Abstract
Background:
Aberrant white matter (WM) microstructure has been proposed as a mechanism underlying bipolar disorder (BD). Given the strong genetic underpinnings of both WM microstructure and BD, such WM aberrations may be not only a disease marker, but also an endophenotype of BD. If so, they should be observable in individuals at risk (AR; i.e. first-degree relatives). This meta-analysis integrates evidence on perturbed WM microstructure in individuals with, or at risk for, BD.
Methods:
A comprehensive literature search up to April 2020 identified diffusion tensor imaging studies that used a voxel-based approach to compare fractional anisotropy (FA) and radial diffusivity (RD) between BD and/or AR and healthy volunteers. Effect size comparison and conjunction analysis allowed identification of endophenotypes and disease markers of BD. Effects of age, sex, mood state and psychotropic medication were explored using meta-regressions.
Results:
57 studies in BD (N=4631) and 10 in AR (N=753) were included. Both BD and AR were associated with lower FA in the body and splenium of the corpus callosum compared to healthy volunteers. In BD, decreased FA and increased RD comprised the entire corpus callosum, anterior thalamic radiation, fronto-orbito-polar tracts, and superior longitudinal fasciculus, and were influenced by age, sex and mood state. Lithium and antipsychotics were positively correlated with FA.
Conclusion:
Findings suggest that abnormalities in the body and splenium of the corpus callosum may be an endophenotype for BD, and associate BD with WM tracts relevant for working memory performance, attention, and reward-processing.
INTRODUCTION
Bipolar disorder (BD) is a psychopathological condition that can be severely disabling due to early onset, severity, and chronicity (1). Limited understanding of the mechanisms underlying BD hampers early, precise diagnosis and the development of more effective treatments (2). BD is characterized by episodes of elevated and depressed mood accompanied by related changes in cognition, motivation, and behavior (3). Neurobiological models of BD emphasize structural and functional aberrancies in prefrontal-limbic circuits relevant for emotion processing and emotion regulation (4). Accumulating evidence has shown aberrancies in the microstructure of white matter (WM) tracts interconnecting these circuits in BD and individuals at risk for BD (i.e., first-degree relatives, AR) (5,6). This meta-analysis integrates the available evidence regarding the role of WM microstructure in the risk-architecture of BD.
This work differs from prior meta-analyses (7–12) of WM microstructure in BD in several ways. First, AR individuals have not been included in prior meta-analyses. Second, prior analyses had a more limited focus in terms of age (e.g., excluding those under 18), BD subtype (e.g., excluding BD-II), processing pipeline (e.g., tract based spatial statistics), or metric quantifying WM microstructure (i.e., fractional anisotropy). Finally, the sample size of this meta-analysis is three times that of previous reports, allowing for exploration of the effects of age, sex, mood state, and psychotropic medications.
It has been proposed that altered WM microstructure represents an endophenotype of BD (13) as WM microstructure is heritable (14), and aberrancies in WM microstructure have been associated with BD independent of the disease state and are more prevalent in unaffected relatives compared to the general population (15–18). This is relevant, as it suggests that WM aberrancies might play an antecedent, possibly causal, role in the pathophysiology of BD. However, more work integrating findings in individuals with BD and AR is needed to establish WM microstructure as endophenotype and vulnerability marker.
The microstructure of WM tracts can be investigated using diffusion tensor imaging (DTI), a technique that quantifies the restricted diffusion of water in WM through scalars, such as fractional anisotropy (FA), which is known to be positively correlated with the directionality and coherence of WM bundles (19). In previous meta-analyses, reduced FA in the anterior corpus callosum (CC) and cingulum bundle have emerged as the most robust findings in individuals with BD (7–12). Studies in AR have also reported reduced FA in the CC (16,20–22). However, individual reports of reduced FA also include the uncinate fasciculus (UNC), a tract that connects the ventral prefrontal cortex with the amygdala (e.g., BD: (15,17,20,21,23–26), AR: (15,21)), and the anterior thalamic radiation (ATR), which connects the prefrontal cortex, striatum and thalamus (BD: (15,17,26–28), AR: (15,20,21)). Further, lower FA-values in the inferior longitudinal fasciculus (ILF), superior longitudinal fasciculus (SLF), inferior fronto-occipital fasciculus (IFOF), superior corona radiata (SCR), and corticospinal tract (CST) have been reported in individuals with BD (16,22,23,26) or AR (16,20,22). To date, only five studies directly compared individuals with BD to AR, with heterogeneous results including comparable FA reductions in the CC (16,22), ATR (15), UNC (15), CST (16,29), or SLF (22) as well as null findings (30). Meta-analytic group comparisons that leverage all available studies, regardless of whether BD and AR were directly compared, can help to determine which tracts may represent an endophenotype (9).
Alterations in FA cannot be traced back to specific tissue characteristics such as the degree of myelination, which has been argued to underlie aberrant FA in BD (6). Thus, researchers have started to analyze additional metrics such as radial diffusivity (RD), which is more sensitive to the degree of myelination (31). Reports include both RD increases (15,16,23,32) and decreases (32,33) across numerous tracts in individuals with BD and AR, warranting meta-analytic integration to test the hypothesis of aberrant myelin plasticity in BD (6).
Heterogeneity in subject characteristics (e.g., age, mood state, or psychotropic medication) may explain inconsistencies in FA and RD findings in BD. WM abnormalities in BD could reflect deviations from the normal developmental myelination of fiber tracts (6), which continues until the third decade of life (34). Indeed, studies suggest less pronounced age-related FA increases, or even age-related FA decreases, particularly in the CC and UNC (16,21,24,25,35) in BD. WM aberrancies in BD might also represent consequences of affective episodes, as myelination changes in response to experiences (36) such as altered activity levels or cognitive biases associated with depression and mania (3). Finally, findings may be influenced by medication such as lithium (37,38) or second-generation antipsychotic usage (39), but currently evidence supporting this assumption is sparse and contradictory.
The primary goal of the present meta-analysis is to determine which aberrancies in WM microstructure represent an endophenotype and thus a vulnerability marker of BD (i.e., are present in both individuals with BD or AR). We further investigate if any WM changes may be disease markers, meaning they are only present in individuals with BD. Second, by extending our analysis from FA to RD, we aim to elucidate whether altered myelination may be the mechanism underlying WM alterations in BD. Third, we investigate how age, sex, mood state, and medication status relate to heterogeneity in DTI-findings in BD, but not in AR given the limited number of studies in this population (n=10).
METHODS AND MATERIALS
Literature Searches
DTI literature in BD published up to April 23, 2020 were identified using PubMed, Embase, and Web of Science with the following search terms: diffusion tensor OR white matter AND bipolar OR mania. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (40), we included studies that 1) compared individuals with BD or AR (i.e. siblings and offspring) to HV, 2) used a whole-brain approach, and 3) reported one metric of interest (FA or RD). For longitudinal trials, only data from the baseline period were included (41–43). In case of overlapping samples, solely the study with the largest sample size or without missing data was included (18,20,22,28,44–46).
Data extraction
From each study, we extracted participants’ mean age, sex ratio, mean scores of the Young Mania Rating Scale (YMRS) (47) and Hamilton Depression Rating Scale (HDRS) (48), and percentage of individuals that were prescribed lithium or antipsychotic medication. To incorporate different HDRS scales, the “equivalent HDRS-17” score was calculated by dividing the total scores of HDRS-21 and HDRS-25 by the number of items and multiplying by 17. We chose the HDRS as it was the most commonly reported clinician-rated measure of depression. In general, we focused on observer-ratings as they might be less biased than self-reports. Given our hypotheses, we gathered peak voxel coordinates and test-statistics for FA and if available, RD. We did not have hypotheses regarding the two other commonly used diffusion metrics, mean diffusivity and axial diffusivity, so they were not extracted.
Two email requests were sent to corresponding authors for any missing information (e.g. peak voxel coordinates, test statistics). We obtained t-statistic maps from 12 studies (15–17,26,27,44,49–52), which are preferable over peak coordinates as they provide information for each voxel (53). The quality of each study was assessed using the Newcastle-Ottawa assessment scale (NOS), which evaluates the quality of studies based on the selection of participants, comparability of cases and controls, and ascertainment of exposure (Table S1) (54). All data were initially extracted by RH and cross-checked independently by JL and CS.
Whole-brain analyses
All analyses were conducted using anisotropic effect size-signed differential mapping (AES-SDM v5.142). AES-SDM can combine peak coordinates and statistical parametric maps and uses standard effect size and variance-based meta-analytic calculations (53,55). This method implements random-effects models in which each study is weighted according to its sample size and variability. The multimodal feature of AES-SDM allows for conjunction analyses that compare abnormalities between study groups (here, BD and AR) based on the evaluation of effect sizes, even if the groups have not been directly compared in primary studies.
To test whether WM abnormalities represent an endophenotype of BD, we first conducted whole-brain effect size comparisons to identify brain regions where individuals with BD or AR differed from HV in FA. Next, conjunction analyses were performed to identify tracts showing similar abnormalities in individuals with BD or AR (compared to HV), thus meeting criteria for an endophenotype. Brain regions indicating a disease marker, where meta-analytically computed effect sizes differed between BD and AR, were identified using the linear model feature in AES-SDM. To test whether altered myelination measured via RD represents the mechanism underlying altered WM microstructure in BD, we first conducted whole-brain effect size comparisons to identify brain regions where individuals with BD differed from HV in RD, followed by a conjunction analysis of FA and RD maps. Sociodemographic (i.e., age, sex) and clinical factors (i.e. YMRS score, HDRS score, lithium or antipsychotic status) potentially contributing to cross-study heterogeneity in clusters identified during the initial whole-brain effect size comparison were examined with simple meta-regressions. Meta-regressions can only be expected to produce robust results when ≥ 20 studies are available, and thus are only reported for FA maps. (see Tables S6–S7 for meta-regressions on RD and in AR).
For statistical inference, we used the recommended threshold of p = 0.005 with peak Z > 1 and cluster extent of > 20 voxels (53). The robustness of results was explored with jackknife sensitivity analysis to assess the contribution of each study to the overall results. Results that lost significance in >10% of iterations were considered non-robust and are only reported in Table S5. Publication bias was examined with Egger’s test for asymmetry of the funnel plot for each significant peak voxel derived from the AES-SDM meta-analyses.
RESULTS
Literature Searches and Quality Assessment
The initial literature search identified 1750 studies (Figure S1). After screening based on title and abstract review, 169 studies were selected for full text review. A total of 57 distinct studies were included in the whole-brain FA and RD meta-analyses comparing BD or AR to HV (BD: n=57 FA studies, n=15 RD studies; AR: n=10 FA studies, n=5 RD studies). Summaries of overall sample characteristics are described in Table 1. According to the NOS quality assessment criteria, all studies were determined to be of sufficient quality (score > 5) to be included (Table S2). For details of all individual studies, see Tables S3–4.
Table 1.
Overall sample characteristics for the meta-analyses of FA and RD data
| BD vs. HV |
AR vs. HV |
|||
|---|---|---|---|---|
| FA | RD | FA | RD | |
|
| ||||
| Number of studies | 57 | 15 | 10 | 5 |
| Total sample size | 4631 | 981 | 753 | 240 |
| BD or AR sample size | 2054 | 462 | 378 | 121 |
| Mean Age (years) | 36.3 | 34.1 | 27.4 | 16.4 |
| Female (%) | 55.3 | 60 | 54.8 | 50.4 |
| Illness Duration (years) | 11.7 | 15.4 | ||
| Medicated (%) | 80.8 | 71 | ||
| Lithium (%) | 42.7 | 31 | ||
| Antipsychotics (%) | 47.1 | 39 | ||
| Mean YMRS | 5.5 | 5.3 | ||
| Mean HDRS-17 | 9.1 | 10.9 | ||
Abbreviations: AR, at-risk; BD, bipolar disorder; FA, fractional anisotropy; HDRS-17, Hamilton Depression Rating Scale, 17-item; HV, healthy volunteer; RD, radial diffusivity; YMRS, Young Mania Rating Scale.
The role of WM aberrancies in the risk architecture of BD
Bipolar disorder vs. healthy volunteers
Compared to HV, individuals with BD showed robust FA decreases in the CC, right ATR, left SLF and inter-striatal WM, as well as increased RD in the right ATR, CST, fronto-orbito-polar tract (FOP), and left SLF. A conjunction analysis revealed both reduced FA and increased RD in a large cluster encompassing the CC and bilateral ATR, right FOP, and SLF (Figure 1, Table 2). There was no evidence of FA increases or RD decreases in BD compared to HV.
Figure 1. Depiction of differences and communalities in white matter microstructure between individuals with and at risk for BD, and healthy volunteers.
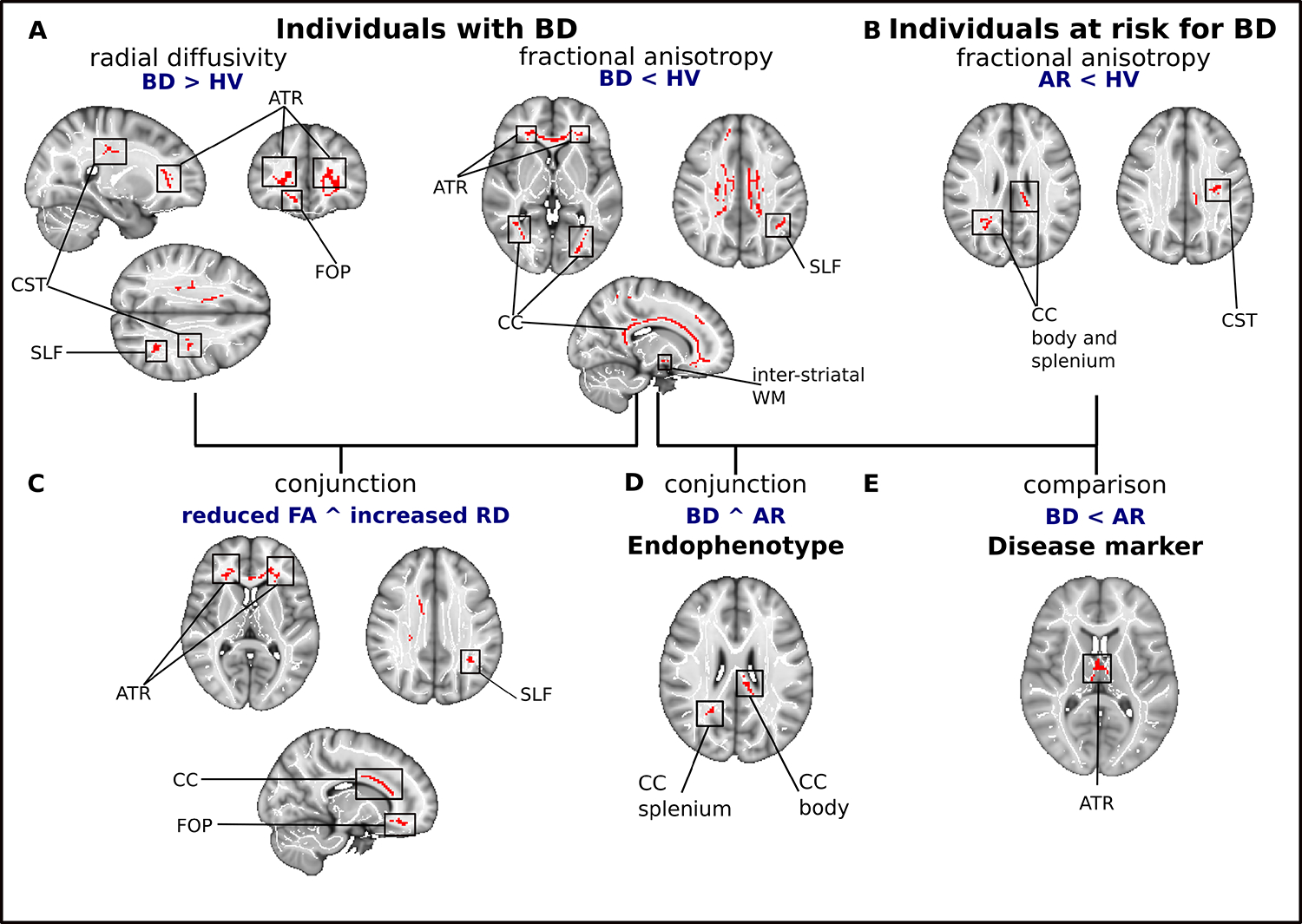
(A) Clusters of reduced fractional anisotropy and increased radial diffusivity in individuals with BD compared to healthy volunteers. (B) Clusters of reduced fractional anisotropy in individuals at risk for BD. (C) Results of multimodal analysis depicting clusters with both reduced fractional anisotropy and increased radial diffusivity in individuals with BD. (D) Regions of reduced fractional anisotropy in individuals with or at risk for BD, compared to HV. (E) WM tracts with more pronounced FA reductions in BD than AR. Significant clusters are overlaid on the MNI template and a standardized fractional anisotropy skeleton.
Abbreviations: AR, individuals at risk for bipolar disorder; ATR, anterior thalamic radiation; BD, bipolar disorder; CC, corpus callosum; CST, corticospinal tract; FOP, fronto-orbito polar tract; PTR, posterior thalamic radiation; SLF, superior longitudinal fasciculus; WM, white matter
Table 2.
Differences and commonalities in white matter microstructure between individuals with or at risk for BD and HV that met criterion for robustness.
| Peak MNI coordinate | Voxels | P | Hemisphere | Regions |
|---|---|---|---|---|
|
| ||||
| Individuals with BD | ||||
| Fractional anisotropy: BD < HV | ||||
| 24, 34, 8 | 2089 | <.00001 | R | CC, ATR |
| −42, −50, 36 | 42 | .00007 | L | SLF |
| 14, 40, 36 | 33 | .00001 | R | CC |
| 20, −46, 54 | 22 | .00001 | R | CC |
| 12, 0, −10 | 31 | .00025 | R | Inter-striatal WM |
| Radial diffusivity: BD > HV | ||||
| 24, 34, 8 | 603 | <.00001 | R | ATR, CC |
| 26, −20, 34 | 101 | <.00001 | R | CST |
| −30, −18, 34 | 48 | .00013 | L | SLF |
| 16, 32, −16 | 38 | .00008 | R | FOP |
| Conjunction: Reduced FA ^ Increased RD | ||||
| 23, 34, 8 | 458 | <.005 | R | ATR, CC |
| 21, −19, 36 | 25 | <.005 | R | SLF |
| 15, 32, −11 | 21 | <.005 | R | FOP |
| Individuals at risk for BD | ||||
| Fractional anisotropy: AR < HV | ||||
| 26, −56, 24 | 80 | <.00001 | R | CC body |
| −12, −32, 26 | 52 | .00024 | L | CC splenium |
| −24, −20, 34 | 37 | .00047 | L | CST |
| Endophenotype | ||||
| Decreased fractional anisotropy: (BD < HV) ^ (AR < HV) | ||||
| −12, −31, 26 | 52 | <.005 | L | CC splenium |
| 25, −53, 20 | 39 | <.005 | R | CC body |
| Disease marker | ||||
| Fractional anisotropy: BD < AR = HV | ||||
| 2, −10, 6 | 83 | <.00001 | R | ATR |
Abbreviations: AR, at risk; ATR, anterior thalamic radiations; BD, bipolar disorder; CC, corpus callosum; CST, corticospinal tract; FA, fractional anisotropy; FOP, fronto-orbito-polar tract; HV, healthy volunteers; L, left; R, right; RD, radial diffusivity; SLF, superior longitudinal fasciculus.
Individuals at risk vs. healthy volunteers
AR individuals showed lower FA in the body and splenium of the CC and the CST (Figure 1b, Table 2), but no abnormalities in RD. However, RD analyses comprised only five studies, so this finding should be interpreted cautiously.
Endophenotype vs. disease marker
Conjunction analysis indicated similar FA reductions in BD and AR in the splenium and body of the CC, suggesting that aberrant FA in these regions represents an endophenotype of BD. The comparison of effect size maps showed significantly larger FA decreases in BD (compared to HV) than in AR (compared to HV) in the ATR suggesting that they are associated with a symptomatic state and hence represent a disease marker (Figure 1d and e, Table 2).
Factors influencing heterogeneity in WM aberrancies in BD
Sociodemographic factors
Studies that included older BD individuals reported stronger FA decreases in the CC, whereas reports of FA reductions in the SLF and FOP were more likely in studies with younger BD participants (Figure 2, Table 3). FA decreases in the anterior CC, FOP and left SLF were more pronounced in studies with a higher proportion of females with BD, whereas reports of reduced FA in the posterior CC and the arcuate fasciculus were associated with a higher proportion of male individuals with BD (Figure 2, Table 3).
Figure 2. Illustration of meta-regression findings regarding age, medication status, and current mood in individuals with bipolar disorder.
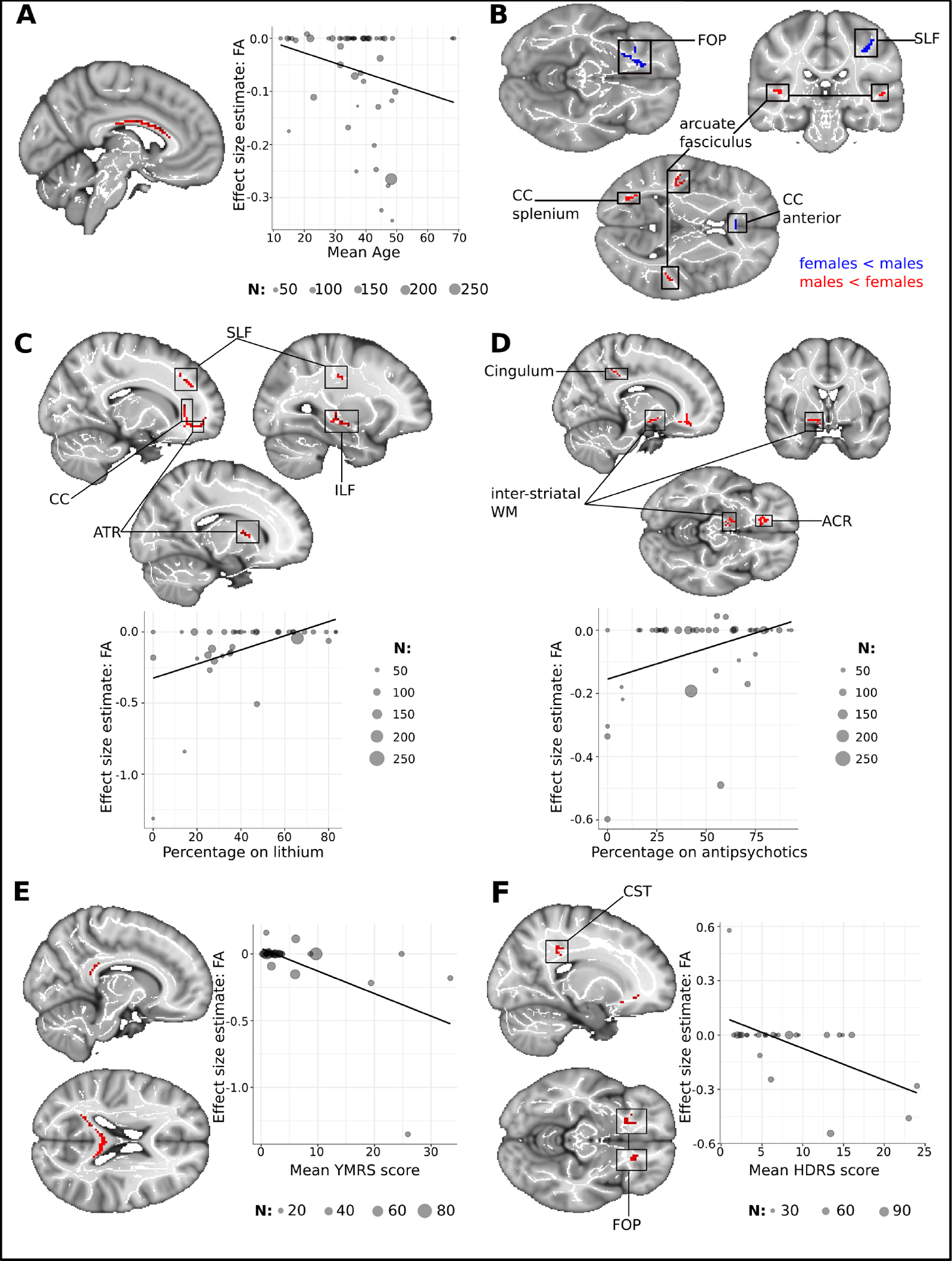
(A) Shows the cluster of age-related FA increases in the corpus callosum. Estimates of regional FA were extracted from the peak voxel coordinates (x = −6, y = −14, z = 26) (B) Depiction of the sex effect in BD (C) Positive lithium effect on FA. FA-values shown in the scatterplot were extracted from the largest cluster in the anterior thalamic radiation (x = 20, y = 38, z = −6). (D) Positive effect of antipsychotics on FA, FA-values were extracted from the peak voxel of the cluster in the ACR (x = 14, y = 28, z = −12) (E) Cluster showing FA decreases related to manic symptoms in the splenium of the CC (F) Clusters of decreased FA associated with depressed mood. FA-values were extracted from the CST-cluster (x = 26, y = −22, z = 36). N indicates the number of individuals with BD in the studies.
Abbreviations: ACR, anterior corona radiata; ATR, anterior thalamic radiation; CST, corticospinal tract; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus
Table 3.
Meta-regression findings on effect of mean age, sex (% female), affective symptoms, and medication status on FA in BD individuals compared to HV. Number of studies and total sample size are reported in parentheses.
| Peak MNI coordinate | Voxels | P | Regions | Peak MNI coordinate | Voxels | P | Regions | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| AGE | Positive age effect (57 studies, N = 4631) | Negative age effect (57 studies, N = 4631) | ||||||
| 28, −18, 14 | 29 | .00008 | CST, SLF | −6, −14, 26 | 158 | <.00001 | CC | |
| 32, −14, 34 | 28 | .00022 | SLF | −32, −60, 14 | 62 | <.00001 | CC | |
| −20, 22, −2 | 26 | .00033 | FOP | |||||
| SEX | Males < Females (57 studies, N = 4631) | Males > Females (57 studies, N = 4631) | ||||||
| 32, −66, 10 | 52 | .00001 | CC | 18, 30, −10 | 63 | .00041 | FOP | |
| 50, −20, 2 | 50 | .00001 | AF | 16, 36, 32 | 40 | .00004 | CC | |
| −28, −66, 14 | 25 | .00001 | CC | 4, 30, 8 | 24 | .00075 | CC | |
| −46, −32, 2 | 28 | .00003 | AF | −36, −28, 44 | 24 | .00016 | SLF | |
| SYMPTOMS | Negative relation with manic symptoms (33 studies, N = 2118) | Negative relation with depressive symptoms (30 studies, N = 1994) | ||||||
| 26, 16, 30 | 177 | <.00001 | CC | 26, −22, 36 | 165 | <.00001 | CST | |
| 18, −50, 58 | 42 | .00037 | CC | −20, 28, −10 | 107 | .00003 | FOP | |
| −20, −50, 34 | 30 | .00058 | CC |
22, 36, 0 | 63 | .00128 | ATR | |
| MEDICATIONS | Positive lithium effect (40 studies, N = 3159) | Positive antipsychotic effect (47 studies, N = 3785) | ||||||
| 20, 38, −6 | 205 | .00012 | CC, ATR | 14, −6, −12 | 57 | <.00001 | Inter-striatal WM | |
| −22, 6, 18 | 26 | .00006 | ATR | 14, 28, −12 | 53 | .00001 | ACR | |
| −16, 10, 6 | 20 | .00015 | ATR | −50, −58, 6 | 40 | .00006 | AF, ILF | |
| −28, −26, −8 | 44 | .00003 | ILF | 14, −40, 40 | 21 | .00002 | Cingulum | |
| 20, 32, 30 | 33 | .00002 | SLF | |||||
| −6, −38, −32 | 65 | .00018 | ATR | |||||
| 4, −18, −30 | 30 | <.00001 | CST | |||||
| −30, −18, 32 | 24 | .00027 | SLF | |||||
| −46, −60, 8 | 22 | .00016 | SLF | |||||
Abbreviations: AF, arcuate fasciculus; ATR, anterior thalamic radiations; BD, bipolar disorder; CC, corpus callosum; CST, corticospinal tract; FA, fractional anisotropy; FOP, fronto-orbito-polar tract; HV, healthy volunteers; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus; WM, white matter.
Affective symptoms
FA decreases in the posterior CC were more pronounced in studies where participants with BD reported more manic symptoms at the time of scanning. In contrast, studies where participants with BD reported more depressive symptoms at the time of scanning showed more pronounced FA decreases in the right CST, ATR, and bilateral FOP. (Figure 2, Table 3).
Medication status
Studies with a greater proportion of BD individuals currently taking lithium showed less pronounced FA changes compared to HV across the CC, bilateral ATR, left SLF and right CST. We also observed less pronounced FA decreases in studies where high proportions of individuals with BD were on a regimen of antipsychotics. These effects manifested in the anterior CC, inter-striatal WM, and left arcuate fasciculus (Figure 2, Table 3).
DISCUSSION
This meta-analysis comprises three times the number of individuals with BD than previous meta-analyses and is the first one to include studies of AR individuals; as such it extends the existing literature on WM abnormalities in BD in several ways. First, we find similar FA reductions in the body and splenium of the CC in BD and AR, suggesting an endophenotype of BD, whereas abnormalities in the ATR and body of the CC were observed only in BD indicating a disease marker. Second, we provide evidence that WM abnormalities in individuals with BD are not restricted to lower FA in the anterior CC and cingulum bundle, as suggested by previous meta-analyses (7–12). Instead, we find both reduced FA and increased RD in the entire CC, ATR, FOP, and SLF in individuals with BD. Third, our findings indicate that age, sex, and mood state contribute to the heterogeneity of DTI findings in individuals with BD. Finally, we confirm a positive effect of lithium and antipsychotics on FA.
The role of WM aberrancies in the risk architecture of BD
The primary question of the present meta-analysis was whether WM abnormalities represent an endophenotype of BD and thus are expressed similarly in both individuals with BD and AR. Our findings suggest that reduced FA in the body and splenium of the CC may represent an endophenotype of BD. Callosal fibers connect both hemispheres, with the splenium receiving input from the occipital lobes and the body interconnecting parietal and temporal cortices. To date, little is known regarding the role of callosal WM in interhemispheric information integration. Animal studies suggest that posterior callosal fibers modulate thalamocortical input via both inhibitory and excitatory signals in a stimulus-specific manner (56,57) relevant for the synchronization of alpha-band oscillations in response to task demands (58). Alpha-band oscillations, which appear to be less synchronized in BD (59), are highly relevant for attentional processes identified by meta-analyses as an impaired domain in both individuals with BD (60) and AR (61). While, our findings highlight visual-thalamo-cortical circuitry, relevant for attentional processes as a risk factor for BD, they also associate the posterior callosal fibers with manic symptoms, underlining the need for more studies investigating this circuitry and related neuro-cognitive functions in the context of BD.
Our second question concerned the cellular mechanism underlying WM aberrancies in BD. Deviations in the microstructure of tracts relevant for working memory performance and attention (CC, SLF) (62,63), as well as reward-processing (ATR, FOP) (15,64) were quantifiable by FA and RD. Increased RD in particular indicates lower levels of axonal myelination (31), which might impair the conduction of action potentials (36) and thereby potentially contribute to impairments in working memory and attention in BD (60). To this end, our results are consistent with the hypothesis of aberrant myelin plasticity in BD that suggests a dysfunction in the oligodendrocyte population, which may affect myelin formation and remodeling (6).
While sparse myelination comes at the cost of reduced processing speed, it also preserves plasticity in neural networks (65). Thus, we propose that increased plasticity of cognitive and reward circuitry resulting from low levels of myelination would allow for faster reconfiguration of these networks. Reduced myelination may therefore represent the neurobiological basis for switches between elated and depressed mood states characteristic of BD (3), as well as the increased creativity frequently reported in BD (66). However, studies testing this specific hypothesis are needed.
Factors influencing heterogeneity in WM aberrancies in BD
Evidence suggests that delayed maturation of WM tracts may be a relevant mechanism, perhaps specifically for early-onset BD (16,21,24,25,35). We found indirect support for this hypothesis in the CST and SLF, where FA decreases were more pronounced in younger BD samples. In contrast, FA decreases in the body of the CC were more pronounced in older BD samples, suggesting that abnormalities worsen over time. To test such hypotheses, more longitudinal studies in BD are needed.
Sex appears to contribute to the regional heterogeneity of findings in the CC. Females drive FA decreases in the anterior CC, associated with working-memory performance and attention, which is consistent with reports of more pronounced deficits of women with BD in these cognitive domains (67,68). Further, studies in BD with a higher proportion of male participants observed lower FA in the arcuate fasciculus, which is primarily associated with language processing and speech. In general, sex-differences are scarcely studied in BD. One would hope that future studies will focus more on sex-specific neurobiological mechanisms and associated behavioral or symptom profiles, which might then be used to personalize treatment.
Depressive symptoms were associated with WM aberrancies in the right CST and bilateral FOP. The CST is a major motor pathway, and reduced FA in this region has been associated with processing speed (69) and motor retardation during bipolar depression (70), whereas the FOP may be more relevant for reward processing (63). Both are symptom domains highly relevant in the context of depression (71,72). However, it remains an open question whether these WM abnormalities reflect plastic changes induced by the experience of depressive symptoms or characterize a subgroup of patients that tends to experience more depressive episodes. Such questions can only be answered in a longitudinal design and underscore the need for studies following individuals with BD and AR over time.
Consistent with the literature (37–39), we show a positive effect of lithium and antipsychotics on WM. Overall, the effect of lithium appeared to be widespread and comprised most tracts associated with BD (i.e., CC, ATR, SLF/CST, ILF, IFOF). In contrast, antipsychotics appear to exert more specific effects on WM tracts associated with the dopaminergic neurotransmitter system (inter-striatal WM, FOP, prefrontal WM) and the arcuate fasciculus. In sum, these findings support the hypothesis that alterations in WM microstructure might be one mechanism of change underlying the positive effects of lithium and antipsychotics, and underscore the need of control for medication effects when investigating WM microstructure.
Limitations
Analyses in AR had less power than in individuals with BD due to the smaller sample size (BD:AR=5:1). Similarly, only a subset of the studies reported RD, affective symptoms and medication status, which should be considered when interpreting the findings. Second, AR varied with regard to their relationship with the index case (i.e., sibling, offspring), and their own mental health. Jackknife analysis and Eggers test suggested that no single study was driving the reported endophenotype effect. However, we were unable to investigate these sources of heterogeneity given the limited number of studies in AR. Third, as we only included the bipolar phenotype, questions regarding the specificity of these findings for BD cannot be answered. Fourth, DTI has limitations when delineating and quantifying WM tracts with complex architecture (i.e. crossing-fibers) (73). However, this is unlikely to apply to our main findings in the corpus callosum, known for its small number of crossing fibers. Future studies utilizing high angular resolution diffusion imaging (HARDI) or diffusion spectrum magnetic resonance imaging (DSI) can overcome limitations with DTI concerning WM tracts with complex architecture. Finally, we regret that due to ongoing technical difficulties, we were unable to use the most recent version of SDM, which does not test for the spatial convergence of findings, but instead tests whether the effects are different from null and presumably provides greater power to detect group differences in the presence of multiple effects (74).
Conclusion
The present study underlines the relevance of abnormal WM microstructure in the risk-architecture of BD. Findings suggest abnormalities in the WM of the visual-thalamo-cortical circuitry as an endophenotype for BD; associate BD with reduced myelination of the CC and SLF relevant for working memory performance and attention, as well as the ATR and FOP thought to play a role in reward-processing; and confirm positive effects of lithium and antipsychotics on WM microstructure.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the NIMH (ZIA: MH002778–18). Author RH was also supported by the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, the American Association for Dental Research, the Colgate-Palmolive Company, Elsevier, alumni of the student research programs, and other individual supporters.
Footnotes
DISCLOSURES
The authors report no conflicts of interest.
REFERENCES
- 1.Ferrari AJ, Stockings E, Khoo J-P, Erskine HE, Degenhardt L, Vos T, Whiteford HA (2016): The prevalence and burden of bipolar disorder: findings from the Global Burden of Disease Study 2013. Bipolar Disord 18: 440–450. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell PB, Loo CK, Gould BM (2010): Diagnosis and monitoring of bipolar disorder in general practice. Med J Aust 193. 10.5694/j.1326-5377.2010.tb03890.x [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. American Psychiatric Association. 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- 4.Phillips ML, Ladouceur CD, Drevets WC (2008): A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 13: 833–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wessa M, Kanske P, Linke J (2014): Bipolar disorder: A neural network perspective on a disorder of emotion and motivation. Restor Neurol Neurosci 32: 51–62. [DOI] [PubMed] [Google Scholar]
- 6.Bellani M, Boschello F, Delvecchio G, Dusi N, Altamura CA, Ruggeri M, Brambilla P (2016): DTI and Myelin Plasticity in Bipolar Disorder: Integrating Neuroimaging and Neuropathological Findings. Front Psychiatry 7. 10.3389/fpsyt.2016.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vederine F-E, Wessa M, Leboyer M, Houenou J (2011): A meta-analysis of whole-brain diffusion tensor imaging studies in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 35: 1820–1826. [DOI] [PubMed] [Google Scholar]
- 8.Nortje G, Stein DJ, Radua J, Mataix-Cols D, Horn N (2013): Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. J Affect Disord 150: 192–200. [DOI] [PubMed] [Google Scholar]
- 9.Wise T, Radua J, Nortje G, Cleare AJ, Young AH, Arnone D (2016): Voxel-Based Meta-Analytical Evidence of Structural Disconnectivity in Major Depression and Bipolar Disorder. Biol Psychiatry 79: 293–302. [DOI] [PubMed] [Google Scholar]
- 10.Dong D, Wang Y, Chang X, Jiang Y, Klugah-Brown B, Luo C, Yao D (2017): Shared abnormality of white matter integrity in schizophrenia and bipolar disorder: A comparative voxel-based meta-analysis. Schizophr Res 185: 41–50. [DOI] [PubMed] [Google Scholar]
- 11.Yang C, Li Lei, Hu X, Luo Q, Kuang W, Lui S, et al. (2018): Psychoradiologic abnormalities of white matter in patients with bipolar disorder: diffusion tensor imaging studies using tract-based spatial statistics. J Psychiatry Neurosci JPN 43: 170221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Favre P, Pauling M, Stout J, Hozer F, Sarrazin S, Abé C, et al. (2019): Widespread white matter microstructural abnormalities in bipolar disorder: evidence from mega- and meta-analyses across 3033 individuals. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 44: 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK (2006): Toward Constructing an Endophenotype Strategy for Bipolar Disorders. Biol Psychiatry 60: 93–105. [DOI] [PubMed] [Google Scholar]
- 14.Kochunov P, Glahn DC, Lancaster JL, Winkler AM, Smith S, Thompson PM, et al. (2010): Genetics of microstructure of cerebral white matter using diffusion tensor imaging. NeuroImage 53: 1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linke J, King AV, Poupon C, Hennerici MG, Gass A, Wessa M (2013): Impaired Anatomical Connectivity and Related Executive Functions: Differentiating Vulnerability and Disease Marker in Bipolar Disorder. Biol Psychiatry 74: 908–916. [DOI] [PubMed] [Google Scholar]
- 16.Linke JO, Stavish C, Adleman NE, Sarlls J, Towbin KE, Leibenluft E, Brotman MA (2019): White matter microstructure in youth with and at risk for bipolar disorder. Bipolar Disord. 10.1111/bdi.12885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maller JJ, Thaveenthiran P, Thomson RH, McQueen S, Fitzgerald PB (2014): Volumetric, cortical thickness and white matter integrity alterations in bipolar disorder type I and II. J Affect Disord 169: 118–127. [DOI] [PubMed] [Google Scholar]
- 18.Magioncalda P, Martino M, Conio B, Piaggio N, Teodorescu R, Escelsior A, et al. (2016): Patterns of microstructural white matter abnormalities and their impact on cognitive dysfunction in the various phases of type I bipolar disorder. J Affect Disord 193: 39–50. [DOI] [PubMed] [Google Scholar]
- 19.Basser PJ, Mattiello J, LeBihan D (1994): MR diffusion tensor spectroscopy and imaging. Biophys J 66: 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaddock CA, Barker GJ, Marshall N, Schulze K, Hall MH, Fern A, et al. (2009): White matter microstructural impairments and genetic liability to familial bipolar I disorder. Br J Psychiatry 194: 527–534. [DOI] [PubMed] [Google Scholar]
- 21.Sprooten E, Sussmann JE, Clugston A, Peel A, McKirdy J, Moorhead TWJ, et al. (2011): White Matter Integrity in Individuals at High Genetic Risk of Bipolar Disorder. Biol Psychiatry 70: 350–356. [DOI] [PubMed] [Google Scholar]
- 22.Sprooten E, Brumbaugh MS, Knowles EEM, McKay DR, Lewis J, Barrett J, et al. (2013): Reduced White Matter Integrity in Sibling Pairs Discordant for Bipolar Disorder. Am J Psychiatry 170: 1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.the IMAGEN consortium (http://www.imagen-europe.com), Paillère Martinot M-L, Lemaitre H, Artiges E, Miranda R, Goodman R, et al. (2014): White-matter microstructure and gray-matter volumes in adolescents with subthreshold bipolar symptoms. Mol Psychiatry 19: 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Versace A, Ladouceur CD, Romero S, Birmaher B, Axelson DA, Kupfer DJ, Phillips ML (2010): Altered Development of White Matter in Youth at High Familial Risk for Bipolar Disorder: A Diffusion Tensor Imaging Study. J Am Acad Child Adolesc Psychiatry 49: 1249–1259.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weathers J, Lippard ETC, Spencer L, Pittman B, Wang F, Blumberg HP (2018): Longitudinal Diffusion Tensor Imaging Study of Adolescents and Young Adults With Bipolar Disorder. J Am Acad Child Adolesc Psychiatry 57: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yip SW, Chandler RA, Rogers RD, Mackay CE, Goodwin GM (2013): White matter alterations in antipsychotic- and mood stabilizer-naïve individuals with bipolar II/NOS disorder. NeuroImage Clin 3: 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alves GS, Knöchel C, Paulitsch MA, Reinke B, Carvalho AF, Feddern R, et al. (2018): White Matter Microstructural Changes and Episodic Memory Disturbances in Late-Onset Bipolar Disorder. Front Psychiatry 9: 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sussmann JE, Lymer GKS, McKirdy J, Moorhead TWJ, Maniega SM, Job D, et al. (2009): White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disord 11: 11–18. [DOI] [PubMed] [Google Scholar]
- 29.Skudlarski P, Schretlen DJ, Thaker GK, Stevens MC, Keshavan MS, Sweeney JA, et al. (2013): Diffusion Tensor Imaging White Matter Endophenotypes in Patients With Schizophrenia or Psychotic Bipolar Disorder and Their Relatives. Am J Psychiatry 170: 886–898. [DOI] [PubMed] [Google Scholar]
- 30.Teixeira AMA, Kleinman A, Zanetti M, Jackowski M, Duran F, Pereira F, et al. (2014): Preserved white matter in unmedicated pediatric bipolar disorder. Neurosci Lett 579: 41–45. [DOI] [PubMed] [Google Scholar]
- 31.Song S-K, Sun S-W, Ramsbottom MJ, Chang C, Russell J, Cross AH (2002): Dysmyelination Revealed through MRI as Increased Radial (but Unchanged Axial) Diffusion of Water. NeuroImage 17: 1429–1436. [DOI] [PubMed] [Google Scholar]
- 32.Mahon K, Burdick KE, Ikuta T, Braga RJ, Gruner P, Malhotra AK, Szeszko PR (2013): Abnormal Temporal Lobe White Matter as a Biomarker for Genetic Risk of Bipolar Disorder. Biol Psychiatry 73: 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haarman BCM (‘Benno’), Riemersma – Van der Lek RF, Burger H, de Groot JC, Drexhage HA, Nolen WA, Cerliani L (2016): Diffusion tensor imaging in euthymic bipolar disorder – A tract-based spatial statistics study. J Affect Disord 203: 281–291. [DOI] [PubMed] [Google Scholar]
- 34.Lebel C, Beaulieu C (2011): Longitudinal Development of Human Brain Wiring Continues from Childhood into Adulthood. J Neurosci 31: 10937–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cabeen RP, Laidlaw DH, Ruggieri A, Dickstein DP (2018): Preliminary mapping of the structural effects of age in pediatric bipolar disorder with multimodal MR imaging. Psychiatry Res Neuroimaging 273: 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sampaio-Baptista C, Johansen-Berg H (2017): White Matter Plasticity in the Adult Brain. Neuron 96: 1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makoukji J, Belle M, Meffre D, Stassart R, Grenier J, Shackleford G, et al. (2012): Lithium enhances remyelination of peripheral nerves. Proc Natl Acad Sci 109: 3973–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarrazin S, Cachia A, Hozer F, McDonald C, Emsell L, Cannon DM, et al. (2018): Neurodevelopmental subtypes of bipolar disorder are related to cortical folding patterns: An international multicenter study. Bipolar Disord 20: 721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szeszko PR, Robinson DG, Ikuta T, Peters BD, Gallego JA, Kane J, Malhotra AK (2014): White Matter Changes Associated with Antipsychotic Treatment in First-Episode Psychosis. Neuropsychopharmacology 39: 1324–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Group PRISMA-P, Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, et al. (2015): Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganzola R, McIntosh AM, Nickson T, Sprooten E, Bastin ME, Giles S, et al. (2018): Diffusion tensor imaging correlates of early markers of depression in youth at high-familial risk for bipolar disorder. J Child Psychol Psychiatry 59: 917–927. [DOI] [PubMed] [Google Scholar]
- 42.Delaloye C, Moy G, de Bilbao F, Weber K, Baudois S, Haller S, et al. (2011): Longitudinal analysis of cognitive performances and structural brain changes in late-life bipolar disorder. Int J Geriatr Psychiatry 26: 1309–1318. [DOI] [PubMed] [Google Scholar]
- 43.Favre P, Houenou J, Baciu M, Pichat C, Poupon C, Bougerol T, Polosan M (2015): White Matter Plasticity Induced by Psychoeducation in Bipolar Patients: A Controlled Diffusion Tensor Imaging Study. Psychother Psychosom 85: 58–60. [DOI] [PubMed] [Google Scholar]
- 44.Abramovic L, Boks MPM, Vreeker A, Verkooijen S, van Bergen AH, Ophoff RA, et al. (2018): White matter disruptions in patients with bipolar disorder. Eur Neuropsychopharmacol 28: 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emsell L, Langan C, Van Hecke W, Barker GJ, Leemans A, Sunaert S, et al. (2013): White matter differences in euthymic bipolar I disorder: a combined magnetic resonance imaging and diffusion tensor imaging voxel-based study. Bipolar Disord 15: 365–376. [DOI] [PubMed] [Google Scholar]
- 46.Magioncalda P, Martino M, Tardito S, Sterlini B, Conio B, Marozzi V, et al. (2018): White matter microstructure alterations correlate with terminally differentiated CD8+ effector T cell depletion in the peripheral blood in mania: Combined DTI and immunological investigation in the different phases of bipolar disorder. Brain Behav Immun 73: 192–204. [DOI] [PubMed] [Google Scholar]
- 47.Young RC, Biggs JT, Ziegler VE, Meyer DA (1978): A Rating Scale for Mania: Reliability, Validity and Sensitivity. Br J Psychiatry 133: 429–435. [DOI] [PubMed] [Google Scholar]
- 48.Hamilton M (1960): A rating scale for depression. J Neurol Neurosurg Psychiat 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.James A, Hough M, James S, Burge L, Winmill L, Nijhawan S, et al. (2011): Structural brain and neuropsychometric changes associated with pediatric bipolar disorder with psychosis: Imaging pediatric bipolar disorder. Bipolar Disord 13: 16–27. [DOI] [PubMed] [Google Scholar]
- 50.Roybal DJ, Barnea-Goraly N, Kelley R, Bararpour L, Howe ME, Reiss AL, Chang KD (2015): Widespread white matter tract aberrations in youth with familial risk for bipolar disorder. Psychiatry Res Neuroimaging 232: 184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tønnesen S, Kaufmann T, Doan NT, Alnæs D, Córdova-Palomera A, van der Meer D, et al. (2018): White matter aberrations and age-related trajectories in patients with schizophrenia and bipolar disorder revealed by diffusion tensor imaging. Sci Rep 8: 14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tuozzo C, Lyall AE, Pasternak O, James ACD, Crow TJ, Kubicki M (2018): Patients with chronic bipolar disorder exhibit widespread increases in extracellular free water. Bipolar Disord 20: 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Radua J, Mataix-Cols D, Phillips ML, El-Hage W, Kronhaus DM, Cardoner N, Surguladze S (2012): A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry 27: 605–611. [DOI] [PubMed] [Google Scholar]
- 54.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2019): The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Retrieved from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 55.Radua J, Rubia K, Canales-Rodríguez EJ, Pomarol-Clotet E, Fusar-Poli P, Mataix-Cols D (2014): Anisotropic Kernels for Coordinate-Based Meta-Analyses of Neuroimaging Studies. Front Psychiatry 5. 10.3389/fpsyt.2014.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Makarov VA, Schmidt KE, Castellanos NP, Lopez-Aguado L, Innocenti GM (2008): Stimulus-Dependent Interaction between the Visual Areas 17 and 18 of the 2 Hemispheres of the Ferret (Mustela putorius). Cereb Cortex 18: 1951–1960. [DOI] [PubMed] [Google Scholar]
- 57.Schmidt KE, Lomber SG, Innocenti GM (2010): Specificity of Neuronal Responses in Primary Visual Cortex Is Modulated by Interhemispheric CorticoCortical Input. Cereb Cortex 20: 2776–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knyazeva MG (2013): Splenium of Corpus Callosum: Patterns of Interhemispheric Interaction in Children and Adults. Neural Plast 2013: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moeini M, Khaleghi A, Mohammadi MR (2015): Characteristics of Alpha Band Frequency in Adolescents with Bipolar II Disorder: A Resting-State QEEG Study. Iran J Psychiatry 10: 8–12. [PMC free article] [PubMed] [Google Scholar]
- 60.Dickinson T, Becerra R, Coombes J (2017): Executive functioning deficits among adults with Bipolar Disorder (types I and II): A systematic review and meta-analysis. J Affect Disord 218: 407–427. [DOI] [PubMed] [Google Scholar]
- 61.Bora E, Özerdem A (2017): A meta-analysis of neurocognition in youth with familial high risk for bipolar disorder. Eur Psychiatry 44: 17–23. [DOI] [PubMed] [Google Scholar]
- 62.Yamada S, Takahashi S, Ukai S, Tsuji T, Iwatani J, Tsuda K, et al. (2015): Microstructural abnormalities in anterior callosal fibers and their relationship with cognitive function in major depressive disorder and bipolar disorder: A tract-specific analysis study. J Affect Disord 174: 542–548. [DOI] [PubMed] [Google Scholar]
- 63.Kantarci K, Senjem ML, Avula R, Zhang B, Samikoglu AR, Weigand SD, et al. (2011): Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology 77: 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kringelbach ML (2005): The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 6: 691–702. [DOI] [PubMed] [Google Scholar]
- 65.McGee AW (2005): Experience-Driven Plasticity of Visual Cortex Limited by Myelin and Nogo Receptor. Science 309: 2222–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson SL, Murray G, Fredrickson B, Youngstrom EA, Hinshaw S, Bass JM, et al. (2012): Creativity and bipolar disorder: Touched by fire or burning with questions? Clin Psychol Rev 32: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bücker J, Popuri S, Muralidharan K, Kozicky J-M, Baitz HA, Honer WG, et al. (2014): Sex differences in cognitive functioning in patients with bipolar disorder who recently recovered from a first episode of mania: Data from the Systematic Treatment Optimization Program for Early Mania (STOP-EM). J Affect Disord 155: 162–168. [DOI] [PubMed] [Google Scholar]
- 68.Ibrahim S, Salama H, Aly H, El-Shestawy H (2015): Sex differences in cognitive dysfunction among bipolar disorder patients. Egypt J Psychiatry 36: 1. [Google Scholar]
- 69.Dev SI, Nguyen TT, McKenna BS, Sutherland AN, Bartsch H, Theilmann RJ, Eyler LT (2017): Steeper Slope of Age-Related Changes in White Matter Microstructure and Processing Speed in Bipolar Disorder. Am J Geriatr Psychiatry 25: 744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bracht T, Steinau S, Federspiel A, Schneider C, Wiest R, Walther S (2018): Physical activity is associated with left corticospinal tract microstructure in bipolar depression. NeuroImage Clin 20: 939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liberg B, Rahm C (2015): The Functional Anatomy of Psychomotor Disturbances in Major Depressive Disorder. Front Psychiatry 6. 10.3389/fpsyt.2015.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ng TH, Alloy LB, Smith DV (2019): Meta-analysis of reward processing in major depressive disorder reveals distinct abnormalities within the reward circuit. Transl Psychiatry 9: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berman JI, Lanza MR, Blaskey L, Edgar JC, Roberts TPL (2013): High angular resolution diffusion imaging probabilistic tractography of the auditory radiation. AJNR Am J Neuroradiol 34: 1573–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Albajes-Eizagirre A, Solanes A, Vieta E, Radua J (2019): Voxel-based meta-analysis via permutation of subject images (PSI): Theory and implementation for SDM. NeuroImage 186: 174–184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


