Abstract
Although early antibiotic treatment of patients with septic arthritis eradicates bacteria, joint destruction commonly results from the unregulated host inflammatory responses to infection. The spin trap compound phenyl-N-tert-butyl nitrone (PBN) has been shown to have both anti-inflammatory and antioxidant effects. The aim of this study was to assess the effect of combined systemic administration of PBN and cloxacillin on the development of Staphylococcus aureus arthritis.
Three days after Naval Medical Research Institute (NMRI) mice were infected intravenously with S. aureus LS-1, daily treatment was started with cloxacillin alone, PBN alone, or cloxacillin and PBN. Arthritis, weight loss and general condition were evaluated for each mouse, and joints were analyzed histopathologically. Systemic administration of PBN in conjunction with cloxacillin ameliorated the course of experimental S. aureus arthritis, as evidenced by an increased cure rate. Thus, combinatorial antioxidant plus antibiotic anti-inflammatory therapies represent a potentially efficacious approach to the management of septic arthritis.
Keywords: arthritis, murine, spin-trap, Staphylococcus aureus, treatment
Introduction
Staphylococcus aureus is the most common causative agent of septic arthritis [1-3], a severe, rapidly progressing, erosive disease with high morbidity and mortality. Inflammatory processes during septic arthritis erode articular cartilage, destroy bone and promote joint destruction leading to irreversible loss of joint function in 25–50% of patients [4,5]. Early administration of antibiotics eradicates the bacteria, but does not stop joint destruction. We have previously shown that antibacterial therapy combined with systemic corticosteroid administration ameliorated S. aureus arthritis in mice [6]. The compound α-phenyl-N-tert-butyl nitrone (PBN) was originally developed as a means of trapping and detecting free radical intermediates [7]. PBN and related nitrones have a variety of anti-inflammatory and antioxidant properties. Therefore we considered whether PBN might be an effective therapeutic in septic arthritis. In this study, we evaluated the efficacy of a combined PBN and antibiotic (cloxacillin) treatment in reducing joint destruction during staphylococcal arthritis in a murine model of hematogenously spread S. aureus sepsis and septic arthritis [8,9].
Materials and methods
The arthritis model
Female 5–8 week-old Naval Medical Research Institute (NMRI) mice were injected intravenously in the tail vein with an arthritogenic dose of S. aureus LS-1 [6]. Limbs were inspected (by a blinded observer) at various time-points after bacterial inoculation. A system of clinical scoring (arthritic index; 0–3 scale) was used to assess the severity of arthritis in each limb [10]. The total index was calculated by adding the individual limb scores for each animal tested. The cure rate was estimated by subtracting the arthritic index at day 10 (i.e. seven days after treatment commencement) from that at day three (i.e. just prior to treatment commencement).
Histopathological examination
The animals were sacrificed ten days after inoculation of bacteria and the joints were examined histologically [6] for synovial hypertrophy (synovial membrane thickness of more than two cell layers [11]), pannus formation and destruction of cartilage and subchondral bone. To evaluate the severity of synovitis and cartilage and/or bone destruction, a histological scoring system (histological index) was employed [12]. The total histological index was calculated by totaling all the scores for each animal tested.
Bacteriological examination
After sacrifice, kidneys were aseptically removed, homogenized manually at 4°C, diluted in PBS, and inoculated on horse blood agar in serial dilutions to estimate the bacterial load in each organ.
Treatment procedures
Cloxacillin (Astra, Södertälje, Sweden) was dissolved in sterile PBS, and mice were injected intraperitoneally with 0.1 ml of the solution, corresponding to 500 mg/kg body weight, every 12 hours, starting on day three after inoculation of bacteria and continuing until the animals were sacrificed.
Phenyl-N-tert-butyl nitrone (PBN) (Sigma, St Louis, MO, USA) was diluted in 0.1 ml of sterile PBS and injected intraperitoneally (40 mg/kg body weight) every 12 hours, starting on day zero or day three after inoculation of bacteria and continuing until the animals were sacrificed. PBN is not bactericidal for S. aureus.
Statistical analyses
The differences between parametric and non-parametric values in all treatment groups were tested for significance by use of the two-tailed Student's t-test and the Mann-Whitney U-test, respectively. Differences between groups in the incidence of arthritis and mortality were analyzed by the Fisher's exact test. Results are presented as the mean ± SEM. A P value of less than 0.05 was considered statistically significant.
Results
The effect of PBN-alone treatment on sepsis and septic arthritis
The treatment with PBN alone started on day zero, and had no effect either on the prevalence or severity of arthritis. Thus, 21 days after the treatment was started, six out of nine mice in the control group, and four out of seven in the PBN-treated group exhibited symptoms of arthritis The mean arthritic index was 1.7 ± 0.1 in both groups. However, we observed a moderate increase in the mortality rate in the PBN-treated animals; 30% of the PBN-treated animals died, compared to 10% of control animals (n = 10 per group) by day 21 post-infection (data not shown).
The effect of combined PBN-cloxacillin treatment on the clinical course of sepsis and septic arthritis
In order to evaluate the effect of combined PBN-cloxacillin treatment, forty 5–6-week-old female NMRI mice were injected intravenously with arthritogenic doses of S. aureus LS-1. The mice were subdivided into four groups and treatment began three days after infection. The first (control) group was given no treatment, the second group was treated with cloxacillin alone, the third group was given PBN plus cloxacillin, and the fourth group received PBN only. The experiment was performed three times. The fourth group was excluded in the last experiment. Since all three experiments displayed similar outcomes, the clinical, bacteriologic and histologic results were pooled.
Three days after inoculation of bacteria, 56–70% of all groups had developed symptoms of arthritis. The frequency and severity of arthritis are depicted in Fig. 1. On day 10 after inoculation of bacteria (seven days after treatment was initiated), the frequency and severity of arthritis increased in all the groups, except for the group receiving combined treatment. Thus, 59% of mice receiving combined treatment exhibited symptoms of arthritis, compared to 64% in the cloxacillin-alone treated group and 70% in the controls. The prevalence of arthritis was greatest in the PBN-alone treated mice (74%). The severity of arthritis followed a similar pattern: mean arthritic index in mice receiving combined treatment was 0.9 ± 0.2, compared to 1.1 ± 0.2 in the cloxacillin-alone treated group, 1.6 ± 0.3 in the controls and 2.0 ± 0.4 in PBN-alone treated animals. Mice receiving combined PBN-cloxacillin treatment exhibited significantly improved cure rates compared to groups receiving treatments with either PBN alone (P < 0.05), cloxacillin alone (P < 0.05), or untreated controls (P < 0.01) (Fig. 2).
Figure 1.
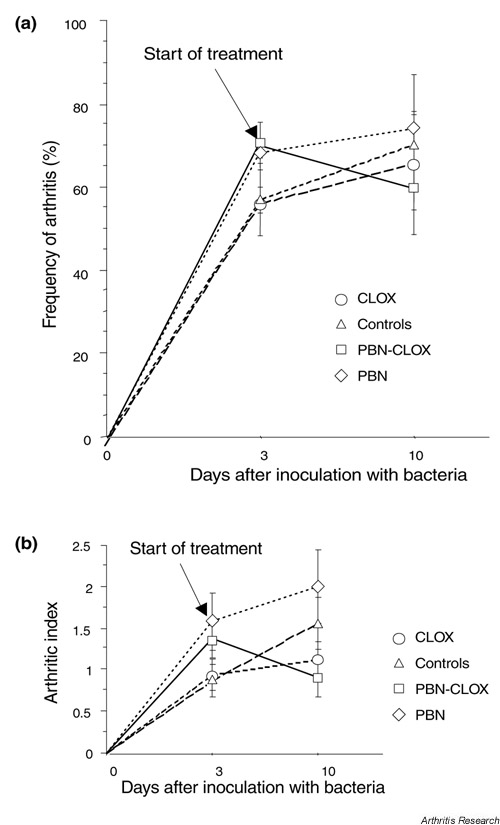
The effects of different treatment modalities on the prevalence and the severity of septic arthritis. (a) The prevalence and (b) the severity of septic arthritis in NMRI mice (n = 19–30) on days 3 and 10 following intravenous infection with S. aureus strain LS-1 were evaluated. To evaluate the severity of arthritis, a system of clinical scoring (arthritic index) was employed, where macroscopic inspection yielded a score of 0 to 3 points for each limb. The total index was calculated by adding the individual limb scores for each animal tested. Results are expressed as the mean ± SEM. CLOX, cloxacillin; PBN, phenyl-N-tert-butyl nitrone.
Figure 2.
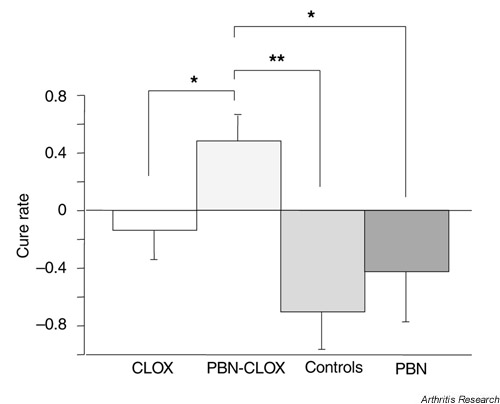
The curative effects of different modalities to treat septic arthritis. The cure rate is expressed as the difference in the severity of arthritis between days 3 and 10 following S. aureus infection in NMRI mice (n = 19–30). All treatments were initiated on day 3 following intravenous inoculation of bacteria and continued until day 10. Positive values indicate amelioration of arthritis and negative values indicate increased severity of arthritis between days 3 and 10. Results are expressed as the mean ± SEM. *P < 0.05; **P < 0.005. CLOX, cloxacillin; PBN, phenyl-N-tert-butyl nitrone.
Histopathological findings
The frequency of synovitis was 85–90% in all treatment groups. Fifty-five percent of mice receiving the PBN-cloxacillin treatment displayed cartilage destruction, compared to 70% in the cloxacillin-alone and PBN-alone groups and 74% in the control group. The severity of histological changes was lowest in the animals receiving combined treatment (Fig. 3).
Figure 3.
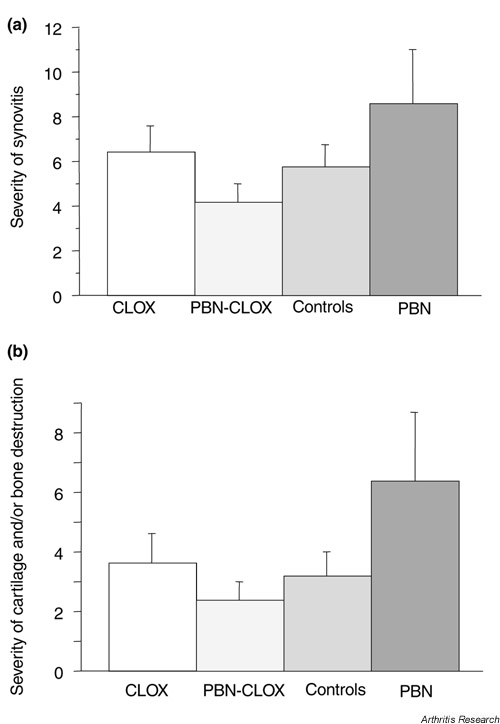
The effects of different treatment modalities on the severity of the symptoms of septic arthritis. (a) The severity of synovitis and (b) cartilage and/or bone destruction in NMRI mice inoculated with S. aureus strain LS-1 (n = 10–20) is expressed as the mean histological index ± SEM. The total index was calculated by totaling all the scores for each animal tested. CLOX, cloxacillin; PBN, phenyl-N-tert-butyl nitrone.
Bacteriological findings
All of the control and PBN-alone treated mice harbored bacteria in the kidneys 10 days after infection, compared to 58% of mice treated with combined PBN-cloxacillin therapy, and 68% of mice treated with cloxacillin alone. The numbers of bacteria in kidneys were significantly lower in the combined PBN-cloxacillin and cloxacillin-alone groups than in the control or PBN-alone treatment groups (Fig. 4).
Figure 4.
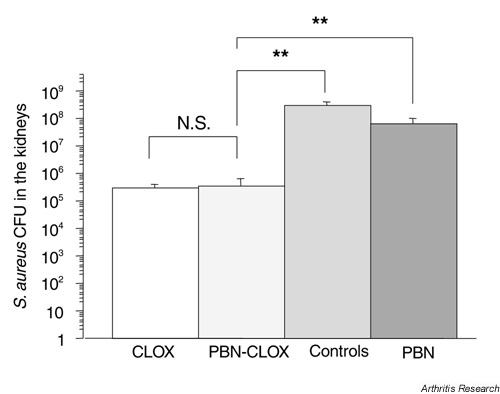
The effects of different treatments on the number of bacteria isolated from the kidneys of NMRI mice, 10 days after they were inoculated intravenously with S. aureus strain LS-1 (n = 9–19). **P < 0.005; NS, not significant (*P < 0.05). CFU, colony-forming units; CLOX, cloxacillin; PBN, phenyl-N-tert-butyl nitrone.
Discussion
We used a murine model of hematogenously acquired S. aureus arthritis to evaluate the effects of PBN treatment, given alone or in combination with cloxacillin. Treatment efficacy was expressed as the cure rate (i.e. arthritic index changes following treatment of infected mice). The combined PBN-cloxacillin treatment significantly (P < 0.05) increased the cure rate, compared to cloxacillin-alone treatment. Histological investigation confirmed these results. Joint destruction develops early (within 48 hours) in S. aureus infection [12,13]. Therefore, the occurrence, even in the treated animals, of erosions is not surprising, bearing in mind that the treatment was started three days after inoculation of bacteria. This was done in an attempt to reflect the clinical situation when patients present with staphylococcal infections. It is important to note that despite the higher prevalence and severity of arthritis at the start of the treatment modalities, the joints of mice receiving combined treatment exhibited less severe histological changes. Therefore, PBN appears to exert an ameliorating effect on arthritis progression and joint destruction when given in combination with an antibiotic.
Treatment with PBN alone had no beneficial effect on disease. Indeed, there was a tendency towards increased mortality in animals receiving PBN from the first day of infection. Phagocytes manufacture large amounts of reactive oxidants that participate in the destruction of invading microorganisms [14]. PBN scavenges radicals and probably decreases phagocyte bactericidal capacity, thereby increasing the bacterial burden and contributing to sepsis-induced mortality. On the other hand, the ability of PBN to spin trap free radicals could have directly diminished joint tissue damage. The role of oxygen-derived free radicals in inflammation and tissue damage is well established [15]. Interestingly, another nitrone, tempol, has been shown to have beneficial effects on collagen-induced arthritis in rats [16].
PBN suppresses proinflammatory cytokine production (e.g. interleukin-1 and tumor necrosis factor-α) by monocytes in vitro[17]. The involvement of proinflammatory cytokines in the pathogenesis of S. aureus infection has been previously established [18-22] Thus, both direct and indirect effects of PBN on immune cells and on the production of cytokines might have contributed to the observed amelioration of arthritis.
Conclusions
This is the first infectious model of inflammatory disease in which PBN has been tested as a potential therapeutic agent. We have shown that systemic administration of PBN concomitant to antibiotic therapy improves the cure rate in S. aureus-induced arthritis. The pleiotropic effects of PBN in modulating macrophage and neutrophil activities within the joint may reduce destructive arthritis. Therefore, PBN might have therapeutic applications to nonseptic as well as septic inflammatory disease.
Abbreviations
NMRI = Naval Medical Research Institute; PBN = phenyl-N-tert-butyl nitrone; PBS = phosphate-buffered saline.
Acknowledgments
Acknowledgments
We thank Ing-Marie Nilsson and Liu Zai-Qing for excellent technical assistance. This work was supported by grants from the Gothenburg Medical Society, Nanna Svartz Foundation, the Swedish Medical Research Council, the King Gustaf V 80 Years Foundation, the Swedish Association against Rheumatism, Börje Dahlins Foundation, and Rune and Ulla Amlövs Foundation for Neurological, Rheumatological and Audiological Research.
References
- Studahl M, Bergman B, Kälebo P, Lindberg J. Septic arthritis of the knee: a 10-year review and long-term follow-up using a new scoring system. Scand J Infect Dis. 1994;26:85–93. doi: 10.3109/00365549409008595. [DOI] [PubMed] [Google Scholar]
- Kaandorp CJ, Dinant HJ, van de Laar MA, Moens HJ, Prins AP, Dijkmans BA. Incidence and sources of native and prosthetic joint infection: a community based prospective survey. Ann Rheum Dis. 1997;56:470–475. doi: 10.1136/ard.56.8.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy DW, Kehl DK. Increasing prevalence of Kingella kingae in osteoarticular infections in young children. J Pediatr Orthop. 1998;18:262–267. [PubMed] [Google Scholar]
- Goldenberg DL. Septic arthritis. Lancet. 1998;351:197–202. doi: 10.1016/S0140-6736(97)09522-6. [DOI] [PubMed] [Google Scholar]
- Goldenberg DL, Reed JI. Bacterial arthritis. N Engl J Med. 1985;312:764–771. doi: 10.1056/NEJM198503213121206. [DOI] [PubMed] [Google Scholar]
- Sakiniene E, Bremell T, Tarkowski A. Addition of corticosteroids ameliorate the course of experimental Staphylococcus aureus arthritis. Arthritis Rheum. 1996;39:1596–1605. doi: 10.1002/art.1780390921. [DOI] [PubMed] [Google Scholar]
- Janzen EG, Blackburn BJ. Detection and identification of short-lived free radicals by en electron spin resonance trapping technique. J Am Chem Soc. 1968;90:5909–5910. [Google Scholar]
- Bremell T, Lange S, Yacoub A, Rydén C, Tarkowski A. Experimental Staphylococcus aureus arthritis in mice. Infect Immun. 1991;59:2615–2623. doi: 10.1128/iai.59.8.2615-2623.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkowski A, Collins LV, Gjertsson I, Hultgren OH, Jonsson I, Sakiniene E, Verdrengh M. Model systems: Modeling human staphylococcal arthritis and sepsis in the mouse. Trends Microbiol. 2001;9:321–326. doi: 10.1016/s0966-842x(01)02078-9. [DOI] [PubMed] [Google Scholar]
- Abdelnour A, Arvidson S, Bremell T, Rydén C, Tarkowski A. The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect Immun. 1993;61:3879–3885. doi: 10.1128/iai.61.9.3879-3885.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg DL, Cohen AS. Synovial membrane histopathology in the different diagnosis of rheumatoid arthritis, gout, pseudogout, systemic lupus erythematosus, infectious arthritis and degenerative joint disease. Medicine. 1978;57:239–252. doi: 10.1097/00005792-197805000-00004. [DOI] [PubMed] [Google Scholar]
- Sakiniene E, Bremell T, Tarkowski A. Complement depletion aggravates Staphylococcus aureus septicemia and septic arthritis. Clin Exp Immunol. 1999;115:95–102. doi: 10.1046/j.1365-2249.1999.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremell T, Abdelnour A, Tarkowski A. Histopathological and serological progression of experimental Staphylococcus aureus arthritis. Infect Immun. 1992;60:2976–2985. doi: 10.1128/iai.60.7.2976-2985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- McCord JM. Oxygen-derived free radicals. New Horiz. 1993;1:70–76. [PubMed] [Google Scholar]
- Cuzzocrea S, McDonald MC, Mota-Filipe H, Mazzon E, Costantino G, Britti D, Mazzullo G, Caputi AP, Thiemermann C. Beneficial effects of tempol, a membrane-permeable radical scavenger, in a rodent model of collagen-induced arthritis. Arthritis Rheum. 2000;43:320–328. doi: 10.1002/1529-0131(200002)43:2<320::AID-ANR11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Sang H, Wallis GL, Stewart CA, Kotake Y. Expression of cytokines and activation of transcription factors in lipopolysaccharide-administered rats and their inhibition by phenyl N-tert-butylnitrone (PBN). Arch Biochem Biophys. 1999;363:341–348. doi: 10.1006/abbi.1998.1086. [DOI] [PubMed] [Google Scholar]
- Hultgren O, Eugster HP, Sedgwick JD, Korner H, Tarkowski A. TNF/lymphotoxin-alpha double-mutant mice resist septic arthritis but display increased mortality in response to Staphylococcus aureus. J Immunol. 1998;161:5937–5942. [PubMed] [Google Scholar]
- Zhao YX, Nilsson IM, Tarkowski A. The dual role of interferon-gamma in experimental Staphylococcus aureus septicaemia versus arthritis. Immunology. 1998;93:80–85. doi: 10.1046/j.1365-2567.1998.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77:1627–1652. [PubMed] [Google Scholar]
- Brennan FM. Role of cytokines in experimental arthritis. Clin Exp Immunol. 1994;97:1–3. doi: 10.1111/j.1365-2249.1994.tb06570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren O, Stensson M, Tarkowski A. Role of IL-12 in Staphylococcus aureus-triggered arthritis and sepsis. Arthritis Res. 2001;3:41–47. doi: 10.1186/ar138. [DOI] [PMC free article] [PubMed] [Google Scholar]


