Abstract
Background:
X-linked hypophosphatemia (XLH) is a rare, progressive disorder characterized by excess fibroblast growth factor 23 (FGF23), causing renal phosphate-wasting and impaired active vitamin D synthesis. Burosumab is a recombinant human monoclonal antibody that inhibits FGF23, restoring patient serum phosphate levels. Safety data on long-term burosumab treatment are currently limited.
Objectives:
This post-authorization safety study (PASS) aims to monitor long-term safety outcomes in children and adolescents (1–17 years) treated with burosumab for XLH. This first interim analysis reports the initial PASS safety outcomes.
Design:
A 10-year retrospective and prospective cohort study.
Methods:
This PASS utilizes International XLH Registry (NCT03193476) data, which includes standard diagnostic and monitoring practice data at participating European centers.
Results:
At data cut-off (13 May 2021), 647 participants were included in the International XLH Registry; 367 were receiving burosumab, of which 67 provided consent to be included in the PASS. Mean (SD) follow-up time was 2.2 (1.0) years. Mean (SD) age was 7.3 (4.3) years (range 1.0–17.5 years). Mean duration of burosumab exposure was 29.7 (25.0) months. Overall, 25/67 participants (37.3%) experienced ⩾1 adverse event (AE) during follow-up; 83 AEs were reported. There were no deaths, no AEs leading to treatment withdrawal, nor serious AEs related to treatment. The most frequently reported AEs were classified as ‘musculoskeletal and connective tissue disorders’, with ‘pain in extremity’ most frequently reported, followed by ‘infections and infestations’, with ‘tooth abscess’ the most frequently reported.
Conclusion:
In this first interim analysis of the PASS, covering the initial 2 years of data collection, the safety profile of burosumab is consistent with previously reported safety data. The PASS will provide long-term safety data over its 10-year duration for healthcare providers and participants with XLH that contribute to improvements in the knowledge of burosumab safety.
Trial registration:
European Union electronic Register of Post-Authorisation Studies: EUPAS32190.
Keywords: burosumab, patient registry, post-authorization safety study, safety, X-linked hypophosphatemia
Introduction
X-linked hypophosphatemia (XLH) is a rare, chronic, and progressive genetic disease caused by mutations in the phosphate-regulating endopeptidase homolog X-linked (PHEX) gene.1,2 Loss of function of PHEX results in excess levels of circulating fibroblast growth factor 23 (FGF23), causing excessive renal phosphate-wasting in addition to decreased activation of vitamin D to calcitriol, which leads to chronic hypophosphatemia.1,3,4 Children with XLH usually present with signs and symptoms of rickets and osteomalacia, bowing of the legs, disproportionate and short stature, and may experience undesirable dental conditions, including tooth abscess.2,5–8 A 2009 Danish study reported that the estimated incidence of hereditary hypophosphatemic rickets in children was 3.9 per 100,000 per year. 9 More recent UK and Norway studies have reported a lower estimated incidence of 1.4 and 1.7 per 100,000 per year, respectively.7,10 Conventional therapy for XLH typically involves the use of oral phosphate supplementations and active vitamin D, with the aim of improving disease symptoms and complications, such as correcting rickets, improving growth and dental health, and reducing bone pain.5,6 However, in most cases, this treatment is only partially successful; supplementation is unable to correct renal phosphate-wasting to normalize plasma phosphate levels and can be associated with clinically significant side effects. 5
Burosumab (Crysvita®; Kyowa Kirin) is a recombinant human immunoglobulin G1 monoclonal antibody that binds to and inhibits the biological activity of excess FGF23, thereby minimizing the clinical consequences of XLH by restoring normal serum phosphate levels. Burosumab received a conditional marketing authorization in the EU in 2018, for the treatment of XLH with radiographic evidence of bone disease in children aged 1 year and older and adolescents with a growing skeleton, which was subsequently converted to a standard marketing authorization in 2022. Later in 2018, burosumab was approved by the US Food and Drug Administration for the treatment of XLH in adults and children aged 1 year and older, and subsequently expanded to children aged 6 months and older in 2019 11 ; burosumab has also been approved for the treatment of both children and adults in several other countries, including Japan, Canada, and Switzerland. 12 In 2020, burosumab was approved for use in older adolescents and adults with XLH in the EU. 13 The recommended starting dose in children and adolescents with XLH aged 1–17 years is 0.8 mg/kg of body weight given every 2 weeks subcutaneously, which may be increased stepwise by 0.4 mg/kg up to a maximum dose of 2.0 mg/kg (maximum dose of 90 mg), to ensure that fasting serum phosphate is maintained within the reference range for age. 13 In accordance with good pharmacovigilance practices, a risk management plan was required for burosumab in children and adolescents. As part of the risk management plan agreed upon at the time of the conditional marketing authorization, Kyowa Kirin initiated a post-authorization safety study (PASS) utilizing data collected from participants included in the existing International XLH Registry [ClinicalTrials.gov identifier: NCT03193476], of which the protocol and methods have been described previously. 1 Here, we present the first interim analysis results of the PASS.
Methods
Objectives
The rationale and description of this PASS of burosumab use in patients with XLH are covered in detail elsewhere. 14 Briefly, this study aims to monitor long-term safety outcomes and determine the frequency and severity of any adverse events (AEs) during burosumab treatment of XLH in children and adolescents in clinical practice. Of note, burosumab is contraindicated in the setting of severe kidney impairment or end-stage kidney disease.11,13 Use of burosumab in patients with XLH and mild to moderate chronic kidney disease (CKD) is a key safety interest to be studied in this long-term PASS.
As a secondary objective, this PASS aims to compare safety outcomes in participants exposed to burosumab, to those in participants receiving other treatments for XLH; this objective will be addressed in future reports and is not included in this first interim analysis.
Study design
This is a 10-year retrospective and prospective cohort study using data embedded within the International XLH Registry. The methods for the International XLH Registry have been reported previously. 1 In brief, the International XLH Registry is a multicenter, noninterventional, observational registry of participants with XLH, collecting data retrospectively data at study entry, and prospectively at follow-up. 1 The International XLH Registry captures standard diagnostic and monitoring practice data in adult and pediatric participants with XLH. 1 Only data collected during standard routine examinations are recorded in the International XLH Registry. The PASS is a substudy embedded within the International XLH Registry. At the time of writing, the currently approved version of the PASS is Version 2.0, which includes adult participants in addition to children older than 1 year, including adolescents. The data cut-off for this first interim analysis was before the implementation of Version 2.0; thus, no adult participants are included in this analysis. This analysis includes data on participants residing in France, Italy, the Netherlands, Norway, Spain, and the United Kingdom. AEs were recorded from the time of enrollment in the International XLH Registry; AEs related to treatment before enrollment were not evaluated.
Participants
Participants in the PASS included children aged ⩾1 year and adolescents with a growing skeleton (aged <18 years). Included participants required a clinical presentation, along with radiological, biochemical, or genetic investigation results in support of a diagnosis of XLH, as determined by the treating physician. Written informed consent (parental/legal guardian, or adolescent assent, where applicable) was required for participation in the International XLH Registry and inclusion in the PASS analysis.
As a noninterventional study, all data were collected during the usual clinical management of these participants. All participants investigated for the primary objective were expected to receive treatment with burosumab in line with the stipulations of the marketing authorization. Therefore, all participants investigated for the primary objective comprise children aged ⩾1 year and adolescents with a growing skeleton, receiving burosumab for the treatment of XLH and enrolled in the International XLH Registry via 1 of the 18 participating European countries.
Study size
As the PASS utilized data from a prospective observational rare disease patient registry, the sample size was not based on statistical considerations, but on evidence provided by XLH clinical experts across Europe. This suggested that a recruitment target of 1200 patients with XLH would be sufficient to enable robust research to be conducted. 1 It is estimated that approximately 400 participants will be exposed to burosumab in the 10-year duration of the PASS and approximately 800 participants will receive either an alternative, or no treatment, which will form the comparator cohort for the secondary objective. 14
The data cut-off point for the current analysis was based on a predetermined milestone; at least 50 pediatric participants must have achieved at least 6 months on the PASS, that is, at least 6 months of burosumab treatment. This milestone for the first interim analysis was reached on 13 May 2021.
Data analysis
Data were summarized using descriptive statistics. For continuous variables, the number of reported values and number of ‘not reported’ values [i.e., n (not reported), arithmetic mean, standard deviation (SD), median, first and third quartiles, minimum, and maximum] were presented. Categorical variables were summarized by frequency counts (n) and percentages (%) of participants in each category, unless otherwise specified. The safety analysis set (SAF) included all screened participants enrolled in the International XLH Registry, where the participant’s hospital site had agreed to participate in the PASS; the participant’s reported age at the index date was ⩾1 and <18 years; and the participant (or legal guardian) had signed informed consent for enrollment in the International XLH Registry and the PASS, had confirmed re-consent in the electronic data capture form, and the participant had received burosumab treatment between 30 days before signing the informed consent form and the data cut-off date of the first interim analysis in the PASS (13 May 2021).
Analyses were conducted for the overall SAF population and stratified according to the following age groups at first informed consent date: toddler (1 to <5 years), children (5 to <12 years), and adolescents (12 to <18 years).
Results
Participant disposition and characteristics
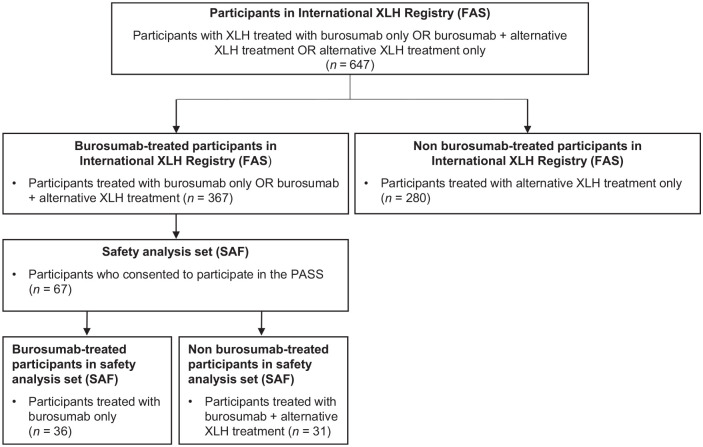
A total of 647 participants in the International XLH Registry were screened at the date of data cut-off (13 May 2021), of whom 367 were receiving burosumab alone or with other treatment. Among these 367 participants, 67 consented to participate in the PASS and were included in the SAF. Among participants in the SAF population (n = 67), 36 received burosumab as their only treatment for XLH during the study period; 31 participants received burosumab plus other treatment at some point during the study period, either sequentially or concomitantly with burosumab (Figure 1). Treatments recorded in addition to burosumab, though not necessarily concomitantly, were phosphate, active vitamin D, growth hormone, calcium carbonate, calcium carbonate and cholecalciferol, cholecalciferol, and ‘other’. The mean (SD) follow-up time was 2.2 (1.0) years. The mean (SD) age of the overall participant population was 7.3 (4.3) years; range 1.0–17.5 years. Overall, 31 males and 36 females (53.7%) were included. Of these 67 participants, 22 children (32.8%) were aged 1 to <5 years, 34 (50.7%) were aged 5 to <12 years, and 11 (16.4%) were adolescents (aged 12 to <18 years). An overview of participant characteristics can be found in Table 1. Of note, although one female participant was of child-bearing potential, no pregnancies were reported over the course of the study.
Figure 1.
Participant flow diagram (children ⩾1 year and adolescents).
FAS, full analysis set; PASS, post-authorization safety study; SAF, safety analysis set; XLH, X-linked hypophosphatemia.
Table 1.
Demographic characteristics by age group at baseline (safety analysis set).
| Characteristic | Overall population, n = 67 | (1 to <5 years), n = 22 | (5 to <12 years), n = 34 | (12 to <18 years), n = 11 |
|---|---|---|---|---|
| Age at informed consent, years | ||||
| Mean (SD) | 7.3 (4.3) | 2.6 (1.1) | 8.0 (2.0) | 14.3 (2.0) |
| Median (min, max) | 6.8 (1.0, 17.5) | 2.4 (1.0, 4.2) | 7.3 (5.0, 11.8) | 13.3 (12.3, 17.5) |
| Sex, n (%) | ||||
| Male | 31 (46.3) | 11 (50.0) | 15 (44.1) | 5 (45.5) |
| Female | 36 (53.7) | 11 (50.0) | 19 (55.9) | 6 (54.5) |
| If female, child-bearing potential, n | 34 | 11 | 18 | 5 |
| Yes, n (%) | 1 (2.9) | 0 | 0 | 1 (20.0) |
| No, n (%) | 33 (97.1) | 11 (100) | 18 (100) | 4 (80.0) |
| Missing, n (%) | 2 | 0 | 1 | 1 |
| Race, n (%) | ||||
| White | 48 (71.6) | 17 (77.3) | 23 (67.6) | 8 (72.7) |
| Black/African | 3 (4.5) | 1 (4.5) | 2 (5.9) | 0 |
| Asian | 2 (3.0) | 0 | 2 (5.9) | 0 |
| Other | 9 (13.4) | 2 (9.1) | 6 (17.6) | 1 (9.1) |
| Not applicable/not collected | 2 (3.0) | 0 | 0 | 2 (18.2) |
| Unknown | 3 (4.5) | 2 (9.1) | 1 (2.9) | 0 |
Max, maximum; min, minimum; SD, standard deviation.
Study medication exposure
During the study period, a total of 36 participants received burosumab only; 31 participants received other therapy either sequentially or concomitantly with burosumab. The majority of participants who received burosumab plus other treatment during the study period received oral phosphate supplements (n = 28/67; 41.8%) and/or active vitamin D (n = 24/67; 35.8%). Recorded concomitant use of either phosphate supplements or active vitamin D consisted of six participants who did not discontinue active vitamin D treatment before initiation of burosumab. These participants were flagged, in accordance with the Summary of Product Characteristics of burosumab, as taking a contraindicated combination of treatment. It was not possible to determine if the participants who did not discontinue active vitamin D treatment experienced AEs during this study or were included in the CKD cohort. In this study population, no treatment with growth hormone was reported during the study period.
The mean (SD) duration of exposure to burosumab was 29.7 (25.0) months. The mean (SD) drug dose was 32.9 (20.5) mg every 2 weeks subcutaneously [1 to <5 years: 20.5 (8.6) mg; 5 to <12 years: 32.7 (15.5) mg; 12 to <18 years: 58.2 (27.9) mg]. In participants who used phosphate during the treatment period, the mean (SD) duration of exposure to phosphate was 20.7 (19.9) months; the mean (SD) individual dose was 178.5 (184.9) mg. In participants who received treatment with active vitamin D during the treatment period, the mean (SD) duration of exposure to active vitamin D was 23.4 (21.3) months. As this is a noninterventional study collecting real-world data, patients were not excluded from the SAF if a dose was missed or if they required a dose suspension. In addition, interruptions and compliance were not considered for the duration of cumulative exposure.
Abnormal phosphate and parathyroid hormone levels, hypercalciuria, and nephrocalcinosis
Serum phosphate levels were reported for 14 participants at baseline (of which six were abnormal and clinically significant), and for two participants prospectively (of which one was abnormal and clinically significant). No instances of hyperphosphatemia were recorded. Parathyroid hormone levels were reported for 22 participants at baseline and five prospectively. In total, four baseline and zero prospective results were reported to be abnormal and clinically significant. Nephrocalcinosis was reported in one patient at baseline, with one patient reported as ‘diagnosed or treated’ during follow-up. No AEs of ectopic mineralization, including nephrocalcinosis, hyperphosphatemia, increased parathyroid hormone, or hypercalciuria, were reported during the study.
Safety
Overall participant population
Overall, 25 of 67 participants (37.3%) experienced ⩾1 AE prospectively during the study period (Table 2). In total, 83 AEs were reported. No deaths, no serious AEs (SAEs) related to XLH treatment, and no AEs leading to XLH treatment withdrawal were reported in the study at data cut-off.
Table 2.
Overview of AEs, overall and by age group (safety analysis set).
| N (%) | Participants receiving burosumab | |||
|---|---|---|---|---|
| Overall population, n = 67 | (1 to <5 years), n = 22 | (5 to <12 years), n = 34 | (12 to <18 years), n = 11 | |
| Any AE | 25 (37.3) | 7 (31.8) | 13 (38.2) | 5 (45.5) |
| Any AE possibly/probably related to XLH treatment a | 13 (19.4) | 4 (18.2) | 7 (20.6) | 2 (18.2) |
| Any AE leading to death | 0 | 0 | 0 | 0 |
| Any AE leading to death and possibly/probably related to XLH treatment a | 0 | 0 | 0 | 0 |
| Any AE leading to XLH treatment withdrawn | 0 | 0 | 0 | 0 |
| Any AE leading to XLH treatment withdrawn and possibly/probably related to XLH treatment a | 0 | 0 | 0 | 0 |
| Any severe AE | 4 (6.0) | 0 | 3 (8.8) | 1 (9.1) |
| Any SAE | 2 (3.0) | 0 | 2 (5.9) | 0 |
| Any SAE possibly/probably related to XLH treatment a | 0 | 0 | 0 | 0 |
Percentages are calculated using the number of participants in the safety analysis set as denominator.
The relationship of the AE with XLH treatment as reported by the investigator.
AE, adverse event; SAE, serious adverse event; XLH, X-linked hypophosphatemia.
Sixteen participants (23.9%) reported 30 AEs classified as ‘musculoskeletal and connective tissue disorders’ (Table 3). Within this category, the most frequently reported AEs were ‘pain in extremity’ (18 events in 12 participants) and ‘arthralgia’ (four events in four participants). Eleven participants (16.4%) reported 12 AEs classified as ‘infections and infestations’. The most frequently reported AE was ‘tooth abscess’, reported seven times in seven participants. Seven participants (10.4%) reported 11 AEs classified as ‘general disorders and administration site conditions’. The most frequently reported AEs in this category were ‘pain’, which was reported four times in three participants, and ‘fatigue’ and ‘injection site erythema’, which were each reported in two participants. Six participants (9.0%) reported eight AEs classified as ‘gastrointestinal disorders’, of which ‘toothache’ was reported three times in two participants.
Table 3.
AEs in participants with XLH treated with burosumab (safety analysis set).
| MedDRA SOC/PTa,b,c | Burosumab, n = 67 | |
|---|---|---|
| Participants | AEs | |
| Participants with any AE, n (%) | 25 (37.3) | 83 |
| Congenital, familial, and genetic disorders | 1 (1.5) | 1 |
| Craniosynostosis | 1 (1.5) | 1 |
| Ear and labyrinth disorders | 1 (1.5) | 1 |
| Ear pain | 1 (1.5) | 1 |
| Gastrointestinal disorders | 6 (9.0) | 8 |
| Toothache | 2 (3.0) | 3 |
| Abdominal pain | 1 (1.5) | 1 |
| Diarrhea | 1 (1.5) | 1 |
| Infrequent bowel movements | 1 (1.5) | 1 |
| Loose tooth | 1 (1.5) | 1 |
| Vomiting | 1 (1.5) | 1 |
| General disorders and administration site conditions | 7 (10.4) | 11 |
| Pain | 3 (4.5) | 4 |
| Fatigue | 2 (3.0) | 2 |
| Injection site erythema | 2 (3.0) | 2 |
| Chest pain | 1 (1.5) | 1 |
| Injection-site mass | 1 (1.5) | 1 |
| Peripheral swelling | 1 (1.5) | 1 |
| Infections and infestations | 11 (16.4) | 12 |
| Tooth abscess | 7 (10.4) | 7 |
| Abscess jaw | 1 (1.5) | 1 |
| COVID-19 | 1 (1.5) | 1 |
| Gastroenteritis | 1 (1.5) | 1 |
| Viral infection | 1 (1.5) | 1 |
| Viral upper respiratory tract infection | 1 (1.5) | 1 |
| Injury, poisoning, and procedural complications | 2 (3.0) | 3 |
| Accidental poisoning | 1 (1.5) | 1 |
| Head injury | 1 (1.5) | 1 |
| Procedural pain | 1 (1.5) | 1 |
| Investigations | 1 (1.5) | 1 |
| Blood alkaline phosphatase increased | 1 (1.5) | 1 |
| Musculoskeletal and connective tissue disorders | 16 (23.9) | 30 |
| Pain in extremity | 12 (17.9) | 18 |
| Arthralgia | 4 (6.0) | 4 |
| Knee deformity | 2 (3.0) | 2 |
| Limb discomfort | 2 (3.0) | 2 |
| Neck pain | 1 (1.5) | 2 |
| Back pain | 1 (1.5) | 1 |
| Limb deformity | 1 (1.5) | 1 |
| Nervous system disorders | 3 (4.5) | 3 |
| Headache | 2 (3.0) | 2 |
| Restless legs syndrome | 1 (1.5) | 1 |
| Psychiatric disorders | 3 (4.5) | 3 |
| Emotional distress | 1 (1.5) | 1 |
| Irritability | 1 (1.5) | 1 |
| Sleep terror | 1 (1.5) | 1 |
| Respiratory, thoracic, and mediastinal disorders | 2 (3.0) | 2 |
| Asthma | 1 (1.5) | 1 |
| Dyspnea | 1 (1.5) | 1 |
| Skin and subcutaneous tissue disorders | 2 (3.0) | 2 |
| Rash | 2 (3.0) | 2 |
| Uncoded | 6 (9.0) | 6 |
Percentages are calculated using the number of participants in the safety analysis set as denominator.
SOC terms ordered alphabetically; PT sorted in order of frequency of the total column within each SOC.
A participant can have more than one SOC and more than one PT reported under a given SOC.
If a participant experiences the same AE (same SOC or same PT) more than once, participant is only counted once.
AE, adverse event; MedDRA, Medical Dictionary for Regulatory Activities version 23.1; PT, preferred term; SOC, system organ class; XLH, X-linked hypophosphatemia.
Among the 25 participants with ⩾1 AE, 13 participants (19.4%) had AEs possibly/probably related to XLH treatment, as reported by the investigator (Table 2). Among these 13 participants, 12 had AEs possibly/probably related to burosumab specifically, with a total of 23 AEs. In the 13 participants who had AEs possibly/probably related to XLH treatment, seven participants reported nine AEs that were classified as ‘musculoskeletal and connective tissue disorders’. Within this category, the most frequently reported AEs were ‘pain in extremity’ (reported seven times in six participants) (Supplemental Table 1). Five participants reported five AEs classified as ‘infections and infestations’, for which the reported AE was ‘tooth abscess’. Four participants reported five AEs classified as ‘general disorders and administration site conditions’. Within this category, ‘injection site erythema’ was reported two times in two participants.
Two participants (3.0%) reported SAEs, with two SAEs in total (Table 4). Of these, one SAE was classified as ‘congenital, familial, and genetic disorders’, for which ‘craniosynostosis’ was the event, and one SAE was classified as ‘musculoskeletal and connective tissue disorders’, for which ‘knee deformity’ was the event.
Table 4.
SAEs in participants with XLH treated with burosumab (safety analysis set).
| MedDRA SOC/PTa,b,c | Burosumab, n = 67 | |
|---|---|---|
| Participants | AEs | |
| Participants with any SAE, n (%) | 2 (3.0) | 2 |
| Congenital, familial, and genetic disorders, n (%) | 1 (1.5) | 1 |
| Craniosynostosis, n (%) | 1 (1.5) | 1 |
| Musculoskeletal and connective tissue disorders, n (%) | 1 (1.5) | 1 |
| Knee deformity, n (%) | 1 (1.5) | 1 |
Percentages are calculated using the number of participants in the safety analysis set as denominator.
SOC ordered alphabetically; PT ordered in order of frequency of the total column within each SOC.
A participant can have more than one SOC and more than one PT reported under a given SOC.
If a participant experiences the same AE (same SOC or same PT) more than once, participant is only counted once.
AE, adverse event; MedDRA, Medical Dictionary for Regulatory Activities version 23.1; PT, preferred term; SAE, serious adverse event; SOC, system organ class; XLH, X-linked hypophosphatemia.
AEs stratified by age cohort
In the 1- to <5-year age group (n = 22), seven participants (31.8%) experienced a total of 22 AEs. Four participants (18.2%) experienced eight AEs that were possibly/probably related to XLH treatment, as reported by the investigator (Supplemental Table 2), of whom three participants had six AEs that were possibly/probably related to burosumab specifically. No severe AEs, SAEs, deaths, or AEs leading to XLH treatment withdrawal were reported in this age group (Table 2).
In the 5- to <12-year age group (n = 34), 13 participants (38.2%) experienced a total of 53 AEs. Seven participants (20.6%) experienced AEs that were possibly/probably related to XLH treatment, as reported by the investigator (Supplemental Table 2). Three participants had four severe AEs: three events of ‘pain in extremity’ (classified as ‘musculoskeletal and connective tissue disorders’) and one ‘procedural pain’ (classified as ‘injury, poisoning, and procedural complication’). Two participants experienced SAEs: one event of ‘craniosynostosis’ (classified as ‘congenital, familial, and genetic disorders’) and one event of ‘knee deformity’ (classified as ‘musculoskeletal and connective tissue disorders’). These SAEs were not considered to be related to XLH treatment. No deaths or AEs leading to XLH treatment withdrawal were reported in this age group (Table 2).
In the 12- to <18-year age group (n = 11), five participants (45.5%) experienced a total of eight AEs. Two participants (18.2%) in the adolescent cohort had a total of four AEs that were reported as possibly/probably related to XLH treatment by the investigator (Supplemental Table 2). One adolescent reported two severe AEs; ‘limb discomfort’ and ‘pain in extremity’, both classified as ‘musculoskeletal and connective tissue disorders’. No SAEs, deaths, or AEs leading to XLH treatment withdrawal were reported in this age group (Table 2).
Effects in participants with mild to moderate CKD
CKD is defined as abnormalities of kidney structure or function, present for >3 months, with implications for health. 15 In total, five participants had normal–mild CKD. Participants with normal CKD (Grade 1; G1) present with a normal estimated glomerular filtration rate (eGFR) ⩾90 mL/min/1.73 m2, participants with mild CKD (Grade 2; G2) present with a mildly reduced eGFR between 60 and 89 mL/min/1.73 m2. Among these five participants, three (60.0%) had ⩾1 AE reported, with a total of nine AEs (Table 5). In these three participants, two (40.0%) had AEs that were reported as possibly/probably related to XLH treatment by the investigator, of whom one had an AE possibly/probably related to burosumab specifically. No SAEs, severe AEs, deaths, or AEs leading to XLH treatment withdrawal were reported in participants with CKD in the study.
Table 5.
AEs in participants with CKD with XLH treated with burosumab (safety analysis set).
| MedDRA SOC/PTa,b,c | Burosumab, n = 67 | |
|---|---|---|
| Participants | AEs | |
| Total (N) | 5 | |
| Participants with any AE, n (%) | 3 (60.0) | 9 |
| Gastrointestinal disorders | 2 (40.0) | 3 |
| Abdominal pain | 1 (20.0) | 1 |
| Diarrhea | 1 (20.0) | 1 |
| Infrequent bowel movements | 1 (20.0) | 1 |
| General disorders and administration site conditions | 1 (20.0) | 1 |
| Peripheral swelling | 1 (20.0) | 1 |
| Infections and infestations | 1 (20.0) | 1 |
| COVID-19 | 1 (20.0) | 1 |
| Musculoskeletal and connective tissue disorders | 2 (40.0) | 2 |
| Pain in extremity | 2 (40.0) | 2 |
| Skin and subcutaneous tissue disorders | 1 (20.0) | 1 |
| Rash | 1 (20.0) | 1 |
| Uncoded | 1 (20.0) | 1 |
| Normal stage CKD (N) | 4 | |
| Participants with any AE, n (%) | 2 (50.0) | 5 |
| Gastrointestinal disorders | 2 (50.0) | 3 |
| Abdominal pain | 1 (25.0) | 1 |
| Diarrhea | 1 (25.0) | 1 |
| Infrequent bowel movements | 1 (25.0) | 1 |
| Musculoskeletal and connective tissue disorders | 1 (25.0) | 1 |
| Pain in extremity | 1 (25.0) | 1 |
| Skin and subcutaneous tissue disorders | 1 (25.0) | 1 |
| Rash | 1 (25.0) | 1 |
| Mild stage CKD (N) | 1 | |
| Participants with any AE, n (%) | 1 (100.0) | 4 |
| General disorders and administration site conditions | 1 (100.0) | 1 |
| Peripheral swelling | 1 (100.0) | 1 |
| Infections and infestations | 1 (100.0) | 1 |
| COVID-19 | 1 (100.0) | 1 |
| Musculoskeletal and connective tissue disorders | 1 (100.0) | 1 |
| Pain in extremity | 1 (100.0) | 1 |
| Uncoded | 1 (100.0) | 1 |
Percentages are calculated using the number of CKD participants as denominator.
SOC terms ordered alphabetically; PT sorted in order of frequency of the total column within each SOC.
A participant can have more than one SOC and more than one PT reported under a given SOC.
If a participant experiences the same AE (same SOC or same PT) more than once, participant is only counted once.
AE, adverse event; CKD, chronic kidney disease; MedDRA, Medical Dictionary for Regulatory Activities version 23.1; PT, preferred term; SOC, system organ class; XLH, X-linked hypophosphatemia.
Among the five participants with CKD, four were category G1. Of these four participants, two reported ⩾1 AE; the total number of AEs was five. One participant had AEs that were possibly/probably related to XLH treatment (phosphate and calcitriol), as reported by the investigator.
The one remaining participant presented with CKD category G2. This participant reported a total of four AEs, with one AE possibly/probably related to XLH treatment (burosumab), as reported by the investigator.
No participants in the SAF had CKD with a GFR category >G2 (i.e., G3a: mildly to moderately decreased eGFR 45–59 mL/min/1.73 m2; G3b: moderately to severely decreased eGFR 30–44 mL/min/1.73 m2; G4: severely decreased eGFR 15–29 mL/min/1.73 m2; G5: kidney failure eGFR <15 mL/min/1.73 m2). 15
Discussion
This PASS aims to evaluate the frequency and severity of AEs in children and adolescents using burosumab for the treatment of XLH; a rare, debilitating genetic disease, with a current lack of real-world long-term data. The International XLH Registry was established to address this paucity of evidence, aiming to recruit 1200 pediatric and adult patients with XLH, running for 10 years. As part of the conditional marketing authorization for burosumab, this PASS was mandated and is embedded in the International XLH Registry.
In total, 67 participants receiving ⩾1 dose of burosumab were included in this study between 12 September 2017 and the interim data cut-off date of 13 May 2021, with 37.3% experiencing ⩾1 AE, and 19.4% experiencing ⩾1 AE possibly/probably related to XLH treatment during this follow-up time. Analysis by age group showed that 31.8%, 38.2%, and 45.5% of participants experienced ⩾1 AE in the age cohorts 1 to <5, 5 to <12, and 12 to <18 years, respectively.
The most frequently reported AEs were classified as ‘musculoskeletal and connective tissue disorders’, with ‘pain in extremity’ the most frequently reported event, followed by ‘infections and infestations’, with ‘tooth abscess’ as the most frequently reported event. Notably, extremity pain and tooth abscesses are known features of XLH; thus, these AEs may be more attributable to the disease itself rather than specifically due to burosumab treatment per se. No deaths, no SAEs related to XLH treatment, and no AEs leading to XLH treatment withdrawal were observed.
A 2019 US clinical trial evaluating burosumab in children aged 1–4 years with XLH reported that all 13 enrolled participants experienced ⩾1 AE; the investigators reported that five children experienced 14 treatment-related AEs, most of which were injection-site reactions. 16 A 2019 US Phase III trial of burosumab in children reported that participants in the burosumab group experienced treatment-emergent AEs, most of which were related to injection-site reactions (15/29; 51.7%). These reactions were mild in severity and resolved within a few days. 17 A 2018 US Phase II trial also reported injection-site reaction as a common AE in children with XLH receiving burosumab [17/26 patients (65.4%) in the every-2-week dosing group and 13/26 (50.0%) in the every-4-week dosing group]. These reactions were mild, limited to the skin, and lasted no longer than 1–2 days. 18 All three US clinical trials concluded that the safety profile of burosumab was consistent across the published literature.16–18 Notably, in this current study, only two participants (3%) reported ‘injection-site erythema’.
In this PASS, five participants had been diagnosed with CKD. Burosumab treatment for XLH is contraindicated in patients with severe or end stage renal disease and burosumab has not been studied in patients with renal impairment. 13 A known homeostatic response in CKD is the elevation of FGF23 in order to maintain physiological phosphate levels. 19 In addition, potential risks for patients treated with conventional therapy or burosumab are nephrocalcinosis, hypercalciuria, or hyperparathyroidism, and these may negatively affect renal function.2,10,14 Therefore, a key safety interest of this PASS was the use of burosumab in patients with XLH and mild to moderate CKD. A greater frequency of participants with CKD reported AEs compared with all participants (60% and 37.3%, respectively). Thus, the safety of burosumab in this participant group warrants further investigation.
A limitation of this PASS is the generalizability of results. Not all countries that participated in the International XLH Registry were included/in scope in this first interim analysis; as such, the results may not be entirely representative of the broader population of patients with XLH. In future analyses, other countries that participate in the International XLH Registry will be included.
A second limitation of this PASS is the limited number of biochemical parameters collected. A greater number of biochemical parameters collected will help to inform, and understand in greater detail, the safety outcomes of patients treated with burosumab. It is therefore important to ensure collection of these data to provide more detailed analyses in the future.
Conclusion
Over a 2-year follow-up, the most common AEs reported in this first interim analysis of the burosumab PASS were typical of a pediatric population or were frequent manifestations of XLH, with most AEs reported in ‘musculoskeletal and connective tissue disorders’ or ‘infections and infestations’ Medical Dictionary for Regulatory Activities system organ class. Therefore, the safety profile of burosumab observed in this interim analysis is consistent with previously reported safety data for burosumab. The PASS will continue to follow up participants for a period of at least 10 years, with the goal of providing long-term safety data for healthcare providers and people living with XLH. This knowledge will contribute to the overall understanding of burosumab safety and will help ultimately inform the future clinical management of XLH.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223241247643 for Real-world non-interventional post-authorization safety study of long-term use of burosumab in children and adolescents with X-linked hypophosphatemia: first interim analysis by Annemieke M. Boot, Gema Ariceta, Signe Sparre Beck-Nielsen, Maria Luisa Brandi, Karine Briot, Carmen de Lucas Collantes, Sandro Giannini, Dieter Haffner, Richard Keen, Elena Levtchenko, M. Zulf Mughal, Outi Makitie, Ola Nilsson, Dirk Schnabel, Liana Tripto-Shkolnik, M. Carola Zillikens, Jonathan Liu, Alina Tudor and Francesco Emma in Therapeutic Advances in Chronic Disease
Acknowledgments
The authors thank the participants/guardians who agreed to take part in the International XLH Registry and in this embedded PASS. Medical writing support for this manuscript was provided by Rosalind Bonomally, MSc, Edward Maguire, PhD, and Dionne Turnbull, PhD (90TEN), funded by Kyowa Kirin International plc. The authors maintained full editorial control of the paper and provided their final approval of all content. The International XLH Registry was implemented by Kyowa Kirin International plc, supported by Medialis Ltd, a contract research organization (CRO), for the first year of the registry. In March 2019, Kyowa Kirin International plc commissioned a new CRO, IQVIA, to support the International XLH Registry, taking over duties from the previous CRO. As part of the transition, Kyowa Kirin International plc updated the core documents to refer to the burosumab PASS as a sub-study embedded within the main International XLH Registry and reassigned the role of data controller from the patient to the sponsor. GA, EL, and FE are members of the ERKNet consortium; SG and ON are members of ERN BOND; MCZ is a member of ERN BOND and ERN Rare Endocrine Diseases.
Footnotes
ORCID iDs: Annemieke M. Boot  https://orcid.org/0000-0002-2426-3222
https://orcid.org/0000-0002-2426-3222
Maria Luisa Brandi  https://orcid.org/0000-0002-8741-0592
https://orcid.org/0000-0002-8741-0592
M. Carola Zillikens  https://orcid.org/0000-0001-9186-3423
https://orcid.org/0000-0001-9186-3423
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Annemieke M. Boot, Department of Pediatric Endocrinology, University Medical Center Groningen, University of Groningen, Hanzeplein 1, Groningen 9713 GZ, The Netherlands.
Gema Ariceta, Department of Pediatric Nephrology, University Hospital Vall d’Hebron, Autonomous University of Barcelona, Barcelona, Spain.
Signe Sparre Beck-Nielsen, Centre for Rare Diseases, Pediatric Department, Aarhus University Hospital, Aarhus, Denmark; Department of Clinical Medicine, Aarhus University, Aarhus, Denmark.
Maria Luisa Brandi, FIRMO Foundation, Florence, Italy.
Karine Briot, AP-HP, Department of Rheumatology, Cochin Hospital, Paris, France.
Carmen de Lucas Collantes, Pediatric Nephrology, Hospital Infantil Universitario Niño Jesús, Universidad Autónoma de Madrid, Madrid, Spain.
Sandro Giannini, Department of Medicine, Clinica Medica 1, University of Padova, Padova, Italy.
Dieter Haffner, Department of Pediatric Kidney, Liver and Metabolic Diseases, Hannover Medical School, Hannover, Germany.
Richard Keen, Royal National Orthopaedic Hospital, Stanmore, UK.
Elena Levtchenko, Department of Pediatric Nephrology, Amsterdam University Medical Centre, Amsterdam, The Netherlands.
M. Zulf Mughal, Department of Pediatric Endocrinology, Royal Manchester Children’s Hospital, Manchester, UK; Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK.
Outi Makitie, Children’s Hospital, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
Ola Nilsson, Division of Pediatric Endocrinology, Center for Molecular Medicine, Department of Women’s and Children’s Health, Karolinska Institutet and University Hospital, Stockholm, Sweden; Department of Pediatrics and School of Medical Sciences, Örebro University and University Hospital, Örebro, Sweden.
Dirk Schnabel, Center for Chronically Sick Children, Pediatric Endocrinology, Charitè, University Medicine, Berlin, Germany.
Liana Tripto-Shkolnik, The Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel; Division of Endocrinology, Diabetes and Metabolism, Sheba Medical Center, Tel HaShomer, Israel.
M. Carola Zillikens, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands.
Jonathan Liu, Kyowa Kirin International, Marlow, UK.
Alina Tudor, Kyowa Kirin International, Marlow, UK.
Francesco Emma, Division of Nephrology, Bambino Gesù Children’s Hospital IRCCS, Rome, Italy.
Declarations
Ethics approval and consent to participate: Written informed consent (parental/legal guardian, or adolescent assent, where applicable) was obtained for participation in the International XLH Registry and inclusion in the PASS analysis. The International XLH Registry is run in accordance with the recommendations from the Declaration of Helsinki (1964) and has received ethical, regulatory, and institutional approvals at national, regional, and site level for each participating country, as required. EMA Ethics Submission Procedure Number: EMEA/H/C/004275/MEA/004.2, approval date: 20 May 2021.
Consent for publication: Not applicable.
Author contributions: Annemieke M. Boot: Conceptualization; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Gema Ariceta: Conceptualization; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Signe Sparre Beck-Nielsen: Conceptualization; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Maria Luisa Brandi: Conceptualization; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Karine Briot: Conceptualization; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Carmen de Lucas Collantes: Conceptualization; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Sandro Giannini: Conceptualization; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Dieter Haffner: Conceptualization; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Richard Keen: Conceptualization; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Elena Levtchenko: Conceptualization; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
M. Zulf Mughal: Conceptualization; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Outi Mӓkitie: Conceptualization; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Ola Nilsson: Conceptualization; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Dirk Schnabel: Conceptualization; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Liana Tripto-Shkolnik: Conceptualization; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
M. Carola Zillikens: Conceptualization; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Jonathan Liu: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Alina Tudor: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Francesco Emma: Conceptualization; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Kyowa Kirin International plc is the sponsor of the International XLH Registry and the PASS, and facilitated the development of this document, although its creation was led entirely and independently by the authors. Kyowa Kirin did not provide any direct financial support.
Competing interests: AMB has received research grants and received honoraria as a consultant and speaker, paid to her institution, from Kyowa Kirin and Ultragenyx. CdLC has received honoraria as a consultant or speaker from Alexion, Chiesi, and Kyowa Kirin. MCZ is an unpaid member of the International XLH Registry steering committee and reports that her institution has received a research grant from Kyowa Kirin. DH has received a research grant and/or honoraria as a consultant and speaker from Amgen, Chiesi, and Kyowa Kirin. DS has received honoraria as a consultant from BioMarin, Kyowa Kirin, Novo Nordisk, and Sandoz. EL has received honoraria as a consultant from Advicenne, Chiesi, Kyowa Kirin, Novartis, and Recordati. FE declares competing interests with Avrobio, Chiesi, Kyowa Kirin, Otsuka, and Recordati Rare Diseases. GA has received personal fees and non-financial support from Advicenne, Alexion, Kyowa Kirin, Recordati Rare Diseases, and received personal fees from Alnylam and Dicerna, and received personal fees and other from Chiesi. KB has received honoraria as a consultant from Amgen, Kyowa Kirin, Theramex, and UCB. LT-S has received honoraria as a consultant from Amgen and Kyowa Kirin. MLB has received research grants or honoraria as a consultant or speaker from Abiogen, Alexion, Amgen, Bruno Farmaceutici, Calilytix, Echolight, Eli Lilly, Kyowa Kirin, SPA, Theramex, and UCB. ON has received honoraria as a consultant from BioMarin and Kyowa Kirin. OM has received honoraria as a consultant from BridgeBio, Kyowa Kirin, and Ultragenyx. RK has received honoraria as a consultant and for advisory boards from Kyowa Kirin. SSB-N received honoraria as a consultant from Inozyme and Kyowa Kirin in addition to honoraria as speaker and research funding from Kyowa Kirin. MZM has received honoraria as a consultant or speaker from Inozyme Pharma, Ipsen, and Kyowa Kirin. AT is an employee of Kyowa Kirin International plc. JL is a former employee of Kyowa Kirin International plc. SG declares no conflict of interests.
Availability of data and materials: The data sets used and analyzed that support the findings of this manuscript are available from the corresponding author and Kyowa Kirin International on reasonable request.
References
- 1. Padidela R, Nilsson O, Makitie O, et al. The international X-linked hypophosphataemia (XLH) registry (NCT03193476): rationale for and description of an international, observational study. Orphanet J Rare Dis 2020; 15: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schindeler A, Biggin A, Munns CF. Clinical evidence for the benefits of burosumab therapy for X-linked hypophosphatemia (XLH) and other conditions in adults and children. Front Endocrinol (Lausanne) 2020; 11: 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dahir K, Roberts MS, Krolczyk S, et al. X-linked hypophosphatemia: a new era in management. J Endocr Soc 2020; 4: bvaa151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Imel EA, White KE. Pharmacological management of X-linked hypophosphataemia. Br J Clin Pharmacol 2019; 85: 1188–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carpenter TO, Imel EA, Holm IA, et al. A clinician’s guide to X-linked hypophosphatemia. J Bone Miner Res 2011; 26: 1381–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haffner D, Emma F, Eastwood DM, et al. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat Rev Nephrol 2019; 15: 435–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hawley S, Shaw NJ, Delmestri A, et al. Prevalence and mortality of individuals with X-linked hypophosphataemia: a United Kingdom real world data analysis. J Clin Endocrinol Metab 2020; 105: e871–e878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pettifor JM. What’s new in hypophosphataemic rickets? Eur J Pediatr 2008; 167: 493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beck-Nielsen SS, Brock-Jacobsen B, Gram J, et al. Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. Eur J Endocrinol 2009; 160: 491–497. [DOI] [PubMed] [Google Scholar]
- 10. Rafaelsen S, Johansson S, Raeder H, et al. Hereditary hypophosphatemia in Norway: a retrospective population-based study of genotypes, phenotypes, and treatment complications. Eur J Endocrinol 2016; 174: 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. US Food and Drug Administration. (Crysvita) burosumab, highlights of prescribing information. Ultragenyx Pharmaceutical Inc., https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761068s004lbl.pdf (2018, accessed May 2022). [Google Scholar]
- 12. Kyowa Kirin receives positive CHMP opinion for the expanded use of CRYSVITA® (burosumab) to include older adolescents and adults for the treatment of X-linked hypophosphataemia (XLH), https://www.kyowakirin.com/media_center/news_releases/2020/e20200727_01.html (2020, accessed May 2022).
- 13. European Medicines Agency. (Crysvita) burosumab, summary of product characteristics. Kyowa Kirin Holdings B.V., https://www.ema.europa.eu/en/documents/product-information/crysvita-epar-product-information_en.pdf (2018). [Google Scholar]
- 14. Brandi ML, Ariceta G, Beck-Nielsen SS, et al. Post-authorisation safety study of burosumab use in paediatric, adolescent and adult patients with X-linked hypophosphataemia: rationale and description. Ther Adv Chronic Dis 2022; 13: 20406223221117471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150. [Google Scholar]
- 16. Whyte MP, Carpenter TO, Gottesman GS, et al. Efficacy and safety of burosumab in children aged 1–4 years with X-linked hypophosphataemia: a multicentre, open-label, phase 2 trial. Lancet Diabetes Endocrinol 2019; 7: 189–199. [DOI] [PubMed] [Google Scholar]
- 17. Imel EA, Glorieux FH, Whyte MP, et al. Burosumab versus conventional therapy in children with X-linked hypophosphataemia: a randomised, active-controlled, open-label, phase 3 trial. Lancet 2019; 393: 2416–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carpenter TO, Whyte MP, Imel EA, et al. Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med 2018; 378: 1987–1998. [DOI] [PubMed] [Google Scholar]
- 19. Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 2011; 305: 2432–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223241247643 for Real-world non-interventional post-authorization safety study of long-term use of burosumab in children and adolescents with X-linked hypophosphatemia: first interim analysis by Annemieke M. Boot, Gema Ariceta, Signe Sparre Beck-Nielsen, Maria Luisa Brandi, Karine Briot, Carmen de Lucas Collantes, Sandro Giannini, Dieter Haffner, Richard Keen, Elena Levtchenko, M. Zulf Mughal, Outi Makitie, Ola Nilsson, Dirk Schnabel, Liana Tripto-Shkolnik, M. Carola Zillikens, Jonathan Liu, Alina Tudor and Francesco Emma in Therapeutic Advances in Chronic Disease