Abstract
Idiopathic inflammatory myopathy (IIM) summarizes rare, systemic autoimmune conditions primarily characterized by inflammatory damage to the skeletal muscle. Although primary damage occurs to the muscle, these IIM-related conditions involve other organs, including the skin, lungs, upper gastrointestinal tract, joints, and heart. While many patients have an adequate response to immunosuppressive treatment, some patients develop rapidly progressive and treatment-resistant life-threatening courses. Treatment-resistant IIM is challenging for the treating physician and requires interdisciplinary and individualized treatment approaches. Extracorporeal therapy is one option for rescue therapy, with immunoadsorption (IA) having proven more effective than plasma exchange regarding the removal of circulating antibodies. Despite its efficacy and desirable safety profile, the clinical value of IA use in IIM is understudied with no controlled trials reported. Here, we present a review of the current knowledge regarding the management of treatment-resistant IIM and the cases of three patients with treatment-resistant IIM (two with dermatomyositis and one with immune-mediated necrotizing myopathy) who have successfully been treated with IA. All patients responded well to the therapy and experienced no IA-related complications. Taken together, we found IA to be a safe and effective treatment option in treatment-resistant IIM.
Keywords: case report, dermatomyositis, idiopathic inflammatory myopathy, immunoadsorption, myositis
Introduction
Idiopathic inflammatory myopathy (IIM) is a group of rare autoimmune-mediated diseases that are characterized by inflammatory damage to the skeletal muscle. Although IIM patients traditionally present with muscle weakness, IIM diseases can affect other organ systems, such as the skin, in the case of dermatomyositis (DM). The lung is the most commonly involved major organ. Severe cases of IIM can be resistant to standard treatments and can have lethal outcomes.
Treatment options for IIM include eliminating potentially reversible causes, immunosuppressive and immunomodulating medication, and extracorporeal therapies. While addressing potentially reversible causes highlights that some IIMs are triggered by malignancies or drugs such as statins, immunosuppressive and immunomodulating drugs are still the standard of IIM treatment. There is evidence suggesting that at least some of the autoantibodies associated with IIM are pathogenic agents rather than solely diagnostic bystanders. 1 Strategies to suppress these antibodies are attractive therapeutic approaches. For example, B-cell depletion with rituximab has emerged as a valuable option for treatment-resistant IIM cases but has shown varying success.
However, cell-depleting and antiproliferative therapies need up to several weeks or even months to take full effect and do not influence currently circulating pathogenic antibodies. This is a particularly important issue in severe, rapidly progressive courses with tetraparesis, severe respiratory muscle weakness, or severe dysphagia where immediate action is required.
As such an immediate means to remove circulating antibodies, extracorporeal therapy is a valuable treatment option for several immune-mediated conditions and can be divided into two common procedures: plasma exchange (PLEX) and immunoadsorption (IA). While recent studies have featured the use of PLEX in immunological diseases,2,3 this article focuses on the understudied use of IA in IIM.
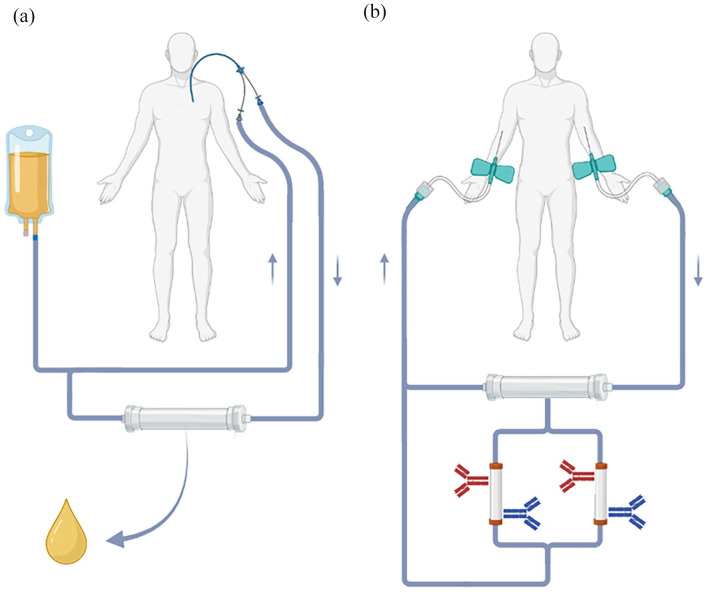
IA can selectively filter antibodies out of the blood. Plasma is removed from the patient’s blood via a plasma filter and subsequently passed through columns coated with protein-binding molecules. For example, columns can be coated with staphylococcal protein A, which binds to the Fc-part of most antibodies. Such columns unselectively remove antibodies from the plasma while other components like albumin and coagulation factors can be reinfused. This negates the need for replacement fluids. Modifying the coatings of the IA columns allows for more selective removal of antibodies that fuel the underlying disease. Figure 1 schematically summarizes the concepts of PLEX and IA.
Figure 1.
Schematic overview of PLEX and IA. (a) In PLEX, plasma is separated from the cellular phase via centrifugation or filtering and mostly discarded afterward. Blood cells are reinfused into the patient after the blood volume has been re-expanded with a replacement fluid, for example, human albumin or allogeneic plasma. (b) In IA, the plasma is not discarded but directed through columns that are coated with molecules that remove certain proteins (e.g. antibodies) from the bloodstream. Along with the blood cells, the remaining plasma is reinfused into the patient. Thus, no exogenous replacement fluids are needed. The depiction of venous access is exemplary. Both procedures can be performed with central or peripheral large-diameter intravenous catheters.
Source: Created with BioRender.
IA, immunoadsorption; PLEX, plasma exchange.
IA has been successfully used in several antibody-mediated conditions spanning neuroimmunological disorders (Guillain–Barré syndrome, myasthenia gravis, multiple sclerosis, chronic inflammatory demyelinating polyneuropathy, neuromyelitis optica spectrum disorders),4–6 preparation of ABO-incompatible kidney recipients, 7 humoral rejection of allogeneic organ transplants, 8 and conditions in internal medicine like anti-glomerular basement membrane (anti-GBM) disease 9 or catastrophic antiphospholipid syndrome. 10
Despite bearing the potential of direct action, there is very little literature and no controlled trials on the effectiveness and safety of IA in IIM. In order to address this sparse evidence, we present a review of the literature on the use of IA in IIM and a case series of three female patients with treatment-resistant IIM [one with immune-mediated necrotizing myositis (IMNM) and two with DM] who were successfully treated with IA. Since most publications are limited to an individual patient case, we believe that our study of three patients is a valuable addition to this underestimated treatment approach and emphasizes the necessity for further investigation through controlled trials.
Methods
Case presentations
We used Case Report guidelines (CARE) 11 as a reference for the creation of our case reports. No genetic testing was performed on the patients, which is why we do not mention this aspect throughout the case reports.
Immunoadsorption
IA was performed in the dialysis unit of the University Hospital of Cologne. Circulatory access was achieved via central venous catheters. TheraSorb® – Ig omni 5 (Miltenyi Biotec, Bergisch Gladbach, Germany) columns were used with the LIFE 21® apheresis unit (Miltenyi Biotec). Patients were treated daily (except for Sundays) for a total amount of sessions mentioned in the respective section of the case presentations. Patient 3 was treated as an outpatient and received the first 10 IA treatments on all weekdays of two consecutive weeks and an additional five treatments performed every other day.
Calculation of the myositis intention-to-treat activity index score
The myositis intention-to-treat activity index (MITAX) is a validated tool for the assessment of disease activity in myositis12,13 that is based on the British Isles Lupus Assessment Group scoring system for disease activity in patients with lupus. 14 The index takes the disease activity in seven organ systems into consideration (constitutional, cutaneous, skeletal, gastrointestinal, pulmonary, cardiovascular, and muscular disease activity) by assigning each organ system a score of 0, 1, 3, or 9, with higher values corresponding to more disease activity. The final MITAX score is calculated by dividing the sum of these values by the maximum possible score of the organ systems considered (63 in our case). We retrospectively calculated the MITAX score based on detailed clinical notes and discharge letters.
Literature review
We queried two independent databases (PubMed and Cochrane) for publications investigating IA in idiopathic inflammatory myositis on 7 August 2023. We used the following search strategies: Medline via PubMed: (‘myositis’ [MeSH Terms] OR ‘myositis’ [Title/Abstract] OR ‘myopathy’ [Title/Abstract]) AND (‘immunoadsorption’ [Title/Abstract] OR ‘antibody removal therapy’ [Title/Abstract] OR ‘extracorporeal immunoadsorption’ [Title/Abstract] OR ‘immunoadsorbent therapy’ [Title/Abstract]). CENTRAL via Cochrane Library: MeSH descriptor: [Dermatomyositis] explode all trees OR MeSH descriptor: [Myositis] explode all trees OR (‘myositis’): ti, ab, kw OR (dermatomyositis): ti, ab, kw AND MeSH descriptor: [Plasmapheresis] explode all trees OR (immunoadsorption): ti, ab, kw. This resulted in 12 articles that were manually screened by the authors for content relevant for this article. Four articles were used for the literature presented in the manuscript.
Results
Case presentations
Case 1
The first patient was a 74-year-old woman with metabolic syndrome, atrial fibrillation, an aortic aneurysm, and no relevant family history. She was admitted to our department in 07/2018 for optimization of her immunosuppressive treatment of biopsy-proven myositis with skin involvement that had been diagnosed a year prior. Her antinuclear antibodies (ANA) titer was highly elevated (1:32,000; performed in the referring center, pattern not reported), and further subclassification revealed positivity for anti-Mi2-autoantibodies. Histopathological evaluation of her muscle biopsy (performed in the referring center) presented necrotizing muscular atrophy and upregulation of major histocompatibility complex (MHC) class I molecules on muscle fibers. These findings are typically seen in anti-Mi2-positive DM. 15
On admission, she presented with a proximal tetraparesis with an emphasis on the lower extremity, erythema of the face and the upper trunk, and Gottron papules. The combination of the typical clinical presentation, findings in laboratory testing, the results from histopathological examination of her muscle biopsy, and the positivity for anti-Mi2-antibodies were consistent with the diagnosis of DM.
In the referring center, the patient underwent treatment with high-dose prednisolone (1 mg/kg per os) and azathioprine (dosage unknown), which was discontinued due to hepatotoxicity. In our facility, the patient’s creatine kinase (CK) was initially slightly elevated (299 U/L; ref <170 U/L) but ultimately rose to a maximum of 5045 U/L. We started the patient on methotrexate (10 mg s.c. weekly), which was discontinued after three applications due to rising liver enzymes. Simultaneously, she underwent treatment with 3 days of 30 g of intravenous immunoglobulins (IVIG, cumulative dosage 1.2 g/kg bodyweight). This yielded temporary relief of fatigue. We repeated the 3-day-IVIG treatment twice with increasing dosages (60 and 100 g; cumulative dosages of 1.6 and 2.7 g/kg body weight, respectively).
As the patient’s tetraparesis continued to progress (mobilization into the standing position was only possible with the help of two), we decided to treat the patient with IA as a rescue option (six sessions) to ameliorate the acute situation. We did not see any complications related to IA treatment. Days after initiation, we saw an increase in muscle strength and a decrease in the MITAX disease activity score (see Table 1). CK normalized to a minimum of 231 U/L. After the termination of IA, the patient was administered two infusions of rituximab (1000 mg each) to prevent an overshooting rebound in antibody synthesis and to keep the patient in long-term remission.
Table 1.
Patient characteristics.
| Characteristic | Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|---|
| Sex | Female | Female | Female | |
| Age (at the time of IA) | 74 | 55 | 55 | |
| Diagnosis | DM (06/2017) | DM (04/2020) | IMNM (2010) | |
| Clinical presentation | Skin • Gottron papules • Erythema of face and décolleté |
Skin • Angioedema of the face • Heliotrope erythema • Gottron papules • Holster sign • Hypomimia |
Muscle • Proximal muscle weakness |
|
| Muscle • Tetraparesis |
Muscle • Tetraparesis |
Extra-muscular • None |
||
| Lung • None |
Lung • None |
|||
| Other • None |
Other • Dysphagia • Angina pectoris |
|||
| ANA (ICAP pattern) | 1:32,000 (not reported) | 1:1000 (AC-4) | 1:1000 (AC-20) | |
| Myositis-specific antibodies | αMi-2 | αTIF-1γ, Ro-52 | αHMG-CoA-reductase | |
| Histopathological findings in biopsies | Muscle Necrotizing muscular atrophy, sarcolemmal upregulation of MHC-I 15 |
Not performed | Muscle Necrotizing myopathy, upregulation of MHC-I on muscle fibers, phagocytosis by CD4+ cells and macrophages, very few CD4+ and CD8+ cells in between intact muscle fibers |
|
| MITAX (organ system scores) | Before IA | 07/2018: 0.24 (constitutional: 3, cutaneous: 3, muscles: 9) |
06/2020: 0.33 (constitutional: 3, cutaneous: 3, GI: 9, pulmonary: 3, muscles: 3) |
02/2021: 0.19 (constitutional: 3, muscles: 9) |
| After termination of IA | 03/2019: 0.02 (constitutional: 1) |
09/2020: 0.03 (constitutional: 1, muscles: 1) |
10/2021: 0.06 (constitutional: 1, muscles: 3) |
|
| During follow-up | 12/2019: 0.06 (constitutional: 1, cutaneous: 3) |
01/2021: 0.00 | 03/2023: 0.06 (constitutional: 1, muscles: 3) |
|
| Pharmacological therapy | Prednisolone, AZA, IVIG, MTX, RTX | Prednisolone, AZA, RTX, IVIG, MMF, BNB, TOF | Prednisolone, AZA, MTX, RTX, MMF, IVIG, TAC, TOF | |
| IA sessions | 6 sessions (08/2018) | 10 sessions (06–07/2020) | 15 sessions (02–03/2021) | |
| IA-related complications | None | None | None | |
| Intravenous device | Shaldon catheter | Shaldon catheter | Demers catheter | |
| Further course | • Recovery of everyday competence • 01/2020: aggravation of myopathy, increase in CK • Progressive dyspnea due to aortic aneurysm and inoperability; best supportive care • Death of pneumonia (2020) |
• Stable remission of DM • Invasive carcinoma of the breast 01/2021; neoadjuvant chemotherapy with epirubicin, paclitaxel, cyclophosphamide, carboplatin + surgery |
Stable partial remission under ongoing treatment with IVIG and MMF | |
ANA, antinuclear antibodies; AZA, azathioprine; BNB, baricitinib; CK, creatine kinase; CRP, C-reactive protein; DM, dermatomyositis; GI, gastrointestinal; αHMG-CoA-reductase, anti-3-hydroxy-3-methylglutaryl coenzyme A reductase antibody; IA, immunoadsorption; ICAP, international consensus on ANA patterns; IMNM, immune-mediated necrotizing myopathy; IVIG, intravenous immunoglobulin; LDH, lactate dehydrogenase; MMF, mycophenolate mofetil; MTX, methotrexate; RTX, rituximab; TAC, tacrolimus; αTIF-1γ, anti-transcriptional intermediary factor 1 gamma antibody; TOF, tofacitinib.
In order to test for a possible trigger for the disease, a comprehensive screening for malignancies was completed and found negative. However, a thoracic computed tomography (CT) scan showed rapid progress of an aortic aneurysm (8 cm), which was not surgically treated due to the reduced general constitution of the patient. Furthermore, a potential operation was refused by the patient and her family. In the follow-up visits over the next year, the patient did not show signs of myositis activity. Eventually, she was admitted to another hospital and subjected to best supportive care as she reported progressive dyspnea due to the growing aneurysm as well as signs of myopathy with rising levels of CK (01/2020). Patient 1 died of pneumonia in 2020. Figure 2(a) shows the timeline of the patient’s treatment.
Figure 2.
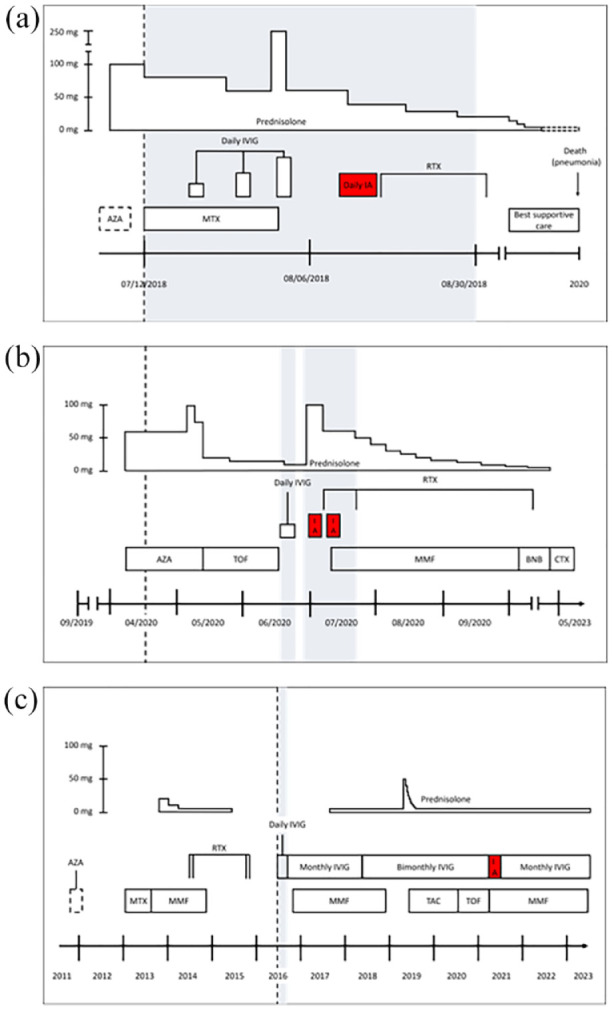
Schematic overview of the treatment course of patient 1 (a), patient 2 (b), and patient 3 (c). Gray boxes mark inpatient treatment periods. The dotted line indicates first presentation to our clinic. Dotted boxes mark uncertain information as it was not fully reported in previous medical reports.
AZA, azathioprine; BNB baricitinib; CTX, chemotherapy; IA, immunoadsorption; IVIG, intravenous immunoglobulins; MMF, mycophenolate mofetil; MTX, methotrexate; RTX, rituximab; TAC, tacrolimus; TOF, tofacitinib.
Case 2
The second patient was a 55-year-old female who was initially treated with breast-conserving therapy (surgery and adjuvant radiotherapy) for a small ductal carcinoma in situ of her right breast (09/2019). No other relevant medical history was reported. The patient’s sister was diagnosed with multiple sclerosis, and her father with rheumatoid arthritis.
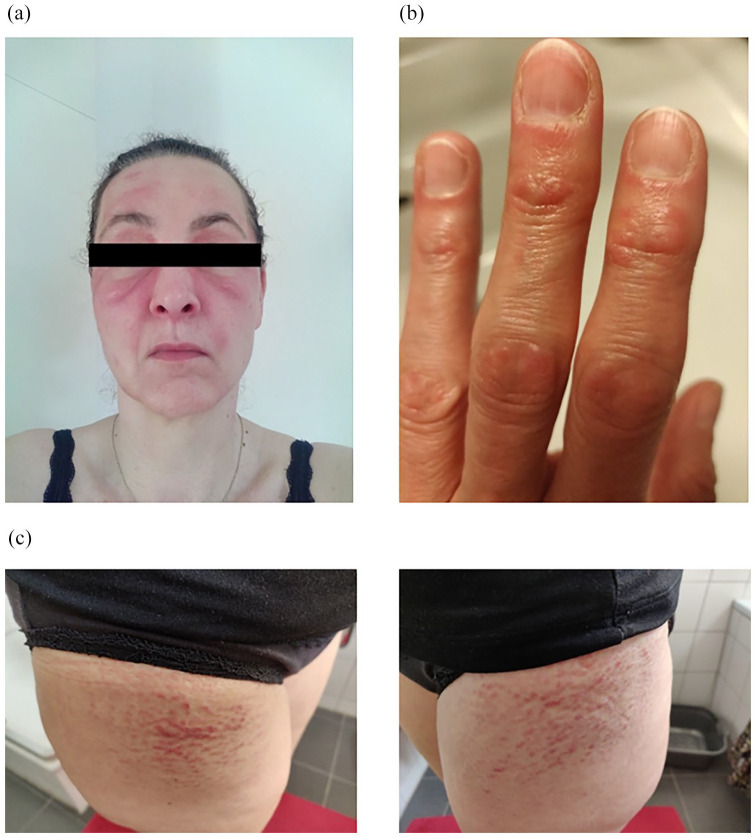
Immediately after surgery, the patient developed erythema of the face and trunk, as well as Gottron papules. Seven months later, severe myalgia, muscle weakness, and fatigue followed. Her primary care physician suspected DM, started her on high-dose prednisolone (1 mg/kg p.o.), and referred her to our outpatient rheumatology clinic (04/2020). Here, she presented with the full clinical picture of DM, including ongoing proximal muscle weakness of all extremities, numbing fatigue, recurrent angina pectoris in the morning, erythema of the face and trunk, Gottron papules, slight scaling of the digital skin, and a positive Holster sign (Figure 3). Laboratory testing was remarkable for slightly elevated levels of LDH (298 U/L, ref < 250 U/L), ANA titers of 1:1000 (AC-4 pattern) and positivity for anti-TIF-1γ- and anti-Ro-52-autoantibodies. Further workup also showed a restrictive ventilation disorder of the lung (total lung capacity 3.68 L, 65% of ref). A high-resolution CT scan of the lung ruled out interstitial lung disease but showed minor fibrotic lesions probably caused by the previous radiotherapy. Given the severe muscle weakness, insufficiency of muscles supporting respiration is the most plausible cause for the ventilation disorder. Taken together, we confirmed the diagnosis of DM.
Figure 3.
Skin manifestations of patient 2 with (a) heliotrope erythema, (b) Gottron papules, and (c) bilateral Holster sign.
We initially started the patient on prednisolone (1 mg/kg p.o.) and azathioprine (50 mg twice daily). We tapered the steroid dosage quickly as we suspected steroid-induced myopathy as an additional factor of her weakness. We also started treatment with tofacitinib (05/2020, 5 mg twice daily) since azathioprine had no effect. However, her muscle weakness progressed, now also involving the pharyngo-esophageal system with worsening dysphagia. This prompted us to discontinue tofacitinib and start IVIG treatment (120 g cumulative; 2.0 g per kg bodyweight). This did not show any effect on her clinical status. Instead, she developed severe facial swelling while dyspnea and dysphagia further worsened as documented by daily logopedic evaluation (progressive weakness of the tongue, velum, and pharyngeal muscles, severely impaired laryngeal elevation). Electromyography showed myopathic changes in proximal muscles of the arms and legs.
To stabilize her acutely deteriorating condition (progressive muscle weakness involving vital systems, high MITAX activity score of 0.33), we decided to treat her with IA, which was performed over a total of 10 sessions in 06–07/2020. IA was well tolerated without complications associated with the treatment. As soon as 1 day after the first IA session, she responded with improvement of the skin manifestations and muscle affection. This was reflected in a marked amelioration of dyspnea and improvement of strength, both subjectively reported by the patient and objectified with improvements in logopedic evaluation and a drop in the MITAX score.
Five days after IA initiation, we added rituximab (1000 mg i.v., second dose 14 days later) and mycophenolate (500 mg twice daily) 2 days later to support induction and maintenance of remission. We paused IA treatment for 3 days to avoid removing rituximab from the circulation and continued with another five sessions afterward. Mycophenolate had to be substituted for baricitinib (10/2020, 4 mg daily) due to another flare of skin involvement. Her muscle affection, however, has been very well-controlled during the follow-up. This was reflected in normal muscle strength with no signs of IIM activity in laboratory parameters. Prednisolone was discontinued completely in 06/2021. Yet, her anti-TIF-1γ-autoantibody remained positive. Unfortunately, the measurement of the antibody was non-quantitative, so it was not possible to determine whether the titer was affected by the treatment.
Underlining the association of her DM subtype with malignancies, she was regularly reevaluated (skeletal scintigraphy 04/2020, gastroscopy and colonoscopy 04/2020, thoracic CT and whole-body positron emission tomography (PET-CT) scan 05/2020, magnetic resonance imaging (MRI) of the head 05/2020, mammography 11/2020) and finally diagnosed with an invasive triple-negative ductal carcinoma of her right breast in 01/2021, which was successfully treated with neoadjuvant chemotherapy and surgery. With treatment of her cancer, she went into complete remission of her DM (normal strength, no skin manifestations), and there is still no sign of recurrence during her regular follow-up visits until 03/2023. The treatment timeline of patient 2 is shown in Figure 2(b).
Case 3
The third case was a 55-year-old woman who was diagnosed with IMNM at the age of 45 after experiencing proximal muscle weakness in the arms and legs. The remaining medical and family history was unremarkable.
At the time of diagnosis (being treated in another clinic), she presented with highly elevated levels of CK (>20,000 U/L). Histopathological examination of a biopsy taken from the quadriceps femoris muscle showed necrotizing myopathy, strong upregulation of MHC class I on muscle fibers, phagocytosis by CD4+ cells and macrophages, and low numbers of CD4+ and CD8+ cells in between healthy muscle fibers. Initially, screening for myositis-specific autoantibodies was unremarkable, but she later tested positive for anti-3-hydroxy-3-methylglutaryl coenzyme A reductase antibody reductase antibodies. Immunosuppressive treatment with prednisolone was reported to have brought only slight symptomatic relief, while azathioprine (12/2011, dosage unknown) was discontinued quickly due to intolerable gastrointestinal side effects. With only partial success, recurrence of the disease was met with intensified supportive therapy and combinations of high-dose steroids, methotrexate (01–09/2013, 7.5–15 mg p.o. weekly), mycophenolate (09/2013–01/2015, 720 mg twice daily), and a total of four treatments with rituximab (twice in 08/2014, 10/2015, 11/2015; 1000 mg each).
On admission to our clinic in 06/2016, the patient reported a progressive loss of muscle strength. We initiated IVIG treatment (30 g daily for 5 days), followed by regular outpatient IVIG infusions (40 g every month and later every other month). This led to partial remission for the following 3 years. She then suffered from progressive muscle wasting despite ongoing and more frequent IVIG treatment (40 g monthly), which was briefly ameliorated by treatment with tacrolimus (07/2019, 1 mg twice daily).
As neither an increase in tacrolimus dosage (3 mg twice daily) nor a temporary switch to tofacitinib (08/2020, 5 mg twice daily) improved the symptoms, we subjected her to IA. Fifteen sessions were performed in an outpatient setting in 02–03/2021 and yielded great improvements in subjective symptom load and muscle strength. CK fell from levels persistently elevated >4000 U/L before IA to a minimum of 244 U/L but later climbed to a plateau of 600–800 U/L. No IA-related complications were observed throughout the treatment period.
Under ongoing outpatient IVIG treatment and medication with mycophenolate, the disease has been well-controlled in a state of partial remission. Figure 2(c) shows the treatment course of patient 3.
Table 1 summarizes the clinical data of our three patients.
Literature review
A systematic search querying two established medical databases (PubMed and Cochrane) for articles investigating IA in IIM resulted in only 12 articles, which were manually screened for relevance. Four of these were deemed relevant as they specifically mention IA in the context of IIM. However, one of the articles was published in Japanese with only the abstract available in English. This sparsity can partially be explained by the fact that IA is only in use in certain countries, excluding the United States.
A 1991 case report from Japan reports successful IA treatment of a polymyositis patient with progressing proximal muscle weakness that was refractory to high-dose steroids and azathioprine. 16 As only the abstract was available in English, it is unclear what the authors considered to be ‘successful’.
In another case report, severe skin manifestation (necrotizing lesions of the hands, Gottron papules) was ameliorated with a combination of IA and thalidomide in one DM patient. In this patient, prior treatment with prednisolone, methotrexate, IVIG, rituximab, and MMF was either not tolerated or ineffective. 17
Further, another patient with refractory muscle weakness and dyspnea due to cryptogenic organizing pneumonia in the context of anti-signal recognition particle (SRP)-positive myositis benefited from a combination of IA and PLEX. However, IA alone was unable to achieve satisfactory treatment success in this context (the authors do not specify which parameters were used to deem the response unsatisfactory). Months later, the authors found a decrease in muscle edema in MRI. Also, CK decreased gradually as the combination of IA and PLEX was continued for 2 more years. No IA-related side effect was seen. 18
In the fourth study, a patient with interstitial pneumonia in the context of anti-synthetase syndrome (ASS) was initially treated with PLEX. However, he developed transfusion-related acute lung injury in response to the plasma transfusions mandatory in PLEX. He was then switched to nine sessions of daily IA. In combination with cyclosporine A treatment, the patient recovered and was taken off mechanical ventilation 6 days later. 19
In addition to our systematic search, we found an abstract published by a group from Vienna on 24 patients collected over more than two decades. This study suggests that PLEX and IA are both effective strategies as they significantly reduce CK and enable quicker steroid tapering. 20 However, using the decrease of serological parameters like CK as an outcome in patients treated with PLEX is not ideal as this decrease is to be expected since it is the nature of the procedure to remove these parts from the patient’s blood. Indeed, a controlled trial from 1992 showed that these endpoints might not be clinically relevant as it found no difference in patients with PM and DM undergoing PLEX or sham apheresis. 21 The limited data available in the abstract does not specifically mention the safety aspects of the treatment modalities.
Discussion
Here, we used IA as a rescue option in three critically ill patients with progressive treatment-resistant IIM without any intervention-associated complications and complemented our data with a review of the literature on the use of IA in IIM. In line with the limited data available, we found that IA improved clinical symptoms and scoring in a disease activity index, as well as decreased CK. However, it needs to be mentioned that CK levels are not a reliable parameter to measure clinical improvement, but might be helpful in individual cases. 22 Most studies across the subgroups of IIM found that CK levels do not correlate with muscular function.23–26
Extracorporeal therapies have several advantages and disadvantages compared with drug therapy alone. The major advantage of extracorporeal therapies, such as IA, is the immediate effect due to the direct, physical removal of circulating pathogenic antibodies. Except glucocorticoids, all other therapies, such as proliferation inhibitors or cell-depleting antibodies, can take weeks or months to take full effect. In refractory cases, glucocorticoids are required in high doses, which causes significant side effects such as susceptibility to infections, acute psychosis, depression, hyperglycemia, arterial hypertension, or steroid-induced muscle weakness. In addition, these treatment options are not sufficiently effective in some acute, progressive cases.
Concerning the question of which procedure to use as rescue therapy, we favored IA over PLEX for two reasons. First, IA is more effective in removing antibodies than PLEX. The efficacy of PLEX is dependent on the blood concentration of the antibody to be removed, which makes it a saturating procedure (first-order kinetics). Thus, efficacy decreases over time. The removal of antibodies in IA, on the other hand, is based on column-antibody affinity. This means that antibodies are effectively filtered out of the blood even if it is present in very small concentrations (zero-order kinetics). Usually, two columns are used in an IA session: While the patient’s plasma is guided through one of them, the other can be regenerated by washing the bound antibodies away. This setup allows for the ongoing removal of antibodies with – at least theoretically – no upper limit. A retrospective study compared the efficacy of PLEX and IA in the removal of donor-specific antibodies (DSA) before kidney transplantation or for treatment of antibody-mediated rejection of a kidney transplant. The analysis of 440 serum samples revealed that a single session of IA reduced the mean fluorescent intensity of DSA by 69%, while three sessions of PLEX only led to a reduction of 58%. 27 To achieve a reduction of 90% of IgG antibodies with PLEX, the 1.5-fold plasma volume must be treated at least five times within a period of 7–10 days, while a reduction of >90% is very hard to achieve with this procedure. IA can reduce the levels of circulating IgG by 95% in only two sessions. 28 Therefore, IA allows ongoing and more effective removal of antibodies. In contrast to PLEX, the efficacy of IA does not depend on periods of waiting for the pathological antibodies to build up again, with the risk of potentially fatal relapses in this patient cohort that is critically ill to begin with. Thus, IA is the better option if the goal is to keep antibody titers consistently at a low level.
Second, IA is considered to be a safe procedure. Of course, every kind of extracorporeal circulation harbors the risk of complications, which include the need for anticoagulation, hemodynamic alterations, and infectious complications. However, both IA and PLEX rarely cause serious adverse events and several studies report superior safety of IA compared to PLEX. IA circumvents the need for supplementation with recombinant albumin or transfusion products. This negates the risk of complications by transfusions and reduces the risk of allergic reactions in comparison to PLEX. As mentioned in our literature review section, one patient with ASS developed transfusion-related lung injury in the course of PLEX treatment, a very concerning complication in a patient that already suffered from severe lung involvement of the autoimmune condition. The switch to IA avoided this danger, as no replacement fluids were needed. Allergic reactions to components of the IA circulatory system, like filters and columns, can occur and pose the most frequent complication of the treatment. Yet, they are less frequent compared to reactions to the replacement fluids in PLEX. We did not find any IA-related complications in our three patients or the literature review.
There are no trials specifically investigating the safety of IA in IIM. However, IA is a very safe intervention in other disease settings. A prospective study investigated the safety of IA in a range of diseases (mostly neuroinflammatory disorders). 29 In this mixed cohort of 81 patients, the authors found electrolyte imbalances (hypokalemia and hypocalcemia) to be the most frequently observed adverse effects. No serious adverse events or deaths were reported. A 2020 meta-analysis analyzed the safety of IA using data from more than 1300 patients with demyelinating diseases. Bleeding, allergic reactions, and respiratory infections were reported. Yet, the majority of these events were mild. 30 One recent randomized controlled trial comparing the safety of IA versus PLEX in 61 patients with multiple sclerosis found both procedures to be safe, with one deep vein thrombosis without long-term consequences having occurred in both groups. 4
Taken together, IA is a safe option for extracorporeal removal of antibodies in several autoimmune conditions. The limited data available indicates that the same is true for the use of IA in IIM.
Despite the superior efficacy, the good safety profile, and the independence from access to replacement fluids like albumin or transfusion products, IA is rarely used. High cost, difficulties in reimbursement, and, hence, its rare application limit the required technical knowledge to specialized centers.
Another important point to consider is that the effect of IA is not persistent and that a combination with drug therapy is essential. The mere removal of circulating antibodies can rapidly reduce the load of pathogenic antibodies. However, the antibody-producing cells remain active and unaffected. Indeed, an overshooting rebound of (auto-)antibody production is a well-known phenomenon after PLEX, which can worsen the disease that was supposed to be treated.31,32 Therefore, extracorporeal treatments are inherently time-limited and are not supposed to be used alone in refractory autoimmune conditions but should always be combined with other immunosuppressive treatments to prevent overshooting rebound effects and to preserve the state of remission. Thus, extracorporeal therapies should be seen as a valuable adjunct in induction therapy, which can serve as a bridge in rapidly progressing, refractory cases to reduce disease activity until drug therapies take effect.
From an evidence-based point of view, the major drawback of trying to distill evidence for a single treatment from limited case reports of critically ill patients is that individual effects cannot be well separated in these ethically imperative multimodal treatment concepts. Thus, all our patients were under ongoing strong immunosuppressive treatment once IA was started. Nevertheless, the addition of IA did have a substantial effect on the clinical situation of the three patients reported here. This was mirrored in breaking plateaus in objective testing (MITAX, physiotherapy, logopedic evaluation) and laboratory values. Still, further studies are needed to evaluate the role of IA as a rescue therapy in treatment-resistant IIM.
Conclusion
In summary, we report that IA represents a safe and effective rescue option for treatment-resistant IIM. Since the published evidence on this topic is very sparse, we sought to draw more attention to this option by reviewing the literature and presenting three cases of patients with severe, deteriorating IIM who were successfully treated with IA without any IA-associated complications. The major drawbacks of IA treatment are reimbursement issues due to its high cost and limited availability, both on a global (IA is only approved in certain countries) and on a local (know-how is limited to specialized centers) scale. Controlled studies are urgently needed to further evaluate this type of therapy for severely ill patients with treatment-resistant IIM.
Acknowledgments
None.
Footnotes
ORCID iD: Jasper F. Nies  https://orcid.org/0000-0003-3751-4008
https://orcid.org/0000-0003-3751-4008
Contributor Information
Jasper F. Nies, Department II of Internal Medicine and Center for Molecular Medicine Cologne, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany
Claudia Hendrix, Department II of Internal Medicine and Center for Molecular Medicine Cologne, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
Malte P. Bartram, Department II of Internal Medicine and Center for Molecular Medicine Cologne, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany
Ryan Spear, Department of Internal Medicine, Rush University Medical Center, Chicago, IL, USA.
Henning Hagmann, Department II of Internal Medicine and Center for Molecular Medicine Cologne, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
Thomas Benzing, Department II of Internal Medicine and Center for Molecular Medicine Cologne, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
Torsten Kubacki, Department II of Internal Medicine and Center for Molecular Medicine Cologne, Faculty of Medicine and University Hospital Cologne, University of Cologne, Kerpener Straße 62, Cologne 50924, Germany.
Declarations
Ethics approval and consent to participate: The Ethics Committee of the Medical Faculty of the University Medical Center Cologne waived the requirement for approval (reference number 24-1009-retro). Consent to participate: Not applicable.
Consent for publication: All patients or their legal guardians (patient 1) gave written consent for publication of their data. Patient 2 gave written informed consent to the publication of the photographs in Figure 3 that she kindly provided us with.
Author contributions: Jasper F. Nies: Data curation; Formal analysis; Methodology; Visualization; Writing – original draft; Writing – review & editing.
Claudia Hendrix: Data curation; Formal analysis; Writing – review & editing.
Malte P. Bartram: Conceptualization; Data curation; Supervision; Writing – review & editing.
Ryan Spear: Methodology; Writing – review & editing.
Henning Hagmann: Conceptualization; Data curation; Supervision; Writing – review & editing.
Thomas Benzing: Conceptualization; Supervision; Writing – review & editing.
Torsten Kubacki: Conceptualization; Data curation; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Galindo-Feria AS, Wang G, Lundberg IE. Autoantibodies: pathogenic or epiphenomenon. Best Pract Res Clin Rheumatol 2022; 36: 101767. [DOI] [PubMed] [Google Scholar]
- 2. Jacob S, Mazibrada G, Irani SR, et al. The role of plasma exchange in the treatment of refractory autoimmune neurological diseases: a narrative review. J Neuroimmune Pharmacol 2021; 16: 806–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kronbichler A, Brezina B, Quintana LF, et al. Efficacy of plasma exchange and immunoadsorption in systemic lupus erythematosus and antiphospholipid syndrome: a systematic review. Autoimmun Rev 2016; 15: 38–49. [DOI] [PubMed] [Google Scholar]
- 4. Dorst J, Fangerau T, Taranu D, et al. Safety and efficacy of immunoadsorption versus plasma exchange in steroid-refractory relapse of multiple sclerosis and clinically isolated syndrome: a randomised, parallel-group, controlled trial. EClinicalMedicine 2019; 16: 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yasuda M, Uzawa A, Ozawa Y, et al. Immunoadsorption apheresis versus intravenous immunoglobulin therapy for exacerbation of myasthenia gravis. Scand J Immunol 2022; 95: e13122. [DOI] [PubMed] [Google Scholar]
- 6. Oji S, Nomura K. Immunoadsorption in neurological disorders. Transfus Apher Sci 2017; 56: 671–676. [DOI] [PubMed] [Google Scholar]
- 7. Tiwari AK, Aggarwal G, Arora D, et al. Immunoadsorption in ABO-incompatible kidney transplantation in adult and pediatric patients with follow-up on graft and patient survival: first series from India. Asian J Transfus Sci 2020; 14: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schinstock CA, Mannon RB, Budde K, et al. Recommended treatment for antibody-mediated rejection after kidney transplantation: the 2019 Expert Consensus from the Transplantion Society Working Group. Transplantation 2020; 104: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Biesenbach P, Kain R, Derfler K, et al. Long-term outcome of anti-glomerular basement membrane antibody disease treated with immunoadsorption. PLoS One 2014; 9: e103568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kronbichler A, Frank R, Kirschfink M, et al. Efficacy of eculizumab in a patient with immunoadsorption-dependent catastrophic antiphospholipid syndrome: a case report. Medicine (Baltimore) 2014; 93: e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. J Med Case Rep 2013; 7: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Isenberg DA, Allen E, Farewell V, et al. International consensus outcome measures for patients with idiopathic inflammatory myopathies. Development and initial validation of myositis activity and damage indices in patients with adult onset disease. Rheumatology (Oxford) 2004; 43: 49–54. [DOI] [PubMed] [Google Scholar]
- 13. Sultan SM, Allen E, Oddis CV, et al. Reliability and validity of the myositis disease activity assessment tool. Arthritis Rheum 2008; 58: 3593–3599. [DOI] [PubMed] [Google Scholar]
- 14. Hay EM, Bacon PA, Gordon C, et al. The BILAG index: a reliable and valid instrument for measuring clinical disease activity in systemic lupus erythematosus. Q J Med 1993; 86: 447–458. [PubMed] [Google Scholar]
- 15. Fornaro M, Girolamo F, Cavagna L, et al. Severe muscle damage with myofiber necrosis and macrophage infiltrates characterize anti-Mi2 positive dermatomyositis. Rheumatology (Oxford) 2021; 60: 2916–2926. [DOI] [PubMed] [Google Scholar]
- 16. Maruyama T, Koh CS, Inoue A, et al. [Successful treatment of ‘steroid-immunosuppressant resistant’ polymyositis with immunoadsorption]. Rinsho Shinkeigaku 1991; 31: 1208–1213. [PubMed] [Google Scholar]
- 17. Sebastiani M, Puccini R, Manfredi A, et al. Staphylococcus protein A-based extracorporeal immunoadsorption and thalidomide in the treatment of skin manifestation of dermatomyositis: a case report. Ther Apher Dial 2009; 13: 225–228. [DOI] [PubMed] [Google Scholar]
- 18. Wantke F, Kneussl M, Hubner M, et al. Signal recognition particle (SRP) positive myositis in a patient with cryptogenic organizing pneumonia (COP). Rheumatol Int 2010; 30: 1361–1365. [DOI] [PubMed] [Google Scholar]
- 19. Eller P, Flick H, Schilcher G, et al. Successful treatment of severe interstitial pneumonia by removal of circulating autoantibodies: a case series. BMC Pulm Med 2021; 21: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kastrati K, Rajab H, Rader A, et al. AB0718 immune-apheresis in patients with inflammatory myopathies, a case series. Ann Rheum Dis 2022; 81: 1486–1487.36008130 [Google Scholar]
- 21. Miller FW, Leitman SF, Cronin ME, et al. Controlled trial of plasma exchange and leukapheresis in polymyositis and dermatomyositis. N Engl J Med 1992; 326: 1380–1384. [DOI] [PubMed] [Google Scholar]
- 22. Weeding E, Tiniakou E. Therapeutic management of immune-mediated necrotizing myositis. Curr Treatm Opt Rheumatol 2021; 7: 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arcani R, Rey L, Mazziotto A, et al. Anti-Jo-1 autoantibodies: biomarkers of severity and evolution of the disease in antisynthetase syndrome. Arthritis Res Ther 2023; 25: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saygin D, Rockette-Wagner B, Oddis C, et al. Consumer-based activity trackers in evaluation of physical activity in myositis patients. Rheumatology (Oxford) 2022; 61: 2951–2958. [DOI] [PubMed] [Google Scholar]
- 25. Benveniste O, Drouot L, Jouen F, et al. Correlation of anti-signal recognition particle autoantibody levels with creatine kinase activity in patients with necrotizing myopathy. Arthritis Rheum 2011; 63: 1961–1971. [DOI] [PubMed] [Google Scholar]
- 26. Benveniste O, Goebel HH, Stenzel W. Biomarkers in inflammatory myopathies – an expanded definition. Front Neurol 2019; 10: 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maillard N, Absi L, Claisse G, et al. Protein A-based immunoadsorption is more efficient than conventional plasma exchange to remove circulating anti-HLA antibodies. Blood Purif 2015; 40: 167–172. [DOI] [PubMed] [Google Scholar]
- 28. Schmaldienst S, Mullner M, Goldammer A, et al. Intravenous immunoglobulin application following immunoadsorption: benefit or risk in patients with autoimmune diseases? Rheumatology (Oxford) 2001; 40: 513–521. [DOI] [PubMed] [Google Scholar]
- 29. Fuchs K, Rummler S, Ries W, et al. Performance, clinical effectiveness, and safety of immunoadsorption in a wide range of indications. Ther Apher Dial 2022; 26: 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lipphardt M, Wallbach M, Koziolek MJ. Plasma exchange or immunoadsorption in demyelinating diseases: a meta-analysis. J Clin Med 2020; 9: 1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ching J, Richards D, Lewis RA, et al. Myasthenia gravis exacerbation in association with antibody overshoot following plasmapheresis. Muscle Nerve 2021; 64: 483–487. [DOI] [PubMed] [Google Scholar]
- 32. Derksen RH, Schuurman HJ, Gmelig Meyling FH, et al. Rebound and overshoot after plasma exchange in humans. J Lab Clin Med 1984; 104: 35–43. [PubMed] [Google Scholar]