Abstract
Objective.
The Manhattan Lupus Surveillance Program (MLSP) is a population-based registry designed to determine the prevalence of systemic lupus erythematosus (SLE) in 2007 and the incidence from 2007 to 2009 among residents of New York County (Manhattan), New York, and to characterize cases by race/ethnicity, including Asians and Hispanics, for whom data are lacking.
Methods.
We identified possible SLE cases from hospital records, rheumatologist records, and administrative databases. Cases were defined according to the American College of Rheumatology (ACR) classification criteria, the Systemic Lupus International Collaborating Clinics (SLICC) classification criteria, or the treating rheumatologist’s diagnosis. Rates among Manhattan residents were age-standardized, and capture–recapture analyses were conducted to assess case underascertainment.
Results.
By the ACR definition, the age-standardized prevalence and incidence rates of SLE were 62.2 and 4.6 per 100,000 person-years, respectively. Rates were ~9 times higher in women than in men for prevalence (107.4 versus 12.5) and incidence (7.9 versus 1.0). Compared with non-Hispanic white women (64.3), prevalence was higher among non-Hispanic black (210.9), Hispanic (138.3), and non-Hispanic Asian (91.2) women. Incidence rates were higher among non-Hispanic black women (15.7) compared with non-Hispanic Asian (6.6), Hispanic (6.5), and non-Hispanic white (6.5) women. Capture–recapture adjustment increased the prevalence and incidence rates (75.9 and 6.0, respectively). Alternate SLE definitions without capture–recapture adjustment revealed higher age-standardized prevalence and incidence rates (73.8 and 6.2, respectively, by the SLICC definition and 72.6 and 5.0 by the rheumatologist definition) than the ACR definition, with similar patterns by sex and race/ethnicity.
Conclusion.
The MLSP confirms findings from other registries on disparities by sex and race/ethnicity, provides new estimates among Asians and Hispanics, and provides estimates using the SLICC criteria.
Systemic lupus erythematosus (SLE) is a potentially fatal, heterogeneous, chronic, systemic autoimmune disease of unknown etiology (1). Given widely varying estimates of the incidence and prevalence of SLE in the US (2) and the absence of available data for certain demographic groups, we sought to obtain a fundamental epidemiologic understanding of SLE across racial/ethnic groups. Under the auspices of the National Arthritis Action Plan (3), the Centers for Disease Control and Prevention (CDC) funded 4 state or city health departments as well as the Indian Health Service (IHS) to more robustly define the incidence and prevalence of SLE. Results from the 2 initial sites, the Georgia Lupus Registry (GLR) and the Michigan Lupus Epidemiology and Surveillance (MILES) program, and the IHS site have been recently published (4–6). However, their estimates for Asians and Hispanics were limited. The Manhattan Lupus Surveillance Program (MLSP) was designed, along with the California Lupus Surveillance Project (CLSP), to provide estimates of the incidence and prevalence of SLE overall and specifically among Hispanic and Asian populations.
We launched the MLSP in 2009 as a collaboration between the New York City Department of Health and Mental Hygiene (DOHMH) and New York University School of Medicine (NYUSoM). Following methods similar to those used at the other CDC-funded sites (2,5,6), we designed the MLSP as a retrospective descriptive project to identify all cases of diagnosed SLE among residents of New York County (Manhattan), New York, 2007–2009, to determine the prevalence and incidence of SLE in this population.
PATIENTS AND METHODS
The Manhattan Lupus Surveillance Program.
The MLSP was designed to be similar to the GLR and MILES programs and, as described elsewhere (5,6), was conducted as a public health surveillance project by the New York City DOHMH, with NYUSoM acting as a public health agent on behalf of the DOHMH. No patients were contacted for this project. Medical records were collected under the health surveillance exemption to the Health Insurance Portability and Accountability Act (HIPAA) privacy rules (45 CFR § 164.512[b]) and as authorized by New York City Charter Sections 556(c)(2) and (d)(2). The CDC deemed the MLSP to be public health practice that did not require review by the CDC Institutional Review Board (IRB). IRBs at both the New York City DOHMH and NYUSoM reviewed and deemed the MLSP to be a surveillance activity. Additional IRB applications were completed and submitted to independent case-finding sources as requested.
Study population and study period.
The MLSP surveillance period was January 1, 2007 through December 31, 2009. New York County (Manhattan) was selected as the program catchment area because of its racial/ethnic diversity and because it is an island on which inhabitants largely remain for their health care, thus making access to medical records easier. We used data from lupus specialty clinics across New York City during initial planning for the MLSP and found that few Manhattan residents seek care in outer boroughs and that residents from other boroughs were more likely to seek care across a wide geographic range. Based on US Census data, there were 1,585,873 persons residing in Manhattan in 2010 (48% non-Hispanic white, 13% non-Hispanic black, 25% Hispanic, 11% non-Hispanic Asian) (7).
Case definitions.
Our primary American College of Rheumatology (ACR) case definition required ≥4 of the 11 criteria for the classification of SLE (8,9). Under the ACR classification criteria, patients with evidence of lupus nephritis (by biopsy report or specific documentation by a rheumatologist and/or nephrologist) are considered to have met the renal criteria for SLE, even without information on the degree of proteinuria or a description of the sediment. We also used 2 secondary case definitions of SLE: 1) the Systemic Lupus Erythematosus Collaborating Clinics (SLICC) classification criteria, which require the presence of at least 4 of 17 criteria, at least 1 of which must be clinical and 1 immunologic, or requires the presence of biopsy-proven lupus nephritis as well as antinuclear antibodies or anti–double-stranded DNA antibodies (10); or 2) the treating rheumatologist’s diagnosis of SLE. The SLICC case definition was included as a recently derived set of classification criteria with greater sensitivity and less specificity than the ACR classification criteria (10). The rheumatologist case definition was included because there is no gold standard for diagnosing SLE, and diagnosis is usually made by a physician who is familiar with the disease, often a rheumatologist.
Initial case-finding.
We used information from administrative databases, hospitals, and private rheumatologists to identify possible cases from as far back as 2004, when records were available. Administrative databases included the State of New York Department of Health Statewide Planning and Research Cooperative System, with information on hospitalization discharges in New York State, and the New York City DOHMH Vital Records, with information on all deaths in New York City. We included only hospitals and private rheumatologists based in Manhattan. We queried these sources to identify records with International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes indicating SLE (710.0), discoid lupus (695.4), or a related condition that may evolve into SLE or might have related symptoms (sicca syndrome [710.2], other specified connective tissue disease [CTD] [710.8], unspecified CTD [710.9]). If residence information was available from the case-finding source, we further restricted these records to include only those with evidence of Manhattan residence. Final screening of records was completed by trained MLSP abstractors to confirm physician diagnosis or suspicion of SLE or a related CTD and Manhattan residence during the surveillance period.
Data collection.
After initial case-finding, abstractors collected and entered information from the medical records into a New York City DOHMH database, with database and data dictionary materials adapted from those used by the GLR. When necessary, we corroborated Manhattan residence using the LexisNexis online database service (11). Our abstractors entered any ambiguous information into open text notes, which were later reviewed with the NYUSoM principal investigator (PMI) to correctly code it in the database.
All MLSP abstractors were trained under the GLR model (5) before abstraction began and underwent routine quality assurance reviews throughout the project. These reviews provided the opportunity for abstractors and the NYUSoM principal investigator to discuss any issues arising in the field and to address questions from the abstractors. Each abstractor had a medical degree and consistently achieved the required minimum interobserver agreement of 90% on all elements and 95% on the ACR classification criteria, using as the gold standard abstraction by the NYUSoM principal investigator. The average performance of the abstractors during training and reviews was 95.6% on all elements, 97.2% on the ACR classification criteria elements, and 97.5% on the unique elements in the SLICC classification criteria that were not already captured as part of the ACR classification criteria.
Statistical analysis.
We defined prevalent cases as new or existing cases meeting the ACR, SLICC, or rheumatologist case definition and residing in Manhattan at some time from January 1, 2007 through December 31, 2007. We defined incident cases as those meeting at least 1 of the case definitions, having their first diagnosis from January 1, 2007 through December 31, 2009, and residing in Manhattan. Population denominators were taken from the New York City DOHMH–interpolated intercensal population estimates for Manhattan (12). We calculated rates overall, by sex, and by race/ethnicity per 100,000 person-years and age-standardized to the 2000 standard population of the US using 10-year age categories for each racial/ethnic group (13). Information on race was collected separately from Hispanic ethnicity during abstraction. For analysis, we assigned cases to 1 of 5 mutually exclusive race/ethnicity categories: non-Hispanic white, non-Hispanic black, Hispanic, non-Hispanic Asian, and non-Hispanic other. Non-Hispanic cases identified as >1 race were categorized as non-Hispanic other.
We conducted capture–recapture analyses (14,15) to estimate case underascertainment from our primary ACR case definition. We fit log-linear models separately for incident and prevalent cases by sex and race/ethnicity to estimate the number of cases missed in our catchment area. Specifically, we fit various models that addressed potential violation of the homogeneity assumption of capture probability and identified the best fitting model using the Akaike information criterion. We then used estimates from these models to calculate revised prevalence and incidence rates.
Chi-square tests, or Fisher’s exact tests when needed, were used to assess univariate differences in SLE and ACR manifestations by race/ethnicity and sex. We compared differences between estimates by case definition using 95% confidence intervals (95% CIs) of the age-standardized rates, with non-overlapping 95% CIs considered to be significantly different. All analyses were completed using SAS version 9.3 (SAS Institute) and R version 3.3.0 (R Foundation for Statistical Computing) software.
RESULTS
Case-finding results.
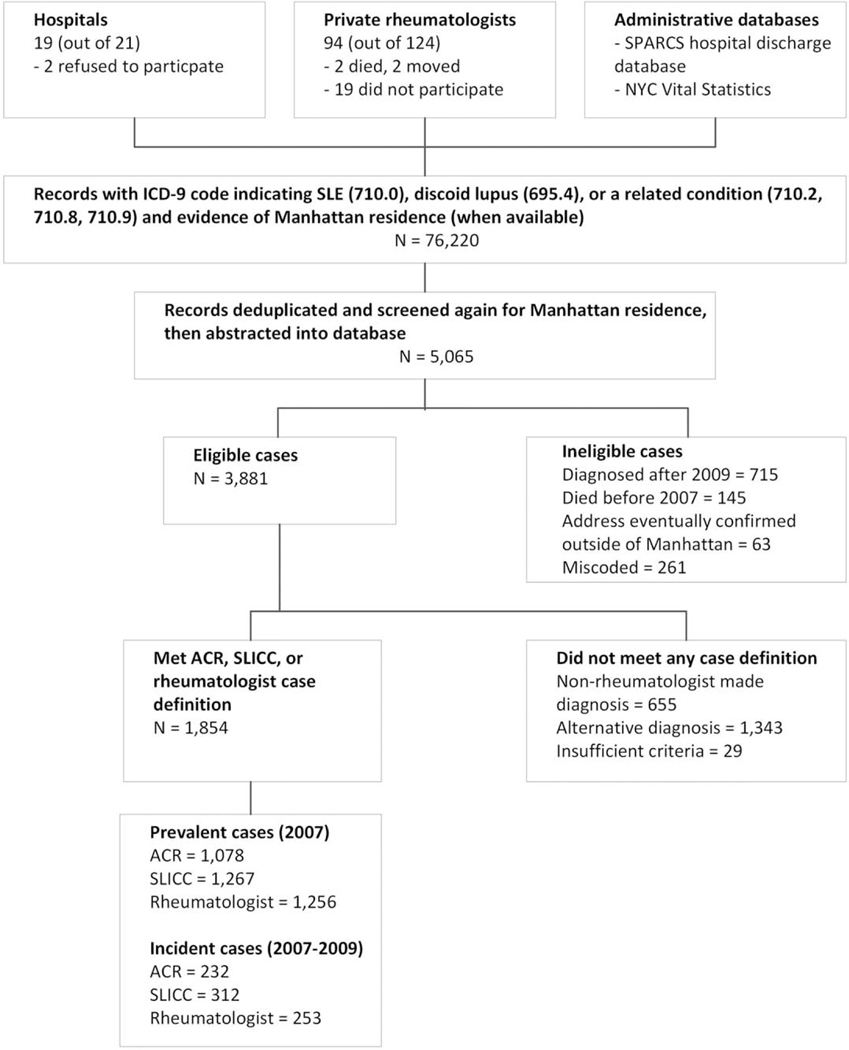
Case-finding and abstraction were completed in 19 of 21 hospitals (90.5%) (Figure 1), with 2 hospitals declining to participate (a cancer specialty hospital and a Veterans Affairs hospital). Case-finding and abstraction were performed from records of 94 of 124 private rheumatologists identified in the catchment area (75.8%). Of the 30 rheumatologists who did not participate, 19 did not respond to repeated requests or declined to participate, 2 died, 2 had retired and relocated, and 7 agreed to participate but abstraction could not be arranged despite repeated attempts before the data abstraction period ended.
Figure 1.
Flow chart showing the Manhattan Lupus Surveillance Program case-finding procedure for systemic lupus erythematosus (SLE). Cases of SLE were defined according to the American College of Rheumatology (ACR) criteria (met ≥4 of the 11 classification criteria), the Systemic Lupus International Collaborating Clinics (SLICC) criteria (met at least 4 of 17 criteria, at least 1 of which must be clinical and 1 immunologic, or the presence of biopsy-proven lupus nephritis as well as antinuclear antibodies or anti–double-stranded DNA antibodies), or the treating rheumatologist’s diagnosis. SPARCS = Statewide Planning and Research Cooperative System (of the New York State Department of Health); NYC = New York City; ICD-9 = International Classification of Diseases, Ninth Revision Clinical Modification.
Initial lists provided from the various case-finding sources identified 76,220 records (Figure 1). We removed duplicate records and records that did not have a Manhattan address, resulting in 5,065 possible cases with records for abstraction. During abstraction and data cleaning, we deemed 1,184 cases ineligible due to miscoded diagnosis or non-Manhattan residence. Of the remaining 3,881 possible cases, 1,854 met at least 1 of the case definitions.
Primary ACR case definition of prevalence.
In 2007, a total of 1,078 cases (307 non-Hispanic white, 282 non-Hispanic black, 344 Hispanic, 111 non-Hispanic Asian, and 34 non-Hispanic other race/ethnicity) fulfilled the ACR case definition for SLE (Table 1). The overall crude and age-standardized prevalence rates were 68.2 (95% CI 64.1–72.2) and 62.2 (95% CI 58.4–66.0) per 100,000 person-years. Age-standardized rates were ~9 times higher for women compared with men (107.4 versus 12.5).
Table 1.
Crude and age-standardized prevalence of SLE among residents of New York County (Manhattan), New York, 2007, according to the ACR, SLICC, and rheumatologist case definitions overall and by race/ethnicity and sex*
| ACR | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| Capture–recapture |
SLICC |
Rheumatologist |
|||||||||
| No. of patients | Crude rate (95% CI) | Age-standardized rate (95% CI) | No. missed | Adjusted rate (95% CI) | No. of patients | Crude rate (95% CI) | Age-standardized rate (95% CI) | No. of patients | Crude rate (95% CI) | Age-standardized rate (95% CI) | |
|
| |||||||||||
| Overall | 1,078 | 68.2 (64.1–72.2) | 62.2 (58.4–66.0) | 122.4 | 75.9 (70.6–81.2) | 1,267 | 80.1 (75.7–84.5) | 73.8 (69.6–77.9) | 1,256 | 79.4 (75.0–83.8) | 72.6 (68.5–76.7) |
| Men | 101 | 13.6 (10.9–16.2) | 12.5 (10.0–15.0) | 8.3 | 14.7 (12.5–16.9) | 110 | 14.8 (12.0–17.6) | 13.8 (11.1–16.4) | 98 | 13.2 (10.7–16.1) | 12.0 (9.7–14.7) |
| Women | 977 | 116.7 (109.3–124.0) | 107.4 (100.5–114.4) | 114.1 | 130.3(122.1–138.4) | 1,157 | 138.2 (130.2–146.1) | 128.3 (120.7–135.9) | 1,158 | 138.3 (130.3–146.2) | 127.5 (119.9–135.1) |
| Non-Hispanic white | 307 | 40.5 (36.0–45.0) | 34.7 (30.7–38.8) | 82.4 | 51.4 (45.0–57.7) | 373 | 49.2 (44.2–54.2) | 42.7 (38.1–47.3) | 352 | 46.4 (41.6–51.3) | 39.7 (35.3–44.0) |
| Men | 17 | 4.7 (2.7–7.5) | 3.7 (2.2–6.0) | 5.9 | 6.3 (3.2–9.4) | 23 | 6.3 (4.0–9.5) | 5.3 (3.3–8.0) | 24 | 6.6 (4.2–9.8) | 5.3 (3.4–7.8) |
| Women | 290 | 73.4 (64.9–81.8) | 64.3 (56.4–72.2) | 76.5 | 92.7 (83.4–102.1) | 350 | 88.6 (79.3–97.8) | 78.2 (69.4–86.9) | 328 | 83.0 (74.0–92.0) | 72.0 (63.7–80.4) |
| Non-Hispanic black | 282 | 131.4 (116.1–146.8) | 124.9 (110.3–139.6) | 3.6 | 133.1 (130.6–135.7) | 326 | 151.9 (135.5–168.4) | 144.7 (128.9–160.5) | 312 | 145.4 (129.3–161.6) | 137.7 (122.3–153.1) |
| Men | 28 | 28.5 (18.9–41.2) | 26.7 (17.7–38.7) | 0 | 28.5 (28.1–28.9) | 31 | 31.6 (21.4–44.8) | 29.7 (20.2–42.3) | 24 | 24.4 (15.7–36.4) | 22.6 (14.5–33.7) |
| Women | 254 | 218.4 (191.5–245.2) | 210.9 (184.8–237.1) | 3.6 | 221.4 (217.1–225.8) | 295 | 253.6 (224.7–282.5) | 244.4 (216.3–272.6) | 288 | 247.6 (219.0–276.2) | 237.2 (209.5–264.8) |
| Hispanic | 344 | 84.2 (75.3–93.1) | 82.8 (74.0–91.7) | 1.4 | 84.6 (83.8–85.3) | 372 | 91.1 (81.8–100.3) | 90.2 (81.0–99.5) | 396 | 97.0 (87.4–106.5) | 96.2 (86.7–105.8) |
| Men | 38 | 19.7 (13.9–27.0) | 19.4 (13.6–26.9) | 0.1 | 19.7 (19.4–20.0) | 38 | 19.7 (13.9–27.0) | 19.5 (13.7–26.9) | 33 | 17.1 (11.8–24.0) | 16.7 (11.4–23.6) |
| Women | 306 | 142.1 (126.2–158.0) | 138.3 (122.7–153.9) | 1.3 | 142.7 (141.5–143.9) | 334 | 155.1 (138.4–171.7) | 151.7 (135.3–168.1) | 363 | 168.5 (151.2–185.9) | 165.3 (148.2–182.5) |
| Non-Hispanic Asian | 111 | 64.0 (52.1–75.9) | 56.2 (44.7–67.7) | 20.0 | 75.5 (66.0–85.0) | 145 | 83.6 (70.0–97.2) | 75.1 (61.7–88.5) | 118 | 68.0 (55.7–80.3) | 62.2 (49.7–74.6) |
| Men | 15 | 19.3 (10.8–31.9) | 14.2 (7.6–24.0) | 2.3 | 22.3 (17.0–27.6) | 15 | 19.3 (10.8–31.9) | 14.2 (7.6–24.0) | 13 | 16.8 (8.9–28.7) | 12.5 (6.2–22.6) |
| Women | 96 | 100.0 (81.0–122.2) | 91.2 (72.1–113.8) | 17.7 | 118.5 (105.6–131.3) | 130 | 135.5 (112.2–158.7) | 125.9 (102.0–149.9) | 105 | 109.4 (88.5–130.3) | 103.6 (81.5–125.8) |
| Non-Hispanic other | 34 | – | – | 15.0 | – | 51 | – | – | 78 | – | – |
Systemic lupus erythematosus (SLE) cases were defined according to the American College of Rheumatology (ACR) criteria (met ≥4 of the 11 classification criteria), the Systemic Lupus International Collaborating Clinics (SLICC) criteria (met at least 4 of 17 criteria, at least 1 of which must be clinical and 1 immunologic, or the presence of biopsyproven lupus nephritis as well as antinuclear antibodies or anti–double-stranded DNA antibodies), or the treating rheumatologist’s diagnosis. Rates are per 100,000 person-years as a New York County (Manhattan) resident. Denominator data are based on 2007 intercensal population estimates from the New York City Department of Health and Mental Hygiene Bureau of Epidemiology Services (2000–2014 files). Data are standardized for age and race/ethnicity to the US standard population, 2000. Cases were assigned to 1 of 5 mutually exclusive race/ethnicity categories: non-Hispanic white, non-Hispanic black, Hispanic, non-Hispanic Asian, and non-Hispanic other. Non-Hispanic cases identified as being of >1 race were categorized as non-Hispanic other.
Age-standardized rates also differed by race/ethnicity among both women and men. The highest age-standardized prevalence rate was seen among non-Hispanic black women (210.9 per 100,000 personyears) followed by Hispanic women (138.3), non-Hispanic Asian women (91.2), and non-Hispanic white women (64.3). The age-standardized prevalence among men followed a similar pattern, with the highest estimate among non-Hispanic blacks (26.7) followed by Hispanics (19.4), non-Hispanic Asians (14.2), and non-Hispanic whites (3.7).
Capture–recapture estimates showed an additional 122 cases of SLE, indicating that 10.2% of cases may have been missed. Almost two-thirds (62.5%) of the estimated cases missed were non-Hispanic white women. With capture–recapture adjustment, the prevalence rate increased to 75.9 per 100,000 person-years (95% CI 70.6–81.2).
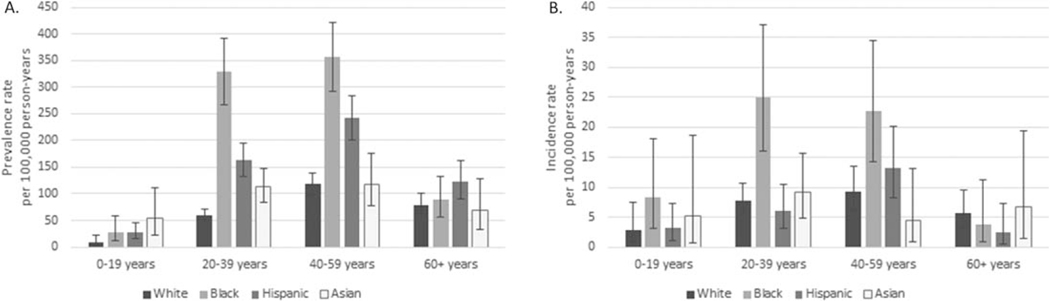
The mean ± SD age of women and men with SLE living in Manhattan in 2007 was 43.3 ± 15.5 years and 40.7 ± 16.9 years, respectively. The average age by race/ethnicity was 47.0 ± 16.5 years among non-Hispanic whites, 41.5 ± 13.7 years among non-Hispanic blacks, 42.9 ± 15.6 years among Hispanics, and 37.3 ± 15.4 years among non-Hispanic Asians. Figure 2A shows the age-specific prevalence for women by race/ethnicity. Prevalence was higher among non-Hispanic black and Hispanic women ages 20–59 years as compared to non-Hispanic white women of the same age group. Prevalence among non-Hispanic Asian women was not significantly different from that among non-Hispanic white women for any age group. Numbers among men were too small to assess age-specific rates by race/ethnicity.
Figure 2.
Age-specific prevalence and incidence rates (with 95% confidence intervals) of systemic lupus erythematosus among female residents of New York County (Manhattan) in 2007 and during 2007–2009, respectively, according to the American College of Rheumatology case definition (met ≥4 of the 11 classification criteria), categorized by age group. Cases were assigned to 1 of 5 mutually exclusive race/ethnicity categories: non-Hispanic white, non-Hispanic black, Hispanic, non-Hispanic Asian, and non-Hispanic other. Non-Hispanic cases identified as being of >1 race were categorized as non-Hispanic other and are not shown here.
Among the 344 Hispanic cases, 82.6% were also identified as white, 11.0% as black, and 6.4% as other race/ethnicity. Information on Hispanic ethnicity was often absent, with 239 (69.5%) having no further details, but Hispanic case ethnicities included Central or South American, Cuban, Dominican, Mexican, Puerto Rican, and Spanish. There were 111 non-Hispanic Asian cases as well as 5 identified as non-Hispanic other due to multiple race/ethnicity but with evidence of Asian race. More than one-fourth (26.7%) of these cases had no further classification for Asian ethnicity, but ethnicities among cases with information available included Chinese, Filipino, Hawaiian, Indian or Pakistani, Japanese, Korean, Pacific Islander not otherwise specified, South Asian, and Vietnamese.
Table 2 shows the occurrence of the 11 ACR criteria overall and by race/ethnicity among prevalent ACR cases. Renal disease was more common among non-Hispanic Asians (53.2%), non-Hispanic blacks (50.7%), and Hispanics (49.4%) compared with nonHispanic whites (25.4%). Neurologic manifestations were more common among Hispanics (26.2%) and nonHispanic blacks (24.5%) compared with non-Hispanic whites (16.6%). Also compared with non-Hispanic whites, discoid lesions were more commonly seen among non-Hispanic blacks (25.9% versus 8.8%) and malar rash was more commonly seen among Hispanics (50.0% versus 35.8%).
Table 2.
Frequency of 11 ACR classification criteria for SLE among prevalent and incident cases defined according to the ACR case definition overall and by race/ethnicity*
| No. (%) of prevalent cases (2007) | No. (%) of incident cases | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| ACR criterion | Overall | Non-Hispanic white | Non-Hispanic black | Hispanic | Non-Hispanic Asian | Overall | Non-Hispanic white | Non-Hispanic black | Hispanic | Non-Hispanic Asian |
|
| ||||||||||
| Overall | 1,078 (100) | 307 (28.5) | 282 (26.2) | 344 (31.9) | 111 (10.3) | 232 (100) | 92 (39.7) | 62 (26.7) | 49 (21.1) | 22 (9.5) |
| Antinuclear antibody | 996 (92.4) | 284 (92.5) | 262 (92.9) | 316 (91.9) | 103 (92.8) | 213 (91.8) | 82 (89.1) | 58 (93.5) | 47 (95.9) | 22 (100) |
| Hematologic disorder | 893 (82.8) | 255 (83.1) | 238 (84.4) | 278 (80.8) | 98 (88.3) | 188 (81.0) | 71 (77.2) | 56 (90.3)† | 37 (75.5) | 19 (86.4) |
| Arthritis | 813 (75.4) | 246 (80.1) | 204 (72.3) | 255 (74.1) | 80 (72.1) | 159 (68.5) | 66 (71.7) | 41 (66.1) | 30 (61.2) | 17 (77.3) |
| Immunologic disorder | 781 (72.4) | 213 (69.4) | 204 (72.3) | 253 (73.5) | 89 (80.2)† | 170 (73.3) | 66 (71.7) | 46 (74.2) | 37 (75.5) | 18 (81.8) |
| Renal disorder | 457 (42.4) | 78 (25.4) | 143 (50.7)† | 170 (49.4)† | 59 (53.2)† | 81 (34.9) | 22 (23.9) | 27 (43.5)† | 21 (42.9)† | 10 (45.5)† |
| Serositis | 449 (41.7) | 117 (38.1) | 127 (45.0) | 156 (45.3) | 36 (32.4) | 84 (36.2) | 25 (27.2) | 33 (53.2)† | 18 (36.7) | 5 (22.7) |
| Malar rash | 428 (39.7) | 110 (35.8) | 82 (29.1) | 172 (50.0)† | 46 (41.4) | 86 (37.1) | 31 (33.7) | 20 (32.3) | 22 (44.9) | 8 (36.4) |
| Photo sensitivity | 370 (34.3) | 121 (39.4) | 76 (27.0)† | 132 (38.4) | 30 (27.0)† | 74 (31.9) | 32 (34.8) | 11 (17.7)† | 21 (42.9) | 7 (31.8) |
| Oral ulcers | 333 (30.9) | 104 (33.9) | 64 (22.7)† | 115 (33.4) | 37 (33.3) | 81 (34.9) | 42 (45.7) | 16 (25.8)† | 12 (24.5)† | 7 (31.8) |
| Neurologic disorder | 230 (21.3) | 51 (16.6) | 69 (24.5)† | 90 (26.2)† | 11 (9.9) | 43 (18.5) | 15 (16.3) | 15 (24.2) | 11 (22.4) | 1 (4.5) |
| Discoid rash | 179 (16.6) | 27 (8.8) | 73 (25.9)† | 58 (16.9)† | 17 (15.3) | 32 (13.8) | 9 (9.8) | 16 (25.8)† | 5 (10.2) | 1 (4.5) |
Systemic lupus erythematosus (SLE) cases were defined according to the American College of Rheumatology (ACR) criteria (met ≥4 of the 11 classification criteria). Cases were assigned to 1 of 5 mutually exclusive race/ethnicity categories: non-Hispanic white, non-Hispanic black, Hispanic, non-Hispanic Asian, and non-Hispanic other. Non-Hispanic cases identified as being of >1 race were categorized as non-Hispanic other.
P< 0.05 versus non-Hispanic whites, by univariate logistic regression.
Primary ACR case definition of incidence rates.
From 2007 to 2009, a total of 232 incident cases met the ACR case definition (Table 3) for SLE (92 non-Hispanic white, 62 non-Hispanic black, 49 Hispanic, 22 non-Hispanic Asian, and 7 non-Hispanic other race/ethnicity). The overall crude and age-standardized incidence rates were 4.9 (95% CI 4.3–5.5) and 4.6 (95% CI 4.0–5.2) per 100,000 person-years, respectively. Age-standardized rates differed by sex and were almost 8 times higher in women than in men (7.9 versus 1.0). Age-standardized rates also differed by race/ethnicity among both women and men. The highest agestandardized incidence rates among women were among non-Hispanic blacks (15.7) followed by non-Hispanic Asians (6.6), Hispanics (6.5), and non-Hispanic whites (6.5). Similarly, the highest age-standardized incidence rates among men were among non-Hispanic blacks (2.4) followed by Hispanics (1.3), non-Hispanic Asians (0.5), and non-Hispanic whites (0.5).
Table 3.
Crude and age-standardized incidence rates of SLE among residents of New York County (Manhattan), New York, 2007–2009, according to the ACR, SLICC, and rheumatologist case definitions overall and by race/ethnicity and sex*
| ACR | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| Capture–recapture |
SLICC |
Rheumatologist |
|||||||||
| No. of patients | Crude rate (95% CI) | Age-standardized rate (95% CI) | No. missed | Adjusted rate (95% CI) | No. of patients | Crude rate (95% CI) | Agestandardized rate (95% CI) | No. of patients | Crude rate (95% CI) | Agestandardized rate (95% CI) | |
|
| |||||||||||
| Overall | 232 | 4.9 (4.3–5.5) | 4.6 (4.0–5.2) | 52.4 | 6.0 (4.6–7.4) | 312 | 6.6 (5.8–7.3) | 6.2 (5.5–6.9) | 253 | 5.3 (4.7–6.0) | 5.0 (4.4–5.7) |
| Men | 23 | 1.0 (0.7–1.5) | 1.0 (0.6–1.5) | 3.3 | 1.2 (0.7–1.7) | 38 | 1.7 (1.2–2.3) | 1.7 (1.2–2.3) | 28 | 1.3 (0.8–1.8) | 1.2 (0.8–1.8) |
| Women | 209 | 8.3 (7.2–9.4) | 7.9 (6.8–9.0) | 49.1 | 10.3 (8.0–12.5) | 274 | 10.9 (9.6–12.2) | 10.3 (9.1–11.6) | 225 | 8.9 (7.8–10.1) | 8.6 (7.4–9.7) |
| Non-Hispanic white | 92 | 4.0 (3.2–4.9) | 3.6 (2.8–4.5) | 36.7 | 5.6 (4.2–7.1) | 124 | 5.4 (4.5–6.4) | 4.8 (3.9–5.8) | 94 | 4.1 (3.3–5.0) | 3.8 (3.0–4.8) |
| Men | 7 | 0.6 (0.3–1.3) | 0.5 (0.2–1.0) | 1.6 | 0.8 (0.4–1.2) | 13 | 1.2 (0.6–2.0) | 1.0 (0.5–1.7) | 9 | 0.8 (0.4–1.6) | 0.7 (0.3–1.3) |
| Women | 85 | 7.1 (5.7–8.8) | 6.5 (5.0–8.3) | 35.1 | 10.1 (7.7–12.5) | 111 | 9.3 (7.6–11.1) | 8.5 (6.7–10.3) | 85 | 7.1 (5.7–8.8) | 6.8 (5.2–8.6) |
| Non-Hispanic black | 62 | 9.8 (7.5–12.6) | 9.3 (7.1–12.0) | 1.8 | 10.1 (9.1–11.0) | 79 | 12.5 (9.9–15.5) | 12.0 (9.5–15.0) | 61 | 9.6 (7.4–12.4) | 9.2 (7.0–11.8) |
| Men | 7 | 2.4 (1.0–5.0) | 2.4 (1.0–5.0) | 1.0 | 2.8 (1.6–3.9) | 11 | 3.8 (1.9–6.8) | 3.8 (1.9–6.8) | 7 | 2.4 (1.0–5.0) | 2.3 (0.9–4.7) |
| Women | 55 | 16.0 (12.1–20.9) | 15.7 (11.8–20.5) | 0.8 | 16.3 (15.5–17.0) | 68 | 19.8 (15.4–25.1) | 19.3 (14.9–24.5) | 54 | 15.7 (11.8–20.5) | 15.5 (11.6–20.3) |
| Hispanic | 49 | 4.0 (3.0–5.3) | 4.0 (3.0–5.4) | 1.3 | 4.1 (3.8–4.5) | 64 | 5.2 (4.0–6.7) | 5.3 (4.1–6.7) | 50 | 4.1 (3.0–5.4) | 4.2 (3.1–5.5) |
| Men | 7 | 1.2 (0.5–2.5) | 1.3 (0.5–2.7) | 0.4 | 1.3 (1.0–1.5) | 8 | 1.4 (0.6–2.7) | 1.6 (0.7–3.2) | 6 | 1.0 (0.4–2.3) | 1.1 (0.4–2.5) |
| Women | 42 | 6.5 (4.7–8.8) | 6.5 (4.7–8.8) | 0.9 | 6.7 (6.2–7.1) | 56 | 8.7 (6.6–11.3) | 8.6 (6.5–11.2) | 44 | 6.8 (5.0–9.2) | 7.0 (5.1–9.4) |
| Non-Hispanic Asian | 22 | 4.2 (2.6–6.3) | 3.8 (2.3–6.0) | 6.7 | 5.4 (3.3–7.5) | 31 | 5.8 (4.0–8.3) | 5.3 (3.4–7.7) | 27 | 5.1 (3.4–7.4) | 4.5 (2.9–6.9) |
| Men | 1 | 0.4 (0.0–2.4) | 0.5 (0.0–2.7) | 0.3 | 0.6 (0.0–1.1) | 2 | 0.8 (0.1–3.1) | 1.0 (0.1–3.5) | 2 | 0.8 (0.1–3.1) | 1.0 (0.1–3.7) |
| Women | 21 | 7.1 (4.4–10.9) | 6.6 (3.8–10.5) | 6.4 | 9.3 (6.0–12.7) | 29 | 9.9 (6.6–14.2) | 8.8 (5.6–13.1) | 25 | 8.5 (5.5–12.6) | 7.5 (4.5–11.6) |
| Non-Hispanic other | 7 | 5.9 | 14 | 21 | |||||||
Systemic lupus erythematosus (SLE) cases were defined according to the American College of Rheumatology (ACR) criteria (met ≥4 of the 11 classification criteria), the Systemic Lupus International Collaborating Clinics (SLICC) criteria (met at least 4 of 17 criteria, at least 1 of which must be clinical and 1 immunologic, or the presence of biopsyproven lupus nephritis as well as antinuclear antibodies or anti–double-stranded DNA antibodies), or the treating rheumatologist’s diagnosis. Rates are per 100,000 person-years as a New York County (Manhattan) resident. Denominator data are based on 2007–2009 intercensal population estimates from the New York City Department of Health and Mental Hygiene Bureau of Epidemiology Services (2000–2014 files). Data are standardized for age and race/ethnicity to the US standard population, 2000. Cases were assigned to 1 of 5 mutually exclusive race/ethnicity categories: non-Hispanic white, non-Hispanic black, Hispanic, non-Hispanic Asian, and non-Hispanic other. Non-Hispanic cases identified as being of >1 race were categorized as non-Hispanic other.
Capture–recapture adjustment estimated 284 incident cases of SLE, indicating that 18.4% of cases were missed; 67.0% of these were non-Hispanic white women. The resulting capture–recapture adjusted incidence rate increased to 6.0 per 100,000 person-years (95% CI 4.6–7.4).
The mean ± SD age at diagnosis was 40.1 ± 16.6 years among women and 42.9 ± 20.4 years among men, with values of 42.2 ± 17.7 years among non-Hispanic whites, 39.2 ± 16.3 years among non-Hispanic blacks, 39.6 ± 17.0 years among Hispanics, and 37.9 ± 16.0 years among non-Hispanic Asians. Figure 2B shows the age-specific incidence rates for women by race/ethnicity. The only age-specific difference was between non-Hispanic black and non-Hispanic white women who were 20–39 years old. Otherwise, due to small numbers within each stratum, no age-specific differences were found.
Among the 49 incident Hispanic cases, 77.6% were also identified as non-Hispanic white, 16.3% as non-Hispanic black, and 6.1% as non-Hispanic other race/ethnicity. As with the prevalent cases, Hispanic ethnicity information for incident cases was often absent, with 71.4% having no further ethnicity information available. Among the 22 incident non-Hispanic Asian cases, 32% had no further data available.
Table 2 shows the occurrence of the 11 ACR criteria overall and by race/ethnicity among incident ACR cases. Evidence of renal disease was found among 34.9% of incident cases, but was more common among non-Hispanic Asians (45.5%), non-Hispanic blacks (43.5%), and Hispanics (42.9%) compared with non-Hispanic whites (23.9%). Discoid lesions were more common among non-Hispanic blacks (25.8%) compared with non-Hispanic whites (9.8%).
Secondary case definitions.
Prevalence and incidence rates calculated using the SLICC case definition for SLE were significantly higher than those calculated using the primary ACR case definition. Using the SLICC case definition generated crude and agestandardized prevalence rates of 80.1 (95% CI 75.7–84.5) and 73.8 (95% CI 69.6–77.9) per 100,000 person-years, respectively, which were 17–19% higher than those calculated using the ACR case definition. The SLICC crude and age-standardized incidence rates (6.6 [95% CI 5.8–7.3] and 6.2 [95% CI 5.5–6.9], respectively) were nearly 35% higher than the ACR incidence rates.
The rheumatologist case definition yielded crude and age-standardized prevalence rates that were ~17% higher than the ACR case definition rates (79.4 [95% CI 75.0–83.8] and 72.6 [95% CI 68.5–76.7] per 100,000 person-years, respectively). Crude and age-standardized incidence rates using the rheumatologist case definition were similar to rates using the ACR case definition (5.3 [95% CI 4.7–6.0] and 5.0 [95% CI 4.4–5.7], respectively). For both secondary case definitions, differences in rates by sex and race/ethnicity were similar to those identified by the ACR case definition.
Of the 1,538 incident and prevalent cases meeting either the ACR or SLICC case definition, 75.6% met both ACR and SLICC definitions, 4.3% met only the ACR definition, and 20.2% met only the SLICC definition. Table 4 displays information on the unique SLICC criteria that are not part of the ACR classification criteria among incident and prevalent cases meeting the SLICC case definition only. The most common unique SLICC criteria among these cases were low complement levels, alopecia, and different definitions for lymphopenia. In addition, 5.5% of cases meeting the SLICC case definition had ANA or anti–double-stranded DNA antibody and biopsy findings consistent with lupus nephritis. Reasons that cases met the ACR and not the SLICC case definition were largely due to having ≥4 clinical criteria but no immunologic criteria, differences in categorization of photosensitivity and malar rash (which were separate in the ACR criteria and combined in the SLICC criteria), and differences in defining lymphopenia and anticardiolipin antibody (data not shown).
Table 4.
Unique criteria among 310 incident and prevalent SLE cases meeting the SLICC, but not the ACR, case definitions*
| Unique SLICC criteria | No. (%) of patients |
|---|---|
|
| |
| Immunologic criteria | |
| Low complement levels | 151 (48.7) |
| Anti—β2-glycoprotein antibodies (IgG or IgM) | 16 (5.2) |
| Positive direct Coombs’ test result in the absence of hemolytic anemia | 5 (1.6) |
| Clinical criteria | |
| Acute cutaneous lupus | |
| Bullous lupus | 1 (0.3) |
| Toxic epidermal necrolysis variant of SLE | 0 (0.0) |
| Maculopapular lupus rash | 13 (4.2) |
| Subacute cutaneous lupus | 4 (1.3) |
| Chronic cutaneous lupus | |
| Hypertrophic (verrucous) lupus | 3 (1.0) |
| Lupus panniculitis (profundus) | 4 (1.3) |
| Mucosal lupus | 0 (0.0) |
| Lupus erythematosus tumidus | 1 (0.3) |
| Chilblains lupus | 1 (0.3) |
| Discoid lupus/lichen planus overlap | 4 (1.3) |
| Nonscarring alopecia | 122 (39.4) |
| Neurologic criteria | |
| Mononeuritis multiplex | 3 (1.0) |
| Myelitis | 2 (0.6) |
| Peripheral or cranial neuropathy | 53 (17.1) |
| Acute confusional state | 3 (1.0) |
| Lymphopenia | 147 (47.4) |
| ANA or anti-dsDNA antibody and biopsy-proven lupus nephritis | 17 (5.5) |
More than 1 criterion may have been manifested by a given systemic lupus erythematosus (SLE) case. Data on the IgA isotype of anti–β2-glycoprotein I and anticardiolipin antibodies were not collected. For anti–double-stranded DNA (anti-dsDNA) determined by enzyme-linked immunosorbent assay, the results were reported as positive or negative; thus, it is possible that in some cases, this criterion was overcounted in the Systemic Lupus International Collaborating Clinics (SLICC) system if the positive result was not specifically double the upper cutoff for the negative value. Finally, CH50 was not captured, and thus, it is possible that the SLICC criterion for complement was undercounted. ACR = American College of Rheumatology; ANA = antinuclear antibody.
DISCUSSION
Our analysis of the MLSP data provides prevalence and incidence rate estimates of SLE among Manhattan residents using methods similar to other CDC-funded SLE registries. Our analysis confirms evidence of a higher prevalence of SLE among non-Hispanic blacks compared with non-Hispanic whites and adds evidence of a higher prevalence of SLE among Hispanics and non-Hispanic Asians. The MLSP is the first among the CDC-funded SLE registries to report using the SLICC classification criteria, which were recently validated (10), to describe cases of SLE.
The age-standardized prevalence and incidence rates of SLE in Manhattan were 62.2 (95% CI 58.4–66.0) and 4.6 (95% CI 4.0–5.2) per 100,000 person-years using the ACR case definition. Compared with previous reports by the CDC-funded sites, we estimated slightly lower overall age-standardized prevalence than did the GLR (73.0 [95% CI 68.9–77.4]) (5) and MILES (72.8 [95% CI 70.8–74.8]) studies (6), but we found similar disparities by sex and race/ethnicity for non-Hispanic whites and non-Hispanic blacks. The MLSP prevalence estimates increased with capture–recapture adjustment (75.9 [95% CI 70.6–81.2]) and were comparable to the capture–recapture–adjusted estimates from the GLR (83.0 [95% CI 78.6–87.7]). Our age-standardized incidence rates using the ACR case definition were similar to those from the GLR and MILES.
We found the highest prevalence and incidence rates among non-Hispanic blacks, consistent with the GLR and MILES studies and with preliminary data from the CLSP study. However, unlike the GLR and MILES studies, we found elevated prevalence among non-Hispanic Asians and Hispanics compared with nonHispanic whites. Compared with preliminary crude estimates from the CLSP study (16), the MLSP showed similar elevated rates among Hispanics (84.2 [95% CI 75.3–93.1] versus 87.7 [95% CI 72.1–106.8] per 100,000 person-years) but slightly lower rates among nonHispanic Asians (64.0 [95% CI 52.1–75.9] versus 95.8 [95% CI 84.9–108.1] per 100,000 person-years). These MLSP findings are particularly important, given the few published studies on the prevalence and incidence of SLE among Asians and Hispanics in the US. A review published in 1973 presented estimates among New York City residents from 1956 to 1965 but focused only on whites, blacks, and Puerto Ricans (17). Another study published in 2001 estimated the prevalence of SLE among Hispanics in Arizona to be 103.0 per 100,000 persons, slightly higher than the rate found by the MLSP among Hispanics in Manhattan (18). A more recent study using Medicaid data estimated an even higher prevalence of SLE among Hispanics (126.5 per 100,000 persons) with Medicaid coverage in the US from 2000 to 2004 (19).
The study using Medicaid data is one of the few to estimate rates of SLE among Asians in the US, reporting a prevalence ~3 times that estimated by the MLSP (175.1 versus 56.2 per 100,000 persons) (19). The only other studies known to assess rates of SLE among Asians in the US focused on SLE prevalence. One study identified cases in Hawaii based on physician diagnosis at 5 medical centers and outpatient practices in 1989. The overall SLE prevalence identified in that study (41.8 per 100,000 persons) was similar to the MLSP estimate for non-Hispanic Asians, and the age-standardized rates for women of specific Asian ethnic groups (Chinese, Filipino, Hawaiian, Japanese) was found to be higher compared with that among white women (20). Another study, using hospital discharge data, reported that Asian/Pacific Islander women had a lower rate of prevalent SLE compared with white women (21). Less is known about the incidence of SLE among Asians. In England, new diagnoses of SLE are more common among Asians, specifically South Asians from India and Pakistan, compared with whites (22,23), but to our knowledge, there are no other published reports on the incidence of SLE among Asians in the US.
In this analysis, we also provide information on manifestations among SLE cases. Clinical or serologic manifestations among prevalent cases approximated those from the GLR and MILES registries. The MLSP found a high burden of nephritis overall, with nearly half (42.4%) of prevalent cases developing nephritis. The proportion of those with nephritis was higher among non-white prevalent cases, specifically 50.7% among non-Hispanic blacks, 49.4% among Hispanics, and 53.2% among non-Hispanic Asians as compared with 25.4% among non-Hispanic whites, results that are consistent with those of other studies (5,6,19,24,25).
The SLICC case definition of SLE yielded higher incidence and prevalence estimates than the ACR case definition. Unique criteria which substantiated the classification of SLE based on SLICC, but not ACR, criteria included low complement levels, alopecia, and different definitions of lymphopenia (10). The small number of cases that met the ACR but not the SLICC case definition is reassuring, as it suggests that few cases met ACR criteria for SLE without the presence of autoantibodies. However, given the descriptive nature of the MLSP and the absence of a gold standard test that would unambiguously identify SLE, this project could not assess which set of classification criteria is more sensitive or specific. In addition, non-overlapping confidence intervals were used to conservatively assess differences among rates (26).
There were several limitations to this project. First, we may have underestimated cases, as 2 hospitals and one-fourth of rheumatologists in the catchment area declined to participate. Most of the practices that did not participate were in neighborhoods with a majority white population, which is consistent with our capture–recapture analysis that estimated 67.3% of prevalent cases and 70.0% of incident cases missed were non-Hispanic white. However, the exclusion of the Veterans Affairs hospital may have resulted in under-identification of men diagnosed as having SLE. We also did not include nephrology, dermatology, or primary or alternative care practices among our case-finding sources. Though when possible we did query hospital pathology databases for relevant kidney or skin biopsies, we still may have missed milder cases that were not hospitalized or seen by a rheumatologist during the surveillance period. It is also possible that we missed cases if they lived in Manhattan but sought care in other boroughs or a neighboring state.
Second, medical systems differed tremendously, and any difficulty navigating different electronic medical records or any difficulty with the legibility of paper records could have led to missed or miscoded data. Additionally, medical records are designed for physician use, not for data abstraction and surveillance. Thus, some information of interest may have been missing or ambiguous, depending on what was collected and recorded by the case-finding source.
Third, abstracting occurred several years after the surveillance period, which could have led to missing information if records were put into storage or if data elements were lost during a facility’s migration from paper to electronic records. This lag time may have also affected our ability to find cases of SLE, as some newer systems were unable to query past certain dates. Additionally, many private practices did not retain information on patients’ prior addresses, so we may not have abstracted cases who moved outside of Manhattan since the surveillance period. However, when possible, the software LexisNexis was used to verify patient residence within the catchment area.
Finally, data on race and ethnicity was abstracted from administrative and medical records, which may not accurately represent the patient’s own racial or ethnic identification. Additionally, information on ethnicity was often missing or did not include detail such as country of origin, which limited our ability to describe rates of SLE among specific ethnic groups. Though available information did reflect the major ethnic groups in Manhattan, ethnicity information was missing for most Hispanic cases and more than one-fourth of non-Hispanic Asian cases. Categorized broadly, Hispanic or Asian race encompasses a number of heterogeneous groups and SLE rates among them may differ. Given the already limited number of published studies on SLE among Asians and Hispanics, additional work is needed to better describe and understand the experience of SLE among specific ethnic subpopulations.
Despite these limitations, our analysis benefitted from the design and composition of the MLSP. First, the MLSP was designed as a population-based registry with methods similar to those used for 4 other CDC-funded SLE registries, which allowed us to compare rates across sites. Second, the diverse population within our catchment area allowed us to estimate rates of SLE among the major racial categories, particularly Asians and Hispanics. Third, given the recent publication of the SLICC classification criteria, we were able to estimate rates of SLE by this case definition and compare them to the ACR case definition. Fourth, the partnership with the New York City DOHMH allowed us to collect information from a number of case-finding sources and find complete clinical information on most cases. Finally, our abstractors all had a medical background, which helped during training and provided an advantage during extensive review of medical records to identify SLE criteria.
In conclusion, we found substantial disparities in prevalence, incidence, and manifestations of SLE by sex and race/ethnicity among Manhattan residents. Women consistently had higher prevalence and incidence rates of SLE compared with men, and non-Hispanic blacks, Hispanics, and non-Hispanic Asians had higher rates of diagnosed SLE and a higher proportion had lupus nephritis compared with non-Hispanic whites. The highest rates of SLE were seen among non-Hispanic black women, followed by Hispanic, non-Hispanic Asian, and non-Hispanic white women. Using the SLICC criteria for SLE provided higher prevalence and incidence rates than the ACR criteria.
ACKNOWLEDGMENTS
The authors thank all of the rheumatologists who participated in the MLSP as well as their practice managers who provided assistance. We would also like to thank all of the administrators in the medical records departments of the participating hospitals for their assistance in providing lists and obtaining medical records. We would like to acknowledge the contributions of past and current members at the New York City DOHMH, including Tamira Collins-Bowers, Manasi Joshi, Bonnie Kerker, Maushumi Mavinkurve, Angela Merges, Kyyon Nelson, Viren Shah, Joseph Slade, Lorna Thorpe, Talytha Utley, and Elizabeth Waddell. In addition, we would like to acknowledge the hard work of the MLSP abstractors, Drs. Janice McFarlane, Nick Stefanopoulos, Zahira Zahid, Rukayatu Ibrahim, Saleh Massasati, and Simone Shrestha. Finally, we would like to acknowledge the support and contributions of the principal investigators of the other CDC-funded surveillance sites, including Drs. Sam Lim, Cristina Drenkard (who each came to NYUSoM to assist in the development of the MLSP), Emily Somers, Joe McCune, Maria Dall’Era, and Elizabeth Ferucci.
Supported by cooperative agreements between the CDC and the New York City Department of Health and Mental Hygiene (grant U58/DP002827).
Footnotes
The views expressed herein are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Study conception and design. Izmirly, Askanase, Gordon, Helmick, Parton.
Acquisition of data. Izmirly, Wan, Sahl, Buyon, Belmont, Salmon, Askanase, Bathon, Geraldino-Pardilla, Ali, Ginzler, Putterman, Parton.
Analysis and interpretation of data. Izmirly, Wan, Buyon, Belmont, Gordon, Helmick, Parton.
REFERENCES
- 1.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med 2008;358:929–39. [DOI] [PubMed] [Google Scholar]
- 2.Lim SS, Drenkard C, McCune WJ, Helmick CG, Gordon C, DeGuire P, et al. Population-based lupus registries: advancing our epidemiologic understanding. Arthritis Rheum 2009;61:1462–6. [DOI] [PubMed] [Google Scholar]
- 3.Meenan RF, Callahan LF, Helmick CG. The National Arthritis Action Plan: a public health strategy for a looming epidemic. Arthritis Care Res 1999;12:79–81. [PubMed] [Google Scholar]
- 4.Ferucci ED, Johnston JM, Gaddy JR, Sumner L, Posever JO, Choromanski TL, et al. Prevalence and incidence of systemic lupus erythematosus in a population-based registry of American Indian and Alaska native people, 2007–2009. Arthritis Rheumatol 2014;66:2494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C. The incidence and prevalence of systemic lupus erythematosus, 2002–2004: the Georgia Lupus Registry. Arthritis Rheumatol 2014;66:357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somers EC, Marder W, Cagnoli P, Lewis EE, DeGuire P, Gordon C, et al. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol 2014;66:369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Census Bureau. 2010 census. Summary file 1, tables P5, P8, PCT4, PCT5, PCT8, and PCT11. URL: http://factfinder.census.gov.
- 8.Hochberg MC, for the Diagnostic and Therapeutic Criteria Committee of the American College of Rheumatology. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter]. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 9.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 10.Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LexisNexis. URL: https://www.lexisnexis.com/en-us/gateway.page.
- 12.New York City Department of Health and Mental Hygiene population estimates, modified from US Census Bureau intercensal population estimates, 2007–2009. Updated July 22, 2013.
- 13.Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected U.S. population. January 2001. URL: https://www.cdc.gov/nchs/data/statnt/statnt20.pdf. [PubMed]
- 14.Hook EB, Regal RR. Capture-recapture methods in epidemiology: methods and limitations. Epidemiol Rev 1995;17:243–64. [DOI] [PubMed] [Google Scholar]
- 15.Baillargeon S, Rivest LP. Rcapture: loglinear models for capture-recapture in R. J Stat Softw 2007;19:1–31.21494410 [Google Scholar]
- 16.Dall’Era MC, Snipes K, Cisternas M, Gordon C, Helmick CG. Preliminary population-based incidence and prevalence estimates of systemic lupus erythematosus: the California Lupus Surveillance Project [abstract]. Arthritis Rheumatol 2014;66 Suppl: S1217. [DOI] [PubMed] [Google Scholar]
- 17.Siegel M, Lee SL. The epidemiology of systemic lupus erythematosus. Semin Arthritis Rheum 1973;3:1–54. [DOI] [PubMed] [Google Scholar]
- 18.Balluz L, Philen R, Ortega L, Rosales C, Brock J, Barr D, et al. Investigation of systemic lupus erythematosus in Nogales, Arizona. Am J Epidemiol 2001;154:1029–36. [DOI] [PubMed] [Google Scholar]
- 19.Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcón GS, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004. Arthritis Rheum 2013;65: 753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maskarinec G, Katz AR. Prevalence of systemic lupus erythematosus in Hawaii: is there a difference between ethnic groups? Hawaii Med J 1995;54:406–9. [PubMed] [Google Scholar]
- 21.Chakravarty EF, Bush TM, Manzi S, Clarke AE, Ward MM. Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: estimates obtained using hospitalization data. Arthritis Rheum 2007;56:2092–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson AE, Gordon C, Palmer RG, Bacon PA. The prevalence and incidence of systemic lupus erythematosus in Birmingham, England: relationship to ethnicity and country of birth. Arthritis Rheum 1995;38:551–8. [DOI] [PubMed] [Google Scholar]
- 23.Rees F, Doherty M, Grainge M, Davenport G, Lanyon P, Zhang W. The incidence and prevalence of systemic lupus erythematosus in the UK, 1999–2012. Ann Rheum Dis 2016;75:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alarcón GS, McGwin G Jr, Petri M, Reveille JD, Ramsey-Goldman R, Kimberly RP, and the PROFILE Study Group. Baseline characteristics of a multiethnic lupus cohort: PROFILE [published erratum appears in Lupus 2002;11:402]. Lupus 2002; 11:95–101. [DOI] [PubMed] [Google Scholar]
- 25.Hanly JG, O’Keeffe AG, Su L, Urowitz MB, Romero-Diaz J, Gordon C, et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford) 2016;55:252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schenker N, Gentleman JF. On judging the significance of differences by examining overlap between confidence intervals. Am Stat 2001;55:182–6. [Google Scholar]