 2013;17:247–288. doi: 10.1111/1744-9987.12058. [DOI] [PubMed] [Google Scholar]
- 42.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl (2011) 2017;7:1–59. doi: 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evenepoel P., Claes K., Cavalier E., et al. A distinct bone phenotype in ADPKD patients with end-stage renal disease. Kidney Int. 2019;95:412–419. doi: 10.1016/j.kint.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 44.Diaz-Tocados J.M., Rodriguez-Ortiz M.E., Almaden Y., et al. Calcimimetics maintain bone turnover in uremic rats despite the concomitant decrease in parathyroid hormone concentration. Kidney Int. 2019;95:1064–1078. doi: 10.1016/j.kint.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 45.Jorgensen H.S., Cavalier E., Evenepoel P. Clinical evidence of direct bone effects of Cinacalcet. Kidney Int. 2020;98:514–515. doi: 10.1016/j.kint.2020.04.013. [DOI] [PubMed] [Google Scholar]
- 46.Sanchez Navarro M.R., Fernandez-Conde M.E., Blanco Martin S., Samaniego C. Alkaline phosphatase isoenzymes in the serum of patients with renal insufficiency. Article in Spanish. An Med Int. 2002;19:449–452. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Restrictions apply to the availability of the data analyzed in this study to preserve patient confidentiality. Data will be shared on request to the corresponding author with permission from the DOPPS investigators.