See Clinical Research on Page 1072
Idiopathic hypercalciuria (IH) was described for the first time by Albright et al.1 as a condition of increased urinary calcium excretion without evident metabolic or endocrine causes, to differentiate it from disorders with known underlying mechanisms such as hyperparathyroidism, malignancy, sarcoidosis, and vitamin D poisoning. Since then, numerous regulatory pathways have been explored to clarify metabolic factors, cytokines, and genes contributing to IH in animal models and patient samples.2,3 Despite that, we still lack a better understanding of mechanisms leading to the increased urinary calcium excretion in patients with IH and explaining the unbalance among the determinants of calcium excretion, such as intestinal calcium absorption, bone metabolism, and tubular calcium reabsorption.
Due to its characteristics, IH is considered a multifactorial disorder involving multiple environmental factors and genes in its pathogenesis.2 Patients with IH show a complex phenotype, including alterations of bone remodeling, intestinal calcium absorption, and urinary calcium reabsorption. Because of its role in divalent ion homeostasis, exploring vitamin D regulatory pathways has become a wise approach in IH.4 Earliest studies hypothesized an increase of 1,25(OH)2D levels and a higher conversion of 25(OH)D to 1,25(OH)2D supporting calcium intestinal absorption in hypercalciuric patients.5
In addition to IH, monogenic forms of stone disease with hypercalciuria are known and mutation or polymorphisms at these genes may account for a proportion of nephrolithiasis and could contribute to the causal events and the phenotype of hypercalciuric patients.2 Idiopathic infantile hypercalcemia is a rare autosomal recessive monogenic disorder due to mutations in the CYP24A1 gene.6 The product encoded by CYP24A1 is an enzyme that can inactivate 25(OH)D and 1,25(OH)2D by hydroxylation of their molecules at the carbon in position 24. Mutations or polymorphisms at CYP24A1 gene may influence the activity of its enzymatic product and the distribution of vitamin D metabolites. Carriers of genetic variants as homozygotes or double heterozygotes develop the typical phenotype of idiopathic infantile hypercalcemia, including kidney stones and nephrocalcinosis and are characterized by hypercalciuria, hypercalcemia, low serum parathyroid hormone, and high serum concentrations of 1,25(OH)2D, and 25(OH)D. Heterozygous carriers for CYP24A1 variants may develop higher serum calcium and 25(OH)D concentrations compared to wild type and may be predisposed to kidney stone production. Studies in patients carrying these mutations showed that 25(OH)D-to-24,25(OH)2D ratio may express the activity of CYP24A1; the increase of this ratio occurs when the enzyme activity decreases and could identify patients carrying mutations at the CYP24A1 gene.
In this regard, the study by Dhayat et al.7 hypothesized a role of the vitamin D-inactivating enzyme (CYP24A1) in the pathogenesis of IH. In their observational, cross-sectional, cohort study, the authors used the vitamin D metabolite diagnostic ratio (25(OH)D/24,25(OH)2D ratio; VMDR) to estimate the vitamin D-inactivating enzyme (CYP24A1) activity (the higher VMDR, the lower CYP24A1 activity) and explored the association of VMDR with the variables involved in the phenotype of IH. The study included 974 adults aged ≥18 years having a clinical history with 2 or more kidney stones or 1 stone combined with additional risk factors for stone recurrence. Patients were recruited from the Swiss Kidney Stone Cohort and the Bern Kidney Stone Registry and had their bone densitometry and blood metabolic workup performed. Fractional excretion of calcium and mean 24-hour urinary calcium excretion were also determined. Age, sex (male/female), body mass index, estimated glomerular filtration rate, and baseline plasma 25(OH)D were addressed as potential confounders due to their capacity to interact with the total plasma 24,25(OH)D concentration. A sensitivity analysis was performed to address medications in use, urinary sodium excretion, and monthly fluctuations of VMDR because these factors can potentially affect urinary calcium excretion and total calcium balance. A VMDR <25 was considered normal, whereas VMDR between 25 and 80 identified monoallelic carriers of pathogenic CYP24A1 variants and VMDR >80 biallelic carriers of pathogenic CYP24A1. The study population was stratified in 2 subgroups according to their baseline levels of 25(OH)D < or ≥ 20 ng/ml, to further assess the association between clinical traits of IH and the VMDR model. Stone formers with low 25(OH)D (<20 ng/ml) had significantly higher VMDR compared to stone formers in the normal range. Interestingly, the association between VMDR and serum levels of 1,25(OH)2D was observed when the analysis was adjusted for fibroblast growth factor 23, a key factor in the regulation of 1,25(OH)2D synthesis. After univariate and multivariate linear regression analysis, the authors found an association between the CYP24A1 activity, estimated by VMDR, and serum calcium, urine calcium excretion, kidney stone composition, and bone mineral density at the femoral neck in patients with IH. The study captured a direct association of VMDR with both total and ionized plasma calcium, and an association between reduced CYP24A1 activity and a lower bone mineral density at the femoral neck.
Nephrolithiasis is the most common clinical expression of IH; however, it may also predispose to hematuria in children and bone mass loss in adults.8 This may explain the frequent association of IH and nephrolithiasis with osteopenia-osteoporosis and bone fracture. Due to its complex nature and its variable clinical presentation, diagnosis of IH usually occurs after its complications have already arisen, making the treatment of IH and the prevention of complications challenging.
Patients with nephrolithiasis usually undergo regular monitoring and follow-up schemes for years with therapy based on high fluid intake, potassium citrate, thiazide diuretic agents, and reduced sodium diet. These measures are usually prescribed for the prevention of recurrent calcium renal stones9 aiming at decreasing calcium excretion. However, due to the variability in symptoms and patient characteristics, and the unknown pathogenetic pathways involved in IH, developing individualized treatment plans remains a challenge.
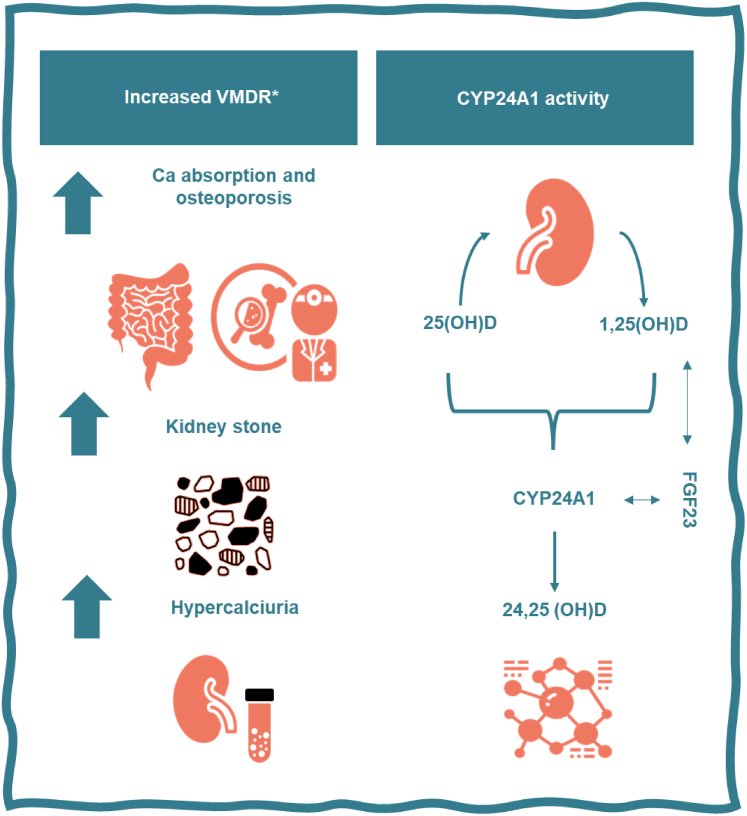
Given that the phenotype in patients with IH is sustained by different mechanisms and is influenced by a mixture of multiple factors acting in calcium metabolism, assessing treatment efficacy, adjusting interventions, and monitoring for complications are currently still very much conditional on the phenotypic expression of the urinary calcium excretion rather than in the correction of the dysfunctional pathways leading to the IH. The study by Dhayat et al.7 was featured not only by a detailed phenotype analysis, which included stone and bone mineral density measurements, but also by an in-depth investigation of the vitamin D cascade allowing a better understanding of its role in the pathogenesis of the IH. It is currently known that CYP24A1 expression is influenced by a complex array of substances, including inflammatory cytokines, diet, and genetic factors.2,3 The concept supported in the current study suggests an elevated VMDR to be secondary by either monoallelic or biallelic mutations in CYP24A1. These mutations would theoretically lead to increased levels of 1,25(OH)2D (relative to 25(OH)D and 24,25(OH)2D), increased intestinal calcium absorption, increased plasma calcium, suppressed parathyroid hormone, hypercalciuria and calcium stone formation (Figure 1). In the present study, high 1,25(OH)2D and low or suppressed parathyroid hormone were not commonly seen in patients, possibly reflecting its limitations due to the observational and cross-sectional nature; and the yet not fully understood complex chain of feedback elements involved, such as vitamin D metabolites, fibroblast growth factor 23, serum calcium, and phosphate levels. In addition, VMDR was used in this study as a surrogate marker for the activity of CYP24A1 expression limiting the casual implications of these findings.
Figure 1.
The 24-hydroxylation of 1,25(OH)2D and 25(OH)D is the first step in the catalytic pathway of these vitamin D metabolites. A deficiency in CYP24A1 leads to an increased expression of 1,25(OH)2D and 25(OH)D that increased the risk of kidney stone recurrence and osteoporosis though an increase of serum and urinary calcium. The effect on 1,25(OH)2D synthesis is hampered by the increase in fibroblast growth factor 23 production associated with CYP24A1 decreased activity.7
Despite the need for further validation, the study by Dhayat et al7 shed light on the contributions of the dysfunctional vitamin D pathway in the development of IH allowing us to envision a future where VMDR could be used both as a diagnostic tool and as a follow-up marker in the metabolic work-up of kidney stone formers. This will be especially useful in the prospect of the predictive capacity of VMDR in foretelling the risk of kidney stone recurrence and osteoporosis as such and in response to current treatment strategies. Finally, this study once again shows that monogenic diseases may be a rich source of information for understanding metabolic pathways in multifactorial disorders such as IH and calcium nephrolithiasis.
Disclosure
All the authors declared no competing interests.
Footnotes
Supplementary References
Supplementary Material
Supplementary References
References
- 1.Albright F., Henneman P., Benedict P.H., Forbes A.P. Idiopathic hypercalciuria: a preliminary report. Proc R Soc Med. 1953;46:1077–1081. doi: 10.1177/003591575304601218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vezzoli G., Terranegra A., Arcidiacono T., Soldati L. Genetics and calcium nephrolithiasis. Kidney Int. 2011;80:587–593. doi: 10.1038/ki.2010.430. [DOI] [PubMed] [Google Scholar]
- 3.Santos A.C.S., Lima E.M., Oliveira E.A., Silva A.C.S. Bone disease and cytokines in idiopathic hypercalciuria: a review. J Pediatr Endocrinol Metab. 2011;24:405–410. doi: 10.1515/JPEM.2011.243. [DOI] [PubMed] [Google Scholar]
- 4.Chmiel J.A., Stuivenberg G.A., Al K.F., et al. Vitamins as regulators of calcium-containing kidney stones — new perspectives on the role of the gut microbiome. Nat Rev Urol. 2023;20:615–637. doi: 10.1038/s41585-023-00768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Insogna K., Broadus A., Dreyer B., Ellison A., Gertner J. Elevated production rate of 1,25-dihydroxyvitamin D in patients with absorptive hypercalciuria∗. J Clin Endocrinol Metab. 1985;61:490–495. doi: 10.1210/jcem-61-3-490. [DOI] [PubMed] [Google Scholar]
- 6.Schlingmann K.P., Kaufmann M., Weber S., et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med. 2011;365:410–421. doi: 10.1056/NEJMoa1103864. [DOI] [PubMed] [Google Scholar]
- 7.Dhayat N., Mattmann C., Seeger H., et al. The vitamin D metabolite diagnostic ratio associates with phenotypic traits of idiopathic hypercalciuria. Kidney Int Rep. 2024;9:1072–1082. doi: 10.1016/j.ekir.2024.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan L.E., Ing S.W. Idiopathic hypercalciuria: can we prevent stones and protect bones? Cleve Clin J Med. 2018;85:47–54. doi: 10.3949/ccjm.85a.16090. [DOI] [PubMed] [Google Scholar]
- 9.Coe F.L., Worcester E.M., Evan A.P. Idiopathic hypercalciuria and formation of calcium renal stones. Nat Rev Nephrol. 2016;12:519–533. doi: 10.1038/nrneph.2016.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.