Key Points
Question
What is the association of consuming ultraprocessed foods (UPFs) with cardiometabolic risk factors in children?
Findings
In this cross-sectional study of 1426 children, higher consumption of UPFs was positively associated with body mass index, waist circumference, fat mass index, and fasting plasma glucose and negatively associated with high-density lipoprotein cholesterol concentrations.
Meaning
These findings highlight the need for public health initiatives to promote the replacement of UPFs with unprocessed or minimally processed foods.
This cross-sectional study investigates the association between ultraprocessed food consumption and cardiometabolic risk factors in preschool-aged children in Spain.
Abstract
Importance
High intake of ultraprocessed foods (UPFs) has been associated with higher cardiometabolic risk in adults; however, the evidence in children is limited.
Objective
To investigate the association between UPF consumption and cardiometabolic risk factors in the Childhood Obesity Risk Assessment Longitudinal Study (CORALS).
Design, Setting, and Participants
This baseline cross-sectional analysis was conducted using the data of CORALS participants recruited between March 22, 2019, and June 30, 2022. Preschool children (aged 3-6 years) were recruited from schools and centers in 7 cities in Spain. Inclusion criteria included informed consent signed by parents or caregivers and having a completed a set of questionnaires about the child’s prenatal history at home. Exclusion criteria included low command of Spanish or unstable residence.
Exposure
Energy-adjusted UPF consumption (in grams per day) from food frequency questionnaires and based on the NOVA food classification system.
Main Outcomes and Measures
Age- and sex-specific z scores of adiposity parameters (body mass index [BMI], fat mass index, waist-to-height ratio, and waist circumference) and cardiometabolic parameters (diastolic and systolic blood pressure, fasting plasma glucose, homeostasis model assessment for insulin resistance, high-density and low-density lipoprotein cholesterol, and triglycerides) were estimated using linear regression models.
Results
Of 1509 enrolled CORALS participants, 1426 (mean [SD] age, 5.8 [1.1] years; 698 boys [49.0%]) were included in this study. Mothers of children with high UPF consumption were younger, had a higher BMI, were more likely to have overweight or obesity, and had lower education levels and employment rates. Compared with participants in the lowest tertile of energy-adjusted UPF consumption, those in the highest tertile showed higher z scores of BMI (β coefficient, 0.20; 95% CI, 0.05-0.35), waist circumference (β coefficient, 0.20; 95% CI, 0.05-0.35), fat mass index (β coefficient, 0.17; 95% CI, 0.00-0.32), and fasting plasma glucose (β coefficient, 0.22; 95% CI, 0.06-0.37) and lower z scores for HDL cholesterol (β coefficient, −0.19; 95% CI, −0.36 to −0.02). One-SD increments in energy-adjusted UPF consumption were associated with higher z scores for BMI (β coefficient, 0.11; 95% CI, 0.05-0.17), waist circumference (β coefficient, 0.09; 95% CI, 0.02-0.15), fat mass index (β coefficient, 0.11; 95% CI, 0.04-1.18), and fasting plasma glucose (β coefficient, 0.10; 95% CI, 0.03-0.17) and lower HDL cholesterol (β coefficient, −0.07; 95% CI, −0.15 to −0.00). Substituting 100 g of UPFs with 100 g of unprocessed or minimally processed foods was associated with lower z scores of BMI (β coefficient, −0.03; 95% CI, −0.06 to −0.01), fat mass index (β coefficient, −0.03; 95% CI, −0.06 to 0.00), and fasting plasma glucose (β coefficient, −0.04; 95% CI, −0.07 to −0.01).
Conclusions and Relevance
These findings suggest that high UPF consumption in young children is associated with adiposity and other cardiometabolic risk factors, highlighting the need for public health initiatives to promote the replacement of UPFs with unprocessed or minimally processed foods.
Introduction
The presence of abnormal cardiometabolic risk factors often begins in childhood, highlighting the importance of identifying and controlling them early to delay or prevent cardiovascular disease (CVD) in the future.1 Modifiable risk factors (eg, diet and physical activity) may contribute to the development of recognized cardiometabolic risk factors.2,3,4 Emerging studies have shed light on the potential role of ultraprocessed foods (UPFs) in determining the risk of chronic diseases,5,6,7 independent of their nutritional profiles.8
Commonly, UPFs represent a category of food products that undergo extensive industrial processing, often containing multiple ingredients, additives, and preservatives to make them not only convenient (ready to eat) but also palatable and appealing. This approach has been used to create the most widely used UPF classification, the NOVA Food Classification system.9,10 Ultraprocessed foods are typically rich in saturated fats, sugars, sodium, and other substances (eg, additives) and lower in essential nutrients, all of which are associated with cardiometabolic health.11,12 Due to their high availability and affordability and wide marketing to children, UPFs have become increasingly frequent in modern diets, particularly among children, adolescents,13,14,15 and their families, and especially among individuals and families with low socioeconomic status and educational levels in which obesity is more prevalent.16 Additionally, the habits established during early childhood often track to later ages17 and carry into adulthood, compounding the risk of CVD and other noncommunicable diseases.18,19,20
Previous observational studies in adults have reported positive associations between UPF consumption and obesity,21 type 2 diabetes,22 CVD,23 and all-cause mortality24; however, the epidemiologic evidence in children remains limited and controversial.25 While the majority of studies have reported unfavorable associations with body mass index (BMI), others did not find this association, and few have focused on cardiometabolic risk factors.25
Given the public health burden of CVDs and the increasing availability of UPFs, having a better understanding of potential associations between UPF consumption and cardiometabolic risk factors in children is essential. Therefore, the aim of this study was to examine the associations between UPF consumption and cardiometabolic risk factors in a population of Spanish preschool children (aged 3-6 years).
Methodology
Population and Study Design
This cross-sectional study was conducted using data from the Childhood Obesity Risk Assessment Longitudinal Study (CORALS), which followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. The ethics committee of each of recruitment center approved the study protocol, which was conducted following the standards of the Declaration of Helsinki.26 Parents or caregivers provided written informed consent.
CORALS is an ongoing prospective multicenter study conducted in 7 Spanish centers aiming to identify potential risk factors for childhood obesity over a 10-year follow-up period. A detailed description of the CORALS is published elsewhere.27 Between March 22, 2019, and June 30, 2022, eligible participants aged 3 to 6 years at enrollment were recruited from schools across 7 cities in Spain. To be enrolled in the study, parents or caregivers had to sign a consent form, attend the inclusion face-to-face visit and complete several questionnaires at home for data collection on leisure time physical activity, 3-day food consumption, and sociodemographics. The exclusion criteria included belonging to a family with difficulty collaborating due to low command of Spanish or unstable residence.
Dietary Intake of UPFs and NOVA Food Classification System
To estimate the dietary intake of UPFs, trained dietitians (B.P.-V., S.d.L.H.D., M.L.M.-B., K.A.P.-V., and R.V.-C.) used the validated, semiquantitative, 125-item food and beverage frequency COME-Kids questionnaire.28 Participants with energy intake below the first percentile or above the 99th percentile were excluded to minimize misreporting (details are provided in the eAppendix in Supplement 1). The NOVA Food Classification system was used to determine the consumption of food depending on its degree of processing27 (details are provided in the eAppendix and eTable 1 in Supplement 1).
Outcomes
Adiposity Measurements
Adiposity measurements and cardiometabolic risk factor assessments were conducted in health care centers. Weight and body fat mass were measured using a precision scale and an octopolar multifrequency bioelectrical impedance device (MC780MAS; Tanita). Height was measured using a portable seca 213 stadiometer according to standard procedures. Body mass index was calculated and categorized as underweight or normal weight or as overweight or obesity based on pediatric cutoffs.29 Waist circumference was determined using a flexible, nonextensible measuring tape. The fat mass index was estimated by dividing body fat mass in kilograms by height in meters squared.30 Waist-to-height ratio was estimated by dividing waist circumference in centimeters by height in centimeters.31
Cardiometabolic Risk Factors Assessment
Blood pressure was measured in the nondominant arm 3 times, with a 5-minute gap between each measurement, using an automatic oscillometer (M3 Intelligence HEM-75051-EV; OMRON Healthcare) equipped with a child-sized cuff. Eight-hour fasting blood samples were collected from participants, and serum total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, plasma glucose, and insulin concentrations were measured using standard procedures. Homeostasis model assessment for insulin resistance (HOMA-IR) was calculated as fasting insulin (μIU/mL) × fasting glucose (mmol/L) / 22.5.
Covariates
Parents or caregivers were provided with a set of questionnaires to complete at home, gathering information on early life factors, maternal characteristics, and lifestyle patterns. To assess physical activity, the total time (in hours) of engagement in sports and physical activities per week was estimated using a previously validated questionnaire.32 An 18-item questionnaire for children was used to assess adherence to the Mediterranean diet, an indicator of diet quality.27
Statistical Analysis
The current analysis was conducted using the CORALS database updated through January 20, 2023. Analysis of descriptive baseline characteristics are reported as mean (SD) or median (IQR) for continuous variables and as numbers with percentages for categorical variables using one-way analysis of variance and χ2 test, respectively.
Consumption of UPFs (in grams per day) was adjusted for total energy intake using the residual regression method.33 Intake of UPFs in grams per day was calculated instead of energy percentage to account for foods with no energy content (eg, artificially sweetened beverages) and for nonnutritional concerns associated with food processing (eg, food additives). Participants were categorized by tertiles of energy-adjusted UPF consumption, ranging from tertile 1 for the lowest intake to tertile 3 for the highest intake.
Age- and sex-specific z scores of each outcome were estimated from standardized residuals conducted using linear regression models. Missing data of less than 5% for covariates were imputed to the mean and to the highest frequency category for quantitative and qualitative confounders, respectively.34 Multivariable linear regression models were fitted to assess the associations (β coefficient and 95% CI) between tertiles of energy-adjusted UPFs and z scores of cardiometabolic risk factors. The first tertile (lowest intake) was considered the reference. Models were adjusted for maternal education level (primary or lower, secondary or university), maternal BMI (underweight, normal overweight, obesity), physical activity (minutes per week), exclusive breastfeeding (yes or no), recruitment center size (<200, 200-400, >400 participants), and NOVA group 1, 2, or 3 (as detailed in eTable 1 in Supplement 1). To assess the linear trend, the median value of each tertile of UPF consumption was modeled as a continuous variable. The analysis was also conducted in a continuous form, with a 1-SD increment and using the same confounders.
Additionally, a simulation model was fitted to substitute 100 g of consumed UPFs with 100 g of unprocessed or minimally processed food to examine the association of healthier food consumption with the outcomes. The theoretical impact of substituting 1 food group for another was assessed by introducing both variables simultaneously as continuous variables into the model. Differences in the β coefficients, variances, and covariance were used to estimate the β coefficients and 95% CIs for the substitution association. Sensitivity analyses were conducted to assess associations according diet quality, maternal education, and socioprofessional level (details provided in the eAppendix in Supplement 1).
Data were analyzed using Stata, version 14 software (StataCorp LLC). All statistical tests were 2-sided, and P < .05 was deemed statistically significant.
Results
A total of 1426 participants (mean [SD] age, of 5.8 [1.1] years; 698 boys [49.0%] and 728 girls [51.0%]) were included in this study after excluding 54 participants lacking the food and beverage frequency questionnaire and 29 with missing data or implausible reported energy intake (eFigure 1 in Supplement 1). The characteristics of the study population across tertiles of energy-adjusted UPF consumption are shown in Table 1. Children in the third tertile (highest UPF consumption) had a higher BMI z score, waist-to-height ratio, fat mass index, systolic blood pressure, and overweight or obesity prevalence and lower HDL and LDL cholesterol. Mothers whose children were categorized in the highest tertile of energy-adjusted UPF consumption were younger, had a higher BMI, were more prone to be living with overweight or obesity, were less likely to have exclusively breastfed their children, and had lower educational achievement and employment rates.
Table 1. General Characteristics of Study Participants Across Tertiles of Energy-Adjusted Ultraprocessed Food Consumption.
| Characteristic | Tertiles of energy-adjusted ultraprocessed food consumption | ||||
|---|---|---|---|---|---|
| 1 (Lowest) (n = 476) | 2 (n = 475) | 3 (Highest) (n = 475) | P valuea | ||
| Child | |||||
| Age, mean (SD), y | 4.8 (1.1) | 5.0 (1.1) | 5.2 (1.1) | <.001 | |
| Sex, No. (%) | |||||
| Girls | 237 (49.8) | 247 (52) | 244 (51.4) | .78 | |
| Boys | 239 (50.2) | 228 (48) | 231 (48.6) | ||
| Adiposity, mean (SD) | |||||
| BMI | 16.1 (1.85) | 16.3 (2.02) | 16.6 (2.21) | .002 | |
| BMI z score | −0.11 (0.91) | 0.00 (1.01) | 0.10 (1.07) | .01 | |
| Weight status, No. (%) | |||||
| Underweight or normal weight | 381 (82.5) | 370 (79.1) | 351 (75.0) | .02 | |
| Overweight or obesity | 81 (17.5) | 98 (20.9) | 115 (25.0) | ||
| Waist circumference, mean (SD), cm | 51.7 (5.93) | 52.3 (6.41) | 52.0 (7.43) | .43 | |
| Waist-to-height ratio, mean (SD) | 0.48 (0.05) | 0.48 (0.05) | 0.47 (0.06) | .01 | |
| Fat mass index, mean (SD) | 3.72 (1.11) | 3.88 (1.25) | 3.97 (1.31) | .02 | |
| Lifestyle | |||||
| Physical activity, mean (SD), min/wk | 178 (110) | 181 (111) | 184 (121) | .69 | |
| Cardiometabolic risk factors | |||||
| Systolic blood pressure, mean (SD), mm Hg | 102 (12.3) | 103 (12.7) | 104 (13.5) | .003 | |
| Diastolic blood pressure, mean (SD), mm Hg | 63.9 (11.6) | 65.2 (11.5) | 64.3 (12.0) | .26 | |
| Fasting plasma glucose, mean (SD), mg/dL | 76.8 (9.24) | 77.6 (9.39) | 77.0 (8.85) | .50 | |
| HDL cholesterol, median (IQR), mg/dL | 58.0 (50.0-66.7) | 58.0 (50.0-66.2) | 55.0 (47.0-64.0) | .01 | |
| LDL cholesterol, median (IQR), mg/dL | 96.0 (84.0-113) | 96.0 (83.0-112) | 93.0 (78.0-110) | .04 | |
| Triglycerides, median (IQR), mg/dL | 51.0 (42.0-62.5) | 51.5.0 (43.0-63.0) | 56.0 (45.0-69.0) | .04 | |
| Maternal | |||||
| Age, mean (SD), y | 39.5 (4.69) | 38.7 (4.73) | 37.6 (5.14) | <.001 | |
| BMI, mean (SD) | 24.1 (4.25) | 24.4 (4.46) | 25.9 (5.49) | <.001 | |
| Exclusive breastfeeding, No. (%) | 194 (40.8) | 37.9 (180) | 157 (33.1) | .046 | |
| Weight status | |||||
| Underweight or normal weight | 322 (67.6) | 310 (65.3) | 273 (57.5) | .003 | |
| Overweight or obesity | 154 (32.3) | 165 (34.7) | 202 (42.5) | ||
| Educational level | |||||
| Primary or lower | 24 (5.04) | 39 (8.21) | 72 (15.2) | <.001 | |
| Secondary | 157 (32.9) | 161 (33.9) | 235 (49.5) | ||
| University | 281 (59.0) | 263 (55.4) | 155 (32.6) | ||
| Not reported | 14 (2.9) | 13 (2.5) | 13 (2.7) | ||
| Socioprofessional category | |||||
| Homemaker, student, retired, or unemployed | 122 (25.6) | 115 (24.2) | 163 (34.3) | .005 | |
| Employed | 354 (74.4) | 360 (75.8) | 312 (65.7) | ||
Abbreviations: BMI, body mass index (as measured by weight in kilograms divided by height in meters squared); HDL, high-density lipoprotein; LDL, low-density lipoprotein.
SI conversions: To convert glucose to mmol/L, multiply by 0.0555; to convert HDL and LDL to mmol/L, multiply by 0.0259; to convert triglycerides to mmol/L, multiply by 0.0113.
P values for comparisons were tested by 1-way analysis of variance or χ2 test, as appropriate, according to tertiles of energy-adjusted ultraprocessed food consumption.
General dietary characteristics of participants are shown in Table 2. Children in the top tertile were more likely to consume higher amounts of total energy, carbohydrates, yogurt, other dairy products, sugar and candy, and sugary beverages and lower amounts of protein, fat, monounsaturated and polyunsaturated fatty acids, fiber, milk, cheese, white meat, unprocessed red meat, eggs, fish, seafood, vegetables, fruits, nuts, legumes, whole and refined cereals, and oils and fat.
Table 2. Baseline Dietary Characteristics of Participants Across Tertiles of Energy-Adjusted Ultraprocessed Food Consumption in the Diet.
| Characteristic | Tertiles of energy-adjusted ultraprocessed food consumption, mean (SD) | |||
|---|---|---|---|---|
| 1 (Lowest) (n = 476) | 2 (n = 475) | 3 (Highest) (n = 475) | P valuea | |
| Energy-adjusted ultraprocessed food (n = 1426), g/d | 192.8 (76.7) | 354.8 (39.9) | 593.4 (167) | <.001 |
| Dietary intake contribution | ||||
| Total energy intake, kcal/d | 1867.3 (420.1) | 1626.4 (388.3) | 1780.8 (470.7) | <.001 |
| Carbohydrates, % of total energy intake | 40.9 (5.7) | 42.9 (4.9) | 46.1 (5.1) | <.001 |
| Proteins, % of total energy intake | 14.9 (2.3) | 15.1 (2.2) | 14.9 (2.1) | .19 |
| Protein intake per body weight, g/kg/d | 3.8 (1.2) | 3.2 (0.9) | 3.3 (1.1) | <.001 |
| Total fat, % of total energy intake | 44.1 (6.4) | 41.9 (5.1) | 38.9 (5.1) | <.001 |
| Saturated fatty acids, % of total energy intake | 13.9 (2.3) | 13.9 (2.0) | 13.8 (2.0) | .71 |
| Monounsaturated fatty acids, % of total energy intake | 20.6 (4.8) | 18.7 (3.8) | 16.3 (3.5) | <.001 |
| Polyunsaturated fatty acids, % of total energy intake | 6.2 (1.7) | 6.3 (1.6) | 6.0 (1.8) | .01 |
| Fiber, g/d | 17.1 (5.3) | 13.6 (3.8) | 13.1 (3.7) | <.001 |
| ≥14 g/1000 kcal, No. (%) | 43 (9.0) | 12 (2.5) | 3 (0.6) | <.001 |
| Sodium, mg/d | 138.8 (1.9) | 138.5 (7.9) | 139.3 (1.9) | .08 |
| Dairy products, g/d | ||||
| Milk | 384.3 (280.6) | 298.2 (197.7) | 286.8 (213/7) | <.001 |
| Yogurt | 92.7 (80.4) | 96.5 (67.5) | 151.7 (130.9) | <.001 |
| Cheese | 16.0 (17.5) | 11.8 (10.6) | 11.6 (14.9) | <.001 |
| Other dairy products | 50.1 (53.9) | 70.9 (66.2) | 155.4 (150.1) | <.001 |
| Protein foods, g/d | ||||
| White meat | 26.7 (14.9) | 24.7 (9.81) | 24.2 (13.3) | .01 |
| Unprocessed red meat | 22.3 (23.6) | 19.9 (14.5) | 18.1 (13.1) | .001 |
| Processed and derivatives meat products | 25.8 (16.6) | 24.1 (13.9) | 28.4 (19.0) | .001 |
| Egg | 26.7 (21.1) | 23.7 (9.6) | 22.5 (17.3) | <.001 |
| Fish and seafood | 36.0 (19.3) | 33.4 (17.5) | 33.1 (21.3) | .04 |
| Vegetables and fruits, g/d | ||||
| Vegetables | 101 (59.4) | 68.3 (43.7) | 58.8 (44.0) | <.001 |
| Tubers | 42.7 (22.8) | 41.2 (19.0) | 40.9 (20.8) | .35 |
| Fruits | 253.9 (160.4) | 180.2 (105.9) | 150.6 (108.5) | <.001 |
| Nuts, g/d | 5.1 (7.6) | 3.2 (4.4) | 2.7 (3.9) | <.001 |
| Cereals and legumes, g/d | ||||
| Legumes | 15.5 (11.9) | 13.4 (6.9) | 13.8 (7.3) | .001 |
| Refined cereals | 81.6 (44.2) | 73.9 (34.8) | 70.8 (35.7) | <.001 |
| Whole cereals | 14.1 (23.9) | 6.59 (12.8) | 4.65 (10.7) | <.001 |
| Miscellaneous, g/d | ||||
| Oil and fats | 34.5 (18.3) | 24.4 (13.5) | 19.7 (12.9) | <.001 |
| Pastries | 42.0 (36.9) | 41.5 (33.4) | 43.6 (32.5) | .60 |
| Sugars and candies | 6.2 (7.2) | 5.7 (6.1) | 6.7 (10.7) | .13 |
| Beverages, mL/d | ||||
| Water | 895.2 (350.5) | 871.9 (385.2) | 840.0 (377.7) | .07 |
| Sugary beverages | 92.8 (126.4) | 81.1 (94.2) | 192.0 (191.3) | <.001 |
| Tea and infusions | 6.7 (26.2) | 7.6 (30.0) | 6.2 (35.1) | .76 |
P values for comparisons were tested by one-way analysis of variance or χ2 test, as appropriate, according to energy-adjusted ultraprocessed food consumption.
Cross-sectional associations between energy-adjusted UPF consumption across tertiles and by 1-SD increment (in grams per day) and cardiometabolic risk factors are shown in Table 3. Compared with participants in the lowest tertile, those in the top tertile had higher z scores of waist circumference (β coefficient, 0.20; 95% CI, 0.05-0.35), BMI (β coefficient, 0.20; 95% CI, 0.05-0.35), fat mass index (β coefficient, 0.17; 95% CI, 0.00-0.32), and fasting plasma glucose (β coefficient, 0.22; 95% CI, 0.06-0.37). Additionally, participants in the highest tertile had a lower z score of HDL cholesterol (β coefficient, −0.19; 95% CI, −0.36 to −0.02). After adjusting for the Mediterranean diet score (12 points), the associations were maintained for the z scores of fasting plasma glucose (β coefficient, 0.17; 95% CI, 0.03-0.31) and HDL cholesterol (β coefficient, −0.20; 95% CI, −0.36 to −0.05) (eTable 2 in Supplement 1). Positive associations were also observed between 1-SD increments of UPF consumption and z scores of waist circumference (β coefficient, 0.09; 95% CI, 0.02-0.15), fat mass index (β coefficient, 0.11; 95% CI, 0.04-1.18), BMI (β coefficient, 0.11; 95% CI, 0.05-0.17), and fasting plasma glucose (β coefficient, 0.10; 95% CI, 0.03-0.17) and negatively associated with HDL cholesterol (β coefficient, −0.07; 95% CI, −0.15 to 0.00) (Table 3). Likewise, after further adjusting for the Mediterranean diet score, the associations between 1-SD increments remained significant for the z scores of fasting plasma glucose (β coefficient, 0.08; 95% CI, 0.02-0.13) and HDL cholesterol (β coefficient, −0.07; 95% CI, −0.13 to −0.01) (eTable 2 in Supplement 1).
Table 3. Association Between Energy-Adjusted Ultraprocessed Food Consumption in Tertiles and 1-SD Increments and Cardiometabolic Risk Factor z Scores.
| Model | Tertiles of energy-adjusted ultraprocessed food consumption, β coefficient (95% CI) | P value for trend | Continuous (1-SD increment), β coefficient (95% CI) | ||
|---|---|---|---|---|---|
| 1 (Lowest) | 2 | 3 (Highest) | |||
| Waist circumference (n = 1390) | |||||
| Crude | 1 [Reference] | 0.05 (−0.07 to 0.18) | −0.04 (−0.17 to 0.08) | .43 | −0.02 (−0.08 to 0.03) |
| Fully adjusteda | 1 [Reference] | 0.11 (−0.02 to 0.24) | 0.20 (0.05 to 0.35) | .01 | 0.09 (0.02 to 0.15) |
| Fat mass index (n = 1219) | |||||
| Crude | 1 [Reference] | 0.09 (−0.04 to 0.22) | 0.16 (0.03 to 0.29) | .02 | 0.08 (0.03 to 0.14) |
| Fully adjusteda | 1 [Reference] | 0.13 (−0.00 to 0.27) | 0.17 (0.00 to 0.32) | .04 | 0.11 (0.04 to 1.18) |
| Waist-to-height ratio (n = 1389) | |||||
| Crude | 1 [Reference] | 0.03 (−0.09 to 0.16) | −0.10 (−0.23 to 0.03) | .09 | −0.04 (−0.09 to 0.01) |
| Fully adjusteda | 1 [Reference] | 0.07 (−0.06 to 0.19) | 0.12 (−0.03 to 0.27) | .12 | 0.06 (−0.01 to 0.12) |
| BMI (n = 1398) | |||||
| Crude | 1 [Reference] | 0.12 (−0.01 to 0.24) | 0.21 (0.09 to 0.34) | .001 | 0.10 (0.05 to 0.15) |
| Fully adjusteda | 1 [Reference] | 0.14 (0.01 to 0.27) | 0.20 (0.05 to 0.35) | .009 | 0.11 (0.05 to 0.17) |
| LDL cholesterol (n = 1120) | |||||
| Crude | 1 [Reference] | 0.03 (−0.11 to 0.17) | −0.13 (−0.27 to 0.01) | .054 | −0.05 (−0.11 to 0.00) |
| Fully adjusteda | 1 [Reference] | 0.01 (−0.13 to 0.16) | −0.06 (−0.23 to 0.11) | .47 | −0.03 (−0.09 to 0.04) |
| HDL cholesterol (n = 1174) | |||||
| Crude | 1 [Reference] | −0.00 (−0.14 to 0.14) | −0.23 (−0.37 to −0.09) | .001 | −0.09 (−0.15 to −0.03) |
| Fully adjusteda | 1 [Reference] | −0.01 (−0.16 to 0.14) | −0.19 (−0.36 to −0.02) | .02 | −0.07 (−0.15 to 0.00) |
| Triglycerides (n = 1175) | |||||
| Crude | 1 [Reference] | 0.09 (−0.05 to 0.23) | 0.21 (0.07 to 0.34) | .004 | 0.07 (0.02 to 0.13) |
| Fully adjusteda | 1 [Reference] | 0.07 (−0.07 to 0.22) | 0.14 (−0.03 to 0.30) | .10 | 0.04 (−0.03 to 0.11) |
| Fasting plasma glucose (n = 1191) | |||||
| Crude | 1 [Reference] | 0.02 (−0.11 to 0.17) | −0.02 (−0.17 to 0.12) | .69 | −0.01 (−0.07 to 0.04) |
| Fully adjusteda | 1 [Reference] | 0.06 (−0.08 to 0.19) | 0.22 (0.06 to 0.37) | .01 | 0.10 (0.03 to 0.17) |
| HOMA-IR (n = 933) | |||||
| Crude | 1 [Reference] | 0.03 (−0.13 to 0.19) | −0.02 (−0.18 to 0.14) | .78 | −0.01 (−0.07 to 0.06) |
| Fully adjusteda | 1 [Reference] | 0.06 (−0.10 to 0.22) | 0.02 (−0.16 to 0.21) | .69 | 0.02 (−0.06 to 0.10) |
| Diastolic blood pressure (n = 1348) | |||||
| Crude | 1 [Reference] | 0.10 (−0.03 to 0.23) | 0.05 (−0.08 to 0.18) | .55 | 0.01 (−0.04 to 0.07) |
| Fully adjusteda | 1 [Reference] | 0.08 (−0.05 to 0.21) | −0.01 (−0.16 to 0.14) | .81 | −0.01 (−0.08 to 0.05) |
| Systolic blood pressure (n = 1346) | |||||
| Crude | 1 [Reference] | 0.14 (0.01 to 0.27) | 0.19 (0.06 to 0.32) | .01 | 0.08 (0.02 to 0.13) |
| Fully adjusteda | 1 [Reference] | 0.12 (−0.02 to 0.25) | 0.12 (−0.03 to 0.28) | .14 | 0.05 (−0.01 to 0.11) |
Abbreviations: BMI, body mass index; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment for insulin resistance; LDL, low-density lipoprotein.
Fully adjusted for maternal education level, maternal BMI, total minutes of physical activity per week, exclusive breastfeeding, center size, and NOVA classification system groups 1, 2, and 3.
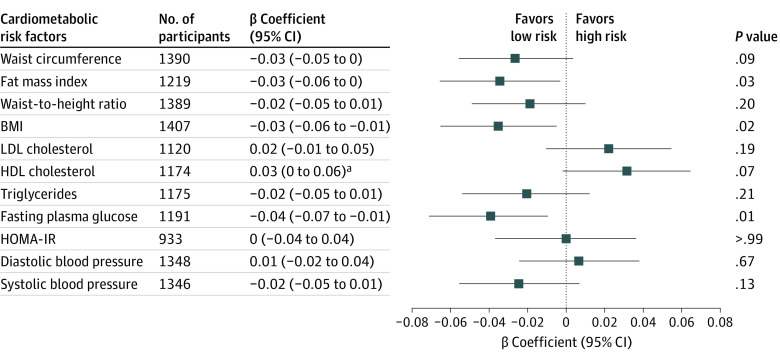
Similar positive associations among fat mass index, BMI, and plasma glucose were observed, irrespective of the animal or vegetable origin of the UPFs consumed. Substitution of 100 g of UPFs with 100 g of unprocessed or minimally processed foods was associated with a decrease in z scores of fat mass index (β coefficient, −0.03; 95% CI, −0.06 to 0.00) and BMI (β coefficient, −0.03; 95% CI, −0.06 to −0.01), and fasting plasma glucose (β coefficient, −0.04; 95% CI, −0.07 to −0.01) (Figure). The same models adjusted for the Mediterranean diet score showed positive associations for z scores of fasting plasma glucose (β coefficient, −0.04; 95% CI, −0.06 to −0.01) and inverse association for z score of HDL cholesterol (β coefficient, 0.03; 95% CI, 0.00-0.07) (eFigure 2 in Supplement 1). No associations were shown for the other outcomes.
Figure. Substitution Analysis of 100 g of Ultraprocessed Food With 100 g of Unprocessed or Minimally Processed Foods and Cardiometabolic Risk Factors.
Linear regression models were fitted and adjusted for maternal education level, maternal body mass index (BMI), total minutes of physical activity per week, breastfeeding, center size, and NOVA classification system groups 2 and 3. HDL indicates high-density lipoprotein; HOMA-IR, homeostasis model assessment for insulin resistance; LDL, low-density lipoprotein.
aFor HDL cholesterol, a positive β coefficient signifies low risk.
In children whose mothers were unemployed, a positive association was found between energy-adjusted UPF consumption and z scores of waist circumference (β coefficient, 0.26; 95% CI, 0.14-0.39), fat mass index (β coefficient, 0.20; 95% CI, 0.07-0.34), waist-to-height ratio (β coefficient, 0.21; 95% CI, 0.09-0.34), BMI (β coefficient, 0.18; 95% CI, 0.04-0.31), fasting plasma glucose (β coefficient, 0.14; 95% CI, 0.03-0.25), and diastolic blood pressure (β coefficient, 0.14; 95% CI, 0.0-0.27). In children with employed mothers, a positive association was observed between 1-SD increments in energy-adjusted UPF consumption and z scores of fasting plasma glucose (β coefficient, 0.09; 95% CI, 0.01-0.17) and a negative association in case of the HDL cholesterol (β coefficient, −0.09; 95% CI, −0.18 to −0.01) (eTable 3 in Supplement 1).
Children whose mothers had a low education level had higher z scores of waist circumference (β coefficient, 0.14; 95% CI, 0.05-0.23), fat mass index (β coefficient, 0.15; 95% CI, 0.06-0.25), BMI (β coefficient, 0.15; 95% CI, 0.06-0.24), and fasting plasma glucose (β coefficient, 0.11; 95% CI, 0.02-0.19). Children whose mothers had a high education level had a lower HDL cholesterol z score (β coefficient, −0.15; 95% CI, −0.26 to −0.03) (eTable 4 in Supplement 1).
Discussion
To our knowledge, this study is the first to assess the associations between UPF consumption and various cardiometabolic risk factors in young children. In this large cross-sectional study, UPF consumption was positively associated with z scores of BMI, waist circumference, fat mass index, and fasting plasma glucose concentration and inversely associated with HDL cholesterol concentration.
Our findings are in line with those of previous studies.35,36,37 Consumption of UPFs at age 4 years was associated with increased BMI z scores at age 10 years in the Generation XXI cohort, while no association was found at age 7 years.35 Another study showed that high UPF consumption in children aged 7 to 13 years was associated with increased BMI growth trajectories.36 Similarly, lower UPF intake in Spanish children aged 4 to 7 years was associated with lower BMI z scores at age 7 years, though this association became nonsignificant after adjusting for maternal factors.37
A global study by Neri et al38 revealed that increased UPF consumption was associated with higher dietary energy density and intake of free sugars, alongside decreased total fiber intake, potentially contributing to childhood obesity. Additionally, findings from the Avon Longitudinal Study of Parents and Children showed that high UPF consumption was associated with unfavorable fat mass index trajectories from age 7 to 24 years.36 Similarly, in a Brazilian cohort, UPF consumption during preschool years was associated with increases in waist circumference from preschool to school age.39 Other studies found no significant association between UPF consumption and HDL cholesterol and fasting plasma glucose concentrations.37,40 Therefore, to our knowledge, our study is the first in children to find significant associations with the aforementioned risk factors and is in line with other studies assessing adult populations.41
Our results provide new insight into the association between UPF consumption and health and the importance of recognizing that early dietary habits in childhood might have future implications on cardiometabolic health. While the magnitude of the associations reported in our study may be considered of limited clinical relevance, it is important to note that our study consisted of young children. Therefore, if such minimal differences can reveal a significant association, they may serve as an early warning of future cardiometabolic conditions.
Our results are in line with previous studies showing that the main UPF products consumed are pastries, sweet beverages, cookies, and candies.42,43,44 In addition, our results support the findings of other European studies that have shown that children of mothers with lower education or with lower socioeconomic status are more likely to consume UPFs. These findings suggest that educational and socioeconomic factors may contribute to the purchase of low-cost and unhealthy foods, such as UPFs, increasing the risk of health disorders.37,45,46
Several possible mechanisms could explain our results. First, UPFs contain higher amounts of sodium, energy, fat, and sugar and lower amounts of fiber, which are well recognized as contributors to cardiometabolic risk factors.47 In addition, some UPFs may be linked to a higher glycemic response, and it has been shown that high consumption of sugar-sweetened beverages may delay the internal satiety signal, leading to excessive calorie intake and higher glycemic load.48,49,50 Moreover, excessive consumption of energy, saturated fat, and sugar contributes to weight gain and a higher risk of obesity, which is an important risk factor in CVD.51 Furthermore, our study showed that children who consumed high amounts of UPFs tended to have lower intakes of fruits and vegetables, which, along with a healthy dietary pattern, are known to be beneficial for cardiometabolic health.52
Most of the associations were maintained in our study after further adjusting the models to Mediterranean diet adherence, suggesting that other intrinsic UPF factors may play an important role in determining these associations (eg, additives). Animal and cellular studies revealed potential cardiovascular risks from authorized additives such as sulfites, monosodium glutamate, and emulsifiers.53,54,55,56,57,58 Food processing generates contaminants such as acrylamide and acrolein, which have been linked, respectively, to increased odds and risk of cardiovascular disease.59,60 Ultraprocessed foods may contain chemicals such as bisphenols and perfluoroalkyl substances that have been associated with a higher risk of cardiometabolic outcomes in children.61,62
The NOVA Food Classification system has sparked debate among researchers due to disagreements over UPF definitions, bias concerns, and the system’s contribution to dietary guidelines.63,64 The NOVA system itself has some limitations, as it does not consider that certain minimal processing could improve the final product (eg, fermentation in milk) and adopts a vague definition of what is considered a cosmetic additive, which has led to considering carotenoids as an additive that increases the potential harmfulness of a product.65 Despite these limitations, NOVA categories have consistently shown associations with cardiometabolic health in adults.
Strengths and Limitations
This study has several strengths. Most importantly, the study was conducted in a large sample size from 7 different geographic areas of Spain. The study also assessed cardiometabolic risk factors not considered in other similar studies.21,22,23,24
Our study also has several limitations. First, because the study is observational, we cannot draw conclusions on cause and effect. Second, our study involved preschool children from Spain, which means that the generalization of our findings to different populations is not appropriate. Third, some grade of misclassification could be present in our study since UPF consumption was estimated from a food and beverage frequency questionnaire that was not specifically developed to assess this type of food, which could result in either an overestimation or underestimation of consumption within various NOVA categories. Additionally, imprecise estimations could also arise from the use of a food and beverage frequency questionnaire, which may be influenced by social desirability bias. Finally, we cannot dismiss that associations may be due to residual confounding or that undetected cardiometabolic disorders in our study population may exist due to age.
Conclusions
In this large cross-sectional study, UPFs consumption was positively associated with fasting plasma glucose levels, BMI, waist circumference, and fat mass index and inversely associated with HDL cholesterol concentration. These findings highlight the importance of promoting unprocessed or minimally processed foods and reducing UPF consumption, particularly starting from early ages. However, further prospective studies are warranted to validate our findings.
eAppendix. Additional Study Assessments
eReference
eTable 1. Distribution of FFQ Items Into 4 Groups According to the Degree of Their Processing Established by NOVA Classification System
eFigure 1. Flow Chart of the Children Included in the Analysis
eTable 2. Association Between Energy-Adjusted Ultraprocessed Food Consumption in Tertiles (in g/day) and CVD Risk Factors at Baseline
eFigure 2. Linear Regression Models Replacing 100 g of UPF With 100 g of Unprocessed/Minimally Processed Foods
eTable 3. Maternal Socioprofessional Stratified Regression Association Between 1-SD Increment of Energy-Adjusted Ultraprocessed Food Consumption (in g/day) and CVD Risk Factors at Baseline
eTable 4. Maternal Education Level Stratified Regression Association Between 1-SD Increment of Energy-Adjusted Ultraprocessed Food Consumption (in g/day) and CVD Risk Factors at Baseline
Data Sharing Statement
References
- 1.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute . Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(suppl 5):S213-S256. doi: 10.1542/peds.2009-2107C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi L, Morrison JA, Wiecha J, Horton M, Hayman LL. Healthy lifestyle factors associated with reduced cardiometabolic risk. Br J Nutr. 2011;105(5):747-754. doi: 10.1017/S0007114510004307 [DOI] [PubMed] [Google Scholar]
- 3.Niebuur J, Vonk JM, Du Y, et al. Lifestyle factors related to prevalent chronic disease multimorbidity: a population-based cross-sectional study. PLoS One. 2023;18(7):e0287263. doi: 10.1371/journal.pone.0287263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng R, Sutradhar R, Yao Z, Wodchis WP, Rosella LC. Smoking, drinking, diet and physical activity-modifiable lifestyle risk factors and their associations with age to first chronic disease. Int J Epidemiol. 2020;49(1):113-130. doi: 10.1093/ije/dyz078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moubarac JC, Parra DC, Cannon G, Monteiro CA. Food classification systems based on food processing: significance and implications for policies and actions: a systematic literature review and assessment. Curr Obes Rep. 2014;3(2):256-272. doi: 10.1007/s13679-014-0092-0 [DOI] [PubMed] [Google Scholar]
- 6.Moodie R, Stuckler D, Monteiro C, et al. ; Lancet NCD Action Group . Profits and pandemics: prevention of harmful effects of tobacco, alcohol, and ultra-processed food and drink industries. Lancet. 2013;381(9867):670-679. doi: 10.1016/S0140-6736(12)62089-3 [DOI] [PubMed] [Google Scholar]
- 7.Pagliai G, Dinu M, Madarena MP, Bonaccio M, Iacoviello L, Sofi F. Consumption of ultra-processed foods and health status: a systematic review and meta-analysis. Br J Nutr. 2021;125(3):308-318. doi: 10.1017/S0007114520002688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romero Ferreiro C, Lora Pablos D, Gómez de la Cámara A. Two dimensions of nutritional value: Nutri-Score and NOVA. Nutrients. 2021;13(8):2783. doi: 10.3390/nu13082783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monteiro CA, Cannon G, Levy RB, et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. 2019;22(5):936-941. doi: 10.1017/S1368980018003762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monteiro CA, Cannon G, Levy R, et al. NOVA. the star shines bright. World Nutr. 2016;7(1-3):28-38. Accessed November 10, 2023. https://worldnutritionjournal.org/index.php/wn/article/view/5 [Google Scholar]
- 11.Monteiro CA, Levy RB, Claro RM, de Castro IRR, Cannon G. Increasing consumption of ultra-processed foods and likely impact on human health: evidence from Brazil. Public Health Nutr. 2011;14(1):5-13. doi: 10.1017/S1368980010003241 [DOI] [PubMed] [Google Scholar]
- 12.Moubarac JC, Martins APB, Claro RM, Levy RB, Cannon G, Monteiro CA. Consumption of ultra-processed foods and likely impact on human health. evidence from Canada. Public Health Nutr. 2013;16(12):2240-2248. doi: 10.1017/S1368980012005009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stuckler D, McKee M, Ebrahim S, Basu S. Manufacturing epidemics: the role of global producers in increased consumption of unhealthy commodities including processed foods, alcohol, and tobacco. PLoS Med. 2012;9(6):e1001235. doi: 10.1371/journal.pmed.1001235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martins APB, Levy RB, Claro RM, Moubarac JC, Monteiro CA. Increased contribution of ultra-processed food products in the Brazilian diet (1987-2009). Rev Saude Publica. 2013;47(4):656-665. doi: 10.1590/S0034-8910.2013047004968 [DOI] [PubMed] [Google Scholar]
- 15.Monteiro CA, Moubarac JC, Cannon G, Ng SW, Popkin B. Ultra-processed products are becoming dominant in the global food system. Obes Rev. 2013;14(suppl 2):21-28. doi: 10.1111/obr.12107 [DOI] [PubMed] [Google Scholar]
- 16.Gutiérrez-González E, Sánchez Arenas F, López-Sobaler AM, Andreu Ivorra B, Rollán Gordo A, García-Solano M. Socioeconomic and gender inequalities in childhood obesity in Spain. An Pediatr (Engl Ed). 2023;99(2):111-121. [DOI] [PubMed] [Google Scholar]
- 17.Luque V, Escribano J, Closa-Monasterolo R, et al. Unhealthy dietary patterns established in infancy track to mid-childhood: the EU Childhood Obesity Project. J Nutr. 2018;148(5):752-759. doi: 10.1093/jn/nxy025 [DOI] [PubMed] [Google Scholar]
- 18.Mikkilä V, Räsänen L, Raitakari OT, Pietinen P, Viikari J. Consistent dietary patterns identified from childhood to adulthood: the cardiovascular risk in Young Finns Study. Br J Nutr. 2005;93(6):923-931. doi: 10.1079/BJN20051418 [DOI] [PubMed] [Google Scholar]
- 19.Mikkilä V, Räsänen L, Raitakari OT, Pietinen P, Viikari J. Longitudinal changes in diet from childhood into adulthood with respect to risk of cardiovascular diseases: The Cardiovascular Risk in Young Finns Study. Eur J Clin Nutr. 2004;58(7):1038-1045. doi: 10.1038/sj.ejcn.1601929 [DOI] [PubMed] [Google Scholar]
- 20.Mikkilä V, Räsänen L, Laaksonen MML, et al. Long-term dietary patterns and carotid artery intima media thickness: the Cardiovascular Risk in Young Finns Study. Br J Nutr. 2009;102(10):1507-1512. doi: 10.1017/S000711450999064X [DOI] [PubMed] [Google Scholar]
- 21.Nardocci M, Polsky JY, Moubarac JC. Consumption of ultra-processed foods is associated with obesity, diabetes and hypertension in Canadian adults. Can J Public Health. 2021;112(3):421-429. doi: 10.17269/s41997-020-00429-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srour B, Fezeu LK, Kesse-Guyot E, et al. Ultraprocessed food consumption and risk of type 2 diabetes among participants of the NutriNet-Santé prospective cohort. JAMA Intern Med. 2020;180(2):283-291. doi: 10.1001/jamainternmed.2019.5942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srour B, Fezeu LK, Kesse-Guyot E, et al. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). BMJ. 2019;365:l1451. doi: 10.1136/bmj.l1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rico-Campà A, Martínez-González MA, Alvarez-Alvarez I, et al. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ. 2019;365:l1949. doi: 10.1136/bmj.l1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petridi E, Karatzi K, Magriplis E, Charidemou E, Philippou E, Zampelas A. The impact of ultra-processed foods on obesity and cardiometabolic comorbidities in children and adolescents: a systematic review. Nutr Rev. Published online August 7, 2023. doi: 10.1093/nutrit/nuad095 [DOI] [PubMed] [Google Scholar]
- 26.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 27.Garcidueñas-Fimbres TE, Paz-Graniel I, Gómez-Martínez C, et al. ; Childhood Obesity Risk Assessment Longitudinal Study (CORALS) Study Investigators . Associations between eating speed, diet quality, adiposity, and cardiometabolic risk factors. J Pediatr. 2023;252:31-39.e1. doi: 10.1016/j.jpeds.2022.08.024 [DOI] [PubMed] [Google Scholar]
- 28.Babio N, de Las Heras-Delgado S, De Miguel-Etayo P, et al. Reproducibility and relative validity of a semi-quantitative food and beverage frequency questionnaire for Spanish children aged 3 to 11 years: the COME-Kids F&B-FQ. Eur J Pediatr. 2023;182(12):5577-5589. doi: 10.1007/s00431-023-05220-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes. 2012;7(4):284-294. doi: 10.1111/j.2047-6310.2012.00064.x [DOI] [PubMed] [Google Scholar]
- 30.VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body’s fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52(6):953-959. doi: 10.1093/ajcn/52.6.953 [DOI] [PubMed] [Google Scholar]
- 31.Muñoz-Hernando J, Escribano J, Ferré N, et al. Usefulness of the waist-to-height ratio for predicting cardiometabolic risk in children and its suggested boundary values. Clin Nutr. 2022;41(2):508-516. doi: 10.1016/j.clnu.2021.12.008 [DOI] [PubMed] [Google Scholar]
- 32.Verbestel V, De Henauw S, Bammann K, et al. ; IDEFICS Consortium . Are context-specific measures of parental-reported physical activity and sedentary behaviour associated with accelerometer data in 2-9-year-old European children? Public Health Nutr. 2015;18(5):860-868. doi: 10.1017/S136898001400086X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17-27. doi: 10.1093/oxfordjournals.aje.a114366 [DOI] [PubMed] [Google Scholar]
- 34.de Waal T, Pannekoek J, Scholtus S. Handbook of Statistical Data Editing and Imputation. Wiley; 2011, . doi: 10.1002/9780470904848 [DOI] [Google Scholar]
- 35.Vedovato GM, Vilela S, Severo M, Rodrigues S, Lopes C, Oliveira A. Ultra-processed food consumption, appetitive traits and BMI in children: a prospective study. Br J Nutr. 2021;125(12):1427-1436. doi: 10.1017/S0007114520003712 [DOI] [PubMed] [Google Scholar]
- 36.Chang K, Khandpur N, Neri D, et al. Association between childhood consumption of ultraprocessed food and adiposity trajectories in the Avon Longitudinal Study of Parents and Children Birth Cohort. JAMA Pediatr. 2021;175(9):e211573. doi: 10.1001/jamapediatrics.2021.1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bawaked RA, Fernández-Barrés S, Navarrete-Muñoz EM, et al. Impact of lifestyle behaviors in early childhood on obesity and cardiometabolic risk in children: results from the Spanish INMA birth cohort study. Pediatr Obes. 2020;15(3):e12590. doi: 10.1111/ijpo.12590 [DOI] [PubMed] [Google Scholar]
- 38.Neri D, Martínez-Steele E, Khandpur N, Levy R. Associations between ultra-processed foods consumption and indicators of adiposity in US adolescents: cross-sectional analysis of the 2011-2016 National Health and Nutrition Examination Survey. J Acad Nutr Diet. 2022;122(8):1474-1487.e2. doi: 10.1016/j.jand.2022.01.005 [DOI] [PubMed] [Google Scholar]
- 39.Costa CS, Rauber F, Leffa PS, Sangalli CN, Campagnolo PDB, Vitolo MR. Ultra-processed food consumption and its effects on anthropometric and glucose profile: a longitudinal study during childhood. Nutr Metab Cardiovasc Dis. 2019;29(2):177-184. doi: 10.1016/j.numecd.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 40.Vilela S, Magalhães V, Severo M, Oliveira A, Torres D, Lopes C. Effect of the food processing degree on cardiometabolic health outcomes: a prospective approach in childhood. Clin Nutr. 2022;41(10):2235-2243. doi: 10.1016/j.clnu.2022.07.034 [DOI] [PubMed] [Google Scholar]
- 41.Donat-Vargas C, Sandoval-Insausti H, Rey-García J, et al. High consumption of ultra-processed food is associated with incident dyslipidemia: a prospective study of older adults. J Nutr. 2021;151(8):2390-2398. doi: 10.1093/jn/nxab118 [DOI] [PubMed] [Google Scholar]
- 42.Ribeiro GJS, de Araújo Pinto A. Consumption of ultra-processed foods in Brazilian Children: an analysis of regional trends. J Pediatr Nurs. 2021;61:e106-e111. doi: 10.1016/j.pedn.2021.06.006 [DOI] [PubMed] [Google Scholar]
- 43.Fangupo LJ, Haszard JJ, Taylor BJ, Gray AR, Lawrence JA, Taylor RW. Ultra-processed food intake and associations with demographic factors in young New Zealand Children. J Acad Nutr Diet. 2021;121(2):305-313. doi: 10.1016/j.jand.2020.08.088 [DOI] [PubMed] [Google Scholar]
- 44.Lauria F, Dello Russo M, Formisano A, et al. ; I.Family Consortium . Ultra-processed foods consumption and diet quality of European children, adolescents and adults: results from the I.Family study. Nutr Metab Cardiovasc Dis. 2021;31(11):3031-3043. doi: 10.1016/j.numecd.2021.07.019 [DOI] [PubMed] [Google Scholar]
- 45.Cronin FM, Hurley SM, Buckley T, et al. Mediators of socioeconomic differences in overweight and obesity among youth in Ireland and the UK (2011-2021): a systematic review. BMC Public Health. 2022;22(1):1585. doi: 10.1186/s12889-022-14004-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El-Sayed AM, Scarborough P, Galea S. Socioeconomic inequalities in childhood obesity in the United Kingdom: a systematic review of the literature. Obes Facts. 2012;5(5):671-692. doi: 10.1159/000343611 [DOI] [PubMed] [Google Scholar]
- 47.Diet, Nutrition, and the Prevention of Chronic Diseases: report of a joint WHO/FAO expert consultation. World Health Organization; 2003. Accessed March 8, 2024. https://apps.who.int/iris/bitstream/handle/10665/42665/WHO_TRS_916.pdf?sequence=1
- 48.Wylie-Rosett J, Segal-Isaacson CJ, Segal-Isaacson A. Carbohydrates and increases in obesity: does the type of carbohydrate make a difference? Obes Res. 2004;12(S11)(suppl 2):124S-129S. doi: 10.1038/oby.2004.277 [DOI] [PubMed] [Google Scholar]
- 49.Drewnowski A, Bellisle F. Liquid calories, sugar, and body weight. Am J Clin Nutr. 2007;85(3):651-661. doi: 10.1093/ajcn/85.3.651 [DOI] [PubMed] [Google Scholar]
- 50.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84(2):274-288. doi: 10.1093/ajcn/84.2.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan SS, Ning H, Wilkins JT, et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3(4):280-287. doi: 10.1001/jamacardio.2018.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133(2):187-225. doi: 10.1161/CIRCULATIONAHA.115.018585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pepino MY, Tiemann CD, Patterson BW, Wice BM, Klein S. Sucralose affects glycemic and hormonal responses to an oral glucose load. Diabetes Care. 2013;36(9):2530-2535. doi: 10.2337/dc12-2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jang W, Jeoung NH, Cho KH. Modified apolipoprotein (apo) A-I by artificial sweetener causes severe premature cellular senescence and atherosclerosis with impairment of functional and structural properties of apoA-I in lipid-free and lipid-bound state. Mol Cells. 2011;31(5):461-470. doi: 10.1007/s10059-011-1009-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shannon M, Green B, Willars G, et al. The endocrine disrupting potential of monosodium glutamate (MSG) on secretion of the glucagon-like peptide-1 (GLP-1) gut hormone and GLP-1 receptor interaction. Toxicol Lett. 2017;265:97-105. doi: 10.1016/j.toxlet.2016.11.015 [DOI] [PubMed] [Google Scholar]
- 56.Bhattacharyya S, O-Sullivan I, Katyal S, Unterman T, Tobacman JK. Exposure to the common food additive carrageenan leads to glucose intolerance, insulin resistance and inhibition of insulin signalling in HepG2 cells and C57BL/6J mice. Diabetologia. 2012;55(1):194-203. doi: 10.1007/s00125-011-2333-z [DOI] [PubMed] [Google Scholar]
- 57.Zhang Q, Bai Y, Yang Z, Tian J, Meng Z. The molecular mechanisms of sodium metabisulfite on the expression of K ATP and L-Ca2+ channels in rat hearts. Regul Toxicol Pharmacol. 2015;72(3):440-446. doi: 10.1016/j.yrtph.2015.05.021 [DOI] [PubMed] [Google Scholar]
- 58.Chassaing B, Koren O, Goodrich JK, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519(7541):92-96. doi: 10.1038/nature14232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeJarnett N, Conklin DJ, Riggs DW, et al. Acrolein exposure is associated with increased cardiovascular disease risk. J Am Heart Assoc. 2014;3(4):e000934. doi: 10.1161/JAHA.114.000934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y, Huang M, Zhuang P, et al. Exposure to acrylamide and the risk of cardiovascular diseases in the National Health and Nutrition Examination Survey 2003-2006. Environ Int. 2018;117:154-163. doi: 10.1016/j.envint.2018.04.047 [DOI] [PubMed] [Google Scholar]
- 61.Canova C, Di Nisio A, Barbieri G, et al. Pfas concentrations and cardiometabolic traits in highly exposed children and adolescents. Int J Environ Res Public Health. 2021;18(24):12881. doi: 10.3390/ijerph182412881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rancière F, Lyons JGY, Loh VH, et al. Bisphenol A and the risk of cardiometabolic disorders: a systematic review with meta-analysis of the epidemiological evidence. Environ Health. 2015;14:46. doi: 10.1186/s12940-015-0036-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Astrup A, Monteiro CA. Does the concept of “ultra-processed foods” help inform dietary guidelines, beyond conventional classification systems? debate consensus. Am J Clin Nutr. 2022;116(6):1489-1491. doi: 10.1093/ajcn/nqac230 [DOI] [PubMed] [Google Scholar]
- 64.Monteiro CA, Astrup A. Does the concept of “ultra-processed foods” help inform dietary guidelines, beyond conventional classification systems? yes. Am J Clin Nutr. 2022;116(6):1476-1481. doi: 10.1093/ajcn/nqac122 [DOI] [PubMed] [Google Scholar]
- 65.Touvier M, da Costa Louzada ML, Mozaffarian D, Baker P, Juul F, Srour B. Ultra-processed foods and cardiometabolic health: public health policies to reduce consumption cannot wait. BMJ. 2023;383:e075294. doi: 10.1136/bmj-2023-075294 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Additional Study Assessments
eReference
eTable 1. Distribution of FFQ Items Into 4 Groups According to the Degree of Their Processing Established by NOVA Classification System
eFigure 1. Flow Chart of the Children Included in the Analysis
eTable 2. Association Between Energy-Adjusted Ultraprocessed Food Consumption in Tertiles (in g/day) and CVD Risk Factors at Baseline
eFigure 2. Linear Regression Models Replacing 100 g of UPF With 100 g of Unprocessed/Minimally Processed Foods
eTable 3. Maternal Socioprofessional Stratified Regression Association Between 1-SD Increment of Energy-Adjusted Ultraprocessed Food Consumption (in g/day) and CVD Risk Factors at Baseline
eTable 4. Maternal Education Level Stratified Regression Association Between 1-SD Increment of Energy-Adjusted Ultraprocessed Food Consumption (in g/day) and CVD Risk Factors at Baseline
Data Sharing Statement