Abstract
Negative pressure wound therapy with instillation and dwell time (NPWTi-d) is increasingly used for a diverse range of wounds. Meanwhile, the topical wound irrigation solution consisting of polyhexamethylene biguanide and betaine (PHMB-B) has shown efficacy in managing wound infections. However, the effectiveness of this solution as a topical instillation solution for NPWTi-d in patients with diabetic foot infections (DFIs) has not been thoroughly studied. The objective of this retrospective study was to evaluate the impact of using PHMB-B as the instillation solution during NPWTi-d on reducing bioburden and improving clinical outcomes in patients with DFIs. Between January 2017 and December 2022, a series of patients with DFIs received treatment with NPWTi-d, using either PHMB-B or normal saline as the instillation solution. Data collected retrospectively included demographic information, baseline wound characteristics, and treatment outcomes. The study included 61 patients in the PHMB-B group and 73 patients in the normal saline group, all diagnosed with DFIs. In comparison to patients treated with normal saline, patients with PHMB-B exhibited no significant differences in terms of wound bed preparation time (P = 0.5034), length of hospital stay (P = 0.6783), NPWTi-d application times (P = 0.1458), duration of systematic antimicrobial administration (P = 0.3567), or overall cost of hospitalization (P = 0.6713). The findings of the study suggest that the use of either PHMB-B or normal saline as an instillation solution in NPWTi-d for DFIs shows promise and effectiveness, yet no clinical distinction was observed between the two solutions.
Keywords: Diabetic foot infection, PHMB, Negative pressure wound therapy, Instillation and dwell time
Subject terms: Endocrine system and metabolic diseases, Metabolic disorders, Skin diseases, Trauma
Introduction
Diabetic foot ulcer (DFU) is a prevalent and dreadful lower-extremity complication that continues to be a significant public health issue due to its high rates of morbidity and mortality, frequently leading to lower limb amputation if not promptly recognized and effectively managed1. Actually, more than half of DFU patients develop infections2,3, which are the principal pathology responsible for the majority of diabetic foot complications and are a leading cause for hospital admissions among individuals with diabetes4,5.
Diabetic foot infections (DFIs) are defined as infections in soft tissue or bone anywhere below the malleoli in a diabetic person6. If the infection advances to the underlying bone, then diabetic foot osteomyelitis develops. Numerous independent predisposing factors contributing to the development of DFIs have been identified, including peripheral neuropathy, immunopathy, wounds that penetrate bones, recurrent foot ulcers, a history of lower extremity amputations, a traumatic etiology, the presence of peripheral arterial disease (PAD) in the involved limb, and renal insufficiency6. Approximately 20% of moderate or severe DFIs lead to lower extremity amputation at various levels and dramatically increase mortality eventually7.
Various organisms, alone or synergistically, can cause DFIs. To make matters worse, a growing body of studies have suggested the presence of biofilms in most DFU wounds, which subsequently impair or deteriorate the healing process and are associated with poor clinical outcomes8–10. In fact, biofilm formation is believed to be one of the most important virulence factors in the pathogenesis of DFIs11.
Since the development of standard commercial negative pressure wound therapy (NPWT), subsequent research has consistently validated its positive physiological effects, establishing it as a crucial tool in wound care12. NPWT involves the application of subatmospheric pressure to the entire wound site through a specialized dressing system. This system comprises four key components: an open-pore foam sponge (typically made of polyurethane or polyvinyl), a semiocclusive adhesive cover, a negative pressure source, and a fluid collection system. NPWT exerts its effectiveness through four primary mechanisms of action: macrodeformation, drainage of fluids, stabilization of the wound environment and microdeformation13. In recent studies, there has been a shift in focus from the four main mechanisms of NPWT to the accompanying secondary effects, such as effects on various cells, bacteria, and surgical wounds14.
A variety of systems with newer features and functions are available that have helped to broaden its applications, including negative pressure wound therapy with instillation and dwell time (NPWTi-d). These systems typically perform a repeating cycle of fluid instillation using a predetermined volume, followed by a dwell time during which the wound is washed, and then fluid removal and resumption of negative pressure suction12. The use of NPWTi-d devices has been shown to promote increased granulation tissue, faster healing times, and decreased hospital length of stay when compared to standard NPWT15,16.
Although a variety of instillation solutions can be used in NPWTi-d, considering the growing diabetic population and the need to achieve more successful outcomes and ultimately avoid amputations, an optimal protocol of care for DFIs integrated with an anti-biofilm strategy is particularly critical. In addition, there has been a focus on measures of wound cleansing whereby debris and exudates are gently and continuously removed to prepare the wound bed for wound closure. For this purpose, physiological solutions or specific disinfectants may be used17.
Prontosan® wound irrigation solutions (B.Braun Medical AG, Melsungen, Germany) are developed for cleansing, rinsing and moisturizing of acute and chronic skin wounds, and for the prevention of biofilm. In vitro study that determined the activity of a polyhexamethylene biguanide-betaine (PHMB-B) solution against collection strains and multidrug-resistant nosocomial isolates shows that PHMB-B presented bactericidal activity against all multidrug-resistant clinical isolates tested, including high-risk clones, at significantly lower concentrations and time of activity18. Numerous clinical studies have also demonstrated the significantly higher efficacy of PHMB-B irrigation solution in terms of tissue compatibility, fast-acting broad spectrum antimicrobial activity, and its benefits for cleaning, moistening and decontaminating the wound bed, reducing inflammatory signs and antimicrobial usage, as well as accelerating the healing of chronic wounds19–23. A systematic review assessed characteristics of various antiseptics and recommended that PHMB-B may be considered as the first-choice agent for infected chronic wounds24.
However, the effect of NPWTi-d with PHMB-B in the management of DFIs wounds has not been well determined. Given the high failure rate of DFI treatment25, and based on the results of the available studies, we hypothesize that the combination of NPWTi-d with anti-biofilm solution PHMB-B has a synergistic effect by enhancing efficacy in the management of DFIs where biofilms present a continuous challenge to effective microbial control and showing advantages in improving the clinical outcomes of the patients.
The purpose of this case study was to evaluate the clinical outcomes of DFI patients treated with NPWTi-d with PHMB-B irrigation solution (Prontosan® Wound Irrigation Solution).
Methods
This retrospective, historical, controlled study compared the efficacy of Prontosan® Wound Irrigation Solution with normal saline (NS) as a topical instillation solution during NPWTi-d in the treatment of DFIs.
This study was conducted at a regional care center specializing in burn injuries and wound repair in Changchun, Jilin Province, with approval from the institution’s Ethical Committee. Informed consent for the academic use of medical records was obtained from patients and their legal guardians who participated in the study during hospitalization. All methods in this research was performed in accordance with relevant guidelines and regulations. Confidentiality was maintained all through the study.
Study populations
A retrospective assessment was conducted on a series of consecutive patients who received treatment and underwent reconstructive surgery for DFIs between April 2018 and December 2020.
Patients who met the inclusion criteria of this study were as follows: (1) Patients aged 18 years or older with a definitive diagnosis of diabetes mellitus and DFU; (2) Diagnosis of DFIs was defined by clinical signs or symptoms of infection (erythema, warmth, tenderness, pain, induration), presence of purulent secretion, elevated white blood cell count, positive culture results from deep wound tissue samples, and/or radiographic evidence of infection; (3) Wound bed preparation was conducted using either Prontosan® Wound Irrigation Solution or NS as the topical wound irrigation solution in the treatment of NPWTi-d. (5) Physical examination techniques such as palpation of pedal pulses or measurement of ankle-brachial index, Doppler ultrasound, and angiography were employed to identify PAD in the affected limbs. NPWTi-d was exclusively administered to patients without apparent vascular compromise as a contraindication; (6) All pertinent data was thoroughly documented and gathered.
The exclusion criteria for patients in both groups included terminal illness, malignancy in the wound, standard negative pressure wound therapy without instillation and dwell time, and incomplete information.
Study products
The Prontosan® Wound Irrigation Solution, as specified by the manufacturer, consists of 0.1% PHMB, 0.1% undecylenamidopropyl betaine, and 99.8% purified water. PHMB is recognized as an effective and rapid-acting broad-spectrum antimicrobial agent, while betaine serves as a surfactant capable of penetrating the skin to disrupt and eliminate biofilm, wound debris, and slough.
The synergistic action of two essential components provides a suitable option for the efficient cleansing, moistening, and decontamination of wounds, particularly in the context of preventing and eliminating biofilms in chronic wounds. The irrigation solution is conveniently packaged in 350 ml bottles and 40 ml ampoules, and is administered directly from the squeeze bottle. In this study, the 350 ml bottle of Prontosan® Wound Irrigation Solution was utilized.
Wound negative pressure wound therapy (NPWT) was administered using either the polyurethane foam-based RENASYS-F Foam Dressing Kit with Soft Port (Smith & Nephew Medical Ltd.) or the V.A.C.® Therapy System (KCI, an Acelity Company, San Antonio, TX, USA). A customized NPWTi-d protocol was devised as a substitute treatment for the surgical wound immediately following the initial debridement, drawing upon existing literature on NPWTi-d.
Study protocol
The study compared the clinical outcomes of patients with DFIs treated with NPWTi-d using either PHMB-B or NS. Matching was achieved in terms of wound characteristics such as location, size, and purulence. Wound dimensions (width, length, and depth) were measured upon admission or during surgery. Additionally, demographic data was collected and the overall patient condition was assessed through clinical diagnoses documented in medical records, including evaluation of comorbidities such as obesity, body mass index, and dyslipidemia, glycemic status and hemoglobin A1c, heart and peripheral vascular diseases, pulmonary disease, hepatic dysfunction, renal insufficiency, and smoking status.
Following assessment, a thorough excisional debridement of necrotic and devitalized tissues was conducted. Subsequently, a NPWTi-d system utilizing either PHMB-B or NS was applied during the initial operative visit immediately post-debridement in the operating room. Specifically, the foam sponge was tailored to conform to the wound shape post-debridement. A tube with a soft port at the end was utilized for suction, while another tube with perforations on each side was inserted into the sponge for instillation solution delivery. The distal end of the suction tube was affixed to the central negative pressure system on the wall, while the distal end of the solution delivery tube was linked to the bag containing either PHMB-B or NS solution.
NPWTi-d was administered using either PHMB-B or NS solution to instill and saturate the sponge. This was followed by a dwell time of 10–15 min, and then a continuous negative pressure suction of -125 mm Hg over a 24-h period, with four cycles per day. The dwell time referred to the period in which the solution remained in the foam/wound interface without negative pressure suction. The NPWTi-d system was changed every 4–6 days.
During the surgical procedure, deep wound culture specimens and biopsies were collected. Following admission, all patients received empirical systemic antibiotics, which were modified according to the findings of bacterial cultures and biopsies. The presence of nonviable tissue and the formation of granulation tissue were evaluated in both patients and wounds at each NPWTi-d system change. Wounds that did not show improvement or continued to exhibit culture growth underwent further debridement until infection was eradicated in both patient groups.
The study's endpoint for both groups included determining readiness for wound closure via split-thickness skin graft, consideration of limb amputation due to life-threatening infection or lack of limb salvage options, or the patient was discharged from the hospital upon clearance of infection. The criteria for split-thickness skin graft closure were consistent across both groups: (1) a minimum of two sets of negative culture results and evaluated by an experienced surgeon; (2) presence of healthy granulation tissue formation in the wound bed; (3) absence of nonviable tissue; and (4) no vital tissue exposure in the wound bed (tendon, bone or cartilage).
Data collection and analysis
Data was gathered from electronic medical records for patients who met the predefined inclusion criteria. The collected data for each patient included demographic information (such as age and sex), baseline wound characteristics, duration of wound bed preparation prior to definitive surgeries, time taken to achieve final healing (in days), length of hospital stay (in days), mean NPWTi-d application times, duration of systematic antimicrobial administration (in days), cost (in CNY), and any existing complications.
The study's findings were presented in numerical and percentage form for categorical variables, and as means ± standard deviation (SD) for continuous variables. Data analysis was conducted using SPSS version 27.0 for Windows (SPSS, Chicago, IL, USA). Student's t-test and Chi-square test or Fisher exact test were utilized for assessing between-group disparities in quantitative or qualitative data, as deemed suitable. Statistical significance was defined as a p-value less than 0.05.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the First Hospital of Jilin University. Written informed consent was obtained from each participating patient or their legal representatives.
Results
A total of 134 consecutive patients with DFIs were included in the analysis, with 73 receiving NS as an irrigation solution for NPWTi-d and 61 receiving PHMB-B instillation for NPWTi-d during the aforementioned time period (Fig. 1). Age, sex, body mass index, current smoking status, and medical comorbidities were comparable and did not show statistically significant differences between the two groups, as shown in Table 1.
Figure 1.
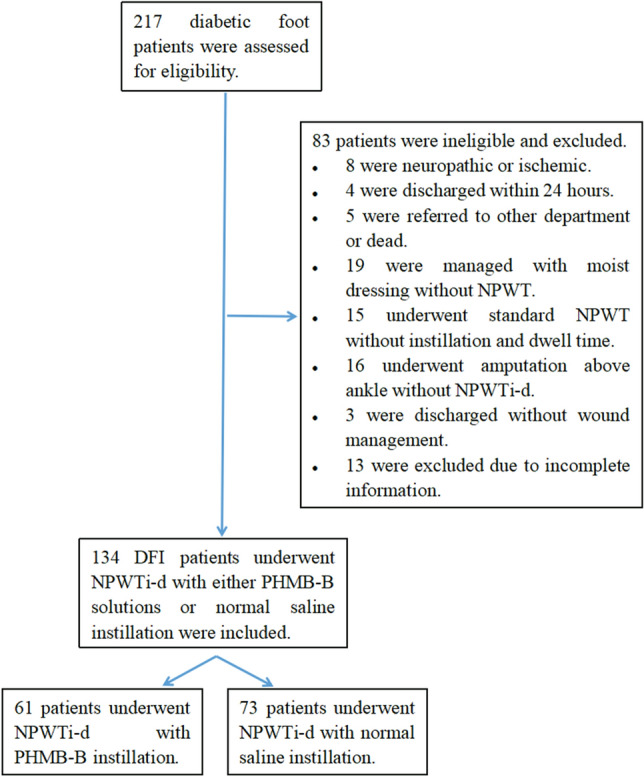
Flowchart of enrollment of study participants. DFI, diabetic foot infection; NPWT, negative pressure wound therapy; NPWTi-d, negative pressure wound therapy with instillation and dwell time; PHMB-B, 0.1% polyhexamethylene and 0.1% biguanide.
Table 1.
Demographic characteristics and baseline laboratory results on admission of diabetic foot infection patients.
| Variable | Polyhexamethylene biguanide instillation (n = 61) | Normal saline instillation (n = 73) | P value |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 49 (80.33%) | 52 (71.23%) | 0.2236 |
| Female | 12 (19.67%) | 21 (28.77%) | |
| Age (years) | 57.8 ± 12.9 | 59.9 ± 12.3 | 0.3316 |
| Blood glucose (mmol/L) | 12.9 ± 7.1 | 10.5 ± 5.3 | 0.0231 |
| Glycosylated hemoglobin (HbA1c, %) | 9.4 ± 2.0 | 9.7 ± 2.2 | 0.5471 |
| C-reactive protein (mg/L) | 81.3 ± 87.2 | 96.1 ± 73.2 | 0.4531 |
| White blood cell (*109/L) | 11.6 ± 5.9 | 12.2 ± 6.1 | 0.5708 |
| Neutrophil (%) | 0.76 ± 0.72 | 0.75 ± 0.11 | 0.5632 |
| Albumin (g/L) | 31.5 ± 9.6 | 30.0 ± 6.3 | 0.2805 |
| Body Mass Index (kg/m2) | 23.8 ± 3.4 | 24.0 ± 3.7 | 0.7912 |
| Procalcitonin (ng/mL) | 1.8 ± 4.1 | 1.1 ± 2.3 | 0.4941 |
Additionally, the laboratory test results upon admission of both groups indicated no significant statistical difference, with the exception of a higher percentage of fasting glucose in the PHMB-B NPWTi-d group compared to the NS NPWTi-d group (p = 0.0231) (Table 1).
The findings indicate that there is no statistically significant difference between the group receiving PHMB-B instillation and the group receiving NS irrigation in various outcomes: (1) length of hospital stay (31.7 ± 17.5 days vs. 33.3 ± 21.7 days; p = 0.6783); (2) duration of wound bed preparation (15.5 ± 8.6 days vs. 17.5 ± 13.3 days, p = 0.5034); (3) NPWTi-d application times (1.9 ± 0.9 times vs. 2.4 ± 1.5 times, p = 0.1458); (4)duration of intravenous antimicrobial administration (14.1 ± 12.9 days vs. 16.1 ± 12.4 days, p = 0.3567); (5) overall cost of hospitalization (89,935 ± 62,245 CNY vs. 95,266 ± 73,282 CNY, p = 6173) ; (6) and complication of NPWTi-d. Two patients (3.3%) in the PHMB-B group and four patients (5.5%) in the NS group experienced complications, specifically skin maceration. However, the disparity in complication rates between the two groups did not reach statistical significance (p = 0.6882).
Discussion
In individuals with diabetes mellitus, foot infections may exhibit prolonged healing times compared to non-diabetic individuals, largely attributed to bacterial biofilm presence within the wound environment.
The evolution of NPWT technology has led to the development of NPWT with instillation and dwell time (NPWTi-d), which involves the application of topical solutions directly onto the wound bed followed by negative pressure removal. This method has shown to enhance wound cleansing, granulation tissue formation, and overall healing in non-responsive wounds compared to traditional NPWT techniques26,27.
The selection of an appropriate instillation solution is a topic of ongoing debate in the treatment of NPWTi-d. The ideal solution for topical instillation should possess both effective wound bioburden reduction properties and minimal local cytotoxicity28. Various solutions have been utilized for instillation in NPWTi-d, with the literature predominantly focusing on active antibacterial agents such as sodium hypochlorite (Dakin's solution), silver nitrate, acetic acid, polyhexanide, povidone-iodine, bacitracin, and sulfur-based solutions29–35. However, some studies have shown favorable results when using NS as an instillation solution in infected wounds, despite its lack of antimicrobial properties27,28,36,37.
Previous research in a porcine skin explant biofilm model has indicated the potential superiority of PHMB over NS in decreasing colony-forming units38. However, despite positive outcomes for both treatments, no significant clinical difference was observed between PHMB-B and NS as topical wound irrigation solution in the management of DFIs. This disparity in research methodologies may be attributed to the distinction between our human study and their ex vivo study. Furthermore, several studies comparing the efficacy of NS and PHMB-B in patients with infected wounds found no significant differences in rates of dehiscence, wound closure, wound recurrence, amputation, or mortality between the two solutions39–41. These findings are consistent with the results of our study. The clinical efficacy, cost-effectiveness, greater accessibility, non-irritating and non-allergenic properties of NS may position it as the preferred choice for expanding the utilization of NPWTi-d in larger patient populations40,41.
In this study, a custom-made NPWTi-d system was utilized in place of commercially available reticulated open-cell foam dressings specifically designed for use with NPWTi-d (V.A.C. VeraFlo Therapy, KCI, an Acelity company, San Antonio, TX), as a result of the unavailability of commercial products at our hospital. It is possible that the use of V.A.C. VeraFlo Therapy could yield different results, given that the NPWTi-d system incorporates topical fluid instillation, programmed dwell time, and a unique foam-wound interface that collectively offer potential benefits to patients with complex wounds42.
Data was collected from two different commercial NPWT devices utilized for NPWTi-d in the current research. Utilizing a single product is expected to mitigate bias and enhance the study's reliability. However, due to the retrospective nature of the study, the device type utilized cannot be altered. A retrospective study compared the efficacy of two NPWT systems (V.A.C. therapy, KCI, Inc., San Antonio, Texas vs. RENASYS NPWT system, Smith & Nephew, Hull, United Kingdom) in treating wounds of various causes. The research findings indicated that there were no significant clinical differences in effectiveness and functionality between the two predominant NPWT devices43.
However, it is important to acknowledge the limitations of this study, such as its retrospective nature and reliance on a single medical center, which hindered the ability to conduct proper randomization of study groups over an extended duration. Additionally, the lack of standardized wounds for comparison and the presence of patient heterogeneity further complicate the interpretation of the results. Given that a historical case series does not meet the criteria of an optimal matched pair or comparative design, our study was restricted to investigating the impact of two topical solutions of NPWTi-d for wound bed preparation before definitive closure, without delving into additional outcomes such as skin graft take rate or suture dehiscence. Moreover, different application systems and handmade components were utilized in the studies. It is important to note that our study primarily focused on the effects of NPWTi-d and did not include a comparison between Renasys-GOTM therapy and V.A.C.® Therapy System. Furthermore, there is a lack of long-term follow-up to evaluate outcomes of these two NPWTi-d solutions. Despite these limitations, our case series demonstrates promising results and contributes valuable insights into the selection of instillation solutions for NPWTi-d in the management of DFIs.
Conclusions
The findings of this study indicate that NPWTi-d is an effective and safe adjunct therapy for DFIs treatment. Nevertheless, the use of NPWTi-d with PHMB-B as a topical instillation does not exhibit superiority over NS in the management of DFIs. Given the limitations of this study, it is recommended that future research endeavors, such as a robust randomized controlled trial designed prospectively with a large sample size, be conducted to further elucidate the efficacy of NPWTi-d with PHMB-B as a topical instillation in the treatment of DFIs.
Abbreviations
- NPWTi-d
Negative pressure wound therapy with instillation and dwell time
- DFIs
Diabetic foot infections
- PHMB-B
Polyhexamethylene biguanide and betaine
- NS
Normal saline
- DFU
Diabetic foot ulcer
- PAD
Peripheral arterial disease
- NPWT
Negative pressure wound therapy
Author contributions
Zhao JC involved in conception and design, literature search, review, drafting and editing. Shi K and Zhang N involved in editing, critical revision, and supervision of the manuscript. Hong L performed data collection, statistical analysis and interpretation. Yu JA involved in the peer reviewing of the manuscript into final form and drafting of research idea of the present study. All authors have read and approved the final manuscript.
Funding
This work received grants from the National Key Research and Development Program of China (2021YFF1201200), the Health Science and Technology Ability Improvement Project of Jilin Province (2023LC004).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bém R, Dubský M, Fejfarová V, Husáková J, Wosková V. Diabetic foot. Vnitr. Lek. 2020;66(2):92–97. doi: 10.36290/vnl.2020.015. [DOI] [PubMed] [Google Scholar]
- 2.Prompers L, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007;50(1):18–25. doi: 10.1007/s00125-006-0491-1. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 2017;376(24):2367–2375. doi: 10.1056/NEJMra1615439. [DOI] [PubMed] [Google Scholar]
- 4.Frykberg RG, Wittmayer B, Zgonis T. Surgical management of diabetic foot infections and osteomyelitis. Clin. Podiatr. Med. Surg. 2007;24(3):469–482. doi: 10.1016/j.cpm.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Hobizal, K.B. & Wukich, D.K. Diabetic foot infections: Current concept review. Diabet. Foot Ankle. 3 (2012). [DOI] [PMC free article] [PubMed]
- 6.Pitocco D, et al. Diabetic foot infections: A comprehensive overview. Eur. Rev. Med. Pharmacol. Sci. 2019;23(2 Suppl):26–37. doi: 10.26355/eurrev_201904_17471. [DOI] [PubMed] [Google Scholar]
- 7.Senneville É, et al. Diagnosis of infection in the foot in diabetes: A systematic review. Diabetes Metab. Res. Rev. 2020;36(Suppl 1):3281. doi: 10.1002/dmrr.3281. [DOI] [PubMed] [Google Scholar]
- 8.MacLeod AS. Bad "Staph" in the wound environment of diabetic foot ulcers. Cell Host Microbe. 2019;25(5):638–640. doi: 10.1016/j.chom.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Kim PJ, Steinberg JS. Wound care: Biofilm and its impact on the latest treatment modalities for ulcerations of the diabetic foot. Semin. Vasc. Surg. 2012;25(2):70–74. doi: 10.1053/j.semvascsurg.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Pouget C, Dunyach-Remy C, Pantel A, Schuldiner S, Sotto A, Lavigne JP. Biofilms in diabetic foot ulcers: Significance and clinical relevance. Microorganisms. 2020;8(10):1580. doi: 10.3390/microorganisms8101580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pugazhendhi S, Dorairaj AP. Appraisal of biofilm formation in diabetic foot infections by comparing phenotypic methods with the ultrastructural analysis. J. Foot Ankle Surg. 2018;57(2):309–315. doi: 10.1053/j.jfas.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Poteet SJ, Schulz SA, Povoski SP, Chao AH. Negative pressure wound therapy: Device design, indications, and the evidence supporting its use. Expert Rev. Med. Devices. 2021;18(2):151–160. doi: 10.1080/17434440.2021.1882301. [DOI] [PubMed] [Google Scholar]
- 13.Normandin S, et al. Negative pressure wound therapy: Mechanism of action and clinical applications. Semin Plast Surg. 2021;35(3):164–170. doi: 10.1055/s-0041-1731792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamashiro T, Kushibiki T, Mayumi Y, Tsuchiya M, Ishihara M, Azuma R. Negative-pressure wound therapy: What we know and what we need to know. Adv. Exp. Med. Biol. 2023;1436:131–152. doi: 10.1007/5584_2023_773. [DOI] [PubMed] [Google Scholar]
- 15.Kim PJ, et al. The impact of negative-pressure wound therapy with instillation compared with standard negative-pressure wound therapy: A retrospective, historical, cohort, controlled study. Plast. Reconstr. Surg. 2014;133(3):709–716. doi: 10.1097/01.prs.0000438060.46290.7a. [DOI] [PubMed] [Google Scholar]
- 16.Gabriel A, Kahn K, Karmy-Jones R. Use of negative pressure wound therapy with automated, volumetric instillation for the treatment of extremity and trunk wounds: Clinical outcomes and potential cost-effectiveness. Eplasty. 2014;14:e41. [PMC free article] [PubMed] [Google Scholar]
- 17.Andriessen AE, Eberlein T. Assessment of a wound cleansing solution in the treatment of problem wounds. Wounds. 2008;20(6):171–175. [PubMed] [Google Scholar]
- 18.López-Rojas R, Fernández-Cuenca F, Serrano-Rocha L, Pascual Á. In vitro activity of a polyhexanide-betaine solution against high-risk clones of multidrug-resistant nosocomial pathogens. Enferm. Infecc. Microbiol. Clin. 2017;35(1):12–19. doi: 10.1016/j.eimc.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Atkin L, Stephenson J, Cooper DM. Wound bed preparation: A case series using polyhexanide and betaine solution and gel-a UK perspective. J. Wound Care. 2020;29(7):380–386. doi: 10.12968/jowc.2020.29.7.380. [DOI] [PubMed] [Google Scholar]
- 20.Bellingeri A, et al. Effect of a wound cleansing solution on wound bed preparation and inflammation in chronic wounds: A single-blind RCT. J. Wound Care. 2016;25(3):160–168. doi: 10.12968/jowc.2016.25.3.160. [DOI] [PubMed] [Google Scholar]
- 21.Durante CM, Greco A, Sidoli O, Maino C, Gallarini A, Ciprandi G. Evaluation of the effectiveness of a polyhexanide and propyl betaine-based gel in the treatment of chronic wounds. Minerva Chir. 2014;69(5):283–292. [PubMed] [Google Scholar]
- 22.Horrocks A. Protosan wound irrigation and gel: Management of chronic wounds. Br. J. Nurs. 2006;15(22):1224–1228. doi: 10.12968/bjon.2006.15.22.22559. [DOI] [PubMed] [Google Scholar]
- 23.Moore M, Dobson N, Cetnarowski W. 0.1% Polyhexanide-betaine solution as an adjuvant in a case-series of chronic wounds. Surg. Technol. Int. 2016;29:85–89. [PubMed] [Google Scholar]
- 24.Kramer A, et al. Consensus on wound antisepsis: Update 2018. Skin Pharmacol. Physiol. 2018;31(1):28–58. doi: 10.1159/000481545. [DOI] [PubMed] [Google Scholar]
- 25.Vardakas KZ, Horianopoulou M, Falagas ME. Factors associated with treatment failure in patients with diabetic foot infections: An analysis of data from randomized controlled trials. Diabetes Res. Clin. Pract. 2008;80(3):344–351. doi: 10.1016/j.diabres.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Gabriel A. Integrated negative pressure wound therapy system with volumetric automated fluid instillation in wounds at risk for compromised healing. Int. Wound J. 2012;9(Suppl 1):25–31. doi: 10.1111/j.1742-481X.2012.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fluieraru S, et al. Sterile-water negative pressure instillation therapy for complex wounds and NPWT failures. J. Wound Care. 2013;22(6):293–299. doi: 10.12968/jowc.2013.22.6.293. [DOI] [PubMed] [Google Scholar]
- 28.De Pellegrin L, et al. Effects of negative pressure wound therapy with instillation and dwell time (NPWTi-d) versus NPWT or standard of care in orthoplastic surgery: A systematic review and meta-analysis. Int. Wound J. 2023;20(6):2402–2413. doi: 10.1111/iwj.14072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raad W, Lantis JC, 2nd, Tyrie L, Gendics C, Todd G. Vacuum-assisted closure instill as a method of sterilizing massive venous stasis wounds prior to split thickness skin graft placement. Int. Wound J. 2010;7(2):81–85. doi: 10.1111/j.1742-481X.2010.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabriel A, et al. Negative pressure wound therapy with instillation: A pilot study describing a new method for treating infected wounds. Int. Wound J. 2008;5(3):399–413. doi: 10.1111/j.1742-481X.2007.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McElroy EF. Use of negative pressure wound therapy with instillation and a reticulated open cell foam dressing with through holes in the acute care setting. Int. Wound J. 2019;16(3):781–787. doi: 10.1111/iwj.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Back DA, Scheuermann-Poley C, Willy C. Recommendations on negative pressure wound therapy with instillation and antimicrobial solutions—when, where and how to use: what does the evidence show? Int. Wound J. 2013;10(1):32–42. doi: 10.1111/iwj.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim PJ, et al. Negative pressure wound therapy with instillation: International consensus guidelines update. Int. Wound J. 2020;17(1):174–186. doi: 10.1111/iwj.13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez LG, Sibaja AP, Kaplan MJ, Sanchez-Betancourt AA, Matthews MR, Cook A. Application of negative pressure wound therapy with instillation and dwell time of the open abdomen: Initial experience. Cureus. 2019;11(9):e5667. doi: 10.7759/cureus.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Timmers MS, Graafland N, Bernards AT, Nelissen RG, van Dissel JT, Jukema GN. Negative pressure wound treatment with polyvinyl alcohol foam and polyhexanide antiseptic solution instillation in posttraumatic osteomyelitis. Wound Repair Regen. 2009;17(2):278–286. doi: 10.1111/j.1524-475X.2009.00458.x. [DOI] [PubMed] [Google Scholar]
- 36.Kim PJ, Silverman R, Attinger CE, Griffin L. Comparison of negative pressure wound therapy with and without instillation of saline in the management of infected wounds. Cureus. 2020;12(7):e9047. doi: 10.7759/cureus.9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brinkert D, Ali M, Naud M, Maire N, Trial C, Téot L. Negative pressure wound therapy with saline instillation: 131 patient case series. Int. Wound J. 2013;10(Suppl 1):56–60. doi: 10.1111/iwj.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips PL, Yang Q, Schultz GS. The effect of negative pressure wound therapy with periodic instillation using antimicrobial solutions on Pseudomonas aeruginosa biofilm on porcine skin explants. Int. Wound J. 2013;10(Suppl 1):48–55. doi: 10.1111/iwj.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim PJ, et al. Comparison of outcomes for normal saline and an antiseptic solution for negative-pressure wound therapy with instillation. Plast. Reconstr. Surg. 2015;136(5):657e–664e. doi: 10.1097/PRS.0000000000001709. [DOI] [PubMed] [Google Scholar]
- 40.Meshkin DH, et al. Long-term outcome assessment between antiseptic and normal saline for negative pressure wound therapy with instillation. Adv. Wound Care (New Rochelle) 2021;10(10):535–543. doi: 10.1089/wound.2021.0023. [DOI] [PubMed] [Google Scholar]
- 41.Akcay K, Ayhan H, Sezer Ceren RE, Simsek C, Abbasoglu O. Comparison of normal saline and antiseptic solution effect on the early peristomal infection rates of patients with percutaneous endoscopic gastrostomy tubes: A randomized double-blind study. Nutr. Clin. Pract. 2023;38(6):1343–1353. doi: 10.1002/ncp.11041. [DOI] [PubMed] [Google Scholar]
- 42.Arowojolu OA, Wirth GA. Sacral and ischial pressure ulcer management with negative-pressure wound therapy with instillation and dwell. Plast. Reconstr. Surg. 2021;147(1S-1):61S–67S. doi: 10.1097/PRS.0000000000007613. [DOI] [PubMed] [Google Scholar]
- 43.Hurd T, Rossington A, Trueman P, Smith J. A retrospective comparison of the performance of two negative pressure wound therapy systems in the management of wounds of mixed etiology. Adv. Wound Care (New Rochelle) 2017;6(1):33–37. doi: 10.1089/wound.2015.0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


