Abstract
The major barrier to eradicating HIV-infection is the generation of tissue-associated quiescent long-lasting viral reservoirs refractory to therapy. Upon interruption of antiretroviral therapy (ART), it can be reactivated. Within the brain, microglia/macrophages and a small population of astrocytes are infected with HIV. However, the role of astrocytes as a potential viral reservoir is becoming more recognized due to improved detection and quantification of HIV viral reservoirs.
In this report, we examined the infectivity of human primary astrocytes in vivo and in vitro, and their capacity to maintain HIV infection, become latently infected, be reactivated, and transfer new HIV virions into neighboring cells. Analysis of human brain tissue sections obtained from HIV-infected individuals under effective and prolonged ART indicates that a small population of astrocytes has integrated HIV-DNA. In vitro experiments using HIV-infected human primary astrocyte cultures confirmed a low percentage of astrocytes had integrated HIV-DNA, with poor to undetectable replication. Even in the absence of ART, long-term culture results in latency that could be transiently reactivated with histone deacetylase inhibitor, TNF-α, or methamphetamine. Reactivation resulted in poor viral production but efficient cell-to-cell viral transfer into cells that support high viral replication. Together, our data provide a new understanding of astrocytes’ role as viral reservoirs within the CNS.
Keywords: Reservoirs, NeuroHIV, Latency, Reactivation, Anti-retroviral, Cure, Astrocytes
Introduction
A key feature of HIV-infection that has made HIV cure elusive is the early generation of latent viral reservoirs in different tissues, including the central nervous system (CNS). By definition, a viral reservoir refers to long-lived HIV-infected cells, most of which can be quiescent and mainly localized in specific anatomical compartments, where the replication-competent virus can persist for a longer period of time compared to the main pool of actively replicating virus (Dufour et al. 2020, De Scheerder et al. 2019, Rose et al. 2018). Apart from well-established and accepted memory CD4+ T lymphocytes and macrophages/microglia, one cell type that still engenders debate as a potential viral reservoir are astrocytes (Li et al. 2020b, Edara et al. 2020, Prevedel et al. 2019, Ko et al. 2019, Barat et al. 2018, Li et al. 2015, Churchill et al. 2015, Eugenin & Berman 2013, Kramer-Hammerle et al. 2005, Gorry et al. 2003).
Astrocytes make up about ~60% of all brain cells (Vasile et al. 2017, von Bartheld et al. 2016). Studies from several groups have shown the detection of multiple viral components (viral DNA, mRNA, or proteins) in astrocytes using postmortem brains obtained from HIV-infected individuals. Moreover, most of these studies were reported in the early days of the introduction of antiretroviral therapy (ART) (Al-Harti et al. 2018, Wiley 2003, Takahashi et al. 1996, Nath et al. 1995, Tornatore et al. 1994, Churchill et al. 2006b). In vitro experiments indicate that human astrocytes are CD4 negative and viral production is low to undetectable (Li et al. 2020b, Zhuang et al. 2014, Liu et al. 2004, Sabri et al. 1999, Speck et al. 1999, Hao et al. 1997). Some groups have also suggested that HIV components are not due to active astrocyte infection; instead, these viral materials correspond to internalized cell debris from dying neighboring infected cells (Chauhan & Khandkar 2015, Chauhan et al. 2014b, Ko et al. 2019). Other studies show that the transfection of astrocytic cell lines with CD4 and CXCR4 or CCR5 resulted in productive HIV infection, suggesting that astrocytes may have an entry restriction (Li et al. 2020b, Al-Harti et al. 2018, Li et al. 2016, Luo & He 2015, Chauhan et al. 2014b, Gray et al. 2014, Liu et al. 2004). Further, it has been demonstrated that the low infectivity observed in astrocytes is due to the alternative mechanism of entry, including mannose receptors and endocytosis; however, the nature of the restriction, in addition to lack of CD4 expression, is unknown (Lutgen et al. 2020, Barat et al. 2018, Russell et al. 2017, Li et al. 2007, Lopez-Herrera et al. 2005, Gorry et al. 2003, Liu et al. 2004, Schweighardt & Atwood 2001). Overall, a poor entry process, lack of productive infection, low to non-expression of CD4, and controversial infectivity data reduced the enthusiasm for exploring astrocytes’ role in neuroHIV further and as potential brain viral reservoirs. Therefore, the debate on the susceptibility of astrocytes to HIV infection persists, and their capacity to become viral reservoirs is unclear but is the main objective of this report.
Recently, our group demonstrated that several viral components, including HIV-DNA, viral mRNA, and proteins, are present in a limited manner in a small population of astrocytes within human brain tissues, SIV infected macaques, and in human primary astrocyte cultures using laboratory-adapted and primary virus isolates (Okafo et al. 2020a, Lutgen et al. 2020, Real et al. 2020, Prevedel et al. 2019). Consistently and irrespective of the biological model used, we identified that HIV infects only a small number of astrocytes in vivo and in vitro (~1 to 3% depending on the model used). Despite the low number of infected astrocytes, HIV-infected astrocytes use connexin and pannexin containing channels to spread toxicity to surrounding uninfected cells in a replication-independent manner (Lutgen et al. 2020, Gajardo-Gomez et al. 2020, Prevedel et al. 2019, Malik et al. 2017, Berman et al. 2016, Castellano & Eugenin 2014, Orellana et al. 2014, Eugenin & Berman 2013, Eugenin et al. 2011, Eugenin & Berman 2007, Okafo et al. 2020a, Valdebenito et al. 2020). One limitation of these studies is that there are based on detecting a single viral component, particularly the expression of HIV-p24 protein or other viral protein or viral genetic material. However, we recently developed an imaging-based method to detect several viral components and cellular markers simultaneously in the same cell and assay. Using our technology, we have detected rare viral reservoirs in multiple tissues obtained from HIV-infected patients under long-term ART, including HIV-infected lymphoid and myeloid cells within urethral tissue and bone marrow (Fernando Real 2020, Prevedel et al. 2019, Ganor et al. 2019). Furthermore, we recently demonstrated that a small population of HIV-infected astrocytes could cause HIV egress from the brain into the periphery, highlighting astrocytes’ potential role as a viral reservoir (Lutgen et al. 2020).
In the current report, we describe and characterize HIV-infection of human astrocytes in vivo using brain tissues from HIV-infected individuals under long-term ART. We also demonstrate the mechanism of HIV-entry into human primary astrocytes in vitro. We equally show that astrocytes’ long-term infection leads to viral latency in a few infected cells, even in the absence of ART. Reactivating agents such as SAHA, TNF-α, and methamphetamine transiently induce viral reactivation without significant changes in the overall numbers of astrocytes with integrated HIV-DNA but with a significant increase in viral mRNA and viral protein expression. Moreover, despite the reactivation of viral replication in vitro, budding and infectivity of the released virions were poor if a cell-free virus infection route was used. In contrast, latently infected astrocytes subjected to viral reactivation and allowed to establish cell-to-cell contact with uninfected cells resulted in efficient viral transfer and spread of infection to surrounding uninfected astrocytes, CD4+ T cells, macrophages, or CEM-SS cells, suggesting that a plasma membrane to plasma membrane contact is essential for an efficient viral transfer. Overall, astrocytes need to be considered an unusual viral reservoir compared to lymphoid and myeloid reservoirs due to their relatively low abundance in the CNS, latency properties in the absence of ART, reactivation mechanism, viral transfer efficiency, and their localization in an immune privilege organ, the brain.
Materials and Methods
Reagents.
The reagents NH4Cl, bafilomycin-A1, methamphetamine, chloroquine, chlorpromazine was purchase from Sigma (St. Louis, MO). HIV isolates, soluble CD4 (sCD4), TAK779, AMD3100, and CEM-SS cells were from the NIH AIDS Research and Reference Reagent Program (Germantown, MD). DMEM and RPMI media, Penicillin/Streptomycin (Pen/Strep), and dyes were obtained from Thermo-Fisher (Waltham, MA). HIV-p24 alphaLISA was obtained from Perkin-Elmer (Waltham, MA). Antibodies to HIV-p24 were obtained from Genetex (Irvine, CA), and all other primary antibodies were purchased from Sigma (St. Louis, MO), Santa Cruz (Santa Cruz, CA) or Abcam (Cambridge, MA). Purified mouse IgG2B and IgG1 myeloma protein were from Cappel Pharmaceuticals, Inc. The Alu repeats and HIV-Nef DNA were synthesized by PNA Bio (Thousand Oaks, CA). The RNAscope probes were purchased from Biotechne/ Advanced Cell Diagnostic (ACD bio, Newark, CA). All experiments were performed under Rutgers University, University of Texas Medical Branch, and the NIH regulations.
Primary Human Astrocytes
Human brain cortical tissue (fetal, 12-20 weeks, and adult without trauma or borderline tumor) was obtained as part of a research protocol approved by Rutgers University and the University of Texas Medical Branch. All tissues were unidentified, and no patient information was collected. Approximately 90% of the population is African American or Hispanic, 7% are Caucasian, and 3% are Asian. The cultures were prepared as described (Eugenin & Berman 2003, King et al. 2010, Eugenin et al. 2007).
Isolation of human PBMCs, CD4+ T lymphocytes, and Macrophages.
Peripheral blood mononuclear cells (PBMCs) were isolated by differential centrifugation using a Ficoll gradient (GE Healthcare, Piscataway, NJ) and according to the procedure described by the manufacturer. PBMCs were isolated within 4 h of blood draw. All described analyses were performed on freshly isolated leukopaks from the New York Blood Bank. CD4+ T cells were isolated from PBMCs with a CD4+ enrichment kit (Stem-cell Technologies) according to the manufacturer’s protocol. Monocyte-derived macrophages (MDMs) were prepared from PBMCs by directly plating the cells on tissue culture dishes with 10 ng/mL macrophage colony-stimulating factor (M-CSF) (Miltenyl Biotec, San Diego, CA) in RPMI 1640 with 10% FBS, 5% human AB serum, 1% Pen/Strep, and 10 mM HEPES for 7 days.
CEM-SS cells.
CEM-SS cells were cultivated in RPMI 1640 with 10% fetal bovine serum, 10mM HEPES, and 1% Pen/Strep as described for the NIH AIDS repository. CEM-SS cells are human T-cell lymphoma, and there are negative for any virus tested, including human retroviruses (https://www.hivreagentprogram.org/Catalog/cellBanks/ARP-776.aspx).
HIV infection and replication.
Primary human astrocytes were infected with viral input (20-80 ng/mL per 1x106 cells) of HIVADA, HIVBAL, HIVJR-CSF, HIVJR-FL, or HIV98UG021, using a described protocol (Eugenin et al. 2011, Eugenin & Berman 2007, Eugenin et al. 2003). Monocyte-derived macrophages, CEM-SS, and CD4+ T cells were inoculated with media from HIV-infected astrocyte cultures. Briefly, astrocytes were exposed to the following viruses HIVADA, HIVBAL, HIVJR-CSF, HIVJR-FL, or HIV98UG021 for 24 hours. Then, extensively washed with 1X PBS to eliminate any unbound virus. We collected the medium every 7 days until 288 days post-infection (dpi) to quantify HIV p24 using alphaLISA from Perkin-Elmer (Waltham, MA) according to the manufacturer’s instructions. Also, to assure that our cells are infected, we performed HIV DNA staining and Alu-PCR to detect HIV-infection and integration.
HIV-1 Viral Entry
To examine the mechanism(s) of viral entry into human primary astrocytes, specific blocking peptides and compounds to CD4 (50 mg/mL, sCD4, blocking peptide), CXCR4 (100 ng/mL, AMD3100), or CCR5 (100 ng/mL, TAK779) were added to the cultured cells to examine if HIV-infection of astrocytes was CD4 or chemokine receptor-dependent. The following inhibitors and concentrations were used to determine if HIV infection of astrocytes is endocytosis-dependent: ammonium chloride (NH4CL, 50 mmol/L), bafilomycin-A1 (50 nmol/L), and chloroquine (100 μg/ml). Chlorpromazine (50 μmol/L) was used specifically to examine clathrin-mediated endocytosis. Astrocyte cultures were pre-treated with: sCD4 peptide, blocking compounds against CXCR4 or CCR5 for 60 min, or the inhibitors mentioned above/chemical blockers for 15 minutes before HIV infection. One to two hours post-infection, astrocytes were washed extensively and placed in fresh media. The astrocyte supernatants were then assessed for HIV p24 antigen using an alphaLISA protocol described previously after different time points.
Immunofluorescence.
HIV-infected and non-infected human brain tissues were mounted onto glass coverslips for subsequent analysis (frontal cortex and subcortical areas were analyzed). Both were either fixed and permeabilized with cold 70% ethanol for 20 min at −20°C or fixed in 4% paraformaldehyde and permeabilized with 0.01% Triton-X for 2 minutes at room temperature. Cells and tissues were washed three times with PBS at room temperature. First, we performed the HIV-gag pol mRNA staining following the manufacturer’s protocol from ACD/Bio RNAscope 2.5 HD Detection-RED. HIV-gag pol mRNA probe was added to the samples and incubated for 30 min at 42°C and then 50 min at 55°C. Slides were immersed in 1X TBS for 20 sec. The reaction was stopped as soon we observed red color. Samples were washed three times with miliQ water. Slides were kept at 4°C in the dark to minimize the decay of the fluorescence. Later the samples were incubated in DNA probes directed to HIV-Nef DNA conjugated to Alexa488 (Alexa488-GCAGCTTCCTCATTGATGG, PNA Bio), Alu conjugated to Cy5 repeats (Cy5-GCCTCCCAAAGTGCTGGGATTACAG, PNA Bio) and, antibodies to GFAP (an astrocyte marker, 1:500 dilution) overnight at 4°C. Samples were washed three times with PBS at room temperature and incubated with the proper secondary antibodies to detect GFAP (Thermo-Fisher, Carlsbad, CA) for 1 h at room temperature, followed by five washes with 1X PBS. We mounted the samples using an anti-fade reagent with DAPI. This staining method enables us to detect few copies (even a single copy of HIV integrated DNA) of integrated DNA within the host DNA (Alu repeat positive) and cytoplasmic viral mRNA (RNA Scope positive, ACD, cat. no. 322360). For this report, we did not quantify copy numbers; we only quantified negative versus positive cells. Therefore, we regard the samples as positive if we observe colocalization between DAPI, Alu repeats, and the HIV-DNA probe in the infected cells or tissues as recently described (Real et al. 2020, Lutgen et al. 2020, Prevedel et al. 2019, Ganor et al. 2019). Total cell number corresponded to the numbers of nuclei positive for DAPI and Alu-repeat, and cells with integrated HIV-DNA corresponded to the triple staining, DAPI, Alu-repeats, and Nef probe with a Pearson’s index of 0.85 or higher. All calculations were performed using NIS elements and Image J software as we described (Real et al. 2020, Lutgen et al. 2020, Prevedel et al. 2019, Ganor et al. 2019). Overall, 1x105-7 total cells were counted per tissue, but the main focus was on GFAP positive cells. Each tissue was analyzed in different areas with a minimum of 12 sections per area.
Statistical analysis.
Data were analyzed using Origin 8.1 (Northampton, MA, US). For single comparisons, Student’s t-test was performed. For multiple comparisons, mean differences were tested by non-parametric Kruskal–Wallis analysis and adjusted using the Bonferroni–Dunn correction. p values of < 0.05 were considered significant.
Results
In situ detection of HIV-infected astrocytes in human brains obtained from HIV-infected individuals.
Our laboratory recently developed a protocol for the simultaneous in situ detection of HIV integrated DNA, viral mRNA, and viral proteins in the same cell or assay, reducing the ambiguous interpretation of other methods (Okafo et al. 2020a, Lutgen et al. 2020, Real et al. 2020, Ganor et al. 2019, Prevedel et al. 2019). Previous studies from our laboratory have already shown we can detect rare myeloid and lymphoid viral reservoirs in human tissues obtained from HIV-infected individuals under long-term ART (Prevedel et al. 2019, Ganor et al. 2019). Therefore, we decided to examine whether HIV-infected astrocytes are present in human brain tissues of HIV-infected individuals in vivo.
Well-characterized human brain tissue samples were obtained from the National NeuroAIDS Tissue Consortium (NNTC, www.nntc.org) and the neurobiobank (www.neurobiobank.nih.gov) from HIV-infected and uninfected donors, respectively. There were eight HIV seropositive and seven uninfected brain samples used in this study. There were no significant difference between the age of the HIV negative cohort and HIV positive cohort (HIV positive, mean = 58 ± 7 years; HIV negative, mean = 56 ± 14 years; Table 1) and gender (HIV positive = 38% female and 62% male; HIV negative = 43% female and 57% male; Table 1). The HIV positive cohort had an average of 13 ± 3 years living with HIV, an average CD4 count of 310 ± 77 cells/μL, and mean plasma HIV RNA of 160 ± 227 log copies/mL (range from undetectable to 500 log copies/mL). Approximately 50% of participants had an undetectable viral load (see Table 1). Also, we included archival cases of AIDS and encephalitis as a positive control. To assure an unbiased assessment, all samples were received and analyzed blindly. To assure proper scientific rigor, we requested the clinical and HIV status information only after all data had been acquired.
Table 1:
Patient Information
| Patient number | HIV status | Age | Gender | ART Current ARVs (2 y of death) | CD4 counts (cells/mm) | Viral Load (copies/ml) | Years with HIV |
|---|---|---|---|---|---|---|---|
| 1 | + | 49 | M | 3TC, ABC, KTA, RTV, TFV, TZV, ZDV | 273 | N.D. | 10 |
| 2 | + | 64 | M | DRV, EPZ, RTV | 299 | 40 | 18 |
| 3 | + | 60 | F | ATR | 398 | N.D. | 11 |
| 4 | + | 59 | M | 3TC, CBV, D4T, NVP, TRU | 158 | 50 | 13 |
| 5 | + | 71 | F | 3TC, ATV, KTA, RTV, TRU, ZDV | 403 | N.D. | 16 |
| 6 | + | 52 | M | RTV, SQV, TRU | 317 | 50 | 10 |
| 7 | + | 52 | M | RTV, SQV, TRU | 317 | N.D. | 12 |
| 8 | + | 54 | F | EFV, TRU | 317 | 500 | 14 |
| 9 | +HIVE | 5 | F | None | 7 | 750,000 | 5 |
| 10 | +HIVE | 36 | M | None | 12 | 1,250,000 | 3 |
| 11 | +HIVE | 45 | M | None | 24 | 850,000 | 2 |
| 12 | +HIVE | 48 | F | None | 6 | 980,000 | 5 |
| 13 | +HIVE | 51 | M | None | 17 | 1,800,000 | 4 |
| 14 | − | 42 | F | N.A. | N.A. | N.A. | N.A. |
| 15 | − | 69 | M | N.A. | N.A. | N.A. | N.A. |
| 16 | − | 38 | M | N.A. | N.A. | N.A. | N.A. |
| 17 | − | 51 | F | N.A. | N.A. | N.A. | N.A. |
| 18 | − | 54 | M | N.A. | N.A. | N.A. | N.A. |
| 19 | − | 66 | M | N.A. | N.A. | N.A. | N.A. |
| 20 | − | 74 | F | N.A. | N.A. | N.A. | N.A. |
Notes: M: male; F: Female; N.A.: not applicable; N.D.: not detected.
Anti-retrovirals: 3TC, lamivudine (Epivir); ABC, abacavir (Ziagen); ATV, atazanavir (Reyataz); CBV, zidovudine + lamivudine (Combivir); D4T, stavudine (Zerit; EFV, efavirenz (Sustiva); EPZ, ziagen + epivir (Epzicom); KTA, LPV/RTV; lopinavir/ritonavir (Kaletra); NVP, nevirapine (Viramune); RTV, ritonavir (Norvir); FTV, SQV2 or FTV; saquinavir-sgc (Fortovase); SQV, saquinavir (Invirase); TFV, PMPA; tenofivir DF (Viread); DRV, TMC-114, darunavir (Prezista); TRU, emtricitabine + tenofovir (Truvada); TZV, AZT + 3TC + abacavir; zidovudine + lamivudine + abacavir (Trizivir); VCV, vicriviroc; ZDV, AZT, zidovudine (Retrovir).
A small population of astrocytes contains HIV-integrated DNA within the brain of HIV-infected individuals under effective ART.
To identify astrocytes with integrated HIV-DNA, we examined the colocalization between DAPI (nuclear staining), HIV-DNA (probe for Nef), and Alu repeats (Host DNA), as well as astrocyte cell marker, GFAP in the frontal cortex and subcortical areas. To classify a GFAP positive cell as HIV-infected, a colocalization of DAPI, Alu-repeats, and HIV-Nef DNA is required for identification as well as surrounding GFAP staining. We set the nuclear threshold for Pearson’s coefficient index of 0.85 or higher to be considered as a positive HIV-infected astrocyte. Analysis of uninfected cortical brain tissues shows no unspecific staining for the HIV-DNA probe (Fig. 1A) for all the tissues analyzed (see details in Table 1). However, in all the HIV cases analyzed (see Table 1 for details), the HIV-DNA signal colocalized with DAPI and Alu-repeats, indicating a nuclear localization or HIV integration (Fig. 1B). Quantification of the HIV-DNA positive cells that are GFAP positive corresponded to 4.81±3.38 % of all astrocytes (Fig. 1C, HIVAstro). Overall, the numbers of HIV-infected astrocytes observed in the tissues analyzed was lower than those from HIV encephalitis (HIVE) tissues from the pre-ART era (n=5 different individuals), where multiple studies showed that the proportion of HIV infected astrocytes was estimated to be as high as approximately 18% of the GFAP positive cells (Churchill et al. 2009, Ohagen et al. 1999, Nath et al. 1995, Tornatore et al. 1994, Tornatore et al. 1991, Price et al. 1988, Wiley et al. 1986). Our data using human brain tissues from the early stages of ART or pre-ART indicates that 13.83±3.05 % of astrocytes (GFAP positive cells) shows HIV-DNA signal in the nucleus (Fig. 1C, HIVPreAstro) from a total of 37.53±14.65 % of the total numbers of cells with HIV-integrated DNA, including macrophage and microglia (Iba-1 positive cells) (Fig. 1C, HIVPreT, total). Overall, our data indicate two main points: first, the numbers of astrocytes with HIV integrated DNA have decreased in the current long-term ART era as compared to the pre-ART era (up to 18%). Second, GFAP positive astrocytes are a significant CNS viral reservoir in the current ART era. We did not detect any HIV-DNA in GFAP positive cells in the cytoplasm, indicating that the HIV-DNA signal is nuclear in close interaction with host DNA (DAPI and Alu repeats), discarding the possibility of unspecific engouement or endocytosis of dying cells. Overall, a small but stable population of astrocytes within the brain of HIV-infected individuals under long term ART had HIV-integrated DNA supporting that astrocytes are viral reservoirs.
Figure 1:

In situ detection and quantification of HIV-infected astrocytes in human brains obtained from HIV infected individuals under long term ART. (A) In situ staining of uninfected human brain tissues for DAPI (nucleus, blue), HIV-DNA (probe for Nef protein, green), Alu repeats (host DNA, red), and GFAP (to identify astrocytes, white staining, Cy5). DAPI staining colocalized with Alu-repeats, and no HIV-DNA staining was observed. GFAP staining was mostly cytoplasmatic and lineup blood vessels as expected (control). (B) A cluster of HIV-infected astrocytes can be observed with DAPI and Alu-repeat staining that colocalized with HIV-Nef staining (HIV). A higher GFAP expression was identified in all the cases in association with HIV-DNA signal in the nucleus. However, most of the brain was negative for HIV-DNA. (C) Quantify the numbers of GFAP positive cells with HIV-DNA signal colocalized with DAPI and Alu-repeats in brains from individuals in ART for 10-18 years versus the total numbers of cells in large pieces of the frontal cortex and subcortical areas of the brain, 5.45 cm2. The total number of cells with HIV-DNA in the nucleus (HIVPreT) was quantified in samples pre-ART or early ART era. A total of 5 different cases were analyzed (see table 1). HIV-astrocytes (HIV-Astro) corresponds to an important reservoir in the current ART era (n=8, different individuals with 10-12 different large sections per tissue analyzed). No HIV-DNA detection was found in uninfected tissues (n=7, different individuals with 10-12 different large sections per tissue analyzed). In contrast, tissues from the pre-and -early stages of the ART era indicates that the percentage of HIV-astrocytes (HIV-Astro) corresponded to a higher number for both cell types. Also, the % of cells with integrated HIV-DNA was high, reaching up to ~40% of the cells due to the large damage and cell fusion observed. Thus, long term ART reduces the size of the CNS viral reservoirs. (*p≤0.0012 as compared to uninfected conditions. #p≤0.00013 as compared to HIVAstro conditions).
HIV entry and replication in human primary astrocytes have unusual entry and transient replication.
Several groups have reported entry restrictions in astrocytes (Li et al. 2020b, Al-Harti et al. 2018, Li et al. 2016, Luo & He 2015, Chauhan et al. 2014b, Gray et al. 2014, Liu et al. 2004). A critical difference between lymphoid and myeloid cells is the lack of CD4 in astrocytes. Therefore, to characterize the mechanism of HIV entry into human primary astrocytes, we pretreated human primary astrocytes as follows: 60 minutes prior to HIV exposure, we incubated the cells with soluble CD4 (sCD4, 50 μg/ml, to compete for the binding to gp120), α-CCR5 (TAK-779, 100 ng/ml, to block the binding of the virus to CCR5), or α-CXCR4 (AMD3100, 100 ng/ml, to block the binding of the virus to CXCR4). Also, 15 minutes prior to HIV-infection astrocyte cultures were pre-treated with the following agents: bafilomycin-A1 (Bafi, 50 nmol/L, to prevent endocytosis), chloroquine (CQ, 100 μg/ml, to prevent endocytosis), NH4Cl (50 mmol/L, to prevent endocytosis), or Chlorpromazine (Chlorpro, 50 μmol/L, to specifically perturb clathrin-mediated endocytosis) to prevent the use of these pathways for viral entry. Then, we exposed the astrocytes to 20-80ng/mL HIVADA for 6 h, washed away the unbound viruses, and determined HIV replication at different time points using both p24 AlphaLISA and by an automatic screening confocal system to quantify HIV-DNA in the nucleus (DAPI and Alu-repeat positive), as well as HIV-p24 protein staining. The main reason to use HIVADA is their well-described effects on CNS cells, including macrophages and other cell types (Carlson et al. 2004, Ghorpade et al. 1998). We observed that 3.59±2.86 % of the primary human astrocytes became positive for nuclear DNA after 7 days post-infection (Fig. 2A, HIVADA was set to 100% or 1). The preincubation with sCD4 did not alter the presence of HIV-DNA in the host DNA, indicating that HIV entry and subsequent replication is CD4 independent (Fig. 2A, sCD4+HIVADA). Preincubation of the human primary astrocyte cultures with blocking compounds against CCR5 or CXCR4 caused a significant decrease in HIV-DNA levels detected within the nucleus, suggesting that these chemokine receptors are important in viral entry into astrocytes (Fig. 2A, αCCR5+HIVADA or αCXCR4+HIVADA). Furthermore, to assesses the role of endocytosis, several agents that disrupt endocytosis, such as bafilomycin-A1, chloroquine, NH4Cl, or chlorpromazine, were used (Fig. 2A). The concentrations of these reagents used in this study are similar to the ones optimized and used to maximally inhibit the entry of avian leukosis virus (ALV-B) into cells, a virus that is dependent on endocytosis for infection (Diaz-Griffero et al. 2002, Moore et al. 1997). The lysosomotropic agents, NH4Cl (50 mmol/L) and chloroquine (100 μg/ml) are selectively accumulated in endocytic compartments and increase the endosomal pH. Bafilomycin-A1 (50 nmol/L) is a specific blocker of v-type H+-ATPase, and chlorpromazine (50 μmol/L) disrupts clathrin-mediated uptake. Further, HIV-infection of PBMCs was used as a negative control because HIV entry into leukocytes is not mediated by endocytosis but by CD4 receptor and either CCR5 or CXCR4 co-receptor mediated viral entry (Diaz-Griffero et al. 2002, Moore et al. 1997).
Figure 2:
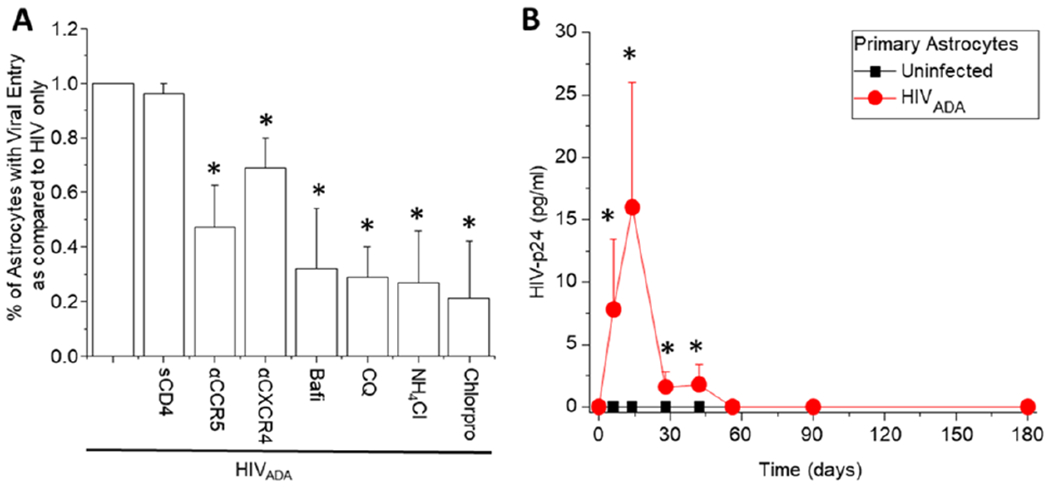
HIV-entry into human primary astrocytes is CD4 independent and chemokine receptor and endocytosis dependent. (A) Quantification of entry and infectivity of primary cultures in the presence and absence of soluble CD4 (sCD4, 50 ng/mL), blocking compounds to CCR5 and CXCR4 (αCCR5, TAK779, and αCXCR4, AMD3100, 100 ng/mL), and blocker for endocytosis (bafilomycin-A1, bafi, 50 nmol/L; chloroquine, CQ, 100 μg/mL; NH4CL, 50 mm/L, and chlorpromazine, Chlorpro, 50 μg/mL). Controls with the vehicle, DMSO, or media alone did not affect the % of astrocytes with viral entry. These data are included in Fig. 2A in the HIVADA data. Infectivity was evaluated by in situ staining for HIV-DNA after 7 days post-infection. (B) Long term time course of HIV-replication upon HIVADA infection. Media was collected at different time points, and HIV-p24 ELISA was performed (n=12 different astrocyte cultures from different individuals with three replicates. *p≤0.00015 compared to HIVADA conditions).
Overall, all blockers of endocytosis significantly decreased the numbers of astrocytes positive for HIV-DNA probe, indicating that endocytosis is required for HIV entry into human primary astrocytes (Fig. 2A, Bafi or CQ or NH4Cl or chlorpro+HIVADA, *p < 0.00015 compared to HIV-infection alone, n =12 different primary cultures with three replicates per experiment). None of these blockers reduced entry into human PBMCs (data not shown). We corroborated our data using Alu-PCR with positive integration, but the numbers or percentage of cells was unknown (data not shown). These data support the hypothesis that HIV entry into human primary astrocytes is CD4 receptor-independent but requires chemokine receptors and an active endocytic process.
HIV infection of human astrocytes results in a low and transient replication that upon long-term culture becomes silent even in the absence of ART.
Our data indicate that HIV-infection of astrocytes is limited but occurs in vivo and in vitro. To examine the degree of viral replication, we examined long-term HIV-p24 production in the media of astrocyte cultures. Published data from our laboratory, using human primary astrocytes, indicates that HIV-p24 production is low to undetectable until 28 days post-infection, the last time point assayed (Berman et al. 2016, Eugenin & Berman 2013, Eugenin & Berman 2007). However, a longer-term time course replication experiment beyond 28 days post-infection was not performed. To evaluate long term infection, we examined HIV replication mainly via HIV-p24 release into the media, up to 180 days post-infection using human primary astrocytes (Fig. 2B, line with red circles). Overall, we observed that HIV-p24 production and release into the supernatant after HIVADA infection was time-dependent. Early after HIV infection, HIV-p24 remained detectable until 60 days post-infection (Fig. 2B, HIVADA, line with circles). Since viral replication was low, all samples were concentrated 5-10 times using Amicon filters to detect HIV-p24 secretion. Surprisingly, after 60 days post-infection, viral replication becomes undetectable, suggesting viral silencing. No HIV-p24 detection was found in uninfected primary astrocyte cultures as expected (Fig. 2B, uninfected, line with black squares). Viral silencing was not associated with cell death or lack of cellular response because TUNEL staining was negative, and cells still produce significant amounts of GFAP and S100 (data not shown). Overall, astrocytes silence viral replication even in the absence of ART.
Long term cultures of HIV-infected astrocytes silence the virus, but viral production can be reactivated.
To expand our long-term HIV-replication experiments in human primary astrocyte cultures, we utilized different HIV isolates (molecular clones and primary HIV isolates) to examine any viral strain dependency on astrocyte infection and long-term replication (Fig. 3A). We examined long term viral replication with HIVADA (see Fig. 2B) but also with HIVBaL, HIVJR-CSF, HIVJRFL, and HIV98UG021 (laboratory-adapted and primary isolates). Overall, we detected similarly low numbers of astrocytes with HIV integrated DNA as previously seen with HIVADA infection, and the time course of HIV-p24 production was not different when we compared these molecular clones and HIV viral isolates replication to HIVADA replication (Fig. 3A). HIV-p24 remains undetected in uninfected cultures of primary astrocytes as expected (Fig. 3A, uninfected). Overall, independent of the viral isolate used, we did not observe any changes in the percentage of astrocytes containing integrated HIV-DNA, nor the long-term viral replication examined. We suggest that HIV infection occurs irrespective of the virus tropism, further showing that entry is not dependent on the canonical receptor-mediated entry as described in CD4+ T cells.
Figure 3:

Long term HIV infection of astrocytes is reactivable. (A) Long term infection time course, up to 120-150 days in the presence of HIVADA, HIVBaL, HIVJRCSF, HIVJRFL, and HIV98UG021. The time course in Fig. 2B was not viral strain-specific and laboratory-adapted, and primary isolates behave similarly after HIV-infection of primary cultures of astrocytes. (B) Viral reactivation (React, see arrows) after 120 days in culture with SAHA (1 μM), TNF-α (10 ng/mL), or Methamphetamine (1 μM). Upon addition of the reactivating agent, HIV-p24 in the supernatant was quantified by ELISA up to 296 days post-infection (*p≤0.0024 as compared to uninfected conditions, in Graph B, all curves were significant as compared to HIV infection alone except 216, 240, and 296 days post-infection with a p≤0.004). (C to J) correspond to a representative example of staining for Uninfected astrocytes (C-F) and HIV infected astrocytes (G-J). Denote the colocalization of the HIV-Nef DNA signal with DAPI and Alu-repeats. Bar: 15 μm. (K) Quantification of the total number of astrocytes with HIV integrated DNA (HIV-DNA) expressing viral mRNA (HIV mRNA) and HIV-p24 (HIV-p24) during long-term culture. (L) Quantification of the numbers of HIV-infected astrocytes after reactivation with SAHA/TNF-α/methamphetamine. All numbers were pooled together due to the low variability. At each time point, three coverslips were fixed and stained for HIV-DNA, mRNA, and p24. Denote the increase in viral mRNA and protein expression n=5-7 independent experiments from different donors with three coverslips per time point.
In addition, after 120-150 dpi, viral replication remains silent, and no HIV-p24 was observed even when we concentrated the media up to 20 times (Fig. 3A and B, red line). However, when we added latency-reversing agents (LRAs) such as SAHA (1 μM), TNF-α (10 ng/ml), or methamphetamine (1 μM) to these long term infected primary astrocyte cultures, 120-150 dpi, we observed a transient HIV reactivation as determined by HIV-p24 ELISA (Fig. 3B, React). The second addition of these LRAs after 230-240 dpi also induced similar transient reactivation of viral replication, suggesting the establishment of latency in these astrocytes (Fig. 3B). Interestingly, reactivation from latency induced by LRAs was transient, and HIV replication became silent again, even without ART. The concentrations of these reactivating compounds were selected based on studies about CNS infection as described (Gorska & Eugenin 2020, Okafo et al. 2017, Velasquez et al. 2016, Castellano et al. 2016, Patel et al. 2013, Eugenin & Berman 2007). Therefore, we conclude that HIV-infected astrocytes are long-term viral reservoirs that can be transiently reactivated to produce new virions.
HIV silencing in astrocytes is due to changes in viral transcription and translation.
To examine the nature of HIV-silencing in astrocytes, we use our imaging system to examine integrated HIV-DNA, mRNA by RNAscope, and HIV-p24 staining. The main goal was to perform a screening that combines integrated HIV-DNA in the host genome (HIV-DNA, as described before), mRNA viral expression using RNAscope (HIV mRNA), and HIV-p24 staining to quantify the percentage of cells for each viral component (Fig. 3C-L).
Quantification of the astrocytes containing HIV-DNA, mRNA, and HIV-p24 indicates that HIV-DNA integration was established early after HIV-infection, approximately 3-7 dpi, without significant changes in the levels of HIV-DNA in the cells afterward, as shown in Fig. 3G-J, even after extended culture times, viral silencing or reactivation. HIV-mRNA and HIV-p24 follow the pattern of viral replication described in Fig. 3A and B. Thus, changes in secreted HIV-p24 were not due to quantitative changes in HIV-DNA but mostly due to increased HIV transcription (mRNA and HIV-p24, Fig. 3K, and L). In addition, upon reactivation, with SAHA (1 μM), TNF-α (10 ng/ml), or methamphetamine (1 μM), both viral mRNA and protein were produced, without changes in the percentage of HIV-DNA positive cells (Fig. 3L). Overall, our data indicate that HIV-infection of astrocytes is different from other cell types like CD4+ T cells or macrophages because, in astrocytes, we observed HIV silencing without the necessity of ART, and it is highly reversible (active versus silencing).
HIV-infected astrocytes efficiently transfer the virus into other astrocytes, T cells, macrophages, or CEM-SS cells, supporting their role as a pivotal CNS reservoir.
We decided to use two different methods to determine if reactivating latently infected astrocytes after 180 dpi with methamphetamine treatment will lead to the transfer of HIV virions from infected astrocytes into neighboring cells that can support high viral replication. First, we examined a cell-free system, using the collected media from methamphetamine reactivated HIV latently infected astrocytes (168 days in the presence of meth, see Fig. 3B). The collected medium was used to infect primary astrocyte cultures, primary CD4+ T cells, macrophages, or CEM-SS cell line cultures to evaluate infection and subsequent viral replication as assessed by HIV-p24 production at 7, 14, and 21 days post-exposure. Second, we utilized a cell-to-cell contact model where astrocyte cultures were reactivated (168 days in the presence of meth, see Fig. 3B). Cells were washed to eliminate the surface or released virus. The cell was then exposed to uninfected cultures of primary astrocytes, primary CD4+ T cells, macrophages, or CEM-SS cells, but separated by an 8 μm filter to enable cell-to-cell communication but prevent the cell body of the cell from moving into another cell type.
In the cell-free experiment, the media collected from the methamphetamine reactivated latently infected astrocytes failed to induce any significant HIV-infection of uninfected primary astrocytes at all times analyzed; 7, 14, and 21 dpi (Fig. 4, soluble, Astro). The use of the same media from the methamphetamine reactivated latently infected astrocytes resulted in a low but significant infection of primary CD4+T lymphocytes (Fig. 4, soluble, T cells), macrophages (Fig. 4, Macro), and CEM-SS cell line (Fig. 4, CEM) as determined by HIV-p24 ELISA (*p≤0.007, n=4, as compared to control, uninfected conditions).
Figure 4:
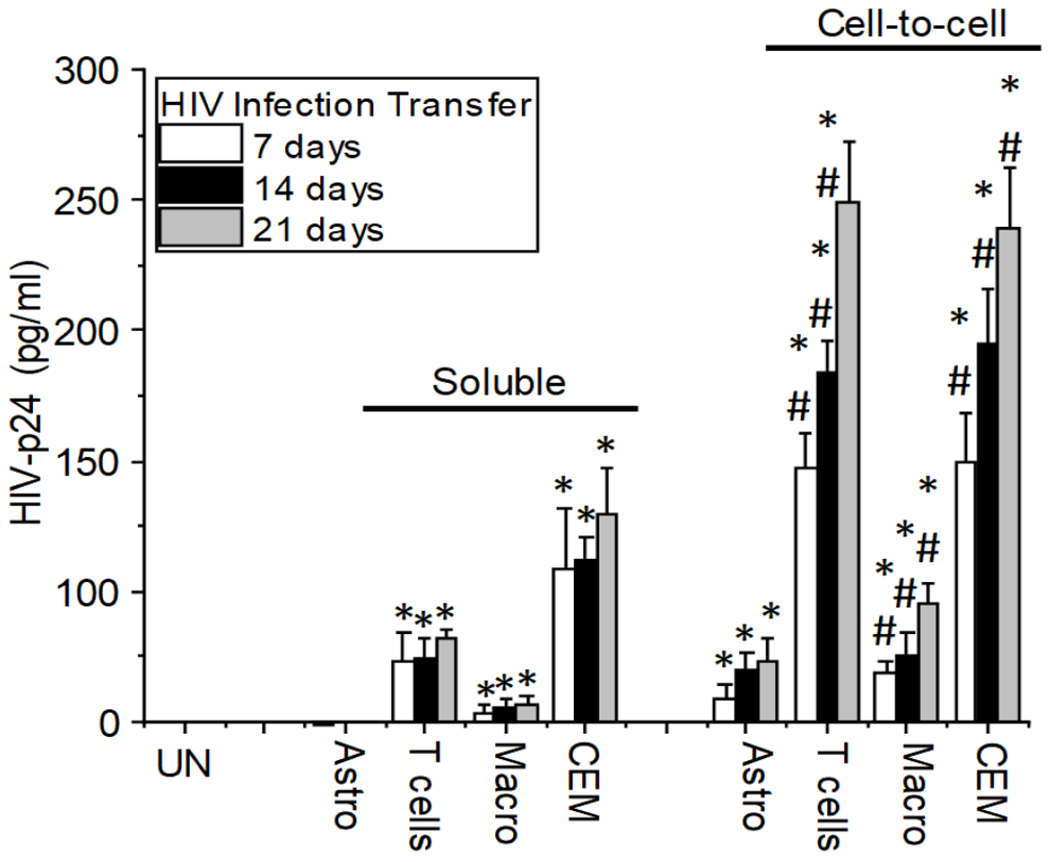
Cell-to-cell contact provides an effective mechanism of astrocyte-glial/myeloid/lymphoid viral infection spread. Upon infection and reactivation of the virus after 168 days in culture as described in Fig. 3B, Media from reactivated cultures with methamphetamine or uninfected cells (Un) was collected and added to uninfected cultures of human primary astrocytes (Astro), CD4 T lymphocytes (T cells), Macrophages (Macro), or CEM-SS cells (CEM) for 2 h, then cultures were washed and viral replication in the supernatant was quantified after 7, 14 and 21 days. However, if the cells could establish cell-to-cell contact through a filter to prevent the mixing of the uninfected and HIV-infected populations, it resulted in greater infection of all the cell types. (*p≤0.007, n=4, as compared to uninfected conditions. #p≤0.0031 as compared to soluble conditions).
In contrast, HIV infection and spread were more efficient in the cell-to-cell contact experiments conducted. In agreement with previous publications in T cells, cell-to-cell contacts enhanced viral transfer into neighboring uninfected cells up to 18,000-fold more efficient than a soluble virus (Chen et al. 2007, Sourisseau et al. 2007, Dimitrov et al. 1993). We observed that cell-to-cell contacts between cultures of methamphetamine reactivated HIV latently infected astrocytes and uninfected cells enhanced HIV transfer into uninfected cultures of primary astrocytes (Fig. 4, cell-to-cell, Astro), primary CD4+ T cells (Fig. 4, cell-to-cell, T cells), macrophages (cell-to-cell, macro), and CEM-SS cell line (Fig. 4, cell-to-cell, CEM). Interestingly, no significant viral transfer was observed without viral reactivation (data not shown), suggesting that reactivation is essential for an efficient viral transfer.
Overall, our data show several critical points; first, HIV-astrocytes are long-lasting viral reservoirs; second, these astrocytes can be reactivated to produce new virions; and lastly, cell-to-cell contact is required for efficient viral transfer from infected astrocytes to other target cells.
Discussion
This study demonstrated that HIV could infect a small proportion of astrocytes both in vivo and in vitro. We identified that viral replication is transient, becoming undetectable after some time, even in the absence of ART. However, viral replication can be transiently reactivated with LRA and methamphetamine treatment. We also show that HIV entry and infection into human primary astrocytes is CD4-independent, but requires to some extent, both chemokine receptors, CCR5 and CXCR4, and most importantly, endocytosis mediated. Furthermore, we demonstrated that the viral infection is transferred from latently HIV-infected astrocytes into uninfected astrocytes, primary CD4+ T cells, primary macrophages, and a T cell line by a cell-to-cell communication mechanism rather than a cell-free infection.
Our in-situ imaging system identified that human astrocytes are infected in vivo using brain tissues from HIV-infected individuals under ART. The overall numbers of infected astrocytes as compared to the total number of astrocytes within the brain is low (3.59±2.86 % of the total number of astrocytes within the brain), but significant, considering that the human brain has approximately 10-55 billion astrocytes (Andrade-Moraes et al. 2013, Azevedo et al. 2009); thus, the overall number of infected astrocytes is high even in the current ART era. Our imaging system requires the colocalization of several hosts and viral markers to detect integrated pro-viral DNA instead of circular DNA or “pieces or debris” of dying infected cells present in the cytoplasm, demonstrating that HIV-DNA is present inside the nucleus with extremely high sensitivity. We did not detect HIV-DNA in the cytoplasm as suggested by several groups discarding the notion that astrocytes engulfed extracellular material containing HIV-DNA, mRNA, or viral proteins (Barat et al. 2018, Churchill et al. 2006a, Fiala et al. 1996, Clarke et al. 2006). Overall, we observed that HIV-infected astrocytes were localized in clusters, and most of the brain was negative for HIV-DNA. The distribution of infected cells in the pre-ART era is significantly different from the current ART era, highly localized, as described in Fig. 1. In the pre-ART era or during the early stages of ART, the presence of HIV-infected cells was widespread and high.
One controversial hypothesis is whether HIV-infection of astrocytes is similar to CD4+ T cell lymphocytes, the major cell-type targeted by HIV infection. This report shows that astrocytes might have an alternative infection mechanism different from the canonical HIV entry, replication, restriction, silencing and reactivation, proliferation, survival, and lifespan. Since astrocytes are the most abundant cell type in the human brain (Vasile et al. 2017, von Bartheld et al. 2016), they can not be considered rare reservoirs due to the high number within the CNS. Several groups have shown that astrocytes do not express CD4 receptors; therefore, HIV-entry into human astrocytes has to be CD4-independent (Chauhan et al. 2014a, Li et al. 2015, Li et al. 2020a, Liu et al. 2004). Our data confirmed that human primary astrocytes become infected in a CD4-independent manner, but HIV co-receptors like CXCR4 and CCR5 can play a role in HIV entry astrocytes. Also, these co-receptors are probably not acting as the established fusion machinery seen in T cells; rather, the effects of CCR5 and CXCR4 observed in this study could be related to trafficking or recycling of these co-receptors through the endocytic pathway that allows for cellular uptake of these co-receptors and allows the virus to “hitch a ride.” In agreement with this hypothesis, our work shows that targeting endocytosis with multiple blockers significantly reduced HIV infection of astrocytes more than when we blocked CXCR4 or CCR5. Thereby supporting the critical role played by endocytosis in HIV entry of astrocytes. Currently, there is no definition of a subpopulation of astrocytes within the human brain; thus, we propose that the astrocytes susceptible to HIV infection could correspond to a particular subpopulation of cells with unique differentiation or localization.
As stated earlier, our in vitro experiments using human primary astrocytes indicates that HIV can infect a small population of astrocytes. Still, these long-term cell cultures result in a spontaneous silencing of the virus, even in the absence of ART. This observation, in itself, is intriguing and needs to be investigated further. In addition, as previously demonstrated, HIV-infection of astrocytes in this study occurs in several stages we have categorized; starting from stage 1 (1 to 7 dpi) early infection that results in HIV-entry and infection, but also bystander apoptosis of neighboring uninfected cells mediated via gap junctions and hemichannel dependent mechanisms; stage 2 (day 7 to 14 dpi), that correspond to proliferation and controlled bystander cytotoxicity; Lastly, stage 3, corresponded to constant inflammation and inter-organelle dysregulation including mitochondria-ER-Golgi and plasma membrane (Malik et al. 2017, Eugenin et al. 2011, Eugenin & Berman 2007). We are expanding our previous findings to include the recent observation in this report, our finding of longer time viral replication, and novel mechanisms of viral silencing and reactivation upon drug abuse and other latency reactivators. We propose that these long-term experiments provide a unique mechanism of extended survival, viral replication control, and reactivation unique even in the absence of ART. The silencing mechanisms are unknown, and it does not involve significant astrocyte apoptosis or significant inflammation but probably involves epigenetic changes that need to be addressed in future experiments.
A similar observation of viral control in the absence of ART has been observed in long-term nonprogress and elite controller patients that mostly correspond to differential LTR methylation preventing viral production and unknown mechanisms involving residual expression of viral proteins (Ding et al. 2019, Casado et al. 2018, Dos Santos et al. 2017, Weber et al. 2014, Palacios et al. 2012, Saksena et al. 2007). Future studies will need to address the mechanism behind viral silencing in the absence of ART. Despite the few cells infected, understanding how a cell type can control the virus opens multiple opportunities to target and eliminate viral reservoirs in the specific cell type, in this case, astrocytes.
The multiple additions of reactivating agents (SAHA, TNF-α, or methamphetamine) resulting in transient reactivation and afterward, re-silencing indicates that these viral reservoirs respond to external signals like (SAHA) and actual patient conditions such as inflammation and drug abuse like (TNF-α or methamphetamine). Normally, when LRA reactivate latently infected cells like memory CD4+ T cells, viral replication resumes. These cells become vulnerable to becoming recognized by the experienced immune system, ART (that only works in cells with active replication), and virus-mediated cytotoxicity (Edara et al. 2020, Marban et al. 2016), which is the basis for the “shock and kill” strategy. In contrast to these latently infected T cells, viral reactivation of latently infected astrocytes occurs transiently without apoptosis or cytotoxicity. Also, reactivation does not increase the viral reservoir pool’s size as the numbers of cells with integrated HIV DNA remain constant; only the transcription of HIV mRNA and translation of viral protein increased upon reactivation. These features are unique because astrocytes seem to control viral replication, prevent apoptosis, and have a relatively long half-life.
Our data indicate that HIV-infection of astrocyte is not only important because it could be a viral reservoir but can amplify apoptosis, inflammation, and cellular dysfunction into neighboring uninfected cells, including neurons, endothelial cells, and other uninfected astrocytes (Malik & Eugenin 2016, Eugenin et al. 2011, Orellana et al. 2014). We have previously demonstrated that HIV-infection but not replication regulates expression of Connexin-43 containing channels resulting in the maintenance of gap junctional communication between the few HIV-infected astrocytes and neighboring cells as well as the opening of hemichannels that contribute to the release of intracellular inflammatory mediators such as ATP and prostacyclin further contributing to the chronic inflammation observed in at least half of the HIV infected population (Velasquez et al. 2020). Also, in vivo studies from our laboratory indicate that infected astrocytes can amplify CNS damage up to 300-500 μm away from the infected astrocytes clusters (Malik & Eugenin 2019, Malik et al. 2017). However, we cannot find a particular association with a brain structure; instead, clusters of HIV-infected cells associated with CNS damage mediated by host proteins such as gap junctions, hemichannels, and tunneling nanotubes but also exosomes supporting local damage and inflammation. Furthermore, we identified that myeloid and astrocytic viral reservoirs could secrete viral proteins that spread cytotoxicity over longer distance due to the activities of both gap junctions and recently discovered tunneling nanotubes, in both in vivo and in vitro experiments, that can amplify toxicity in a targeted manner (Okafo et al. 2020b, Okafo et al. 2017, Eugenin et al. 2009). Thus, HIV-infected astrocytes correspond to a unique and highly flexible viral reservoir, and we propose it is also a major contributor to chronic inflammation within the brain.
A critical characteristic of the viral reservoir is re-seeding viral infection and potentially transferring new virions from the reservoir into cells that support high viral replication, such as CD4+ T lymphocytes (Churchill et al. 2016, Sengupta & Siliciano 2018). Our data indicate that, despite the low numbers of HIV-infected astrocytes, latently infected astrocytes can be induced to reactivate viral replication multiple times and can become silenced multiple times as well. Equally important is the observation that after reactivating these latently infected astrocytes, the media collected at the peak of the reactivation (Fig. 3B, soluble) poorly infects fresh uninfected primary astrocytes, primary CD4+ T cells lymphocytes, primary macrophages, and CEM-SS cell line in the cell-free system. However, when we established these latently infected astrocytes in co-cultures with the same uninfected cell types mentioned above in cell-to-cell contact cellular environments, the infectivity rate was significantly higher and more robust than the cell-free system. This observation clearly shows that cell-to-cell contact is essential to the effective spread of viral infection from astrocytes and further proves that the most efficient model of HIV-infection is via cell-to-cell contact. This is in agreement with multiple studies from our laboratory and others that have all demonstrated that HIV infection utilizes cell-to-cell communications to enhance the spread of the infection, suggesting that in patients under ART, where there are very limited virus replication and mostly inactivated viral reservoirs (Okafo et al. 2020b, Gajardo-Gomez et al. 2020, Hazleton et al. 2010, Hazleton et al. 2012, Orellana et al. 2013), after reactivation, the reservoirs do not just undergo only epigenetic and genetic changes that affect viral gene expression but also changes that affects cell-to-cell communication systems that could shield the produced virions from the immune system.
Our studies in astrocytes also can be extended to a recent publication that characterizes infection of renal cells that are CD4 negative and can spread infection and damage into neighboring cells, including macrophages (Hughes et al. 2020). The role of the virological synapses and transfer into immune cells probably is similar to the mechanism described in astrocytes (Hughes et al. 2020). Thus, instead of proposing that astrocytes and renal tubular epithelial cells/macrophage axis are a rare HIV-reservoirs; we propose that the development of new technologies to identify rare reservoirs will enable the discovery of new kinds of reservoirs. The discovery of these novel reservoirs could provide alternative explanations for the complex reactivation observed in patients that cannot be attributed to CD4 mediated mechanism (Rothenberger et al. 2015, Hill et al. 2016, Chaillon et al. 2020).
Overall, our study shows several novel features in the field of Neuro-HIV and viral reservoirs, including; first, human astrocytes are infected in vivo in the current ART era; second, our in-situ analysis of both in vivo and in vitro experiments indicates that HIV-DNA is seen in the nucleus of HIV infected astrocytes; third, HIV enters into human primary astrocytes, independent of CD4 receptor but with the help chemokine receptors (CCR5 and CXCR4), and mainly through endocytosis; fourth, long-term infection of astrocytes silences viral replication without ART; fifth, viral replication can be reactivated and silenced multiple times in astrocytes without cellular apoptosis or cytotoxicity; sixth, induced viral reactivation does not expand the numbers of astrocytes with integrated HIV DNA, but increased viral mRNA transcription and viral protein translation; and seventh, HIV-transfer into uninfected cells is more reliant on cell-to-cell contacts.
In conclusion, astrocytes are viral reservoirs with the unique characteristic of viral silencing, reactivation, cell-free/viral contact transfer, and the long term survival of these cells makes them more important for further research to identify the mechanisms of viral silencing, survival, and reactivation in comparison to other cell types like CD4+ T cells. We propose that HIV infection of astrocytes can provide unique mechanisms of viral-mediated survival and silencing that can be used to prevent the onset of Neuro-HIV and cure HIV.
Acknowledgments:
We would like to thank National NeuroAIDS Tissue Consortium (NNTC) for providing all human samples. The NNTC is made possible through funding from the NIMH and NINDS by the following grants: Manhattan HIV Brain Bank (MHBB): U24MH100931; Texas NeuroAIDS Research Center (TNRC): U24MH100930; National Neurological AIDS Bank (NNAB): U24MH100929; California NeuroAIDS Tissue Network (CNTN): U24MH100928; and Data Coordinating Center (DCC): U24MH100925.
Funding:
This work was funded by The National Institute of Mental Health grant, MH096625, the National Institute of Neurological Disorders and Stroke, NS105584, and UTMB internal and State of Texas funding (to E.A.E).
Footnotes
Ethics declarations: Competing for interest. The authors declare no competing interest.
References
- Al-Harti L, Joseph J and Nath A (2018) Astrocytes as an HIV CNS reservoir: highlights and reflections of an NIMH-sponsored symposium. Journal of neurovirology, 24, 665–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Moraes CH, Oliveira-Pinto AV, Castro-Fonseca E et al. (2013) Cell number changes in Alzheimer’s disease relate to dementia, not to plaques and tangles. Brain : a journal of neurology, 136, 3738–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R and Herculano-Houzel S (2009) Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. The Journal of comparative neurology, 513, 532–541. [DOI] [PubMed] [Google Scholar]
- Barat C, Proust A, Deshiere A, Leboeuf M, Drouin J and Tremblay MJ (2018) Astrocytes sustain long-term productive HIV-1 infection without establishment of reactivable viral latency. Glia, 66, 1363–1381. [DOI] [PubMed] [Google Scholar]
- Berman JW, Carvallo L, Buckner CM, Luers A, Prevedel L, Bennett MV and Eugenin EA (2016) HIV-tat alters Connexin43 expression and trafficking in human astrocytes: role in NeuroAIDS. Journal of neuroinflammation, 13, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson KA, Ciborowski P, Schellpeper CN, Biskup TM, Shen RF, Luo X, Destache CJ and Gendelman HE (2004) Proteomic fingerprinting of HIV-1-infected human monocyte-derived macrophages: a preliminary report. Journal of neuroimmunology, 147, 35–42. [DOI] [PubMed] [Google Scholar]
- Casado C, Marrero-Hernandez S, Marquez-Arce D et al. (2018) Viral Characteristics Associated with the Clinical Nonprogressor Phenotype Are Inherited by Viruses from a Cluster of HIV-1 Elite Controllers. MBio, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano P and Eugenin EA (2014) Regulation of gap junction channels by infectious agents and inflammation in the CNS. Frontiers in cellular neuroscience, 8, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano P, Nwagbo C, Martinez LR and Eugenin EA (2016) Methamphetamine compromises gap junctional communication in astrocytes and neurons. Journal of neurochemistry, 137, 561–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaillon A, Gianella S, Dellicour S et al. (2020) HIV persists throughout deep tissues with repopulation from multiple anatomical sources. The Journal of clinical investigation, 130, 1699–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A and Khandkar M (2015) Endocytosis of human immunodeficiency virus 1 (HIV-1) in astrocytes: a fiery path to its destination. Microbial pathogenesis, 78, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A, Mehla R, Vijayakumar TS and Handy I (2014a) Endocytosis-mediated HIV-1 entry and its significance in the elusive behavior of the virus in astrocytes. Virology, 456-457, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A, Tikoo A, Patel J and Abdullah AM (2014b) HIV-1 endocytosis in astrocytes: a kiss of death or survival of the fittest? Neuroscience research, 88, 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Hubner W, Spinelli MA and Chen BK (2007) Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J Virol, 81, 12582–12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill MJ, Cowley DJ, Wesselingh SL, Gorry PR and Gray LR (2015) HIV-1 transcriptional regulation in the central nervous system and implications for HIV cure research. Journal of neurovirology, 21, 290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill MJ, Deeks SG, Margolis DM, Siliciano RF and Swanstrom R (2016) HIV reservoirs: what, where and how to target them. Nat Rev Microbiol, 14, 55–60. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Gorry PR, Cowley D et al. (2006a) Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J Neurovirol, 12, 146–152. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Gorry PR, Cowley D et al. (2006b) Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. Journal of neurovirology, 12, 146–152. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ and Gorry PR (2009) Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Annals of neurology, 66, 253–258. [DOI] [PubMed] [Google Scholar]
- Clarke JN, Lake JA, Burrell CJ, Wesselingh SL, Gorry PR and Li P (2006) Novel pathway of human immunodeficiency virus type 1 uptake and release in astrocytes. Virology, 348, 141–155. [DOI] [PubMed] [Google Scholar]
- De Scheerder MA, Vrancken B, Dellicour S et al. (2019) HIV Rebound Is Predominantly Fueled by Genetically Identical Viral Expansions from Diverse Reservoirs. Cell host & microbe, 26, 347–358 e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Griffero F, Hoschander SA and Brojatsch J (2002) Endocytosis is a critical step in entry of subgroup B avian leukosis viruses. J Virol, 76, 12866–12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov DS, Willey RL, Sato H, Chang LJ, Blumenthal R and Martin MA (1993) Quantitation of human immunodeficiency virus type 1 infection kinetics. J Virol, 67, 2182–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Ma L, Zhao J, Xie Y, Zhou J, Li X and Cen S (2019) An integrative genomic analysis of transcriptional profiles identifies characteristic genes and patterns in HIV-infected long-term non-progressors and elite controllers. Journal of translational medicine, 17, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos JS, de Almeida SM, Ferreira GS, Bordignon J, Maia Teixeira SL, Martins Lima AC and Raboni SM (2017) Host Factor Predictors in Long-term Nonprogressors HIV-1 Infected with Distinct Viral Clades. Current HIV research, 15, 440–447. [DOI] [PubMed] [Google Scholar]
- Dufour C, Gantner P, Fromentin R and Chomont N (2020) The multifaceted nature of HIV latency. The Journal of clinical investigation, 130, 3381–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edara VV, Ghorpade A and Borgmann K (2020) Insights into the Gene Expression Profiles of Active and Restricted Red/Green-HIV(+) Human Astrocytes: Implications for Shock or Lock Therapies in the Brain. Journal of virology, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA and Berman JW (2003) Chemokine-dependent mechanisms of leukocyte trafficking across a model of the blood-brain barrier. Methods, 29, 351–361. [DOI] [PubMed] [Google Scholar]
- Eugenin EA and Berman JW (2007) Gap junctions mediate human immunodeficiency virus-bystander killing in astrocytes. J Neurosci, 27, 12844–12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA and Berman JW (2013) Cytochrome C dysregulation induced by HIV infection of astrocytes results in bystander apoptosis of uninfected astrocytes by an IP3 and calcium-dependent mechanism. J Neurochem, 127, 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Clements JE, Zink MC and Berman JW (2011) Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism. J Neurosci, 31, 9456–9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, D’Aversa TG, Lopez L, Calderon TM and Berman JW (2003) MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem, 85, 1299–1311. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Gaskill PJ and Berman JW (2009) Tunneling nanotubes (TNT): A potential mechanism for intercellular HIV trafficking. Commun Integr Biol, 2, 243–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, King JE, Nath A, Calderon TM, Zukin RS, Bennett MV and Berman JW (2007) HIV-tat induces formation of an LRP-PSD-95- NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Natl Acad Sci U S A, 104, 3438–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando Real CC, Sennepin Alexis, Arrigucci Riccardo, Zhu Aiwei, Sannier Geremy, Zheng Jonathan, Xu Lin, Massé Jean-Marc, Greffe Ségolène, Cazabat Michelle, Donoso Maribel, Delobel Pierre, Izopet Jacques, Eugenin Eliseo, Gennaro Maria Laura, Rouveix Elisabeth, Bordé Elisabeth Cramer, Bomsel Morgane (2020) Platelets from cART-suppressed HIV-infected patients with poor CD4+T cell recovery carry infectious HIV. Science Translational Medicine. [DOI] [PubMed] [Google Scholar]
- Fiala M, Rhodes RH, Shapshak P, Nagano I, Martinez-Maza O, Diagne A, Baldwin G and Graves M (1996) Regulation of HIV-1 infection in astrocytes: expression of Nef, TNF-alpha and IL-6 is enhanced in coculture of astrocytes with macrophages. Journal of neurovirology, 2, 158–166. [DOI] [PubMed] [Google Scholar]
- Gajardo-Gomez R, Santibanez CA, Labra VC, Gomez GI, Eugenin EA and Orellana JA (2020) HIV gp120 Protein Increases the Function of Connexin 43 Hemichannels and Pannexin-1 Channels in Astrocytes: Repercussions on Astroglial Function. Int J Mol Sci, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganor Y, Real F, Sennepin A et al. (2019) HIV-1 reservoirs in urethral macrophages of patients under suppressive antiretroviral therapy. Nature microbiology, 4, 633–644. [DOI] [PubMed] [Google Scholar]
- Ghorpade A, Nukuna A, Che M, Haggerty S, Persidsky Y, Carter E, Carhart L, Shafer L and Gendelman HE (1998) Human immunodeficiency virus neurotropism: an analysis of viral replication and cytopathicity for divergent strains in monocytes and microglia. Journal of virology, 72, 3340–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, Vesselingh SL and Purcell DF (2003) Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Current HIV research, 1, 463–473. [DOI] [PubMed] [Google Scholar]
- Gorska AM and Eugenin EA (2020) The Glutamate System as a Crucial Regulator of CNS Toxicity and Survival of HIV Reservoirs. Frontiers in cellular and infection microbiology, 10, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LR, Turville SG, Hitchen TL et al. (2014) HIV-1 entry and trans-infection of astrocytes involves CD81 vesicles. PLoS One, 9, e90620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao HN, Chiu FC, Losev L, Weidenheim KM, Rashbaum WK and Lyman WD (1997) HIV infection of human fetal neural cells is mediated by gp120 binding to a cell membrane-associated molecule that is not CD4 nor galactocerebroside. Brain research, 764, 149–157. [DOI] [PubMed] [Google Scholar]
- Hazleton JE, Berman JW and Eugenin EA (2010) Novel mechanisms of central nervous system damage in HIV infection. HIV AIDS (Auckl), 2, 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazleton JE, Berman JW and Eugenin EA (2012) Purinergic receptors are required for HIV-1 infection of primary human macrophages. J Immunol, 188, 4488–4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AL, Rosenbloom DI, Goldstein E, Hanhauser E, Kuritzkes DR, Siliciano RF and Henrich TJ (2016) Real-Time Predictions of Reservoir Size and Rebound Time during Antiretroviral Therapy Interruption Trials for HIV. PLoS pathogens, 12, e1005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K, Akturk G, Gnjatic S, Chen B, Klotman M and Blasi M (2020) Proliferation of HIV-infected renal epithelial cells following virus acquisition from infected macrophages. AIDS, 34, 1581–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JE, Eugenin EA, Hazleton JE, Morgello S and Berman JW (2010) Mechanisms of HIV-tat-induced phosphorylation of N-methyl-D-aspartate receptor subunit 2A in human primary neurons: implications for neuroAIDS pathogenesis. Am J Pathol, 176, 2819–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko A, Kang G, Hattler JB, Galadima HI, Zhang J, Li Q and Kim WK (2019) Macrophages but not Astrocytes Harbor HIV DNA in the Brains of HIV-1-Infected Aviremic Individuals on Suppressive Antiretroviral Therapy. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology, 14, 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE and Brack-Werner R (2005) Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus research, 111, 194–213. [DOI] [PubMed] [Google Scholar]
- Li GH, Anderson C, Jaeger L, Do T, Major EO and Nath A (2015) Cell-to-cell contact facilitates HIV transmission from lymphocytes to astrocytes via CXCR4. AIDS, 29, 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GH, Henderson L and Nath A (2016) Astrocytes as an HIV Reservoir: Mechanism of HIV Infection. Current HIV research, 14, 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GH, Maric D, Major EO and Nath A (2020a) Productive HIV infection in astrocytes can be established via a non-classical mechanism. AIDS (London, England). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GH, Maric D, Major EO and Nath A (2020b) Productive HIV infection in astrocytes can be established via a nonclassical mechanism. AIDS, 34, 963–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Bentsman G, Potash MJ and Volsky DJ (2007) Human immunodeficiency virus type 1 efficiently binds to human fetal astrocytes and induces neuroinflammatory responses independent of infection. BMC neuroscience, 8, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu H, Kim BO, Gattone VH, Li J, Nath A, Blum J and He JJ (2004) CD4-independent infection of astrocytes by human immunodeficiency virus type 1: requirement for the human mannose receptor. Journal of virology, 78, 4120–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Herrera A, Liu Y, Rugeles MT and He JJ (2005) HIV-1 interaction with human mannose receptor (hMR) induces production of matrix metalloproteinase 2 (MMP-2) through hMR-mediated intracellular signaling in astrocytes. Biochimica et biophysica acta, 1741, 55–64. [DOI] [PubMed] [Google Scholar]
- Luo X and He JJ (2015) Cell-cell contact viral transfer contributes to HIV infection and persistence in astrocytes. Journal of neurovirology, 21, 66–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgen V, Narasipura SD, Barbian HJ et al. (2020) HIV infects astrocytes in vivo and egresses from the brain to the periphery. PLoS pathogens, 16, e1008381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S and Eugenin EA (2016) Mechanisms of HIV Neuropathogenesis: Role of Cellular Communication Systems. Curr HIV Res, 14, 400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S and Eugenin EA (2019) Role of Connexin and Pannexin containing channels in HIV infection and NeuroAIDS. Neuroscience letters, 695, 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, Theis M and Eugenin EA (2017) Connexin43 Containing Gap Junction Channels Facilitate HIV Bystander Toxicity: Implications in NeuroHIV. Frontiers in molecular neuroscience, 10, 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marban C, Forouzanfar F, Ait-Ammar A, Fahmi F, El Mekdad H, Daouad F, Rohr O and Schwartz C (2016) Targeting the Brain Reservoirs: Toward an HIV Cure. Frontiers in immunology, 7, 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, Trkola A and Dragic T (1997) Co-receptors for HIV-1 entry. Curr Opin Immunol, 9, 551–562. [DOI] [PubMed] [Google Scholar]
- Nath A, Hartloper V, Furer M and Fowke KR (1995) Infection of human fetal astrocytes with HIV-1: viral tropism and the role of cell to cell contact in viral transmission. Journal of neuropathology and experimental neurology, 54, 320–330. [DOI] [PubMed] [Google Scholar]
- Ohagen A, Ghosh S, He J et al. (1999) Apoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type 1 isolates: evidence for a role of the envelope. Journal of virology, 73, 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okafo G, Prevedel L and Eugenin E (2017) Tunneling nanotubes (TNT) mediate long-range gap junctional communication: Implications for HIV cell to cell spread. Sci Rep, 7, 16660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okafo G, Valdebenito S, Donoso M, Luu R, Ajasin D, Prideaux B, Gorantla S and Eugenin EA (2020a) Role of Tunneling Nanotube-like Structures during the Early Events of HIV Infection: Novel Features of Tissue Compartmentalization and Mechanism of HIV Spread. Journal of immunology, 205, 2726–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okafo G, Valdebenito S, Donoso M, Luu R, Ajasin D, Prideaux B, Gorantla S and Eugenin EA (2020b) Role of Tunneling Nanotube-like Structures during the Early Events of HIV Infection: Novel Features of Tissue Compartmentalization and Mechanism of HIV Spread. Journal of immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, Saez JC, Bennett MV, Berman JW, Morgello S and Eugenin EA (2014) HIV increases the release of dickkopf-1 protein from human astrocytes by a Cx43 hemichannel-dependent mechanism. Journal of neurochemistry, 128, 752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, Velasquez S, Williams DW, Saez JC, Berman JW and Eugenin EA (2013) Pannexin1 hemichannels are critical for HIV infection of human primary CD4+ T lymphocytes. J Leukoc Biol, 94, 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios JA, Perez-Pinar T, Toro C, Sanz-Minguela B, Moreno V, Valencia E, Gomez-Hernando C and Rodes B (2012) Long-term nonprogressor and elite controller patients who control viremia have a higher percentage of methylation in their HIV-1 proviral promoters than aviremic patients receiving highly active antiretroviral therapy. Journal of virology, 86, 13081–13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D, Desai GM, Frases S, Cordero RJ, DeLeon-Rodriguez CM, Eugenin EA, Nosanchuk JD and Martinez LR (2013) Methamphetamine enhances Cryptococcus neoformans pulmonary infection and dissemination to the brain. MBio, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevedel L, Ruel N, Castellano P, Smith C, Malik S, Villeux C, Bomsel M, Morgello S and Eugenin EA (2019) Identification, Localization, and Quantification of HIV Reservoirs Using Microscopy. Current protocols in cell biology, 82, e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RW, Brew B, Sidtis J, Rosenblum M, Scheck AC and Cleary P (1988) The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science, 239, 586–592. [DOI] [PubMed] [Google Scholar]
- Real F, Capron C, Sennepin A et al. (2020) Platelets from HIV-infected individuals on antiretroviral drug therapy with poor CD4(+) T cell recovery can harbor replication-competent HIV despite viral suppression. Science Translational Medicine, 12. [DOI] [PubMed] [Google Scholar]
- Rose R, Nolan DJ, Maidji E, Stoddart CA, Singer EJ, Lamers SL and McGrath MS (2018) Eradication of HIV from Tissue Reservoirs: Challenges for the Cure. AIDS research and human retroviruses, 34, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberger MK, Keele BF, Wietgrefe SW et al. (2015) Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc Natl Acad Sci U S A, 112, E1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RA, Chojnacki J, Jones DM, Johnson E, Do T, Eggeling C, Padilla-Parra S and Sattentau QJ (2017) Astrocytes Resist HIV-1 Fusion but Engulf Infected Macrophage Material. Cell Rep, 18, 1473–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri F, Tresoldi E, Di Stefano M et al. (1999) Nonproductive human immunodeficiency virus type 1 infection of human fetal astrocytes: independence from CD4 and major chemokine receptors. Virology, 264, 370–384. [DOI] [PubMed] [Google Scholar]
- Saksena NK, Rodes B, Wang B and Soriano V (2007) Elite HIV controllers: myth or reality? AIDS reviews, 9, 195–207. [PubMed] [Google Scholar]
- Schweighardt B and Atwood WJ (2001) HIV type 1 infection of human astrocytes is restricted by inefficient viral entry. AIDS research and human retroviruses, 17, 1133–1142. [DOI] [PubMed] [Google Scholar]
- Sengupta S and Siliciano RF (2018) Targeting the Latent Reservoir for HIV-1. Immunity, 48, 872–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourisseau M, Sol-Foulon N, Porrot F, Blanchet F and Schwartz O (2007) Inefficient human immunodeficiency virus replication in mobile lymphocytes. J Virol, 81, 1000–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck RF, Esser U, Penn ML, Eckstein DA, Pulliam L, Chan SY and Goldsmith MA (1999) A trans-receptor mechanism for infection of CD4-negative cells by human immunodeficiency virus type 1. Current biology : CB, 9, 547–550. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Wesselingh SL, Griffin DE, McArthur JC, Johnson RT and Glass JD (1996) Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Annals of neurology, 39, 705–711. [DOI] [PubMed] [Google Scholar]
- Tornatore C, Chandra R, Berger JR and Major EO (1994) HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology, 44, 481–487. [DOI] [PubMed] [Google Scholar]
- Tornatore C, Nath A, Amemiya K and Major EO (1991) Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. Journal of virology, 65, 6094–6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdebenito S, Audia A, Bhat KPL, Okafo G and Eugenin EA (2020) Tunneling Nanotubes Mediate Adaptation of Glioblastoma Cells to Temozolomide and Ionizing Radiation Treatment. iScience, 23, 101450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasile F, Dossi E and Rouach N (2017) Human astrocytes: structure and functions in the healthy brain. Brain Struct Funct, 222, 2017–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez S, Malik S, Lutz SE, Scemes E and Eugenin EA (2016) Pannexin1 Channels Are Required for Chemokine-Mediated Migration of CD4+ T Lymphocytes: Role in Inflammation and Experimental Autoimmune Encephalomyelitis. Journal of immunology, 196, 4338–4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez S, Prevedel L, Valdebenito S, Gorska AM, Golovko M, Khan N, Geiger J and Eugenin EA (2020) Circulating levels of ATP is a biomarker of HIV cognitive impairment. EBioMedicine, 51, 102503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bartheld CS, Bahney J and Herculano-Houzel S (2016) The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. J Comp Neurol, 524, 3865–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber S, Weiser B, Kemal KS et al. (2014) Epigenetic analysis of HIV-1 proviral genomes from infected individuals: predominance of unmethylated CpG’s. Virology, 449, 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CA (2003) Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain pathology, 13, 415; author reply 415-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CA, Schrier RD, Nelson JA, Lampert PW and Oldstone MB (1986) Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A, 83, 7089–7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang K, Leda AR, Tsai L, Knight H, Harbison C, Gettie A, Blanchard J, Westmoreland S and Cheng-Mayer C (2014) Emergence of CD4 independence envelopes and astrocyte infection in R5 simian-human immunodeficiency virus model of encephalitis. Journal of virology, 88, 8407–8420. [DOI] [PMC free article] [PubMed] [Google Scholar]


