Abstract
Previous research has shown that training of a novel task can improve subsequent performance in the opposite arm owing to anticipation of the previously learned task conditions. Interestingly, we recently reported preliminary evidence that such transfer might also include modulation of feedback-mediated responses. We now test interlimb transfer of load compensation responses, measured through kinematic and EMG recordings during rapid 20° elbow flexion movements. Two subject groups, LR and RL, each comprising six right-handed subjects, first performed using either the left (LR) or right (RL) arm, followed by opposite arm performance. After 30 trials of consistent performance, five random trials within a background of 50 trials were loaded with a 2-kg mass prior to the “go” signal. We compared load compensation responses for naïve performance with those following opposite arm exposure. Under naïve conditions, the resulting load compensation responses began about 50 ms following movement onset, and were substantially more effective for the nondominant arm. Opposite arm exposure substantially improved the accuracy of only dominant arm responses. This, however, did not occur through changes in the short latency components of the load compensation response. Instead, changes in muscle activities, associated with interlimb transfer, began some 150 ms following movement onset. We expect that these changes represent transfer in the “volitional” component of the load compensation response. Because the shorter latency response was unaffected by opposite arm exposure, modulation of this component likely requires prior experience with limb specific effectors.
Keywords: Load compensation response, Opposite arm exposure, Interlimb transfer
Introduction
Previous research has shown that practice of a novel activity with one arm can affect subsequent performance with the other arm (Laszlo et al. 1970; Latash 1999; Thut et al. 1996; Morton et al. 2001; Dizio and Lackner 1995; Criscimagna-Hemminger et al. 2003; Elliott and Roy 1981; Imamizu and Shimojo 1995; Sainburg and Wang 2002; Wang and Sainburg 2003). Moreover, specific aspects of performance can show transfer in different directions. For example, we showed that following adaptation to a visuomotor rotation task, initial direction accuracy only transfers to the dominant arm (Sainburg and Wang 2002), whereas final position accuracy only transfers to the nondominant arm. Interestingly, dominant and nondominant arm control has previously been shown to differentiate according to these different features of task accuracy (Sainburg 2002). Thus, the direction of interlimb transfer that occurs for a given task might depend on asymmetries in the underlying control mechanisms. These previous studies have focused on adaptation to novel task conditions, and as such, transfer was reflected by anticipation of the novel conditions during subsequent opposite arm performance. However, we recently reported preliminary evidence that such transfer may not be limited to anticipatory mechanisms associated with adaptation, but may also include feedforward modulation of feedback-mediated responses (Bagesteiro and Sainburg 2003).
Compensation for unexpected loads during voluntary movement is arguably an important requirement for the skillful performance of many daily activities (Bagesteiro and Sainburg 2003; Bizzi et al. 1978; Bock 1990; Crago et al. 1976; Lacquaniti et al. 1982; Prochazka et al. 1997a, 1997b). We recently reported interlimb asymmetries in the efficacy of such responses. During randomly loaded elbow flexion movements, compensatory responses were initiated some 50 ms following movement onset. We showed that the nondominant arm response was substantially more effective at achieving accurate movements in the face of the unpredictable load. We also reported preliminary evidence for improved dominant arm responses following opposite arm exposure to the task. The purpose of the current study is to examine whether, under random perturbation conditions, opposite arm exposure can improve the load compensation response. If it can, this would suggest that modulation of such responses is not specific to a particular set of limb effectors.
Previous studies have shown that loads applied during voluntary movements evoke short and longer latency reactions that function to resist the load (Brown and Cooke 1981, 1986; Day et al. 1983; Shapiro et al. 2002). The shortest latency responses occur at about 50 ms following stimulus onset. This latency is consistent with that of the functional stretch reflex, which is thought to be mediated through a combination of both multisynaptic spinal and long-loop circuits (Day et al. 1983; Ghez and Shinoda 1978; Marsden et al. 1976; Rothwell et al. 1980). Because these responses have previously been shown to be highly modifiable according to task and et instructional conditions (Evarts and Tanji 1974, 1976; Lacquaniti al. 1991), we hypothesized that interlimb asymmetries in load compensation might be due to differential modulation of these circuits by each hemisphere/limb system. It is thus plausible that such modulation might transfer from the more efficient nondominant arm response to the less efficient dominant arm response. This would be expected if modulation of the load compensation response were to occur upstream to the generation of limb-specific commands. In contrast, such modulation might be specific to a particular set of limb effectors, and thus not amenable to interlimb transfer.
In order to differentiate between these alternatives, we compared load compensation responses for naïve performance with those following opposite arm exposure. For naïve conditions, our results were consistent with those of our previous study, demonstrating a distinct nondominant arm advantage (Bagesteiro and Sainburg 2003). We confirmed our prediction of interlimb transfer by showing substantial improvement in dominant arm responses following nondominant arm exposure. However, this improvement did not occur through changes in the short latency components of the responses. Instead, substantial changes in muscle activities were measured beginning some 150 ms following movement onset. We expect that these changes characterize transfer in the “volitional” component of the load compensation response, which has been shown to be most malleable in response to variations in instructional set and task conditions (Crago et al. 1976; Evarts and Tanji 1974; Gielen et al. 1988). In contrast, because the shorter latency component was unaffected by opposite arm exposure, it is likely that modulation of this component is dependent on prior experience with limb-specific effectors.
Methods
The methods are similar to those previously reported in Bagesteiro and Sainburg (2003), and thus are only briefly discussed here.
Subjects
Subjects were 12 neurologically intact right-handed adult (six males and six females), aged from 23 to 35 years old. Only right-handers were recruited; handedness was determined using a 12-item version of the Edinburgh inventory (Oldfield 1971). Subjects were recruited from the University community and were paid for their participation. Informed consent was solicited prior to participation, which was approved by the Office of Regulatory Compliance of the Pennsylvania State University. Each subject performed two 80-trial (fast point-to-point elbow flexion movements) experimental sessions (one with each arm). Six subjects performed with their left arm first (LR group) and the other six with their right arm first (RL group).
Experimental setup
Subjects sat facing a projection screen with either the right or left arm supported over a horizontal table top, positioned just below shoulder height (adjusted to the subject’s comfort), by a frictionless air-jet system. A cursor representing finger position, a start circle, and a target were projected on a horizontal back-projection screen positioned above the arm. All joints distal to the elbow were immobilized using an adjustable brace. This virtual reality environment assured that subjects had no visual feedback of their arm, or the mass condition, during an experimental session. Position and orientation of the segments proximal and distal to the elbow joint were sampled using a Flock of birds (Ascension-Technology) magnetic six-degree-of-freedom movement recording system. A single sensor was attached to the upper arm segment via an adjustable plastic cuff, while another sensor was fixed to the air-sled where the forearm was fitted. A vertical rod was mounted on the transverse piece of the air-sled, allowing the attachment of a 2-kg mass placed 20 cm lateral to the forearm. The air-sled arrangement prevented somatosensory information about the mass through gravitational or frictional effects.
Digital data were collected at 103 Hz using a Macintosh computer, which controlled the sensors through separate serial ports, and stored on disk for further analysis. EMG activity was recorded from four representative muscles of the elbow joint: biceps brachii and brachioradialis (elbow flexors) and long head of triceps brachii and anconeus (elbow extensors). The EMG signals were digitized at 1 kHz using a Macintosh computer equipped with an A/D board (National Instruments PCI-MIO-16XE-50). The EMG signals were full-wave rectified and bin integrated every 10 ms. This bin integration was done to reduce high-frequency components of the EMG that might be functionally insignificant (Hodges and Bui 1996). We employed a 10-ms resolution, in accordance with that recommended by De Luca (1997) for comparison of EMG timing between electrodes and/or subjects.
Experimental task
The elbow angle (angle formed between upper arm and forearm) established the start and end locations, which were 120° and 100°, respectively. The upper arm was immobilized by a brace; arm movements were thus restricted to the elbow joint. Subjects were to hold the cursor within the starting circle for 5 s to initiate each trial. They were instructed to move the finger to the target using a single, uncorrected motion in response to an audiovisual “go” signal. In addition, subjects were to match their hand velocity within a 1.1–1.4 m/s range. Peak hand velocity feedback was shown as a screen gauge after each trial. Feedback of the fingertip position (cursor display) was given to allow subjects to position the hand in the start circle, and then was removed at the go signal. The first 30 trials were given as practice to familiarize the subject with the task. The following 50 trials were then randomly perturbed. At the final position, subjects were given knowledge of results and points were awarded when meeting the task requirements, as well as to provide motivation for task performance.
Data analysis
All kinematic data were low-pass filtered at 8 Hz (third order, dual-pass Butterworth), and differentiated to obtain angular velocity and acceleration values. Movement accuracy was measured as the final position error, which was calculated as the distance between the index finger location at movement termination and the center of the target.
EMG signals were recorded from 300 ms before to 1 s after the trigger signal (trial start). For averaging, we synchronized the digitized EMG data to elbow peak acceleration. The integrated EMG data were normalized to percent of maximum EMG of each muscle, within subjects. The maximum EMG was found using a computer algorithm to locate the highest integrated EMG magnitude for each muscle within the experimental session. In order to analyze the differences in muscle activity signals between loaded and unloaded (baseline) trials, and to identify the timing of the EMG response to the unexpected load, we used two analyses. First, we quantified the EMG impulse over the first and second 100-ms intervals following peak tangential acceleration. The rationale for these intervals was to capture the duration of EMG bursts initiated from 0 to 50 ms following peak tangential acceleration, which thus might represent fairly short latency responses. The next interval would then capture EMG activity after this period. This analysis allowed comparison of EMG amplitude over the duration of the interval. Second, in order to specifically identify the timing of EMG responses to the load, we modified a technique introduced by Shapiro et al. (2002, 2004). For each muscle, five EMG trials against the unexpected load were compared with five baseline EMG trials using ANOVA and Newman-Keuls post hoc test. This test was repeated for every point, thus generating a P value time series. The EMG signals were considered statistically different if P fell below 0.05. This procedure is illustrated in Fig. 1 for biceps and anconeus muscle activities. We modified this analysis from that of Shapiro et al. in two important ways: First, the frequency of our bin-integrated EMG was 100 Hz, consistent with that recommended by De Luca for temporal comparisons between electrodes and subjects. Second, rather than compare each data point separately, we used a 30-ms integral defined by a moving window. This is because we expect that changes in EMG must be sustained for greater than 20 ms to effect appreciable changes in joint torque (Hodges and Bui 1996). This is most notable by the fact that the short latency component of the stretch reflex (M1) typically has a high amplitude, but its short duration precludes appreciable force generation (Ghez and Shinoda 1978; Houk 1976).
Fig. 1.
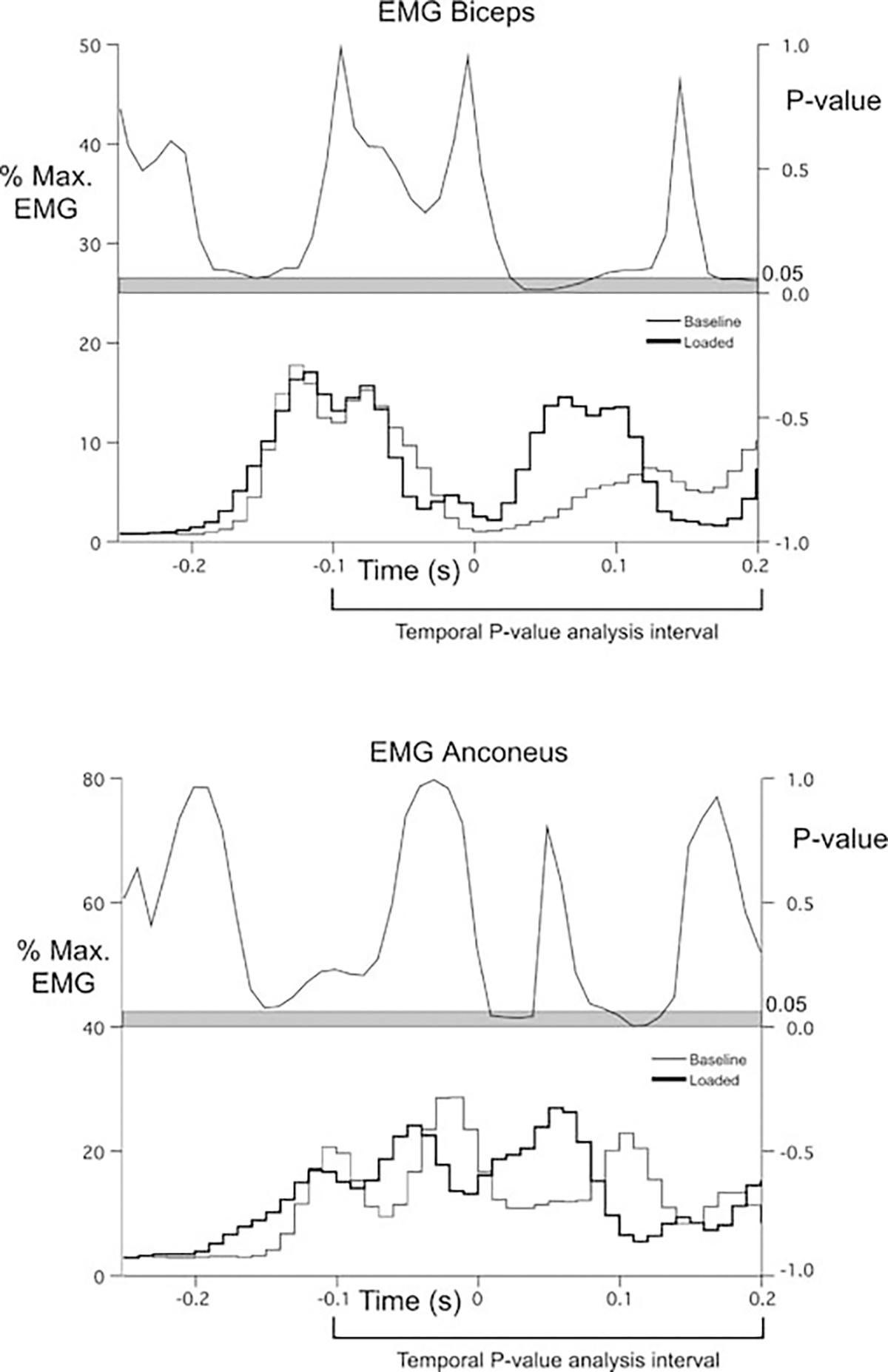
EMG timing analysis procedure. P value time series. Baseline and loaded averaged EMG recordings (five trials each condition) for biceps and anconeus. Data were synchronized to elbow peak acceleration. Data were normalized to the maximum muscle activity
Separate ANOVAs were conducted to test for statistical significance, with the criterion level set at P<0.05. In order to assess load compensation, interlimb differences were quantified to test for main effects of load and order of performance, as well as interaction between arm and condition. (Baseline trials were taken as the trials prior to perturbation.)
Results
Nondominant arm advantage for load compensation
Figure 2 shows ensemble averages of nondominant (left) and dominant (right) elbow displacement profiles from baseline (thin line) and loaded trials (thick line) for two representative subjects from each subject group performing under naïve conditions. All data have been synchronized to peak acceleration, which occurred an average of 48±3.7 ms (SE) following movement onset. The insets show the corresponding elbow velocity profiles. The amplitudes of the displacement and velocity profiles have been normalized to baseline performance in order to focus on the change from baseline as an indication of the load compensation response (see Bagesteiro and Sainburg 2003). The effect of the load in reducing movement velocity is reflected by the decreased amplitude of the peak velocity profiles, which corresponds to the decreased slope of the displacement/time profiles. For the loaded trials of both arms, a distinct reversal in slope of the velocity profile followed its peak and resulted in a delayed zero crossing, from flexor to extensor velocity (see arrows in Fig. 2 insets). This compensated the effect of the load by allowing greater displacement. As is readily apparent in Fig. 2 (top), the nondominant arm loaded trials were accurate, whereas those of the dominant arm were substantially hypermetric. These effects were consistent across all subjects, as indicated by the bar plots. For the nondominant arm, the final positions of loaded trials were not significantly different than those of baseline trials (P=0.73), whereas dominant arm loaded trials were significantly hypermetric (P=0.03). This was due to an exaggerated delay in the cross zero velocity profile, which was on average 60±22 ms (SE) longer than that for the nondominant arm (P=0.02). As a result, final position errors during loaded trials were substantially greater than those of the nondominant arm (P<0.01).
Fig. 2.
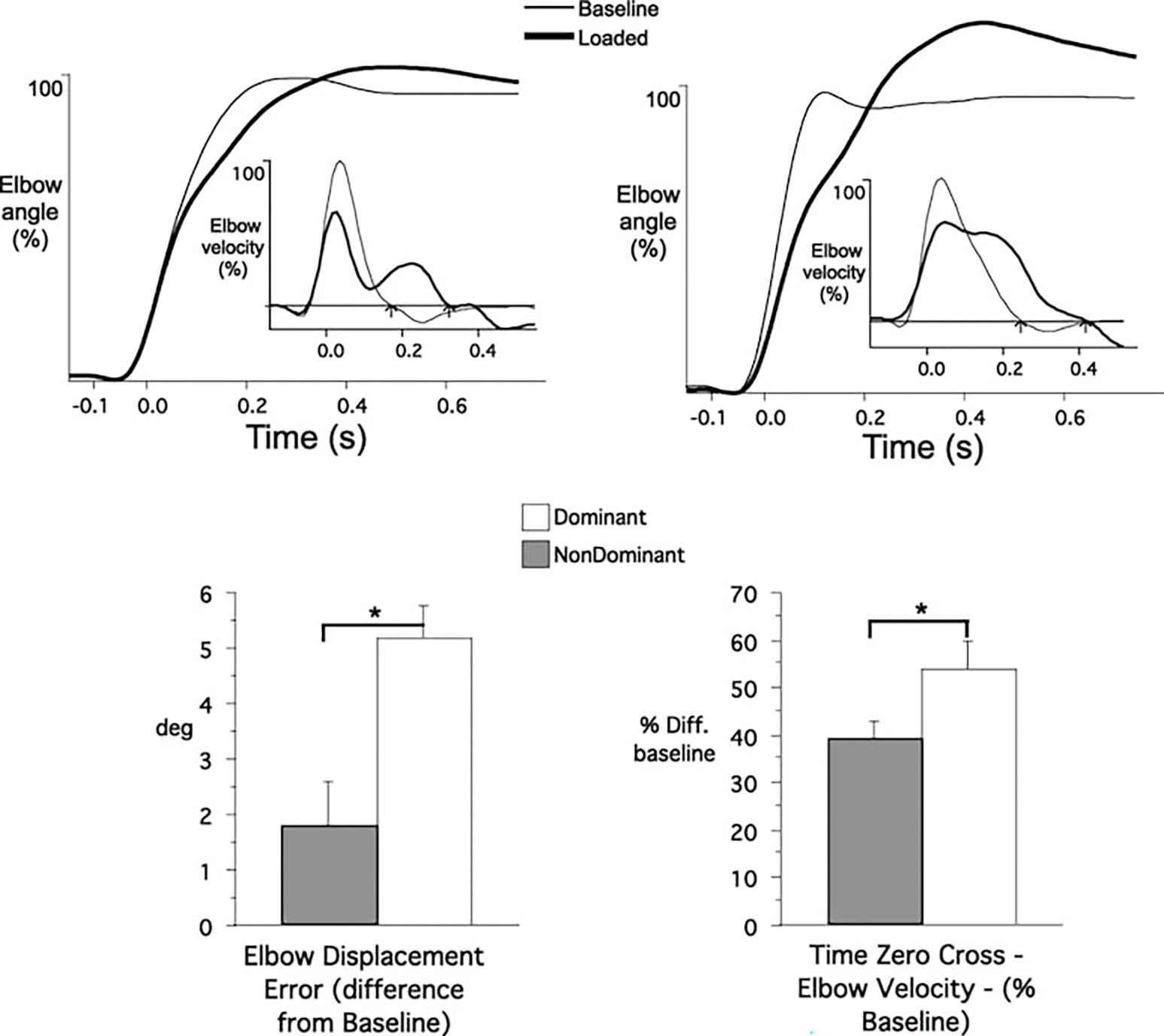
Two representative profiles from the nondominant (left) and dominant (right) arm groups under naïve performances. Averaged elbow displacement. The insets depict the respective elbow velocity profiles. Data were synchronized to elbow peak acceleration. Bar graphs: Kinematic comparisons: elbow displacement error and time of zero crossing (elbow velocity) for dominant and nondominant arm groups across subjects and conditions. *Results from statistical analysis were significant
Figure 3 shows ensemble averaged EMG profiles (biceps and anconeus) corresponding to the baseline (thin line) and loaded trials (thick line) shown in Fig. 2 (naïve performance). Under baseline conditions, these rapid elbow flexion movements were initiated by a large burst of agonist activity, which was followed by an antagonist burst, initiated just prior to peak acceleration. For the nondominant arm loaded trials, antagonist activities were reduced, whereas no apparent change in agonist activities occurred. For the dominant arm loaded trials, a substantial early burst in agonist activities (biceps) also occurred. These findings were consistent across subjects, as quantified by EMG impulse measured during the first 100-ms interval following peak acceleration. During loaded trials, nondominant arm agonist activities (biceps and brachioradialis) were not significantly different than those of baseline, whereas anconeus activity showed a significant decrease (P=0.04). EMG timing analysis revealed that this response occurred, on average, 5±25 ms (SD) following peak acceleration and lasted for about 30±35 ms (SD). It is this reduction in extensor activity that corresponds to the prolonged flexor velocity that resulted in load compensation.
Fig. 3.
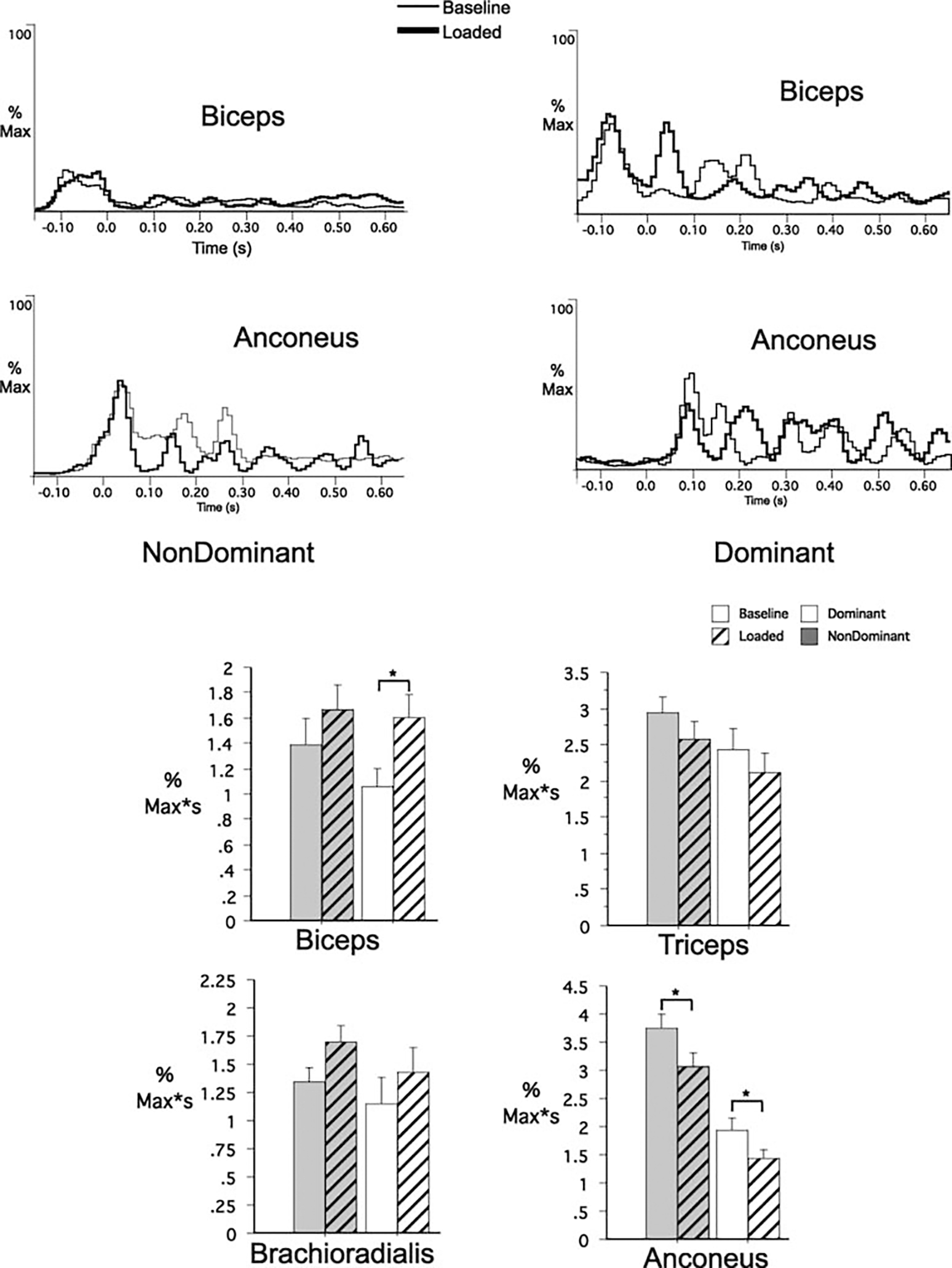
EMG recordings (individual trials) for biceps brachii and anconeus for the nondominant (left) and the dominant (right) arm under naïve performances. Data were synchronized to elbow peak acceleration. Data were normalized to the maximum muscle activity. Bar graphs: EMG impulse comparisons for dominant and nondominant arm groups for all recorded muscles. *Results from statistical analysis were significant
Across subjects, dominant arm extensor muscles showed a similar decrease in anconeus activity (P=0.04), which started 25±30 ms (SD) following peak acceleration with a duration of 55±25 ms (SD). However, dominant arm biceps also showed a large increase in activity (P=0.02). This response began, on average, 15±15 ms (SD) following peak acceleration and was sustained for 50±30 ms (SD). This response reflects the early second peak in flexor activity shown in Fig. 3 (top right corner). As a result, flexor velocity was markedly prolonged, resulting in the overshoot in elbow flexion previously quantified for the dominant arm.
Interlimb transfer of load compensation to the dominant arm
Figure 4 shows ensemble averaged nondominant (left) and dominant (right) elbow displacement profiles from baseline (thin line) and loaded trials (thick line) for two representative subjects from each subject group, performing following opposite arm exposure to the task (insets show elbow velocity profiles). For the nondominant arm, no change in response occurred, relative to the naïve conditions shown in Fig. 2 (left). Across subjects, neither final position error nor time of zero crossing was significantly different following opposite arm exposure from that of naïve performance (elbow displacement error: P=0.88; zero crossing: P=0.90).
Fig. 4.
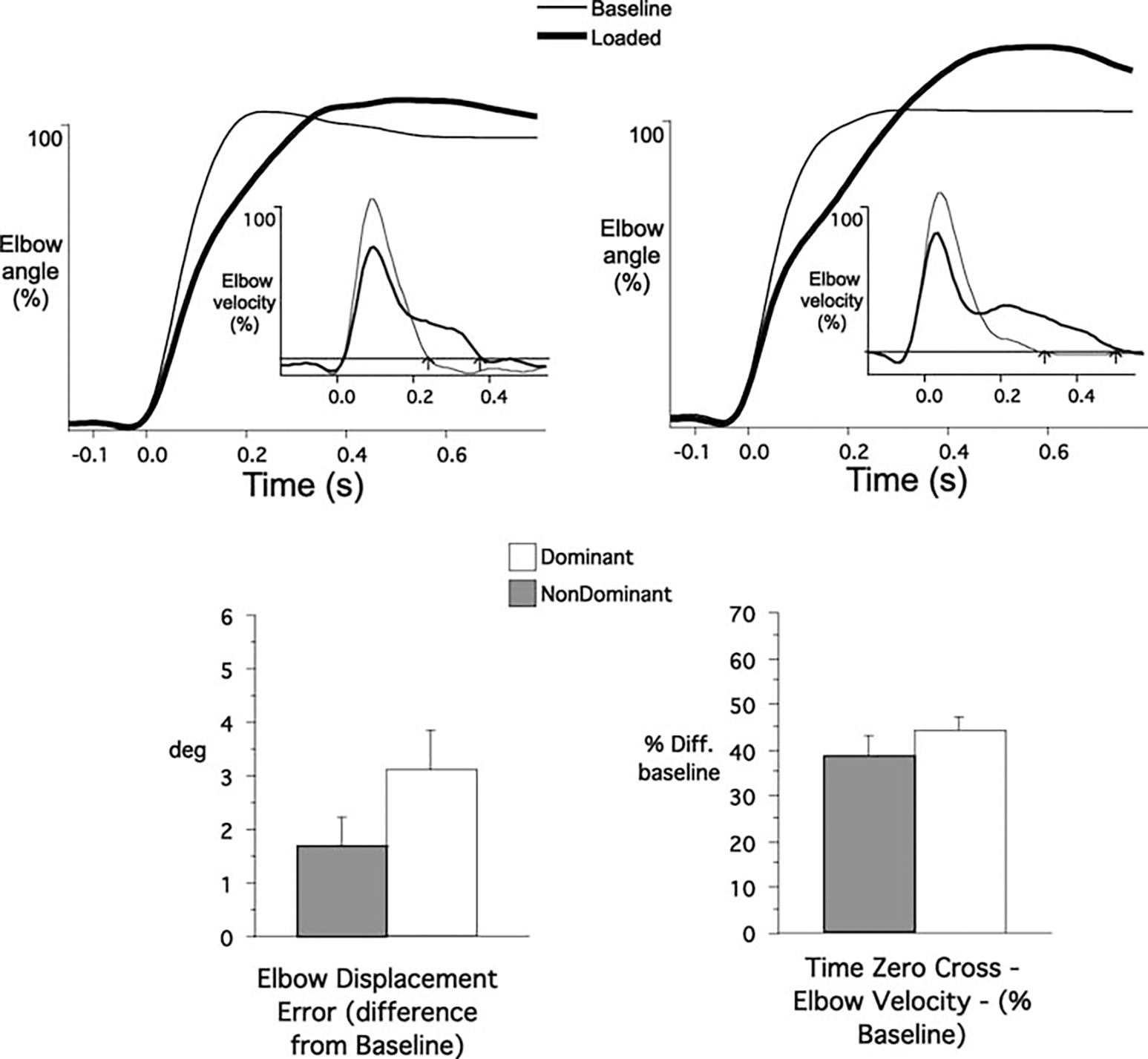
Two representative profiles from the nondominant (left) and dominant (right) arm groups after opposite arm performances. Averaged elbow displacement. The insets depict the respective elbow velocity profiles. Data were synchronized to elbow peak acceleration. Bar graphs: Kinematic comparisons: elbow displacement error and time of zero crossing (elbow velocity) for dominant and nondominant arm groups across subjects and conditions
In contrast, load compensation responses for the dominant arm improved following opposite arm exposure to the task. This is reflected by the improvements in final position accuracy shown in Fig. 4 (right), as compared with Fig. 2 (right). Across subjects, the dominant arm response remained less accurate than that of the nondominant arm, but accuracy was improved relative to that of naïve performance (P=0.02). The overshoot in final position was diminished by nearly 40% following opposite arm performance. The zero crossing for elbow velocity was also significantly reduced in time by some 25 ms (P=0.03). The consistency of these findings across subjects is illustrated in the bar plots in Fig. 4. After opposite arm exposure conditions, the dominant arm continued to overshoot nondominant arm performance, although this difference no longer achieved significance (P=0.12) nor the interlimb difference in timing of zero crossing for elbow velocity (P=0.30) was still significant. Thus, opposite arm exposure significantly reduced final position errors by reducing the duration of elbow flexor acceleration, relative to that of näive performance.
In order to further explore the mechanisms through which opposite arm exposure improved the accuracy of load compensation responses, we examined whether improvements in accuracy could be attributed to improved extensor corrections late in the movement. We quantified both the peak elbow angle and the final elbow angle for the dominant arm (difference from baseline), as displayed by the bar graphs in Fig. 5. These measures are shown for naïve performance (open bar), and that following opposite arm performance (hatched bar). If improvements in performance are due to the late extensor response, the changes in peak elbow angle, representing initial overshoot of elbow flexion, should not be different for naïve and opposite arm trials. However, across subjects, the mean improvement in the initial peak elbow flexor angle (P=0.02) was 49%, whereas that for final position accuracy (P=0.01) was only 40%, indicating that the greatest effect of opposite arm performance occurred by the time of peak elbow flexion excursion. Peak elbow acceleration occurred, on average, 10±10 ms (SE) following peak acceleration. We conclude that improvements in response following opposite arm exposure occurred through shortening of the flexor velocity phase of motion, rather than through corrections for extensor overshoot.
Fig. 5.
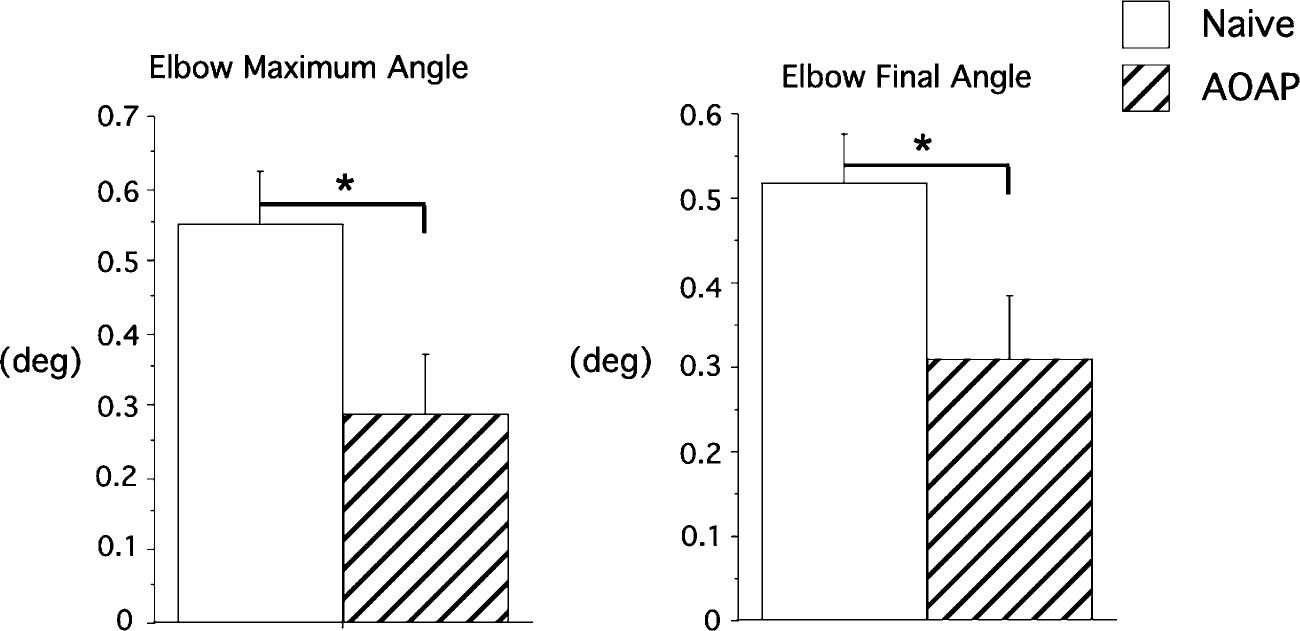
Maximum and final elbow displacement angles across subjects for the dominant arm, under naïve performance and after opposite arm performance (AOAP). *Results from statistical analysis were significant
The EMG correlates of these improvements are shown in Fig. 6 (individual trial samples). We first quantified recorded EMG impulse for 100 ms following elbow peak acceleration, as this interval was shown to reflect load compensation under naïve conditions. The bar plots of Fig. 6 show the mean EMG impulse, across subjects, for this interval for both dominant and nondominant arms under baseline and loaded conditions. Similar to naïve performance, the nondominant arm flexors showed no change in activity from that of baseline performance (biceps, P=0.98; brachioradialis, P=0.88). Nondominant arm extensor muscles showed a trend toward reduced activity, although this trend was not significant (triceps, P=0.09; anconeus P=0.07). Even though the dominant arm showed a substantial reduction in overshoot following opposite arm performance, the exaggerated increases in biceps activity remained, as was the case for naïve performance (P=0.03). This difference was initiated on average 30±10 ms (SD) and remained significant for 50±20 ms (SD). For both arms, the recorded EMG impulse during this 100-ms interval, relative to that of baseline performance, was not significantly different than that of naïve conditions (Table 1). We conclude that the improvements in load compensation noted above did not occur due to changes in the short latency EMG activity recorded during the first 100 ms following elbow peak acceleration.
Fig. 6.
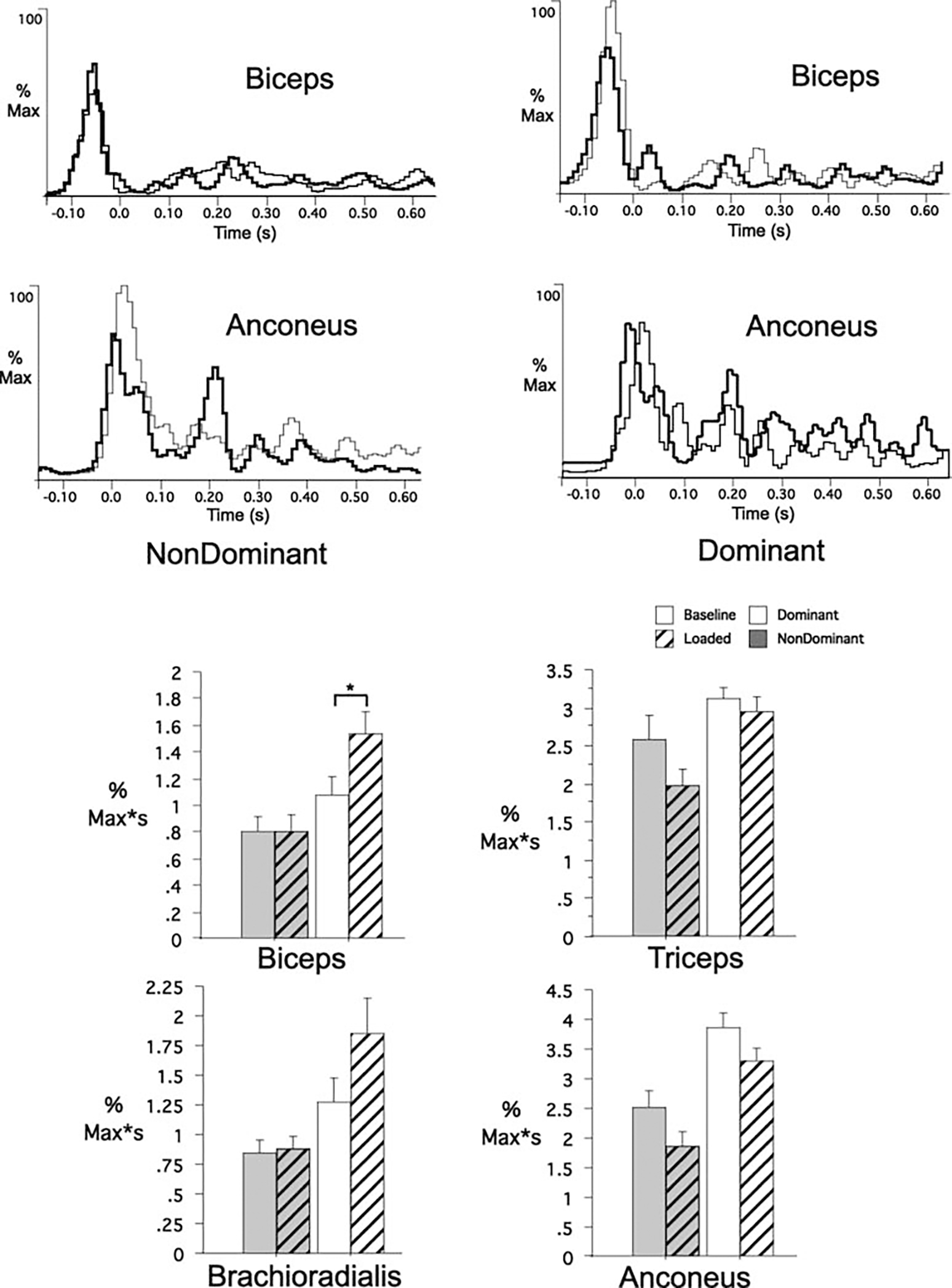
EMG recordings (individual trials) for biceps brachii and anconeus for nondominant (left) and dominant (right) arm after opposite arm performances. Data were synchronized to elbow peak acceleration. Data were normalized to the maximum muscle activity. Bar graphs: EMG impulse comparisons for dominant and nondominant arm groups for all recorded muscles. *Results from statistical analysis were significant
Table 1.
ANOVA (P values): EMG impulse fromAmax (elbow peak acceleration) to 100 ms
| Nondominant | Dominant | |
|---|---|---|
|
| ||
| Biceps | 0.3231 | 0.2892 |
| Brachioradialis | 0.3249 | 0.2795 |
| Triceps | 0.1186 | 0.7056 |
| Anconeus | 0.5913 | 0.8617 |
We next quantified EMG data as a percentage change from baseline activity for the two 100 ms intervals following peak acceleration. As stated above, for the first interval (Fig. 7, left bars), no significant differences between naïve and opposite arm exposed trials occurred for any recorded muscle. However, for the next 100-ms interval, a consistent and substantial change occurred in both biceps (P=0.01) and anconeus (P<0.01) activities. EMG timing analysis revealed that these differences began, on average, 115±25 ms (SD) and 145±35 ms (SD), with durations of 45±25 ms (SD) and 55±25 ms (SD) for biceps and anconeus, respectively. During this interval (100–200 ms following peak acceleration, Amax), under baseline conditions flexor activity was reduced and extensor activity was increased. We conclude that interlimb transfer of the load compensation response did not occur during the first 100 ms following elbow peak acceleration. Instead, longer latency mechanisms that began more than 100 ms following elbow peak acceleration were responsible for the improvements in performance following opposite arm exposure. These improvements consisted of both an increase in extensor activity, which should directly reduce flexor acceleration, and persistence of flexor activity, which likely contributed to decreased joint compliance through coactivation of muscles. During flexor motion, this effect should contribute to extensor torques, thus reducing flexor overshoot.
Fig. 7.
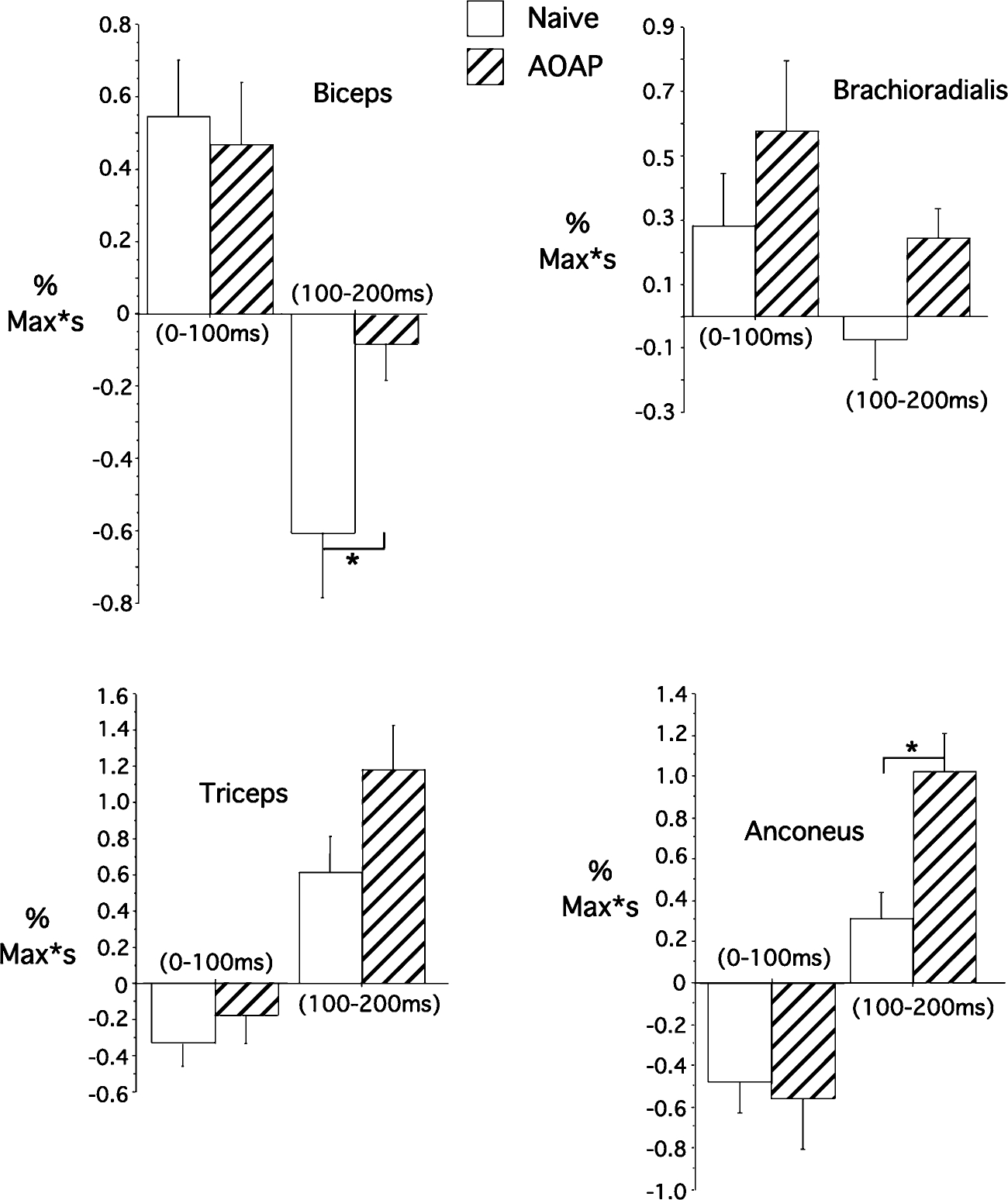
Dominant arm EMG impulse comparisons for all recorded muscles for two time intervals: 0 (Amax) to 100 ms (left) and 100–200 ms (right). *Results from statistical analysis were significant
Discussion
In this study we tested interlimb transfer of load compensation responses, measured through kinematic and EMG recordings during horizontal plane single-joint elbow flexion movements. Subjects made repetitive movements that were targeted in both velocity and amplitude. Following 30 trials of consistent performance, five loaded trials within a background of 50 trials were presented, with a 2-kg mass attached to the forearm splint prior to the “go” signal. We previously showed that the resulting load compensation responses begin about 50 ms following movement onset, and are substantially more effective for the nondominant arm (Bagesteiro and Sainburg 2003). While the neural mechanisms that underlie load compensations are not fully understood, they are thought to rely on sensory feedback from muscle spindle afferents (Ghez and Shinoda 1978; Prochazka 1986, 1989; Prochazka et al. 1979, 2000). When a force perturbation is applied to the stationary limb of an animal or human, three distinct bursts of activity in the stretched muscles have been observed. The different latencies of each burst have been attributed to the lengths of the underlying neural pathways. The specific latencies of these bursts vary with the animal and the muscle stimulated (Day et al. 1983; Ghez and Shinoda 1978; Marsden et al. 1976; Rothwell et al. 1980), so only response ranges can be extracted from the literature. The smallest latency burst, termed M1, occurs from some 7 to 45 ms. This activity occurs in response to a brief “tendon jerk” and is thought to occur primarily through the monosynaptic component of the stretch reflex. The force generated by this brief burst of activity is very small. The intermediate latency response, termed M2, requires a larger force perturbation and occurs at some 30–60 ms following the stimulus onset. This response results in measurable force and is thus referred to as the functional stretch reflex. M2 is thought to occur through a transcortical loop (Evarts and Tanji 1974, 1976; Ghez and Shinoda 1978; Godaux and Desmedt 1979) because (1) the latency varies with the distance of the involved spinal segment from the cortex (Marsden et al. 1973, 1976), (2) activity in sensory and motor cortices corresponds temporally to the EMG event (Evarts and Tanji 1976; Tatton et al. 1975), and (3) lesions of sensory cortex in monkeys abolish this response component (Tatton et al. 1975). However, Ghez and Shinoda (1978) have shown persistence of the M2 response in spinalized cats, suggesting that cortical circuits are not necessary for the response. Thus, spinal circuits triggered from ongoing afferent activity may also contribute to these longer latency responses (McCloskey and Prochazka 1994). The longest latency burst of EMG, M3, occurs at some 60–100 ms and is thought to reflect volitional responses to proprioceptive stimuli. Significantly, several studies have shown that the amplitude and occurrence of M2 and M3 are dependent on the instructions provided to the subject (Day et al. 1983; Evarts and Tanji 1974; Rothwell et al. 1980); furthermore, Laquaniti et al. (1991) showed that these responses can be transiently reversed in order to reduce limb compliance in anticipation of catching a ball.
When load perturbations are applied during an ongoing voluntary movement, instead of to a stationary limb, the load compensation responses are more difficult to characterize because the response must be extracted from the background muscle activity associated with the voluntary movement (Bagesteiro and Sainburg 2003; Bennett et al. 1994; Bizzi et al. 1978; Bock 1993; Brown and Cooke 1981; Shapiro et al. 2002). Under these circumstances, the short latency M1 response is rarely seen. Instead, only the two longer latency responses occur, which are observable as changes in EMG, as compared with those of unperturbed movements. The initial responses occur some 50–60 ms following the onset of the stimulus (Bagesteiro and Sainburg 2003; Brown and Cooke 1981). In the current study, inertial loads were applied prior to movement onset on random trials. Under these conditions, the effect of the load is zero prior to movement and varies with acceleration following movement initiation (Shapiro et al. 2002; Bagesteiro and Sainburg 2003). This experimental paradigm is thus ambiguous with respect to stimulus onset, as it is not known when the threshold stimulus associated with the load occurred. In a previous study (Bagesteiro and Sainburg 2003), we showed that when movements were randomly loaded during repeated voluntary reaches, observable changes in EMG occurred some 50 ms following movement onset. This is consistent with the findings of Brown and Cooke (1981) for discrete torque perturbations and suggests that, in our paradigm, the stimulus reaches threshold very early in the movement. In our current study, we confirm that load-dependent changes in EMG began some 50 ms following movement onset. These responses included a reduction in extensor EMG for both arms, and a concurrent increase in flexor EMG for the dominant arm. The additional flexor EMG in the dominant arm led to excessive elbow flexion, and overshoot of the target. Thus, the response in the nondominant arm was smaller, yet more effective at achieving an accurate final position.
In our previous study, we showed preliminary evidence that prior task experience with the nondominant arm improved the efficacy of subsequent responses with the dominant arm. In the current study, we specifically examined whether these responses show transfer between the limbs. In order to test this hypothesis, comparisons were made between load compensation responses under naïve conditions and following opposite arm exposure to the task. Our results indicated a substantial improvement in dominant arm responses following nondominant arm exposure. We showed that the dominant arm response to the load included a discrete burst of flexor muscle activity concurrent with a reduction in extensor activity. This resulted in prolongation of the flexor velocity phase of motion, and ultimately in overshoot of the target. The elbow trajectory tended to show a peak in flexion, followed by slight extension back toward the target before coming to a stop. Following nondominant arm exposure to the task, a reduction in flexor overshoot occurred, measured at peak angular displacement and final position. Analysis of EMG revealed that there was no difference in measured EMG from that of naïve conditions, for the 100-ms interval that began at elbow peak acceleration. This was the interval for which the load compensation response was measured under naïve conditions, which likely corresponds to the intermediate latency responses identified as M2 in previous perturbation studies (Day et al. 1983; Ghez and Shinoda 1978; Marsden et al. 1976; Rothwell et al. 1980). Instead, a substantial change in muscle activities was measured during the following 100-ms interval. We expect that this interval corresponds to the “volitional” component of the load compensation response, identified as M3 in previous studies.
The lack of change in the shorter latency load compensation response, following opposite arm performance, indicates three important facts: First, our paradigm remained sufficiently random as to prevent subjects from modifying their responses by anticipating the loaded trials. Second, and most important, prior opposite arm exposure to the task does not affect the shorter latency component of the response. Third, the change in the long latency response reveals that feedforward modulation of feedback-mediated responses can be transferred across limb effectors, but only for the long latency component of the response. If this response were simply a volitional reaction to the error generated during the first 150 ms of the task, analogous performance would be expected under naïve conditions. Instead, the response was improved only following opposite arm exposure. These findings extend those of previous studies (Laszlo et al. 1970; Latash 1999; Thut et al. 1996; Morton et al. 2001; Dizio and Lackner 1995; Criscimagna-Hemminger et al. 2003; Elliott and Roy 1981; Imamizu and Shimojo 1995; Sainburg and Wang 2002; Wang and Sainburg 2003) by indicating that interlimb transfer is not limited to anticipatory mechanisms associated with adaptation, but can also include feedforward modulation of feedback-mediated responses. Such modulation must arise fairly high in the control hierarchy, prior to separation of limb-specific commands. It remains to be determined whether such transfer would occur across effectors that are not analogous, such as from elbow flexion movements to elbow extension movements.
Acknowledgements
This research was supported by National Institute of Child Health and Human Development Grant R01HD-39311.
References
- Bagesteiro LB, Sainburg RL (2003) Nondominant arm advantages in load compensation during rapid elbow joint movements. J Neurophysiol 90:1503–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, Gorassini M, Prochazka A (1994) Catching a ball: contributions of intrinsic muscle stiffness, reflexes, and higher order responses. Can J Physiol Pharmacol 72:525–534 [DOI] [PubMed] [Google Scholar]
- Bizzi E, Dev P, Morasso P, Polit A (1978) Effect of load disturbances during centrally initiated movements. J Neurophysiol 41:542–556 [DOI] [PubMed] [Google Scholar]
- Bock O (1990) Load compensation in human goal-directed arm movements. Behav Brain Res 41:167–177 [DOI] [PubMed] [Google Scholar]
- Bock O (1993) Early stages of load compensation in human aimed arm movements. Behav Brain Res 55:61–68 [DOI] [PubMed] [Google Scholar]
- Brown SH, Cooke JD (1981) Responses to force perturbations preceding voluntary human arm movements. Brain Res 220:350–355 [DOI] [PubMed] [Google Scholar]
- Brown SH, Cooke JD (1986) Initial agonist burst is modified by perturbations preceding movement. Brain Res 377:311–322 [DOI] [PubMed] [Google Scholar]
- Crago PE, Houk JC, Hasan Z (1976) Regulatory actions of human stretch reflex. J Neurophysiol 39:925–935 [DOI] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Donchin O, Gazzaniga MS, Shadmehr R (2003) Learned dynamics of reaching movements generalize from dominant to nondominant arm. J Neurophysiol 89:168–176 [DOI] [PubMed] [Google Scholar]
- Day BL, Rothwell JC, Marsden CD (1983) Interaction between the long-latency stretch reflex and voluntary electromyographic activity prior to a rapid voluntary motor reaction. Brain Res 270:55–62 [DOI] [PubMed] [Google Scholar]
- De Luca CJ (1997) The use of surface electromyography in biomechanics. J Appl Biomechanics 13:135–163 [Google Scholar]
- Dizio P, Lackner JR (1995) Motor adaptation to Coriolis force perturbations of reaching movements: endpoint but not trajectory adaptation transfers to the nonexposed arm. J Neurophysiol 74:1787–1792 [DOI] [PubMed] [Google Scholar]
- Elliott D, Roy EA (1981) Interlimb transfer after adaptation to visual displacement: patterns predicted from the functional closeness of limb neural control centers. Perception 10:383–389 [DOI] [PubMed] [Google Scholar]
- Evarts EV, Tanji J (1974) Gating of motor cortex reflexes by prior instruction. Brain Res 71 (2–3):479–494 [DOI] [PubMed] [Google Scholar]
- Evarts EV, Tanji J (1976) Reflex and intended responses in motor cortex pyramidal tract neurons of monkey. J Neurophysiol 39:1069–1080 [DOI] [PubMed] [Google Scholar]
- Ghez C, Shinoda Y (1978) Spinal mechanisms of the functional stretch reflex. Exp Brain Res 32:55–68 [DOI] [PubMed] [Google Scholar]
- Gielen CC, Ramaekers L, van Zuylen EJ (1988) Long-latency stretch reflexes as co-ordinated functional responses in man. J Physiol 407:275–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godaux E, Desmedt JE (1979) Long loop reflexes during ballistic movements (proceedings). Arch Int Physiol Biochim 87:346–347 [PubMed] [Google Scholar]
- Hodges PW, Bui BH (1996) A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr Clin Neurophysiol 101:511–519 [DOI] [PubMed] [Google Scholar]
- Houk JC (1976) An assessment of stretch reflex function. Prog Brain Res 44:303–314 [DOI] [PubMed] [Google Scholar]
- Imamizu H, Shimojo S (1995) The locus of visual-motor learning at the task or manipulator level: implications from intermanual transfer. J Exp Psychol Hum Percept Perform 21:719–733 [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Soechting JF, Terzuolo CA (1982) Some factors pertinent to the organization and control of arm movements. Brain Res 252:394–397 [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Borghese NA, Carrozzo M (1991) Transient reversal of the stretch reflex in human arm muscles. J Neurophysiol 66:939–954 [DOI] [PubMed] [Google Scholar]
- Laszlo JI, Baguley RA, Bairstow PJ (1970) Bilateral transfer in tapping skill in the absence of peripheral information. J Mot Behav 2:261–271 [DOI] [PubMed] [Google Scholar]
- Latash ML (1999) Mirror writing: learning, transfer, and implications for internal inverse models. J Mot Behav 31:107–111 [DOI] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB (1973) Latency measurements compatible with a cortical pathway for the stretch reflex in man. J Physiol 230 (1):58P–59P [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB (1976) Stretch reflex and servo action in a variety of human muscles. J Physiol 259:531–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DI, Prochazka A (1994) The role of sensory information in the guidance of voluntary movement: reflections on a symposium held at the 22nd annual meeting of the Society for Neuroscience. Somatosens Mot Res 11:69–76 [DOI] [PubMed] [Google Scholar]
- Morton SM, Lang CE, Bastian AJ (2001) Inter- and intra-limb generalization of adaptation during catching. Exp Brain Res 141:438–445 [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 9:97–113 [DOI] [PubMed] [Google Scholar]
- Prochazka A (1986) Proprioception during voluntary movement. Can J Physiol Pharmacol 64:499–504 [DOI] [PubMed] [Google Scholar]
- Prochazka A (1989) Sensorimotor gain control: a basic strategy of motor systems? Prog Neurobiol 33:281–307 [DOI] [PubMed] [Google Scholar]
- Prochazka A, Stephens JA, Wand P (1979) Muscle spindle discharge in normal and obstructed movements. J Physiol 287:57–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Gillard D, Bennett DJ (1997a) Implications of positive feedback in the control of movement. J Neurophysiol 77:3237–3251 [DOI] [PubMed] [Google Scholar]
- Prochazka A, Gillard D, Bennett DJ (1997b) Positive force feedback control of muscles. J Neurophysiol 77:3226–3236 [DOI] [PubMed] [Google Scholar]
- Prochazka A, Clarac F, Loeb GE, Rothwell JC, Wolpaw JR (2000) What do reflex and voluntary mean? Modern views on an ancient debate. Exp Brain Res 130:417–432 [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Traub MM, Marsden CD (1980) Influence of voluntary intent on the human long-latency stretch reflex. Nature 286:496–498 [DOI] [PubMed] [Google Scholar]
- Sainburg RL (2002) Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res 142:241–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Wang J (2002) Interlimb transfer of visuomotor rotations: independence of direction and final position information. Exp Brain Res 145:437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro MB, Gottlieb GL, Moore CG, Corcos DM (2002) Electromyographic responses to an unexpected load in fast voluntary movements: descending regulation of segmental reflexes. J Neurophysiol 88:1059–1063 [DOI] [PubMed] [Google Scholar]
- Shapiro MB, Gottlieb GL, Corcos DM (2004) EMG responses to an unexpected load in fast movements are delayed with an increase in the expected movement time. J Neurophysiol 91:2135–2147 [DOI] [PubMed] [Google Scholar]
- Tatton WG, Forner SD, Gerstein GL, Chambers WW, Liu CN (1975) The effect of postcentral cortical lesions on motor responses to sudden upper limb displacements in monkeys. Brain Res 96:108–113 [DOI] [PubMed] [Google Scholar]
- Thut G, Cook ND, Regard M, Leenders KL, Halsband U, Landis T (1996) Intermanual transfer of proximal and distal motor engrams in humans. Exp Brain Res 108:321–327 [DOI] [PubMed] [Google Scholar]
- Wang J, Sainburg RL (2003) Mechanisms underlying interlimb transfer of visuomotor rotations. Exp Brain Res 149:520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]


