Abstract
Introduction
Posttransplant thrombotic microangiopathy (PT-TMA) is an uncommon event that characterizes approximately 3% to 14% of kidney transplants (KTs), and that is associated with a higher risk of delayed graft function and graft loss. PT-TMA occurs more frequently within the first 3 months after transplant and can be a manifestation of de novo disease or the recurrence of previous atypical hemolytic uremic syndrome (aHUS). Abnormalities in complement regulation genes could explain the increased susceptibility of some patients to PT-TMA. Eculizumab is a humanized monoclonal antibody that inhibits the formation of the membrane attack complex C5b-9. The aim of this study is to evaluate the efficacy of eculizumab as treatment for PT-TMA.
Methods
We retrospectively analyzed clinical records of 45 KT patients who received eculizumab immediately after the clinical diagnosis of PT-TMA.
Results
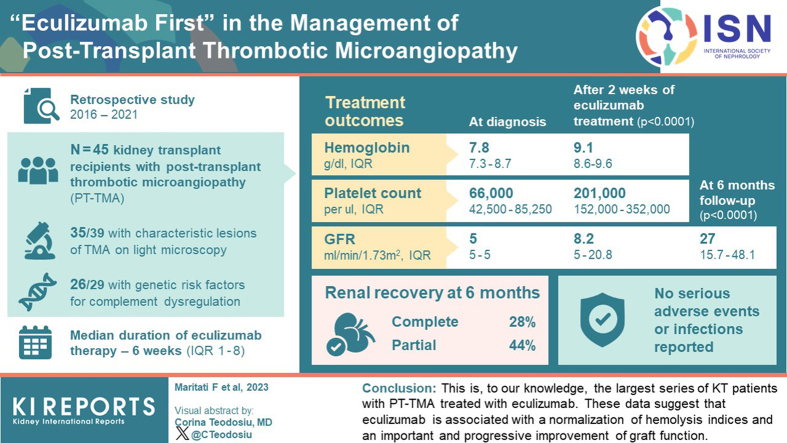
Kidney biopsy was performed in 91.1% of patients, and complement genetic study was performed in 64.4%. Of the kidney biopsies, 85.4% showed signs of TMA; genetic analysis revealed 1 pathogenetic variant, 2 variants of uncertain significance, 1 likely benign variant, 8 risk polymorphisms, and 27 risk haplotypes. After 2 weeks from the treatment starting, hemoglobin and platelets significantly increased. A remarkable improvement in kidney function was also observed. After 6 months, 28.8% of patients had a complete renal recovery whereas 44.4% had a partial recovery.
Conclusion
This is, to our knowledge, the largest series of KT patients with PT-TMA treated with eculizumab. These data suggest that eculizumab is associated with a normalization of hemolysis indices and an important and progressive improvement of graft function.
Keywords: thrombotic microangiopathy, atypical hemolytic uremic syndrome, kidney transplant, eculizumab
Graphical abstract
PT-TMA is a rare condition associated with delayed graft function, which can lead to a severe worsening of graft and patient’s prognosis. It is detected in 3% to 14% of KTs and can manifest as a systemic or localized form. Systemic PT-TMA is characterized by the onset of hemolytic anemia (i.e., reduction in hemoglobin levels, increase in lactate dehydrogenase [LDH] levels and presence of schistocytes in peripheral blood smear), thrombocytopenia, and ischemic organ damage, whereas the localized form is mainly confined to the graft. PT-TMA can develop at any time after transplant, but it is more frequent in the first 3 months along with the presence of complement activating triggers.1 It can occur as a de novo disease, related to ischemia-reperfusion injury, immunosuppressive drugs, antibody-related rejection or infections (i.e., Cytomegalovirus disease), or as recurrence after KT in patients with a previous aHUS, being the transplant itself a trigger of the pathogenetic mechanisms of disease.2,3 It is generally accepted that a significant number of patients with secondary TMA, actually have a misdiagnosed aHUS, as cause of primary end-stage renal disease (ESRD). However, because of the rarity of aHUS, many authors affirm that the majority of PT-TMA should be considered as a de novo disease.4, 5, 6 The incidence of graft loss in the de novo PT-TMA ranges from 30% to 60% over a 3-year period. The presence of complement regulatory genes abnormalities could explain why only a small percentage of KT patients develop a PT-TMA.2
Eculizumab is a humanized monoclonal antibody that prevents the cleavage of the C5 molecule, inhibiting the formation of the membrane attack complex C5b-9. Many observational studies have reported its efficacy in the treatment and prevention of recurrent aHUS after transplantation.7, 8, 9, 10, 11, 12 Conversely, few data are available on the use of eculizumab for treating PT-TMA.13,14
Here, we describe clinical characteristics, short-term and long-term outcomes of a large series of patients who developed PT-TMA and were early treated with eculizumab.
Methods
Patients and Treatment
We retrospectively reviewed clinical records of all patients with a clinical diagnosis of PT-TMA performed at our Center (Sant’Orsola University Hospital, Bologna) from June 2016 to June 2021. To the aim of this project, we included KT patients with the following criteria: (i) low platelet count (less than 150 × 103 /ul) or a decrease of >25% from baseline values; (ii) microangiopathic hemolytic anemia (low hemoglobin, high LDH levels, decreased serum haptoglobin, and evidence of schistocytes in peripheral blood examination); (iii) delayed graft function or deterioration of kidney function, fulfilling the criteria of acute kidney injury; (iv) negative Coombs test; and (v) normal ADAMST-13 activity. Exclusion criteria were as follows: (i) patients with any other type of solid organ transplant and (ii) patients with a known diagnosis of aHUS or C3 glomerulopathies in the native kidneys.
All patients received eculizumab according to the label instructions. Particularly, eculizumab administration schedule includes 4 weekly intravenous doses of 900 mg and then 1200 mg every 2 weeks. Antimeningococcal vaccination and antibiotic prophylaxis were administered at the time of the first eculizumab dose. The number of administrations and the duration of the treatment were determined by the treating physician based on patient’s characteristics and disease’s response. Hematological complete remission was defined as a normalization of platelet counts and hemoglobin values, with the disappearance of the hemolysis markers. Renal response was considered in case of recovery of graft function and was defined as: (i) partial response (estimated glomerular filtration rate [eGFR] between 15 and 45 ml/min per 1.75 m2) and (ii) complete response (eGFR >45 ml/min per 1.75 m2).
The study was performed in accordance with the Declaration of Helsinki and the protocol was approved by the ethical committee of the Sant’Orsola University Hospital of Bologna (Protocol number 832/2022/Sper/AOUBo). Patients provided informed consent for study participation.
Data Collection
We retrieved data about patient demographics and clinical history, including the etiology of chronic kidney disease, the characteristics of KT and the time from KT to PT-TMA. Laboratory tests at the time of PT-TMA onset and during the follow-up, including platelet counts, hemoglobin levels, schistocytes, LDH values, serum creatinine and eGFR were collected. We also recorded the treatments received before eculizumab, the duration of eculizumab therapy and any side effects.
Kidney Biopsies
When possible, kidney biopsy was performed. For histological evaluation, kidney biopsies were fixed in Serra fluid, paraffin-embedded, and stained with periodic acid-Schiff reagent, Masson trichrome, and acid fuchsine orange G. Immunohistochemistry for C4d was always performed for the evaluation of antibody-mediated rejection (ABMR). When enough tissue was available, immunofluorescence and electron microscopy were also performed. TMA was recognized for the presence of glomerular and arteriolar microthrombi, fibrin in the glomeruli, thickening and double contours of the glomerular basement membrane, fibrinoid necrosis of the glomeruli, and arteriolar endothelial swelling. With electron microscopy, TMA signs were the subendothelial widening by flocculent material and the endothelial cell swelling.1,15
Genetic Analysis and Complement Activation Test
Once a PT-TMA was diagnosed, the genetic study of complement was performed in order to identify patients with genetic predisposition. Genetic analysis was performed using a next-generation sequencing platform, including the coding regions together with the 2 intronic bases as well as the exons of the genes: CFH, MCP, CFI, C3, CFB, THBD, DGKE, C5, CFHR1, CFHR2, CFHR3, CFHR4, CFHR5, MMACHC, and ADAMST13.16
Alternative assays including an in vitro test of complement activation on human microvascular endothelial cell and the dosage of plasmatic levels of sc5b9 (Enzyme-linked immunosorbent assay test) were also assessed before starting eculizumab treatment.17
Statistical Analyses
Continuous variables were expressed as mean and SD or median and interquartile range (IQR), according to variable distribution. Categorical variables were depicted as number (n) and percentage (%). Mean change of continuous variables across different time points (baseline, eculizumab starting, month 1, month 3, and last follow-up visit) were assessed using Friedman’s test. P values were corrected using Dunn’s multiple comparison test. Multiple comparison tests were shown if statistically significant. P values <0.05 were considered statistically significant. Statistical analysis was performed using STATA version 16 and GraphPad Prism 5.
Results
Patients and Graft Characteristics
From June 2016 to June 2021, we performed a total of 579 KTs at our kidney transplant unit. Among them, 45 patients (7.8%) received a diagnosis of PT-TMA and were included in the study. The main demographic and clinical characteristics of the study population are summarized in Table 1. Of the 45 patients included, 24 (53.3%) were men; median age at KT was 59 years (IQR: 53–66). The most frequent etiologies of the ESRD were the autosomal dominant polycystic disease (7 patients, 15.6%) and vascular nephropathy (7 patients, 15.6%). In 12 patients (26.7%) the etiology of kidney disease was not known. However, none of these patients had a clinical history consistent with aHUS, and in particular, 6 of them had undergone a late kidney biopsy that showed sclerotic not diagnostic lesions, but no signs of TMA were found. The median value of panel-reactive antibody was 18% (IQR: 0–78). All patients received a deceased donor kidney; among them, 4 (8.9%) received the transplant from a donor after circulatory death (DCD) Maastricht III, and 31 (68.9%) from an extended criteria donor. Twenty-one patients (46.7%) underwent a dual kidney transplant. The median kidney donor profile index was 85 (IQR: 65–98) and the median cold ischemia time was of 12.3 hours (IQR: 10.6–15.6). The majority of patients received induction therapy with basiliximab (27 patients, 60%) and all of them a standard immunosuppressive treatment, based on prednisone, tacrolimus, and mycophenolate mofetil. Thirty-six patients (80%) experienced a delayed graft function.
Table 1.
Main characteristics of the 45 patients at the initiation of eculizumab treatment
| Male sex, n (%) | 24 (53.3) |
| Age, median (IQR) yrs | 59 (53–66) |
| CKD etiology, n (%) | |
| ADPKD | 7 (15.6) |
| FSGS | 3 (7.7) |
| IgA Nephropathy | 1 (2.2) |
| Urological problems | 6 (13.3) |
| Vascular nephropathy | 7 (15.6) |
| Other | 9 (20.0) |
| Unknowna | 12 (26.7) |
| Panel-reactive antibody, median (IQR) | 18 (0–78) |
| Kidney transplant characteristics | |
| Deceased kidney transplant, n (%) | 45 (100) |
| Donor after circulatory death, n (%) | 4 (8.9) |
| Extended criteria donor, n (%) | 31 (68.9) |
| Dual kidney transplant, n (%) | 21 (46.7) |
| KDPI, median (IQR) | 85 (65–98) |
| Cold ischemia time, hours, median (IQR) | 12.3 (10.6–15.6) |
| Induction therapy | |
| Basiliximab, n (%) | 27 (60.0) |
| Thymoglobulin, n (%) | 18 (40.0) |
| Immunosuppressive therapy | |
| Tacrolimus, n (%) | 45 (100) |
| Mycophenolate mofetil, n (%) | 45 (100) |
| Prednisone, n (%) | 45 (100) |
| Delayed graft function, n (%) | 36 (80.0) |
ADPKD, autosomal dominant polycystic disease; FSGS, focal segmental glomerulosclerosis; IQR, interquartile range; KDPI, kidney donor profile index.
Except where indicated otherwise, values are the number (%).
“unknown etiology” includes those patients in which the origin of kidney disease could not be completely defined. In this group, 6 patients had undergone a nondiagnostic kidney biopsy (because of late referral), which showed chronic lesions, without clear signs of TMA. Overall, no patient had a clinical or laboratory history consistent with aHUS.
TMA Characteristics
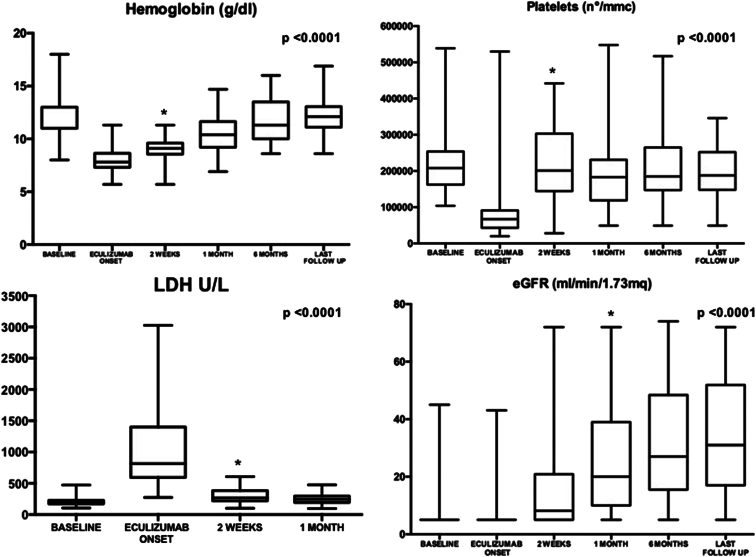
The clinical diagnosis of PT-TMA occurred at a median time of 3 days from KT (IQR: 2–4). At that time, 39 patients (86.7%) were still dialysis-dependent and eGFR was not different from that at the time of KT (baseline), with a median of 5 ml/min per 1.73 m2 (IQR: 5–5, P = 1.00) (Figure 1). Compared with baseline, we observed an abrupt and significant reduction in hemoglobin and platelets values at PT-TMA diagnosis. Hemoglobin was significantly lower (7.8 g/dl; IQR: 7.3–8.7) as compared to baseline (11.4 g/dl; IQR: 10.8–12.7; P<0.001) and platelet count was decreased (66,000/ul; IQR: 42,500–85,250 vs. 208,000/ul at baseline; IQR: 163,000–253,000; P < 0.001). LDH values rapidly raised, with a median of 815 U/l (IQR: 618–1391) versus 199 at baseline (IQR 172–223; P < 0.001) (Figure 1). Once hemolysis was suspected, we performed haptoglobin test, which resulted often below the detection level (median 30 mg/dl; IQR: 30–30).
Figure 1.
Changes in the distribution of hemoglobin, platelets, LDH, and eGFR values in patients treated with eculizumab after diagnosis of PT-TMA. Compared to baseline (day of transplant), the diagnosis of PT-TMA was associated with a worsening of hemoglobin, platelets and LDH values, whereas the eGFR value did not undergo significant changes with a median value of 5 ml/min per 1.73 m2. The asterisks indicate the time point at which the difference became statistically significant. eGFR, estimated glomerular filtration rate; LDH, lactate dehydrogenase; PT-TMA, posttransplant thrombotic microangiopathy.
At the diagnosis of TMA, the median tacrolimus trough levels was 9.4 ng/ml (IQR: 6.7–13.6).
Kidney Biopsies
Renal biopsies were performed in 41 patients (91.1%) (Table 2), at a median time of 8 days (IQR: 6–11) from the diagnosis of TMA. Two biopsies were not diagnostic due to inadequate samples (4.8%). Light microscopy analysis was performed in all the remaining biopsies. In 18 patients (43.9%), samples were available also for immunofluorescence; and in 20 patients (48.7%) electron microscopy was performed. With light microscopy, characteristic lesions of TMA were found in 35 patients (85.4%). In 22 patients with TMA these lesions were associated with acute tubular necrosis (53.6%); and in 4 patients (9.7%), the lesions were associated with signs of cortical necrosis. Four patients with TMA had lesions of rejection. In 1 patient (2.4%), an acute antibody-mediated rejection was found, with signs of microvascular inflammation and positivity of C4d staining. In the other 3 patients was diagnosed an acute cellular rejection. In 4 patients, kidney biopsy did not show TMA findings (9.7%). In 2 of them, light microscopy showed an acute humoral rejection; in the other 4, there were signs of tubular necrosis. However, we could not exclude the diagnosis of TMA because clinical and laboratory data were strongly consistent with PT-TMA whereas differential diagnosis (i.e., thrombotic thrombocytopenic purpura) were excluded.
Table 2.
Key findings of renal biopsy performed on 91.1% of patients at a median time of 8 days after PT-TMA diagnosis
| Kidney biopsy, n (%) | 41/45 (91.1) |
| Time from TMA to kidney biopsy, days, median (IQR) | 8 (6–11) |
| Inadequate sample, n (%) | 2/41 (4.8) |
| Light microscopy, n (%) | 39/41 (95.1) |
| TMA | 35 (85.4) |
| ABMR | 3 (7.3) |
| TCMR | 3 (7.3) |
| ATN | 24 (58.5) |
| Cortical necrosis | 4 (9.7) |
| Combined lesions at light microscopy, n (%) | 25 (60.9) |
| TMA+ABMR | 1 (2.4) |
| TMA+TCMR | 3 (7.3) |
| TMA+ATN | 22 (53.6) |
| TMA+ cortical necrosis | 4 (9.7) |
| Immunofluorescence, n (%) | 18/41 (43.9) |
| IgM, C3 and C1q vascular deposition | 2 (11.1) |
| C3 glomerular deposition | 2 (11.1) |
| Fibrinogen vascular deposition | 2 (11.1) |
| Negative | 12 (66.6) |
| Electron microscopy, n (%) | 20/41 (48.7) |
| TMA | 13 (65.0) |
| ABMR | 2 (10.0) |
| Negative | 5 (25.0) |
ABMR, antibody-mediated rejection; ATN, acute tubular necrosis; PT, posttransplant; TCMR, T cell mediated rejection, TMA, thrombotic microangiopathy.
Light microscopy analysis is available for 95.1% of the performed biopsies. Immunofluorescence and electron microscopy analysis were respectively available for 43.9% and 51.3% of patients.
Immunofluorescence was available in 18 patients (43.9%) and was negative in 13 of them. In 2 patients, immunofluorescence was positive for IgM, C3, and C1q vascular deposition; and in two others, it was positive for C3 glomerular deposition.
Finally, 20 patients (48.7%) had electron microscopy. In 15 of them, the diagnosis performed at light microscopy was confirmed; whereas in 5 patients, no significant lesions were found.
Genetic Analysis and Complement Activation Tests
After the clinical diagnosis of TMA, genetic analysis for complement mutations was performed in 29 patients (64.4%) at Mario Negri Research Institute. Of these patients, 26 (89.6%) carried 1 or more genetic risk factor for the dysfunction of complement pathway. In particular, we found 4 rare variants (MAD <0.001): 1 likely pathogenic variant in CFI encoding complement factor I; 2 variants of uncertain significance (VUS) encoding the membrane cofactor protein and ADAMTS13, respectively; and 1 likely benign variant in CFHR5. All rare variants were heterozygous (Table 3), including the ADAMTS13 VUS, which together with ADAMT13 activity >10% excluded a TTP. Twenty-two patients carried at least 1 risk haplotype (CFH-H3, the MCP C.∗897 T>C that tags the CD46ggaac haplotype and the CFHR1B) (Table 3). The most common genetic risk factor was the haplotype MCP C.∗897 T>C (rs7144), which was found in 16 (64%) patients. Finally, in 5 patients, we found the p.R102G polymorphism in C3.
Table 3.
Complement genetic risk factors found in 26 of the 29 patients (86.2%) tested at Mario Negri Research Institute
| Pt | Complement protein | Genetic risk factor | Coding | Inheritance | MAF | In silico prediction | Kidney allograft outcome at 6 months fu |
|---|---|---|---|---|---|---|---|
| 1 | CFI CFH |
Rare variant Polymorphism |
p.M1V c.−332C>T (rs3753394) |
Heterozygous Heterozygous |
1x10-5 | Likely pathogenic variant | CR |
| 2 | MCP | Rare variant | C.424G>C, p.(E142Q) | Heterozygous | 4.6x10-5 | Variant of unknown significance | CR |
| 3 | MCP ADAMTS13 |
Risk haplotype Rare variant |
C.∗897 T>C (rs7144) p.D187H |
Heterozygous Heterozygous |
4x10-4 | Variant of unknown significance | CR |
| 4 | CFHR5 | Rare variant | p.G101E | Heterozygous | 1x10-4 | Likely benign variant | PNF |
| 5 | MCP | Risk haplotype | C.∗897 T>C (rs7144) | Heterozygous | PR | ||
| 6 | CFH | Risk haplotype | CFH-H3 | Heterozygous | PR | ||
| 7 | MCP C3 |
Risk haplotype Polymorphism |
C.∗897 T>C (rs7144) p.R102G |
Heterozygous Heterozygous |
PR | ||
| 8 | MCP | Risk haplotype | C.∗897 T>C (rs7144) | Heterozygous | PR | ||
| 9 | CFH C3 |
Risk haplotype Polymorphism |
CFH-H3 p.R102G |
Heterozygous Heterozygous |
PR | ||
| 10 | MCP CFHR1 |
Risk haplotype Risk haplotype |
C.∗897 T>C (rs7144) CFHR1B |
Heterozygous Homozygous |
CR | ||
| 11 | MCP | Risk haplotype | C.∗897 T>C (rs7144) | Heterozygous | PR | ||
| 12 | MCP | Risk haplotype | C.∗897 T>C (rs7144) | Heterozygous | PR | ||
| 13 | CFH MCP |
Risk haplotype Risk haplotype |
CFH-H3 C.∗897 T>C (rs7144) |
Heterozygous Heterozygous |
PNF | ||
| 14 | MCP C3 |
Risk haplotype Polymorphism |
C.∗897 T>C (rs7144) p.R102G |
Heterozygous Homozygous |
PR | ||
| 15 | CFH MCP C3 |
Polymorphism Risk haplotype Polymorphism |
c.-332C>T (rs3753394) C.∗897 T>C (rs7144) p.R102G |
Heterozygous Homozygous Heterozygous |
Patient deceased | ||
| 16 | CFH CFHR1 |
Risk haplotype Risk haplotype |
CFH-H3 CFHR1B |
Heterozygous Homozygous |
PR | ||
| 17 | MCP | Risk haplotype | C.∗897 T>C (rs7144) | Heterozygous | CR | ||
| 18 | CFH MCP |
Risk haplotype Risk haplotype |
CFH-H3 C.∗897 T>C (rs7144) |
Heterozygous Homozygous |
PNF | ||
| 19 | CFH | Risk haplotype | CFH-H3 | Heterozygous | CR | ||
| 20 | MCP | Risk haplotype | C.∗897 T>C (rs7144) | Heterozygous | PR | ||
| 21 | MCP | Risk haplotype | C.∗897 T>C (rs7144) | Heterozygous | PR | ||
| 22 | MCP CFHR3/R1 |
Risk haplotype Deletion |
C.∗897 T>C (rs7144) | Heterozygous Homozygous |
PNF | ||
| 23 | CFH CFHR1 |
Risk haplotype Risk haplotype |
CFH-H3 CFHR1B |
Heterozygous Homozygous |
CR | ||
| 24 | C3 | Polymorphism | p.R102G | Heterozygous | CR | ||
| 25 | MCP CFH |
Risk haplotype Polymorphism |
C.∗897 T>C (rs7144) c.−332C>T (rs3753394) |
Heterozygous Heterozygous |
PR | ||
| 26 | CFH | Risk haplotype | CFH-H3 | Heterozygous | PR |
CFH, complement factor H; CFHR1, Factor H- related protein 1; CFI, complement factor I; CR, complete response; MAF, minor allele frequency; MCP, membrane co-factor protein; PNF, primary nonfunction; PR, partial response; Pt, patient.
Partial response is defined as eGFR between 15 and 45 ml/min per 1.73 m2, and complete response is defined as eGFR >45 ml/min per 1.73 m2.
Thirteen patients had a combination of complement genetic risk factors. The patient with the CFI likely pathogenetic variant also carried a single-nucleotide variation (SNV, formerly SNP) in CFH. The MCP risk haplotype was associated with a CFH risk haplotype in four patients. The SNV in C3 was also found in patients with MCP or CFH risk haplotypes.
The in vitro test of complement activation on human microvascular endothelial cell-1 was available in 25 patients. It revealed elevated C5b-9 deposits both on unstimulated and activated endothelium in almost the totality of patients (84% and 92%, respectively); and this data was consistent with the diagnosis of active TMA. At variance plasma levels of C5b-9 were high only in 5 patients (17.2%). (Supplementary Table S1)
Treatment and Safety
After the diagnosis of TMA, eculizumab was immediately started (Table 4). In 41 patients, this drug was discontinued after resolution, with a median duration of therapy of 6 (IQR: 1–8) weeks and a median of 4 (IQR: 1–6) doses. In 4 patients, the treating physicians decided to maintain eculizumab longer after TMA resolution due to disease severity. In particular, in 2 patients this decision was made on the basis of histology (one patient had TMA associated with ABMR and another one had cortical necrosis) whereas in the other 2, this decision was made on the basis of a slower laboratory remission. Eculizumab therapy was well-tolerated. No serious adverse events or infections related to this treatment in these patients were observed.
Table 4.
Time from transplantation to TMA diagnosis and eculizumab initiation and management of the PT-TMA in terms of eculizumab doses and duration and tacrolimus reduction
| Time from KT to TMA, days, median (IQR) | 3 (2–4) |
| Time from KT to eculizumab | 3 (2–4) |
| Eculizumab duration, weeks, median (IQR) | 6 (1–8) |
| Eculizumab n° of doses, median (IQR) | 4 (1–6) |
| Eculizumab discontinuation, no. of patients (%) | 41 (91.1) |
| Tacrolimus, trough levels reduction >25%, n (%) | 28 (62.2) |
| Tacrolimus, trough levels before TMA diagnosis, median (IQR) | 9.4 (6.7–13.6) |
| Tacrolimus, trough levels 1 week after TMA diagnosis, median (IQR) | 6.4 (4.3–7.8) |
IQR, interquartile range; KT, kidney transplant; PT, posttransplant; TMA, thrombotic microangiopathy.
In the majority of patients, the treatment with the complement inhibitor was associated with the reduction in tacrolimus doses. In particular, a reduction of tacrolimus doses >25% was achieved in 62.2% of patients, with a significant reduction in the median tacrolimus trough levels than that at the diagnosis of TMA (median 6.4 ng/ml; IQR: 4.3–7.8; P < 0.001) (Table 4). However, in this series, no patient completely discontinued the calcineurin inhibitor (CNI). Patients with ABMR associated with TMA were treated in addition, with plasma exchange (PE) and rituximab; those with TCMR received 3 consecutive bolus of methylprednisolone 500 mg.
Outcome
After 2 weeks since the start of PT-TMA treatment, hemoglobin values and platelets significantly increased (to 9.1 g/dl; IQR: 8.6–9.6; P < 0.0001 and to 201,000/ul, IQR: 152,000–352,000; P < 0.0001, respectively). Hemolysis indices, including LDH and haptoglobin also improved. LDH values significantly decreased (median 265 U/l; IQR: 220–378; P < 0.0001) and haptoglobin was increased (median 103; IQR: 69–155; P < 0.0001). One month after eculizumab, 30 patients (66.6%) had obtained a full hematologic response with platelets, hemoglobin, and LDH normalization. During the follow-up, which lasts for a median of 13.8 months (IQR: 6.8–35.9), hematological parameters further ameliorated or remained within the normal ranges (Figure 1).
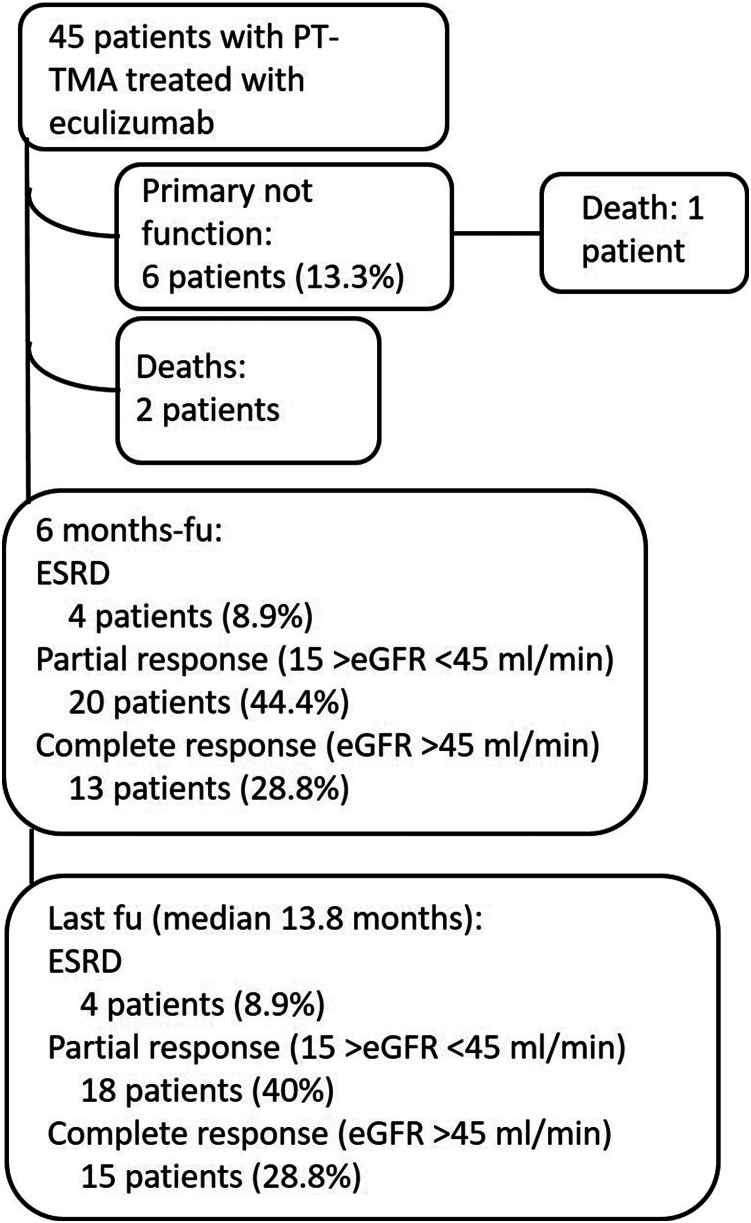
Renal function improvement also was remarkable (Figure 1). After 2 weeks from the starting of treatment, the eGFR significantly improved (8.2 ml/min per 1.73 m2; IQR: 5–20.8; P < 0.0001). eGFR continued to increase during the first 6 months of follow-up (median 27 ml/min per 1.73 m2; IQR 15.7–48.1; P < 0.0001) (Figure 1). Six patients (13.3%) had a primary nonfunction. At 6 months from starting eculizumab, 28.8% of patients had a complete renal response and 44.4% had a partial response. Four patients (8.9%) were dialysis-dependent. At the last follow-up, eGFR was stable compared with eGFR at 6 months (median 31 ml/min per 1.73 m2; IQR: 18–50.1; P = 0.14); 33.3% of patients had an eGFR >45 ml/min per 1.73 m2 and 40% an eGFR between 15 and 45 ml/min/1.73 m2, and 4 patients remained dialysis-dependent. Three patients (6.7%) died during follow-up, at 3.5, 1.9, and 1.8 months from KT, respectively. All patients died for sepsis-related complications. Patients and grafts’ outcomes are schematized in Figure 2.
Figure 2.
Graft and patients’ outcomes at 6 months of follow-up from the start of treatment and at the last follow-up visit.
Associations Between Hemolysis Markers and Other Risk Factors and Renal Outcome
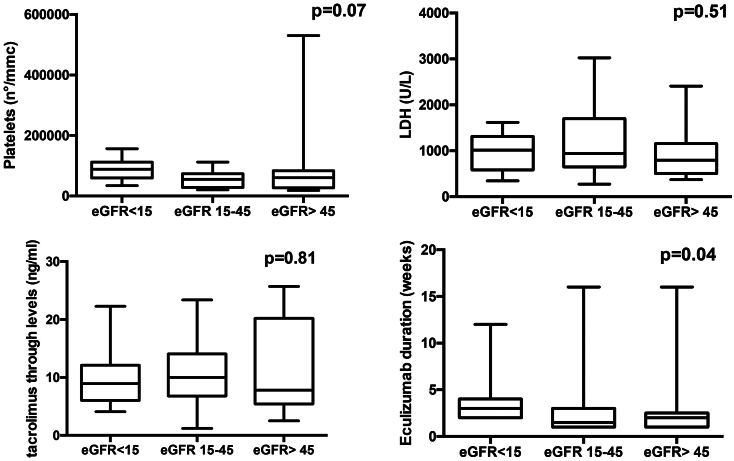
In order to evaluate any difference between the severity of hemolysis indices at the time of starting eculizumab in patients with different outcome of kidney function, we compared platelets and LDH levels at that time among patients who achieved a complete response, a partial response, and patients who needed hemodialytic treatment at 6 months of follow-up. No significant differences were found in terms of platelet counts and LDH levels at starting therapy among these 3 groups of patients. We also analyzed tacrolimus trough levels at eculizumab starting between patients who achieved partial and complete renal response and in those with ESRD at 6 months. Again, no significant differences were found between these patients (Figure 3). Then, we analyzed the duration of eculizumab therapy in patients who had different renal outcomes in terms of eGFR achieved at 6 months of follow-up (complete, partial recovery, or ESRD). Patients with the worse renal function significantly had a higher duration of eculizumab therapy (P = 0.04) (Figure 3).
Figure 3.
Values of platelets, LDH, and tacrolimus trough levels at the starting of eculizumab in patients who achieved a complete or partial response and in those who continued dialysis treatment at 6 months of follow-up. The levels of these 3 parameters were not different among the 3 outcome groups. In the last picture of the panel is shown the duration of treatment with eculizumab (number of weeks of therapy) in the 3 outcome groups at 6 months of follow-up: a significant higher duration of eculizumab was found in patients with the worse renal function (hemodialysis group) (P = 0.04). LDH, lactate dehydrogenase.
Finally, we retrospectively reviewed patients’ and kidney outcomes of the 4 patients with a rare complement genetic variant. The patient with CFI pathogenetic variant, the patient with MCP VUS and the one with ADAMST13 VUS had achieved a complete renal response at the last follow-up (Table 3). The patient with the CFHR5 likely benign variant had a primary nonfunction. We were not able to demonstrate any associations among risk haplotypes or polymorphisms and graft outcome.
Discussion
PT-TMA is a severe condition observed in up to 14% of KTs. The de novo PT-TMA is much more frequent than recurrent aHUS (90% vs. 10% of all cases), even though PT-TMA is more likely to develop in patients with a history of aHUS (29 vs. 0.8%).4 It is well known that the early detection and treatment of PT-TMA have a relevant impact on prognosis. However, the lack of diagnosis awareness and the absence of clear therapeutic strategies often cause a delay that can lead to the graft loss.18,19
The treatment of PT-TMA is based on correcting the potential cause of the condition. The first step is to avoid the complement activation before donation by preventing renal hypoperfusion during organ procurement and reducing the ischemia time.20 However, it should be considered that most of donors are actually marginal donors or DCD with an intrinsic risk of renal ischemic damage. Second, all causes of TMA should be corrected (by reducing or discontinuing CNI, treating ABMR or infections).21 Therefore, in the pre-eculizumab era, PT-TMAs unresponsive to the previous measures were treated with PE.22 However, studies performed in large series of patients demonstrated that the use of PE does not improve kidney outcome.23 Several case reports and, more recently, 2 larger Spanish case series reported the efficacy of eculizumab in this condition.13,14,24, 25, 26, 27, 28 Cavero et al.14 described 29 patients with secondary TMA, including 7 KT patients, in which eculizumab was effective in hematological remission and renal function improvement. Portoles et al. in a series of 22 PT-TMA patients, have shown the efficacy of eculizumab even in patients previously treated with PE and who did not benefit from that.13
Overall, eculizumab use is still limited by the lack of experience and the high costs of the drug. In this paper, we reported our experience with 45 consecutive patients with PT-TMA, treated with eculizumab as first-line therapy, during the past 5 years. This is, to our knowledge, the largest series with an accompanying genetic and histologic study performed in a single kidney transplant unit, with a median follow-up of 13.8 months (IQR: 6.8–35.9). Here, we provide more information regarding this condition in KT and, particularly, with respect to efficacy, safety, and outcome of kidney allograft and patients.
We did not include patients with a previous diagnosis of aHUS because in this case, patients are treated with eculizumab before KT, as prophylaxis.29,30 However, we are aware that a high percentage of patients included in this study have an chronic kidney disease of unknown etiology. Thus, preexisting TMA could be misdiagnosed. Nevertheless, the revision of clinical history of these patients could reasonably exclude an aHUS in the native kidneys.
It should also be considered that the genetic study of complement system, performed in the 86.2% of patients, have revealed that only 2 patients with this condition could be related to genetic variants (the CFI likely pathogenic variant and the MCP VUS). The majority of patients only carried polymorphisms or risk haplotypes.16,31 Interestingly, we found that the 64% of patients carried the MCP aHUS risk haplotype C.∗897 T>C (rs7144). This condition affects the promoter region of the MCP gene and results in decreased gene transcription, suggesting a decrease expression of MCP on the cell surfaces. It was initially described as a common genetic variant that increases aHUS penetrance in carriers of other complement mutations. However, recent evidence have emphasized its role in the predisposition to secondary HUS under strong complement-activating conditions, such as kidney transplant, even in patients without other genetic variants.18,32,33 Our data could confirm this hypothesis, because among patients with the haplotype MCP C.∗897 T>C (rs7144), only the half of them had a combination of this condition with other complement risk factors (polymorphisms or risk haplotypes).
Patients who underwent genetic test, also performed the test of serum-induced C5b-9 deposition on cultured endothelial cells. The test demonstrated elevated C5b-9 deposits in many patients, both on unstimulated and activated endothelium (84% and 92%, respectively). This condition further confirms a high TMA activity, revealing how eculizumab should be an effective therapy.17
The association between CNIs and PT-TMA has been well-documented in the literature, especially in patients with genetic predisposition and in the early posttransplant period, when the levels of these drugs are high.34, 35, 36 Many case reports or case series have documented TMA resolution after CNI discontinuation, but no controlled studies have prospectively evaluated the effectiveness of this approach in the treatment of PT-TMA. Mechanistic target of rapamycin inhibitors could be a good alternative to CNIs in patients with high risk for PT-TMA but data are still not consistent.37,38 On the other hand, a few case reports have demonstrated the efficacy of shifting from CNI to the inhibitor of T cell costimulatory protein CTLA-4, belatacept in the treatment of CNI-induced TMA after KT.39,40 We preferred not to switch to mechanistic target of rapamycin inhibitors in the first days after kidney transplant due to the side effects of these drugs and we could not use belatacept because it is not provided by the Italian National Health System. Thus, we decided to only maintain low tacrolimus trough levels in the majority of patients in order to reduce the risk of CNI-induced TMA. However, we are not able to quantify how this clinical decision have modified renal outcome; also considering that, in our patients, the median of tacrolimus trough levels at the diagnosis of TMA was on target and that we did not find an association between tacrolimus trough levels and kidney outcome (P = 0.81).
In our series, most patients received a transplant from suboptimal donors. In particular, ischemia-reperfusion damage has been associated with an early injury of the renal allograft. In this context, complement may be activated by the classical, lectin, and alternative pathway; and consequently the injury can be amplified by the release of C3a, C5a as anaphylotoxines and MAC.41 Regarding the use of kidneys from DCDs, the prolonged warm and cold ischemia time have been associated with a higher risk of PT-TMA. In these cases, the activation of the complement pathway is the result of the altered cell phenotype due to the hemodynamic instability, hormone dysregulation, and inflammatory responses.42 In our series, we had a high percentage of suboptimal donors, such as extended criteria donors, DCDs, or donors with high kidney donor profile index. Thus, it is not surprising that we found a high percentage of acute tubular necrosis, which was often associated with TMA, in the kidney biopsies of our patients.
Another risk factor for PT-TMA is acute ABMR.43 As with the ischemia-reperfusion mechanism, this condition may induce endothelial injury by the activation of the classical complement pathway and the resulting generation of the MAC.44,45 A nonnegligible percentage of our patients received a histological diagnosis of ABMR. In these patients, the association of eculizumab with the standard therapy, including PE and rituximab, led to a progressive recovery of renal function.
Overall, we can affirm that our patients that had a sort of genetic predisposition that probably was not able to induce an aHUS in native kidneys but could have determined a TMA after the endothelial injury induced by ischemia-reperfusion or rejection occurred after transplantation. Genetic predisposition and ischemia-reperfusion damage are factors that cannot be modified. Rejection was recognized only after kidney biopsies could be performed. Thus, we may assume that eculizumab started immediately after the diagnosis of TMA, have led to a rapid resolution of hemolysis indices and, more importantly, to a remarkable improvement of renal function. The median eGFR showed a significant increase after 2 weeks of starting eculizumab and a further progressive recovering of renal function at 6 months of follow-up, when 28.8% of patients had achieved an eGFR >45 ml/min per 1.73 m2 and 44.4% of patients had an eGFR between 15 and 45 ml/min per 1.73 m2. These data are confirmed also at the last follow-up, with the treatment that appears to be more effective during the first months after diagnosis.
Therefore, even if the usual real-world scenario still treats PT-TMA with a step-wise fashion, our experience favors the early use of complement inhibitor, because the delay in this therapy may lead to suboptimal outcomes.
Eculizumab duration was up to physician decision. Patients with a worse renal outcome had a significant eculizumab therapy duration. This is not surprising, maybe due to a higher propensity to overtreat patients who did not achieve a kidney function recovery early after starting therapy. Overall, considering the median short time-to-remission of this real-life experience, we suggest that the optimal duration of treatment includes at least 4 eculizumab administrations, with the exception of those cases in which genetic analysis has revealed pathogenetic variants. Furthermore, eculizumab was well-tolerated and no serious treatment-related adverse events were recorded.
We looked for any risk factors which could predict a worse renal outcome. However, hemolysis markers, that is, lower platelet counts or higher LDH levels were not different among the groups of patients who achieved a complete or a partial renal response or who still had an ESRD at 6 months of follow-up.
Our study has limitations. First, this is an observational, and thus hypothesis generating (rather than hypothesis testing) study. In this context, the treatment effect cannot be measured by accounting for several confounding factors. In this cohort, many patients were managed with a reduction of tacrolimus trough levels and we cannot exclude that this approach may have determined, at least partially, TMA resolution. Nevertheless, this study represents the largest reported experience on the use of eculizumab as first-line therapy in PT-TMA. We have observed that the clinical findings of TMA are associated with the typical TMA histological lesions in kidney biopsies in the majority of patients and that the traditional TMA risk factors such as ischemia or reperfusion injury, drugs, or conditions related to nonoptimal donors (elderly, DCD, or high kidney donor profile index) may induce PT-TMA, especially in patients with complement genetic risk factors. We found that the early use of eculizumab after PT-TMA diagnosis is safe and may lead to a complete or partial recovery of the renal function. However, larger, controlled studies are warranted to further clarify the role of eculizumab in the management of this condition; and cost-effectiveness studies are needed to define the weight of this therapeutic option on the National Health System.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Acknowledgments
Funding
The work reported in this publication was funded by the Italian Ministry of Health, RC-2023-2778808.
Footnotes
Table S1. Results from the test of complement activation on human microvascular endothelial cell-1 and the dosage of serum C5b-9 levels performed in 25 of the 45 patients (55.5%) after PT-TMA diagnosis.
STROBE Statement.
Supplementary Material
Table S1. Results from the test of complement activation on human microvascular endothelial cell-1 and the dosage of serum C5b-9 levels performed in 25 of the 45 patients (55.5%) after PT-TMA diagnosis
STROBE Statement.
References
- 1.Chiurchiu C., Ruggenenti P., Remuzzi G. Thrombotic microangiopathy in renal transplantation. Ann Transplant. 2002;7:28–33. [PubMed] [Google Scholar]
- 2.Garg N., Rennke H.G., Pavlakis M., Zandi-Nejad K. De novo thrombotic microangiopathy after kidney transplantation. Transplant Rev (Orlando) 2018;32:58–68. doi: 10.1016/j.trre.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Palma L.M.P., Sridharan M., Sethi S. Complement in secondary thrombotic microangiopathy. Kidney Int Rep. 2021;6:11–23. doi: 10.1016/j.ekir.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avila A., Gavela E., Sancho A. Thrombotic microangiopathy after kidney transplantation: an underdiagnosed and potentially reversible entity. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.642864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds J.C., Agodoa L.Y., Yuan C.M., Abbott K.C. Thrombotic microangiopathy after renal transplantation in the United States. Am J Kidney Dis. 2003;42:1058–1068. doi: 10.1016/j.ajkd.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Schwimmer J., Nadasdy T.A., Spitalnik P.F., Kaplan K.L., Zand M.S. De novo thrombotic microangiopathy in renal transplant recipients: a comparison of hemolytic uremic syndrome with localized renal thrombotic microangiopathy. Am J Kidney Dis. 2003;41:471–479. doi: 10.1053/ajkd.2003.50058. [DOI] [PubMed] [Google Scholar]
- 7.Matar D., Naqvi F., Racusen L.C., Carter-Monroe N., Montgomery R.A., Alachkar N. Atypical hemolytic uremic syndrome recurrence after kidney transplantation. Transplantation. 2014;98:1205–1212. doi: 10.1097/TP.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 8.Legendre C.M., Licht C., Muus P., et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 9.Licht C., Greenbaum L.A., Muus P., et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 2015;87:1061–1073. doi: 10.1038/ki.2014.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noris M., Remuzzi G. Managing and preventing atypical hemolytic uremic syndrome recurrence after kidney transplantation. Curr Opin Nephrol Hypertens. 2013;22:704–712. doi: 10.1097/MNH.0b013e328365b3fe. [DOI] [PubMed] [Google Scholar]
- 11.Zuber J., Fakhouri F., Roumenina L.T., Loirat C., Frémeaux-Bacchi V., French Study Group for aHUS/C3G Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol. 2012;8:643–657. doi: 10.1038/nrneph.2012.214. [DOI] [PubMed] [Google Scholar]
- 12.Zuber J., Frimat M., Caillard S., et al. Use of highly individualized complement blockade has revolutionized clinical outcomes after kidney transplantation and renal epidemiology of atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2019;30:2449–2463. doi: 10.1681/ASN.2019040331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Portoles J., Huerta A., Arjona E., et al. Characteristics, management and outcomes of atypical haemolytic uraemic syndrome in kidney transplant patients: a retrospective national study. Clin Kidney J. 2021;14:1173–1180. doi: 10.1093/ckj/sfaa096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavero T., Rabasco C., López A., et al. Eculizumab in secondary atypical haemolytic uraemic syndrome. Nephrol Dial Transplant. 2017;32:466–474. doi: 10.1093/ndt/gfw453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Afrouzian M., Kozakowski N., Liapis H., et al. Thrombotic microangiopathy in the renal allograft: results of the TMA Banff working group consensus on pathologic diagnostic criteria. Transpl Int. 2023;36 doi: 10.3389/ti.2023.11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Quintrec M., Zuber J., Moulin B., et al. Complement genes strongly predict recurrence and graft outcome in adult renal transplant recipients with atypical hemolytic and uremic syndrome. Am J Transplant. 2013;13:663–675. doi: 10.1111/ajt.12077. [DOI] [PubMed] [Google Scholar]
- 17.Galbusera M., Noris M., Gastoldi S., et al. An ex vivo test of complement activation on endothelium for individualized eculizumab therapy in hemolytic uremic syndrome. Am J Kidney Dis. 2019;74:56–72. doi: 10.1053/j.ajkd.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Imanifard Z., Liguori L., Remuzzi G. TMA in kidney transplantation. Transplantation. 2023;107:2329–2340. doi: 10.1097/TP.0000000000004585. [DOI] [PubMed] [Google Scholar]
- 19.George J.N., Nester C.M. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:654–666. doi: 10.1056/NEJMra1312353. [DOI] [PubMed] [Google Scholar]
- 20.Ponticelli C. De novo thrombotic microangiopathy. An underrated complication of renal transplantation. Clin Nephrol. 2007;67:335–340. doi: 10.5414/cnp67335. [DOI] [PubMed] [Google Scholar]
- 21.Langer R.M., Van Buren C.T., Katz S.M., Kahan B.D. De novo hemolytic uremic syndrome after kidney transplantation in patients treated with cyclosporine-sirolimus combination. Transplantation. 2002;73:756–760. doi: 10.1097/00007890-200203150-00017. [DOI] [PubMed] [Google Scholar]
- 22.Loirat C., Garnier A., Sellier-Leclerc A.L., Kwon T. Plasmatherapy in atypical hemolytic uremic syndrome. Semin Thromb Hemost. 2010;36:673–681. doi: 10.1055/s-0030-1262890. [DOI] [PubMed] [Google Scholar]
- 23.Fremeaux-Bacchi V., Fakhouri F., Garnier A., et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8:554–562. doi: 10.2215/CJN.04760512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devresse A., de Meyer M., Aydin S., Dahan K., Kanaan N. De novo atypical haemolytic uremic syndrome after kidney transplantation. Case Rep Nephrol. 2018;2018 doi: 10.1155/2018/1727986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duineveld C., Verhave J.C., Berger S.P., van de Kar N.C.A.J., Wetzels J.F.M. Living donor kidney transplantation in atypical hemolytic uremic syndrome: a case series. Am J Kidney Dis. 2017;70:770–777. doi: 10.1053/j.ajkd.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Dedhia P., Govil A., Mogilishetty G., Alloway R.R., Woodle E.S., Abu Jawdeh B.G. Eculizumab and Belatacept for de novo atypical hemolytic uremic syndrome associated with CFHR3-CFHR1 deletion in a kidney transplant recipient: a case report. Transplant Proc. 2017;49:188–192. doi: 10.1016/j.transproceed.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda T., Okumi M., Unagami K., et al. Two cases of kidney transplantation-associated thrombotic microangiopathy successfully treated with eculizumab. Nephrol (Carlton) 2016;21(suppl 1):35–40. doi: 10.1111/nep.12768. [DOI] [PubMed] [Google Scholar]
- 28.Safa K., Logan M.S., Batal I., Gabardi S., Rennke H.G., Abdi R. Eculizumab for drug-induced de novo posttransplantation thrombotic microangiopathy: a case report. Clin Nephrol. 2015;83:125–129. doi: 10.5414/CN108163. [DOI] [PubMed] [Google Scholar]
- 29.Goodship T.H., Cook H.T., Fakhouri F., et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2017;91:539–551. doi: 10.1016/j.kint.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Siedlecki A.M., Isbel N., Vande Walle J., James Eggleston J., Cohen D.J., Global aHUS Registry Eculizumab use for kidney transplantation in patients with a diagnosis of atypical hemolytic uremic syndrome. Kidney Int Rep. 2019;4:434–446. doi: 10.1016/j.ekir.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren Z., Perkins S.J., Love-Gregory L., Atkinson J.P., Java A. Clinicopathologic implications of complement genetic variants in kidney transplantation. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.775280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Clech A., Simon-Tillaux N., Provôt F., et al. Atypical and secondary hemolytic uremic syndromes have a distinct presentation and no common genetic risk factors. Kidney Int. 2019;95:1443–1452. doi: 10.1016/j.kint.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez-Moreno A., de la Cerda F., Rodríguez-Barba A., et al. Is the atypical hemolytic uremic syndrome risk polymorphism in Membrane Cofactor Protein MCPggaac relevant in kidney transplantation? A case report. Pediatr Transplant. 2021;25 doi: 10.1111/petr.13903. [DOI] [PubMed] [Google Scholar]
- 34.Mulgaonkar S., Kaufman D.B. Conversion from calcineurin inhibitor-based immunosuppression to mammalian target of rapamycin inhibitors or Belatacept in renal transplant recipients. Clin Transpl. 2014;28:1209–1224. doi: 10.1111/ctr.12453. [DOI] [PubMed] [Google Scholar]
- 35.Sahin G., Akay O.M., Bal C., Yalcin A.U., Gulbas Z. The effect of calcineurin inhibitors on endothelial and platelet function in renal transplant patients. Clin Nephrol. 2011;76:218–225. [PubMed] [Google Scholar]
- 36.Renner B., Klawitter J., Goldberg R., et al. Cyclosporine induces endothelial cell release of complement-activating microparticles. J Am Soc Nephrol. 2013;24:1849–1862. doi: 10.1681/ASN.2012111064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nava F., Cappelli G., Mori G., et al. Everolimus, cyclosporine, and thrombotic microangiopathy: clinical role and preventive tools in renal transplantation. Transplant Proc. 2014;46:2263–2268. doi: 10.1016/j.transproceed.2014.07.062. [DOI] [PubMed] [Google Scholar]
- 38.Keller K., Daniel C., Schöcklmann H., et al. Everolimus inhibits glomerular endothelial cell proliferation and VEGF, but not long-term recovery in experimental thrombotic microangiopathy. Nephrol Dial Transplant. 2006;21:2724–2735. doi: 10.1093/ndt/gfl340. [DOI] [PubMed] [Google Scholar]
- 39.Koppula S., Yost S.E., Sussman A., Bracamonte E.R., Kaplan B. Successful conversion to Belatacept after thrombotic microangiopathy in kidney transplant patients. Clin Transpl. 2013;27:591–597. doi: 10.1111/ctr.12170. [DOI] [PubMed] [Google Scholar]
- 40.Cicora F., Paz M., Mos F., Roberti J. Use of belatacept as alternative immunosuppression in three renal transplant patients with de novo drug-induced thrombotic microangiopathy. Case Rep Med. 2013;2013 doi: 10.1155/2013/260254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamel M.H., Jaberi A., Gordon C.E., Beck L.H., Francis J. The complement system in the modern era of kidney transplantation: mechanisms of injury and targeted therapies. Semin Nephrol. 2022;42:14–28. doi: 10.1016/j.semnephrol.2022.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Biglarnia A.R., Huber-Lang M., Mohlin C., Ekdahl K.N., Nilsson B. The multifaceted role of complement in kidney transplantation. Nat Rev Nephrol. 2018;14:767–781. doi: 10.1038/s41581-018-0071-x. [DOI] [PubMed] [Google Scholar]
- 43.Lefaucheur C., Viglietti D., Hidalgo L.G., et al. Complement-activating anti-HLA antibodies in kidney transplantation: allograft gene expression profiling and response to treatment. J Am Soc Nephrol. 2018;29:620–635. doi: 10.1681/ASN.2017050589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horwitz J.K., Chun N.H., Heeger P.S. Complement and transplantation: from new mechanisms to potential biomarkers and novel treatment strategies. Clin Lab Med. 2019;39:31–43. doi: 10.1016/j.cll.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhalla A., Alachkar N., Alasfar S. Complement-based therapy in the management of antibody-mediated rejection. Adv Chronic Kidney Dis. 2020;27:138–148. doi: 10.1053/j.ackd.2019.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.