Abstract
Purpose
To characterize clinical outcomes following a single administration of bimatoprost SR in eyes with glaucoma in a real-world setting implanted at the slit-lamp.
Setting
Tertiary care Glaucoma practice, Glaucoma Associates of Texas, Dallas, Texas.
Design
Retrospective interventional case series.
Methods
Data were analyzed from consecutive patients receiving a single bimatoprost SR implant from the time of its approval to the time of data collection. All eyes were implanted at the slit-lamp. Eyes with less than 1 month of follow-up were excluded. The primary outcome was median time to next intraocular pressure (IOP)-lowering intervention. Mean IOP and medication use, and changes from baseline, were also assessed through 12 months of follow-up.
Results
Overall 129 eyes of 81 patients were analyzed. Following bimatoprost SR administration (replacing a topical prostaglandin analogue [PGA] in most eyes), the median survival time without any further IOP-lowering interventions was between 6–9 months. Mean IOP remained unchanged from baseline at month 1 (consistent with switch from topical to intracameral PGA therapy) and began to rise at month 3. At month 12, 40.5% of eyes (52 eyes) remained intervention-free, mean medication reduction was 0.5 medications per eye, and 27.8% of eyes (36 eyes) were medication-free. Adverse events were uncommon and most were transient and resolved with or without intervention.
Conclusion
This real-world analysis of bimatoprost SR use for glaucoma therapy complements Phase 3 study findings and demonstrates that the implant can safely provide medication reduction through 6 months in most eyes and through 12 months in almost 40% of eyes.
Keywords: glaucoma, bimatoprost SR, durysta, sustained release
Introduction
Topical medical therapy for glaucoma has been the first-line standard of care for more than 150 years.1 This approach has been validated in multiple landmark clinical trials as an effective and safe means of preventing the development and/or progression of glaucoma.2–6 However, first-line medical therapy does have important limitations. Nonadherence is high, with rates of nonadherence ranging from 30–80%.7–11 Side effects are common, the most important of which is ocular surface disease, likely attributable to the preservative benzalkonium chloride, with prevalence ranging from 30–70% in published studies.12–18 In recent years, there has been growing interest in therapies that do require daily self-dosing of topical medications by patients. These include laser procedures such as selective laser trabeculoplasty (SLT), an array of minimally invasive glaucoma surgeries (MIGS), and sustained-release drug delivery (SRDD) systems to obviate the need for daily self-dosing.
The bimatoprost sustained release (SR) implant (Durysta, Allergan, an AbbVie company) is a small, rod-shaped, biodegradable implant incorporating 10 µg of bimatoprost designed to release the drug in a slow, steady fashion for 3–4 months.19,20 In Phase 3 clinical trials, intraocular pressure (IOP) lowering with bimatoprost SR was noninferior to that seen with timolol through 12 weeks.19,20 A post hoc analysis of data from a Phase 2 trial demonstrated IOP-lowering efficacy of a single administration of bimatoprost SR through up to 24 months in up to 28% of patients.21 Common side effects included transient conjunctival hyperemia associated with the administration procedure. Because of concerns related to ongoing corneal endothelial cell loss with repeated administrations, the implant was approved for use only once.22
Despite having been approved for use in the United States more than 3 years ago, there are few if any post-marketing publications to complement the findings of Phase 3 studies. Such studies are important to characterize the efficacy and safety of bimatoprost SR in real-world settings in diverse patient populations less homogenous than those enrolled in registry trials. Specifically, because the Phase 3 trials of bimatoprost SR required readministration at weeks 16 and 3219,20 but the product’s approval limited its use to a single administration,22 there is an unmet need for efficacy data following a single administration. In this report, we describe the clinical outcomes in the first 132 consecutive eyes treated with a single administration of bimatoprost SR by a single provider delivered at the slit-lamp.
Methods
This was a retrospective, interventional case series of patients with open-angle glaucoma or ocular hypertension. Data were drawn from existing health records and were de-identified prior to analysis. The study protocol was reviewed by a central institutional review board (Sterling IRB, #8545-DSGrover) and deemed exempt with waiver of consent on December 7, 2020. The study was conducted in accordance with the tenets of the Declaration of Helsinki.
This analysis included data from all adult patients (18 years or older) with glaucoma or ocular hypertension who received the bimatoprost SR implant in one or both eyes between July 2020 and October 2021 with at least 1 month of follow-up. Indications for treatment included primary therapy, replacement for topical PGA use, adjunctive to other medications, or adjunctive to prior glaucoma laser or surgery. Patients were excluded if the implantation process was unsuccessful or if no on-treatment data were available.
Bimatoprost SR implantation was performed according to the manufacturer’s instructions for use by a single surgeon (DG).22 The implant is provided preloaded into an inserter with a 28-gauge needle for intraocular injection. After application of diluted betadine as well as one drop of ofloxacin ophthalmic solution and under sterile conditions, topical anesthesia, lid speculum exposure, and illuminated magnification at the slit-lamp, the needle was inserted through the peripheral clear cornea, with the bevel oriented anteriorly, and advanced until the needle tip was two bevel lengths deep into the anterior chamber and not overlying the pupil. The actuator button was depressed until an audible or palpable click was detected, indicating release of the implant. The needle was then removed, the track placed under tamponade briefly, and then the injection site checked for leaks and confirmation of self-sealing. The patient was instructed to remain upright for 1 hour to ensure settling of the implant into the inferior chamber angle. All implantations were performed at the slit-lamp in a clinical environment. During implantation, an assistant helped stabilize the patients head to ensure safety and minimize the risk of the patient moving or pulling back. All patients received a 3-day course of topical antibiotics dosed four times daily.
As this was a retrospective study, no prespecified schedule of visits or assessments was followed. Instead, patients were managed according to routine clinical practice and were seen approximately 1–2 weeks, 1 month, and 3, 6, 9, and 12 months after the implantation procedure. Where available, data for each time point were drawn from the visits closest to the specified time point; if no visits occurred close to the specified time point, no data were recorded for that time point. At each visit, visual acuity, IOP, and IOP-lowering medication use were assessed. IOP was assessed using Goldmann tonometry.
The primary outcome measure of the study was successful control of IOP, with failure defined as any subsequent IOP-lowering intervention (medication or surgery). Additional outcomes included mean IOP and IOP reduction at each time point among eyes remaining controlled following implantation, and mean medication use and medication reductions at each time point among all eyes not requiring surgery at each time point. Median survival time (time to any subsequent IOP-lowering intervention) was assessed using Kaplan-Meier survival analysis. Changes from baseline in IOP and medication use were assessed using paired t-tests, with p = 0.05 taken as the level of significance. Means are presented with their standard deviations. As no formal hypothesis was prespecified or tested, no a priori power/sample size analysis was conducted. Instead, the sample size was determined by the number of eyes available for analysis based on the eligibility criteria described above; the resulting sample size was deemed adequate to characterize the key outcomes as it included approximately 65–75% of the sample sizes of the Phase 3 bimatoprost SR 10 µg treatment groups.19,20
Results
Overall, 129 eyes of 81 subjects were included in this analysis. Demographic and prior glaucoma treatment data are given in Table 1. Subjects’ mean age was 73.7 (11.5) years, 75.3% were female (61 subjects), and most (75.3%, 61 subjects) were Caucasian. Most eyes (86.0%, 111 eyes) had primary open-angle glaucoma (POAG) and half (50.4%, 65 eyes) were using 2 or more IOP-lowering medications. Approximately 2/3 (64.3%, 83 eyes) were pseudophakic, and the majority (53.5%, 69 eyes) had previously undergone one or more laser or surgical intervention for IOP control, the most common of which were laser trabeculoplasty (31.8%, 41 eyes) or one of several MIGS procedures.
Table 1.
Demographics and Baseline Glaucoma Data
| Parameter | Value |
|---|---|
| Subject-level (N=81) | |
| Age (yr), mean (SD) | 73.7 (11.5) |
| Sex, n (%) | |
| Male | 20 (24.7) |
| Female | 61 (75.3) |
| Ethnicity, n (%) | |
| Caucasian | 61 (75.3) |
| Black | 11 (13.6) |
| Hispanic | 4 (4.9) |
| Other | 5 (6.2) |
| Eye-level (N=129) | |
| Glaucoma diagnosis, n (%) | |
| Primary open-angle | 111 (86.0) |
| Normal tension | 9 (7.0) |
| Other | 9 (7.0) |
| Mean (SD) IOP, mmHg | 16.0 (4.0) |
| No. of glaucoma medications at baseline, n (%) | |
| 0 | 11 (8.5) |
| 1 | 53 (41.1) |
| 2 | 32 (24.8) |
| 3 | 26 (20.2) |
| >3 | 7 (5.4) |
| Prior glaucoma procedures, n (%) | |
| Any | 69 (53.5) |
| Laser trabeculoplasty | 41 (31.8) |
| Laser iridotomy | 8 (6.2) |
| Goniotomy (excisional or by ablation) | 12 (9.3) |
| Trabecular micro-bypass implant or canal stent | 10 (7.8) |
| Cilioablation | 11 (8.5) |
| Tube-shunt | 5 (3.9) |
| Trabeculectomy | 5 (3.9) |
| Gel stent | 7 (5.4) |
| Gonioscopy-assisted transluminal trabeculotomy | 7 (5.4) |
| Phakic status, n (%) | |
| Phakic | 46 (35.7) |
| Pseudophakic | 83 (64.3) |
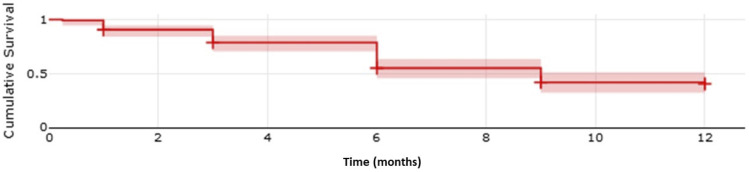
The median time to treatment failure after bimatoprost SR implantation was between 6 and 9 months (Figure 1). Survival at 12 months was 40.5%; at 3, 6, and 9 months, survival was 69.2%, 55.0%, and 42.2%. Treatment failure occurred in 67 eyes, of which 59 were medication additions, 3 were selective laser trabeculoplasty, and 5 were surgical procedures (3 tube-shunts, 1 gel stent, and 1 trabeculectomy).
Figure 1.
Kaplan-Meier survival plot of survival to next IOP-lowering intervention. Shaded region represents 95% confidence interval.
Mean IOP and medication use, as well as changes in each, are given in Table 2. To characterize the magnitude of bimatoprost SR effect, IOP reductions are reported only for eyes without further IOP-lowering interventions at each time point. Mean IOP was unchanged (up by 0.2 mmHg [p=0.656]) at month 1, consistent with the exchange of a topical PGA for bimatoprost SR in most eyes. At month 3 and again at month 6, mean IOP rose significantly (by 1.2 mmHg [p=0.013] and 1.7 mmHg [p<0.001], respectively) in the expected timeframe for bimatoprost SR drug depletion. Among eyes surviving beyond month 6, mean IOP was insignificantly changed from baseline at month 9 (by 0.8 mmHg [p=0.150]) and only marginally significantly changed (by 1.3 mmHg, p=0.047) at month 12.
Table 2.
Intraocular Pressure (IOP) and Medication Data Through 12 Months of Follow-Up.
| Month 1 | Month 3 | Month 6 | Month 9 | Month 12 | |
|---|---|---|---|---|---|
| IOP | |||||
| N | 109 | 80 | 86 | 48 | 26 |
| Baseline IOP, mean (SD) | 16.2 (4.1) | 16.2 (3.2) | 15.4 (3.4) | 14.7 (3.7) | 14.5 (3.6) |
| Mean (SD) | 16.3 (4.4) | 17.4 (5.1) | 17.0 (4.9) | 15.5 (4.7) | 15.7 (4.3) |
| CFB (SD) | 0.2 (3.6) | 1.2 (4.2) | 1.7 (3.9) | 0.8 (3.7) | 1.3 (3.5) |
| P | 0.656 | 0.013 | <0.001 | 0.150 | 0.047 |
| Medications | |||||
| N | 110 | 88 | 101 | 88 | 79 |
| Baseline (n-adjusted) | 1.8 (1.1) | 1.6 (1.0) | 1.7 (1.0) | 1.7 (0.9) | 1.7 (0.9) |
| Mean (SD) | 0.7 (0.9) | 0.7 (0.9) | 0.8 (1.0) | 1.1 (1.0) | 1.2 (1.2) |
| CFB | −1.0 | −1.0 | −0.9 | −0.6 | −0.5 |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Medication-free, n (%) | 61 (55.5) | 51 (58) | 51 (50.5) | 24 (27.2) | 22 (27.8) |
Notes: Because Treatment Failures Received Additional IOP-Lowering Interventions, Only Treatment Successes are Included in the IOP Analysis, and to Account for the Diminishing Sample Size Over Time as Treatment Failures Occur, Baseline IOP Values for Each Post-Treatment Time Point Have Been Adjusted to Include Only Those Eyes Still Deemed Treatment Successes at the Respective Time Points. In Contrast, the Medication Analysis Includes All Eyes Evaluated at Each Time Point Regarding of Success/Failure Status to Reflect the Addition of Medications in Eyes Failing Treatment.
Abbreviations: CFB, change from baseline; SD, standard deviation.
Mean medication reduction of 1.1 medication was seen as soon immediately after treatment, reflecting the replacement of topical PGA with bimatoprost SR in most eyes. By month 6, a mean reduction of 0.8 medications per eye persisted, while by month 12, the mean medication reduction has decreased to 0.5 medications per eye. More than half of the eyes remained medication-free through 6 months (Table 2).
Complications of bimatoprost SR implantation were uncommon. Ten eyes experienced IOP spikes > 10 mmHg, occurring at months 3 (n = 2), 6 (n = 2), 9 (n = 2), and 12 (n = 4), all consistent with depletion of the drug from the implant. Adverse events were uncommon: two eyes developed anterior chamber inflammation (at months 1 and 12), and one eye developed a corneal edema at 6 months which resolved once the implant was removed. There were no reported cases of corneal delivery of the implant or of a patient moving away from the slit lamp during implantation.
Discussion
This report is among the first to characterize the clinical performance of the bimatoprost SR implant for IOP reduction in real-world use. In this sample, the median time to next IOP-lowering intervention following a single implant was between 6 and 9 months, and 12-month survival with no further IOP-lowering interventions was 40.5%.
Mean IOP reduction at month 1 was insignificant compared to baseline, which was expected as bimatoprost SR was used as replacement therapy for a topical PGA in all but 11 eyes that were untreated at baseline. By month 3 and at months 6 and 12, mean IOP was significantly higher than baseline, indicative of drug depletion and loss of implant effectiveness over time. These IOP reduction outcomes cannot be benchmarked to the phase 3 trial outcomes, as subjects in the Phase 3 study underwent medication washout at baseline before receiving the first implant,19,20 a practice that is common in research settings but not in real-world clinical practice. Because no washout was undertaken in this study and patients were using a mean of 1.7 medications at baseline, many patients continued to use adjunctive medications after the bimatoprost SR implantation. Also, the 6-, 9- and 12-month time points after bimatoprost SR administration in the current study have no correlates in the Phase 3 studies, as all eyes received additional implants at months 4 and 8. The median survival time (the first time survival drops below 50%) was between 6 and 9 months, and the relatively low incremental failure rate thereafter (40.5% still successes at month 12) suggests that eyes surviving to 6–9 months have a high probability of surviving through month 12. Interestingly, the mean baseline IOP of eyes with prolonged responses (9 and 12 months) were substantially lower than mean baseline IOP of shorter-term responders (mean baseline IOP 16.2 mmHg in eyes surviving to months 1 and 3 versus 14.5–14.7 mmHg in eyes surviving to months 9 and 12). This suggests that lower baseline IOP may be associated with longer duration of effect, perhaps because lower baseline IOP was indicative of a good response to the topical PGA that the implant replaced and matched.
The early reduction of medications by a mean of 1.0 at month 1 was expected given that the implant was given as replacement therapy for topical PGAs in most eyes. A small proportion of eyes were able to discontinue both the topical PGA and at least one additional medication for the first few months, as 50.4% of eyes were using 2 or more medications at baseline and medication-free rates were greater than this (55.5–58%) at months 1 and 3. The medication-free rate at month 12 was 27.8%, substantially higher than the 8.5% of eyes that were medically untreated at study entry. On average, half of eyes were using 1 less medication at month 12 compared to baseline.
The implant was well tolerated in the current study. Adverse events were uncommon (a total of 3 events in 129 eyes) and most were transient and resolved with or without intervention. Benchmarking the safety outcomes of this study to the Phase 3 studies is limited by key differences in study design. First, this was a retrospective study in which mild or clinically insignificant adverse events may not have been robustly documented. Second, the Phase 3 studies utilized repeat dosing, with cumulative risk of adverse events, while the current study did not. However, the low rate and severity of adverse events in the current study support the safe administration of this treatment in real-world use. Corneal endothelial cell density was not routinely assessed in this study as this is not the standard of care in real-world practice.
The data derived from bimatoprost SR’s Phase 3 studies are inadequate to guide clinical use consistent with the product’s final labeling. At the time the Phase 3 studies were designed, it was anticipated that repeat dosing would be appropriate after the expected 4-month duration of effect. However, evidence of corneal endothelial cell loss with repeated dosing led to labelling for single administration only.19,20,23 Key outcomes characterized in the current study—including median survival time, intervention-free survival through month 12, and IOP and medication changes beyond 4 months post-dosing—could not be assessed in the Phase 3 study analyses. Therefore, the current study’s design—characterizing long-term IOP after a single administration—adds clinically relevant data on long-term on-label product use. One other real-world study has evaluated the efficacy of a single administration of bimatoprost SR. In 122 eyes, both mean IOP and mean medication use was reduced through a mean follow-up of 28 weeks,24 and in a subset of eyes with prior glaucoma surgery (minimally invasive or filtration surgery), mean medication use was decreased at 28 weeks.25
This study is strengthened by several aspects of its design. First was the analysis of all consecutive patients receiving the bimatoprost SR implant from the time of its approval to the time of study commencement, with the goal of minimizing selection bias. The sample size for this analysis was at least 65% of the sample sizes of the phase 3 trials and only a small handful of patients were excluded for limited follow-up (<1 month); these were referral patients who returned to their referring providers post-procedure for ongoing care. The endpoint assessed—the time to next IOP-lowering intervention—is a meaningful outcome meant to be more practical to real-world care than utilizing an arbitrary IOP threshold to define success/failure, particularly as treatment targets vary between patients. Limitations included the fairly homogeneous sample (mostly Caucasians, mostly POAG) and the single-provider/single-practice nature of the care setting.
Conclusions
In summary, this real-world analysis of bimatoprost SR use for glaucoma therapy complements Phase 3 study findings and demonstrates that the implant can safely provide medication reduction through 6 months in most eyes and through 12 months in ~40% of eyes.
Funding Statement
This study was supported by an Investigator Initiated grant from AbbVie.
Disclosure
DSG is a consultant for Allergan and reports grants/personal fees for Medical Advisory Board from Belkin Vision, CATS tonometer, Reichert, Sanoculis, Versant Health and iStar Medical; grants/personal fees from New World Medical and Regeneron; personal fees and minority equity ownership from Nova Eye Medical and Olleyes, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Realini T. A history of glaucoma pharmacology. Optom Vis Sci. 2011;88(1):36–38. doi: 10.1097/OPX.0b013e3182058ead [DOI] [PubMed] [Google Scholar]
- 2.Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713. doi: 10.1001/archopht.120.6.701 [DOI] [PubMed] [Google Scholar]
- 3.Miglior S, Zeyen T, Pfeiffer N, Cunha-Vaz J, Torri V, Adamsons I. Results of the European glaucoma prevention study. Ophthalmology. 2005;112(3):366–375. doi: 10.1016/j.ophtha.2005.06.020 [DOI] [PubMed] [Google Scholar]
- 4.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–1279. doi: 10.1001/archopht.120.10.1268 [DOI] [PubMed] [Google Scholar]
- 5.Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the collaborative initial glaucoma treatment study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108(11):1943–1953. doi: 10.1016/S0161-6420(01)00873-9 [DOI] [PubMed] [Google Scholar]
- 6.Laser Trial Research Group G, Glaucoma Laser Trial Research Group. The Glaucoma Laser Trial (GLT) and Glaucoma Laser Trial Follow-Up Study: 7. Results. Am J Ophthalmol. 1995;120(6):718–731. doi: 10.1016/S0002-9394(14)72725-4 [DOI] [PubMed] [Google Scholar]
- 7.Rossi GC, Pasinetti GM, Scudeller L, Radaelli R, Bianchi PE. Do adherence rates and glaucomatous visual field progression correlate? Eur J Ophthalmol. 2011;21(4):410–414. doi: 10.5301/EJO.2010.6112 [DOI] [PubMed] [Google Scholar]
- 8.Sleath B, Blalock S, Covert D, et al. The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Research Support, N.I.H. extramuralresearch support, Non-U.S. Gov’t. Ophthalmology. 2011;118(12):2398–2402. doi: 10.1016/j.ophtha.2011.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olthoff CM, Schouten JS, van de Borne BW, Webers CA. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology. 2005;112(6):953–961. doi: 10.1016/j.ophtha.2004.12.035 [DOI] [PubMed] [Google Scholar]
- 10.Stewart WC, Chorak RP, Hunt HH, Sethuraman G. Factors associated with visual loss in patients with advanced glaucomatous changes in the optic nerve head. Am J Ophthalmol. 1993;116(2):176–181. doi: 10.1016/S0002-9394(14)71282-6 [DOI] [PubMed] [Google Scholar]
- 11.Kass MA, Gordon M, Morley RE, Meltzer DW, Goldberg JJ. Compliance with topical timolol treatment. Am J Ophthalmol. 1987;103(2):188–193. doi: 10.1016/s0002-9394(14)74225-4 [DOI] [PubMed] [Google Scholar]
- 12.Labbe A, Terry O, Brasnu E, Van Went C, Baudouin C. Tear film osmolarity in patients treated for glaucoma or ocular hypertension. Cornea. 2012;31(9):994–999. doi: 10.1097/ICO.0b013e31823f8cb6 [DOI] [PubMed] [Google Scholar]
- 13.O’Hare F, Ghosh S, Lamoureux E, Vajpayee RB, Crowston JG. Prevalence of signs and symptoms of ocular surface disease in individuals treated and not treated with glaucoma medication. Clin Exp Ophthalmol. 2012;40(7):675–681. doi: 10.1111/j.1442-9071.2012.02781.x [DOI] [PubMed] [Google Scholar]
- 14.Valente C, Iester M, Corsi E, Rolando M. Symptoms and signs of tear film dysfunction in glaucomatous patients. J Ocul Pharmacol Ther. 2011;27(3):281–285. doi: 10.1089/jop.2010.0133 [DOI] [PubMed] [Google Scholar]
- 15.Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350–355. doi: 10.1097/IJG.0b013e31815c5f4f [DOI] [PubMed] [Google Scholar]
- 16.Fechtner RD, Godfrey DG, Budenz D, Stewart JA, Stewart WC, Jasek MC. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29(6):618–621. doi: 10.1097/ICO.0b013e3181c325b2 [DOI] [PubMed] [Google Scholar]
- 17.Rossi GC, Pasinetti GM, Scudeller L, Raimondi M, Lanteri S, Bianchi PE. Risk factors to develop ocular surface disease in treated glaucoma or ocular hypertension patients. Eur J Ophthalmol. 2013;23(3):296–302. doi: 10.5301/ejo.5000220 [DOI] [PubMed] [Google Scholar]
- 18.Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with Glaucoma. Am J Ophthalmol. 2011;153(1):1–9.e2. doi: 10.1016/j.ajo.2011.05.033 [DOI] [PubMed] [Google Scholar]
- 19.Bacharach J, Tatham A, Ferguson G, et al. Phase 3, randomized, 20-month study of the efficacy and safety of bimatoprost implant in patients with open-angle glaucoma and ocular hypertension (ARTEMIS 2). Drugs. 2021;81(17):2017–2033. doi: 10.1007/s40265-021-01624-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medeiros FA, Walters TR, Kolko M, et al. Phase 3, randomized, 20-month study of bimatoprost implant in open-angle Glaucoma and ocular hypertension (ARTEMIS 1). Ophthalmology. 2020;127(12):1627–1641. doi: 10.1016/j.ophtha.2020.06.018 [DOI] [PubMed] [Google Scholar]
- 21.Craven ER, Walters T, Christie WC, et al. 24-month phase I/II clinical trial of bimatoprost sustained-release implant (Bimatoprost SR) in Glaucoma patients. Drugs. 2020;80(2):167–179. doi: 10.1007/s40265-019-01248-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allergan, an AbbVie company. Durysta Prescribing Information. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211911s000lbl.pdf. Accessed June 4, 2020.
- 23.Drugs@FDA: FDA-Approved Drugs. Durysta. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=211911. Accessed April 22, 2020.
- 24.Wong MK, Bowers ME, Ventimiglia J, et al. Short-term outcomes of bimatoprost sustained-release intracameral implant in Glaucoma. J Glaucoma. 2023;32(9):738–743. doi: 10.1097/IJG.0000000000002271 [DOI] [PubMed] [Google Scholar]
- 25.Bowers ME, Wong MK, Ventimiglia J, et al. Effect of bimatoprost sustained-release intracameral implant on intraocular pressure and medication burden in patients with prior glaucoma surgery. J Fr Ophtalmol. 2024;47(2):103996. doi: 10.1016/j.jfo.2023.07.016 [DOI] [PubMed] [Google Scholar]