Abstract
Background
Hyperkalaemia is a common electrolyte abnormality seen in critically ill patients. In haemorrhagic shock, it may contribute to cardiac arrest and has been identified as a potential marker for tissue hypoxia. However, the significance of its role in haemorrhagic shock and its contribution to mortality remains unclear. This study aimed to examine the potential underlying pathophysiology and evaluate the incidence and characteristics of patients with hyperkalaemia on hospital arrival in bleeding trauma patients before transfusions and its mortality.
Methods
A retrospective cohort study was conducted on adult patients with traumatic bleeding admitted to a European Major Trauma Centre between January 2016 and December 2021. Patients were classified according to their serum potassium levels on arrival, and relevant clinical parameters between non-hyperkalaemic and hyperkalaemic patients were compared.
Results
Among the 83 patients in this study, 8 (9.6 %) presented with hyperkalaemia on arrival. The median shock index showed a higher tendency in the hyperkalaemic group. Hyperkalaemia was found to be more common among younger patients who sustained penetrating trauma. Mortality rates were higher in the hyperkalaemic group, but the difference was not statistically significant.
Conclusion
Our results suggest that hyperkalaemia occurs frequently in bleeding trauma patients on hospital arrival pre-transfusions, indicating a more severe illness. Our findings provide insights into the pathophysiology and characteristics of hyperkalaemia in bleeding trauma patients. Further studies are required to investigate the mechanisms by which hyperkalaemia contributes to mortality in haemorrhagic shock patients.
Keywords: Shock, Haemorrhage, Ischemia, Multiple trauma, Potassium
Highlights
-
•
Approximately 10 % of haemorrhaging adult trauma patients presented with hyperkalaemia upon arrival, before transfusions.
-
•
Younger patients and those suffering from penetrating trauma were more likely to exhibit hyperkalaemia.
-
•
The study emphasises the importance of early recognition and management of hyperkalaemia in bleeding trauma patients.
1. Introduction
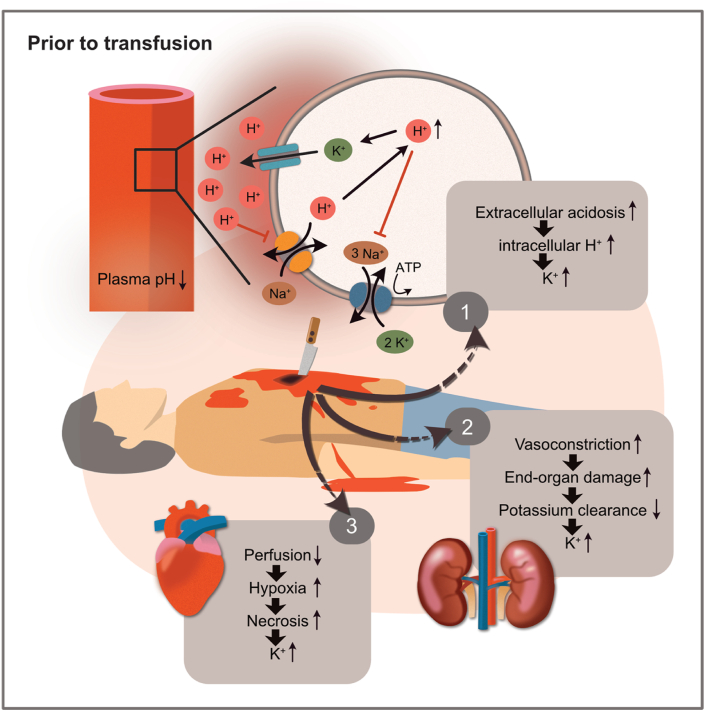
Traumatic haemorrhagic shock (THS) remains a significant challenge in trauma care, responsible for approximately one-third of trauma deaths [1]. In non-compressible torso haemorrhage (NCTH), invasive techniques such as prehospital blood products or resuscitative endovascular balloon occlusion of the aorta (REBOA) are often required to manage haemodynamics in critically ill patients with multiple side effects that might even obscure potential beneficial effects of the procedure like with REBOA [2]. However, in the initial treatment phase, these techniques have led to an increase in the number of patients who would have likely not survived a few years ago, posing significant challenges for trauma centres [3,4]. THS represents a delicate and competitive equilibrium between autonomic activation for bleeding reduction through mechanisms like vasoconstriction and the reduction of cellular oxygen consumption to avoid insufficient oxygen delivery to the cells for metabolic and nervous regulatory circuits. Oxygen debt due to inadequate delivery has multiple effects on metabolism, which can account for refractory shock and increased mortality [5]. In hypoxic conditions, such as shock, cellular stress response consists of increased secretion of signalling metabolites, such as lactate or damage-associated molecular patterns (DAMPs), known as alarmins [6]. These molecules have been shown to initiate a dysregulated immune response. At the same time, blood loss causes sympathetic activation, leading to under-perfused peripheral tissues and accumulating acidotic products [7]. Consequently, metabolic acidosis develops, and the potassium concentration in the extracellular space increases before potential transfusions, mainly through three mechanisms: 1) increased amounts of H+ ions (acidosis) slow down the Na+/H+ antiporter of the cellular membranes. Consequently, potassium cannot shift back into the cells, leading to extracellular hyperkalaemia. 2) Vasoconstriction-induced end-organ damage to the kidneys causes decreased renal excretion of potassium ions, contributing to the evolution of hyperkalaemia in haemorrhagic shock [5]. 3) Lastly, necrosis occurs in peripheral under-perfused tissues, whose degree depends on the time until reperfusion and total blood loss. Necrosis is a chaotic cellular process leading to the leakage of cellular contents, such as potassium, into the surrounding area (Fig. 1) [8].
Fig. 1.
Pathophysiologic factors contributing to hyperkalaemia in haemorrhagic shock before transfusions. 1) Cellular level: increasing extracellular and intracellular hydrogen ions (H+) concentrations lead to extracellular hyperkalaemia through various cellular mechanisms. 2) Adrenergic vasoconstriction leads to end-organ damage, and renal underperfusion can significantly lead to lower potassium clearance. 3) Underperfused tissues due to insufficient perfusion lead to hypoxia-induced cell necrosis, increasing extracellular potassium concentrations—graph by Ariane Pessentheiner, licensed under CC BY-NC.
Hyperkalaemia has been shown to have a negative impact on the cardiovascular system, leading to life-threatening arrhythmias [9] and cardiac dysfunction [10]. Previous literature investigated the prevalence and risk factors of hyperkalaemia in military trauma patients following blood product use, finding a high prevalence of 29 % in the population studied. It underscored the importance of monitoring potassium levels in trauma patients, particularly those with initial plasma potassium levels >4.0 mmol/L and those undergoing aggressive transfusion support [11]. Also, shock index (SI), the ratio of heart rate to systolic blood pressure, has been widely used to indicate the severity of shock and prognosis in trauma patients [12]. Recent studies have suggested a possible association between the glucose/potassium ratio and shock index in trauma patients [13,14].
Empirically, hyperkalaemia can be observed in patients in extremis despite high levels of catecholamines known to shift potassium back into the cells. This may contribute to severe trauma-associated cardiac failure (STAC), a phenomenon seen by multiple services dealing with such patients prehospital [15]. In haemorrhagic shock, hyperkalaemia has been identified as a potential marker for tissue hypoxia in a swine model [16]. It was postulated that hyperkalaemia in haemorrhagic shock may significantly contribute to cardiac arrest as a consequence of STAC and may even cause it [17]. Consequently, it might be a significant contributing factor to evaluation in patients with therapy-resistant haemorrhagic shock or arrest.
Hypokalaemia in trauma has already been studied and was associated with increased mortality [18,19]. Early recognition and appropriate management of hyperkalaemia may improve outcomes and reduce mortality in this population, as indicated in a swine model [20].
The clinical significance of identifying and addressing hyperkalaemia alongside established markers such as the SI or lactate levels in traumatic haemorrhage is not fully understood but lies in its potential to offer an early, additional indicator of cellular damage and metabolic distress, especially for patients in extremis at risk of developing STAC. Unlike the SI, which reflects circulatory efficiency, or lactate, which indicates metabolic stress, hyperkalaemia can provide insights into the extent of cellular injury and membrane dysfunction. This understanding could enable clinicians to tailor interventions more precisely, potentially mitigating the cascade of complications that follow severe trauma and STAC. Finally, this retrospective study should help clinicians to comprehensively understand the potential background of developing hyperkalaemia in traumatic haemorrhage and make them aware of its possible complications.
Therefore, our primary aim was to investigate the incidence and characteristics of patients in haemorrhagic shock with hyperkalaemia (>5.0 mmol/L) on hospital arrival before transfusions. The secondary aims were 1) to associate potassium with shock index as a parameter for physiologic decompensation and 2) to assess the 24-h and 30-day mortality between the groups after admission in bleeding trauma patients to categorise the potential impact of this physiologic response and help investigate STAC further.
2. Methods
2.1. Study design, data source and setting
This study presents a retrospective cohort analysis of bleeding trauma patients admitted to the resuscitation room of a large Level 1 Trauma Centre in Europe, the University Hospital of Graz, Austria, between January 1, 2016 and December 31, 2021. This Trauma Centre participates in the German trauma registry (DGU) and treats 250 patients with an Injury Severity Score (ISS) of more than 15 per year. The ethics committee at the Medical University of Graz, Austria, approved the study (1223-2022). Due to the retrospective design and pseudonymised data, informed consent was not necessary.
We confirm that all methods were performed in accordance with the scientific guideline of good clinical practice (GCP) and the Declaration of Helsinki. To ensure the reproducibility of our results, the data used for this analysis were published at https://data.mendeley.com/datasets/2t8zv3b6k5/1.
2.2. Patient selection, data extraction and preparation
The cohort of patients with STAC is generally small and difficult to study. Therefore, we selected a patient group that we assumed near to decompensation based on resuscitation needs, like blood product use, to assess our study aims. The study size was chosen based on our institution's data availability. We aimed to include a broad spectrum of trauma patients to ensure that our findings are representative of similar settings.
Adult trauma patients (age ≥18 years) who received at least five units of packed red blood cells (PRBCs) within the first 24 h after admission and who had complete admission documentation, including blood gas analyses (BGAs), were included in the study. Patients with an isolated traumatic brain injury (TBI) were excluded. The traditional definition of massive transfusion, typically more than ten units of PRBCs within 24 h, is widely recognised in trauma care. However, this definition is evolving, with a trend towards adopting lower thresholds within shorter time frames, reflecting current clinical practices [21]. In light of this, we opted for a reduced threshold for our study, aiming to encompass a broader range of trauma patients at risk of decompensation. This approach allows for the inclusion of patients who may not meet the conventional criteria for massive transfusion but are nonetheless experiencing significant haemorrhagic shock.
2.3. Data collection and variables
The regional medical documentation and communication programme was used to collect data on performed blood gas analyses (BGAs), including pH, pO2, pCO2, K+, Ca2+, Na+, Base Excess (BE), HCO3−, Lactate, Haemoglobin (Hb), and Haematocrit (Hct). All probes were analysed by the ABL90 FLEX Plus blood gas analyser (Radiometer Medical ApS, Copenhagen, Denmark). Demographic and interventional characteristics of the patients were also collected, including age, sex, mechanism of injury, injuries, and blood products received. Outcome data were determined 24 h and 30 days post-admission.
In our study, patients were classified into three groups based on serum potassium levels: normokalaemia (3.5–5.0 mmol/L), hyperkalaemia (>5.0 mmol/L), and hypokalaemia (<3.5 mmol/L). Although our investigation covered all categories to assess the implications of potassium imbalances comprehensively, we mainly focused on the hyperkalaemia group due to the already-known consequences of hypokalaemia in trauma [18,19].
Hyperkalaemia was defined according to the local thresholds of our laboratory, which also aligns with other studies investigating the threshold for increased risk of cardiac complications due to hyperkalaemia [9]. Exposures and predictors examined included the volume of packed red blood cells (PRBCs) administered within the first 24 h post-admission and the presence of hyperkalaemia. Potential confounders identified included age, gender, mechanism of injury, and new injury severity score (NISS), each accounted for in our analytical models to mitigate their impact on the observed relationships. Effect modifiers considered were factors such as the type of injury (blunt vs. penetrating) and advanced haemorrhagic control interventions, including REBOA, which could influence the relationship between hyperkalaemia and patient outcomes.
2.4. Statistical analysis
Continuous variables, such as Injury Severity Score (ISS), age, and Na+ were analysed using the One-way Analysis of Means for data normally distributed and the Kruskal-Wallis rank sum test for those not meeting normality distribution. For categorical variables, such as sex and the mechanism of injury, Fisher's Exact test for Count Data was employed. Mortality was compared using the Fisher's Exact test. Spearman's rank correlations were utilised to correlate vital parameters with Potassium. Finally, we constructed a survival model for in-hospital mortality as the event of interest and analysed it using a Cox proportional hazards model. The model was adjusted for serum potassium levels, and four categories were used: patient age in years, patient sex, pH value, and NISS. The JASP software package performed all statistical analyses, and the statistical significance level was set at p < 0.05.
3. Results
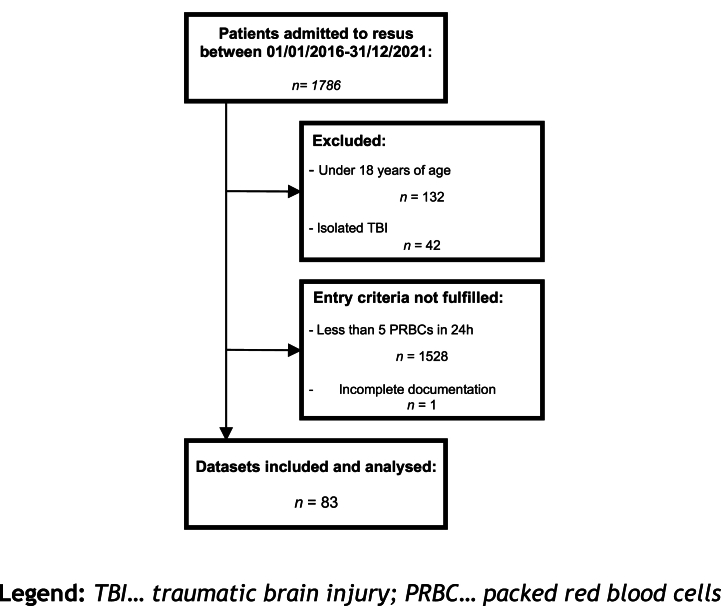
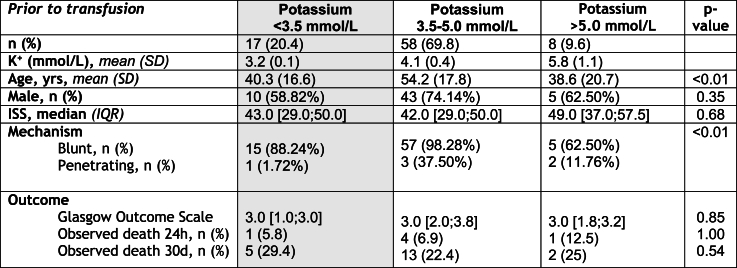
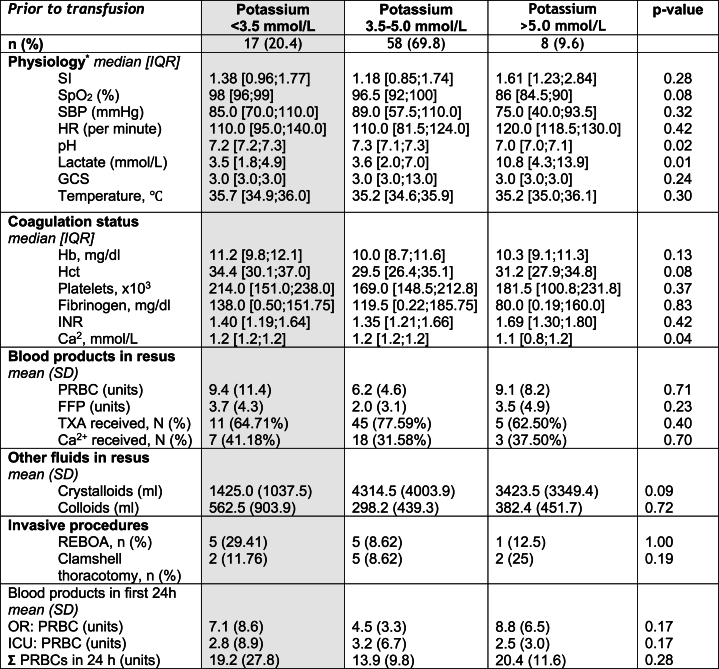
A total of 83 patients met the inclusion criteria (Fig. 2), and 8 of them (9.6 %) presented with hyperkalaemia, while the remaining 75 patients had values ≤ 5 mmol/L (Table 1).
Fig. 2.
Flowchart of patients included and analysed.
Table 1.
Descriptives and between-group comparisons.
Legend: Level of significance was set at p < 0.05; CI, confidence interval; K+, potassium; ISS, injury severity score; RTC, road traffic accident.
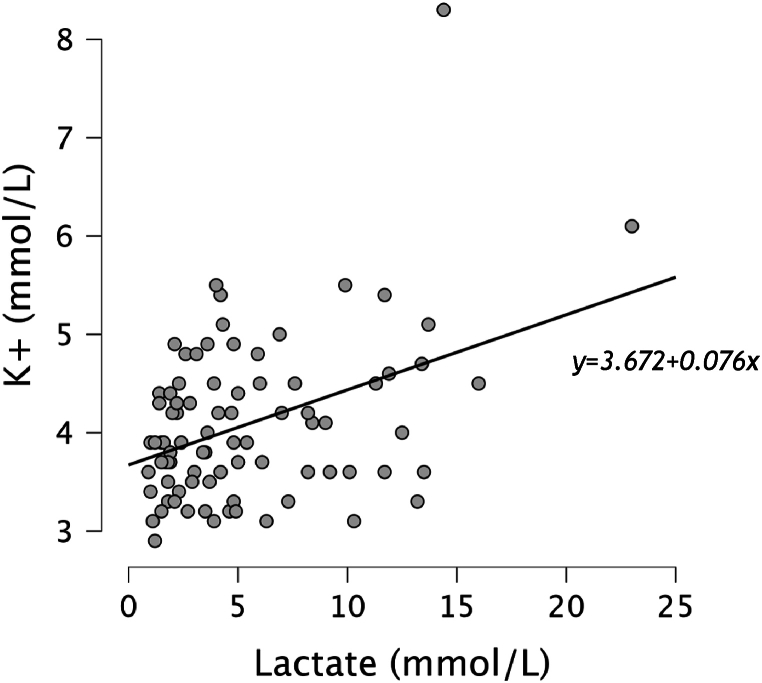
The hyperkalaemic group exhibited significantly higher lactate (p = 0.01) and lower pH levels (p = 0.02) compared to the other group, suggesting a more severe illness. Lactate and Potassium showed a weakly positive correlation (r = 0.27) (Fig. 3). The majority of injuries in both groups were due to blunt trauma, with road traffic accidents being the most common cause. However, the hyperkalaemic group had a higher proportion of penetrating injuries, primarily from stabbings and shootings, which was statistically significant (p < 0.01). The mean age was significantly higher in the not hyperkalaemic (54.2 years) compared to the hyperkalaemic group (38.6 years) with a p-value of <0.01. The gender distribution and injury severity score (ISS) was not significantly different between the groups (Table 1).
Fig. 3.
Scatter plot with a linear regression line of potassium (mmol/L) and Lactate (mmol/L) showing a weakly positive correlation between these variables (ρ = 0.27).
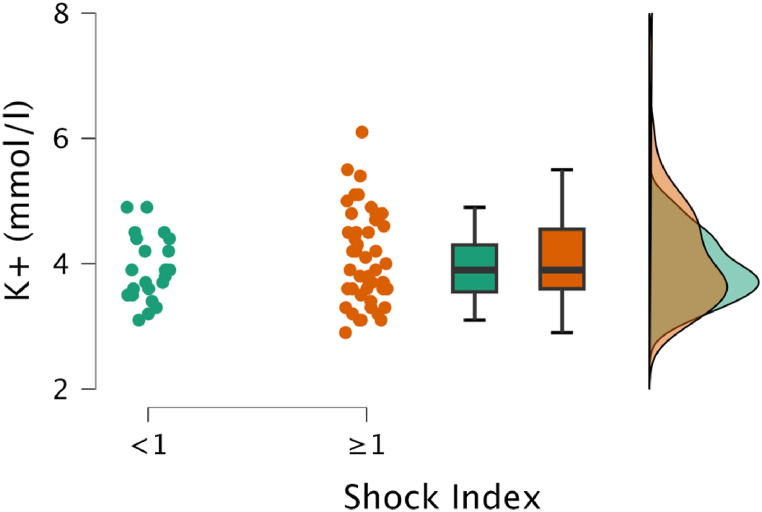
As a secondary aim, the association between hyperkalaemia and shock index (SI), a widely used clinical marker of illness severity in trauma patients, was of particular interest. The median SI value did not significantly differ between the two groups (Table 2, Fig. 4). Spearman's rank correlation analysis revealed no significant positive correlation between potassium levels and SI (r = 0.13). The use of crystalloids in resuscitation was higher in the non-hyperkalaemic group. Invasive procedures such as resuscitative endovascular balloon occlusion of the aorta (REBOA) and clamshell thoracotomy were more frequently performed in the hyperkalaemic group, but the differences were not statistically significant.
Table 2.
Patient characteristics.
Legend: parameters were measured on arrival to the hospital before the first blood transfusion was administered; Level of significance was set at p < 0.05; SI, shock index; SpO2, Oxygen saturation; SBP, Systolic blood pressure; HR, heart rate; GCS, Glasgow Coma Scale; Hb, Haemoglobin; Hct Haematocrit; INR, International normalised ratio; PRBC, packed red blood cells; FFP, fresh frozen plasma; TXA, tranexamic acid; REBOA, resuscitative endovascular balloon occlusion of the aorta; OR, operation room; ICU, intensive care unit. Crystalloids used were exclusively ‘ELO-MEL isotonic’ by Fresenius Kabi Austria GmbH, ATC code: B05BB01.
Fig. 4.
Raincloud plot shows the relationship between potassium (mmol/L) and Shock Index (SI). No significant difference between patients with high SI (≥1) and normal SI (<1) can be detected (p = 0.26).
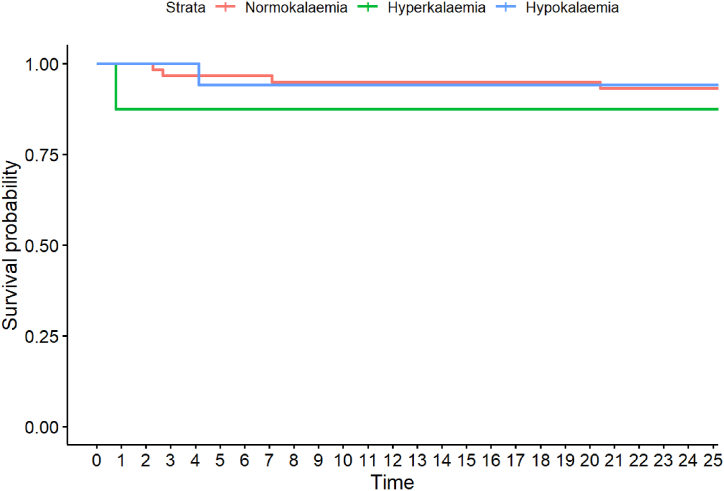
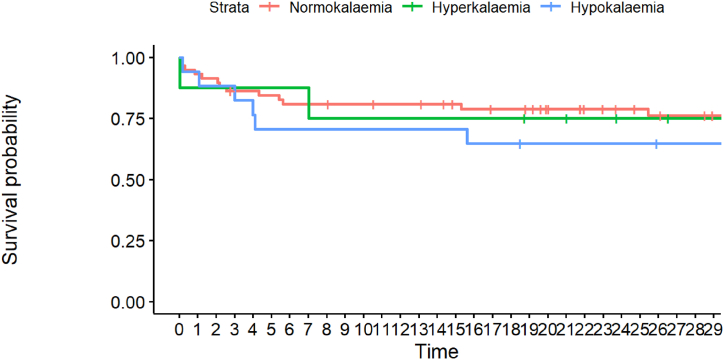
Finally, we investigated mortality rates (Table 1, Fig. 5, Fig. 6). Although the hyperkalaemic group had higher mortality rates at 24 h and 30 days post-admission, the differences did not reach statistical significance.
Fig. 5.
Kaplan-Meier curve for 24 h mortality, categorised into normo-, hyper- and hypokalaemia. Every point on x denotes 1 h.
Fig. 6.
Kaplan-Meier curve for 30d mortality, categorised into normo-, hyper- and hypokalaemia. Every point on x denotes one day.
4. Discussion
The present study suggests that hyperkalaemia on arrival before transfusions is relevant in this patient population as every tenth haemorrhaging trauma patient seems to suffer from it, according to our data. Younger age and penetrating trauma were patient characteristics significantly associated with this condition.
Haemorrhaging trauma patients develop different pathophysiologies depending on the mechanism of injury. ‘Arterial shock’ through penetrating vessel injury will have other consequences than blunt trauma after a fall from height, for example. Thus, differences in the types of injury could explain the observed dissimilarities in age and the tendency of hyperkalaemia to occur more frequently in penetrating trauma. These differences could also be attributed to decreased compensation strategies of the body to deal with the rapidly escalating situation in younger patients with penetrating trauma, potentially even after invasive procedures like REBOA.
The age differences seem paradoxical as we can assume that older patients might suffer from chronic kidney injury more often. However, this alteration could be explained by the different types of the mechanism of injury; younger patients suffer from penetrating trauma more frequently. Consequently, a more rapid evolution of a life-threatening condition leading to hyperkalaemia can be assumed.
We could not demonstrate a significant association between hyperkalaemia and SI as an indicator of the extent of tissue hypoperfusion, which might play an essential role in hyperkalaemia in these patients. In other studies, the shock index was presented as a predictor of the severity of the physiological response to haemorrhage, with values greater than 1 indicating a higher likelihood of mortality [7,8]. An animal model suggested that the extent of higher lactate and lower pH in this patient group might be unrelated to arterial acidaemia [16]. Vital parameters may not be optimal to evaluate the extent of decompensation in patients in extremis, whereas potassium offers an additional insight into a patient's metabolic state (Fig. 4). In this light, hyperkalaemia should not replace but rather complement the use of SI or lactate levels in the clinical setting, providing a more comprehensive assessment of a trauma patient's condition and guiding targeted therapeutic strategies.
Interestingly, even though the hyperkalaemic group showed higher early mortality, we did not observe significant differences in mortality between patients with and without hyperkalaemia on arrival in our cohort. This finding may be due to the relatively small sample size and the heterogeneity of the patient population. Moreover, the impact of hyperkalaemia on mortality may be influenced by the speed of correction and the underlying trauma pathophysiology. It is possible that centres with higher numbers of penetrating trauma patients in extremis could observe a significant effect and higher incidence, like in the United States or the United Kingdom.
5. Limitations
Our study has several limitations, including its retrospective design and reliance on data collected from medical records. In addition, the definition of hyperkalaemia used in this study may not be generalisable to other populations, and the impact of confounding factors, such as the extent of tissue injury and the speed of resuscitation, could not be thoroughly evaluated.
The relatively small sample size was mentioned already and could lead to overestimating the observed effects. Our data might not represent trauma patients from other areas where penetrating trauma and ‘arterial shock’ are more prevalent. However, these data represent an average of Trauma Centres in central Europe.
Due to the retrospective design, no prehospital data were included, as data quality was insufficient for scientific purposes. Therefore, this study did not consider prehospital analyses to reduce data bias. Finally, no causality can be concluded; the associations found do not necessarily mean clinical usefulness. However, attention should be drawn to this parameter, which can add a new perspective to such a complex and fragile system.
6. Conclusion
Overall, our findings indicate that hyperkalaemia is frequently present in bleeding trauma patients, especially in penetrating injury and younger age, which could contribute to STAC. Although we did not find a significant association between hyperkalaemia and shock index, our study highlights the importance of early recognition and management of hyperkalaemia in these patients. Further research is needed to investigate the impact of hyperkalaemia on mortality in bleeding and especially penetrating trauma patients.
Data availability statement
The data used for this analysis have been deposited into a publicly available repository called Mendeley Data, which can be accessed here: https://data.mendeley.com/datasets/2t8zv3b6k5/1.
Ethics statement
The Medical University of Graz, Austria's ethics committee approved the study (1223-2022). Due to the retrospective design and pseudonymised data, written informed consent was not necessary. We confirm that all methods were performed in accordance with the Declaration of Helsinki and comply with all regulations.
Consent for publication
Not applicable.
Funding
None.
Author’s information
All authors are involved in prehospital and in-hospital trauma care. ME also worked at a MTC in the United Kingdom where penetrating injuries occur more often. This led to the development of this study to evaluate the realistic situation at other MTCs less often confronted with this type of injury but highlight the potential harm by hyperkalaemia in bleeding trauma patients.
CRediT authorship contribution statement
Michael Eichinger: Writing – review & editing, Writing – original draft, Visualization, Validation, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Martin Rief: Writing – review & editing, Supervision, Methodology, Formal analysis, Conceptualization. Michael Eichlseder: Writing – review & editing, Validation, Supervision, Formal analysis, Data curation. Alexander Pichler: Writing – review & editing, Validation, Supervision, Data curation. Philipp Zoidl: Writing – review & editing, Validation, Supervision, Project administration. Barbara Hallmann: Writing – review & editing, Validation, Supervision. Paul Zajic: Writing – review & editing, Validation, Supervision.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work, the authors used ChatGPT to check the grammar of English. After using this tool/service, the authors reviewed and edited the content as needed and took full responsibility for the content of the publication.
Declaration of competing interest
ME, MR, ME2, AP, PZ, HB and PZ1 report no conflicts of interest.
Acknowledgements
We would like to name Anna Loeschnig MD, who participated in the study and contributed to the data generation.
References
- 1.Evans J.A., Van Wessem K.J.P., McDougall D., Lee K.A., Lyons T., Balogh Z.J. Epidemiology of traumatic deaths: comprehensive population-based assessment. World J. Surg. 2010;34(1):158–163. doi: 10.1007/s00268-009-0266-1. [DOI] [PubMed] [Google Scholar]
- 2.Jansen J.O., Hudson J., Cochran C., Maclennan G., Lendrum R., Sadek S., et al. Emergency department resuscitative endovascular balloon occlusion of the aorta in trauma patients with exsanguinating hemorrhage: the UK-REBOA randomized clinical trial. JAMA. 2023;330(19) doi: 10.1001/jama.2023.20850. https://pubmed.ncbi.nlm.nih.gov/37824132/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braverman M.A., Smith A., Pokorny D., Axtman B., Shahan C.P., Barry L., et al. Prehospital whole blood reduces early mortality in patients with hemorrhagic shock. Transfusion (Paris) 2021;61(S1) doi: 10.1111/trf.16528. https://onlinelibrary.wiley.com/doi/full/10.1111/trf.16528 [DOI] [PubMed] [Google Scholar]
- 4.Castellini G., Gianola S., Biffi A., Porcu G., Fabbri A., Ruggieri M.P., et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA) in patients with major trauma and uncontrolled haemorrhagic shock: a systematic review with meta-analysis. World J. Emerg. Surg. 2021;16(1):1–12. doi: 10.1186/s13017-021-00386-9. https://link.springer.com/articles/10.1186/s13017-021-00386-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon J.W., Shock Hemorrhagic, Longo D.L., editors. vol. 378. 2018. pp. 370–379. (New England Journal of Medicine). [Google Scholar]
- 6.Chan J.K., Roth J., Oppenheim J.J., Tracey K.J., Vogl T., Feldmann M., et al. Science in medicine Alarmins : awaiting a clinical response. J. Clin. Invest. 2012;122(8):2711–2719. doi: 10.1172/JCI62423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore E.E., Moore H.B., Kornblith L.Z., Neal M.D., Hoffman M., Mutch N.J., et al. Trauma-induced coagulopathy. Nat. Rev. Dis. Primers [Internet] 2021;7(1):30. doi: 10.1038/s41572-021-00264-3. https://www.nature.com/articles/s41572-021-00264-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenihan C.R., Taylor C.T. The impact of hypoxia on cell death pathways. Biochem. Soc. Trans. 2013;41(2):657–663. doi: 10.1042/BST20120345. [DOI] [PubMed] [Google Scholar]
- 9.McCullough P.A., Beaver T.M., Bennett-Guerrero E., Emmett M., Fonarow G.C., Goyal A., et al. Acute and chronic cardiovascular effects of hyperkalemia: new insights into prevention and clinical management. Rev. Cardiovasc. Med. 2014;15:11–23. MedReviews LLC. [PubMed] [Google Scholar]
- 10.Simpson R., Praditsuktavorn B., Wall J., Morales V., Thiemermann C., Tremoleda J.L., et al. Myocardial alterations following traumatic hemorrhagic injury. J. Trauma Acute Care Surg. 2023;95(4):481–489. doi: 10.1097/TA.0000000000003987. [DOI] [PubMed] [Google Scholar]
- 11.Perkins R.M., Aboudara M.C., Abbott K.C., Holcomb J.B. Resuscitative hyperkalemia in noncrush trauma: a prospective, observational study. Clin. J. Am. Soc. Nephrol. 2007;2(2):313–319. doi: 10.2215/CJN.03070906. [DOI] [PubMed] [Google Scholar]
- 12.Jehan F., Con J., McIntyre M., Khan M., Azim A., Prabhakaran K., et al. Pre-hospital shock index correlates with transfusion, resource utilization and mortality; the role of patient first vitals. Am. J. Surg. 2019;218(6):1169–1174. doi: 10.1016/j.amjsurg.2019.08.028. [DOI] [PubMed] [Google Scholar]
- 13.Turan E. Turkish Journal of Trauma and Emergency Surgery; 2022. Role of Glucose/Potassium Ratio and Shock Index in Predicting Mortality in Patients with Isolated Thoracoabdominal Blunt Trauma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torabi M., Moeinaddini S., Mirafzal A., Rastegari A., Sadeghkhani N. Shock index, modified shock index, and age shock index for prediction of mortality in Emergency Severity Index level 3. Am. J. Emerg. Med. 2016 Nov;34(11):2079–2083. doi: 10.1016/j.ajem.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Dietrich M., Weilbacher F., Katzenschlager S., Weigand M.A., Popp E. Severe trauma associated cardiac failure. Scand. J. Trauma Resuscitation Emerg. Med. 2024;32(1):1–3. doi: 10.1186/s13049-024-01175-4. https://link.springer.com/articles/10.1186/s13049-024-01175-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocha Filho J.A., Nani R.S., D'Albuquerque L.A.C., Malbouisson L.M.S., Carmona M.J.C., Rocha-e-Silva M., et al. Potassium in hemorrhagic shock: a potential marker of tissue hypoxia. J. Trauma. 2010 Jun;68(6):1335–1341. doi: 10.1097/TA.0b013e3181dbbb36. https://journals.lww.com/00005373-201006000-00010 Available from: [DOI] [PubMed] [Google Scholar]
- 17.Rocha Filho J.A., Nani R.S., D'Albuquerque L.A.C., Holms C.A., Rocha J.P.S., Malbouisson L.M.S., et al. Hyperkalemia accompanies hemorrhagic shock and correlates with mortality. Clinics. 2009;64(6):591–597. doi: 10.1590/S1807-59322009000600016. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1807-59322009000600016&lng=en&nrm=iso&tlng=en [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlögl M., Käch I., Beeler P.E., Pape H.C., Neuhaus V. Trauma patients with hypokalemia have an increased risk of morbidity and mortality. Surg. Pract. Sci. 2021 Dec 1;7 [Google Scholar]
- 19.Ookuma T., Miyasho K., Kashitani N., Beika N., Ishibashi N., Yamashita T., et al. The clinical relevance of plasma potassium abnormalities on admission in trauma patients: a retrospective observational study. J. Intensive Care. 2015;3(1):1–7. doi: 10.1186/s40560-015-0103-6. https://jintensivecare.biomedcentral.com/articles/10.1186/s40560-015-0103-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith S., Behrens B., McCully B., Murphy J., Bommiasamy A., Goodman A., et al. Aggressive treatment of acute kidney injury and hyperkalemia improves survival in a combat relevant trauma model in swine. Am. J. Surg. 2020;219(5):860–864. doi: 10.1016/j.amjsurg.2020.02.058. https://pubmed.ncbi.nlm.nih.gov/32245610/ [DOI] [PubMed] [Google Scholar]
- 21.Lin V.S., Sun E., Yau S., Abeyakoon C., Seamer G., Bhopal S., et al. Definitions of massive transfusion in adults with critical bleeding: a systematic review. Crit. Care. 2023;27(1):1–12. doi: 10.1186/s13054-023-04537-z. https://ccforum.biomedcentral.com/articles/10.1186/s13054-023-04537-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for this analysis have been deposited into a publicly available repository called Mendeley Data, which can be accessed here: https://data.mendeley.com/datasets/2t8zv3b6k5/1.