Abstract
Introduction: Metabolic acidosis is very common amongst critically ill sepsis patients partly due to the presence of unmeasured ions in serum. These ions can be detected by anion gap (AG) or strong ion gap (SIG) concentration values. The purpose of this study is to assess the correlation and potential agreement of the two methods in critically ill patients with sepsis. Materials and Methods: The present is a retrospective study including septic patients admitted to the Intensive Care Unit from December 2014 to July 2016. The [SIG] and the [AG] corrected for albumin and lactate ([AGcl]) were calculated on admission and on sepsis remission or deterioration. The correlation of the two parameters was assessed in all patient groups using the Pearson correlation coefficient and linear regression analysis and the agreement with Bland-Altman plots. ROC survival curves were also generated for the patients in relation to the values of [AGcl], [SIG] and inorganic [SIG] ([SIGi]) on admission. Results: There was a strong correlation linking [AGcl] and [SIG] values (r>0.9, P<0.05) in all patient groups. The results from all three linear regression equations were statistically significant as the models predicted the [AGcl] value from the [SIG] value with high accuracy. The mean difference of the two methods (i.e. [AGcl] - [SIG] in every patient separately) in septic patients on admission was 11.75 mEq/l with 95% limits of agreement [9.7-13.8]; in patients with sepsis deterioration, it was 11.8 mEq/l with 95% limits of agreement [9.8-13.7] and in patients with sepsis remission, it was 11.5 mEq/l with 95% limits of agreement [10.4-12.7]. ROC survival curves demonstrated a small area under the curve (AUC): [SIG] AUC: 0.479, 95% CI [0.351, 0.606], [SIGi] AUC: 0.581, 95% CI [0.457, 0.705], [AGcl] AUC: 0.529, 95% CI [0.401, 0.656]. Conclusion: [AGcl] and [SIG] demonstrate excellent correlation in septic patients, with a mean difference of about 12 mEq/l. Both parameters failed to demonstrate any predictive ability regarding patient mortality.
Keywords: Sepsis, metabolic acidosis, anion gap, strong ion gap, physicochemical approach
Introduction
In simple terms, sepsis is a syndrome in which an infection leads to organ dysfunction/failure (one or more) that is life-threatening to the patient [1]. Pathophysiologically, these organ dysfunctions are caused by a dysregulated host response to the infection. Main consequences of this response are disturbances in macrocirculation, microcirculation and even in the ability of the cells themselves to utilize oxygen, resulting in tissue hypoxia/dysoxia and multiple organ dysfunction syndrome [2]. Thus, the functionality of the respiratory and cardiovascular system, the kidneys, the liver, the coagulation system, as well as the central nervous system can be affected, resulting, for the latter, in altered mental status. Dysfunction severity for the various organs and systems is assessed by particular criteria, specific to each of them; for example the severity of respiratory failure can be expressed by the reduction of the PaO2/FiO2 ratio, while the perturbation of the coagulation system from the reduction in the number of platelets. Septic shock, specifically, refers to a condition in which the underlying circulatory and cellular/metabolic abnormalities are severe enough to further increase mortality. Patients with septic shock present with refractory hypotension requiring vasopressors in order to maintain a mean arterial pressure (MAP) ≥65 mmHg despite adequate volume resuscitation; they also have a serum lactate level >2 mmol/L [1].
Finally, in addition to the specific clinical and laboratory findings observed in each infection, in a septic condition, the same applies as a corollary of the organism’s systemic inflammatory response (SIRS) itself [3], i.e. tachypnoea, tachycardia, fever or hypothermia, leukocytosis or leukopenia. Occasionally, impaired level of consciousness may be the only clinical sign of sepsis, particularly in elderly individuals. Apart from lactate, certain biomarkers may also be deranged and measured. These include markers of the hyper-inflammatory response (pro-inflammatory cytokines and chemokines), as well as markers of the immunosuppressive phase of sepsis (anti-inflammatory cytokines) and also proteins such as C-reactive protein and procalcitonin, which are synthesized in response to infection and inflammation [4].
Overall, septic patients often present with a wide range of complex pathophysiological disorders, which the clinician (and most often the ICU physician) is called upon to recognize and treat. The spectrum of these disorders also includes acid-base balance disorders, in which metabolic acidosis predominates. Metabolic acidosis is very common in patients with sepsis and septic shock in the ICU. It is also an important prognostic indicator and has even been shown to be related with mortality in patients who retain the ability to compensate for this disorder [5]. Whether this correlation is primarily due to the acidosis or the underlying disorder (e.g. renal failure) has been debated in the recent medical literature. Nevertheless, it appears that patients who do not survive, have lower pH values compared to survivors, and indeed the aetiology of acidosis in non-survivors appears to be metabolic rather than respiratory [6]. On the other hand, if acidemia per se was an important factor influencing patient mortality, one would expect that treating it with e.g. direct administration of sodium bicarbonate (NaHCO3) would lead to significant reduction in patient mortality. However, the studies that have addressed this question did not show any benefit in terms of mortality [7], which also reflects on the fact that the recommendation for the administration of NaHCO3 concerns, at the time of writing, mainly severe cases of acidemia (pH≤7.10) due to metabolic acidosis. Thus, a cornerstone of the treatment of metabolic acidosis in the ICU is the treatment of the causal disorder, which can be extremely complex in critically ill patients, in which many different factors may contribute to acidemia. Factors such as disturbances in water homeostasis, repletion with normal saline solutions, renal failure, disturbances of the renin-angiotensin-aldosterone system, lactic acidosis, as well as the presence of “unmeasured ions”, other than lactate, may contribute to the observed metabolic acidosis through different pathways. The presence of unmeasured ions is of particular interest, as they can reveal important clinical implications, while their timely finding often acts as a guide for a targeted therapeutic approach.
The concept of unmeasured ions is often misleading and can be confusing to the clinician. Any ion not included in the anion gap formula is designated as “unmeasured” and is included in the gap. The gap contains a number of anions and cations that have been difficult historically to measure in daily clinical practice, but many of them are now routinely measured, especially in the ICU setting. Characteristic examples of common cations that are not included in the formula are Mg2+ and Ca2+, while respectively the anions not included in the original gap formulas are L-lactate, PO3-4 and albumin. L-lactate specifically was the main “unmeasured” anion during the introduction of the “ion gap concept” and indeed the capacity of the most accurate lactate estimation has been used as a measure of comparison among the various methods of the “gaps” calculation [8]. Nowadays, measuring L-lactate is easy and widely available on most blood gas analyzers. The utility of unmeasured ion detection methods now lies in the range and clinical implications of the remaining substances included in this category. Many of these are involved in common poisonings such as salicylic acid (aspirin), glycols (ethylene glycol and propylene glycol from antifreeze and disinfectants), pyroglutamate (a metabolite of acetaminophen involved in acetaminophen poisoning), and methanol. Other ions are products of metabolism and accumulate in pathological conditions, such as ketones, phosphate and sulfate ions, as well as D-lactate. The latter is a product of bacterial metabolism in the gastrointestinal tract and it is usually increased in gastrointestinal diseases, especially in short bowel syndrome. Of particular interest is the fact that in many septic patients, there is a disproportionate increase of the unmeasured ions’ values relative to the values of L-lactate, phosphate, and albumin [9]. This observation has been attributed to an accumulation of Krebs’ cycle ions such as citrate, isocitrate and α-ketoglutarate [10]. This may be due to the assumption that Krebs’ cycle intermediates are generated rather than consumed.
There are two main approaches to identify acid-base disorders in clinical practice; the physiological approach, based on the application of the Henderson-Hasselbach equation for the bicarbonate buffering system, and the physicochemical approach which was introduced by Stewart and views serum pH as a product of three main factors: the strong ion difference (SID), the CO2 content, consequently the partial pressure of CO2 in blood, and the total concentration of non-volatile acids ([Atot]). An extensive review of both methods and their advantages and disadvantages is beyond the scope of this article. Nevertheless, both of these approaches incorporate a method to estimate the number of unmeasured ions present in the blood. The physiological approach uses the concept of anion gap, which is represented by the equation:
[AG] = [Na+] + [K+] - ([Cl-] + [HCO3-])
The anion gap is now virtually always corrected for the value of albumin (anion gap corrected - AGc) as follows:
[AGc] = [AG] + 2.5 × (4 - [Albumin g/dl])
Further, the anion gap can be adjusted to also incorporate L-lactate measurements (anion gap corrected for lactate - AGcl), as follows [11]:
[AGcl] = [AGc] - [L-lactate (mmol/l)]
Stewart’s approach, on the other hand, estimates unmeasured ions by using the difference between the apparent strong ion difference:
[SIDa] = [Na+] + [K+] + [Ca2+] + [Mg2+] - [Cl-] - [L - Lactate-]
And the effective strong ion difference:
[SIDe] = [HCO3-] + [A-]
Their difference is termed the Strong Ion Gap ([SIG] = [SIDa] - [SIDe]). This approach effectively incorporates the additional relative contribution of phosphate ions in the anion gap, in addition to accounting for the ionized forms of albumin, as:
[A-] = [Albumin g/l] × (0.123 × pH - 0.631) + [Phosphate mmol/l] × (0.309 × pH - 0.469)
At this point, it would be essential to mention the effects of albumin and lactate in both the [AG] and the [SIG]. A decrease in serum albumin is accompanied by an equal increase in the remainder pool of unmeasured weak anions, mainly bicarbonates, provided that [SIDa] does not change. This leads to [SIDe] and [SIG] values ([SIDa] - [SIDe]) both remaining steady. On the other hand, an increase in lactate is accompanied by an equivalent decrease in [HCO3-]. Thus, if we include [Lac-] in the calculation of [SIDa], [SIDa] will decrease, corresponding to a similar decrease in [SIDe]; the net effect is a constant [SIG] value. This is expected, since [SIG] corresponds to the concentration of the unknown anions and cannot account for a possible increase in [Lac-] in case we include it in the calculation of [SIDa] - making it, thus, a measured/known anion. In case of lactate alterations, the same applies for [AGcl] calculation, which remains steady. However, in case we do not include [Lac-] in [SIDa] calculation (inorganic [SIDa]), [SIDa] will not change, [SIDe] will decrease due to the lower [HCO3-] and, thus, [SIG] will increase, accounting for the increased [Lac-]. The same applies for the [AGc] calculation. In the case of albumin, the normal [AG] is affected, since albumin mainly (along with phosphates) contributes most of the normal [AG] charge. Thus, in case of hypoalbuminemia, a decrease in normal [AG] is expected. This is why we should always correct [AG] for [Alb-], by calculating what [AG] would be if [Alb-] was normal. Only the albumin-corrected anion gap can be compared with the considered normal (corresponding to normal [Alb-]).
Regarding the clinical relevance of the calculated gaps, it should be emphasized that the presence of unmeasured ions, especially exogenous ones, can have significant effects on the human organism, with the main example being the metabolites of methanol and ethylene glycol which can lead to visual loss and brain oedema. Accordingly, the finding of an increased [SIG] seems to have prognostic value in some patient populations such as post-cardiac arrest [12], in patients with acute renal failure [13], in insecticide poisoning [14] and in paediatric populations with malaria [15]. A recent study also demonstrated the importance of [SIG] as a prognostic factor in medical and surgical ICU patients [16]. On the other hand, some studies have failed to find a correlation between [SIG] and mortality [17]. Nevertheless, the rapid detection of unmeasured ions is of particular importance, especially in cases where there is specific treatment or they can be removed by renal replacement therapy.
The ideal paraclinical method of identification of unmeasured ions has not been clarified. However, the fact that [SIG] includes more parameters and accounts for the effect of pH on the phosphate and albumin content, makes it a theoretically more potent method, although it is limited by the complexity of the calculations. Studies have demonstrated a satisfactory correlation between [SIG] and [AGc] values, especially when corrected for phosphate and/or lactate values [14,18]. Thus, as the measurement of [AGc] is easier compared to the calculation of [SIG], the former could possibly replace the calculation of [SIG] in clinical practice. Physiologically, [SIG] and [AGc] represent different concepts. The former represents the charge difference between all anions and cations, the normal value of which is zero, while the latter represents a concentration difference whose normal value is usually close to 12 mEq/l and is mostly due to the unaccounted presence of phosphate and albumin in serum. As such, conceptually, the presence of unmeasured ions is represented by a divergence of SIG from zero and of [AGcl] above its normal value ([ΔAGcl]).
The purpose of this study is to examine the potential association and accordance between the [AGcl] and the strong ion gap [SIG] in ICU patients with sepsis. While extensive research has been held regarding this association in a general ICU population, to our knowledge there is no study regarding specifically septic patients. Our hypothesis is that in septic patients, [SIG] and the increase of [AGcl] can be used interchangeably to account for the presence of unmeasured ions.
Materials and methods
This is a retrospective study conducted using data from patients who were admitted to the Intensive Care Unit of the 1st Department of Respiratory Medicine of the National and Kapodistrian University of Athens, of Athens General Hospital for Thoracic Diseases “Sotiria”, during the time period between December 2014 and July 2016 and had a diagnosis of severe sepsis or septic shock on admission. The data was retrieved from the prospective observational study “Buffering Capacity in Sepsis: A Prospective Cohort Study in Critically Ill Patients” [19] that had taken place in “Sotiria” Hospital.
Inclusion and exclusion criteria
All patients admitted to the ICU, diagnosed with severe sepsis or septic shock according to the criteria in effect at the time of the original study’s initiation [20], were considered eligible for being enrolled in the study. Of note, the revised sepsis definitions of 2016 [1] did not affect patient recruitment. In addition, patients who took part in the original study - and were also included in the present study - had to have a central venous catheter placed in the subclavian or jugular vein, in case this was deemed necessary by the attending physician. Patients admitted to the ICU with a different diagnosis, as well as those without a subclavian or jugular central venous line (usually patients with less severe disease in whom placement of a central venous line was deemed unnecessary) were excluded from the study.
Most particularly, regarding the diagnosis of severe sepsis and septic shock that was done following the international guidelines in effect at the time of initiation of the sampling [20]: Sepsis diagnosis was based on the presence of a suspected infection and clinical or microbiological evidence of infection in the presence of at least two of the four SIRS criteria, i.e. 1) body temperature above 38°C or below 36°C, 2) heart rate greater than 90 beats per minute, 3) respiratory rate greater than 20 beats per minute or carbon dioxide partial pressure below 35 mmHg, and 4) neutrophilia above 12000/mm3 or neutropenia below 4000/mm3 with 10% or more of non-segmented peripheral blood neutrophils. Septic shock was defined as sepsis-induced hypotension despite adequate fluid resuscitation in the presence of perfusion abnormalities. During the time period of the study, a revision of the above guidelines [1] was published, which, as noted, did not affect sampling.
Assessed clinical and laboratory variables
On admission to the ICU, patient demographics and body measurements, medical history, and APACHE II and SOFA scores were recorded, the latter having been calculated daily during hospitalization. Arterial blood samples were obtained - through an arterial line placed in the radial or brachial artery - from which measurements for common biochemical markers and acid-base balance analyses were performed. The samples for the blood gas analyser were taken anaerobically in 2 ml heparinized syringes, while the samples for the other analyses were taken in non-heparinized syringes. The measurement of PaCO2, PaO2, lactate, pH and sodium (Na+), potassium (K+) and chloride (Cl-) ion concentration values was done using a blood gas analyser (RapidLab®1200 Systems, 2009, Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA). Calculation of [HCO3-] was conducted automatically by the gas analyser using the Henderson-Hasselbach equation. Calcium (Ca2+), albumin and phosphate concentrations were measured using a Dimension®EXL. 200 Integrated Chemistry System analyser (2011, Siemens Healthineers, Newark, Delaware, USA). All measurements were repeated, within the study, during clinical improvement or deterioration of each patient.
Definition of the clinical stages of the septic episode
Clinical improvement was defined as: 1. Restoration of hemodynamic stability (systolic blood pressure ≥90 mmHg and mean blood pressure ≥65 mmHg) for more than 24 hours, without the need for vasopressors or inotropic drugs and without markers of tissue hypoxia (lactic acid levels <2 mEq/l and no prolongation of capillary refill time) and 2. Normal temperature (T, 36°C≤Θ≤38 C) and white blood cell levels (WBC 4000/μl≤WBC≤12000/μl with <10% immature forms) and 3. Reduction of SOFA score ≥2 points.
Alternatively, if, at the discretion of the treating physician the patient was discharged from the ICU, the parameters on the day of discharge from the ICU were recorded as clinical remission of sepsis.
Deterioration was defined as: 1. Significant aggravation of hemodynamic status with SOFA cardiovascular score ≥3, increase in lactic acid ≥1 mEq/l with a total value ≥2 mEq/l or increase in the need for administration of vasoactive drugs (increase in norepinephrine ≥0.1 μg/kg/min or addition of other vasoactive drugs), as long as these were not attributed to a form of shock other than septic (e.g. obstructive) and 2. SOFA score increase ≥1 since admission.
Estimated acid-base parameters
The following equations were used to estimate the required parameters:
•[SIDa] = [Na+] + [K+] + [Ca2+] - [Cl-] - [L-Lactate-]
•[SIDai](Inorganic SID) = [Na+] + [K+] + [Ca2+] - [Cl-]
The present equation takes into account only the inorganic strong ions and excludes the organic lactate
•[SIDe] = [HCO3-] + [Albumin g/l] × (0.123 × pH - 0.631) + [Phosphate mmol/l] × (0.309 × pH - 0.469)
•[AGcl] = [Na+] + [K+] - ([Cl-] + [HCO3-] + [L-Lactate-]) + 2.5 × (4 - [Albumin g/dl])
Strong ion gap is calculated by the difference of [SIDe] from [SIDa]. Thus,
[SIG] = [SIDa] - [SIDe]
From the equation above also arises the next equation:
[SIGi] (Inorganic [SIG]) = [SIDai] - [SIDe]
Definition of acid-base disorders
For the purposes of this research paper, acidemia was defined as a serum pH<7.35 and alkalemia as a serum pH>7.45. Hyperchloremic acidosis was defined using a serum [Cl-] measurement >107 mEq/l and dilutional acidosis as a serum [Na+]<138 mEq/l. Hypochloremic alkalosis was defined as a serum [Cl-]<102 mEq/l and concentrational alkalosis as a serum [Na+]>144 mEq/l. The normal limits for [SIDa] were defined between 35 mEq/l and 42 mEq/l, while a [SIG] value above 6 mEq/l was considered abnormal.
Statistical analysis
The normal distribution of the data was checked by the Kolmogorov-Smirnoff or Shapiro-Wilkes methods, where appropriate. Comparison of the data of the three groups (i.e. all patients on admission, the improving patients and the deteriorating patients) was performed with the t-test for independent samples for parameters that followed a normal distribution and Mann-Whitney U for non-parametric data. After confirmation of normal distribution, Pearson’s correlation test was performed for [AGcl] and [SIG] for the three groups. At the same time, linear regression was performed and R2 was calculated, while a scatter plot of the data with a regression line was also created. Bland-Altman plots were drawn to investigate agreement of the two methods. Finally, ROC survival curves were generated for the patients in relation to the values (continuous) of [AGcl], [SIG] and [SIGi] at admission.
Parameter processing for the calculations of the requested variables, correlation analysis, linear regression and ROC curves were performed using IBM SPSS software (Version 26.0.0.0). MedCalc (Version 20.104) was used to create the Bland-Altman graphs. Tables were drawn on Microsoft Office Word 2016. The threshold of statistical significance for this study and for all analyses was considered to be P<0.05.
Institutional approval
This study was approved by the Scientific Council of the Athens General Hospital for Thoracic Diseases “Sotiria”, identified by the protocol number 29990/29-11-2021; all the while, the anonymity, privacy and confidentiality of the patients’ personal data were maintained. It was not deemed necessary to obtain patient consent for the use of the data.
Results
From the total sample of the initial study of 114 patients, 17 patients belonged to the control group so they were not included in the present study and 8 patients did not have sufficient data recorded to calculate the requested parameters so they were also excluded. The flow chart of the study is presented in Figure 1. The final sample included 89 patients.
Figure 1.
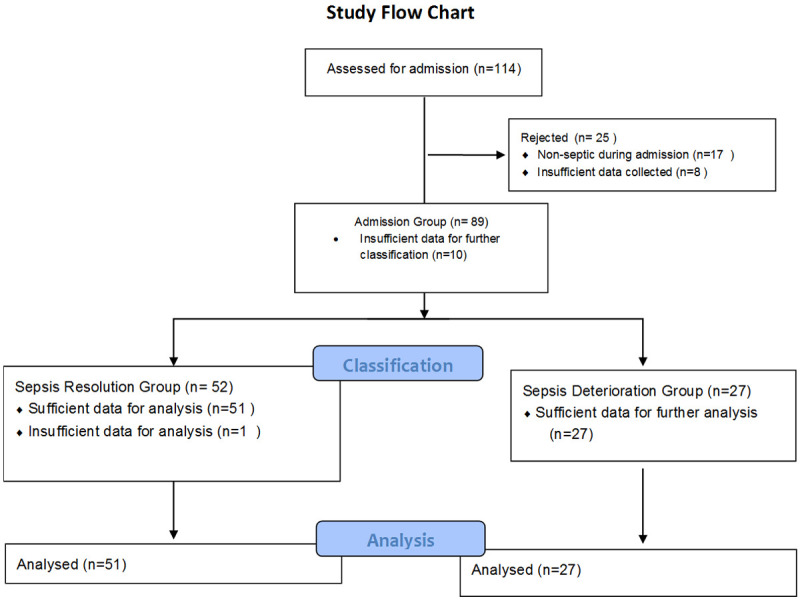
Study flow chart.
Sepsis aetiology
Of these, 78 (87.6%) had lower respiratory tract infection as the main cause of sepsis, two patients were diagnosed with abdominal infections (liver abscess and peritonitis caused by rupture of the hollow viscera), one patient with surgical wound infection, two patients with mediastinitis, one patient with infectious pericarditis, one patient with lower extremity gangrene associated with pneumonia, one patient with pyelonephritis, one patient with mitral valve endocarditis, and two patients with primary bacteremia.
Clinical variables
Of the 89 patients in the study, 27 presented sepsis deterioration, while 52 recovered and were categorized in the sepsis remission group. For 10 of the patients in the sample, insufficient data were collected during sepsis remission or exacerbation, but they were nevertheless included in the analysis of the admission data.
Patient characteristics at admission are reported in Table 1, while Table 2 lists the characteristics of the exacerbation and remission groups. Patients who recovered from sepsis had longer ICU stay, which is likely due to higher survival compared to patients who deteriorated. Accordingly, patients whose sepsis aggravated had statistically significantly higher severity scores (APACHEII and SOFA) at admission. The two groups did not differ significantly in terms of age, sex, BMI, duration of mechanical ventilation and basic acid-base indices. At the sepsis remission group 4 out of the 51 (7.8%) patients in the group needed continuous renal replacement therapy (CRRT), while in the exacerbation group CRRT was needed for 15 out of 27 patients (55%).
Table 1.
General characteristics of study patients
| N | 89 |
| Age (years) | 68 [55-77] |
| Male | 56 (62.9%) |
| BMI (Kg/m2) | 26.51 [23.67-29.41] |
| Duration of ICU stay (days) | 13 [7-28] |
| Duration of Mechanical Ventilation (days) | 13 [7-28] |
| Admission APACHEII | 23.73 (7.71) |
| Admission SOFA | 9.13 (3.25) |
| Mortality | 38.2% |
| Admission pH | 7.364 (0.083) |
| Admission PaCO2 (mmHg) | 45 (9.9) |
| Admission [HCO3-] (mEq/l) | 25.03 (4.99) |
Values are reported as Median [Interquartile range] or Mean (Confidence Interval). BMI - Body Mass Index, APACHEII - Acute Physiology and Chronic Health Evaluation II, SOFA - Sequential Organ Failure Assessment, ICU - Intensive Care Unit.
Table 2.
Characteristics of patients in the sepsis deterioration and resolution groups
| Deterioration Group | Resolution Group | p-value | |
|---|---|---|---|
| N | 27 | 52 | |
| Male:Female | 16:11 | 32:20 | >0.1 |
| Age (years) | 73 [66-77] | 63 [50-79] | 0.125 |
| BMI (Kg/m2) | 26.67 [23.45-29.41] | 26.17 [24.22-30.55] | 0.992 |
| Length of ICU stay (days) | 11 [5-20] | 19 [9-34] | 0.006 |
| Duration of Mechanical Ventilation (days) | 13 [6-21] | 16 [8-34] | 0.103 |
| Admission APACHEII | 26.6 (7) | 21.6 (7.7) | <0.05 |
| Admission SOFA | 10.3 (2.9) | 8.4 (3.1) | <0.05 |
| Admission pH | 7.35 (0.09) | 7.37 (0.07) | 0.27 |
| Admission PaCO2 (mmHg) | 43.3 (7.7) | 45.3 (10.3) | 0.38 |
| Admission [HCO3-] (mEq/l) | 23.6 (5.4) | 25.5 (4.8) | 0.11 |
| Mortality | N=25 (93%) | N=6 (12%) | <0.05 |
Values are reported as Median [Interquartile range] or Mean (Confidence Interval). The comparison of the two groups was done in the first case using the Mann-Whitney U test and in the second using the t-test for independent samples. BMI - Body Mass Index, APACHEII - Acute Physiology and Chronic Health Evaluation II, SOFA - Sequential Organ Failure Assessment, ICU - Intensive Care Unit.
Acid-base variables on admission
A total of 36 (40.4%) patients had acidemia on admission (mean pH 7.27, range [7.047, 7.344]), while 16 (17.9%) of them had pure metabolic acidosis (pH<7.35 and PaCO2<45 mmHg). The mean value of [AGcl] at admission was 14.88 (±2.85) mEq/l, [SIDa] 36.44 (±5) mEq/l and [SIG] 3.12 (±2.72) mEq/l. A total of 35 of 89 patients (39%) had metabolic acidosis due to low [SIDa] (<35 mEq/l): in 23 of them acidosis was attributed to water dilution ([Na+]<138 mEq/l), 21 had hyperchloremic acidosis and 10 presented mixed [SIDa] acidosis. 12 (13.5%) of the patients showed [SIDa] alkalosis ([SIDa]>42 mEq/l). Of these, 4 (33.3%) showed concentration alkalosis and 7 (58.3%) of 11 out of 89 (12.3%) patients had an elevated [SIG] (>6 mEq/l) on admission, 2 (18.18%) of whom had concomitant [SIDa] acidosis, 3 (27.27%) had [SIDa] alkalosis and the rest had normal [SIDa].
Acid-base variables at sepsis deterioration
A total of 19 (70.3%) patients in the deterioration group had acidemia (mean pH 7.17, range [6.7, 7.34]), with 11 (40.7%) of them having pure metabolic acidosis. In these patients the mean value of [AGcl] was 13.54 mEq/l (±4.77), [SIDa] 28.28 mEq/l (±6.27) and [SIG] 1.75 mEq/l (±4.22). Out of the 27 patients with worsening sepsis, 21 (77%) presented [SIDa] acidosis, while none presented [SIDa] alkalosis. Of the patients who developed [SIDa] acidosis, 13 (61.9%) had dilutional acidosis, 17 (80.9%) hyperchloremic acidosis, with 11 (52%) having mixed acidosis. Four patients in the worsening sepsis group had an elevated [SIG] (>6 mEq/l), 3 of whom had concurrent [SIDa]<35 mEq/l, while the last one had [SIDa] within normal limits.
Acid-base variables at sepsis remission
Of the 52 patients who recovered from sepsis, one had insufficient data to calculate [SIDa] and [SIG]. One (1.9%) patient in this group had acidemia (pH 7.34) which was purely of metabolic origin. The mean value of [AGcl] in the recovered patients was 14.19 (±3.31) mEq/l, [SIDa] 37.97 (±5.28) mEq/l and [SIG] 2.61 (±3.23) mEq/l. A total of 14 of 51 patients (27.4%) had [SIDa] acidosis: in 6 (42.8%) of the mit was dilutional acidosis, in 10 (71.4%) hyperchloremic and in 2 (14.2%) mixed. No patient with [SIDa]<35 mEq/l had elevated [SIG] (>6 mEq/l). On the other hand, 12 of the 51 patients (23.5%) presented [SIDa] alkalosis, with 4 (33.3%) of them presenting concentration alkalosis and 5 (41.6%) presenting hypochloremic alkalosis, while 1 patient presented, simultaneously, increased [SIDa] and [SIG]>6 mEq/l. All patients in the group had [Atot] alkalosis due to hypoalbuminemia. Five of the 51 (9.8%) patients in the remission group had [SIG]>6 mEq/l.
Patient acid-base characteristics are listed in Table 3.
Table 3.
Acid-base characteristics
| Admission (N=89) | Resolution (N=51) | Deterioration (N=27) | |
|---|---|---|---|
| [SIDa] Acidosis ([SIDa]<35 mEq/l) | 35 (39%) | 14 (27.4%) | 21 (77%) |
| Dilutional ([Na+]<138 mEq/l) | 23 (65.7%) | 6 (42.8%) | 13 (61.9%) |
| Hyperchloremic ([Cl-]>107 mEq/l) | 21 (60%) | 10 (71.4%) | 17 (80.9%) |
| Mixed | 10 (28.6%) | 2 (14.2%) | 11 (52%) |
| [SIDa] Alkalosis ([SIDa]>42 mEq/l) | 12 (13.5%) | 12 (23.5%) | 0 (0%) |
| Concentrational ([Na+]>144 mEq/l) | 4 (33.3%) | 4 (33.3%) | - |
| Hypochloremic ([Cl-]<102 mEq/l) | 7 (58.3%) | 5 (41.6%) | - |
| [SIG] Acidosis ([SIG]>6 mEq/l) | 11 (12.3%) | 5 (9.8%) | 4 (14.8%) |
| [Atot] Acidosis | 0 | 0 | 0 |
| Hyperalbunemic ([30]>4.9 g/dl) | - | - | - |
| Hyperphosphatemic ([17]≥2 mmol/l) | - | - | - |
| [Atot] Alkalosis | |||
| Hypoalbunemic ([30]<3.8 g/dl) | 89 (100%) | 51 (100%) | 27 (100%) |
| Pure Metabolic Acidosis | 16 (17.9%) | 1 (1.9%) | 11 (40.7%) |
AGcl: Anion gap corrected for albumin and lactate, Atot: Total concentration of non-volatile acids and conjugate bases, SIG: Strong Ion Gap, SIDa: Apparent Strong Ion Difference.
It is noted that all study patients met the threshold for hypoalbuminemia in all three analyses. The mean value of albumin at admission was 2.23 (±0.5) g/dl, in the sepsis remission group 2.19 (±0.485) g/dl and in the sepsis exacerbation group 1.71 (±0.52) g/dl.
Correlations among acid-base variables
[AGcl] and [SIG] were very well correlated, with r>0.9 (P<0.05) in all patient groups. Linear regression models were also created for each patient group in order to investigate whether [AGcl] values could predict those of [SIG]. [AGcl] was defined as the independent variable and [SIG] as the dependent variable. The results from all three linear regression equations were statistically significant, with the models predicting the [AGcl] value from [SIG] very accurately. Detailed characteristics of the models are listed in Tables 4 and 5. An indicative scatterplot with regression line for the patient group at admission is shown in Figure 2.
Table 4.
Pearson’s r correlation index for AGcl and SIG in the different patient groups
| Group | [AGcl] (mEq/l) | [SIG] (mEq/l) | Pearson r | p-value |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Admission (N=89) | 14.88 (2.85) | 3.12 (2.72) | 0.929 | <0.05 |
| Deterioration (N=27) | 13.54 (4.77) | 1.75 (4.22) | 0.982 | <0.05 |
| Resolution (N=52) | 14.19 (3.31) | 2.61 (3.23) | 0.985 | <0.05 |
SD: Standard Deviation, AGcl: Anion gap corrected for albumin and lactate, SIG: Strong Ion Gap.
Table 5.
Linear regression analysis data
| Group | F | p-value | R2 | Constant (Standard Error) | Coefficient (Standard Error) |
|---|---|---|---|---|---|
| Admission (N=89) | F (1.87)=549.4 | <0.05 | 0.863 | -10.101 (0.574) | 0.929 (0.038) |
| Deterioration (N=27) | F (1.25)=679,8 | <0.05 | 0.963 | -10.101 (0.481) | 0.982 (0.034) |
| Resolution (N=51) | F (1.49)=1645.3 | <0.05 | 0.970 | -10.894 (0.342) | 0.985 (0.024) |
Figure 2.
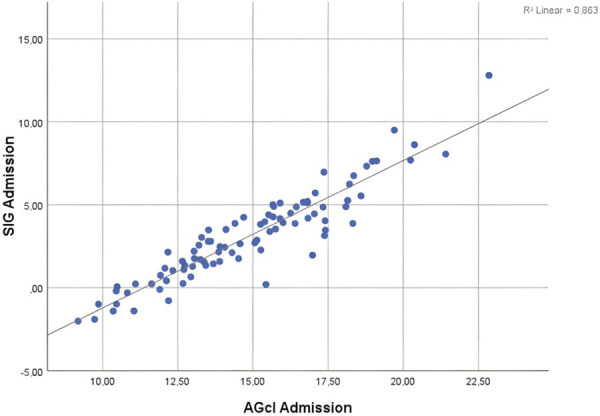
The regression line scatterplot of the values of [AGcl] and [SIG] at input is plotted. The linear regression equation is y = -10.101 + 0.929x. P<0.05.
Agreement of the compared measurements
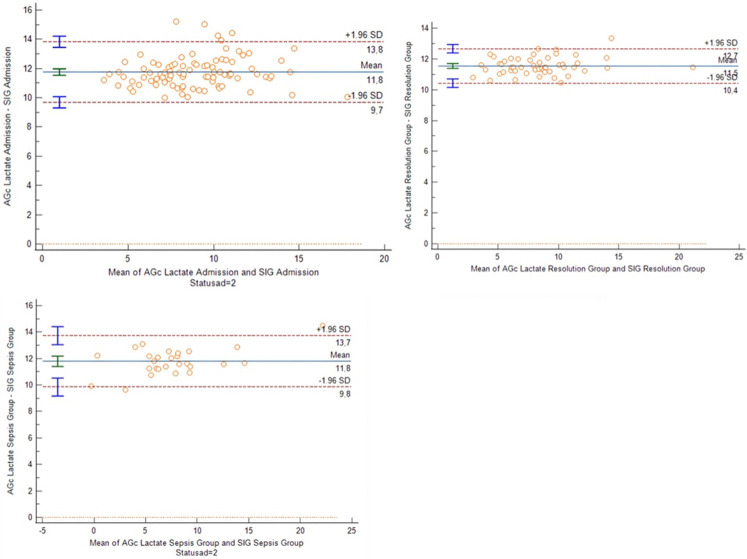
In addition, the agreement of the two methods measurements was investigated. Bland-Altman plots were drawn for the three groups (Figure 3), which demonstrated overall good agreement of [AGcl] and [SIG] for all patients. The mean difference of the two methods in septic patients at admission was 11.75 mEq/l with 95% limits of agreement [9.7-13.8]; in patients with sepsis deterioration, it was 11.8 mEq/l, with 95% limits of agreement [9.8-13.7]; in patients with sepsis remission, it was 11.5 mEq/l with 95% limits of agreement [10.4-12.7]. Visually the data appeared to have a rather normal distribution, which was statistically confirmed. The variance of data was also constant over the measurement range. The sensitivity and specificity of [AGcl] in predicting a high [SIG] value in the sampled patients was examined (Table 6). An [AGcl] value >17 mEq/l had a sensitivity of 100% and a specificity of 91.16% for ascertaining an elevated [SIG] (>6 mEq/l); higher thresholds appeared to lead to an increase in specificity and positive predictive value, but with a gradual decrease in sensitivity and negative predictive value.
Figure 3.
Bland-Altman plots of [AGcl] and [SIG] for patients during admission and on sepsis deterioration or remission. Note the mean difference of the two methods being around 12 mEq/l and similar in all 3 groups. The 95% limits of agreement are also almost identical in all patient groups. Visually the data appears to have a rather normal distribution, which was statistically confirmed. The variance of the data also was constant over the measurement range.
Table 6.
Sensitivity and specificity of [AGcl] in predicting increased [SIG] for different thresholds
| [AGcl] Increased | [AGcl] Increased | ||
|---|---|---|---|
| [AGcl]>16 mEq/l | SIG Normal | 122 | 25 |
| SIG Increased | 0 | 20 | |
| Sensitivity: 100% | Specificity: 82.9% | ||
| PPV: 44.4% | NPV: 100% | ||
| [AGcl]>17 mEq/l | SIG Normal | 134 | 13 |
| SIG Increased | 0 | 20 | |
| Sensitivity: 100% | Specificity: 91.16% | ||
| PPV: 60.6% | NPV: 100% | ||
| [AGcl]>18 mEq/l | SIG Normal | 143 | 4 |
| SIG Increased | 2 | 18 | |
| Sensitivity: 90% | Specificity: 97.28% | ||
| PPV: 81.82% | NPV: 98.62% |
AGcl: Anion gap corrected for albumin and lactate, SIG: Strong Ion Gap, PPV: Positive Predictive Value, NPV: Negative predictive value.
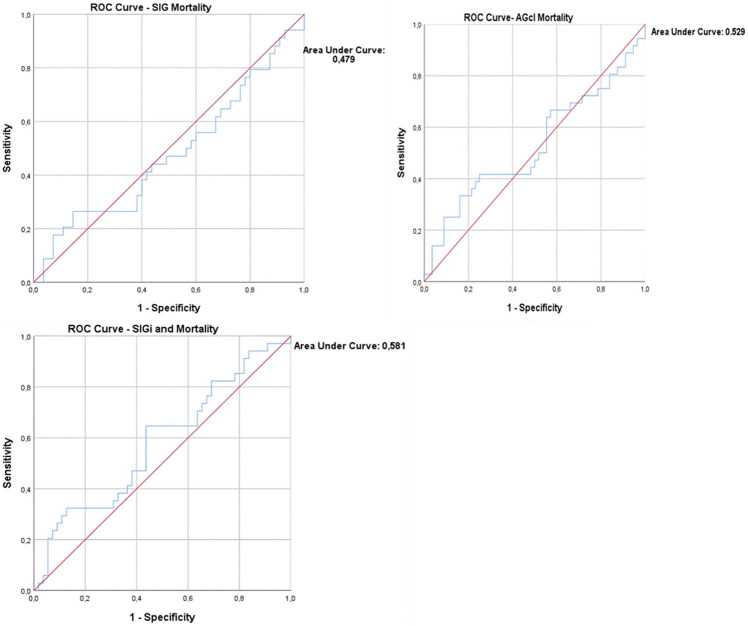
Finally, ROC curves were drawn to investigate whether [AGcl], [SIG] and [SIGi] at admission could predict mortality (Figure 4). All curves showed a small area under the curve (AUC), with that of [SIG] having AUC: 0.479, 95% CI [0.351, 0.606], [AGcl] AUC: 0.529, 95% CI [0.401, 0.656] and [SIGi] AUC: 0.581, 95% CI [0.457, 0.705].
Figure 4.
ROC mortality curves for [AGcl], [SIG] and [SIGi]. Note the small Areas Under the Curve (AUC) for all 3 parameters. For [SIG] AUC: 0.479, 95% CI [0.351, 0.606], for [SIGi] AUC: 0.581, 95% CI [0.457, 0.705] and for [AGcl] AUC: 0.529, 95% CI [0.401, 0.656]. None of the parameters demonstrated a significant predictive ability for mortality.
Discussion
This study analysed the correlation and possible alignment between [SIG] and [AGcl] in patients with sepsis and septic shock in an adult ICU. A primary question of the study was whether [AGcl] can replace the measurement of [SIG], since the calculation of the latter is clearly more difficult. For this purpose, it was initially considered necessary, in addition to the usual correction made to the [AG] values for albumin, to also account for the effect of [L-lactate], as its measurement is now widely available in all ICUs. No correction was made for phosphate; adding more measured parameters increases the complexity of [AG] measurement, depriving it of its advantage over [SIG], namely wide availability and faster measurement with fewer parameters. Furthermore, the correction for the physiologically occurring anions (such as phosphate and albumin), as it has been used in certain studies [17] is conceptually different from the meaning of the proposed classical correction of [AG] for albumin, and may lead to interpretive confusion or errors. Pertinent comments follow.
Of particular interest are certain patient characteristics in all three groups. Initially, all patients in all study analyses met criteria for [Atot] alkalosis due to low albumin values. Although hypoalbuminemia is an extremely common finding in critically ill patients and, even more so, in patients with sepsis and septic shock, the incidence generally reported in the literature, and depending on the diagnostic criteria used, is usually ranging between 70-80% [21]. In our study, all patients without exception were characterized by hypoalbuminemia using the laboratory cut-off value of 3.8 g/dl. In fact, the albumin concentration values in our patients were so low that even if more conservative limits were used to define hypoalbuminemia (e.g. <3.4 g/dl), their classification would be unchanged. This finding also may explain the disproportionately low incidence of acidemia in the study compared to that of metabolic acidosis disorders, as it is possible that the strongly alkalinizing effect of hypoalbuminemia overshadowed the effects of metabolic acidosis.
Regarding the acid-base characteristics of the patients, the very high prevalence of [SIDa] acidosis in the group of patients with sepsis deterioration is of note, while at the same time none of them showed [SIDa] alkalosis. On the contrary, patients who demonstrated sepsis remission had a much lower percentage of metabolic acidosis disorders, with the exception of [SIG] disorders, which were found in similar rate in the three groups of patients. The general observation, that patients who managed to cope with the initial septic episode improved their metabolic profile of acid-base balance, is in agreement with similar studies in the literature. Similarly, in a prospective observational study including sixty septic patients, Noritomi et al. [22] observed that patients who survived severe sepsis and septic shock managed to provoke a more effective compensation for metabolic acidosis disorders, i.e. clear unmeasured anions, compared with non-survivors.
At this point, a slight deviation from the main topic is in order to provide a comment: unfortunately, the authors, misunderstanding the mass conservation concept by Gattinoni et al. [23], misinterpreted the findings of their own study concerning the value of urinary [SID] in patients who did not survive compared to those who survived. Their finding, a consistently lower urinary [SID] in the former than in the latter, could be seen as completely expected, being indicative of a more intense effort by the body (kidneys) to cope with acidosis in non-survivors (acidosis was more severe in this patient group, as demonstrated by their lower plasma [SID]), with elimination of the excess acid in the urine, and does not constitute a “disparity” that “might be explained by the renal dysfunction in the non-survivors’ group, which did not allow them to develop an expected compensation”. A comparative review of the importance of [SID] (or [AG]) in urine exceeds the purposes of the present article, but has been the subject of a recent study that has instigated physiology debates [24].
Surprisingly, in our study, there was no significant difference among the percentage of patients with high [SIG] or the absolute values of [SIG] in the three groups. This phenomenon is probably attributed to the combination of two main factors, the small number of patients in the sepsis exacerbation group (27 patients) and the disproportionately greater use of CRRT, as more than half of the patients were undergoing CRRT on deterioration, in contrast to the remission group in which a very small percentage of patients were on CRRT. We hypothesized that the use of renal replacement therapy in this group may have reduced the [SIG] in some patients that would otherwise have been elevated. This hypothesis is also supported by literature pointing to unmeasured ion concentration reduction following CRRT [25].
In the present group of patients, the two parameters ([SIG] and [AGcl]) had an excellent correlation with each other, while the linear regression models created predicted the [SIG] value from [AGcl] with excellent accuracy in all patient groups. This finding is in agreement with the majority of studies in the literature showing a good correlation between the two parameters, which is further improved by adding lactate and albumin to the calculation of [AG] [17,18]. On the other hand, increased attention should be paid towards the use of uncorrected [AG] as a surrogate for [SIG], as it is known that in this case the two parameters do not correlate well with each other [26].
From the Bland-Altman diagrams, a systematic error in the differences between the two values emerged, with a mean difference of approximately 12 mEq/l for all groups. This finding is expected, given that the normal value of [SIG] is theoretically close to zero, while that of [AGcl] is close to 12 (±4) mEq/l. Another study that used Bland-Altman plots to compare the two methods was that of Zampieri et al. [17]. In this study, the [AG] was corrected for the values of phosphate ions, in addition to albumin and lactate; the systematic error between the two methods was 1.4 with limits of agreement -0.75 to 3.57. Nevertheless, in this study the mean value of [AG] corrected for albumin, lactate and phosphate was 6.69 with a standard deviation of 4.34-9.34, significantly lower than our own study and which is not justified only by the addition of phosphate in the anion gap calculation. The reason this difference is observed is because in that study the authors corrected the [AG] for albumin and phosphate charge, according to the methodology proposed by Kellum [26], i.e. by “correcting” for lactate, instead of adjusting the calculated value of [AG] according to the difference of albumin from its normal value. This methodology requires further clarification. Kellum labels as “corrected” anion gap ([AGc]) the “delta” anion gap ([ΔAG]); the [ΔAG] is what he actually calculated, correctly pointing out that its normal value should be close to zero [26]. Thus, in the study of Zampieri et al. [14], 6.69 is actually the [ΔAG] (difference between the calculated and what is considered as expected for the patient - with the further subtraction of [lactate]):
([Na+] + [K+] - [Cl-] - [HCO3-]) - (2 × [Albumin g/dl] + 0.5 × [phosphate mg/dl]) - [lactate mmol/l] [14].
The 2 × [Albumin g/dl] + 0.5 × [phosphate mg/dl] calculates the negative charges provided by the albumin and phosphate that are physiologically present in plasma, and should cover the deficit of the negative charges that the calculated anion gap indicates (charge difference of the measured anions compared to the measured cations), i.e. it corresponds to the expected anion gap for the patient, assuming no other unmeasured anions are present.
Some explanatory comments would be relevant here. The traditionally calculated [AG] (estimated through the concentrations of the most important, quantitatively, ions in plasma) gives an estimate of the concentration of unmeasured anions or, in other words, physiologically, the grade of unmeasured anion prevalence to unmeasured cations. In normal conditions, the main unmeasured anions are albumin and phosphate, especially albumin, doubling the concentration of which can lead to a 75% reduction in the normal anion gap. The 12 (mEq/l), which is considered a normal value (or any normal value), corresponds to a normal concentration of albumin (together with that of phosphates), and it is this value of [AG] that we use as a measure of comparison to conclude whether the [AG] we calculated is increased, i.e. if there are other, besides normal, anions in the plasma. This is also why we correct for albumin (the concentration of which is usually quite low in critically ill patients). To remind the corresponding formula:
[AGc] = [AG] + 2.5 × (4 - [Albumin g/dl])
Thus, the corrected for albumin [AG] estimates what the [AG] would be if the patient had normal albumin; consequently, in case of hypoalbuminemia, it increases the calculated value. This corrected value can now be compared with the considered normal value (e.g. 12 mEq/l). For example, if an [AG] value of 16 mEq/l is calculated and the patient’s normal [AG], for the given albumin levels, is 4 mEq/l, a significant high [AG] metabolic acidosis disorder will be missed, which may be considered unimportant, if correction for albumin levels is not conducted and it is assumed that the calculated [AG] is within the normal range (12±4 mEq/l). In Kellum’s method there is no correction in this sense (which is noted to be widely recommended); simply, from the calculated [AG] we subtract the expected [AG] for the patient - that is, that which corresponds to the normally present albumin and phosphate anions, the concentration of which are measured. Thus, the result of the difference gives a number regarding the concentration of ions that are not normally present (other than albumin and phosphate) and increase the [AG] above the expected normal value for the patient (it is conceptually similar to [SIG]). In addition, the correction for [lactate], excludes the concentration of lactate anions from the calculations, and the remaining difference concerns ‘unmeasured ions’ of another kind.
A study that used a similar methodology to ours was that of Moviat et al. [18]. This study had several similarities with ours in that it tested the association of [SIG] with [AG] corrected for albumin and lactate. However, the study involved a general population of critically ill patients in the ICU and not specifically patients with sepsis and septic shock. In addition to the strong correlation between [AGcl] and [SIG], which is also a finding of our own study, a very good agreement between the two methods was shown, with a systematic error of 1.81 and an accuracy of 0.96. In that study, however, [SIG] values >0 mEq/l were considered abnormal, in contrast to the cut-off 6 mEq/l presented in our study. At the same time, to assess the agreement, the measured value of [AGcl] was subtracted by 12 mEq/l (the normal [AG] value). If we had followed the same methodology, having subtracted the normal values from the upper normal limits of the two variables in order to eliminate this systematic “error”, we would have had similar results. Nevertheless, we felt that capturing this expected error provides useful clinical information, given that the normal limits of [SIG] and [AGcl] are unclear and vary significantly between studies.
Using 6 mEq/l as the upper normal limit of [SIG], based on the methodology of respective studies by Antonogiannaki et al. [27] and Zampieri et al. [17], the sensitivity and specificity of [AGcl] (at three different value ranges) to predict elevated [SIG] values were analysed. The analysis highlighted 17 mEq/l as an “ideal” limit for the study patients, which showed a sensitivity of 100% and a specificity of 91.2%. Using higher cut-offs for [AGcl] (e.g. 18 mEq/l) increased its specificity but led to a gradual decrease in its sensitivity. Given the clinical importance of calculating unmeasured ions, and if one considers [SIG]>6 mEq/l as the method of choice for this purpose, in this group of patients a cut-off of [AGcl]>17 mEq/l was considered better as a screening method. Of course, this limit was set with important assumptions and is not recommended to be widely used. As will be mentioned below, the reference method for finding unmeasured ions can only include their direct measurement. Therefore, these limits certainly cannot be generalized to other patient groups or if other electrolyte measuring devices are used.
Finally, we analyzed the predictive ability of [SIG] and [AGcl] on admission. None of the two variables showed any predictive ability for the patients’ mortality, with the AUCs being characteristically small. This finding is similar to that of Zampieri et al. [14], who found no association between [SIG] and patient mortality in a general ICU population. On the other hand, some other studies, such as the one by Mohr et al. [28], have concluded that there may be some prognostic value in the quantification of unmeasured ions, although in this particular one, the analysis was done using [AG] without correction for albumin or lactate. A more recent study [13] confirms the aforementioned findings.
Regarding the study sample, it is worth noting that all study patients were mechanically ventilated. Due to the limited ICU beds in the country during the data collection period, it was common practice to admit almost exclusively patients requiring mechanical ventilation to the hospital’s ICU. Although mechanical ventilation and sedation drugs have not been widely reported to affect [AG] or [SIG], one should consider the patient characteristics of this study before generalizing its results to all patients with septic shock. At the same time, due to the fact that the hospital in which the study was conducted specializes in diseases of the respiratory system, the study population is mainly represented by patients with respiratory diseases and this is also reflected in the fact that the main cause of sepsis in our sample is pneumonia. Meanwhile, there is only one patient in the study who presented sepsis due to urinary tract infection, few patients with sepsis due to surgical causes and no patient with central nervous system infection. Another limitation, mentioned earlier, is the broad use of CRRT in the sepsis aggravation group, which may have led to misinterpretation of their acid-base balance. In addition, the final study sample (89 patients) is relatively small, especially for statistical analyses involving mortality, the result of which may not be safe to generalize.
Finding the correct upper normal limit of [AGcl] and [SIG] values is a challenge. In the present study the threshold of SIG was defined as above at >6 mEq and this value was utilized to define the ideal threshold for [AGcl]. This decision has important limitations, however, as the upper physiological values of the [AG] reported in the literature have a wide range (ranging from 10 to 18 mEq/l [29,30]), while adding lactate to the equation further increases the uncertainty. The reason this happens is because both variables include certain substances as components, the normal concentrations of which vary greatly depending on the reagent and the method used to measure them. As a consequence, the more variables added to the equation, the wider the range of normal values. Thus, in order to define the normal range of both values, one should test [AGcl] and [SIG] in a group of hospital control patients. This was unattainable in this particular study, which was conducted in an ICU patient population, as by definition solely critically ill patients were included, while no similar study has been done in the hospital in order to determine the SIG reference values. Therefore, a corresponding study conducted in another Greek hospital and involving Emergency Department patients was used as a reference point [25]. Finally, a reliable comparison of [AGcl] and [SIG] in terms of their ability to detect “unmeasured ions” can be made only by using methods of direct measurement of these ions. As conducting such a study would be costly and the range of possible exogenous ions is large, to the best of our knowledge, this has not been reported in the international literature as of yet. In the past, the two methods were compared for their ability to detect elevated lactate values. However, the widespread availability of measuring this anion with modern gas analyzers has rendered this practice obsolete.
Based on the data of the present study, [AGcl] is a reliable and faster alternative method for detecting unmeasured ions in septic and non-septic (at sepsis resolution) patients in the ICU. However, the use of each method is subject to some limitations, which the clinician must be aware of. The main disadvantages of [AGcl] mainly arise from the impact the non-volatile acids have on its calculation. Although the method includes a correction for albumin values, this is a simplified one and does not take into account the effect of pH on the imidazole groups, which is included in the [SIDe] calculation. In addition, it does not take into account the influence of phosphorus ions. These disadvantages make [AGcl] “vulnerable” to extreme values of pH. However, these flaws did not appear to affect the ability of [AGcl] to detect unmeasured ionsin the population under study, provided that the correct upper normal limit is selected. At the same time, the excellent correlation and agreement of the two methods was confirmed in septic and non-septic intubated ICU patients, while it appeared that in our group of patients [AGcl] can be safely used as an alternative method of [SIG] with a positivity limit of 17 mEq/l and, in case of increased values, further investigation can be done with Stewart’s method. It is clear that there is a need to further compare [AGcl] with [SIG] and base deficit in terms of their ability to detect the presence of unmeasured ions, a need created by the potential complications these substances can pose and the existence of therapeutic options if recognized early. For this purpose, further studies involving the measurement of “non-measured ions” are necessary.
Finally, we should also mention another limitation of the study, regarding the definition of hyperchloremic acidosis as in plasma chloride concentration ([Cl-]>107 mEq/l). Due to the interdependence of [Cl-] with the corresponding concentration of sodium, it would be perhaps more appropriate to use another criterion, e.g. the [Cl-]/[Na+]>0.75, as in other studies [31]. Nevertheless, this limitation does not affect the primary object of the study.
Conclusion
According to our study, there is excellent correlation between [AGcl] and [SIG] in patients with sepsis. The mean difference in every specific team is between 11.5-11.8 mEq/l. While both parameters failed to demonstrate any predictability for patient mortality, this may be due to the relatively low number of patients included. Further investigation is needed to explore the possible correlation of the [AGcl]/[SIG] difference and mortality.
Disclosure of conflict of interest
None.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pecchiari M, Pontikis K, Alevrakis E, Vasileiadis I, Kompoti M, Koutsoukou A. Cardiovascular responses during sepsis. Compr Physiol. 2021;11:1605–1652. doi: 10.1002/cphy.c190044. [DOI] [PubMed] [Google Scholar]
- 3.Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372:1629–1638. doi: 10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- 4.Barichello T, Generoso JS, Singer M, Dal-Pizzol F. Biomarkers for sepsis: more than just fever and leukocytosis-a narrative review. Crit Care. 2022;26:14. doi: 10.1186/s13054-021-03862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wernly B, Heramvand N, Masyuk M, Rezar R, Bruno RR, Kelm M, Niederseer D, Lichtenauer M, Hoppe UC, Bakker J, Jung C. Acidosis predicts mortality independently from hyperlactatemia in patients with sepsis. Eur J Intern Med. 2020;76:76–81. doi: 10.1016/j.ejim.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 6.Kellum JA, Song M, Subramanian S. Acidemia: good, bad or inconsequential? In: Vincent JL, editor. Yearbook of Intensive Care Medicine. 2002. pp. 510–516. [Google Scholar]
- 7.Lo KB, Garvia V, Stempel JM, Ram P, Rangaswami J. Bicarbonate use and mortality outcome among critically ill patients with metabolic acidosis: a meta analysis. Heart Lung. 2020;49:167–174. doi: 10.1016/j.hrtlng.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Tuhay G, Pein MC, Masevicius FD, Kutscherauer DO, Dubin A. Severe hyperlactatemia with normal base excess: a quantitative analysis using conventional and Stewart approaches. Crit Care. 2008;12:R66. doi: 10.1186/cc6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mecher C, Rackow EC, Astiz ME, Weil MH. Unaccounted for anion in metabolic acidosis during severe sepsis in humans. Crit Care Med. 1991;19:705–711. doi: 10.1097/00003246-199105000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Hussain M, Zaki KE, Asef MA, Song H, Treger RM. Unmeasured organic anions as predictors of clinical outcomes in lactic acidosis due to sepsis. J Intensive Care Med. 2023;38:975–982. doi: 10.1177/08850666231177602. [DOI] [PubMed] [Google Scholar]
- 11.Chawla LS, Shih S, Davison D, Junker C, Seneff MG. Anion gap, anion gap corrected for albumin, base deficit and unmeasured anions in critically ill patients: implications on the assessment of metabolic acidosis and the diagnosis of hyperlactatemia. BMC Emerg Med. 2008;8:18. doi: 10.1186/1471-227X-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funk GC, Doberer D, Sterz F, Richling N, Kneidinger N, Lindner G, Schneeweiss B, Eisenburger P. The strong ion gap and outcome after cardiac arrest in patients treated with therapeutic hypothermia: a retrospective study. Intensive Care Med. 2009;35:232–239. doi: 10.1007/s00134-008-1315-1. [DOI] [PubMed] [Google Scholar]
- 13.Zheng CM, Liu WC, Zheng JQ, Liao MT, Ma WY, Hung KC, Lu CL, Wu CC, Lu KC. Metabolic acidosis and strong ion gap in critically ill patients with acute kidney injury. Biomed Res Int. 2014;2014:819528. doi: 10.1155/2014/819528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gil HW, Hong M, Lee H, Cho NJ, Lee EY, Park S. Impact of acid-base status on mortality in patients with acute pesticide poisoning. Toxics. 2021;9:22. doi: 10.3390/toxics9020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dondorp AM, Chau TT, Phu NH, Mai NT, Loc PP, Chuong LV, Sinh DX, Taylor A, Hien TT, White NJ, Day NP. Unidentified acids of strong prognostic significance in severe malaria. Crit Care Med. 2004;32:1683–1688. doi: 10.1097/01.ccm.0000132901.86681.ca. [DOI] [PubMed] [Google Scholar]
- 16.Altun HI, Altun G, Altas OF, Aran G. Prognostic significance of the strong ion gap in patients in medical and surgical intensive care units. Cureus. 2023;15:e47964. doi: 10.7759/cureus.47964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zampieri FG, Park M, Ranzani OT, Maciel AT, de Souza HP, da Cruz Neto LM, da Silva FP. Anion gap corrected for albumin, phosphate and lactate is a good predictor of strong ion gap in critically ill patients: a nested cohort study. Rev Bras Ter Intensiva. 2013;25:205–211. doi: 10.5935/0103-507X.20130036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moviat M, van Haren F, van der Hoeven H. Conventional or physicochemical approach in intensive care unit patients with metabolic acidosis. Crit Care. 2003;7:R41–45. doi: 10.1186/cc2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasileiadis I, Kompoti M, Rovina N, Tripodaki ES, Filis C, Alevrakis E, Kyriakoudi A, Kyriakopoulou M, Koulouris N, Koutsoukou A. Buffering capacity in sepsis: a prospective cohort study in critically ill patients. J Clin Med. 2019;8:1759. doi: 10.3390/jcm8111759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 21.Hu J, Lv C, Hu X, Liu J. Effect of hypoproteinemia on the mortality of sepsis patients in the ICU: a retrospective cohort study. Sci Rep. 2021;11:24379. doi: 10.1038/s41598-021-03865-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noritomi DT, Soriano FG, Kellum JA, Cappi SB, Biselli PJ, Libório AB, Park M. Metabolic acidosis in patients with severe sepsis and septic shock: a longitudinal quantitative study. Crit Care Med. 2009;37:2733–2739. doi: 10.1097/ccm.0b013e3181a59165. [DOI] [PubMed] [Google Scholar]
- 23.Gattinoni L, Carlesso E, Cadringher P, Caironi P. Strong ion difference in urine: new perspectives in acid-base assessment. Crit Care. 2006;10:137. doi: 10.1186/cc4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alevrakis E, Gialelis N, Vasileiadis I. Strong ion difference in urine: a measure of proton excretion or of the net plasma charge alteration? Acta Physiol (Oxf) 2020;230:e13559. doi: 10.1111/apha.13559. [DOI] [PubMed] [Google Scholar]
- 25.Naka T, Bellomo R. Bench-to-bedside review: treating acid-base abnormalities in the intensive care unit--the role of renal replacement therapy. Crit Care. 2004;8:108–114. doi: 10.1186/cc2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kellum JA, Kramer DJ, Pinsky MR. Strong ion gap: a methodology for exploring unexplained anions. J Crit Care. 1995;10:51–55. doi: 10.1016/0883-9441(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 27.Antonogiannaki EM, Mitrouska I, Amargianitakis V, Georgopoulos D. Evaluation of acid-base status in patients admitted to ED-physicochemical vs traditional approaches. Am J Emerg Med. 2015;33:378–382. doi: 10.1016/j.ajem.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Mohr NM, Vakkalanka JP, Faine BA, Skow B, Harland KK, Dick-Perez R, Fuller BM, Ahmed A, Simson SQ. Serum anion gap predicts lactate poorly, but may be used to identify sepsis patients at risk for death: a cohort study. J Crit Care. 2018;44:223–228. doi: 10.1016/j.jcrc.2017.10.043. [DOI] [PubMed] [Google Scholar]
- 29.Ayala-Lopez N, Harb R. Interpreting anion gap values in adult and pediatric patients: examining the reference interval. J Appl Lab Med. 2020;5:126–135. doi: 10.1373/jalm.2019.029496. [DOI] [PubMed] [Google Scholar]
- 30.Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol. 2007;2:162–174. doi: 10.2215/CJN.03020906. [DOI] [PubMed] [Google Scholar]
- 31.Vasileiadis I, Kompoti M, Tripodaki ES, Rovina N, Pontikis K, Ntouka E, Stavrou E, Koutsoukou A. Metabolic acidosis in patients with sepsis. Intensive Care Med Exp. 2017;5(Suppl 2):1084. [Google Scholar]