Abstract
Tissue factor (TF) is a transmembrane glycoprotein and the main triggering element of blood coagulation. TF expression on monocytes and endothelial cells is induced by exposure to endotoxin, tumor necrosis factor, and IL-1 and is considered to appear in consequence of inflammation. In order to assess the proinflammatory capacity of TF itself, the recombinant extracellular domain of TF was injected intra-articularly into healthy mice. To characterize the role of immune cells in the TF-induced arthritis, mice deprived of lymphocytes, neutrophils and monocytes were used. Histomorphological analysis of the joints with respect to inflammatory cell infiltration, pannus formation and erosion formation revealed development of arthritis in 80% of animals injected with TF. In most of the cases synovial proliferation was accompanied by pannus formation and cartilage destruction. Inflammatory cell infiltrate consisted of CD4-Mac1+ macrophages. Depletion of monocytes was, however, not enough to abolish inflammation. Indeed, combined deficiency of monocytes and lymphocytes was required to prevent inflammation following the injection of TF. We observed that TF induced chemokine production (MIP-1α and RANTES), but did not induce a proliferative response nor cytokine release by mouse spleen cells. TF has strong inflammatogenic properties mediated predominantly by monocytes and their release of chemokines. Our study shows that TF can simultaneously trigger the immune and coagulation systems.
Keywords: arthritis, inflammation, monocyte, tissue factor
Introduction
Tissue factor (TF) is a transmembrane glycoprotein and the major cellular trigger of blood coagulation. Assembly of a complex between TF and factor VII (FVII) initiates fibrin formation. Besides its role in blood coagulation, TF is also important for the vascular development regulating embryonic angiogenesis and supporting proliferative and invasive capacities of cells [1-4]. Expression of TF on the cell surface and its appearance as a soluble molecule are characteristic features of acute and chronic inflammation in conditions such as sepsis, atherosclerosis, Crohn's desease, systemic lupus erythematosus, and rejection reactions [5-9].
The role of TF in the inflammatory process is a matter of discussion. A variety of inflammatory stimuli, including mitogens, bacterial cell products, components of the complement system and cytokines, are known to promote the expression of TF on the surface of endothelial cells and monocytes [10]. T cells regulate TF expression: upregulation is achieved by Th1 cytokines, while Th2-derived cytokines are mostly inhibitory [11]. TF, in turn, may participate in cellular interactions promoting leukocyte adhesion and transendothelial migration [12,13].
In the present study we investigated the capacity of TF to induce inflammation by injecting human recombinant TF (rTF) into joint cavities of healthy mice. Histomorphological investigation of the injected joints showed a remarkable cellular infiltration of synovia and occasionally cartilage destruction, indicating that TF possesses strong proinflammatory properties. Expression of TF on monocytes and synovial cells may be crucial for potentiating the initial stages of inflammation in joints.
Materials and methods
Mice and reagents
BALB/c and NMRI mice were purchased from ALAB (Stockholm, Sweden). Severe combined immunodeficient (SCID) mice and their congenic strain CB17 were purchased from M&B (Bomholtvej, Denmark). All mice were housed in the animal facility of the Department of Rheumatology, University of Göteborg. Female mice 6–8 weeks of age were used in all the experiments.
Recombinant tissue factor
This version of rTF contains the extracellular domain of human TF, amino acid residues 1–219 [14], which retains the ability to bind to FVII and FVIIa and to enhance enzymatic activity of FVIIa. rTF preparation was dissolved in Hepes buffered saline (Hepes 30 mM, NaCl 100 mM, 0.02% Na-azide; pH7.5) and kept in aliquots at -70°C until use. Two preparations of TF were used that contained 3.7 μg/ml and 32 ng/ml of lipopolysaccharide (LPS), respectively. No significant difference in the results obtained with these two preparations was observed and the results were thus pooled.
Injection protocol and cell depletion procedure
NMRI strain mice were used in the experiments unless stated otherwise. Induction of arthritis was performed by injection of rTF in a volume of 20 μl intra-articularly into the right knee joint. Control experiments were performed by injecting an equivalent amount of LPS preparation in Hepes buffered saline into the contralateral knee joint.
Neutrophil depletion was performed by intraperitoneal injection of the monoclonal antibody RB6-8C5 (hybridoma was kindly provided by Dr R Coffman, DNAX Research Institute, Palo Alto, CA, USA) two hours before the rTF injection, as described previously [15]. The IgG rat antiovalbumin monoclonal antibody (kindly provided by Dr E Telemo, Department of Clinical Immunology, University of Göteborg, Sweden) was injected into the control group. Monocyte depletion was induced by subcutaneous injection of etoposide (Bristol-Myers Squibb, Bromma, Sweden; 12.5 mg/kg body weight, in a volume of 100 μl [16]) on three consecutive days before and three consecutive days after injection of rTF. The impact of lymphocytes was studied by injecting rTF into SCID mice lacking B and T lymphocytes and their congenic strain, CB17.
Systemic defibrination
This was achieved by using mini-osmotic pumps (model 2002, Alza Corp, Palo Alto, CA, USA) filled with buffered solution containing 200 U/ml ancrod (Sigma Chemical, St Louis, MO, USA) implanted subcutaneously three days before the injection of rTF [17].
Histopathological and immunohistological examination of joints
Histological examination of joints was done after paraffin embedding, cutting, and staining with hematoxylin and eosin. All the slides were coded and evaluated blindly. The specimens were evaluated with respect to occurrence of synovial hypertrophy, inflammatory cells in synovial sublining compartment, pannus formation and cartilage and/or subchondral bone destruction. Intensity of synovial inflammation (arthritis index) was graded arbitrarily from 0 to 3.
For immunohistochemical examination, the knee joints were removed and demineralized as described [18]. Serial cryosections of 6 μm thickness were stained with rat monoclonal antibodies directed against mouse CD11b (Mac-1; M 1/70) or CD4 (GK1.5) (both antibodies from PharMingen, San Diego, CA, USA), then incubated with biotinylated secondary antibodies (DAKO A/S, Gosulp, Denmark) and avidin-biotin-peroxidase complexes (ABC). All sections were counterstained with Mayer's hematoxylin.
Splenocyte stimulation
Murine splenocyte suspension (cell density 2 × 106/ml) in Iscove's medium supplemented with 10% fetal calf serum (FCS), 2 nM L-glutamine, mercaptoethanol and 50 μg/ml gentamicin was stimulated with rTF at a final concentration of 0.1–10 μg/ml. At defined time points, supernatants were collected for determination of cytokine and chemokine levels.
Lymphocyte proliferation
Proliferation was determined by the incorporation of [3H]-thymidine (specific activity, 42 Ci/mmol; Amersham International, Buckinghamshire, UK) into splenocyte suspension (cell density 4 × 105/ml), stimulated for 72 hours with either rTF or LPS at a final concentration of 0.1–10 μg/ml. The results were expressed as a stimulation index (counts per minute, mean ± standard deviation).
IL-6 determination
The level of interleukin (IL)-6 in the rTF-stimulated supernatants was determined by a bioassay measuring the effect of test samples on the growth of the IL-6-dependent cell line B13.29 [19]. The results were analyzed by incorporation of [3H]-thymidine after 72 hours stimulation and compared to results with the standard dilutions of recombinant mouse IL-6 (Genzyme, Cambridge, MA, USA).
RANTES and MIP-1α determination
The levels of macrophage inflammatory protein (MIP)-1α and RANTES (regulated on activation normal T-cell expressed and secreted) were measured by an enzyme-linked immunosorbent assay using Quantikine M kits (R&D Systems, Minneapolis, MN, USA) and expressed in pg/ml.
Statistical analysis
The differences in the incidence and the severity of arthritis in the groups were analyzed by the Fisher's test and Mann–Whitney U test, respectively.
Results
rTF induces arthritis
The ability of rTF to induce arthritis was evaluated by injection of rTF in the doses 0.2, 2.0, and 20 μg (0.004, 0.04, and 0.4 nmol) into the knee joint of NMRI mice. Four days after the inoculation, morphological signs of arthritis (synovial tissue hypertrophy, and inflammatory cell infiltrates) were found in 33%, 56% and 83% of joints, respectively. Control mice injected with the amount of LPS equivalent to its concentration in the rTF preparation exhibited arthritis only in 28% of cases (13/15 versus 4/14; P < 0.01). The arthritis index of the rTF-induced arthritis was significantly higher in joints injected with 20 μg of rTF than in the controls (1.73 versus 0.23; P < 0.01). Moreover, 6/13 joints injected with 20 μg of rTF developed extrasynovial features of arthritis (pannus formation, n = 2; cartilage destruction, n = 3 and periarticular bone destruction, n = 1) compared to only one in the control group. In all further experiments the dose of 20 μg of TF per knee was used.
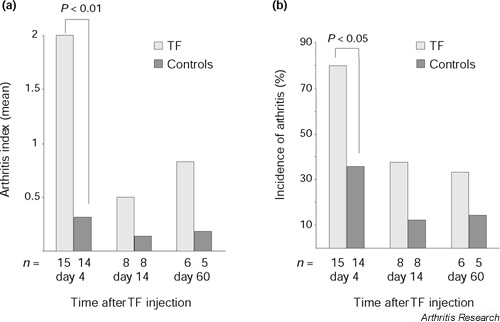
Dynamics of rTF-induced arthritis were assessed morphologically on days 4, 14 and 60 after the rTF injection. The highest frequency of arthritis and severity of inflammation was observed on day 4 after injection and it diminished significantly by days 14 and 60 (13/15 versus 2/8 and 2/6, P < 0.05; Fig. 1a,b). Notably, erosion and/or pannus formation were always found in cases of long lasting arthritis but in none of the controls.
Figure 1.
Measurements of arthritis in murine knee joints. (a) Arthritis index and (b) incidence of arthritis after intra-articular injection of TF (20 μg/joint) as evaluated by histological examination. Arthritis index was assessed as described in the Materials and methods section. Asterisks indicate significant differences of the means between the mice receiving TF and the controls.
Sensitivity to rTF varied between the healthy mouse strains. Four days after the injection of rTF (20 μg/knee) into NMRI (n = 15), CB17 (n = 8) and BALB/c mice (n = 8), morphological signs of arthritis were registered in 80%, 75% and 50% of knee joints, respectively. These results indicate that susceptibility to inflammatory potential of rTF is dependent on genetic background of the host.
Participation of various immune cells in the rTF-induced inflammation
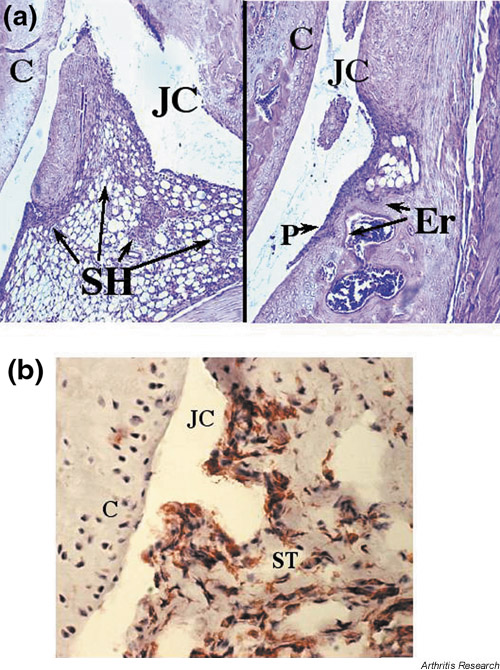
Immunochemical staining of joint sections revealed dense infiltrates consisting of Mac-1+ mononuclear cells in the synovial tissue. In contrast, all the sections were negative for staining with CD4-specific antibodies. This allowed us to conclude that the inflammatory infiltrate observed after the injection of rTF consisted predominantly of macrophages accompanied by few if any neutrophils (Fig. 2).
Figure 2.
Morphological changes in the joint after injection of TF. (a)Histopathology of an arthritic knee joint four days after injection of TF (20 μg/joint). Infiltration of mononuclear cells in synovial tissue is apparent. Original magnification ×20. (b) Immunohistochemical staining of an arthritic knee joint, showing cells expressing Mac-1. Original magnification ×40. JC, joint cavity; C, cartilage; SH, synovial hyperplasy; P, pannus; Er, bone erosion; ST, synovial tissue. Arrows indicate inflammatory cells in synovia, pannus formation and cartilage destruction.
To evaluate the role of different immune cells in the development of rTF-induced inflammation, cell depletion procedures were performed. Mice pretreated with lysing antineutrophil antibodies showed no reduction in the frequency or intensity of arthritis compared to controls that received antiovalbumin antibodies (5/8 versus 7/8, respectively). Mice injected with etoposide, and thereby deprived of monocyte/macrophage cell population, demonstrated a tendency to a reduction in frequency but not the severity of arthritis (6/8 versus 4/7; not significant; Fig. 3). Intra-articular injection of rTF into SCID mice deficient for T- and B-lymphocytes revealed no difference in the frequency of arthritis compared to congenic CB17 mice strain (11/15 versus 12/15, not significant). The results of experiments indicated that isolated depletion of neither monocyte nor lymphocyte cell populations was enough to abolish the induction of rTF-induced arthritis. To test if a combined lymphocyte and monocyte cell depletion was efficient in prevention of rTF-induced cell infiltration, SCID mice treated with etoposide were intra-articularly injected with rTF. Only one of seven mice in this group developed arthritis in response to injection of rTF, demonstrating that interaction between macrophages and lymphocytes is essential for cellular infiltration of synovium following rTF injection (Fig. 3).
Figure 3.
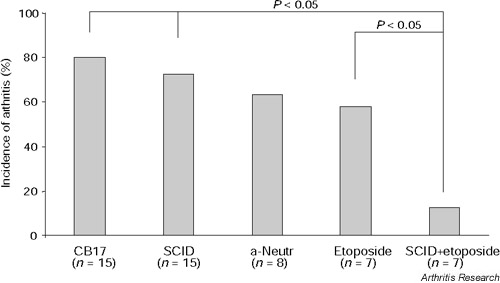
Lymphocytes and monocytes are mandatory for the development of TF-induced arthritis. Incidence of arthritis was assessed in mice depleted of various immune cells. Significant differences of incidence of arthritis between the groups are indicated.
Systemic depletion of fibrinogen with ancrod prior to rTF injection did not reduce the incidence of rTF-induced arthritis in 6/8 NMRI mice.
In vitro cell stimulation with rTF
Effects of rTF on lymphoid cells were investigated by incubating mouse splenocytes with rTF (0.1 μg/ml, 1 μg/ml, 5 μg/ml, or 10 μg/ml). Investigation of supernatants after 48 hours of stimulation for the level of IL-6, RANTES, and MIP-1α demonstrated a dose-dependent increase in the level of chemokines. The increase was pronounced for MIP-1α (73 ± 33 pg/ml in non-stimulated cells versus 139 ± 32 pg/ml after stimulation with 5 μg/ml of rTF; n = 3) but was only marginal for RANTES (256 ± 46 pg/ml versus 365 ± 32 pg/ml; n = 3). In contrast, neither change in the level of IL-6 nor proliferation of lymphocytes was observed.
Discussion
Inflammation and thrombosis are linked in many clinical conditions. It is presumed that proinflammatory mediators potentiate activation of blood coagulation and serine proteases of the coagulation system, especially the terminal enzyme thrombin, known for proinflammatory and mitogenic/chemotactic effects both in circulation and locally in synovial tissue [20-22]. An indication of the importance of TF in the inflammatory process was obtained in the animal model of sepsis, in which TF-dependent procoagulant activity correlated with the level of tumor necrosis factor-α [23]. In addition, modulation of TF-dependent coagulation by administration of either TF-pathway inhibitor or anti-TF antibodies decreased circulating levels of IL-6 and IL-8 [24,25], and diminished proliferation and perivascular cell infiltration [26,27]. This study shows that the TF molecule possesses strong proinflammatory properties, causing arthritis. Histological analysis revealed that TF-induced synovitis could be attributed mostly to infiltrating macrophages. Mononuclear cell influx into synovia is an early finding in the majority of asymptomatic subjects, preceding clinically overt arthritis in RA [28,29]. Furthermore, synovial macrophage infiltration is a characteristic feature of both autoimmune and bacterial arthritis [30,31] and is a determinant of joint erosions [32,33]. Our observation, in combination with an increased TF-dependent procoagulant activity in blood and synovial fluid of patients with RA, implies participation of TF in triggering the initial steps of inflammation during RA and/or in rendering the inflammation chronic.
Composition of inflammatory infiltrate and cell depletion studies showed that TF exerts its proinflammatory properties in a cell-specific manner targeting the macrophage/ monocyte population. This is not surprising taking into consideration that macrophages/monocytes are the only cells in the blood circulation compartment which express TF on their surface [8,34]. Moreover, we demonstrated that an interaction between monocytes and lymphocytes was a prerequisite of the TF-induced arthritis. Activated T cells were able to induce TF production by stimulating monocytes through CD40 ligand [35,36]. This mechanism may contribute to a positive feedback essential for maintenance of TF-induced inflammation. Interestingly, neutrophils, other principal inflammatory cells, were not sensitive to TF stimulation – these cells were neither found in synovial tissues nor affected arthritis frequency in the depletion experiments.
Mononuclear cells composing the inflammatory infiltrate in synovia were mostly Mac-1+ CD4- cells. Abundant expression of Mac-1 molecule on the monocyte surface indicates a principal role of β2-integrins in cell activation following exposure to TF. Two methods of such activation may be considered. TF may attract Mac-1+ cells indirectly by increasing formation of coagulation proteins known as Mac-1 ligands, e.g. Factor X and fibrinogen. However, the high incidence of TF-induced arthritis in defibrinated mice favors a direct stimulatory effect of TF on sensitive cells. Treatment of macrophages with TF in vitro has been shown to increase the expression of β2-chain-containing adhesion molecules [37] and supports the idea of direct TF-induced Mac-1 expression. Lack of CD4+ cells responsible for peptide presentation through MHC class II molecules shows that this mechanism is not compulsory for TF-induced synovial infiltration, in contrast to experimental glomerulonephritis where simultaneous expression of TF and MHC class II molecules was observed and efficiently blocked by anti-TF antibodies [38,39].
The way in which TF interacts with macrophages is not clear. Exposure of spleen cell cultures to TF did not induce the release of proinflammatory cytokines. These results favor the suggestion that TF acts directly on inflammatory cells and not by a cytokine-mediated mechanism. TF-mediated release of chemoattractant molecules, which recruit inflammatory cells from the circulation or surrounding tissues, is an alternative possibility. We found that in vitro splenocyte stimulation with TF was associated with selective release of the monocyte chemoattractant molecule MIP-1α. This observation may explain the dominance of mononuclear cells in the synovial inflammatory infiltrates of the joints injected with TF.
Altogether our observations suggest that TF may play an active role during arthritis by direct and/or indirect stimulation of influx of monocytes into the synovial tissue.
Abbreviations
FCS = fetal calf serum; FVII = factor VII; IL = interleukin; LPS = lipopolysaccharide; MIP = macrophage inhibitory protein; RA = rheumatoid arthritis; RANTES = regulated on activation normal T-cell expressed and secreted; RTF = recombinant TF; SCID = severe combined immunodeficient; TF = tissue factor.
Acknowledgments
Acknowledgements
The study was approved by the Ethics Committee of Sahlgrenska Hospital and animal experimentation guidelines were followed. The work was supported by the Göteborg Medical Society, the Swedish Association against Rheumatism, the King Gustaf V's Foundation, the Swedish Medical Research Council, the Nanna Svartz' Foundation, National Inflammation Network and the University of Göteborg.
References
- Carmeliet P, Mackman N, Moons L, Luther T, Gressens P, Van Vlaenderen I, Demunck H, Kasper M, Breier G, Evrard P, Muller M, Risau W, Edgington T, Collen D. Role of tissue factor in embryonic blood vessel development. Nature. 1996;383:73–75. doi: 10.1038/383073a0. [DOI] [PubMed] [Google Scholar]
- Lopez-Pedrera C, Jardi M, del Mar Malagon M, Ingles-Esteve J, Dorado G, Torres A, Felez J, Velasco F. Tissue factor (TF) and urokinase plasminogen activator receptor (uPAR) and bleeding complications in leukemic patients. Thromb Haemost. 1997;77:62–70. [PubMed] [Google Scholar]
- Inufusa H, Nakatani Y, Adachi T, Wakano T, Nakajima A, Nakamura M, Suzuki M, Ando O, Kurimoto M, Miyake M, Shindo K, Yasutomi M. Correlation of prognosis of breast cancer patients and expression of Ley which acts as a cofactor of tumor procoagulant. Int J Oncol. 1998;13:481–487. doi: 10.3892/ijo.13.3.481. [DOI] [PubMed] [Google Scholar]
- Ueno T, Toi M, Koike M, Nakamura S, Tominaga T. Tissue factor expression in breast cancer tissues: its correlation with prognosis and plasma concentration. Br J Cancer. 2000;83:164–170. doi: 10.1054/bjoc.2000.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor FB, Jr, Chang AC, Esmon CT, Hinshaw LB. Baboon model of Escherichia coli sepsis: description of its four stages and the role of tumor necrosis factor, tissue factors, and the protein C system in septic shock. Curr Stud Hematol Blood Transfus. 1991;58:8–14. doi: 10.1159/000419328. [DOI] [PubMed] [Google Scholar]
- Wakita Y, Wada H, Nakase T, Nakasaki T, Shimura M, Hiyoyama K, Mori Y, Gabazza EC, Nishikawa M, Deguchi K, Shiku H. Aberrations of the tissue factor pathway in patients positive for lupus anticoagulant. Clin Appl Thromb Hemost. 1999;5:10–15. doi: 10.1177/107602969900500103. [DOI] [PubMed] [Google Scholar]
- Weinberg JB, Wortham TS, Misukonis MA, Patton KL, Chitneni SR. Synovial mononuclear phagocytes in rheumatoid arthritis and osteoarthritis: quantitative and functional aspects. Immunol Invest. 1993;22:365–374. doi: 10.3109/08820139309063415. [DOI] [PubMed] [Google Scholar]
- Tremoli E, Camera M, Toschi V, Colli S. Tissue factor in atherosclerosis. Atherosclerosis. 1999;144:273–283. doi: 10.1016/s0021-9150(99)00063-5. [DOI] [PubMed] [Google Scholar]
- More L, Sim R, Hudson M, Dhillon AP, Pounder R, Wakefield AJ. Immunohistochemical study of tissue factor expression in normal intestine and idiopathic inflammatory bowel disease. J Clin Pathol. 1993;46:703–708. doi: 10.1136/jcp.46.8.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broze GJ. in "Haemostasis and Thrombosis," 3d ed, Churchill Livingstone, 1994.
- Del Prete G, De Carli M, Lammel RM, D'Elios MM, Daniel KC, Giusti B, Abbate R, Romagnani S. Th1 and Th2 T-helper cells exert opposite regulatory effects on procoagulant activity and tissue factor production by human monocytes. Blood. 1995;86:250–257. [PubMed] [Google Scholar]
- Randolph GJ, Luther T, Albrecht S, Magdolen V, Muller WA. Role of tissue factor in adhesion of mononuclear phagocytes to and trafficking through endothelium in vitro. Blood. 1998;92:4167–4177. [PubMed] [Google Scholar]
- Ott I, Fischer EG, Miyagi Y, Mueller BM, Ruf W. A role for tissue factor in cell adhesion and migration mediated by interaction with actin-binding protein 280. J Cell Biol. 1998;140:1241–1253. doi: 10.1083/jcb.140.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey JH, Fakhrai H, Edgington TS. Molecular cloning of the cDNA for tissue factor, the cellular receptor for the initiation of the coagulation protease cascade. Cell. 1987;50:129–135. doi: 10.1016/0092-8674(87)90669-6. [DOI] [PubMed] [Google Scholar]
- Verdrengh M, Tarkowski A. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect Immun. 1997;65:2517–2521. doi: 10.1128/iai.65.7.2517-2521.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calame W, Douwes-Idema AE, van den Barselaar MT, van Furth R, Mattie H. Influence of cytostatic agents on the pulmonary defence of mice infected with Klebsiella pneumoniae and on the efficacy of treatment with ceftriaxone. J Infect. 1994;29:53–66. doi: 10.1016/s0163-4453(94)95087-3. [DOI] [PubMed] [Google Scholar]
- Busso N, Peclat V, Van Ness K, Kolodziesczyk E, Degen J, Bugge T, So A. Exacerbation of antigen-induced arthritis in urokinase-deficient mice. J Clin Invest. 1998;102:41–50. doi: 10.1172/JCI2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson R, Tarkowski A, Klareskog L. A demineralization procedure for immunohistopathological use. EDTA treatment preserves lymphoid cell surface antigens. J Immunol Methods. 1986;88:109–114. doi: 10.1016/0022-1759(86)90058-x. [DOI] [PubMed] [Google Scholar]
- Helle M, Boeije L, Aarden LA. Functional discrimination between interleukin 6 and interleukin 1. Eur J Immunol. 1988;18:1535–1540. doi: 10.1002/eji.1830181010. [DOI] [PubMed] [Google Scholar]
- Bone RC. Modulators of coagulation. A critical appraisal of their role in sepsis. Arch Intern Med. 1992;152:1381–1389. doi: 10.1001/archinte.152.7.1381. [DOI] [PubMed] [Google Scholar]
- Cicala C, Cirino G. Linkage between inflammation and coagulation: an update on the molecular basis of the crosstalk. Life Sci. 1998;62:1817–1824. doi: 10.1016/s0024-3205(97)01167-3. [DOI] [PubMed] [Google Scholar]
- Shin H, Kitajima I, Nakajima T, Shao Q, Tokioka T, Takasaki I, Hanyu N, Kubo T, Maruyama I. Thrombin receptor mediated signals induce expressions of interleukin 6 and granulocyte colony stimulating factor via NF-kappa B activation in synovial fibroblasts. Ann Rheum Dis. 1999;58:55–60. doi: 10.1136/ard.58.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor FB, Jr, Chang A, Ruf W, Morrissey JH, Hinshaw L, Catlett R, Blick K, Edgington TS. Lethal E. coli septic shock is prevented by blocking tissue factor with monoclonal antibody. Circ Shock. 1991;33:127–134. [PubMed] [Google Scholar]
- Goldfarb RD, Glock D, Johnson K, Creasey AA, Carr C, McCarthy RJ, Matushek M, Akhter I, Trenholme G, Parrillo JE. Randomized, blinded, placebo-controlled trial of tissue factor pathway inhibitor in porcine septic shock. Shock. 1998;10:258–264. doi: 10.1097/00024382-199810000-00005. [DOI] [PubMed] [Google Scholar]
- Carr C, Bild GS, Chang AC, Peer GT, Palmier MO, Frazier RB, Gustafson ME, Wun TC, Creasey AA, Hinshaw LB, Taylor Jr FB, Galluppi GR. Recombinant E. coli-derived tissue factor pathway inhibitor reduces coagulopathic and lethal effects in the baboon gram-negative model of septic shock. Circ Shock. 1994;44:126–137. [PubMed] [Google Scholar]
- Sato Y, Asada Y, Marutsuka K, Hatakeyama K, Sumiyoshi A. Tissue factor induces migration of cultured aortic smooth muscle cells. Thromb Haemost. 1996;75:389–392. [PubMed] [Google Scholar]
- Annex BH, Davies MG, Fulton GJ, Huynh TT, Channon KM, Ezekowitz MD, Hagen PO. Local delivery of a tissue factor antibody reduces early leukocyte infiltration but fails to limit intimal hyperplasia in experimental vein grafts. J Surg Res. 1998;80:164–170. doi: 10.1006/jsre.1998.5438. [DOI] [PubMed] [Google Scholar]
- Koizumi F, Matsuno H, Wakaki K, Ishii Y, Kurashige Y, Nakamura H. Synovitis in rheumatoid arthritis: scoring of characteristic histopathological features. Pathol Int. 1999;49:298–304. doi: 10.1046/j.1440-1827.1999.00863.x. [DOI] [PubMed] [Google Scholar]
- Kraan MC, Versendaal H, Jonker M, Bresnihan B, Post WJ, t Hart BA, Breedreld FC, Tak PP. Asymptomatic synovitis precedes clinically manifest arthritis. Arthritis Rheum. 1998;41:1481–1488. doi: 10.1002/1529-0131(199808)41:8<1481::AID-ART19>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Tak PP, Smeets TJ, Daha MR, Kluin PM, Meijers KA, Brand R, Meinders AE, Breedveld FC. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity [see comments]. Arthritis Rheum. 1997;40:217–225. doi: 10.1002/art.1780400206. [DOI] [PubMed] [Google Scholar]
- Bremell T, Abdelnour A, Tarkowski A. Histopathological and serological progression of experimental Staphylococcus aureus arthritis. Infect Immun. 1992;60:2976–2985. doi: 10.1128/iai.60.7.2976-2985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LE, Justen HP, Scholmerich J, Straub RH. The loss of sympathetic nerve fibers in the synovial tissue of patients with rheumatoid arthritis is accompanied by increased norepi-nephrine release from synovial macrophages. FASEB J. 2000;14:2097–2107. doi: 10.1096/fj.99-1082com. [DOI] [PubMed] [Google Scholar]
- Yanni G, Whelan A, Feighery C, Bresnihan B. Synovial tissue macrophages and joint erosion in rheumatoid arthritis. Ann Rheum Dis. 1994;53:39–44. doi: 10.1136/ard.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesen PL, Rauch U, Bohrmann B, Kling D, Roque M, Fallon JT, Badimon JJ, Himber J, Riederer MA, Nemerson Y. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci U S A. 1999;96:2311–2315. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach F, Schonbeck U, Bonnefoy JY, Pober JS, Libby P. Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of CD40: induction of collagenase, stromelysin, and tissue factor. Circulation. 1997;96:396–399. doi: 10.1161/01.cir.96.2.396. [DOI] [PubMed] [Google Scholar]
- Miller DL, Yaron R, Yellin MJ. CD40L-CD40 interactions regulate endothelial cell surface tissue factor and thrombomodulin expression. J Leukoc Biol. 1998;63:373–379. doi: 10.1002/jlb.63.3.373. [DOI] [PubMed] [Google Scholar]
- Cunningham MA, Romas P, Hutchinson P, Holdsworth SR, Tipping PG. Tissue factor and factor VIIa receptor/ligand interactions induce proinflammatory effects in macrophages. Blood. 1999;94:3413–3420. [PubMed] [Google Scholar]
- Erlich JH, Holdsworth SR, Tipping PG. Tissue factor initiates glomerular fibrin deposition and promotes major histocompatibility complex class II expression in crescentic glomerulonephritis [see comments]. Am J Pathol. 1997;150:873–880. [PMC free article] [PubMed] [Google Scholar]
- Labarrere CA, Esmon CT, Carson SD, Faulk WP. Concordant expression of tissue factor and class II MHC antigens in human placental endothelium. Placenta. 1990;11:309–318. doi: 10.1016/s0143-4004(05)80222-x. [DOI] [PubMed] [Google Scholar]