Abstract
Introduction
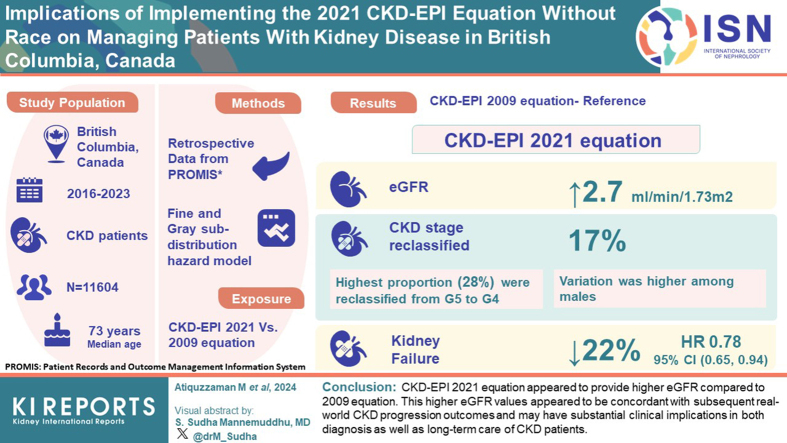
We investigated the implications of implementing race-free Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2021 equation among real-world patients with chronic kidney disease (CKD) from British Columbia (BC), Canada.
Methods
This study included nondialysis-dependent patients with CKD aged ≥19 years who were registered in the Patient Records and Outcome Management Information System (PROMIS) as of March 31, 2016 (index date) with ≥1 serum creatinine measurement within 1 year before the index date. Patients with a history of kidney transplantation before the index date were excluded. CKD-EPI 2021 versus 2009 equation was the exposure variable. Difference in mean estimated glomerular filtration rate (eGFR) and number (%) of patients reclassified to a different eGFR category were estimated. We used Fine and Gray subdistribution hazard model to investigate the association between change in eGFR category and progression to kidney failure (incident maintenance dialysis or kidney transplantation) within 2 years.
Results
A total of 11,604 patients (median age 73 years, 52% male) were included. Compared to the 2009 equation, eGFR from 2021 equation was on average 2.7 ml/min per 1.73 m2 higher. Variation was higher among males. Overall, ∼17% of the study sample were reclassified to a category with higher eGFR by 2021 equation (switchers). The highest proportion (28%) of patients were reclassified from G5 to G4. The risk of progressing to kidney failure was 22% less among switchers compared to nonswitchers; adjusted subdistribution hazard ratio (HR) (95% confidence interval [CI]) is 0.78 (0.65, 0.94).
Conclusion
CKD-EPI 2021 equation appeared to provide higher eGFR compared to 2009 equation. This higher eGFR values appeared to be concordant with subsequent real-world CKD progression outcomes. Higher eGFR from the 2021 equation may have substantial clinical implications in both diagnosis as well as long-term care of patients with CKD.
Keywords: chronic kidney disease, CKD-EPI equation, eGFR, KFRE
Graphical abstract
eGFR is the most commonly used measure of kidney function.1 Several equations, including the Modification of Diet in Renal Disease, European Kidney Function Consortium equation, and the CKD-EPI exist to calculate eGFR from serum creatinine. In 2012, the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guideline for adults with CKD recommended using 2009 CKD-EPI creatinine equation in reporting eGFR.2 This equation included race as a variable (Black vs. non-Black) along with serum creatinine, age, and sex (male vs. female).3
In 2020, the National Kidney Foundation in the United States as well as The American Society of Nephrology created a joint task force to examine the removal of the race variable from GFR estimating equations.4 The task force acknowledged the harms caused by inclusion of race in estimating kidney function and recommended a new race-free equation (CKD-EPI 2021).4, 5, 6
Currently, laboratories in BC, Canada report eGFR using CKD-EPI 2009 equation with coefficient for non-Black race. Recent research suggests that values for eGFR calculated using the new CKD-EPI 2021 equation were higher compared to the eGFR calculated using 2009 equation in non-Black populations.6, 7, 8, 9, 10 The median increase in eGFR was 3.9 (2.9–4.8) ml/min per 1.73 m2.7 This could lead to reclassification of people with CKD to a higher eGFR category in KDIGO staging, potentially resulting in substantial change in patient care, when resource allocation or treatment is based on eGFR.7, 8, 9, 10
The impact of implementing the CKD-EPI 2021 equation without race in Canada was unknown. In this study, our objective was to investigate the difference in eGFR calculated using CKD-EPI 2009 and 2021 equations and its impact on eGFR categories and estimation of risk of progression to kidney failure among real-world patients with CKD from BC, Canada.
Methods
Study Population
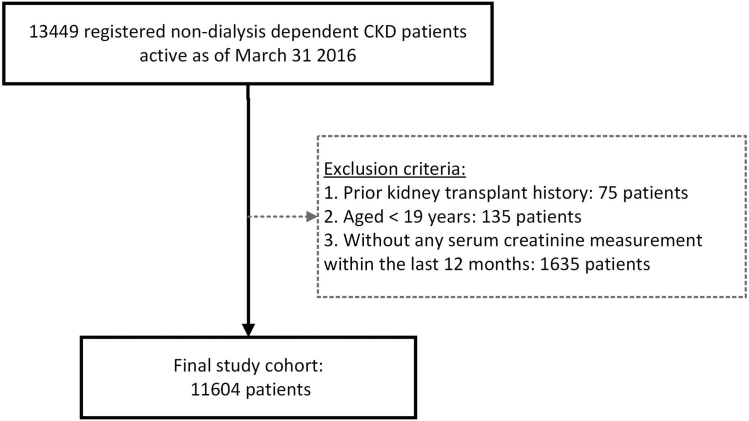
We conducted this retrospective cohort study using data from PROMIS, a population-based integrated registry database for chronic kidney disease patients under the care of nephrologists in BC, Canada.11 Individuals with abnormality in kidney structure or function that is present for ≥3 months with no specific eGFR cutoff are considered to have CKD and are registered into PROMIS. Nondialysis-dependent patients with CKD residing in BC who were actively registered in PROMIS as of March 31, 2016 (study index date), aged ≥19 years and had at least 1 serum creatinine measurement within 1 year before the index date were included. We used a prevalent cohort of patients with CKD from 2016 to investigate the association between possible changes in eGFR categories due to the variation in CKD-EPI 2009 versus 2021 equation and kidney disease outcomes over time. In a sensitivity analysis, we compared the variations in eGFR with a contemporary cohort of patients with CKD assembled as of March 31, 2023. We excluded patients with previous history of kidney transplantation before the index date. This study was approved by the Clinical Research Ethics Board at the University of British Columbia, Canada (H23-03438).
Exposure
CKD-EPI 2021 versus 2009 equation was the independent variable in this study. If a patient had multiple records of serum creatinine within 1 year prior to index date, the one closest to the index date was considered as the index serum creatinine. BC’s practice never factored in the Black race in eGFR calculation using CKD-EPI 2009 equation. So, we calculated the eGFR from serum creatinine using the following 2 equations3,6,9:
CKD-EPI 2021:
eGFR = 142 × min(Scr/k,1)α × max(Scr/k,1)−1.200 × 0.9938age × 1.012 (if female), where Scr is serum creatinine, k is 0.7 for females and 0.9 for males, α is −0.241 for females and −0.302 for males, min indicates the minimum of Scr/k or 1, max indicates the maximum of Scr/k or 1.
CKD-EPI 2009:
eGFR = 141 × min(Scr/k,1)α × max(Scr/k,1)−1.209 × 0.9929age × 1.018(if female), where Scr is serum creatinine, k is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/k or 1, max indicates the maximum of Scr/k or 1.
Outcomes
We explored the impact of using the CKD-EPI 2021 versus 2009 equations on the calculation of kidney failure risk using the Kidney Failure Risk Equation (KFRE), predicting progression to kidney failure, and planning for dialysis education. Because the purpose of the CKD-EPI equation is to estimate GFR, we investigated if the eGFR calculated using 2021 versus 2009 equations were different among the real-world cohort of patients with CKD under the care of nephrologists.7,9,12
In BC, standardized protocols for number and frequency of laboratory testing, clinic visit frequency and timing of modality education, are based on the eGFR category. Thus, differences in eGFR values obtained from 2021 versus 2009 equation, may lead to reclassification of patients to either lower or higher eGFR category. Hence, we investigated the reclassification of patients with CKD in various eGFR categories (G1–G5) based on eGFR calculated using 2021 versus 2009 equation.7,10 Similarly, we investigated the reclassification in various CKD risk groups (low/moderate/high/very high) based on the KDIGO 2012 prognosis of CKD by eGFR and albuminuria categories “heat map.”
We also investigated the association between change in eGFR category and progression to kidney failure over time. Kidney failure was defined as a composite of initiation of maintenance dialysis (≥4 weeks), or incident kidney transplantation.13 Patients with CKD are at an increased risk of death, which often occurs before kidney failure, therefore we accounted for death as a competing event for the outcome of kidney failure.14,15
In BC, the process of dialysis modality choice education and selection starts when eGFR is between 20 and 25 ml/min per 1.73 m2. Therefore, switching from eGFR ≤25 ml/min per 1.73m2 to eGFR >25 ml/min per 1.73 m2 will delay the process of dialysis education and preparation. We investigated the impact of this reclassification on the outcome of progression to kidney failure within 2 years.
Finally, KFRE is frequently used in estimating the short-term (2-year or 5-year) risk of progressing to kidney failure.16, 17, 18 eGFR is one of the 4 or 8 variables used in KFRE.16 We investigated the impact of GFR estimated using 2009 and 2021 equation on the KFRE 2-year risk score.
Covariables
Age was as recorded as a continuous variable in years and sex was recorded as male or female. The self-reported race was recorded as Caucasian, Oriental Asian, South/Southeast Asian, Indigenous, Others, and Unspecified. Time (in years) between registration in PROMIS and the index date, CKD vintage, was recorded as a continuous variable. Cause of CKD was categorized as congenital nephrotic syndrome, diabetic nephropathy, glomerulonephritis, hypertensive nephropathy, polycystic kidney disease, and others. Baseline history of comorbidities were included as binary variables of having diabetes, cardiovascular disease (CVD)-related comorbidities, respiratory diseases, and cancer. Immunosuppressive medications (azathioprine, cyclophosphamide, cyclosporine, mycophenolate, prednisone, rituximab, tacrolimus), renin-angiotensin-aldosterone system inhibitors (RAASi; angiotensin-converting-enzyme inhibitors and angiotensin receptor blockers) and sodium-glucose cotransporter-2 inhibitor usage at the index date were recorded as a binary variable. eGFR was calculated in ml/min per 1.73 m2 and entered as a continuous variable. In PROMIS database, urine albumin-to-creatinine ratio (UACR) was recorded as mg/mmol. Before using this UACR in the KFRE, the unit was converted to mg/g by dividing the UACR value with 0.113.16 The UACR was also taken as the closest one within 1 year before the index date.
Statistical Analyses
For the primary outcome, we estimated GFR using CKD-EPI 2021 and 2009 equations. We then compared the difference in mean eGFR for overall study sample and stratified by age and sex.
In investigating the reclassification in eGFR category, we first grouped the entire study sample using eGFR (ml/min per 1.73 m2) obtained from 2009 equation into 6 CKD categories; G1 (≥90), G2 (60–89), G3a (45–59), G3b (30–44), G4 (15–29), and G5 (<15). Then we grouped the patients with CKD into the same categories using eGFR obtained from 2021 equation. Finally, we calculated the number and proportions of patients in each of the eGFR categories that got reclassified to a different category. We repeated the reclassification analysis using KDIGO risk groups (as per the KDIGO heat map: low risk, moderately increased risk, high risk, very high risk) as the outcome.2
Change in eGFR category was defined as a binary variable if a patient was reclassified to a different eGFR category or not. In investigating the association between change in eGFR category and progression to kidney failure, patients were followed-up with prospectively for 2 years from the index date for incident kidney failure and accounting for death as a competing event. Individuals were censored if they emigrated out of the province. We used Fine and Gray subdistribution hazard model and reported the HR (95% CI) as the measure of association.15,19,20 The covariables included in the multivariate model were age; sex; race; cause and vintage of CKD; baseline comorbidities, including diabetes, CVD-related comorbidities, respiratory diseases, and cancer; baseline history of immunosuppressive medication, RAASi, and sodium-glucose cotransporter-2 inhibitor use to obtain an adjusted HR (aHR). In a sensitivity analysis, we investigated the risk of progressing to kidney failure among patients with CKD in eGFR categories G4 and G5 only, that is, patients with eGFR <30 ml/min per 1.73 m2. We also conducted subgroup analyses by cause of CKD to investigate if the risk of progressing to kidney failure varied by etiology.
Similar to reclassification in eGFR categories, among all patients with an eGFR of ≤25 ml/min per 1.73m2 from CKD-EPI 2009 equation, we first identified the patients who were reclassified to have an eGFR of >25 ml/min per 1.73 m2 by CKD-EPI 2021 equation. Finally, we used the Fine and Gray subdistribution hazard model to investigate the association between this reclassification and progression to kidney failure.
In investigating the impact of eGFR on the KFRE risk score, we calculated the KFRE 2-year score using eGFR obtained from CKD-EPI 2009 and 2021 equations holding the other 3 variables (age, sex, and UACR) constant.16 We investigated if the mean KFRE 2-year risk scores (KFRE-2) were different, for overall study sample and by age group and sex. We then compared the proportion of patients in 4 KFRE score ranges: 0% to 10%, 10% to 20%, 20% to 40% and >40%. We investigated the concordance on KFRE score estimated using eGFR obtained from CKD-EPI 2009 and 2021 equations. Finally, among all patients with KFRE-2 risk of >40% using eGFR from CKD-EPI 2009 equation, we identified the patients who were reclassified to have a KFRE-2 risk of ≤40% using eGFR from CKD-EPI 2021 equation.17 We investigated the association between this reclassification in KFRE risk threshold and progression to kidney failure using Fine and Gray subdistribution hazard model.
UACR was a required variable in creating the KDIGO heat map as well as estimating the KFRE score. In our study sample, 9375 patients (81%) had UACR recorded at baseline, that is, within the last 12 months from the index date. In the primary analyses for KDIGO heat map and KFRE related outcomes, we conducted complete case analyses using data from this subset of 9375 patients. In a sensitivity analysis, we imputed the missing UACR for 2228 patients (19%) by extending the look-back period from 12 months to 24 months before the index date. If UACR was the measure of proteinuria that was closest to the index date, we recorded this as the baseline UACR. If urine protein-to-creatinine ratio was the measure of proteinuria that was closest to the index date, we converted this to UACR using the formula proposed by Sumida et al.21 For the remaining patients without any UACR or urine protein-to-creatinine ratio data within the past 24 months from the index date, we imputed the UACR values with the median UACR calculated from the observed data stratified by age and sex. We grouped the 9375 patients into age groups (in years) of 18 to 29, 30 to 39, 40 to 44, 45 to 49, 50 to 54, 55 to 59, each of the age years from 60 to 89 (for example 60 years is a group, 61 years is another group), and ≥90 years. These grouping ensured sufficient sample size in each of the age-sex strata.
Results
Description of Study Sample
The study cohort included 11,604 nondialysis-dependent patients with CKD who were registered in PROMIS as of March 31, 2016 (Figure 1). Median age of the study sample was 73 years and 52% were male. The highest proportion (60%) of patients were Caucasian followed by 18% of Asians. Majority of the patients (9437, 81%) received care in Kidney Care Clinics, consisting of multidisciplinary healthcare teams, whereas 2167 (19%) received care in a nephrologist’s office. Almost 1 in every 2 patients had either diabetes or CVD-related comorbidities at baseline. Diabetic nephropathy and hypertensive nephropathy were recorded as the most common causes of CKD (19% and 20%, respectively). More than half of the study sample (54%) were taking RAASi, and 1 in every 10 patients (10%) were exposed to immunosuppressive medication at baseline. Only a handful of patients (0.11%) received sodium-glucose cotransporter-2 inhibitors; thus, the numbers are not reported in the table describing patient characteristics due to concern about small cell size.
Figure 1.
Study cohort derivation. CKD, chronic kidney disease.
Variations in eGFR Calculated Using CKD-EPI 2021 Versus 2009 Equations
For the overall study sample, the eGFR calculated using CKD-EPI 2021 equation was on average 2.7 ml/min per 1.73 m2 higher compared to eGFR calculated using 2009 equation. In Table 1, we present the variation in eGFR by age and sex. The variation was higher among males than females. Among males, the eGFR obtained from CKD-EPI 2021 equation was on average 2.85 to 3.07 ml/min per 1.73 m2 higher compared to CKD-EPI 2009 equation. Among females this increase in eGFR was by 1.73 to 2.52 ml/min per 1.73 m2.
Table 1.
Difference in eGFR obtained from CKD-EPI 2009 versus 2021 equations
| Age group, yr | Male |
Female |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | eGFR, Mean (SD) |
Difference, Mean (SD) |
Number of patients | eGFR, Mean (SD) |
Difference, Mean (SD) |
|||||
| CKD-EPI 2009 | CKD-EPI 2021 | CKD-EPI 2021 − CKD-EPI 2009 | P-value | CKD-EPI 2009 | CKD-EPI 2021 | CKD-EPI 2021 − CKD-EPI 2009 | P-value | |||
| Overall | 6058 | 33.5 (18.6) | 36.5 (19.6) | 3.0 (1.2) | <0.0001 | 5546 | 35.8 (21.4) | 38.3 (22.0) | 2.4 (0.9) | <0.0001 |
| 19–29 | 97 | 72.5 (37.7) | 75.4 (38.6) | 2.9 (1.4) | <0.0001 | 99 | 88.3 (36.7) | 90.1 (36.8) | 1.7 (1.0) | <0.0001 |
| 30–39 | 135 | 54.7 (33.0) | 57.6 (34.4) | 2.9 (1.5) | <0.0001 | 200 | 64.7 (36.9) | 66.8 (37.6) | 2.1 (1.0) | <0.0001 |
| 40–49 | 292 | 44.6 (26.5) | 47.5 (27.9) | 2.9 (1.5) | <0.0001 | 269 | 55.8 (31.7) | 58.3 (32.7) | 2.4 (1.1) | <0.0001 |
| 50–59 | 606 | 40.0 (22.5) | 43.0 (23.8) | 3.0 (1.4) | <0.0001 | 570 | 43.3 (24.4) | 45.7 (25.4) | 2.4 (1.1) | <0.0001 |
| 60–69 | 1304 | 35.3 (17.7) | 38.3 (19.0) | 3.1 (1.3) | <0.0001 | 1137 | 35.6 (17.8) | 38.1 (18.8) | 2.4 (1.0) | <0.0001 |
| 70–79 | 1883 | 31.1 (13.4) | 34.2 (14.6) | 3.0 (1.2) | <0.0001 | 1610 | 31.2 (12.5) | 33.6 (13.4) | 2.4 (0.9) | <0.0001 |
| 80–89 | 1518 | 27.3 (10.7) | 30.2 (11.7) | 2.9 (1.0) | <0.0001 | 1363 | 28.7 (10.8) | 31.3 (11.7) | 2.5 (0.9) | <0.0001 |
| ≥90 | 223 | 24.5 (9.5) | 27.4 (10.5) | 2.9 (1.0) | <0.0001 | 298 | 25.1 (10.2) | 27.6 (11.1) | 2.4 (0.9) | <0.0001 |
CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate.
Concordance in eGFR Categories When eGFR was Calculated Using CKD-EPI 2021 Versus 2009 Equations
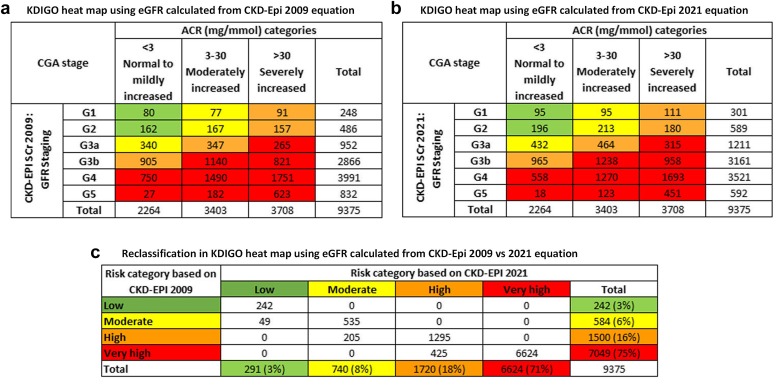
In Table 2, we present the number and proportion of patients in each of the eGFR categories by eGFR calculated using CKD-EPI 2009 versus 2021 equations. At the overall study sample level, compared to CKD-EPI 2009 equation, the proportion of patients with eGFR <30 ml/min per 1.73 m2 (G4 and G5) was significantly lower when eGFR was calculated using CKD-EPI 2021 equation. The proportions (95% CI) were 49.7% (48.7%, 50.7%) and 42.4% (41.4%, 43.3%), respectively (P-value < 0.0001). eGFR calculated using CKD-EPI 2021 equation consistently resulted in higher proportion of patients in categories with higher eGFR (from G1 to G3b) (Table 2).
Table 2.
Distribution of patients in various eGFR categories after calculating eGFR using CKD-EPI 2009 versus 2021 equation
| CKD-EPI 2009: eGFR categories | CKD-EPI 2021: eGFR categories |
||||||
|---|---|---|---|---|---|---|---|
| G1 (≥90) | G2 (60–89) | G3a (45–59) | G3b (30–44) | G4(15–29) | G5 (<15) | Total | |
| G1 (≥90) | 362 [100%] | 0 | 0 | 0 | 0 | 0 | 362 (3%) |
| G2 (60–89) | 86 [12%] | 604 [88%] | 0 | 0 | 0 | 0 | 690 (6%) |
| G3a (45–59) | 0 | 211 [18%] | 987 [82%] | 0 | 0 | 0 | 1198 (10%) |
| G3b (30–44) | 0 | 0 | 533 [15%] | 3059 [85%] | 0 | 0 | 3592 (31%) |
| G4 (15–29) | 0 | 0 | 0 | 846 [18%] | 3924 [82%] | 0 | 4770 (41%) |
| G5 (<15) | 0 | 0 | 0 | 0 | 279 [28%] | 713 [72%] | 992 (9%) |
| Total | 448 (4%) | 815 (7%) | 1520 (13%) | 3905 (34%) | 4203 (36%) | 713 (6%) | 11,604 |
CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate.
Overall, a total of 1955 out of 11,604 patients (17%) of the study sample were reclassified to a category with higher eGFR (from G5 toward G1) when eGFR was calculated using the new 2021 equation (Table 2). Within each eGFR category, G5 had the highest proportion (28%) of patients who were reclassified to G4 by 2021 equation. Among the patients who were classified to be in G4 and G3a categories by eGFR from 2009 equation, approximately 18% were reclassified to a category with higher eGFR when eGFR was calculated using the new 2021 equation without race factor. The proportion of patients who were reclassified from G3b to G3a and from G2 to G1 were 15% and 12%, respectively (Table 2).
In a stratified analysis by sex, compared to females, a slightly higher proportion of males were reclassified to a category with higher eGFR (14% and 19%, respectively). Similar to the overall sample, the highest reclassification occurred from G5 to G4 in both men and women. However, the proportion of patients reclassified was consistently higher among men than women with greater difference at lower categories. For example, 30% versus 25% in G5 to G4 reclassification and 21% versus 13% in G3a to G2 reclassification (Supplementary Table S1).
Concordance in KDIGO Heat Map Categories When eGFR was Calculated Using CKD-EPI 2021 Versus 2009 Equations
Approximately 75% (95% CI: 74%, 76%) of patients were considered to be in the very high-risk group by eGFR from CKD-EPI 2009 equation (Figure 2). This proportion was significantly reduced to 71% (95% CI: 70%, 72%) when eGFR from 2021 equation was considered (P-value < 0.0001) (Figure 2). A total of 679 patients (7%) were reclassified to a lower risk group with highest proportion (14%) being downgraded from high risk to moderate risk group (Figure 2). In a sensitivity analysis, we included 2228 patients after imputing the missing UACR (n = 11,604). The proportion of patients in each of the risk categories and the magnitude and directionality of reclassification were similar to the primary analysis (Supplementary Figure S1).
Figure 2.
Distribution of patients in various KDIGO risk categories by eGFR calculated using CKD-EPI 2009 versus 2021 equation. CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; KDIGO, Kidney Disease Improving Global Outcomes. (Green: low risk; Yellow: moderately increased risk; Orange: high risk; Red, very high risk).
Association Between Change in eGFR Category and Progression to Kidney Failure Over Time
In Table 3, we present the comparison of patient characteristics by change in eGFR category status (yes/no). Age appeared to be similar in both groups. The proportion of men were substantially higher among those who were reclassified to a category of higher eGFR (switchers) compared to patients who remained in the same category (nonswitchers); the proportions were 60% versus 51%, respectively. The self-reported race appeared to be equally distributed among switchers and nonswitchers. Serum creatinine at baseline appeared to be lower among the switchers than nonswitchers, with a median value of 150 and 168 μmol/l, respectively. The etiology of disease appeared to be similar except for a slightly lower proportion of patients with glomerulonephritis in the switcher group. The proportion of patients with comorbidities, including diabetes, CVD-related comorbidities, respiratory disease, and cancer appeared to be comparable. Baseline history of medication use (immunosuppressive and RAASi) was also appeared to be similar.
Table 3.
Comparison of patient characteristics by change in eGFR category status after calculating eGFR using CKD-EPI 2021 equation
| Characteristics | Reclassified to a category with higher eGFR |
|||||
|---|---|---|---|---|---|---|
| Overall study sample (N = 11,604) |
CKD patients in eGFR categories G4 and G5 based on CKD-Epi 2009 (eGFR <30 ml/min per 1.73 m2) |
|||||
| Yes | No | P-value | Yes | No | P-value | |
| Number of patients | 1955 | 9649 | -- | 1125 | 4637 | -- |
| Age at index date, yr | 74 [65, 81] | 73 [62, 81] | <0.0001 | 76 [69, 83] | 76 [66, 83] | 0.034 |
| Male | 1173 (60%) | 4885 (51%) | <0.0001 | 660 (59%) | 2470 (53%) | 0.001 |
| Race/Ethnicity | 0.203 | 0.048 | ||||
| Caucasian | 1170 (60%) | 5821 (60%) | 696 (62%) | 2890 (62%) | ||
| Oriental Asian | 130 (7%) | 770 (8%) | 78 (7%) | 423 (9%) | ||
| South/Southeast Asian | 213 (11%) | 964 (10%) | 128 (11%) | 505 (11%) | ||
| Indigenous | 41 (2%) | 233 (2%) | 20 (2%) | 111 (2%) | ||
| Others | 319 (16%) | 1461 (15%) | 172 (15%) | 597 (13%) | ||
| Unspecified | 82 (4%) | 400 (4%) | 31 (3%) | 111 (2%) | ||
| CKD vintage, yr | 2.9 [1.3, 5.9] | 3.0 [1.3, 6.1] | 0.212 | 3.1 [1.4, 6.1] | 3.3 [1.3, 6.3] | 0.354 |
| Comorbidities | ||||||
| Diabetes | 861 (44%) | 4024 (42%) | 0.056 | 553 (49%) | 2250 (49%) | 0.703 |
| CVD-related comorbidities | 886 (45%) | 4217 (44%) | 0.189 | 585 (52%) | 2336 (50%) | 0.329 |
| Respiratory disease | 254 (13%) | 1263 (13%) | 0.908 | 159 (14%) | 654 (14%) | 0.980 |
| Cancer | 259 (13%) | 1379 (14%) | 0.227 | 172 (15%) | 767 (17%) | 0.308 |
| Serum creatinine on index date, mg/dl | 1.7 [1.4, 2.2] | 1.9 [1.4, 2.5] | <0.0001 | 2.1 [1.8, 2.6] | 2.6 [2.2, 3.2] | <0.0001 |
| Serum creatinine on index date, μmol/l | 150 [125, 192] | 168 [128, 225] | <0.0001 | 187 [156, 231] | 227 [195, 280] | <0.0001 |
| eGFR from CKD-EPI 2009 equation, ml/min per 1.73 m2 | 30 [28, 44] | 31 [21, 39] | <0.0001 | 28 [27, 29] | 21 [17, 24] | <0.0001 |
| eGFR from CKD-EPI 2021 equation, ml/min per 1.73 m2 | 32 [31, 47] | 33 [23, 42] | <0.0001 | 31 [30, 32] | 23 [19, 27] | <0.0001 |
| Urine albumin-to-creatinine ratio, mg/mmol | 10.8 [2.6, 63.0] | 16.6 [3.2, 73.9] | <0.0001 | 16.7 [3.7, 92.9] | 31.2 [6.6, 119.5] | <0.0001 |
| Urine albumin-to-creatinine ratio, mg/g | 95.1 [23.0, 557.5] | 146.5 [28.3, 654.0] | <0.0001 | 147.8 [32.7, 822.1] | 275.7 [58.4, 1057.5] | <0.0001 |
| Cause of CKD | <0.0001 | 0.041 | ||||
| Glomerulonephritis | 215 (11%) | 1426 (15%) | 103 (9%) | 515 (11%) | ||
| Diabetic nephropathy | 353 (18%) | 1850 (19%) | 242 (22%) | 1121 (24%) | ||
| Hypertensive nephropathy | 398 (20%) | 1923 (20%) | 258 (23%) | 968 (21%) | ||
| Polycystic Kidney Disease | 38 (2%) | 225 (2%) | 24 (2%) | 128 (3%) | ||
| Congenital nephrotic syndrome | 19 (1%) | 94 (1%) | 12 (1%) | 54 (1%) | ||
| Others | 932 (48%) | 4131 (43%) | 486 (43%) | 1851 (40%) | ||
| Baseline history of immunosuppressive medication use | 153 (8%) | 981 (10%) | 0.002 | 67 (6%) | 320 (7%) | 0.256 |
| Baseline history of RAASi medication use | 1050 (54%) | 5072 (53%) | 0.356 | 614 (55%) | 2481 (54%) | 0.517 |
CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; RAASi, renin-angiotensin-aldosterone system inhibitors.
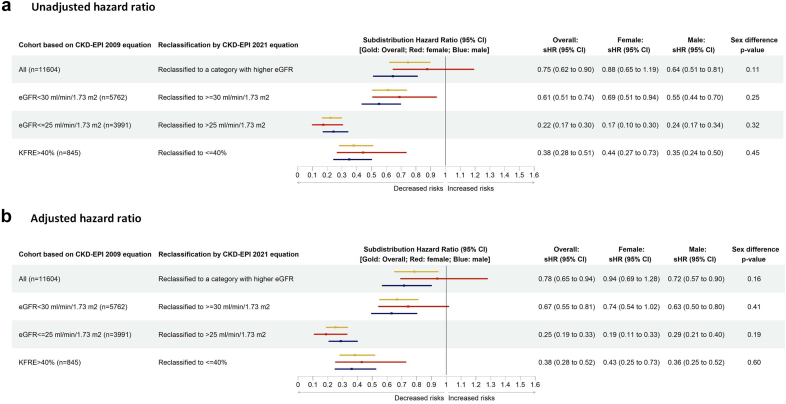
Results from the Fine and Gray subdistribution hazard model indicated that, after calculating eGFR using CKD-EPI 2021 equation, compared to patients whose eGFR category was not changed from eGFR calculated using 2009 equation (nonswitchers), the risk of progressing to kidney failure within 2 years from the index date was 22% less among patients with CKD who were reclassified to a category with higher eGFR (switchers from G5 toward G1 category). After adjusting for age; sex; race; cause and vintage of CKD; baseline comorbidities including diabetes, CVD-related comorbidities, respiratory diseases, and cancer; baseline usage of immunosuppressive medication, and RAASi and sodium-glucose cotransporter-2 inhibitors, the adjusted subdistribution HR (95% CI) was 0.78 (0.65, 0.94) (Figure 3). Compared to nonswitchers, the risk of death before kidney failure, the competing outcome, was 15% less among switchers, adjusted HR (95% CI): 0.85 (0.74, 0.97) (Supplementary Table S2). In a sensitivity analysis, we restricted the analysis among patients with CKD in eGFR categories of G4 and G5. Among patients with CKD with an eGFR <30 ml/min per 1.73 m2, the association was much stronger and the risk of progressing to kidney failure within 2 years was 33% less among switchers compared to nonswitchers (adjusted subdistribution HR = 0.67 [95% CI: 0.55, 0.81]) (Figure 3). Results from the stratified analysis by sex indicated that, the risk of progressing to kidney failure within 2 years was not statistically significantly different among men and women (Figure 3). Subgroup analyses stratified by cause of CKD revealed that the risk of progressing to kidney failure did not vary significantly among patients with diabetes nephropathy, hypertensive nephropathy, and glomerulonephritis. The adjusted HR (95% CI) were 0.83 (0.62, 1.10), 0.82 (0.52, 1.30), and 0.87 (0.56, 1.34), respectively (P-value: 0.765).
Figure 3.
Results from the Fine and Gray subdistribution hazard model stratified by sex.
∗Adjusted for age, sex, race, cause and vintage of CKD, baseline comorbidities including diabetes, CVD-related comorbidities, respiratory diseases and cancer, baseline usage of immunosuppressive medication, Renin-angiotensin-aldosterone system inhibitors (RAASi; angiotensin-converting-enzyme inhibitors and Angiotensin receptor blockers) and sodium-glucose cotransporter-2 (SGLT2) inhibitors. CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; KFRE, Kidney Failure Risk Equation.
Impact of Change in eGFR on the Process for Dialysis Initiation
Among all patients with an eGFR of ≤25 ml/min per 1.73 m2 from CKD-EPI 2009 equation, 724 patients (18%) were reclassified to have an eGFR of >25 ml/min per 1.73 m2 from CKD-EPI 2021 equation. Longitudinal follow-up with these patients demonstrated a 75% lower risk of progressing to kidney failure within 2 years compared to patients who were not reclassified. The adjusted HR and 95% CI from the Fine and Gray subdistribution hazard model was 0.25 (0.19, 0.33) (Figure 3). This risk also did not vary by sex (P-value: 0.19) (Figure 3).
Impact of Change in eGFR on the KFRE Risk Score Predicting the Short-Term Risk of Progressing to Kidney Failure
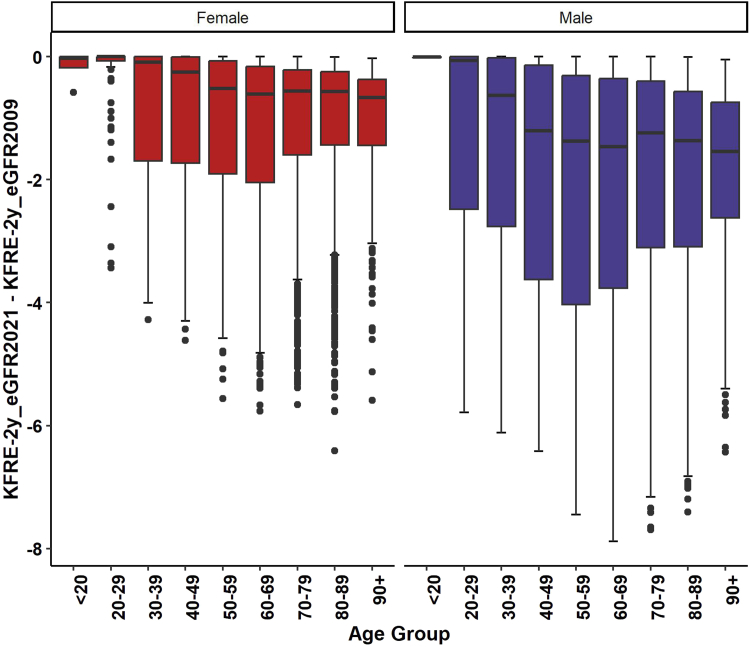
The KFRE-2 score calculated using eGFR from CKD-EPI 2021 equation (KFRE-2_eGFR_CKD-EPI 2021) was less than the KFRE-2 score calculated using eGFR from CKD-EPI 2009 equation (Figure 4). The difference in median (interquartile range) KFRE-2 score was −0.84 (−2.52, −0.26). The difference was larger in males than in females (P-value < 0.0001). The difference in median (interquartile range) KFRE score was −1.33 (−3.37, −0.40) in males and −0.54 (−1.66, −0.17) in females. The difference also varied by age (P < 0.0001) where the difference appeared to be higher in the patients aged between 50 and 80 years (Figure 4).
Figure 4.
Difference in KFRE 2-year risk score calculated using CKD-EPI 2009 and 2021 equations by age and sex. CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; KFRE, Kidney Failure Risk Equation.
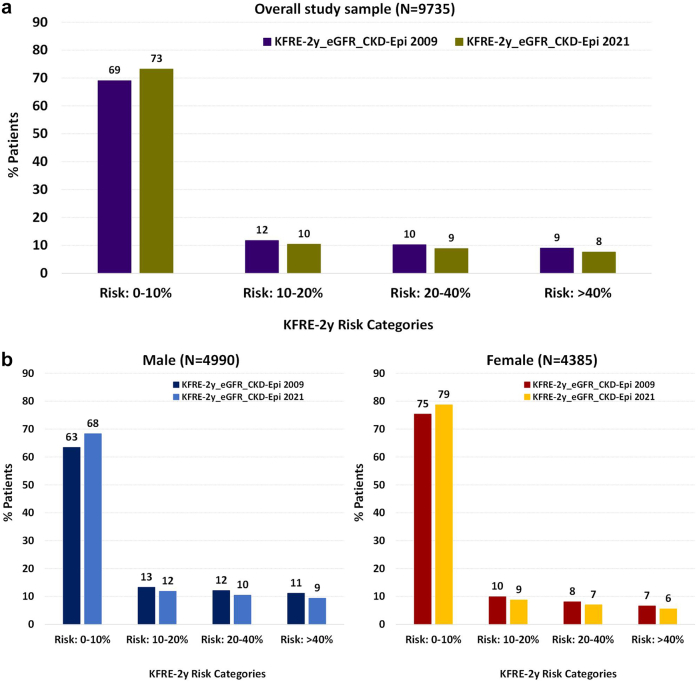
Compared to KFRE-2 score calculated using eGFR from CKD-EPI 2009 equation, KFRE-2_eGFR_CKD-EPI 2021 resulted in greater proportion of patients in lower risk category; the proportions of patients in KRFE score 0% to 10% were 69% and 73%, respectively (Figure 5). The proportion of patients in the other KFRE score ranges (10%–20%, 20%–40%, and >40%) were consistently lower when eGFR from CKD-EPI 2021 equation was used in the KFRE calculation (Figure 5). However, the difference was not substantially different. The proportion of females appeared to be substantially higher in the lower risk group. However, the directionality in reclassification did not differ by sex. Results from a sensitivity analysis after including patients with imputed UACR revealed similar distribution in KFRE risk score (Supplementary Figure S2).
Figure 5.
Distribution of KFRE 2-year risk calculated using eGFR from CKD-EPI 2021 versus 2009 equation. CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; KFRE, Kidney Failure Risk Equation.
A total of 792 (8%; [95% CI: 8%, 9%]) out of 9375 patients were moved to a lower KFRE-2 years risk score range when eGFR from CKD-EPI 2021 equation was used in the KFRE calculation (Table 4). Approximately 16% patients who had the KFRE-2 score calculated using eGFR from CKD-EPI 2009 equation of >40% were reclassified to have KFRE-2 years risk score of ≤40% when eGFR from CKD-EPI 2021 equation was used (KFRE-2_eGFR_CKD-EPI 2021 ≤40%) (Table 4). Results from the sensitivity analysis after including patients with imputed UACR showed similar reclassification (Supplementary Table S3).
Table 4.
Concordance on KFRE-2 risk score when eGFR from CKD-EPI 2009 and CKD-EPI 2021 was used in the equation
| KFRE-2 score by eGFR from CKD-EPI 2009 equation | KFRE-2 risk score by eGFR from CKD-EPI 2021 equation |
||||
|---|---|---|---|---|---|
| 0%–10% | 10%–20% | 20%–40% | >40% | Total | |
| 0%–10% | 6471 [100%] | 0 | 0 | 0 | 6471 |
| 10%–20% | 390 [36%] | 708 [64%] | 0 | 0 | 1098 |
| 20%–40% | 0 | 267 [28%] | 694 [72%] | 0 | 961 |
| >40% | 0 | 0 | 135 [16%] | 710 [84%] | 845 |
| Total | 6861 | 975 | 829 | 710 | 9375 |
CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; KFRE, Kidney Failure Risk Equation.
Among all patients with a the KFRE-2 score calculated using eGFR from CKD-EPI 2009 equation of >40%, patients who were reclassified to have a KFRE-2_eGFR_CKD-EPI 2021 of ≤40% had a 62% reduced risk of progressing to kidney failure within 2 years compared to patients with a KFRE-2_eGFR_CKD-EPI 2021 of >40%. The adjusted HR and 95% CI from the Fine and Gray subdistribution hazard model was 0.38 (0.28, 0.52) (Figure 3). Sensitivity analysis after including patients with imputed UACR revealed similar risk, the HR (95% CI) was 0.41 (0.31, 0.54).
In a separate sensitivity analysis including a contemporary cohort of 16,037 patients with CKD who were registered in PROMIS as of March 31, 2021, the directionality and magnitude of variation in eGFR obtained from CKD-EPI 2009 and 2021 equations resembled to the findings from the primary analysis using a prevalent cohort of patients with CKD from 2016 (Supplementary Table S4).
Discussion
In this observational study, we investigated the impact of implementing CKD-EPI 2021 equation compared to 2009 equation among a population level registry-based cohort of patients with CKD from BC, Canada. We found that, eGFR calculated using CKD-EPI 2021 equation was substantially higher than that estimated using 2009 equation. In the overall study sample, average increase in eGFR by the 2021 equation was 2.7 ml/min per 1.73 m2. Consequently, approximately 17% of the overall study sample, that is, 1 in every 6 patients with CKD would have been reclassified to a higher eGFR category, for example from G5 to G4 or from G4 to G3 and so on. The highest proportion (28%) of patients were reclassified from G5 category (eGFR <15 ml/min per 1.73 m2) to the G4 category. When considering reclassification in categories of kidney failure risk, these findings were attenuated (8% vs. 16%). In all scenarios, patients who were reclassified had a lower risk of progression to kidney failure.
Our findings are concordant with recent findings in the literature. For example, Inker et al.6 found that, among a non-Black study population, the new equation without race overestimated measured GFR by a median of 3.9 ml/min per 1.73 m2. In a retrospective cohort study evaluating the impact of implementing the CKD-EPI 2021 equation in Sweden, Fu et al.7 reported a median increase of 3.9 ml/min per 1.73 m2 in eGFR and the increment was larger in older people and in men. The author also reported that, approximately 10% of the overall study sample was reclassified to a higher eGFR category by CKD-EPI 2021 equation. Although the directionality of the impact was similar in both estimate of GFR and proportion of patients reclassified, the magnitude appeared to vary between studies. This variation could be explained by the differences in the study sample. For example, the Swedish study population was much younger (48 years vs. 73 years) with slightly higher proportion of females (53% vs. 48%). In addition, the author reported that the study population was predominantly White compared to ∼60% in the present study with ∼18% Asian population. Similar findings were also reported by several studies involving various study populations worldwide.8, 9, 10,12 When compared with measured eGFR, CKD-EPI 2021 equation appeared to overestimate the eGFR in elderly patients and in patients with eGFR <60 ml/min per 1.73 m2.12 Gregg et al.8 reported that, compared to CKD-EPI 2009 equation, the new 2021 equation would identify 16,906 fewer US veterans with new CKD stages 3 to 4. In a separate study involving Asian-Indian population, Khandpur et al.9 found that the 2021 equation provided higher values of eGFR in ∼98 % of the study sample with a change in eGFR category in 8% of the patients.
We also found that, compared to those who were not reclassified (stayed at the same category of eGFR obtained from CKD-EPI 2009 equation), patients who were reclassified to a higher eGFR category by 2021 equation had a significantly lower risk of progressing to kidney failure. This risk reduction appeared to be even higher among patients with CKD in G4 and G5 categories (eGFR <30 ml/min per 1.73 m2). Similar to the composite outcome of kidney failure, we observed significantly lower risk of maintenance dialysis initiation among patients who were reclassified to have eGFR >25 versus ≤25 ml/min per 1.73 m2. The increased eGFR obtained from the new CKD-EPI 2021 equation appeared to be concordant with the outcomes observed, in that those with higher eGFR did not progress to dialysis. Our findings were in accord with the similar lower risk of kidney failure with replacement therapy among Swedish patients with CKD who were reclassified to a higher eGFR category by the 2021 CKD-EPI equation.7
Unlike increased risk of death reported by Fu et al.,7 we observed a 15% reduction in the risk of death before kidney failure among patients with CKD who were reclassified to a category with higher eGFR. The difference in the risk estimate for death could be explained by several factors as follows: first, Fu et al.7 follow-up with patients over a 12-year period for all cause mortality compared to our definition of death before kidney failure within 2 years from the index date. The difference in the rate of death among study samples may play a major role in analyses involving competing risk of death. Second, Fu et al.7 used cause-specific Cox proportional hazard model versus the Fine and Gray subdistribution hazard model used in this study. Although, both of these models are frequently used in analyzing survival data in the presence of competing risk, substantial difference exists in how the risk set, that is, population at risk is defined between models. Therefore, the hazards estimated from these models have different interpretations.15,20,22
Adopting the new 2021 CKD-EPI equation would have an impact on routine patient care. For example, the current guidelines recommend blood work for sodium, potassium, chloride, bicarbonate, urea, and creatinine in every 6 months for patients in eGFR category G3a, every 3 months in G3b, every 2 months in G4, and every month in G5. Our findings indicated that, 28% of patients in the current G5 category would be reclassified to have higher eGFR by 2021 equation. Thus, 1 in every 4 patients with current eGFR <15 ml/min per 1.73 m2 would have bloodwork drawn every 2 months instead of monthly. These changes to clinical care have important implications for patients and health resource utilization, especially given that the reclassifications based on CKD-EPI 2021 better correlate with health outcomes. Similarly, it is currently recommended that the process of dialysis modality education is initiated when a patient reaches an eGFR of ≤25 ml/min per 1.73 m2 and referred to create vascular access for dialysis when eGFR reaches at ≤20 ml/min per 1.73 m2. Because the 2021 CKD-EPI equation without race provides higher eGFR, it would potentially delay these referrals for advanced patient care in a substantial proportion of patients with CKD in BC, Canada. However, based on the reduced risk of kidney failure observed in those patients reclassified using the CKD-EPI 2021 equation, this delay may be appropriate.
Our results showed a consistent direction, but reduced magnitude impact of implementing the CKD-EPI 2021 equation of risk-based categories based on the KFRE. This is an expected finding, because the KFRE includes variables other than eGFR, and therefore decisions made on KFRE-based thresholds are less sensitive to change in GFR estimating equations than eGFR-based thresholds. It is also reassuring to note that patients whose risk was downgraded had less than half the risk of those who remained in their original category. These results support the ongoing use of the unchanged KFRE with the CKD-EPI 2021 equation, and further support a move away from eGFR-based thresholds and toward risk-based thresholds for modality education, dialysis planning, transplant referral, and waitlisting. Unlike eGFR category G5 with highest proportion of patients reclassified to a higher eGFR category (G4), the KFRE >40 group was actually affected the least; that is, had the lowest proportion of “switchers” with the 2021 equation. It is reassuring that this clinically relevant KFRE cutoff whereby more intensive activity begins was the least impacted.
Previous studies warranted caution in transitioning from 2009 to 2021 CKD-EPI equation. Ebert et al.12 compared the accuracy of both 2009 and 2021 CKD-EPI equations with measured GFR using invasive iohexol plasma clearance method as a gold standard. The study concluded that, the new 2021 CKD-EPI equation overestimated GFR which would inaccurately decrease the prevalence of CKD among White elderly population.12 In the current study, we compared the new equation with the existing 2009 equation. Both of these equations are well-validated, though they remain estimates of GFR. Note is made that noncreatinine-based estimates using cystatin C may provide additional information, and potentially approximate true GFR more readily.6 The demography of patients with CKD in BC, Canada is unique, with approximately 60% White followed by 18% Asian ethnicity. According to the census by Statistics Canada, only 1.3% of the BC population was identified as self-reported Black. Thus, removal of the race coefficient (Black race) may be less relevant to the BC population. However, our findings are interesting, and given the current recommendations to adopt validated eGFR equations across regions or countries, within a Canadian context, there is a need to consider the implications fully. The findings are hypothesis generating and future prospective research with appropriate gold standard (using measured GFR in specific ethnic groups) is required before adopting the 2021 CKD-EPI equation in estimating GFR in BC laboratories.
Our study has several strengths. The large study sample was created from the population-level registry database for patients with CKD in BC, Canada. Therefore, the observations are expected to be representative for the entire province. Access to individual patient level clinical data enabled us to calculate eGFR using both 2009 and 2021 CKD-EPI equations. The longitudinal nature of the database allowed us to further investigate the impact of difference in those estimates of GFR on the actual kidney outcomes over time. Our study also had a few limitations. We investigated the association between reclassification of patients in eGFR categories by the new CKD-EPI 2021 equation with progression of CKD while the patients have been factually managed with a GFR estimated by the CKD-EPI 2009 equation. Although, in the multivariable model we adjusted for several background factors as well as treatment with various medications, caution should be exercised in interpreting the results due to the observational nature of the study. In addition, although we created a population level cohort of patients with CKD in BC, Canada, the sample size is still small compared to a few contemporary studies in this research area.7,8,10,23 We applied the 2021 CKD-EPI equation in a cohort of patients with CKD from 2016. However, these equations are time invariant. In addition, findings from the sensitivity analysis including patients with CKD from 2023 was similar indicating the robustness of our analyses.
In conclusion, the new CKD-EPI 2021 equation without race provides a higher estimate of GFR compared to 2009 equation among patients with CKD from BC, Canada. The population is multiethnic with a small proportion of Black individuals. This difference in eGFR values appeared to be concordant with subsequent real-world CKD progression outcomes. The higher eGFR from 2021 equation may have substantial clinical implications in both the diagnosis as well as long-term care of patients with CKD. Future prospective research including a gold standard comparison group using alternative filtration markers or measured GFR is recommended before province-wide transitioning from CKD-EPI 2009 to 2021 equation.
Disclosure
All the authors declared no competing interests.
Acknowledgments
An abstract of this study was presented in The American Society of Nephrology, ASN Annual Meeting 2023.
Data Availability Statement
This study was conducted using deidentified data from Patient Records and Outcome Management Information System. Study data can be accessed with prior approval from the Ethics Board and the data steward.
Footnotes
Figure S1. Distribution of patients in various KDIGO risk categories by eGFR calculated using CKD-EPI 2009 versus 2021 equation (sensitivity analysis after including patients with imputed UACR, N = 11,604).
Figure S2. Distribution of KFRE 2-year risk calculated using eGFR from CKD-EPI 2009 versus CKD-EPI 2021 equations (sensitivity analysis after including patients with imputed UACR, N = 11,604).
Table S1. Distribution of patients in various eGFR categories after calculating eGFR using CKD-EPI 2009 versus 2021 equation and stratified by sex.
Table S2. Results from the Fine and Gray subdistribution hazard model for the competing event outcome of death before kidney failure.
Table S3. Concordance on KFRE-2 risk score when eGFR from CKD-EPI 2009 and CKD-EPI 2021 was used in the equation (sensitivity analysis after including patients with imputed UACR, N = 11,604).
Table S4. Difference in eGFR obtained from CKD-EPI 2009 versus 2021 equations (sensitivity analysis including contemporary cohort of patients with CKD registered in PROMIS as of March 31, 2023, N = 16,037).
STROBE Statement.
Supplementary Material
Figure S1. Distribution of patients in various KDIGO risk categories by eGFR calculated using CKD-EPI 2009 versus 2021 equation (sensitivity analysis after including patients with imputed UACR, N = 11,604).
Figure S2. Distribution of KFRE 2-year risk calculated using eGFR from CKD-EPI 2009 versus CKD-EPI 2021 equations (sensitivity analysis after including patients with imputed UACR, N = 11,604).
Table S1. Distribution of patients in various eGFR categories after calculating eGFR using CKD-EPI 2009 versus 2021 equation and stratified by sex.
Table S2. Results from the Fine and Gray subdistribution hazard model for the competing event outcome of death before kidney failure.
Table S3. Concordance on KFRE-2 risk score when eGFR from CKD-EPI 2009 and CKD-EPI 2021 was used in the equation (sensitivity analysis after including patients with imputed UACR, N = 11,604).
Table S4. Difference in eGFR obtained from CKD-EPI 2009 versus 2021 equations (sensitivity analysis including contemporary cohort of patients with CKD registered in PROMIS as of March 31, 2023, N = 16,037).
STROBE statement.
References
- 1.Detection & warning signs. Kidney Foundation. https://kidney.ca/Kidney-Health/Newly-Diagnosed/Detection-Warning-Signs
- 2.CKD evaluation and management. KDIGO. https://kdigo.org/guidelines/ckd-evaluation-and-management/
- 3.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delgado C., Baweja M., Burrows N.R., et al. Reassessing the inclusion of race in diagnosing kidney diseases: an interim report from the NKF-ASN task force. J Am Soc Nephrol. 2021;32:1305–1317. doi: 10.1681/ASN.2021010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delgado C., Baweja M., Crews D.C., et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis. 2022;79:268–288.e1. doi: 10.1053/j.ajkd.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Inker L.A., Eneanya N.D., Coresh J., et al. New creatinine- and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu E.L., Coresh J., Grams M.E., et al. Removing race from the CKD-EPI equation and its impact on prognosis in a predominantly White European population. Nephrol Dial Transplant. 2022;38:119–128. doi: 10.1093/ndt/gfac197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregg L.P., Richardson P.A., Akeroyd J., Matheny M.E., Virani S.S., Navaneethan S.D. Effects of the 2021 CKD-EPI creatinine eGFR equation among a national US veteran cohort. Clin J Am Soc Nephrol. 2022;17:283–285. doi: 10.2215/CJN.10000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khandpur S., Awasthi A., Behera M.R., Purty A.J., Singh N.P., Tiwari S. Kidney disease burden in an Asian Indian population: effect of the new 2021 serum creatinine CKD-EPI equation. Diabetes Res Clin Pract. 2022;193 doi: 10.1016/j.diabres.2022.110120. [DOI] [PubMed] [Google Scholar]
- 10.Meeusen J.W., Kasozi R.N., Larson T.S., Lieske J.C. Clinical impact of the refit CKD-EPI 2021 creatinine-based eGFR equation. Clin Chem. 2022;68:534–539. doi: 10.1093/clinchem/hvab282. [DOI] [PubMed] [Google Scholar]
- 11.PROMIS. BCRenal. http://www.bcrenal.ca/health-professionals/professional-resources/promis
- 12.Ebert N., Pottel H., van der Giet M., Kuhlmann M.K., Delanaye P., Schaeffner E. The impact of the new CKD-EPI equation on GFR estimation in the elderly. Dtsch Ärztebl Int. 2022;119:694–695. doi: 10.3238/arztebl.m2022.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levin A., Agarwal R., Herrington W.G., et al. International consensus definitions of clinical trial outcomes for kidney failure: 2020. Kidney Int. 2020;98:849–859. doi: 10.1016/j.kint.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Tonelli M., Wiebe N., Culleton B., et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 15.Andersen P.K., Geskus R.B., de Witte T., Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol. 2012;41:861–870. doi: 10.1093/ije/dyr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tangri N., Stevens L.A., Griffith J., et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305:1553–1559. doi: 10.1001/jama.2011.451. [DOI] [PubMed] [Google Scholar]
- 17.Kuningas K., Stringer S., Cockwell P., Khawaja A., Inston N. Is there a role of the kidney failure risk equation in optimizing timing of vascular access creation in pre-dialysis patients? J Vasc Access. 2022;24:1305–1313. doi: 10.1177/11297298221084799. [DOI] [PubMed] [Google Scholar]
- 18.Inston N., Lok C.E. Improving precision in prediction: using kidney failure risk equations as a potential adjunct to vascular access planning. J Vasc Access. 2019;20:95–97. doi: 10.1177/1129729818786630. [DOI] [PubMed] [Google Scholar]
- 19.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 20.Austin P.C., Lee D.S., Fine J.P. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sumida K., Nadkarni G.N., Grams M.E., et al. Conversion of urine protein-creatinine ratio or urine dipstick to urine albumin-creatinine ratio for use in CKD screening and prognosis: an individual participant-based meta-analysis. Ann Intern Med. 2020;173:426–435. doi: 10.7326/M20-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau B., Cole S.R., Gange S.J. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan G., Nee R., Scialla J.J., et al. Estimation of black-white disparities in CKD outcomes: comparison using the 2021 versus the 2009 CKD-EPI creatinine equations. Am J Kidney Dis. 2022;80:423–426. doi: 10.1053/j.ajkd.2021.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study was conducted using deidentified data from Patient Records and Outcome Management Information System. Study data can be accessed with prior approval from the Ethics Board and the data steward.