Abstract
Neutrophils play a Janus-faced role in the complex landscape of cancer pathogenesis and immunotherapy. As immune defense cells, neutrophils release toxic substances, including reactive oxygen species and matrix metalloproteinase 9, within the tumor microenvironment. They also modulate the expression of tumor necrosis factor-related apoptosis-inducing ligand and Fas ligand, augmenting their capacity to induce tumor cell apoptosis. Their involvement in antitumor immune regulation synergistically activates a network of immune cells, bolstering anticancer effects. Paradoxically, neutrophils can succumb to the influence of tumors, triggering signaling cascades such as JAK/STAT, which deactivate the immune system network, thereby promoting immune evasion by malignant cells. Additionally, neutrophil granular constituents, such as neutrophil elastase and vascular endothelial growth factor, intricately fuel tumor cell proliferation, metastasis, and angiogenesis. Understanding the mechanisms that guide neutrophils to collaborate with other immune cells for comprehensive tumor eradication is crucial to enhancing the efficacy of cancer therapeutics. In this review, we illuminate the underlying mechanisms governing neutrophil-mediated support or inhibition of tumor progression, with a particular focus on elucidating the internal and external factors that influence neutrophil polarization. We provide an overview of recent advances in clinical research regarding the involvement of neutrophils in cancer therapy. Moreover, the future prospects and limitations of neutrophil research are discussed, aiming to provide fresh insights for the development of innovative cancer treatment strategies targeting neutrophils.
Keywords: Neutrophils, Cancer, Immunotherapy, Antitumor activity, Protumor activity
Introduction
Neutrophils, the most abundant population of white blood cells in circulation, play critical roles in host defense against infection [1]. Traditionally recognized as inflammatory immune cells, neutrophils eliminate pathogens through phagocytosis, degranulation, and neutrophil extracellular trap (NET) formation [2]. Under normal conditions, neutrophils have a brief lifespan, and their activation and mobilization are tightly controlled by the body to prevent potential harm to normal tissues from the highly toxic nature of neutrophil responses [3]. However, in pathological settings, the continuous infiltration of neutrophils can lead to chronic inflammation and tissue damage, which often contribute to tumorigenesis [4]. Recent research has elucidated the multifaceted nature of neutrophils, revealing that they not only serve as effective antibacterial cells but also exhibit phenotypic and functional heterogeneity in pathological contexts such as cancer [5, 6]. Consequently, the impact of neutrophils on tumors has emerged as a prominent area of investigation.
In this work, we present a comprehensive overview of recent research advancements aimed at unraveling the dual mechanisms of neutrophils in tumor pathogenesis and their potential in immunotherapy. We specifically focus on various factors influencing the phenotypic alterations of tumor-associated neutrophils and predict their clinical utility as diagnostic tools and vital targets for antitumor interventions. Additionally, we explore the existing opportunities and challenges in neutrophil research. This work enhances our understanding of the role of neutrophils in cancer and offers systematic insights for the development of cancer immunotherapy strategies targeting neutrophils.
Neutrophils and cancer
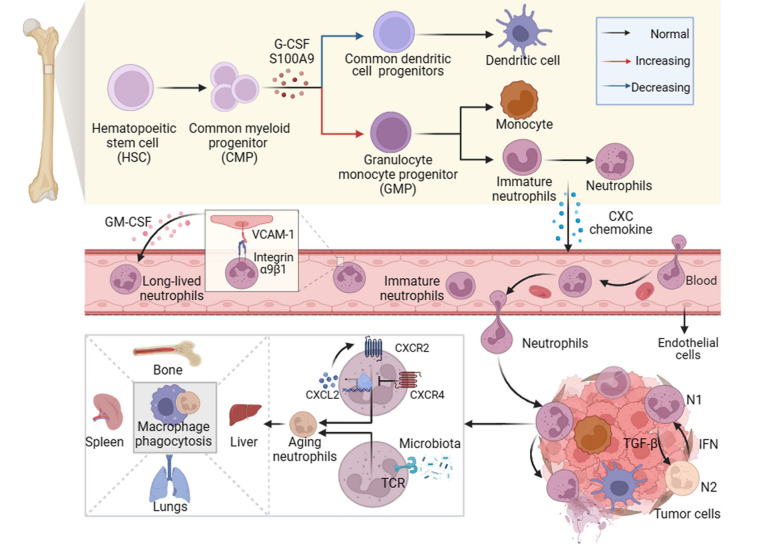
Neutrophils are derived from hematopoietic stem cells (HSCs) located in the bone marrow. The process begins with the differentiation of HSCs into common myeloid progenitors (CMPs), which further develop into granulocyte monocyte progenitors (GMPs) and eventually give rise to mature segmented neutrophils [7]. During carcinogenesis, the hematopoietic system may tend to produce more neutrophils and monocytes [8]. This shift in differentiation is driven by the upregulation of granulocyte colony-stimulating factor (G-CSF) and myeloid-related protein S100 calcium-binding protein A9 (S100A9), inhibiting dendritic cell differentiation while promoting the accumulation of bone marrow-derived suppressor cells [9, 10]. This skewed differentiation process further facilitates immune evasion by tumors, thereby promoting tumor progression and treatment resistance (Fig. 1). Of particular intrigue, polymorphonuclear-myeloid-derived suppressor cells (PMN-MDSCs), exhibit protumor activity akin to that of immunosuppressive neutrophils [11]. Under the stimulation of granulocyte colony-stimulating factor (G-CSF), GM-CSF, pro-inflammatory cytokines interleukin-6 (IL-6), IL-8, TGF-β, and leukotriene B4, immature PMN-MDSCs undergo massive expansion from their precursors in the bone marrow and venture into the peripheral blood and tumor microenvironment [12–17]. While distinguishing PMN-MDSCs from regular neutrophils in mice remains challenging, distinct markers have been identified for human PMN-MDSCs [18]. The biomarkers/features related to classic neutrophils and PMN MDSCs are shown in Table 1.
Fig. 1.
The development, mobilization, and clearance of neutrophils in the tumor microenvironment. Hematopoietic progenitor stem cells (HSCs) in the bone marrow differentiate into common myeloid progenitor cells (CMPs), which give rise to granulocyte-monocyte progenitor cells (GMPs) and eventually mature segmented neutrophils. Tumor-derived mediators such as granulocyte colony-stimulating factor (G-CSF) and S100 calcium-binding protein A9 (S100A9) promote the differentiation of the neutrophil and monocyte lineages while leading to systemic dendritic cell deficiency in vivo. Chemokines trigger the mobilization of mature and immature neutrophils into the circulation. Immature neutrophils, known as polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs), are considered in this context. During transendothelial migration, the interaction between integrin α9β1 on neutrophils and vascular cell adhesion molecule 1 (VCAM-1) on endothelial cells stimulates the release of granulocyte-macrophage colony-stimulating factor (GM-CSF) from the latter, prolonging neutrophil lifespan. Neutrophils that extravasate into the tumor tissue adopt antitumor (type N1) or protumor (type N2) phenotypes, influenced by growth factor-β (TGF-β) and type 1 interferon (IFN), respectively. After fulfilling their functions, neutrophils undergo senescence due to intrinsic programs (CXCR2 or CXCR4) and extrinsic factors (microbiota) and are subsequently cleared by macrophages in the bone marrow, spleen, liver, and lungs. Neutrophils also undergo programmed death to form neutrophil extracellular traps (NETs)
Table 1.
Comparison of basic features of classical neutrophils and polymorphonuclear myeloid derived suppressor cells (PMN-MDSCs)
| Characteristic | Neutrophils | PMN-MDSCs | References |
|---|---|---|---|
| Activation effects | Host infection with bacteria or pathogens | Long-term chronic inflammation, autoimmune diseases and cancer | [11] |
| Mutagenic factors | G-CSF, GM-CSF | G-CSF, GM-CSF, IL-6, IL-8, TGF-β, leukotriene B4 | [12–17, 19, 20] |
| Cell surface marker | CD14−CD15+CD66b+CD16+ | CD14−CD15+CD66b+CD16+CD11b+CD33+HLA-DR− | [21–24] |
| New specific biomarkers | c-KithiCXCR2hiCD101hi | LOX1hiFATP2hiArg-1hiCD300ldhi | [25–30] |
| Nuclear morphology | Segmented nucleus | Banded nucleus | [31] |
| Mature state | Mature | Immaturate | [31] |
| Density centrifugation | High-density cells | Low-density cells | [31] |
Factors like G-CSF and GM-CSF regulate neutrophil development, prompting the release of mature neutrophils from the bone marrow [19, 20]. However, sustained chronic inflammation, autoimmune ailments, and the provocation of cancer may result in neutrophil depletion, setting off an occurrence known as “emergency granulopoiesis,” rapidly facilitating the influx of mature or even immature neutrophils into the circulation [11, 32]. As neutrophils age within the body, they depart from their target sites and are subsequently cleared by macrophages in the bone marrow, spleen, liver, and lungs [33]. Interestingly, both intrinsic and extrinsic factors influence neutrophil aging. Brain and muscle Arnt-like 1 (BMAL1) drives cell aging by inducing the production of CXCL2 and subsequent CXCR2 signaling, while CXCR4 antagonizes this aging effect [34]. Moreover, the microbiome influences neutrophil senescence through a signaling pathway mediated by toll-like receptors and myeloid differentiation factor 88 (MYD88), representing an external mode of regulation [35]. During transendothelial cell migration, the interaction between integrin α9β1 on neutrophils and vascular cell adhesion molecule 1 (VCAM-1) on endothelial cells triggers the release of GM-CSF, thereby prolonging neutrophil survival [36]. Neutrophils also undergo a distinct form of cell death known as NETosis, wherein they release their nuclear DNA and lytic proteins into the extracellular space in the form of NETs [37]. Neutrophils have considerable plasticity and complexity in the tumor microenvironment. The transforming growth factor-β (TGF-β) and type I interferons (IFN 1) respectively induce the polarization of neutrophils towards the N2 and N1 phenotypes [16, 38] (Fig. 1). In the circulation of cancer patients and mice, high-density neutrophils (HDNs) are a uniform group of mature (segmented) neutrophils with anti-tumor properties, while low-density neutrophils (LDNs) are a mix of immaturce (banded) and mature neutrophils with immunosuppressive properties [31].
Therefore, tumor cells have significant regulatory effects on neutrophil recruitment, clearance, and polarization. There is confusion in naming cells with similar or overlapping mechanisms of action, such as PMN-MDSC and N2 neutrophils. Hence, it is necessary to further explore a more appropriate and comprehensive nomenclature.
Anti-tumor effects of neutrophils
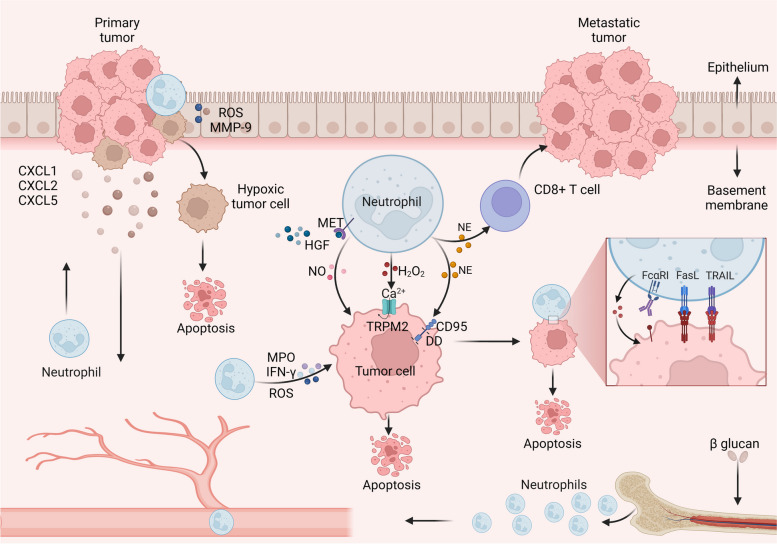
Neutrophils secrete various toxic factors or directly contact tumor cells to exert anti-tumor activity. In a mouse model of uterine cancer, the response to low oxygen levels attracted neutrophils to the tumor area [39]. These neutrophils then induced the separation of tumor cells from the basement membrane, thereby limiting the growth of early tumors [39]. Recent research has illuminated a captivating phenomenon: although reducing the hypoxic tumor environment may lead to fewer neutrophils being recruited, their ability to kill cells is exceptional [40]. This remarkable observation can be attributed to the secretion of reactive oxygen species (ROS) and matrix metalloproteinases (MMP-9) by activated neutrophils, leading to the degradation of the epithelial basement membrane [40]. Additionally, neutrophils treated with β-glucan rely on high ROS levels to fight tumors, a process linked to the development of neutrophil precursors’ memory in the bone marrow of trained mice [41]. Neutrophils secrete hydrogen peroxide (H2O2), which induces apoptosis in tumor cells through the influx of calcium ions (Ca2+) mediated by the transient receptor potential cation channel, subfamily M, member 2 (TRPM2) [42]. Notably, neutrophil elastase (NE) secreted by neutrophils can hydrolyze and release the CD95 death domain (DD), selectively killing cancer cells and minimizing toxicity to noncancer cells [43]. NE also induces distant effects mediated by CD8+ T cells [43]. Inflammatory modulators such as TNF-α induce the expression of MET in neutrophils. When activated by ligand hepatocyte growth factor (HGF), MET+ neutrophils release nitric oxide (NO) to limit tumor growth and metastasis [44]. Neutrophils directly engage with tumor cells to exert their antitumor activity. For instance, neutrophils with enhanced expression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas ligand (FasL) induce apoptosis through direct contact with cancer cells [45, 46]. IL-17 has been shown to increase the expression of TRAIL as well as ROS, and IFN-γ to bolster the direct killing ability of neutrophils [47]. Moreover, antibody-dependent cytotoxicity (ADCC), triggered by the binding of the Fc region of monoclonal antibodies (mAbs) to activated Fc receptors (FcRs) on neutrophils, can exhibit strong antitumor effects [48] (Fig. 2).
Fig. 2.
Direct cytotoxic effects of neutrophils on cancer cells. Neutrophils, when exposed to β-glucan, rely on the memory of bone marrow precursors and exert antitumor effects. The chemokines CXCL1, CXCL2, and CXCL5, secreted by primary tumors, facilitate the recruitment of neutrophils to the tumor site. Under hypoxic conditions, activated neutrophils induce reactive oxygen species (ROS) and matrix metalloproteinase (MMP-9) degradation of the epithelial basement membrane, which ultimately restricts tumor development. Neutrophils directly secrete ROS, myeloperoxidase (MPO), and interferon γ (IFN-γ) to inhibit tumor progression. The interaction between the ligand hepatocyte growth factor (HGF) and receptor tyrosine protein kinase (MET) on neutrophils leads to the release of nitric oxide (NO) by MET+ neutrophils, which exerts antitumor effects. Neutrophils secrete hydrogen peroxide (H2O2), inducing apoptosis in tumor cells through Ca2+ influx via the transient receptor potential cation channel, subfamily M, member 2 (TRPM2). Neutrophil elastase (NE) hydrolyzes and releases the CD95 death structure domain (DD), selectively killing cancer cells. Additionally, NE has distant effects on CD8+ T cells. Neutrophils with enhanced expression of TNF-related apoptosis-inducing ligand (TRAIL) and Fas ligand (FasL) induce apoptosis in cancer cells through direct contact
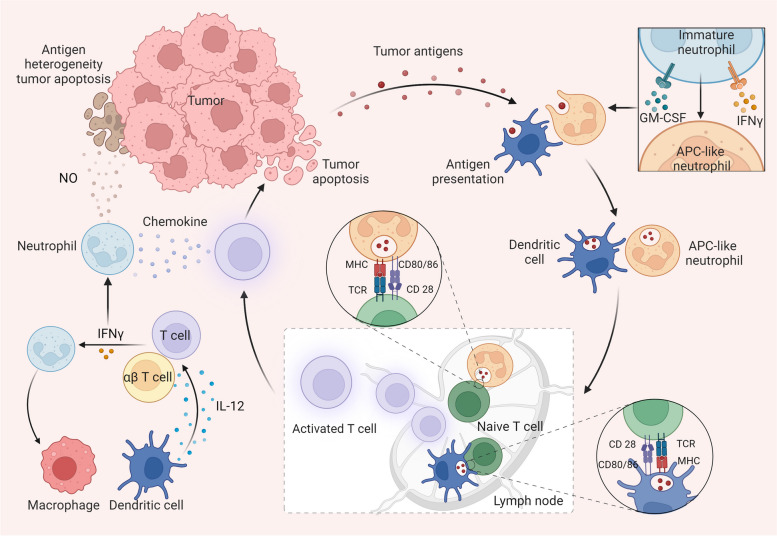
Neutrophils also play crucial roles in activating and regulating both innate and adaptive immunity. Recent findings suggest that T cells perform immune surveillance by recognizing specific antigens expressed by tumor cells. Simultaneously, activated T cells signal neutrophils to eliminate tumor escape variants with antigen heterogeneity [49]. Therefore, neutrophils are indispensable for the complete eradication of tumors, and this effect is partially dependent on iNOS [49]. Early research discovered that under the influence of IFN-γ and GM-CSF, immature neutrophils in the tumor microenvironment differentiate into hybrid neutrophils with antigen-presenting cell (APC) characteristics to stimulate anticancer T-cell responses [50]. Subsequent studies have explained more details of this process. During the pretransfer stage, APC-like neutrophils capture tumor antigens, migrate to the tumor-draining lymph nodes (LNs), and form synapses with T cells, presenting antigens to T cells and triggering antitumor immune responses [51]. Recent research shows that successful immunotherapy triggers a notable increase in tumor-infiltrating neutrophils, defining a distinct antitumor immune state termed the Sellhi state [52]. The production of IL-12, a crucial cytokine, primarily relies on dendritic cells (DCs) and macrophages. Upon IL-12 activation, T cells release IFNγ, which triggers the expression of interferon response transcription factor (IRF1) in neutrophils, thereby exerting antitumor effects [52]. In turn, antitumor neutrophils further drive macrophages to release IL-12, which promotes unconventional αβ T cell type I activation and IFN-γ production, leading to the inhibition of sarcoma progression in mice [53] (Fig. 3).
Fig. 3.
Neutrophils regulate immune cells to drive antitumor immune responses. When exposed to IFN-γ and GM-CSF, immature neutrophils can differentiate into hybrid neutrophils with antigen-presenting cell (APC) characteristics. Thereafter, dendritic cells and APC-like neutrophils can pick up tumor antigens and migrate to lymph nodes (LNs). In LNs, these antigen-presenting cells cross-present tumor antigens to T cells using MHC molecules and costimulatory ligands (CD80/CD86 on dendritic cells and APC-like neutrophils and CD28 on T cells), thereby stimulating antitumor T cell responses. Activated T cells then exit the LN and specifically target and eliminate tumor cells. Tumor cells can undergo antigenic variations, leading to the formation of tumor variants with diverse antigenic profiles. In response, activated T cells can secrete chemokines to recruit neutrophils, which contribute to the elimination of antigenically heterogeneous tumors by releasing NO. Interferons play critical roles in inducing antitumor immune responses in neutrophils. IL-12, secreted by dendritic cells and macrophages, triggers the type I activation of T cells and αβ T cells, resulting in the production of IFN-γ. Subsequently, activated neutrophils can further stimulate macrophages to release IL-12, amplifying the antitumor immune response
Neutrophils express a wide range of cytotoxic factors or apoptosis-related ligands that mediate direct killing effects on tumors. Since neutrophils have a short lifespan, this immune response could be transient. However, neutrophil interactions with other components of the immune system induce long-term adaptive immune responses (refer to Table 2 for details).
Table 2.
The antitumor mechanisms of neutrophils in different cancer models
| Cancer type | Model | Influence factor | Effect | Anticancer mechanism | Reference |
|---|---|---|---|---|---|
| Uterine cancer | PTEN-deficient uterine cancer mice | Hypoxia | Relieving hypoxia decreases neutrophil recruitment while enhancing their cytotoxic effect. | Neutrophils induce ROS and MMP-9 to break down the epithelial basement membrane, which slows the growth of tumors. | [39, 40] |
| Melanoma | B16-F10 tumor–bearing mice | β-glucan | Neutrophils rely on ROS production to exert antitumor activity. | β-glucan induces neutrophil reprogramming into an antitumor phenotype, which depends on the memory of neutrophil precursors in the bone marrow. | [41] |
| Breast cancer | 4 T1 tumor–bearing mice and MDA-MB-231 cells lines | H2O2 | Ca2+ lethal influx kills disseminated tumor cells. | H2O2 secreted by neutrophils induces Ca2+ influx into tumor cells through TRPM2. | [42] |
| TNBC, lung cancer, and melanoma | PDX model mice and genetically engineered mice | NE | NE selectively kills tumor cells and induces distant effects mediated by CD8+ T cells. | The hydrolysis of NE secreted by neutrophils releases the CD95 death domain, selectively killing cancer cells. | [43] |
| Lung cancer, fibrosarcomas, melanomas, and breast tumors | LLC lung cancer mice, T241 and B16F10 tumor–bearing mice, MMTV-PyMT+ transgenic mice, c-Myc-driven hepatocellular carcinomas in mice, and chemically induced colorectal cancers in mice | HGF-MET axis | Neutrophils release NO through the HGF/MET-dependent pathway to exert a killing effect on tumor cells. | HGF activates MET+ neutrophils to kill tumor cells by releasing NO. | [44] |
| Human acute T-lymphocytic leukemia/lung cancer, human epidermoid carcinoma, cervical cancer, and hepatocellular carcinoma | Jurkat cells/A549, A431, HeLa, and HepG2 cell lines | TRAIL/FasL | Neutrophils regulate the expression of their own apoptotic-related ligands to induce tumor cell apoptosis. | Neutrophils expressing TRAIL and FasL induce apoptosis of tumor cells in contact. | [45, 46] |
| Esophageal squamous cell carcinoma | ESCC cell lines (EC109 and KYSE30) and xenograft nude mouse models | IL-17 | IL-17 recruits neutrophils and enhances their antitumor activity. | IL-17 stimulates tumor cells to release CXC chemokines, and promotes TTRAIL, ROS, MPO, and IFN-γ expression in neutrophils. | [47] |
| Melanoma | Human samples and B16: B78H1 tumor-bearing mice | T cell immunotherapies | Neutrophils are activated to completely eradicate tumors. | CD4+T cell therapy co-activated with OX40 or combined with CTLA-4 blocker activate neutrophils to eliminate antigenic heterogeneity in tumors. | [49] |
| Lung cancer | Lung tumor tissue | IFN-γ and GM-CSF | APC-like neutrophil formation stimulates T cell responses in early lung tumors. | Immature neutrophils downregulate the transcription factor Ikaros under the influence of IFN-γ and GM-CSF and thereafter differentiate into APC-like neutrophils for T cell activation. | [50] |
| Lung adenocarcinoma, colon cancer | Mice injected with the G12D Kras mutation carrying oncogene and lacking P53 tumor cells and MC38 tumor–bearing mice | Macrophages and DC and T cells | The expression of IRF1 in neutrophils induces an antitumor immune response. | DCs and macrophages are producers of IL-12, which activates T cells to secrete IFNγ, leading to the induction of IRF1 expression in neutrophils. | [52] |
| Sarcoma | 3-MCA-induced sarcoma mice | Neutrophils and macrophages | IFNγ is produced by unconventional αβ T cells. | Neutrophils drive macrophages to release IL-12, which promotes IFN-γ production by unconventional αβ T cells. | [53] |
Pro-tumor effect of neutrophils
Neutrophils, often drive tumor progression by fostering local tumor initiation and proliferation, promoting angiogenesis, facilitating tumor metastasis, and orchestrating networks of immune suppression within the tumor microenvironment (refer to Table 3 for details).
Table 3.
The protumor mechanisms of neutrophils in different cancer models
| Cancer type | Model | Influence factor | Effect | Anticancer mechanism | Reference |
|---|---|---|---|---|---|
| Colorectal cancer | Recombinase-activating gene-2-deficient (Rag2−/−) mice | Helicobacter pylori | Infection leads to the accumulation of neutrophils in the colon, ultimately promoting intestinal carcinogenesis. | During infectious conditions, TNF-α-driven accumulation of neutrophils in the colon results in the release of abundant NO, promoting intestinal carcinogenesis. | [54] |
| Colon cancer | Gpx4+/Δmye and Gpx4Δ/Δmye mice | ROS released by bone marrow cells | Myeloid cell-derived ROS induce epithelial mutagenesis. | Lack of Gpx4 in bone marrow cells leads to increased ROS production, while increased oxidative stress can induce tumor development. | [55] |
| Colorectal cancer | Human samples, dextran sodium sulfate induced colitis in mice, and acute mucosal injury in mice | Pro-inflammatory particles miR-23a and miR-155 | Neutrophils induce the impairment of colon healing and genomic instability. | Neutrophil-derived miR-23a and miR-155 induce double-strand breaks, leading to the occurrence of cancer. | [56] |
| Lung adenocarcinoma | A549 (K-ras mutant) and K-ras WT 201 T cell lines and LSL-K-ras mice and cell lines | NE | NE derived from neutrophils promotes lung tumor growth. | NE degrades IRS1, leading to activation of PI3K, which ultimately promotes cancer proliferation. | [57] |
| Melanoma | Human samples and RAS driven tumor formation in zebrafish | PGE2 | Neutrophils promote cancer cell proliferation and growth by releasing nutrient factor PGE2. | The PGE2 released by neutrophils induced by wound inflammation can promote the proliferation of pretumor cells. | [58] |
| Prostate cancer | Human samples, Ptenpc−/− mice, and PC3 human prostate cancer cell lines | APOE released by tumor cells | Tumor-induced aging (TREM2+) neutrophils persist in the tumor microenvironment and exert immunosuppressive effects. | The APOE released by tumor cells binds to TREM2 on neutrophils, inducing neutrophil aging. A series of cytokines secreted by TREM2+ neutrophils mediate immune suppression. | [59] |
| Prostate cancer | Human samples, male Il1ra−/− mice, and Pten−/− mouse embryonic fibroblasts (MEFs) | IL-1RA | Tumor-infiltrating neutrophils antagonize tumor cell aging. | IL-1RA secreted by neutrophils can antagonize aging. | [60] |
| Breast cancer | MDA-MB-231 cell line and T47D human breast cancer cell line | OSM | OSM secreted by neutrophils induces VEGF expression in breast cancer cells, promoting angiogenesis and invasion. | GM-CSF derived from breast cancer cells stimulates neutrophils to release OSM, and the latter induces VEGF expression by activating the JAK-STAT pathway after combining with tumor cells. | [61] |
| Colorectal cancer with liver metastasis | Human samples and intrasplenic injection of human colorectal cancer cells into mice | FGF2 | Neutrophils induce metastatic angiogenesis by promoting the production of FGF2. | FGF2 can be produced directly by tumor-associated neutrophils or released by neutrophils secreting heparinase to degrade ECM. | [62] |
| Gastric cancer | Human gastric cancer cell lines BGC-823, MGC80–3, SGC-7901, and HGC-27 | HMGB1 | Exosomes derived from gastric cancer cells carrying HMGB1 induce neutrophil autophagy. | Gastric cancer cell-derived exosomes (HMGB1) induce neutrophil autophagy through the activation of the HMGB1/TLR4/NF-κB pathway, leading to the release of pro-tumor cell migration factors such as IL-1β and OSM. | [63] |
| Breast cancer | Murine D2.0R and human MCF-7 cell lines | NE and MMP-9 | Under the induction of inflammation, NETs induce the recovery of dormant cancer cells. | NE and MMP-9 released by NETs sequentially cut laminin to produce integrin α3β1 activation epitopes. | [64] |
| Melanoma | Inject A375M, 1205 Lu, C8161.Cl9 or UACC 903 M cells into mice | IL-8 | IL-8 secreted by melanoma promotes neutrophil recruitment and interaction with tumor cells. | β2 integrins on neutrophils and intercellular adhesion molecule-1 on tumor cells mediate their interactions. | [65] |
| Breast cancer, lung cancer, and colon cancer | Human samples and 4 T1, LLC, HT29, CT26 tumor-bearing mice | CXCR1 CXCR2 | The barrier of NETs is beneficial for tumor cells to avoid immune toxicity damage mediated by CD8+ T cells and NK cells. | Tumor-derived CXCR1 and CXCR2 induce the formation of NETs, which increase the interception of circulating tumor cells. | [66] |
| Breast cancer | Spontaneous, experimental breast cancer metastasis in mice | Resident mesenchymal cells (MCs) in the lungs | Under the stimulation of lung-resident MC, neutrophils accumulate a large amount of lipids to provide energy for tumor cells. | MCs trigger lipid storage in neutrophils, which then transfer their stored lipids to disseminated tumor cells for survival by releasing vesicles. | [67] |
| Lymphoma, colon cancer, and lung cancer | EL4 lymphoma, and CT26 (colon cancer) and LLC tumor-bearing mice | MPO | Neutrophils limit antigen cross presentation of dendritic cells (DCs). | The MPO of neutrophils drives lipid peroxidation, and the subsequent transfer of this oxidized lipid from neutrophils to DC limits the antigen cross-presentation effect of the latter. | [68] |
| Lewis lung carcinoma, lymphoma, colon carcinoma, and sarcoma | GEM mice for LLC, CT26, and KPC, EL-4 tumor-bearing mice, and the EL4, LLC, CT26, and TC-1 F244 cell lines | FATP2 and PGE2 | Neutrophils expressing FATP2 mediate immune suppression by inducing PGE2 synthesis. | The expression of FATP2 by neutrophils leads to an increase in the uptake of arachidonic acid, thus promoting the biosynthesis of PGE2 and ultimately exerting T cell inhibition. | [25] |
| Lymphoma, Lewis lung carcinoma, and colon carcinoma | Human samples and EL4, EG7, LLC, CT26, and MC-38 tumor-bearing mice | Ferroptosis and PGE2 | Neutrophils undergoing ferroptosis induce tumor immunosuppression. | The number of neutrophils undergoing ferroptosis decrease, but lipid mediators such as PGE2 limit the activity of T cells. | [69] |
| Lewis lung carcinoma | PL and LLC tumor-bearing mice | ARG1 | ARG1 limits the function of T cells by consuming arginine, and its expression is predominant in neutrophils. | Neutrophils actively transcribe ARG1 through the membrane-associated protein A2/TLR2/MYD88 axis, promoting the immunosuppressive effect of neutrophils. | [70] |
| Hepatocellular carcinoma | Human samples and H22 tumor-bearing mice | TNF-α, GM-SCF, and the PD-L1/PD-1 axis | PD-L1 expression on neutrophils negatively regulates adaptive immune T cells. | Tumor-derived cytokines such as TNF-α and GM-SCF help induce PD-L1 expression on neutrophils. | [71] |
| Hepatocellular carcinoma | Human samples | IL-6, PD-L1/PD-1 axis | CAFs promote immune suppression of cancer cells through the IL6-STAT3-PDL1 signaling pathway. | IL-6, secreted by HCC-derived CAF, induced the upregulation of PD-L1 expression in neutrophils through the JAK-STAT3 signaling pathway. | [72] |
| Gastric cancer | Human samples and the human BGC-823 cell line | HMGB1 and the PD-L1/PD-1 axis | Gastric cancer cells activate the immunosuppressive effect of neutrophils through the GM-CSF-PD-L1 pathway. | Tumor cell-derived vesicle transport (HMGB1) upregulates PD-L1 expression in neutrophils by activating the STAT3 pathway. | [73] |
| Melanoma, Lewis lung carcinoma, colon carcinoma, and mammary carcinoma | B16-F10, LLC, CT26, and 4 T1 tumor-bearing mice | Hypoxia and the PD-L1/PD-1 axis | Hypoxia selectively upregulates PD-L1 on neutrophils, affecting T cell antitumor activity. | Hypoxia-induced HIF-1α directly binds to the proximal HRE of PD-L1 and upregulates the expression of PD-L1 on neutrophils. | [74] |
| Burkitt lymphoma, breast cancer, colon adenocarcinoma, and renal carcinoma | Raji, SK-BR-3, DLD-1, LS-174 T, HT-29, HCT-116, Caki-1, RCC4, RCC10, and TK10 cell lines and SRG mice | CD47/SIRPα axis | Tumors impair the antitumor activity of neutrophils and macrophages through the CD47/SIRPα axis. | Tumors express the “don’t eat me” signal CD47, which when combined with the ligand SIRPα on macrophages and neutrophils causes tumors to evade immune injury. | [75] |
| Cervical cancer | TC-1 tumor-bearing mice | C5a, ROS and RNS | C5a induces neutrophil disruption of T cell activity. | C5a promotes the migration of neutrophils to tumors and the release of ROS and RNS from neutrophils to impair T cell activity. | [76] |
| Breast cancer | Metastatic C4 T1 mice | IL-10 and IL-12 | Neutrophils induce macrophage polarization toward the M2 phenotype, promoting the formation of an immunosuppressive environment. | The production of IL-10 by neutrophils leads to a decrease in IL-12 production by macrophages, ultimately leading to polarization to the M2 phenotype. | [77] |
| Lung adenocarcinoma | Autochthonous GEM mice | Commensal Microbiota, γδ T cells and IL-17 | The symbiotic microbiome induces γδ T cells and neutrophils to copromote tumor progression. | The microbiota stimulates the production of IL-1β and IL-23 by neutrophils, which induce the activation of lung-resident gamma-δ T cells. γδ T cells, in turn, produce IL-17 to induce neutrophil infiltration. | [78] |
Neutrophils promote the initiation and proliferation of local tumors
Chronic inflammation and genetic instability caused by neutrophils promote cancer development. In infected mice, neutrophils were observed to gather and release high levels of NO in the colon, speeding up intestinal inflammation and cancer formation [54]. Additionally, neutrophils release harmful substances that damage DNA and heighten cancer risk. In early intestinal tumor stages, ROS from bone marrow cells cause DNA harm, aiding carcinogenic changes [55]. Independent of the ROS mechanism, neutrophil-derived particles with proinflammatory microRNAs (miR-23a and miR-155) also contribute to double-strand breaks in intestinal epithelial cells [56]. Interestingly, depleting the entire neutrophil population using Ly6G antibodies weakens the carcinogenic effects in chemically induced and spontaneous cancer models, underscoring the significant potential of neutrophils in carcinogenesis.
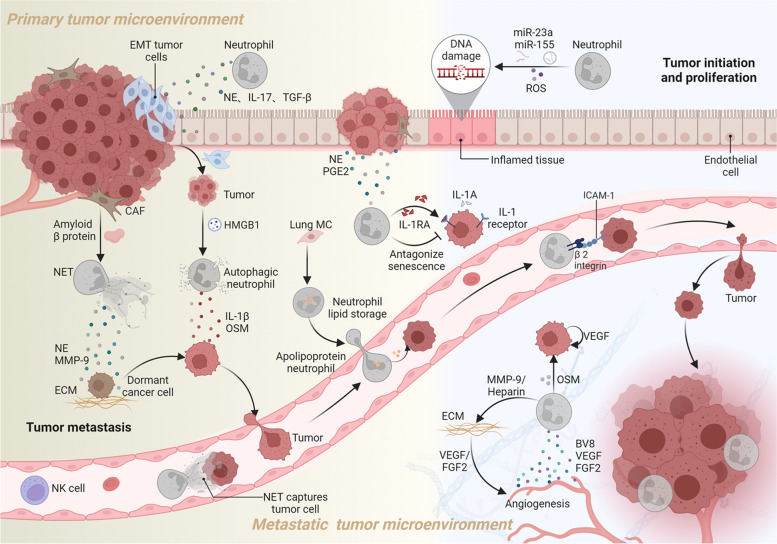
During tumor progression, mediators derived from neutrophils directly or indirectly facilitate tumor growth. NE derived from neutrophils can degrade insulin receptor substrate 1 (IRS1), a negative regulator, leading to the activation of phosphoinositide 3-kinase (PI3K) and promoting the proliferation of lung cancer cells both in vivo and in vitro [57]. In a zebrafish model driven by RAS for tumor formation, neutrophils promoted cancer cell proliferation and growth by releasing PGE2 [58]. In a mouse model of prostate cancer, tumor cell-released apolipoprotein E (APOE) interacts with triggering receptor expressed on myeloid cells 2 (TREM2) on neutrophils, triggering the senescence process in neutrophils [59]. Nevertheless, such senescent-like neutrophils generate a spectrum of bioactive molecules collectively referred to as the senescence-associated secretory phenotype (SASP), can create an immunosuppressive environment and inhibit the activation of NK cells and cytotoxic T lymphocytes, fostering chronic inflammation and driving tumor progression [79]. Tumor-infiltrating neutrophils not only regulate their own senescence but also shield proliferating tumor cells from senescence, leading to sustained tumor cell growth. This antagonistic senescence response is attributed to the release of interleukin-1 receptor antagonist (IL-1RA) by neutrophils [60] (see Fig. 4 for illustration).
Fig. 4.
Neutrophils promote tumor initiation, proliferation, metastasis, and angiogenesis. Neutrophils promote tumor progression by releasing proinflammatory particles (miR-23a, miR-155) and reactive ROS, which cause DNA damage and support carcinogenesis. They contribute to tumor proliferation through the release of NE and prostaglandin E2 (PGE2). Neutrophils also secrete interleukin-1 receptor antagonist (IL-1RA) to protect tumors from senescence and secrete IL-17, TGF-β, and NE, promoting epithelial-mesenchymal transition (EMT) and facilitating metastasis. Tumor-derived HMGB1 exosomes induce neutrophil autophagy, leading to the release of IL-1β and oncostatin M (OSM), which promote tumor migration. Amyloid β from cancer-associated fibroblasts (CAFs) stimulates the formation of NETs, releasing NE and MMP-9 to degrade the extracellular matrix (ECM) and revive dormant cancer cells. Lung mesenchymal cells (MCs) trigger lipid stores in neutrophils, providing nutrients to disseminated tumor cells. NETs also trap circulating tumor cells, protecting them from immunotoxic effects mediated by natural killer (NK) cells. Interactions between neutrophils (via β2 integrin) and tumor cells (through intercellular adhesion molecule-1) enable tumor evasion from blood shear. Additionally, OSM released by neutrophils induces tumor cells to secrete vascular endothelial growth factor (VEGF), promoting angiogenesis. Neutrophils themselves release Bv8, VEGF, and liver fibroblast growth factor 2 (FGF2), directly contributing to tumor angiogenesis. Neutrophil-derived MMP-9 and heparin degrade the ECM, releasing VEGF and FGF2, further supporting tumor angiogenesis
Prolonged chronic inflammation and the resulting tissue damage create a favorable environment for tumor formation, leading to tumors being referred to as “nonhealing wounds.” However, the inflammatory response of neutrophils in the development of tumors still needs further investigation.
Neutrophils promote tumor angiogenesis
Angiogenesis is critical for tumor growth and metastasis, as it supplies nutrients and eliminates metabolites [80]. During tumor development, neutrophils release Bv8 and vascular endothelial growth factor (VEGF), effectively promoting tumor angiogenesis [81, 82]. Moreover, neutrophil-secreted MMP-9 degrades the extracellular matrix (ECM), releasing VEGF [83]. When exposed to GM-CSF from breast cancer cells, neutrophils release large amounts of oncostatin M (OSM), which induces VEGF expression through activation of the Janus-activated kinase/signal transducer and activator of transcription (JAK-STAT) pathway in cancer cells [61]. However, in VEGF-induced tumor models, liver fibroblast growth factor 2 (FGF2) can compensate for angiogenesis [84]. FGF2, found in neutrophils and the ECM in liver metastases, can be released by neutrophil-secreted heparanase, promoting angiogenesis [62]. Therefore, Neutrophils seem to produce FGF2 directly and trigger its release from the ECM (Fig. 4). Chemokines containing the ELR tripeptide motif (CXCL1–3, CXCL5–8) promote angiogenesis, and those lacking this motif (CXCL4, CXCL9–11, and CXCL14) inhibit angiogenesis (see review [85, 86]). The ELR+ subset may promote angiogenesis by directly binding to the receptor CXCR2 expressed on tumor blood vessels or indirectly recruiting leukocyte subsets. Thus, the balance between ELR+ and ELR− chemokines is crucial for regulating tumor angiogenesis.
Overall, Neutrophils can boost proangiogenic factors, making patients less responsive to antiangiogenic treatments. In addition, Antiangiogenic therapy-induced hypoxia triggers mechanisms of drug resistance in tumors, such as promoting invasion and metastasis, coopting the normal vascular system for oxygen and nutrients (co-selection of blood vessels), and the transformation of tumor cells into vascular-like or endothelial-like cells involved in angiogenesis (vascular mimicry) [87–89]. Therefore, further research is necessary to explore the potential mechanisms underlying angiogenesis or its substitution.
Neutrophils promote tumor metastasis
Neutrophils play crucial roles in promoting tumor migration, formation of the pre-metastatic niche and awakening dormant cancer cells in distant sites. Epithelial-mesenchymal transition (EMT) decreases cell adhesion, aiding tumor cells in migrating from the primary site to distant areas [90]. Neutrophils secrete mediators such as IL-17, TGF-β, and NE, all of which bolster tumor EMT [91–93]. Exosome transporter high-mobility group box 1 (HMGB1) derived from gastric cancer cells induces neutrophil autophagy via the HMGB1/Toll-like receptor 4 (TLR4)/nuclear factor-κB (NF-κB) signaling pathway, releasing pro-tumor cell migration factors, such as IL-1β and OSM [63]. Even after successful removal of primary tumors, patients may face renewed cancer spread years later due to dormant cancer cells [94]. In mice model of breast cancer, persistent lung inflammation was found to trigger the transformation of dormant cancer cells into invasive metastatic tumors [64]. Mechanistically, inflammation induces NE and MMP-9 release during NET formation. These enzymes cleave adhesion proteins, generating integrin α3β1-activated epitopes that revive dormant cancer cells [64]. Furthermore, amyloid protein β secreted by cancer-associated fibroblasts (CAFs) also drives the formation of NETs, supporting tumor development [95].
Neutrophils support cancer metastasis by acting as adhesion substrates and energy suppliers. Tumor cells must withstand immune attacks and blood flow shear forces to survive when spreading to distant locations [96, 97]. For breast cancer patients, high levels of circulating tumor cell-neutrophil clusters indicate a greater risk of metastasis [98]. Melanoma cells release IL-8, attracting neutrophils to bind with cancer cells. This process involves β2 integrins on neutrophils and intercellular adhesion molecule-1 on tumor cells, which stabilize the tumor cells and enhance their spread to the lungs [65]. Moreover, NETs trap circulating tumor cells, helping them evade immune cell attacks [66]. Neutrophils also provide energy for metastatic tumor cells. In a breast cancer study, lung mesenchymal cells (MCs) prompted neutrophils to store lipids that could be transferred to tumor cells, supporting their growth and survival, leading to lung metastasis [67] (Fig. 4).
Therefore, neutrophils are pivotal in all phases of tumor spread. Interestingly, organs where metastases occur are not just passive targets; they are actively chosen and modified by the primary tumor before spreading [99]. Common sites for metastasis include the lungs, liver, brain, bones, and lymph nodes, with specific integrins in tumor-derived exosomes guiding organ selection. For example, α6β4 and α6β1 integrins in exosomes are linked to lung metastasis, while αvβ5 integrin is associated with liver metastasis [100]. The precise link between specific exosomes and various metastatic organs is still under active investigation.
Neutrophils regulate other immune cells to drive protumor immune responses
Metabolic abnormalities within the TME, such as abnormal lactate metabolism, lipid metabolism, and amino acid metabolism, significantly impact the antitumor activity of immune cells [69, 101, 102]. Tumors compete with T cells for glucose uptake, leading to T cell depletion and immune evasion [103]. Tumor cells relying on glycolysis produce lactic acid, shaping neutrophils and macrophages towards a pro-tumor state, creating immune-suppressing surroundings [101, 104]. During the development of tumors, lipids are not only energy sources of tumor cells and structural components of membranes but also alter the metabolic crosstalk between different immune cells in the TME [105, 106]. Neutrophil derived myeloperoxidase (MPO) induces lipid peroxidation, hindering DCs’ antigen presentation to CD8+ T cells [68]. Neutrophils expressing FATP2 absorb arachidonic acid, boosting PGE2 production that hampers T cell function [25]. Additionally, in the TME, neutrophils undergoing ferroptosis release lipid mediators, such as PGE2, that limit T cell activity [69]. Tumor-activated neutrophils release arginase 1 (ARG1), reducing L-arginine levels and impacting T cell function [102]. Recent research has shown that neutrophil lineage cells, rather than non-monocytes or macrophages, are the primary sources of ARG1 expression in human non-small cell lung cancer [70].
Neutrophils in the tumor microenvironment induce autoimmune checkpoint ligand expression, exerting immunosuppressive effects. Tumor-driven factors like TNF-α, GM-CSF, HMGB1, IL-6, CCL20, and hypoxia boost PD-L1 levels in neutrophils, dampening immune responses, particularly T cells [71–74, 107–109]. For instance, GM-CSF from gastric cancer, HMGB1 via extracellular vesicles, and IL-6 from HCC-associated CAFs enhance PD-L1 expression in neutrophils through JAK-STAT3 signaling [72, 73, 109]. Moreover, both tumors and healthy tissues express CD47, a “don’t eat me” signal, binding to SIRP receptors on macrophages and neutrophils, aiding tumors in evading immune eradication [75].
Neutrophils influence other immune cells through cytokine secretion, hindering anti-tumor responses. In mice, the complement factor C5a drives neutrophil migration to tumors, releasing ROS and reactive nitrogen species (RNS) that hamper T cell function [76]. Altering TCR recognition and reducing chemokine receptor expression, along with nitrosylation of tyrosine, obstruct T cell movement towards tumors, creating an RNS-based barrier [76, 110]. The RNS-induced immune “cold” environment orchestrates the preferential entry of marrow cells with immunosuppressive characteristics, compromising cancer immunotherapy for various solid tumors, including pancreatic ductal adenocarcinoma [111]. Clearing RNS could enhance current treatment effectiveness. Immunosuppressive neutrophils producing IL-10 lower IL-12 from macrophages, switching them from M1 to M2 [77]. Microbiota TLR signals prompt neutrophils to release IL-1β and IL-23, activating lung-resident γδ T cells [78]. Nevertheless, IL-17 from γδ T cells recruits neutrophils, suppressing CD8+ T cell action in breast tumors [112]. This reciprocal interaction between γδ T cells and neutrophils forms amplification loops within the tumor microenvironment, effectively promoting tumor progression (Fig. 5).
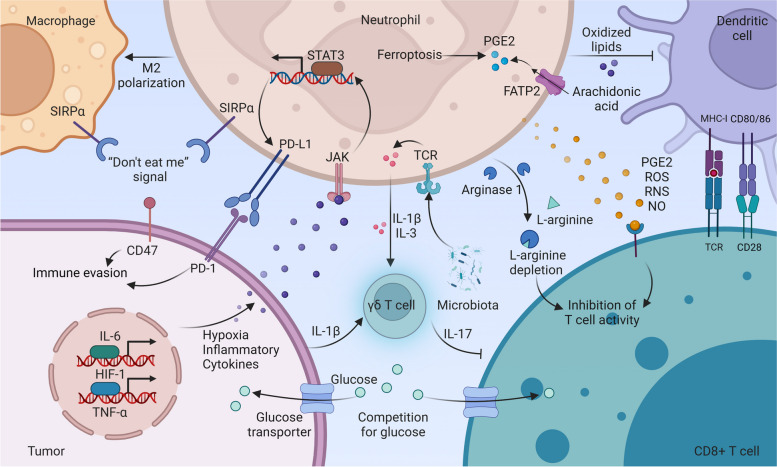
Fig. 5.
Neutrophils regulate immune cells to drive protumor immune responses. Neutrophil-produced IL-10 promotes M2 polarization in macrophages. Tumors evade immune attack through CD47, a “don’t eat me” signal that interacts with SIRPα on macrophages and neutrophils. Tumor-derived mediators (TNF-α, IL-6, GM-CSF, HMGB1, and CCL20) and hypoxia activate JAK-STAT3, upregulating PD-L1 in neutrophils. Microbiota-stimulated neutrophils secrete IL-1β and IL-3, along with tumor-derived IL-1β, activating γδ T cells. Activated γδ T cells produce IL-17, recruiting neutrophils to suppress CD8 T cells. Neutrophils expressing FATP2 enhance PGE2 biosynthesis by promoting arachidonic acid uptake. Neutrophils undergoing ferroptosis release lipid mediators, including PGE2. PGE2, ROS, RNS, and NO released by neutrophils limit T cell activity. Neutrophil-derived MPO drives lipid peroxidation, impairing dendritic cell antigenic cross-presentation. Neutrophil-released ARG1 reduces L-arginine availability, limiting T cell function. Tumors compete with T cells for glucose uptake, depleting T cells and evading the immune response
Thus, neutrophils shape the immune system to aid tumor development, particularly impacting T cells. Essentially, neutrophils assist tumors in evading immune monitoring by changing metabolism, adjusting immune checkpoints, and modulating immune cell subsets via cytokines.
Are neutrophils friends or foes during cancer progression?
Neutrophils exhibit a dual role in the tumor microenvironment, with both antitumor and protumor effects. On the one hand, neutrophils serve as immune system defenders and exert inhibitory effects on tumors. However, under the influence of the tumor microenvironment, the release of immature neutrophils or phenotypic changes in neutrophils promotes tumor growth. The intricate relationship between neutrophils and cancer progression depends on multiple factors, such as tumor characteristics, endogenous influences, exogenous therapeutic interventions, and the overall health status of patients (Fig. 6).
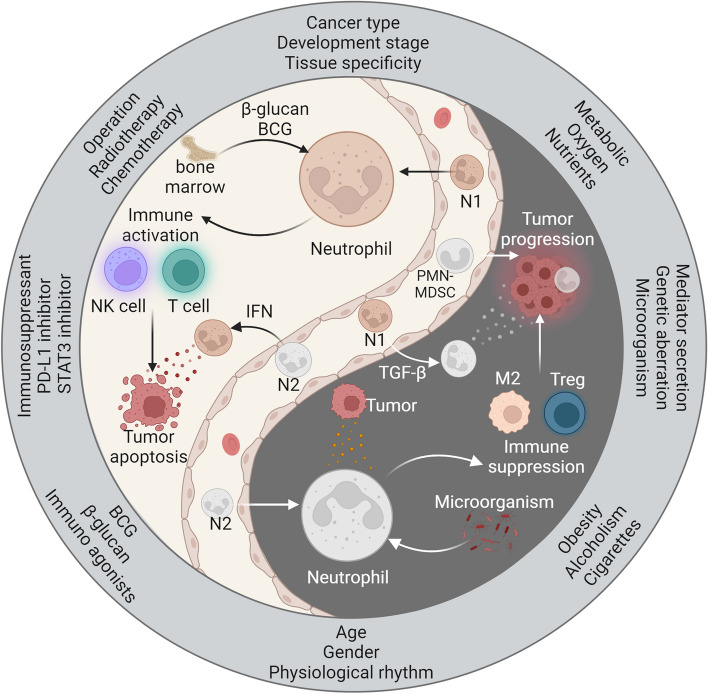
Fig. 6.
The Yin and Yang profiles of neutrophils in the progression of cancer. β-glucan and BCG stimulate neutrophils to develop antitumor innate immune memory in the bone marrow. Antitumor neutrophils (N1) induce tumor apoptosis by activating T cells and NK cells and releasing cytokines. However, tumor-derived factors and microbes can influence neutrophils to adopt a protumorigenic phenotype (N2). N2 neutrophils contribute to an immunosuppressive microenvironment by recruiting M2-type macrophages and Tregs. They also secrete mediators that directly promote tumor progression. IFN and TGF-β drive the formation of N1 and N2 neutrophil phenotypes, respectively. Moreover, neutrophil polarization varies based on tumor characteristics and patient status. Discontinuation of surgery, chemotherapy, and radiotherapy can lead to neutrophil-induced tumor recurrence. Adjuvant therapy using immunoagonists (e.g., β-glucan, BCG vaccine) and immunosuppressants (e.g., PD-L1, STAT3 inhibitors) enhances the antitumor effect of neutrophils. Poor patient habits, endogenous factors, and tumor metabolic reprogramming influence the tumor-promoting effect of neutrophils
The characteristics of tumors within the host, including cancer type, developmental stage, tissue specificity, and metabolic reprogramming, closely influence the phenotypic state of neutrophils [51, 113–117]. The role of neutrophils in cancer is highly intricate, and there may be variations across different cancer types. In most cancer patients, high levels of TAN are generally associated with poorer treatment outcomes. However, in colorectal cancer patients, TAN infiltration is linked to a favorable prognosis [113–115]. We hypothesize that different cancers may exhibit varying levels of inflammation, leading to differences in the recruitment and infiltration of neutrophils into tumor tissue. The developmental stage of cancer, whether early or late, significantly impacts the phenotype and characteristics of neutrophils. For instance, LNs are crucial sites for initiating adaptive immune responses and serve as important locations for tumor cell metastasis [51]. In the early N0 stage, neutrophil infiltration is associated with improved patient survival. Conversely, in the late N1–3 stage, a high presence of neutrophils indicates a decrease in the patient survival rate [51]. The phenotypic transformation of neutrophils may be attributed to changes in the immune microenvironment at different developmental stages. Additionally, neutrophils exhibit distinct functional characteristics in different target tissues within the context of cancer. Neutrophils in the lungs and intestines demonstrate significant angiogenic properties, while they are nearly absent in the intestines and skin under sterile conditions [116]. Metabolic reprogramming plays a beneficial role in regulating energy metabolism and facilitating rapid cell growth and proliferation and is considered an emerging marker of cancer [69, 101]. However, the competitive consumption of abundant oxygen and nutrients by cancer cells typically impairs the metabolic adaptability of immune cells infiltrating tumors [103, 118]. Concurrently, abnormal metabolites or intermediates in tumors ultimately influence the effects and functions of immune cells, thereby promoting immune evasion within the tumor microenvironment [119]. When tumors consume glucose, they generate a substantial amount of lactic acid, leading to the formation of an acidic extracellular tumor microenvironment that hampers the antitumor effects of various immune cells [101, 104]. Furthermore, the establishment of a hypoxic environment promotes the upregulation of PD-L1 in neutrophils [74]. Thus, the presence of high levels of lactic acid, low pH, and hypoxia in the tumor microenvironment exacerbates the negative impact on immune cell functionality.
Endogenous factors such as mediator secretion, genetic aberrations, and microorganisms play crucial regulatory roles in neutrophils [120–122]. Mediators derived from cancer cells and their surrounding stromal cells, such as IL-6, PGE2, TGF-β, hyaluronic acid fragments (HA), and G-CSF/GM-CSF, contribute to the reprogramming of neutrophils into a tumor-promoting phenotype [16, 25, 120, 123, 124]. CircPACRGL, derived from colorectal cancer (CRC), is a circRNA that increases TGF-β expression upon uptake of miR-142-3P or miR-506-3P, leading to the polarization of neutrophils to the N2 phenotype and eventual tumor progression [125]. Conversely, IFN-γ (type II IFN) induces IRF1 expression, which is essential for the tumor-suppressive effect of neutrophils in the context of successful immunotherapy [52]. Moreover, genetic aberrations in the tumor itself alter the cytokine secretion profile, thereby inducing the accumulation of immunosuppressive neutrophils. In a mouse model with Tp53-deficient tumors, WNT ligands secreted by cancer cells induced IL-1β secretion by tumor-associated macrophages, ultimately leading to the aggregation of immunosuppressive neutrophils [121]. Unexpectedly, microorganisms play essential roles in regulating the functional heterogeneity of neutrophils. Local microorganisms not only prolong the lifespan of neutrophils under inflammatory conditions but also promote neutrophil-mediated inflammation and tumor growth [78]. For example, CRC-derived Escherichia coli breaks the intestinal vascular barrier and translocates to the liver, further promoting the accumulation of neutrophils and leading to the formation of an ecological niche before tumor metastasis [122]. The regulatory effects of various endogenous factors on neutrophils are complex and diverse, playing an important role in regulating neutrophil function and inflammatory immunity.
Exogenous therapeutic interventions, including surgery, chemotherapy/radiotherapy, immune agonists (such as β-glucan and BCG), and immunosuppressants, also modulate the functional properties of neutrophils [41, 126–130]. The surgical process may lead to the shedding and dissemination of tumor cells, ultimately resulting in cancer recurrence in postoperative patients [131]. However, surgical trauma-induced inflammatory reactions activate neutrophils and create a tumor microenvironment conducive to tumor growth and spread [126]. Moreover, surgical stress often triggers the formation of NETs, which act as protective barriers, facilitating the metastasis of tumor cells to distant sites [66]. Therefore, precise resection or postoperative adjuvant therapy helps eliminate tumor recurrence. Radiation exposure induces acute lung injury and promotes neutrophilic pro-metastatic responses, enhancing the colonization of tumors at distant sites [127]. Disseminated tumors after chemotherapy cessation induce neutrophil recruitment into the metastatic site of the liver and promote increased metastatic growth [128]. Mechanistically, growth arrest-specific 6 (Gas6) expressed by neutrophils activates AXL receptors on tumor cells, promoting their regeneration [128]. Hence, combining chemotherapy with the inhibition of the Gas6/AXL signaling axis may be effective in reducing cancer recurrence. In fact, β-glucan and BCG vaccines act as agonists of cellular immunity, inducing neutrophils to form antitumor innate immunological memory at the bone marrow level. β-glucan-trained granulocytes possess the ability to limit tumor growth, and this immunological trait is retained at the bone marrow level and can be transferred to untrained recipient mice [41]. BCG vaccination not only promotes the generation of bone marrow lineages biased toward granulocytes but also induces the long-term functional transformation of circulating neutrophils, resulting in enhanced antibacterial function of neutrophils under the co- stimulation of multiple pathogens in vitro [129, 132]. Currently, the BCG vaccine is used as a first-line adjuvant treatment for bladder cancer [133]. Furthermore, targeting key immune checkpoints and immunosuppressive molecules enhances the antitumor activity of neutrophils. Activation of the STAT3 signaling pathway typically leads to the upregulation of PD-L1 in neutrophils, enabling tumors to evade neutrophil surveillance and clearance [73, 109]. Therefore, the development of targeted inhibitors of PD-L1 and STAT3 would be beneficial in restoring the antitumor function of neutrophils.
The state of patients can vary with age, sex, physiological rhythm, and obesity, which have diverse effects on immune cell function [134–139]. Generally, immune function tends to gradually decline with age, which may explain why elderly individuals are more susceptible to infections, cancer, and immune-related diseases [140]. During the aging process, dysfunction of neutrophils occurs due to weakened signal transduction of specific receptors [134]. Additionally, young women exhibit higher levels of mature neutrophils and stronger activation responses than do young men [135]. In pregnant women, the frequency of immature neutrophils in the blood is higher than that in healthy women, and these immature neutrophils may play beneficial roles in maintaining maternal-fetal tolerance [135, 141]. In the process of melanoma-induced metastasis, a clear diurnal pattern has been observed, where many metastatic tumors appear in the lungs during cell injection in the morning, with an almost nonexistence of metastatic lesions during injection at night [136]. This pattern aligns with the circadian rhythm of neutrophil aging [34]. Notably, the depletion of neutrophils leads to the alleviation of tumor metastasis, providing new evidence for targeted neutrophil immunotherapy and time-controlled drug delivery [136]. Obesity and alcoholism exacerbate increases in CXCL1, resulting in the increased infiltration of neutrophils into the liver and synergistically promoting the occurrence of steatohepatitis [139]. In an obese mouse model, adipose tissue was shown to induce an increase in lung neutrophils, thereby facilitating cancer cell metastasis [137]. Smoking exposure is recognized as a risk factor for various cancers. The inflammatory response triggered by smoking induces changes in the number and function of neutrophils, implicating them as potential participants in cancer progression [138]. Nicotine in cigarette smoke stimulates N2-neutrophil polarization through the STAT3 pathway [138]. Subsequently, these neutrophils release exosomal miR-4466, promoting the metabolic transformation of brain cancer cells and ultimately accelerating the metastasis of lung cancer to the brain [138]. Furthermore, persistent lung inflammation caused by tobacco smoke exposure promotes the formation of neutrophil extracellular traps (NETs), which can aid in the recovery of dormant cancer cells [64]. Therefore, the inflammatory response induced by cigarette smoke can enhance the tumor-promoting effect of neutrophils.
In brief, neutrophils play a dual role as both allies and adversaries in cancer development. However, comprehending the complex nature of neutrophils in cancer necessitates considering a myriad of factors. These factors encompass the intrinsic characteristics of the tumor, such as cancer type, stage of progression, tissue specificity, and metabolic alterations. Additionally, endogenous factors such as mediator secretion, genetic abnormalities, and microorganism presence, as well as exogenous therapeutic interventions such as surgery, chemotherapy, radiotherapy, immune agonists, and immunosuppressants, contribute to this intricate dynamic. Furthermore, the individual state of patients, including age, sex, physiological rhythms, obesity, alcoholism, and smoking, also impacts neutrophil behavior. There might be unidentified factors influencing the plasticity of neutrophils in terms of their transcriptional profile and functional attributes.
Cancer immunotherapy strategies targeting neutrophils
The dual nature of neutrophils within the tumor microenvironment presents challenges for targeted therapy. To enhance treatment efficacy, we propose three strategies: 1) blocking the infiltration of neutrophils into local tumors, 2) targeting the immunosuppressive function of neutrophils, 3) improving the adjuvant anticancer efficacy of neutrophils (refer to Table 4 for details).
Table 4.
Clinical trials of targeted neutrophil therapies for cancers
| Targets | Agents | Cancer applications | Phase | NCT trial number | Treatment regimen | References |
|---|---|---|---|---|---|---|
| CXCR1/CXCR2 | Navarixin (MK-7123) | Solid tumor | II | NCT03473925 | Navarixin + Pembrolizumab | NA |
| SX-682 (BKT140) | Resectable pancreatic cancer | II | NCT05604560 | SX-682 + Tislelizumab | NA | |
| Reparixin | Metastatic triple-negative breast cancer | II | NCT02370238 | Reparixin + Paclitaxel | NA | |
| AZD5069 | Metastatic castration-resistant prostate cancer | I/II | NCT03177187 | AZD5069 + Enzalutamide | NA | |
| IL-8 | BMS-986253 | Hepatocellular carcinoma | II | NCT04050462 | BMS-986253 + Nivolumab | NA |
| C5aR | IPH5401 | Solid tumor | I | NCT03665129 | IPH5401 + Durvalumab | NA |
| TJ210001 | Solid tumor | I | NCT04947033 | Monotherapy | NA | |
| CD47-SIRPα | Magrolimab | TP53 mutant acute myeloid leukemia | III | NCT04778397 | Magrolimab + Azacitidine | [142] |
| Lemzoparlimab | Refractory non-Hodgkin’s lymphoma | Ib | NCT03934814 | Lemzoparlimab + Rituximab | [143] | |
| CC-95251 | Relapsed and/or refractory non-Hodgkin lymphoma | I | NCT03783403 | CC-95251 + Rituximab | [144] | |
| BI 765063 | Solid tumors | I | NCT03990233 | BI 765063 + BI 754091 | [145] | |
| STAT3 | TTI-101 | Recurrent or metastatic head and neck squamous cell carcinoma | I/II | NCT05668949 | TTI-101 + Pembrolizumab | NA |
| Napabucasin | Metastatic colorectal cancer | III | NCT02753127 | BBI-608 + FOLFIRI (5-Fluorouracil, Leucovorin, Irinotecan) | [146] | |
| AZD9150 (Danvatirsen) | Advanced pancreatic cancer, non-small cell lung cancer, and mismatch repair deficient colorectal cancer | II | NCT02983578 | AZD9150 + MEDI4736 | NA | |
| TGF-β | LY2157299 | Locally advanced rectal adenocarcinoma | II | NCT02688712 | LY2157299 + Neoadjuvant chemoradiation | [147] |
| M7824 (bintrafusp alfa) | Non-small cell lung cancer | III | NCT03631706 | Monotherapy | NA | |
| NIS793 | Metastatic pancreatic ductal adenocarcinoma | III | NCT04935359 | NIS793 + Nab-paclitaxel/gemcitabine | [148] | |
| G-CSF | Efbemalenograstim alfa (F-627) | Breast cancer | III | NCT04174599 | Monotherapy | [149] |
| IL-6 | Tocilizumab | Melanoma | II | NCT03999749 | Tocilizumab + Ipilimumab and Nivolumab | [150] |
| Siltuximab | High-risk smoldering multiple myeloma | II | NCT01484275 | Monotherapy | [151] |
Targeting pathways that attract neutrophils to tumors shows potential for halting cancer advancement. CXCR1/CXCR2 receptors play a crucial role in neutrophil recruitment throughout the body, responding strongly to IL-8 [15, 152]. Thus, Blocking the IL-8 pathway and its receptors CXCR1/2 emerges as a promising cancer therapy strategy. CXCR1/CXCR2 inhibitors include Navarixin (NCT03473925), SX-682 (NCT05604560), Reparixin (NCT02370238), and AZD5069 (NCT03177187). The anti-IL-8 monoclonal antibody BMS-986253 demonstrated good safety and tolerability in a phase I study as a monotherapy for cancer patients [153]. Currently, BMS-986253 has entered phase II clinical trial (NCT04050462). The C5a receptor (C5aR) on neutrophils is a key therapeutic target. Cancer-produced C5a is a potent neutrophil attractant and contributes to an immunosuppressive tumor environment by upregulating molecules like ARG1, CTLA4, and PD-L1 [154]. Blocking C5a/C5aR signaling holds promise as an effective anti-cancer approach. Clinical trials are underway to assess C5aR antagonists’ efficacy in cancer treatment, including IPH5401 (NCT03665129) and TJ210001 (NCT04947033).
Adjusting the immune-dampening signals of neutrophils in the tumor setting to revive their natural anticancer role holds significant promise. One approach is to target critical immune checkpoints and suppressive molecules to bolster neutrophils’ anticancer functions. Blocking the CD47-SIRPα interaction could amplify neutrophils’ anticancer impact [155]. Promising CD47 antibodies like Magrolimab and Lemzoparimab have shown early clinical benefits and good tolerability [142, 143]. The novel anti-SIRPα antibody CC-95251 (NCT03783403) and the SIRPα inhibitor BI 765063 (NCT03990233) have undergone Phase I study evaluations. Direct STAT3 activation fosters oncogene expression, spurring tumor advancement and immune suppression [72]. Thus, targeting the key regulatory protein STAT3 holds significant promise as an anticancer therapy. Currently effective STAT3 inhibitors include TTI-101, Napabucasin, AZD9150 [146, 156, 157]. TGF-β is a key cytokine in the regulation of immune function and is essential for both neutrophil recruitment and reprogramming [16]. In a phase II trial, the oral TGF-β receptor type I kinase inhibitor LY2157299, when used with neoadjuvant radiochemotherapy, improved complete remission to 32% with good tolerance [147]. M7824 (bintrafusp alfa), a dual-action fusion protein inhibiting TGF-β and PD-L1, displayed promising anticancer effects in a Phase II study [158]. M7824 (NCT03631706) and NIS793 (NCT04935359) have entered Phase III evaluation.
Cancer chemotherapy often causes bone marrow suppression and low neutrophil count, raising infection risks [159]. G-CSF, crucial for boosting neutrophil levels, is frequently used to prevent chemotherapy-induced neutropenia [159]. In a Phase III study with breast cancer patients, F-627, a third-generation long-acting G-CSF drug, lowered infection and febrile neutropenia rates [149]. Although G-CSF doesn’t directly fight cancer, it can improve treatment effectiveness when used alongside chemotherapy. Currently, F-627 has received approval in China for the treatment of chemotherapy-induced neutropenia [160]. IL-6, linked to immune-related adverse events (irAEs) from immune checkpoint blockade (ICB) therapy, is key for neutrophil actions [72, 161]. Anti-CTLA-4 therapy triggers IL-6 release and gut neutrophil buildup, causing an inflammatory response disrupting the gut microbiome balance and promoting irAEs [161]. Combining IL-6 inhibition with antibiotics has shown to reduce irAEs and enhance immune responses [161]. Thus, blocking IL-6 during ICB therapy enhances tumor immunity and relieves irAE symptoms. Tocilizumab and siltuximab are two effective clinical IL-6 inhibitors [150, 151].
In summary, though advancements have been achieved in neutrophil-targeting immunotherapies, challenges remain. Precisely targeting distinct neutrophil groups while maintaining a balance between immune regulation and protective functions is vital for optimal treatment results.
Current challenges and where the field is going
Neutrophils in cancer treatment often lack specificity and have a short duration of actionTargeting neutrophils inadvertently affects other cells or antitumor neutrophils, disrupting the normal immune system and making patients more susceptible to adverse reactions such as infections [162]. For instance, the use of an anti-Gr1 antibody that recognizes Ly6G and Ly6C leads to the depletion of Ly6C-expressing cells such as monocytes and macrophages during treatment [163]. Neutrophil targeting is short-lived due to compensatory mechanisms in the complex immune system that counteract neutrophil blockade within the tumor, resulting in rapid recovery [164]. Anti-Ly6G specifically targets neutrophils, but its depletion triggers compensatory bone marrow release and even induces extramedullary spleen production of neutrophils, leading to a “rebound” effect [165]. Interestingly, newly generated circulating neutrophils express low levels of Ly6G on their membrane, rendering anti-Ly6G treatment inadequate and short-lived [166]. Thus, specific targeting of neutrophils is essential to enhance treatment efficacy and reduce toxic side effects.
Most studies on neutrophil mechanisms have been conducted in mouse models since neutrophils have a short lifespan [167]. Currently, mouse models for inducing tumor formation include genetic engineering models, chemical toxic drug models, implantation models, and humanized mouse models [167]. However, preclinical models often fail to fully replicate the complex immune system environment and disease progression in humans, making the translation of research results from mouse models to clinical treatment a challenging and time-consuming task [167]. Although humanized mouse models can bridge the gap between the human immune system and xenograft mice, they require advanced technical expertise [168]. In the future, it is essential to establish more effective and innovative preclinical models to predict and assess the applicability of various antitumor drugs in humans.
Responses to targeted neutrophil therapy may vary among individuals, highlighting the need for personalized treatment protocols. The effectiveness of treatment can be influenced by various factors, including tumor characteristics, endogenous and exogenous factors, and interpatient differences such as disease type and dietary habits [169]. Additionally, variations in drug metabolism abilities and immune system status can contribute to differences in treatment responses. Therefore, the development of personalized treatment plans is crucial to ease the burden of targeted therapy and improve treatment efficacy.
To optimize the efficacy of targeted neutrophil therapy, it is essential to develop precise methods that specifically target neutrophils while minimizing the impact on other cell types and reducing side effects [170]. Therefore, the identification of highly specific biomarkers for immunosuppressive neutrophils becomes crucial in promoting treatment effectiveness and accuracy [170]. NE, an essential physiological factor in neutrophil-mediated diseases, is exclusively present on the surface of active neutrophils and not in other leukocyte subpopulations [171]. Researchers have discovered that an α1-antitrypsin-derived peptide specifically binds to NE on activated neutrophils. Coating nanoparticles with this peptide enables selective anchoring to activated neutrophils for localized drug delivery [171].
Neutrophils have a dual role in tumor progression and participate in immune evasion and drug resistance in tumors. Thus, combining targeted neutrophil therapy with other treatment methods, such as chemotherapy and immunotherapy, can synergistically breakdown immune suppression and enhance antitumor immune effects [172, 173]. Chimeric antigen receptor (CAR)-T cell therapy based on neutrophils effectively improves therapeutic outcomes in solid tumors with heterogeneous antigen expression without additional immunotoxicity [174]. The multifunctional pro-inflammatory neutrophil activating protein (NAP), derived from Helicobacter pylori, possesses potent immune regulatory properties [174]. Using CAR-T cells carrying NAP can promote the recruitment and activation of neutrophils, leading to the eradication of antigenically heterogeneous tumors [174]. However, primary neutrophils have a short lifespan and are resistant to genome editing, limiting their use in CAR-modified immunotherapy [175]. CRISPR–Cas9 technology can be employed to knock in genes into human pluripotent stem cells, resulting in the generation of CLTX-T-CAR neutrophils that exhibit optimal antitumor activity against glioblastoma [175].
Neutrophils hold potential as carriers for delivering tumor drugs due to their high migratory ability during inflammatory responses and their ability to cross the blood–brain barrier, enhancing drug penetration and anticancer efficacy [176]. Currently, there are two main delivery strategies based on neutrophils: (1) using neutrophils as carriers and (2) using nanovesicles derived from neutrophil membranes as carriers [177]. Neutrophils loaded with paclitaxel liposomes can penetrate the brain and serve as postoperative adjuvant therapy for brain tumors [178]. Neutrophil membrane-derived nanovesicles enhance drug delivery. A nanoscale neutrophil-mimicking drug delivery system (NM-NP) was developed by coating neutrophil membranes onto the surface of polylactic acid glycolic acid nanoparticles (NPs) [179]. This process preserves the biological binding activity of neutrophils. After loading the second-generation proteasome inhibitor carfilzomib, NM-NP-based nanoparticles selectively deplete CTCs, prevent early metastasis, and inhibit the formation of metastatic niches [179]. However, there are limitations to drug delivery using neutrophils as carriers, such as the short in vitro lifespan of neutrophils, potential damage to neutrophil activity caused by drug loading, and challenges in controlling the storage and release profiles of nanoparticles within neutrophils [176].
Overall, targeting neutrophils for cancer therapy presents multiple opportunities and challenges. Current challenges include limitations in drug therapy, preclinical models and individual patient variability. Future studies aim to enhance the effectiveness of targeted therapy by optimizing targeting strategies, combining therapies, and exploring the potential of neutrophils as drug delivery carriers.
Concluding remarks and perspectives
Neutrophils exhibit remarkable plasticity and complexity within the tumor microenvironment. As a subset of lymphocytes with phagocytic and cytotoxic functions, neutrophils can directly eliminate tumor cells by releasing various toxic substances or modulating the expression of apoptosis-related ligands. They also indirectly inhibit tumor progression by regulating immune system activation. However, when exposed to tumor-associated factors or exosomes, neutrophils undergo “functional remodeling,” acquiring biological properties that promote tumor immune escape and angiogenesis, thereby facilitating tumor growth and metastasis. Remodeled neutrophils contribute to tumor growth through various mechanisms and are closely associated with immune-related toxicity caused by immune checkpoint blockade. Therefore, inhibiting neutrophil migration into the tumor region not only enhances antitumor efficacy but also effectively mitigates the strong toxic response induced by immunotherapy.
Determining whether neutrophils act as allies or adversaries in cancer immunotherapy requires considering the combined influence of various factors. Tumor characteristics, including cancer type, stage, tissue specificity, and metabolic reprogramming, influence the dual nature of neutrophils. Endogenous factors such as altered immune status, genetic aberrations, and the microbiota favor the formation of tumor-promoting neutrophils within the tumor microenvironment. Therapeutic interventions, such as surgically induced inflammatory responses, activate immunosuppressive neutrophils, leading to tumor recurrence. However, immune agonists such as β-glucan and BCG can induce antitumor properties in neutrophils. Additionally, individual patient characteristics such as age, sex, and lifestyle further influence the immune landscape. Thus, comprehensively evaluating the complex role of neutrophils in cancer requires considering these multiple factors and exploring other unknown factors to develop personalized and comprehensive treatment strategies.
Neutrophils play a complex role in cancer immunotherapy, acting as a “double-edged sword” by participating in immune defense and immune tolerance maintenance. Targeting neutrophils in cancer treatment poses challenges due to potential immune dysfunction and adverse reactions, such as infections associated with new drugs or formulations. Therefore, a key focus of current clinical research is optimizing chemotherapy regimens to enhance neutrophil vitality while reducing toxicity. In future cancer immunotherapy, comprehensive approaches such as specific immunotherapy, combination therapy, and personalized therapy can be employed to maximize the therapeutic benefits of targeting neutrophils. Furthermore, leveraging cutting-edge technologies and platforms such as gene editing and nanotechnology enhances neutrophil activity and specificity, leading to superior efficacy. Bridging the gap between preclinical mouse models and human pathological environments, as well as developing personalized treatment plans for patients, will facilitate the advancement of the application of neutrophils in cancer treatment. Additionally, researchers should continue exploring the potential of neutrophils as drug delivery carriers in cancer treatment.
Acknowledgments
We would like to thank the National Natural Science Foundation of China and Grant Agency of the Czech Republic for financial support.
Abbreviations
- ADCC
Antibody-dependent cytotoxicity
- APC
Antigen-presenting cell
- APOE
Apolipoprotein E
- ARG1
Arginase 1
- BMAL1
Brain and muscle Arnt-like 1
- C5aR
C5a receptor
- CAFs
Cancer-associated fibroblasts
- CAR
Chimeric antigen receptor
- CCL2
CC motif chemokine ligand 2
- CMP
Common myeloid progenitors
- CXCR4
CXC chemokine receptor 4
- DC
Dendritic cells
- DD
Death domain
- ECM
Extracellular matrix
- FasL
Fas ligand
- FATP2
Fatty acid transporter 2
- FcRs
Fc receptors
- FGF2
Fibroblast growth factor 2
- G-CSF
Granulocyte colony-stimulating factor
- GM-CSF
Granulocyte-macrophage colony stimulating factor
- GMP
Granulocyte monocyte progenitors
- H2O2
Hydrogen peroxide
- HCC
Hepatocellular carcinoma
- HDN
High-density neutrophils
- HGF
Hepatocyte growth factor
- HIF-1α
Hypoxia-inducible factor-1α
- HMGB1
High-mobility group box 1
- HSC
Hematopoietic stem cells
- ICB
Immune checkpoint blockade
- IFN
Interferon
- IL-1RA
Interleukin-1 receptor antagonist
- IL-6
Interleukin-6
- iNOS
Inducible nitric oxide synthase
- irAEs
Immune-related adverse events
- IRF-8
Interferon-regulatory factor-8
- IRS1
Insulin receptor substrate 1
- LDN
Low-density neutrophils
- LNs
Lymph nodes
- LOX1
Low-density lipoprotein receptor 1
- mAbs
Monoclonal antibodies
- MCs
Mesenchymal cells
- MDSC
Myeloid-derived suppressor cells
- MMP-9
Matrix metalloproteinases
- MYD88
Myeloid differentiation factor 88
- NAP
Neutrophil activating protein
- NASH
Nonalcoholic steatohepatitis
- NE
Neutrophil elastase
- NET
Neutrophil extracellular trap
- NF-κB
Nuclear factor-κB
- NPs
Nanoparticles
- OSM
Oncostatin M
- Phd3
Prolyl hydroxylase 3
- PI3K
Phosphoinositide 3-kinase
- ROS
Reactive oxygen species
- S100A9
S100 calcium-binding protein A9
- SASP
Senescence-associated secretory phenotype
- scRNA-seq
Single-cell RNA sequencing
- TAN
Tumor-associated neutrophils
- TGF-β
Transforming growth factor-β
- TLR4
Toll-like receptor 4
- TNF-α
Tumor necrosis factor-α
- TRAIL
Tumor necrosis factor-related apoptosis-inducing ligand
- Tregs
Regulatory T cells
- TREM2
Triggering receptor expressed on myeloid cells 2
- TRPM2
Transient receptor potential cation channel, subfamily M, member 2
- VCAM-1
Vascular cell adhesion molecule 1
- VEGF
Vascular endothelial growth factor
Authors’ contributions
All authors made substantial contributions to the review. QW, VA conceived and conceptualized the work. XH, EN, KK organized and wrote this review. LS and ZH contributed substantially to check the language. MV provided editorial assistance.
Funding
This work was supported by the National Natural Science Foundation of China (Grant no. 32373073); MH CZ-DRO (UHHK, 00179906); the Excellence project PrF UHK 2208/2023–2024 Czech Republic; the Research Program of the University of Granada; and the Scientific Grant Agency (VEGA Project 1/0542/24).
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qinghua Wu, Email: wqh212@hotmail.com.
Kamil Kuca, Email: kamil.kuca@uhk.cz.
References
- 1.Liew PX, Kubes P. The Neutrophil's role during health and disease. Physiol Rev. 2019;99:1223–1248. doi: 10.1152/physrev.00012.2018. [DOI] [PubMed] [Google Scholar]
- 2.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yvan-Charvet L, Ng LG. Granulopoiesis and neutrophil homeostasis: a metabolic, daily balancing act. Trends Immunol. 2019;40:598–612. doi: 10.1016/j.it.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Carnevale S, Di Ceglie I, Grieco G, Rigatelli A, Bonavita E, Jaillon S. Neutrophil diversity in inflammation and cancer. Front Immunol. 2023;14:1180810. doi: 10.3389/fimmu.2023.1180810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raftopoulou S, Valadez-Cosmes P, Mihalic ZN, Schicho R, Kargl J. Tumor-mediated neutrophil polarization and therapeutic implications. Int J Mol Sci. 2022;23 [DOI] [PMC free article] [PubMed]
- 6.Hedrick CC, Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol. 2022;22:173–187. doi: 10.1038/s41577-021-00571-6. [DOI] [PubMed] [Google Scholar]
- 7.Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021;21:345–359. doi: 10.1038/s41568-021-00347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer MA, Baer JM, Knolhoff BL, Nywening TM, Panni RZ, Su X, Weilbaecher KN, Hawkins WG, Ma C, Fields RC, et al. Breast and pancreatic cancer interrupt IRF8-dependent dendritic cell development to overcome immune surveillance. Nat Commun. 2018;9:1250. doi: 10.1038/s41467-018-03600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casbon AJ, Reynaud D, Park C, Khuc E, Gan DD, Schepers K, Passegué E, Werb Z. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci USA. 2015;112:E566–E575. doi: 10.1073/pnas.1424927112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou J, Nefedova Y, Lei A, Gabrilovich D. Neutrophils and PMN-MDSC: their biological role and interaction with stromal cells. Semin Immunol. 2018;35:19–28. doi: 10.1016/j.smim.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorn M, Guha P, Cunetta M, Espat NJ, Miller G, Junghans RP, Katz SC. Tumor-associated GM-CSF overexpression induces immunoinhibitory molecules via STAT3 in myeloid-suppressor cells infiltrating liver metastases. Cancer Gene Ther. 2016;23:188–198. doi: 10.1038/cgt.2016.19. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Zhang X, Chen Y, Xie Y, Liu J, Feng Q, Wang Y, Yuan W, Ma J. G-CSF is a key modulator of MDSC and could be a potential therapeutic target in colitis-associated colorectal cancers. Protein Cell. 2016;7:130–140. doi: 10.1007/s13238-015-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker F, Zhang HH, Matthews V, Weinstock J, Nice EC, Ernst M, Rose-John S, Burgess AW. IL6/sIL6R complex contributes to emergency granulopoietic responses in G-CSF- and GM-CSF-deficient mice. Blood. 2008;111:3978–3985. doi: 10.1182/blood-2007-10-119636. [DOI] [PubMed] [Google Scholar]
- 15.Schimek V, Strasser K, Beer A, Göber S, Walterskirchen N, Brostjan C, Müller C, Bachleitner-Hofmann T, Bergmann M, Dolznig H, Oehler R. Tumour cell apoptosis modulates the colorectal cancer immune microenvironment via interleukin-8-dependent neutrophil recruitment. Cell Death Dis. 2022;13:113. doi: 10.1038/s41419-022-04585-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canetti C, Silva JS, Ferreira SH, Cunha FQ. Tumour necrosis factor-alpha and leukotriene B(4) mediate the neutrophil migration in immune inflammation. Br J Pharmacol. 2001;134:1619–1628. doi: 10.1038/sj.bjp.0704403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol. 2021;21:485–498. doi: 10.1038/s41577-020-00490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masgrau-Alsina S, Wackerbarth LM, Lim DS, Sperandio M. MST1 controls murine neutrophil homeostasis via the G-CSFR/STAT3 axis. Front Immunol. 2022;13:1038936. doi: 10.3389/fimmu.2022.1038936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornish AL, Campbell IK, McKenzie BS, Chatfield S, Wicks IP. G-CSF and GM-CSF as therapeutic targets in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:554–559. doi: 10.1038/nrrheum.2009.178. [DOI] [PubMed] [Google Scholar]
- 21.Dumitru CA, Moses K, Trellakis S, Lang S, Brandau S. Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol Immunother. 2012;61:1155–1167. doi: 10.1007/s00262-012-1294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abeles RD, McPhail MJ, Sowter D, Antoniades CG, Vergis N, Vijay GK, Xystrakis E, Khamri W, Shawcross DL, Ma Y, et al. CD14, CD16 and HLA-DR reliably identifies human monocytes and their subsets in the context of pathologically reduced HLA-DR expression by CD14(hi) /CD16(neg) monocytes: expansion of CD14(hi) /CD16(pos) and contraction of CD14(lo) /CD16(pos) monocytes in acute liver failure. Cytometry A. 2012;81:823–834. doi: 10.1002/cyto.a.22104. [DOI] [PubMed] [Google Scholar]
- 23.Gustafson MP, Lin Y, Maas ML, Van Keulen VP, Johnston PB, Peikert T, Gastineau DA, Dietz AB. A method for identification and analysis of non-overlapping myeloid immunophenotypes in humans. PLoS One. 2015;10:e0121546. doi: 10.1371/journal.pone.0121546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damuzzo V, Pinton L, Desantis G, Solito S, Marigo I, Bronte V, Mandruzzato S. Complexity and challenges in defining myeloid-derived suppressor cells. Cytometry B Clin Cytom. 2015;88:77–91. doi: 10.1002/cytob.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veglia F, Tyurin VA, Blasi M, De Leo A, Kossenkov AV, Donthireddy L, To TKJ. Schug Z, Basu S, Wang F, et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature. 2019;569:73–78. doi: 10.1038/s41586-019-1118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea-Torres K, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol. 2016;1 [DOI] [PMC free article] [PubMed]
- 27.Heuvers ME, Muskens F, Bezemer K, Lambers M, Dingemans AC, Groen HJM, Smit EF, Hoogsteden HC, Hegmans J, Aerts J. Arginase-1 mRNA expression correlates with myeloid-derived suppressor cell levels in peripheral blood of NSCLC patients. Lung Cancer. 2013;81:468–474. doi: 10.1016/j.lungcan.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Rice CM, Davies LC, Subleski JJ, Maio N, Gonzalez-Cotto M, Andrews C, Patel NL, Palmieri EM, Weiss JM, Lee J-M, et al. Tumour-elicited neutrophils engage mitochondrial metabolism to circumvent nutrient limitations and maintain immune suppression. Nat Commun. 2018;9:5099. doi: 10.1038/s41467-018-07505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evrard M, Kwok IWH, Chong SZ, Teng KWW, Becht E, Chen J, Sieow JL, Penny HL, Ching GC, Devi S, et al. Developmental Analysis of Bone Marrow Neutrophils Reveals Populations Specialized in Expansion, Trafficking, and Effector Functions. Immunity. 2018;48:364–379.e368. doi: 10.1016/j.immuni.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Zheng X, Zhang J, Jiang X, Wang J, Li Y, et al. CD300ld on neutrophils is required for tumour-driven immune suppression. Nature. 2023; [DOI] [PubMed]
- 31.Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, Damti P, Lumbroso D, Polyansky L, Sionov RV, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10:562–573. doi: 10.1016/j.celrep.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 32.Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol. 2014;14:302–314. doi: 10.1038/nri3660. [DOI] [PubMed] [Google Scholar]
- 33.Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chèvre R. N AG, Kunisaki Y, Zhang D, van Rooijen N, Silberstein LE, et al: rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153:1025–1035. doi: 10.1016/j.cell.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adrover JM, del Fresno C, Crainiciuc G, Cuartero MI, Casanova-Acebes M, Weiss LA, Huerga-Encabo H, Silvestre-Roig C, Rossaint J, Cossío I, et al. A Neutrophil Timer Coordinates Immune Defense and Vascular Protection. Immunity. 2019;50:390–402.e310. doi: 10.1016/j.immuni.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Zhang D, Chen G, Manwani D, Mortha A, Xu C, Faith JJ, Burk RD, Kunisaki Y, Jang JE, Scheiermann C, et al. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525:528–532. doi: 10.1038/nature15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross EA, Douglas MR, Wong SH, Ross EJ, Curnow SJ, Nash GB, Rainger E, Scheel-Toellner D, Lord JM, Salmon M, Buckley CD. Interaction between integrin α9β1 and vascular cell adhesion molecule-1 (VCAM-1) inhibits neutrophil apoptosis. Blood. 2006;107:1178–1183. doi: 10.1182/blood-2005-07-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 38.Andzinski L, Kasnitz N, Stahnke S, Wu CF, Gereke M, von Köckritz-Blickwede M, Schilling B, Brandau S, Weiss S, Jablonska J. Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int J Cancer. 2016;138:1982–1993. doi: 10.1002/ijc.29945. [DOI] [PubMed] [Google Scholar]
- 39.Blaisdell A, Crequer A, Columbus D, Daikoku T, Mittal K, Dey SK, Erlebacher A. Neutrophils oppose uterine epithelial carcinogenesis via debridement of hypoxic tumor cells. Cancer Cell. 2015;28:785–799. doi: 10.1016/j.ccell.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahiddine K, Blaisdell A, Ma S, Créquer-Grandhomme A, Lowell CA, Erlebacher A. Relief of tumor hypoxia unleashes the tumoricidal potential of neutrophils. J Clin Invest. 2020;130:389–403. doi: 10.1172/JCI130952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalafati L, Kourtzelis I, Schulte-Schrepping J, Li X, Hatzioannou A, Grinenko T, Hagag E, Sinha A, Has C, Dietz S, et al. Innate Immune Training of Granulopoiesis Promotes Anti-tumor Activity. Cell. 2020;183:771–785.e712. doi: 10.1016/j.cell.2020.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gershkovitz M, Caspi Y, Fainsod-Levi T, Katz B, Michaeli J, Khawaled S, Lev S, Polyansky L, Shaul ME, Sionov RV, et al. TRPM2 mediates neutrophil killing of disseminated tumor cells. Cancer Res. 2018;78:2680–2690. doi: 10.1158/0008-5472.CAN-17-3614. [DOI] [PubMed] [Google Scholar]
- 43.Cui C, Chakraborty K, Tang XA, Zhou G, Schoenfelt KQ, Becker KM, Hoffman A, Chang YF, Blank A, Reardon CA, et al. Neutrophil elastase selectively kills cancer cells and attenuates tumorigenesis. Cell. 2021;184:3163–3177.e3121. doi: 10.1016/j.cell.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finisguerra V, Di Conza G, Di Matteo M, Serneels J, Costa S, Thompson AA, Wauters E, Walmsley S, Prenen H, Granot Z, et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature. 2015;522:349–353. doi: 10.1038/nature14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koga Y, Matsuzaki A, Suminoe A, Hattori H, Hara T. Neutrophil-derived TNF-related apoptosis-inducing ligand (TRAIL): a novel mechanism of antitumor effect by neutrophils. Cancer Res. 2004;64:1037–1043. doi: 10.1158/0008-5472.CAN-03-1808. [DOI] [PubMed] [Google Scholar]
- 46.Sun B, Qin W, Song M, Liu L, Yu Y, Qi X, Sun H. Neutrophil suppresses tumor cell proliferation via Fas /Fas ligand pathway mediated cell cycle arrested. Int J Biol Sci. 2018;14:2103–2113. doi: 10.7150/ijbs.29297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen CL, Wang Y, Huang CY, Zhou ZQ, Zhao JJ, Zhang XF, Pan QZ, Wu JX, Weng DS, Tang Y, et al. IL-17 induces antitumor immunity by promoting beneficial neutrophil recruitment and activation in esophageal squamous cell carcinoma. Oncoimmunology. 2017;7:e1373234. doi: 10.1080/2162402X.2017.1373234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Egmond M, Bakema JE. Neutrophils as effector cells for antibody-based immunotherapy of cancer. Semin Cancer Biol. 2013;23:190–199. doi: 10.1016/j.semcancer.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Hirschhorn D, Budhu S, Kraehenbuehl L, Gigoux M, Schröder D, Chow A, Ricca JM, Gasmi B, De Henau O, Mangarin LMB, et al. T cell immunotherapies engage neutrophils to eliminate tumor antigen escape variants. Cell. 2023;186:1432–1447.e1417. doi: 10.1016/j.cell.2023.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singhal S, Bhojnagarwala PS, O'Brien S, Moon EK, Garfall AL, Rao AS, Quatromoni JG, Stephen TL, Litzky L, Deshpande C, et al. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung Cancer. Cancer Cell. 2016;30:120–135. doi: 10.1016/j.ccell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pylaeva E, Korschunow G, Spyra I, Bordbari S, Siakaeva E, Ozel I, Domnich M, Squire A, Hasenberg A, Thangavelu K, et al. During early stages of cancer, neutrophils initiate anti-tumor immune responses in tumor-draining lymph nodes. Cell Rep. 2022;40:111171. doi: 10.1016/j.celrep.2022.111171. [DOI] [PubMed] [Google Scholar]
- 52.Gungabeesoon J, Gort-Freitas NA, Kiss M, Bolli E, Messemaker M, Siwicki M, Hicham M, Bill R, Koch P, Cianciaruso C, et al. A neutrophil response linked to tumor control in immunotherapy. Cell. 2023;186:1448–1464.e1420. doi: 10.1016/j.cell.2023.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ponzetta A, Carriero R, Carnevale S, Barbagallo M, Molgora M, Perucchini C, Magrini E, Gianni F, Kunderfranco P, Polentarutti N, et al. Neutrophils Driving Unconventional T Cells Mediate Resistance against Murine Sarcomas and Selected Human Tumors. Cell. 2019;178:346–360.e324. doi: 10.1016/j.cell.2019.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erdman SE, Rao VP, Poutahidis T, Rogers AB, Taylor CL, Jackson EA, Ge Z, Lee CW, Schauer DB, Wogan GN, et al. Nitric oxide and TNF-alpha trigger colonic inflammation and carcinogenesis in helicobacter hepaticus-infected, Rag2-deficient mice. Proc Natl Acad Sci USA. 2009;106:1027–1032. doi: 10.1073/pnas.0812347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Canli Ö, Nicolas AM, Gupta J, Finkelmeier F, Goncharova O, Pesic M, Neumann T, Horst D, Löwer M, Sahin U, Greten FR. Myeloid Cell-Derived Reactive Oxygen Species Induce Epithelial Mutagenesis. Cancer Cell. 2017;32:869–883.e865. doi: 10.1016/j.ccell.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Butin-Israeli V, Bui TM, Wiesolek HL, Mascarenhas L, Lee JJ, Mehl LC, Knutson KR, Adam SA, Goldman RD, Beyder A, et al. Neutrophil-induced genomic instability impedes resolution of inflammation and wound healing. J Clin Invest. 2019;129:712–726. doi: 10.1172/JCI122085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, Stolz DB, Land SR, Marconcini LA, Kliment CR, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16:219–223. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Antonio N, Bønnelykke-Behrndtz ML, Ward LC, Collin J, Christensen IJ, Steiniche T, Schmidt H, Feng Y, Martin P. The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. EMBO J. 2015;34:2219–2236. doi: 10.15252/embj.201490147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bancaro N, Cali B, Troiani M, Elia AR, Arzola RA, Attanasio G, Lai P, Crespo M, Gurel B, Pereira R, et al. Apolipoprotein E induces pathogenic senescent-like myeloid cells in prostate cancer. Cancer Cell. 2023;41:602–619.e611. doi: 10.1016/j.ccell.2023.02.004. [DOI] [PubMed] [Google Scholar]
- 60.Di Mitri D, Toso A, Chen JJ, Sarti M, Pinton S, Jost TR, D'Antuono R, Montani E, Garcia-Escudero R, Guccini I, et al. Tumour-infiltrating gr-1+ myeloid cells antagonize senescence in cancer. Nature. 2014;515:134–137. doi: 10.1038/nature13638. [DOI] [PubMed] [Google Scholar]
- 61.Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65:8896–8904. doi: 10.1158/0008-5472.CAN-05-1734. [DOI] [PubMed] [Google Scholar]
- 62.Gordon-Weeks AN, Lim SY, Yuzhalin AE, Jones K, Markelc B, Kim KJ, Buzzelli JN, Fokas E, Cao Y, Smart S, Muschel R. Neutrophils promote hepatic metastasis growth through fibroblast growth factor 2-dependent angiogenesis in mice. Hepatology. 2017;65:1920–1935. doi: 10.1002/hep.29088. [DOI] [PubMed] [Google Scholar]
- 63.Zhang X, Shi H, Yuan X, Jiang P, Qian H, Xu W. Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration. Mol Cancer. 2018;17:146. doi: 10.1186/s12943-018-0898-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361 [DOI] [PMC free article] [PubMed]
- 65.Huh SJ, Liang S, Sharma A, Dong C, Robertson GP. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 2010;70:6071–6082. doi: 10.1158/0008-5472.CAN-09-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teijeira Á, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, de Andrea C, Ochoa MC, Otano I, Etxeberria I, et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps that Interfere with Immune Cytotoxicity. Immunity. 2020;52:856–871.e858. doi: 10.1016/j.immuni.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 67.Li P, Lu M, Shi J, Gong Z, Hua L, Li Q, Lim B, Zhang XH, Chen X, Li S, et al. Lung mesenchymal cells elicit lipid storage in neutrophils that fuel breast cancer lung metastasis. Nat Immunol. 2020;21:1444–1455. doi: 10.1038/s41590-020-0783-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ugolini A, Tyurin VA, Tyurina YY, Tcyganov EN, Donthireddy L, Kagan VE, et al. Polymorphonuclear myeloid-derived suppressor cells limit antigen cross-presentation by dendritic cells in cancer. JCI Insight. 2020;5 [DOI] [PMC free article] [PubMed]
- 69.Kim R, Hashimoto A, Markosyan N, Tyurin VA, Tyurina YY, Kar G, Fu S, Sehgal M, Garcia-Gerique L, Kossenkov A, et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature. 2022;612:338–346. doi: 10.1038/s41586-022-05443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang H, Zhu X, Friesen TJ, Kwak JW, Pisarenko T, Mekvanich S, et al. Annexin A2/TLR2/MYD88 pathway induces arginase 1 expression in tumor-associated neutrophils. J Clin Invest. 2022;132 [DOI] [PMC free article] [PubMed]
- 71.He G, Zhang H, Zhou J, Wang B, Chen Y, Kong Y, Xie X, Wang X, Fei R, Wei L, et al. Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;34:141. doi: 10.1186/s13046-015-0256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng Y, Li H, Deng Y, Tai Y, Zeng K, Zhang Y, Liu W, Zhang Q, Yang Y. Cancer-associated fibroblasts induce PDL1+ neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis. 2018;9:422. doi: 10.1038/s41419-018-0458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shi Y, Zhang J, Mao Z, Jiang H, Liu W, Shi H, Ji R, Xu W, Qian H, Zhang X. Extracellular vesicles from gastric Cancer cells induce PD-L1 expression on neutrophils to suppress T-cell immunity. Front Oncol. 2020;10:629. doi: 10.3389/fonc.2020.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ring NG, Herndler-Brandstetter D, Weiskopf K, Shan L, Volkmer JP, George BM, Lietzenmayer M, McKenna KM, Naik TJ, McCarty A, et al. Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci USA. 2017;114:E10578–e10585. doi: 10.1073/pnas.1710877114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, Coukos G, Lambris JD. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9:1225–1235. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 Response1. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 78.Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B, Ameh S, Sandel D, Liang XS, Mazzilli S, et al. Commensal Microbiota Promote Lung Cancer Development via γδ T Cells. Cell. 2019;176:998–1013.e1016. doi: 10.1016/j.cell.2018.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Denk D, Greten FR. Inflammation: the incubator of the tumor microenvironment. Trends Cancer. 2022;8:901–914. doi: 10.1016/j.trecan.2022.07.002. [DOI] [PubMed] [Google Scholar]
- 80.Tazzyman S, Niaz H, Murdoch C. Neutrophil-mediated tumour angiogenesis: subversion of immune responses to promote tumour growth. Semin Cancer Biol. 2013;23:149–158. doi: 10.1016/j.semcancer.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 81.Shojaei F, Singh M, Thompson JD, Ferrara N. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc Natl Acad Sci USA. 2008;105:2640–2645. doi: 10.1073/pnas.0712185105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6:283–287. doi: 10.1023/B:AGEN.0000029415.62384.ba. [DOI] [PubMed] [Google Scholar]
- 83.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 84.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 85.Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 2005;16:593–609. doi: 10.1016/j.cytogfr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 86.Korbecki J, Kojder K, Kapczuk P, Kupnicka P, Gawrońska-Szklarz B, Gutowska I, et al. The effect of hypoxia on the expression of CXC chemokines and CXC chemokine receptors-a review of literature. Int J Mol Sci. 2021;22 [DOI] [PMC free article] [PubMed]
- 87.Scully S, Francescone R, Faibish M, Bentley B, Taylor SL, Oh D, Schapiro R, Moral L, Yan W, Shao R. Transdifferentiation of glioblastoma stem-like cells into mural cells drives vasculogenic mimicry in glioblastomas. J Neurosci. 2012;32:12950–12960. doi: 10.1523/JNEUROSCI.2017-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuczynski EA, Vermeulen PB, Pezzella F, Kerbel RS, Reynolds AR. Vessel co-option in cancer. Nat Rev Clin Oncol. 2019;16:469–493. doi: 10.1038/s41571-019-0181-9. [DOI] [PubMed] [Google Scholar]
- 89.Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annual Review of Pathology: Mechanisms of Disease. 2018;13:395–412. doi: 10.1146/annurev-pathol-020117-043854. [DOI] [PubMed] [Google Scholar]
- 91.Li S, Cong X, Gao H, Lan X, Li Z, Wang W, Song S, Wang Y, Li C, Zhang H, et al. Tumor-associated neutrophils induce EMT by IL-17a to promote migration and invasion in gastric cancer cells. J Exp Clin Cancer Res. 2019;38:6. doi: 10.1186/s13046-018-1003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Z, Bu X, Feng L, Hu J, Shen L, Guan J. Functional and mechanistic study of tumor-associated neutrophils inducing epithelial-mesenchymal transformation,invasion and metastasis of human mucoepidermoid carcinoma cells. Journal of Practical Stomatology. 2021;37:5–9. [Google Scholar]
- 93.Grosse-Steffen T, Giese T, Giese N, Longerich T, Schirmacher P, Hansch GM, et al. Epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma and pancreatic tumor cell lines: the role of neutrophils and neutrophil-derived elastase. Clinical & Developmental Immunology. 2012;2012 [DOI] [PMC free article] [PubMed]
- 94.Cackowski FC, Heath EI. Prostate cancer dormancy and recurrence. Cancer Lett. 2022;524:103–108. doi: 10.1016/j.canlet.2021.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Munir H, Jones JO, Janowitz T, Hoffmann M, Euler M, Martins CP, Welsh SJ, Shields JD. Stromal-driven and amyloid β-dependent induction of neutrophil extracellular traps modulates tumor growth. Nat Commun. 2021;12:683. doi: 10.1038/s41467-021-20982-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Follain G, Osmani N, Azevedo AS, Allio G, Mercier L, Karreman MA, Solecki G, Garcia Leòn MJ, Lefebvre O, Fekonja N, et al. Hemodynamic Forces Tune the Arrest, Adhesion, and Extravasation of Circulating Tumor Cells. Dev Cell. 2018;45:33–52.e12. doi: 10.1016/j.devcel.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 97.Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5:28. doi: 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, Scheidmann MC, Donato C, Scherrer R, Singer J, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566:553–557. doi: 10.1038/s41586-019-0915-y. [DOI] [PubMed] [Google Scholar]
- 99.Correia AL. Locally sourced: site-specific immune barriers to metastasis. Nat Rev Immunol. 2023;23:522–538. doi: 10.1038/s41577-023-00836-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Deng H, Kan A, Lyu N, He M, Huang X, Qiao S, et al. Tumor-derived lactate inhibit the efficacy of lenvatinib through regulating PD-L1 expression on neutrophil in hepatocellular carcinoma. J Immunother Cancer. 2021;9 [DOI] [PMC free article] [PubMed]
- 102.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sukumar M, Roychoudhuri R, Restifo NP. Nutrient competition: a new Axis of tumor immunosuppression. Cell. 2015;162:1206–1208. doi: 10.1016/j.cell.2015.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bohn T, Rapp S, Luther N, Klein M, Bruehl TJ, Kojima N, Aranda Lopez P, Hahlbrock J, Muth S, Endo S, et al. Tumor immunoevasion via acidosis-dependent induction of regulatory tumor-associated macrophages. Nat Immunol. 2018;19:1319–1329. doi: 10.1038/s41590-018-0226-8. [DOI] [PubMed] [Google Scholar]
- 105.Corn KC, Windham MA, Rafat M. Lipids in the tumor microenvironment: from cancer progression to treatment. Prog Lipid Res. 2020;80:101055. doi: 10.1016/j.plipres.2020.101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martin-Perez M, Urdiroz-Urricelqui U, Bigas C, Benitah SA. The role of lipids in cancer progression and metastasis. Cell Metab. 2022;34:1675–1699. doi: 10.1016/j.cmet.2022.09.023. [DOI] [PubMed] [Google Scholar]
- 107.Kwantwi LB, Wang S, Zhang W, Peng W, Cai Z, Sheng Y, Xiao H, Wang X, Wu Q. Tumor-associated neutrophils activated by tumor-derived CCL20 (C-C motif chemokine ligand 20) promote T cell immunosuppression via programmed death-ligand 1 (PD-L1) in breast cancer. Bioengineered. 2021;12:6996–7006. doi: 10.1080/21655979.2021.1977102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shang A, Wang W, Gu C, Chen C, Zeng B, Yang Y, Ji P, Sun J, Wu J, Lu W, et al. Long non-coding RNA HOTTIP enhances IL-6 expression to potentiate immune escape of ovarian cancer cells by upregulating the expression of PD-L1 in neutrophils. J Exp Clin Cancer Res. 2019;38:411. doi: 10.1186/s13046-019-1394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang TT, Zhao YL, Peng LS, Chen N, Chen W, Lv YP, Mao FY, Zhang JY, Cheng P, Teng YS, et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut. 2017;66:1900–1911. doi: 10.1136/gutjnl-2016-313075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kasic T, Colombo P, Soldani C, Wang CM, Miranda E, Roncalli M, Bronte V, Viola A. Modulation of human T-cell functions by reactive nitrogen species. Eur J Immunol. 2011;41:1843–1849. doi: 10.1002/eji.201040868. [DOI] [PubMed] [Google Scholar]
- 111.De Sanctis F, Lamolinara A, Boschi F, Musiu C, Caligola S, Trovato R, et al. Interrupting the nitrosative stress fuels tumor-specific cytotoxic T lymphocytes in pancreatic cancer. J Immunother Cancer. 2022;10 [DOI] [PMC free article] [PubMed]
- 112.Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstegen NJM, Ciampricotti M, Hawinkels L, Jonkers J, de Visser KE. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takakura K, Ito Z, Suka M, Kanai T, Matsumoto Y, Odahara S, Matsudaira H, Haruki K, Fujiwara Y, Saito R, et al. Comprehensive assessment of the prognosis of pancreatic cancer: peripheral blood neutrophil-lymphocyte ratio and immunohistochemical analyses of the tumour site. Scand J Gastroenterol. 2016;51:610–617. doi: 10.3109/00365521.2015.1121515. [DOI] [PubMed] [Google Scholar]
- 114.Zhao JJ, Pan K, Wang W, Chen JG, Wu YH, Lv L, Li JJ, Chen YB, Wang DD, Pan QZ, et al. The prognostic value of tumor-infiltrating neutrophils in gastric adenocarcinoma after resection. PLoS One. 2012;7:e33655. doi: 10.1371/journal.pone.0033655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sconocchia G, Zlobec I, Lugli A, Calabrese D, Iezzi G, Karamitopoulou E, Patsouris ES, Peros G, Horcic M, Tornillo L, et al. Tumor infiltration by FcγRIII (CD16)+ myeloid cells is associated with improved survival in patients with colorectal carcinoma. Int J Cancer. 2011;128:2663–2672. doi: 10.1002/ijc.25609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ballesteros I, Rubio-Ponce A, Genua M, Lusito E, Kwok I, Fernández-Calvo G, Khoyratty TE, van Grinsven E, González-Hernández S, Nicolás-Ávila J, et al. Co-option of Neutrophil Fates by Tissue Environments. Cell. 2020;183:1282–1297.e1218. doi: 10.1016/j.cell.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 117.Bodac A, Meylan E. Neutrophil metabolism in the cancer context. Semin Immunol. 2021;57:101583. doi: 10.1016/j.smim.2021.101583. [DOI] [PubMed] [Google Scholar]
- 118.DePeaux K, Delgoffe GM. Metabolic barriers to cancer immunotherapy. Nat Rev Immunol. 2021;21:785–797. doi: 10.1038/s41577-021-00541-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Harmon C, O'Farrelly C, Robinson MW. The immune consequences of lactate in the tumor microenvironment. Adv Exp Med Biol. 2020;1259:113–124. doi: 10.1007/978-3-030-43093-1_7. [DOI] [PubMed] [Google Scholar]
- 120.Hsieh CC, Hung CH, Chiang M, Tsai YC, He JT. Hepatic stellate cells enhance liver Cancer progression by inducing myeloid-derived suppressor cells through Interleukin-6 signaling. Int J Mol Sci. 2019;20 [DOI] [PMC free article] [PubMed]
- 121.Wellenstein MD, Coffelt SB, Duits DEM, van Miltenburg MH, Slagter M, de Rink I, Henneman L, Kas SM, Prekovic S, Hau C-S, et al. Loss of p53 triggers WNT-dependent systemic inflammation to drive breast cancer metastasis. Nature. 2019;572:538–542. doi: 10.1038/s41586-019-1450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bertocchi A, Carloni S, Ravenda PS, Bertalot G, Spadoni I, Lo Cascio A, Gandini S, Lizier M, Braga D, Asnicar F, et al. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell. 2021;39:708–724.e711. doi: 10.1016/j.ccell.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 123.Wu Y, Zhao Q, Peng C, Sun L, Li XF, Kuang DM. Neutrophils promote motility of cancer cells via a hyaluronan-mediated TLR4/PI3K activation loop. J Pathol. 2011;225:438–447. doi: 10.1002/path.2947. [DOI] [PubMed] [Google Scholar]
- 124.He K, Liu X, Hoffman RD, Shi RZ, Lv GY, Gao JL. G-CSF/GM-CSF-induced hematopoietic dysregulation in the progression of solid tumors. FEBS Open Bio. 2022;12:1268–1285. doi: 10.1002/2211-5463.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shang A, Gu C, Wang W, Wang X, Sun J, Zeng B, Chen C, Chang W, Ping Y, Ji P, et al. Exosomal circPACRGL promotes progression of colorectal cancer via the miR-142-3p/miR-506-3p- TGF-β1 axis. Mol Cancer. 2020;19:117. doi: 10.1186/s12943-020-01235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kanamaru R, Ohzawa H, Miyato H, Matsumoto S, Haruta H, Kurashina K, Saito S, Hosoya Y, Yamaguchi H, Yamashita H, et al. Low density neutrophils (LDN) in postoperative abdominal cavity assist the peritoneal recurrence through the production of neutrophil extracellular traps (NETs) Sci Rep. 2018;8:632. doi: 10.1038/s41598-017-19091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nolan E, Bridgeman VL, Ombrato L, Karoutas A, Rabas N, Sewnath CAN, Vasquez M, Rodrigues FS, Horswell S, Faull P, et al. Radiation exposure elicits a neutrophil-driven response in healthy lung tissue that enhances metastatic colonization. Nat Can. 2022;3:173–187. doi: 10.1038/s43018-022-00336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bellomo G, Rainer C, Quaranta V, Astuti Y, Raymant M, Boyd E, Stafferton R, Campbell F, Ghaneh P, Halloran CM, et al. Chemotherapy-induced infiltration of neutrophils promotes pancreatic cancer metastasis via Gas6/AXL signalling axis. Gut. 2022;71:2284–2299. doi: 10.1136/gutjnl-2021-325272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moorlag S, Rodriguez-Rosales YA, Gillard J, Fanucchi S, Theunissen K, Novakovic B, de Bont CM, Negishi Y, Fok ET, Kalafati L, et al. BCG vaccination induces long-term functional reprogramming of human neutrophils. Cell Rep. 2020;33:108387. doi: 10.1016/j.celrep.2020.108387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Subudhi SK, Siddiqui BA, Aparicio AM, Yadav SS, Basu S, Chen H, et al. Combined CTLA-4 and PD-L1 blockade in patients with chemotherapy-naïve metastatic castration-resistant prostate cancer is associated with increased myeloid and neutrophil immune subsets in the bone microenvironment. J Immunother Cancer. 2021;9 [DOI] [PMC free article] [PubMed]
- 131.Ren J, He J, Zhang H, Xia Y, Hu Z, Loughran P, Billiar T, Huang H, Tsung A. Platelet TLR4-ERK5 Axis facilitates NET-mediated capturing of circulating tumor cells and distant metastasis after surgical stress. Cancer Res. 2021;81:2373–2385. doi: 10.1158/0008-5472.CAN-20-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cirovic B, de Bree LCJ, Groh L, Blok BA, Chan J, van der Velden W, Bremmers MEJ, van Crevel R, Händler K, Picelli S, et al. BCG Vaccination in Humans Elicits Trained Immunity via the Hematopoietic Progenitor Compartment. Cell Host Microbe. 2020;28:322–334.e325. doi: 10.1016/j.chom.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Han J, Gu X, Li Y, Wu Q. Mechanisms of BCG in the treatment of bladder cancer-current understanding and the prospect. Biomed Pharmacother. 2020;129:110393. doi: 10.1016/j.biopha.2020.110393. [DOI] [PubMed] [Google Scholar]
- 134.Fulop T, Larbi A, Douziech N, Fortin C, Guérard KP, Lesur O, Khalil A, Dupuis G. Signal transduction and functional changes in neutrophils with aging. Aging Cell. 2004;3:217–226. doi: 10.1111/j.1474-9728.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 135.Blazkova J, Gupta S, Liu Y, Gaudilliere B, Ganio EA, Bolen CR, Saar-Dover R, Fragiadakis GK, Angst MS, Hasni S, et al. Multicenter systems analysis of human blood reveals immature neutrophils in males and during pregnancy. J Immunol. 2017;198:2479–2488. doi: 10.4049/jimmunol.1601855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Casanova-Acebes M, Nicolás-Ávila JA, Li JL, García-Silva S, Balachander A, Rubio-Ponce A, Weiss LA, Adrover JM, Burrows K, A-González N, et al. Neutrophils instruct homeostatic and pathological states in naive tissues. J Exp Med. 2018;215:2778–2795. doi: 10.1084/jem.20181468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McDowell SAC, Luo RBE, Arabzadeh A, Doré S, Bennett NC, Breton V, Karimi E, Rezanejad M, Yang RR, Lach KD, et al. Neutrophil oxidative stress mediates obesity-associated vascular dysfunction and metastatic transmigration. Nat Can. 2021;2:545–562. doi: 10.1038/s43018-021-00194-9. [DOI] [PubMed] [Google Scholar]
- 138.Tyagi A, Wu SY, Sharma S, Wu K, Zhao D, Deshpande R, Singh R, Li W, Topaloglu U, Ruiz J, Watabe K. Exosomal miR-4466 from nicotine-activated neutrophils promotes tumor cell stemness and metabolism in lung cancer metastasis. Oncogene. 2022;41:3079–3092. doi: 10.1038/s41388-022-02322-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hwang S, Ren T, Gao B. Obesity and binge alcohol intake are deadly combination to induce steatohepatitis: a model of high-fat diet and binge ethanol intake. Clin Mol Hepatol. 2020;26:586–594. doi: 10.3350/cmh.2020.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Solana R, Pawelec G, Tarazona R: Aging and innate immunity. Immunity 2006, 24:491–494. [DOI] [PubMed]
- 141.Köstlin-Gille N, Gille C. Myeloid-derived suppressor cells in pregnancy and the neonatal period. Front Immunol. 2020;11:584712. doi: 10.3389/fimmu.2020.584712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Daver N, Vyas P, Chao M, Xing G, Renard C, Ramsingh G, Sallman DA, Wei AH. A phase 3, randomized, open-label study evaluating the safety and efficacy of Magrolimab in combination with Azacitidine in previously untreated patients with TP53-mutant acute myeloid leukemia. Blood. 2021;138:3426. doi: 10.1182/blood-2021-145208. [DOI] [Google Scholar]
- 143.Mehta A, Harb W, Xu C, Meng Y, Lee L, Yuan V, Wang Z, Song P, Shen JH, Gopal AK. Lemzoparlimab, a differentiated anti-CD47 antibody in combination with rituximab in relapsed and refractory non-Hodgkin's lymphoma: initial clinical results. Blood. 2021;138:3542. doi: 10.1182/blood-2021-150606. [DOI] [Google Scholar]
- 144.Strati P, Hawkes E, Ghosh N, Tuscano JM, Chu Q, Anderson MA, Patel A, Burgess MR, Hege K, Chhagan S, et al. Interim results from the first clinical study of CC-95251, an anti-signal regulatory protein-alpha (SIRPα) antibody, in combination with rituximab in patients with relapsed and/or refractory non-Hodgkin lymphoma (R/R NHL) Blood. 2021;138:2493. doi: 10.1182/blood-2021-147292. [DOI] [Google Scholar]
- 145.Delord J-P, Kotecki N, Marabelle A, Vinceneux A, Korakis I, Jungels C, Champiat S, Huhn RD, Poirier N, Costantini D, et al. A phase 1 study evaluating BI 765063, a first in class selective myeloid Sirpa inhibitor, as stand-alone and in combination with BI 754091, a programmed Death-1 (PD-1) inhibitor, in patients with advanced solid Tumours. Blood. 2019;134:1040. doi: 10.1182/blood-2019-124876. [DOI] [Google Scholar]
- 146.Shah MA, Yoshino T, Tebbutt NC, Grothey A, Tabernero J, Xu RH, Cervantes A, Oh SC, Yamaguchi K, Fakih M, et al. Napabucasin plus FOLFIRI in patients with previously treated metastatic colorectal Cancer: results from the open-label, randomized phase III CanStem303C study. Clin Colorectal Cancer. 2023;22:100–110. doi: 10.1016/j.clcc.2022.11.002. [DOI] [PubMed] [Google Scholar]
- 147.Yamazaki T, Gunderson AJ, Gilchrist M, Whiteford M, Kiely MX, Hayman A, O'Brien D, Ahmad R, Manchio JV, Fox N, et al. Galunisertib plus neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: a single-arm, phase 2 trial. Lancet Oncol. 2022;23:1189–1200. doi: 10.1016/S1470-2045(22)00446-6. [DOI] [PubMed] [Google Scholar]
- 148.O'Reilly EM, Golan T, Ikeda M, Milella M, Taieb J, Wainberg ZA, et al. Phase III study (daNIS-2) of the anti-TGF-ss monoclonal antibody (mAb) NIS793 with nab-paclitaxel/gemcitabine (NG) versus NG alone in patients (pts) with first-line metastatic pancreatic ductal adenocarcinoma (mPDAC). J Clin Oncol. 2022;40
- 149.Daley W, Shao Z, Zhang Q, Li H, Ye C, Wang S, Zhang L, Zhang G, Chen J. Abstract P5–16-14: A randomized, multicenter phase III study of once-per-cycle administration of efbemalenograstim alfa (F-627), a novel long-acting dimeric rhG-CSF, for prophylaxis of chemotherapy-induced neutropenia in patients with breast cancer. Cancer Res. 2022;82:P5–16-14-P15–16-14. doi: 10.1158/1538-7445.SABCS21-P5-16-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Weber JS, Muramatsu T, Hamid O, Mehnert J, Hodi FS, Krishnarajapet S, Malatyali S, Buchbinder E, Goldberg J, Sullivan R, et al. 1040O phase II trial of ipilimumab, nivolumab and tocilizumab for unresectable metastatic melanoma. Ann Oncol. 2021;32:S869. doi: 10.1016/j.annonc.2021.08.1425. [DOI] [Google Scholar]
- 151.Brighton TA, Khot A, Harrison SJ, Ghez D, Weiss BM, Kirsch A, Magen H, Gironella M, Oriol A, Streetly M, et al. Randomized, double-blind, placebo-controlled, multicenter study of Siltuximab in high-risk smoldering multiple myeloma. Clin Cancer Res. 2019;25:3772–3775. doi: 10.1158/1078-0432.CCR-18-3470. [DOI] [PubMed] [Google Scholar]
- 152.Stillie R, Farooq SM, Gordon JR, Stadnyk AW. The functional significance behind expressing two IL-8 receptor types on PMN. J Leukoc Biol. 2009;86:529–543. doi: 10.1189/jlb.0208125. [DOI] [PubMed] [Google Scholar]
- 153.Bilusic M, Heery CR, Collins JM, Donahue RN, Palena C, Madan RA, Karzai F, Marté JL, Strauss J, Gatti-Mays ME, et al. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J Immunother Cancer. 2019;7:240. doi: 10.1186/s40425-019-0706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Corrales L, Ajona D, Rafail S, Lasarte JJ, Riezu-Boj JI, Lambris JD, Rouzaut A, Pajares MJ, Montuenga LM, Pio R. Anaphylatoxin C5a creates a favorable microenvironment for lung cancer progression. J Immunol. 2012;189:4674–4683. doi: 10.4049/jimmunol.1201654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Behrens LM, van den Berg TK, van Egmond M. Targeting the CD47-SIRPα Innate Immune Checkpoint to Potentiate Antibody Therapy in Cancer by Neutrophils. Cancers. 2022;14 [DOI] [PMC free article] [PubMed]
- 156.Tsimberidou AM, Vining DJ, Arora SP, de Achaval S, Larson J, Cartwright C, Avritscher R, Alibhai I, Kaseb AO. Phase 1 trial evaluating TTI-101, a first-in-class, orally bioavailable, small molecule, inhibitor of STAT3, in patients with advanced solid tumors. J Clin Oncol. 2023;41:3018–3018. doi: 10.1200/JCO.2023.41.16_suppl.3018. [DOI] [Google Scholar]
- 157.Nishina T, Fujita T, Yoshizuka N, Sugibayashi K, Murayama K, Kuboki Y. Safety, tolerability, pharmacokinetics and preliminary antitumour activity of an antisense oligonucleotide targeting STAT3 (danvatirsen) as monotherapy and in combination with durvalumab in Japanese patients with advanced solid malignancies: a phase 1 study. BMJ Open. 2022;12:e055718. doi: 10.1136/bmjopen-2021-055718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yoo C, Javle MM, Verdaguer Mata H, de Braud F, Trojan J, Raoul JL, Kim JW, Ueno M, Lee CK, Hijioka S, et al. Phase 2 trial of bintrafusp alfa as second-line therapy for patients with locally advanced/metastatic biliary tract cancers. Hepatology. 2023;78:758–770. doi: 10.1097/HEP.0000000000000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Blayney DW, Schwartzberg L. Chemotherapy-induced neutropenia and emerging agents for prevention and treatment: a review. Cancer Treat Rev. 2022;109:102427. doi: 10.1016/j.ctrv.2022.102427. [DOI] [PubMed] [Google Scholar]
- 160.Blair HA. Efbemalenograstim Alfa: First Approval. Drugs. 2023;83:1125–1130. doi: 10.1007/s40265-023-01911-7. [DOI] [PubMed] [Google Scholar]
- 161.Zhou Y, Medik YB, Patel B, Zamler DB, Chen S, Chapman T, et al. Intestinal toxicity to CTLA-4 blockade driven by IL-6 and myeloid infiltration. J Exp Med. 2023;220 [DOI] [PMC free article] [PubMed]
- 162.Zhang Y, Guoqiang L, Sun M, Lu X. Targeting and exploitation of tumor-associated neutrophils to enhance immunotherapy and drug delivery for cancer treatment. Cancer Biol Med. 2020;17:32–43. doi: 10.20892/j.issn.2095-3941.2019.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 164.Sody S, Uddin M, Grüneboom A, Görgens A, Giebel B, Gunzer M, Brandau S. Distinct Spatio-temporal dynamics of tumor-associated neutrophils in small tumor lesions. Front Immunol. 2019;10:1419. doi: 10.3389/fimmu.2019.01419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pollenus E, Malengier-Devlies B, Vandermosten L, Pham TT, Mitera T, Possemiers H, Boon L, Opdenakker G, Matthys P, Van den Steen PE. Limitations of neutrophil depletion by anti-Ly6G antibodies in two heterogenic immunological models. Immunol Lett. 2019;212:30–36. doi: 10.1016/j.imlet.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 166.Boivin G, Faget J, Ancey PB, Gkasti A, Mussard J, Engblom C, Pfirschke C, Contat C, Pascual J, Vazquez J, et al. Durable and controlled depletion of neutrophils in mice. Nat Commun. 2020;11:2762. doi: 10.1038/s41467-020-16596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Brown ZJ, Heinrich B, Greten TF. Mouse models of hepatocellular carcinoma: an overview and highlights for immunotherapy research. Nat Rev Gastroenterol Hepatol. 2018;15:536–554. doi: 10.1038/s41575-018-0033-6. [DOI] [PubMed] [Google Scholar]
- 168.Morton JJ, Bird G, Refaeli Y, Jimeno A. Humanized mouse xenograft models: narrowing the tumor-microenvironment gap. Cancer Res. 2016;76:6153–6158. doi: 10.1158/0008-5472.CAN-16-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Quail DF, Amulic B, Aziz M, Barnes BJ, Eruslanov E, Fridlender ZG, Goodridge HS, Granot Z, Hidalgo A, Huttenlocher A, et al. Neutrophil phenotypes and functions in cancer: a consensus statement. J Exp Med. 2022;219:e20220011. doi: 10.1084/jem.20220011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Perez C, Botta C, Zabaleta A, Puig N, Cedena MT, Goicoechea I, Alameda D, San José-Eneriz E, Merino J, Rodríguez-Otero P, et al. Immunogenomic identification and characterization of granulocytic myeloid-derived suppressor cells in multiple myeloma. Blood. 2020;136:199–209. doi: 10.1182/blood.2019004537. [DOI] [PubMed] [Google Scholar]
- 171.Cruz MA, Bohinc D, Andraska EA, Alvikas J, Raghunathan S, Masters NA, van Kleef ND, Bane KL, Hart K, Medrow K, et al. Nanomedicine platform for targeting activated neutrophils and neutrophil-platelet complexes using an α(1)-antitrypsin-derived peptide motif. Nat Nanotechnol. 2022;17:1004–1014. doi: 10.1038/s41565-022-01161-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Linde IL, Prestwood TR, Qiu J, Pilarowski G, Linde MH, Zhang X, Shen L, Reticker-Flynn NE, Chiu DK, Sheu LY, et al. Neutrophil-activating therapy for the treatment of cancer. Cancer Cell. 2023;41:356–372.e310. doi: 10.1016/j.ccell.2023.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wisdom AJ, Hong CS, Lin AJ, Xiang Y, Cooper DE, Zhang J, Xu ES, Kuo HC, Mowery YM, Carpenter DJ, et al. Neutrophils promote tumor resistance to radiation therapy. Proc Natl Acad Sci USA. 2019;116:18584–18589. doi: 10.1073/pnas.1901562116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jin C, Ma J, Ramachandran M, Yu D, Essand M. CAR T cells expressing a bacterial virulence factor trigger potent bystander antitumour responses in solid cancers. Nat Biomed Eng. 2022;6:830–841. doi: 10.1038/s41551-022-00875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chang Y, Syahirah R, Wang X, Jin G, Torregrosa-Allen S, Elzey BD, Hummel SN, Wang T, Li C, Lian X, et al. Engineering chimeric antigen receptor neutrophils from human pluripotent stem cells for targeted cancer immunotherapy. Cell Rep. 2022;40:111128. doi: 10.1016/j.celrep.2022.111128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Subhan MA, Torchilin VP. Neutrophils as an emerging therapeutic target and tool for cancer therapy. Life Sci. 2021;285:119952. doi: 10.1016/j.lfs.2021.119952. [DOI] [PubMed] [Google Scholar]
- 177.Chu D, Dong X, Shi X, Zhang C, Wang Z. Neutrophil-based drug delivery systems. Adv Mater. 2018;30:e1706245. doi: 10.1002/adma.201706245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xue J, Zhao Z, Zhang L, Xue L, Shen S, Wen Y, Wei Z, Wang L, Kong L, Sun H, et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol. 2017;12:692–700. doi: 10.1038/nnano.2017.54. [DOI] [PubMed] [Google Scholar]
- 179.Kang T, Zhu Q, Wei D, Feng J, Yao J, Jiang T, Song Q, Wei X, Chen H, Gao X, Chen J. Nanoparticles coated with neutrophil membranes can effectively treat Cancer metastasis. ACS Nano. 2017;11:1397–1411. doi: 10.1021/acsnano.6b06477. [DOI] [PubMed] [Google Scholar]