Introduction
Primary hyperoxaluria is a rare autosomal recessive genetic disorder due to impaired glyoxylate liver metabolism resulting in the accumulation of oxalate. Three types are described with a wide range of severity. Type 1 primary hyperoxaluria is the most common, caused by mutations in the alanine-glyoxylate aminotransferase gene. This results in recurrent nephrolithiasis and nephrocalcinosis from childhood to early adulthood, with the development of chronic and end-stage kidney disease at any age.1
Case Presentation
In 2018, a 19-year-old woman with a history of end-stage kidney disease due to type 1 primary hyperoxaluria was referred to our department for consideration of a combined liver and kidney transplantation. The patient was 16-year-old when the diagnosis was established and was on maintenance dialysis for 3 years. Pretransplantation workup revealed a cardiomyopathy with reduced left ventricular ejection fraction, and the patient had obvious growth retardation and multiple bone deformations. These findings were suggestive of a systemic oxalosis with cardiac and bone involvement, although this could also be partly attributed to complications of chronic kidney disease. Urinary oxalate levels were at 0.36 mmol/mmol of creatinine (normal <0.03) and serum oxalate levels were not measured at that time.
On admission, the initial workup revealed mild laboratory abnormalities, including blood leukocytes count at 3 G/l, hemoglobin level at 8.5 g/dl and platelets count at 151 G/l. Mean globular volume was normal and reticulocytes were at 50 G/l. There was no evidence for hemolysis, iron-deficiency, and vitamin B-deficiency. After multidisciplinary evaluation, the patient underwent a combined liver and kidney transplantation from a deceased donor. Induction immunosuppressive therapy included antithymocytes globulins in addition to glucocorticoids, tacrolimus, and mycophenolate mofetil. During the first weeks following transplantation, hematological parameters progressively decreased with hemoglobin level, platelet, and white blood cell counts at 6.8 g/dl, 65 G/l, and 1.7 G/l, respectively. There was no evidence for thrombotic microangiopathy, nor cytomegalovirus, B19 parvovirus, and Epstein-Barr virus reactivation. A thorough hematological investigation was performed.
Results
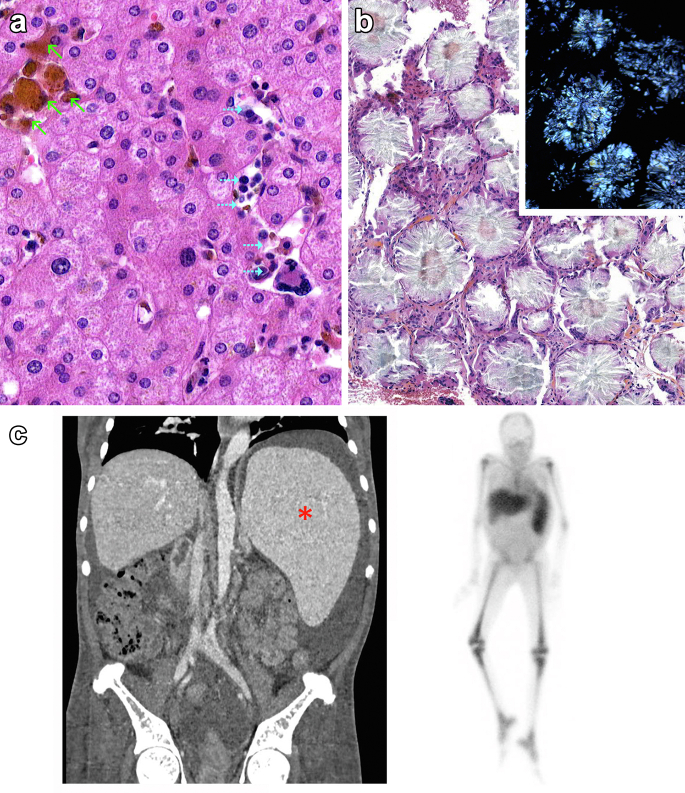
Analysis of the native liver revealed a normal parenchyma with sinusoid capillaries obstructed by small clusters of red blood (green arrows) and myeloid precursors as well as scattered megakaryocytes (dashed blue arrows) consistent with extramedullary liver hematopoiesis (Figure 1a). A bone marrow aspiration revealed a dry tap; thus, a bone marrow biopsy was performed and found the presence of extensive deposition of oxalate crystals that appears highly birefringent on polarized light microscopy (inset) (Figure 1b). Contrast-enhanced abdominal computed tomography-scan (Figure 1c, left) showed massive splenomegaly (red asterisk) without lymphadenopathy, and 111In-chloride scintigraphy confirmed the diagnosis of bone marrow failure and spleen extramedullary erythropoiesis (Figure 1c, right).
Figure 1.
(a) Histopathological analysis of the native liver specimen by hematoxylin and eosin staining revealing a normal parenchyma with sinusoid capillaries obstructed by small clusters of red blood (green arrows) and myeloid precursors as well as scattered megakaryocytes (dashed blue arrows) consistent with extramedullary hematopoiesis. (b) Bone marrow biopsy showing diffuse oxalate crystals deposits that appears highly birefringent on polarized light microscopy (inset) combined with severe alterations of the bone marrow architecture (not shown), consistent with bone marrow failure. (c) (Left) Contrast-enhanced abdominal computed tomography-scan showing massive splenomegaly (red asterisk); (Right) 111In-chloride scintigraphy showed a marked reduction in the uptake of the tracer by the marrow, and a significant uptake by the spleen, consistent with the diagnosis of bone marrow failure and spleen extramedullary erythropoiesis.
Discussion and Follow-Up
The kidney represents the main route of oxalate elimination and is the prime target for oxalate deposition. Systemic oxalosis occurs when the kidneys fail to excrete oxalate into the urine, typically once chronic kidney disease is settled. Oxalate overload may then result in systemic tissue deposition, particularly in the bones, blood vessels, heart, joints, and retina.2 Although the presence of calcium oxalate crystals in the bone marrow is a common finding in the course of primary hyperoxaluria, bone marrow failure and extramedullary hematopoiesis is a rare complication. The association of pancytopenia, splenomegaly, and a dry tap at bone marrow aspiration in this context was indeed highly suggestive of medullary oxalosis. The presence of extensive deposition of oxalate crystals in the bone marrow biopsy confirmed the diagnosis (Table 1).
Table 1.
Teaching points
| The association of pancytopenia and hepatomegaly or splenomegaly in the context of primary hyperoxaluria is suggestive of bone marrow failure due to crystals deposition and extramedullary hematopoiesis. |
| Pancytopenia in the context of medullary oxalosis is usually not responsive to stimulating agents and might require iterative transfusions and be responsible of specific complications as in this case, where leucopenia might have contributed to the occurrence of sepsis. |
| RNA interference therapy should thus be considered as a first-line treatment in every case of primary oxalosis, considering the significant morbidity associated with liver transplantation, and could have prevented the aggravation of bone marrow failure under immunosuppressive drugs as in the case of our patient. |
In the present case, the worsening of hematological features after transplantation is likely multifactorial. Mycophenolate mofetil was discontinued, and the patient underwent supportive blood transfusions in addition to the initiation of erythropoiesis-stimulating agents (darbepoetin alfa, up to 40 μg [1.1 μg/kg] per week) and granulocyte colony-stimulating factor. Unfortunately, there was no substantial improvement, and the patient died shortly after the transplantation because of septic shock.
Combined liver and kidney transplantation is an effective treatment for primary hyperoxaluria, by providing replacement of the deficient enzyme and a new route of excretion for the oxalate crystals. A stabilization or even reversal of systemic oxalosis may be obtained in case of successful treatment, but the clearance of tissular crystals is slow; thus, early diagnosis and treatment of oxalosis are crucial before irreversible organ damages. More recently, new treatment options are available, including RNA interference therapy, and are currently changing the management paradigm, by reducing the development of severe forms; and most likely decreasing the need for liver transplantation, and its associated morbidity, in the future (Table 1).3
Disclosure
All the authors declared no competing interests and no funding was received for conducting this study.
Patient Consent
The authors declare that they have obtained consent from the family of the patient discussed in the report.
References
- 1.Singh P., Viehman J.K., Mehta R.A., et al. Clinical characterization of primary hyperoxaluria type 3 in comparison with types 1 and 2. Nephrol Dial Transplant. 2022;37:869–875. doi: 10.1093/ndt/gfab027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devresse A., Cochat P., Godefroid N., Kanaan N. Transplantation for primary hyperoxaluria Type 1: designing new strategies in the era of promising therapeutic perspectives. Kidney Int Rep. 2020;5:2136–2145. doi: 10.1016/j.ekir.2020.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groothoff J.W., Metry E., Deesker L., et al. Clinical practice recommendations for primary hyperoxaluria: an expert consensus statement from ERKNet and OxalEurope. Nat Rev Nephrol. 2023;19:194–211. doi: 10.1038/s41581-022-00661-1. [DOI] [PubMed] [Google Scholar]